Abstract
Although primarily required for the growth, differentiation, and survival of mast cells, Kit ligand (stem cell factor) is also required for optimal antigen-mediated mast cell activation. Therefore, concurrent inhibition of Kit- and FcεRI-mediated signaling would be an attractive approach for targeting mast cell-driven allergic reactions. To explore this concept, we examined the effects of hypothemycin, a molecule which we identified as having such properties, in human and mouse mast cells. Hypothemycin blocked Kit activation and Kit-mediated mast cell adhesion in a similar manner to the well characterized Kit inhibitor imatinib mesylate (imatinib). In contrast to imatinib, however, hypothemycin also effectively inhibited FcεRI-mediated degranulation and cytokine production in addition to the potentiation of these responses via Kit. The effect of hypothemycin on Kit-mediated responses could be explained by its inhibition of Kit kinase activity, whereas the inhibitory effects on FcεRI-dependent signaling was at the level of Btk activation. As hypothemycin also significantly reduced the mouse passive cutaneous anaphylaxis response in vivo, these data provide proof of principle for a coordinated approach for the suppression of mast cell activation and provides a rationale for the development of compounds with a similar therapeutic profile.
Keywords: Inflammation, Immunopharmacology, Asthma
Introduction
Antigen-dependent mast cell activation is an important step in the initiation of allergic reactions including atopic asthma, allergic rhinitis and conjunctivitis, urticaria (Mekori, 2004; Galli et al., 2005), and the more complex and potentially life-threatening condition, anaphylaxis which is characterized by generalized hives and itching, airway constriction, abdominal cramps, vascular dilation and increased permeability (Brown, 2006). The early cellular responses associated with these conditions are a manifestation of the release of inflammatory mediators following IgE receptor (FcεRI) cross linking (Gilfillan and Tkaczyk, 2006; Rivera and Gilfillan, 2006). Current therapies targeting basophil/mast cell activation have largely focused on FcεRI-dependent pathways. For example, antibodies directed against IgE molecules have been shown to be of benefit in allergy treatment by downregulating the FcεRI numbers on basophils (MacGlashan et al., 1997; Saini et al., 1999) and mast cells (Beck et al., 2004).
Stem cell factor (SCF)-mediated Kit activation is required for mast cell growth and differentiation, and subsequent survival of the mature mast cells (Metcalfe et al., 1997; Kirshenbaum et al., 1999; Okayama and Kawakami, 2006). Furthermore, by the processes of chemotaxis and cell adhesion, SCF may contribute to the homing of mast cells to their eventual sites of residence (Okayama and Kawakami, 2006). It is also now recognized, however, that SCF dramatically enhances antigen-dependent mast cell degranulation, as indicated by studies conducted in both rodent and human mast cells (Bischoff and Dahinden, 1992; Hundley et al., 2004; Tkaczyk et al., 2004). Similarly, in the absence of SCF, antigen only minimally elevates cytokine message and protein levels in human mast cells, whereas in the presence of SCF, the levels are markedly enhanced (Hundley et al., 2004; Tkaczyk et al., 2004). Whether such synergy occurs in vivo is unclear, although due to the absolute requirement for SCF in mast cell homeostasis, this is likely to be the case. Indeed under conditions of low FcεRI aggregation, the relative contribution of SCF to degranulation may be greater than that of antigen (Tkaczyk et al., 2004). Therefore significant degranulation and cytokine production may occur even at the low level of antigen-dependent FcεRI aggregation that could be achieved following the anti-IgE treatment or similar approach to dampen only the FcεRI-mediated response. Thus, concurrent targeting of both Kit- and FcεRI-mediated signaling would be an attractive therapeutic approach for the treatment of mast cell-driven disorders. We, thus, wished to identify a molecule that would concurrently inhibit Kit kinase activity and the pathway described above for FcεRI with the aim of providing a basis for further investigation of this concept and potentially the development and optimization of novel compounds which may have a similar mode of action.
Both FcεRI- and Kit-dependent responses in mast cells are initiated by activation of tyrosine kinases. In the case of FcεRI, the principle tyrosine kinases responsible for these early events are the Src kinase Lyn and Syk. Lyn-dependent activation of Syk results in the phosphorylation of the transmembrane adaptor molecule LAT. This orchestrates the recruitment, and thereby activation of phospholipase (PL)Cγ1, a critical enzyme for the generation of the calcium signal required for degranulation (Gilfillan and Tkaczyk, 2006; Rivera and Gilfillan, 2006). A complementary pathway regulated by the Lyn-related kinase Fyn leads to phosphoinositide 3-kinase (PI3K) activation, thus providing membrane docking sites for signaling molecules such as the tyrosine kinase, Btk and PLCγ1. These two pathways act in conjunction for optimal degranulation and cytokine production. This latter response also requires activation of the Ras/Raf/MAPK cascade. Many of these terminal events are also regulated by Kit. However, inherent tyrosine kinase activity and multiple docking sites on Kit preclude the requirements for recruitment of cytosolic tyrosine kinases such as Syk and the adaptor molecule, LAT-mediated recruitment of PLCγ1 in these responses (Gilfillan and Tkaczyk, 2006; Rivera and Gilfillan, 2006).
Molecules that target Kit activity have been investigated for their potential therapeutic utility in the treatment of mastocytosis and several such compounds have been described in the literature (Zermati et al., 2003; Growney et al., 2005; Petti et al., 2005; Gleixner et al., 2006; Potapova et al., 2006; Schirmer et al., 2006; Schittenhelm et al., 2006; Shah et al., 2006; Verstovsek et al., 2006; Pan et al., 2007). Of these, the most widely used compound is imatinib mesylate (imatinib), also known as Gleevec, Glivec, and STI571 (Schindler et al., 2000; Scheinfeld, 2006). Due to the selective nature of these compounds (Jensen et al., 2007), it is unlikely that they will be similarly efficacious in their ability to inhibit FcεRI-mediated signaling events. We have, however, identified a molecule, the resorcylic acid lactone, hypothemycin (Schirmer et al., 2006), which irreversibly inhibits a specific subset of protein kinases, including Kit, with a conserved cysteine in the ATP-binding site (Schirmer et al., 2006; Winssinger and Barluenga, 2007). Since hypothemycin had the desired pharmacological profile, we have accordingly utilized this compound to examine the manifestations of concurrently inhibiting Kit- and FcεRI-mediated signaling in mast cells in culture and in vivo. As will be reported here, hypothemycin blocks SCF-induced mast cell activation, but, unlike imatinib, hypothemycin also effectively blocks FcεRI-mediated responses. As a consequence, not only is the antigen-mediated component of mast cell degranulation blocked, but also the ability of SCF to potentiate these responses. This profile translated into an inhibition of mast cell-induced anaphylactic responses in vivo. Thus, this study provides proof of principle for a coordinated approach for targeting mast cell-driven allergic reactions and provides a basis for the development and optimization of other compounds with a similar therapeutic profile.
Methods
Cell isolation and culturing of mast cells
Human mast cells (HuMCs) were developed from CD34+ peripheral blood mononuclear cells as described (Kirshenbaum et al., 1999). In brief, CD34+ cells, obtained after informed consent on a protocol approved by the NIAID IRB committee, were cultured in StemPro-34 culture media (Invitrogen, Carlsbad, CA) containing L-glutamine (2 mM), penicillin (100 units/ml), streptomycin (100 μg/ml) (Biofluids, Rockville, MD), IL-6 (100 ng/ml) (PeproTech Inc., Rocky Hill, NJ), and SCF (100 ng/ml) (PeproTech). IL-3 (30 ng/ml) was included for the first week of culture. Experiments were conducted 6-10 weeks after the initiation of culture, at which point, the purity of the mast cell culture was more than 99% as verified by toluidine blue staining, and detection of CD117- and FcεRI-positive cells by FACS analysis.
Mouse bone marrow-derived mast cells (BMMCs) were developed from progenitor cells obtained from the femurs of 2-6 months old C57Bl/6 mice (Jackson Laboratories, Bar Harbor, ME). The cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum, L-glutamine (4 mM), penicillin (100 units/ml), streptomycin (100 μg/ml), sodium pyruvate (1 mM), non-essential amino acids (1 mM), HEPES (25 mM), β-mercaptoethanol (50 μM), and mouse recombinant IL-3 (30 ng/ml) (Peprotech). The cell cultures were spun down4 and resuspended in fresh culture medium twice weekly. Experiments were conducted 4-6 weeks after the initiation of culture, at which point, the purity of the mast cells was more than 99% determined as above.
All animal experiments and protocols were approved by and conducted according to the guidelines of the NIAID Animal Care and Use Committee (ACUC), NIH.
Adhesion assay
HuMCs were cultured overnight in SCF- and IL-6-depleted medium. BMMCs were cultured overnight in IL-3-depleted culture medium. Cells were washed twice in HEPES + 0.04% bovine serum albumin (BSA) and 1×105 human mast cells or 2.5×105 BMMCs were seeded per well in a 96 well tissue culture plate (Falcon, BD Bioscience, San Jose, CA) pre-coated with 5 μg/ml fibronectin (Sigma-Aldrich, St. Louis, MO) and blocked with 4% BSA. The cells were pre-incubated with inhibitors for 15 min and then stimulated with 0.01-100 ng/ml SCF for 1 hour. Non-adherent cells were removed by carefully washing the wells once. Wells used for total cells were not washed. Cells were lysed with 0.1% Triton X-100 and 100 μl of the lysate was then transferred to a 96 well plate for β-hexosaminidase determination (Brown et al., 2007). The percent adherent cells were calculated as ((absorbance of sample - background) / (absorbance of total cell lysates - background)) x 100%.
IgE sensitization of cells for degranulation, cytokine release, signaling studies, and calcium
HuMCs were sensitized overnight in regular culture medium or SCF- and IL-6-depleted medium (for analysis of the SCF effect) with 100 ng/ml biotinylated human myeloma IgE (Furumoto et al., 2006) (IgE from Calbiochem, biotinylation performed by NIAID Custom Antibody Facility, NIH, MD). BMMCs were sensitized overnight in IL-3-depleted culture medium with 100 ng/ml anti-mouse monoclonal dinitrophenyl (DNP)-IgE (Sigma-Aldrich). After sensitization, both types of cells were washed 3 times with HEPES + 0.04% BSA, prior to conducting the experiments.
Degranulation
Degranulation was monitored by β-hexosaminidase release (Brown et al., 2007) preformed in a U-bottom 96 well plate (100 μl final volume). IgE-sensitized HuMCs (7×103 cells per well) or BMMCs (2.5×105 cells per well) were pre-incubated with inhibitors (0.001-10 μM) for 15 minutes prior to the addition of the stimuli. HuMCs were stimulated for 30 min with streptavidin (0-100 ng/ml) for cross linking FcεRI; and/or human SCF (0-100 ng/ml), for triggering Kit. BMMCs were similarly triggered by the addition of DNP-human serum albumin (HSA) (Sigma-Aldrich) (0-100 ng/ml) and/or murine SCF (0-100 ng/ml). The reactions were terminated by centrifugation (1000 rpm) at 4 °C and aliquots of the supernatants were then analyzed for β-hexosaminidase content (Brown et al., 2007). The cell pellets were lysed by adding 0.1% Triton X-100 and then also analyzed for β-hexosaminidase content. Degranulation was calculated as the percentage of total β-hexosaminidase content found in the supernatants following challenge.
Cytokine release and measurement
HuMCs or BMMCs (2 ×105 cells per sample) were sensitized as before, pre-incubated with inhibitors for 15 min and antigen (10 ng/ml) and/or SCF (30 ng/ml) added. The cells were then incubated for 15 hours (HuMCs) or 9 hours (BMMCs). The supernatants were harvested and the cytokine content was measured by using the DuoSet ELISA system (R & D systems, Minneapolis, MN).
Cell lysates and Western blot
BMMCs (IgE-sensitized) were incubated at 1×106 cells per sample in HEPES + 0.04% BSA at 37 °C. Cells were pre-incubated with inhibitors (0.001-10 μM) for 15 min, then stimulated with DNP-HSA (10 ng/ml) and/or SCF (30 ng/ml) for 2 or 30 min. The reaction was terminated by cell lysis as described (Tkaczyk et al., 2002). Aliquots of the lysates were loaded onto 4-12% NuPage BisTris gels (Invitrogen) and the proteins were separated by electrophoresis in MES buffer according to the manufacturer’s protocol. Following transfer onto nitrocellulose membranes, the proteins were probed with the following phospho (p)-antibodies: p-Kit 568/570, p-Kit730, p-Kit 823 and p-PLCγ1 (Tyr(P)-783) from Biosource (Invitrogen); p-Kit 703, p-Kit 721 and p-Kit 936 from Zymed (Invitrogen); p-Kit719, p-Src (Tyr(P)-416), p-AKT (Ser(P)-473), p-ERK (Thr(P)-202 and Tyr(P)-204), p-p38 (Thr(P)-180), p-JNK (Thr(P)-183 and Tyr(P)-185), p-NFκB (Ser(P)-536) and p-c-Jun (Ser(P)-73) from Cell Signaling (Beverly, MA); p-LAT (Tyr(P)-191) from Upstate (Waltham, MA); p-Btk (Tyr(P)-551) from BD Pharmingen (BD Bioscience) and c-Jun from Santa Cruz (Santa Cruz, CA).
The immunoreactive proteins were visualized by probing with horseradish peroxidase-conjugated anti-mouse (Sigma-Aldrich) or anti-rabbit IgG (Amersham Biosciences), then visualized by enhanced chemiluminescence (ECL from PerkinElmer Life Sciences, Shelton, CT). Protein loading of the samples was normalized by probing for β-actin (Sigma-Aldrich). To quantitate changes in protein phosphorylation, membranes were scanned using ChemiDoc XRS and Quantity One software (Bio-Rad Laboratories, Hercules, CA).
Measurement of intracellular calcium
BMMCs (IgE sensitized, 1×106 cells/ml) in culture medium were loaded with Fura-2 AM ester (Molecular Probes, Eugene, OR) for 30 min at 37 °C, rinsed, then resuspended in HEPES buffer containing 0.04% BSA and sulfinapyrazone (0.3 mM) (Sigma-Aldrich). The BMMCs were then placed in a 96 well black culture plate (10,000 cells/well) (CulturPlat-96 F, PerkinElmer Life Sciences), pre-incubated with the inhibitors for 15 min, then stimulated with antigen (10 ng/ml) and or SCF (30 ng/ml). The fluorescence was determined in a Wallac Victor2 1420 Multilabel Counter (PerkinElmer Life Sciences) with excitation wavelengths (340 and 380 nm) and an emission wavelength of 510 nm. After subtraction of back ground fluorescence of non-Fura-2 loaded cells, data were calculated as the ratio of fluorescence at 340 and 380 nm excitation wavelengths.
Passive cutaneous anaphylaxis
Balb/c mice (6-8 weeks old, ∼30 g, Jackson Laboratories) received intradermal injections of 1 μg mouse monoclonal anti-DNP-IgE in 25 μl of PBS in the left ear and 25 μl PBS in the right ear as a control. After 14-16 hours, the mice were injected intraperitoneal with 500 μg hypothemycin in 200 μl of the carrier hydroxylpropyl-β-cyclodextran (Cyclodextrin Technologies Development (CTD Inc, FL) or with 200 μl hydroxylpropyl-β-cyclodextran + DMSO (control for hypothemycin solvent). This concentration and dosage regimen was selected based on personal communication with Dr. Sumati Murli (Kosan Biosciences Incorporated). After 8 hours, the mice were challenged with antigen by intravenous injection of 500 μg/ml DNP-HSA and 0.5% Evans blue in 100 μl saline in the tail vein. The mice were euthanized 30 min after the intravenous injection and the ears were removed and the Evans blue was extracted in 200 μl formamide at 55 °C for 24 hours. Extravasation of Evans blue dye was quantitated by spectrophotometric analysis at 620 nm.
Other materials
Hypothemycin (KOS-1949, kindly provide by Kosan Biosciences Incorporated, CA (Schirmer et al., 2006)). Imatinib was purified by Dr. Elizabeth Greiner from Laboratory of Medical Chemistry NIDDK, NIH (Seggewiss et al., 2005).
Statistics
Data were analyzed by a two-tailed Student’s t-test. The levels of significance were as follows; *: 0.01 < p < 0.05, **: 0.001 < p < 0.01, ***: p < 0.001.
Results
Hypothemycin and Kit phosphorylation
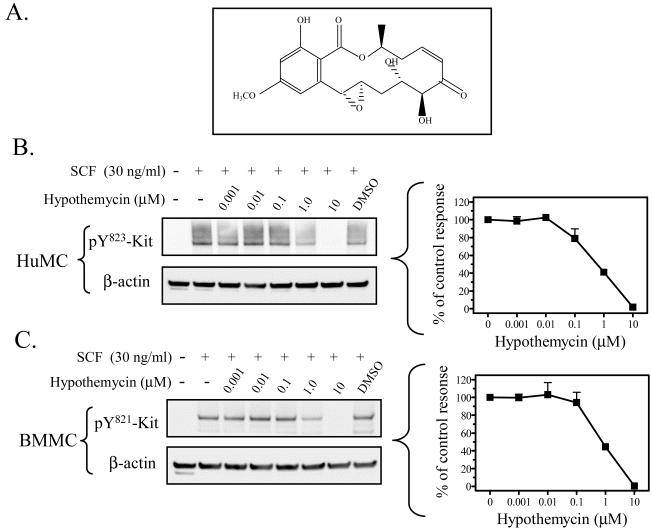
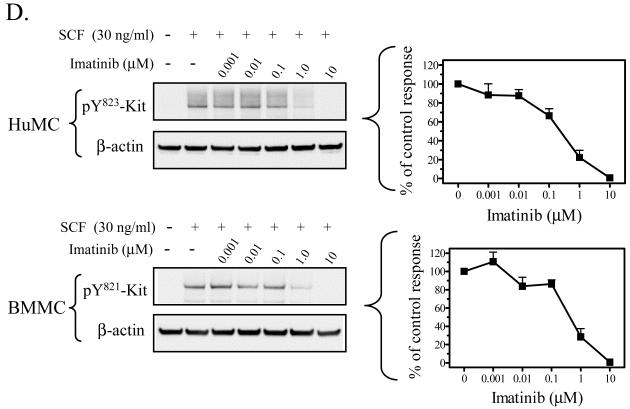
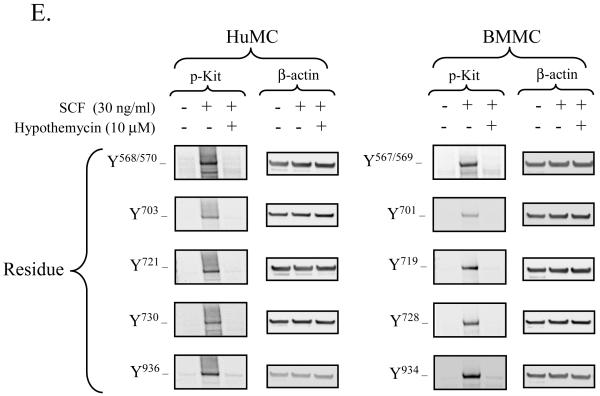
Hypothemycin (Figure 1A) has been reported to inhibit Kit activity in a cell-free assay (Schirmer et al., 2006). We therefore initially confirmed that hypothemycin could block Kit activation and function in mast cells. Kit activation, following ligation by SCF, results in the phosphorylation of several tyrosine residues in the cytosolic tail of Kit which, in human mast cells (or mouse), includes Y568 (567) and Y570 (569) in the juxtamembrane region; 703 (701), 721 (719), 730 (728) in the kinase insert domain; 823 (821), and 900 (898), 936 (934) in the distal kinase domain (Linnekin, 1999; Roskoski, 2005b; Roskoski, 2005a; Reber et al., 2006). We thus compared the effects of hypothemycin to that of imatinib, in its ability to inhibit Kit phosphorylation following SCF challenge in HuMCs and BMMCs. As seen in Figure 1B and C, hypothemycin inhibited the phosphorylation of Kit (tyrosine 823; 821 in mouse) when stimulated with 30 ng/ml SCF in a concentration-dependent manner with complete inhibition observed at 10 μM. These responses were comparable to those obtained with imatinib as shown in Figure 1D, where 10 μM also completely blocked the phosphorylation of Kit. The optimal concentration of hypothemycin (10 μM) also blocked the phosphorylation of the other known tyrosine phosphorylation sites on human (or mouse) Kit: residues 568/570 (567/569), 703 (701), 721 (719), 730 (728) and 936 (934) (Figure 1E).
Figure 1. Hypothemycin and its effect on Kit phosphorylation.
A) The molecular structure of hypothemycin (KOS-1949). Inhibition of the SCF-induced phosphorylation of Kit in HuMCs and BMMCs by hypothemycin (B, C, and E) or imatinib (D). Cells were pre-treated with inhibitors 15 min prior to SCF (30 ng/ml) challenge for 2 min. The blots are representative of n=3. DMSO in a concentration equal to 10 μM hypothemycin is used as solvent control. β-actin was used as loading control. The graphs, which represent the means +/- S.E.M. of n=3 experiments, were generated by scanning of blots and then normalized to the SCF response in the absence of inhibitors.
Hypothemycin and SCF-mediated adhesion
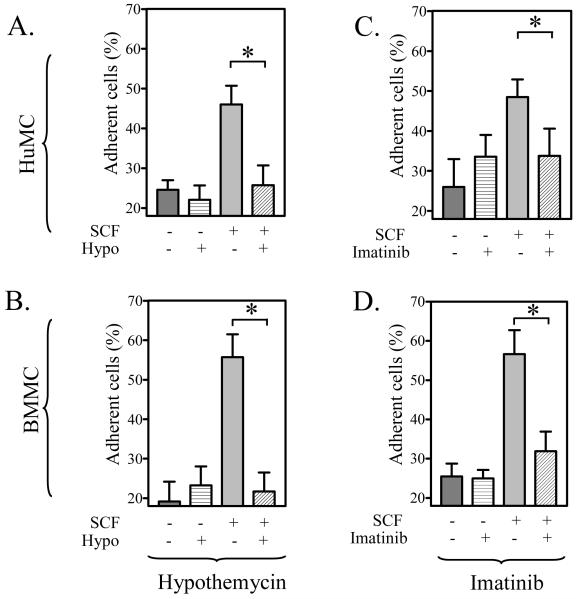
To next establish that hypothemycin could inhibit a recognized SCF-induced mast cell response, we examined its ability to block SCF-mediated adhesion of the cells to fibronectin-coated plates. From Figure 2A and B, it can be seen that hypothemycin significantly inhibited HuMC and BMMC adhesion. This inhibition had a similar concentration dependency (data not shown) as the effects of hypothemycin on Kit phosphorylation. Similarly, we have observed that hypothemycin (10 μM) completely blocks SCF-mediated chemotaxis in BMMCs (M.S. Kim and A.M. Gilfillan, unpublished observations). These results which reflected the data obtained with imatinib (Figure 2C,D) confirmed that hypothemycin could inhibit Kit-activation and a Kit-mediated responses in both human and mouse mast cells.
Figure 2. The comparative effects of hypothemycin and imatinib on mast cell adhesion.
HuMCs (A, C) and BMMCs (B, D) were pre-incubated with hypothemycin (10 μM) (A, B) or imatinib (10 μM) (C, D) 15 min prior to challenging the cells with SCF (10 ng/ml). The adhesion assays were then conducted as described in “Methods”. The data are means +/- S.E.M. of n=4. The effect of hypothemycin or imatinib at 10 μM reached statistical significance (*: 0.01 < p < 0.05). The effects of hypothemycin were observed over a similar concentration range (data not shown) as that required to inhibit Kit phosphorylation (see Figure 1).
The effect of hypothemycin on mast cell degranulation
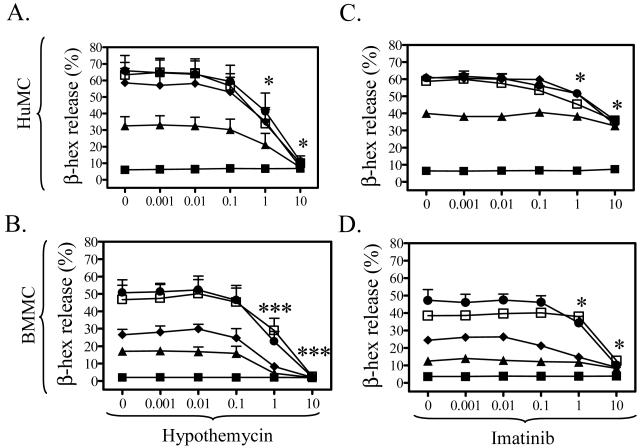
We next explored the ability of this compound to inhibit the enhancement of FcεRI-mediated degranulation by SCF. As described previously (Hundley et al., 2004; Tkaczyk et al., 2004), SCF markedly enhanced FcεRI-mediated degranulation in HuMCs and BMMCs respectively with maximal enhancement being observed between 10 and 100 ng/ml SCF (Figure 3A to D). SCF alone failed to induce degranulation in the absence of other stimuli (data not shown). As shown in Figure 3A and B, hypothemycin blocked the synergistic effects of SCF and streptavidin in HuMCs, and DNP-HSA in BMMCs. At 10 μM, hypothemycin reduced degranulation to basal levels. In contrast, imatinib only reduced degranulation to the level of that produced by antigen alone (Figure 3C,D). These studies also demonstrated that, whereas both hypothemycin and imatinib effectively blocked the Kit-mediated component of the degranulation response, hypothemycin, but not imatinib, also effectively blocked the FcεRI-mediated component.
Figure 3. The comparative effects of hypothemycin and imatinib on mast cell degranulation.
The inhibition by hypothemycin (A, B) and imatinib (C, D) of the synergistic enhancement of the antigen response by SCF in HuMCs (A, C) and BMMCs (B, D). The DMSO concentrations used to solubilize hypothemycin had no effect on degranulation (data not shown). Sensitized cells were pre-treated with inhibitors for 15 min and then HuMCs and BMMCs were challenged with streptavidin or DNP-HSA (1 ng/ml each) (Ag), respectively, in the absence or presence of SCF for 30 min. The data are means +/- S.E.M. of n=3. ∎: No Ag and SCF, ▴: Ag + no of SCF, ◆: Ag + 1 ng/ml SCF. ●: Ag + 10 ng/ml SCF, and ◻: Ag + 100 ng/ml SCF. Where indicated, the values obtained in the presence of hypothemycin were statistically different from those in the absence of hypothemycin (*: 0.01 < p < 0.05, ***: p < 0.001).
The above effects of hypothemycin were determined not to be due to cytotoxicity, as up to 9 hours incubation with hypothemycin did not affect cell viability as assessed by trypan blue exclusion. Furthermore, the effects were also not due to the DMSO carrier, as DMSO at the concentrations used to deliver hypothemycin were without effect. Finally, these effects were also not due to alterations in the expression of FcεRI or Kit as determined by FACS (above data not shown).
The effect of hypothemycin on mast cell cytokine release
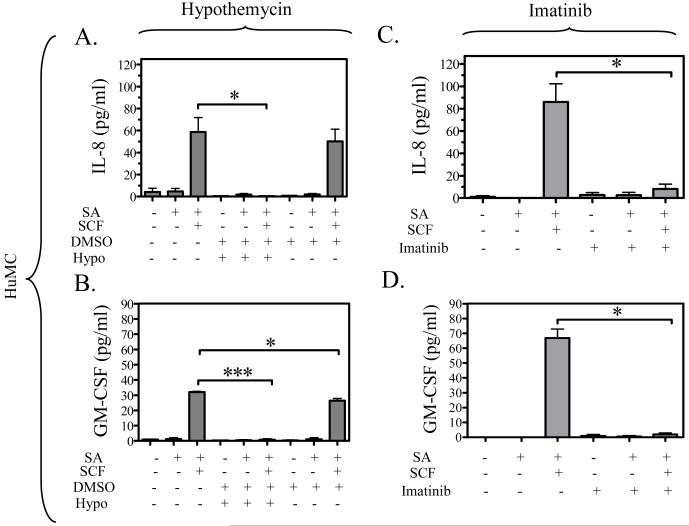
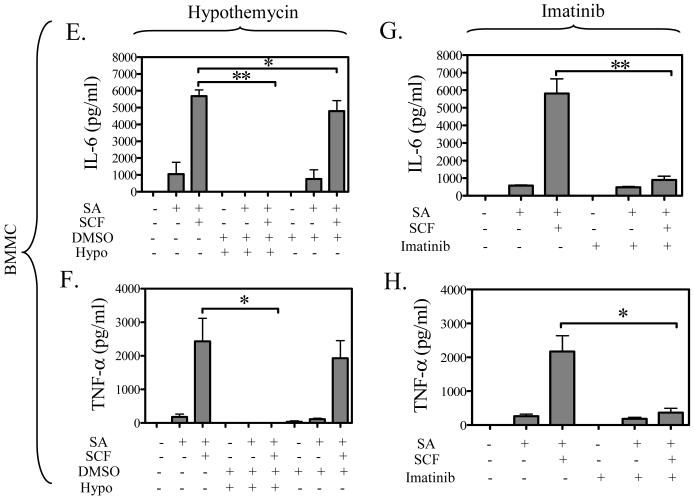
To explore the effect of hypothemycin on cytokine production, we examined the production of IL-8 and GM-CSF in HuMCs, and IL-6 and TNF-α in BMMCs based on previous cytokine expression profiles (Okayama et al., 2001; Hundley et al., 2004). As reported in these studies, FcεRI aggregation in the absence of SCF minimally induces cytokine production in both HuMCs (Figure 4A to D) and BMMCs (Figure 4E,H). However, in the presence of SCF, there is a marked potentiation of the release of IL-8 (Figure 4A,C) and GM-CSF (Figure 4B,D) in the HuMCs, and IL-6 (Figure 4E,G) and TNF-α (Figure 4F,H) in the BMMCs. The release of these cytokines when stimulated with antigen plus SCF was blocked by 10 μM hypothemycin, as shown in Figure 4A, B, E, and F. Again, the DMSO carrier had minimal affect on these responses. Hypothemycin also appeared to reduce the amount of cytokines produced in response to antigen but due to the small magnitude of release, the differences did not reach statistical significance. Imatinib did not appear to affect antigen-induced cytokine release (Figure 4C,D,G,H). The above data thus demonstrate that, unlike imatinib, hypothemycin not only effectively blocks the Kit-mediated component, but also the FcεRI-mediated component of the synergistically enhanced mast cell cytokine production in response to SCF and antigen.
Figure 4. The comparative effects of hypothemycin and imatinib on FcεRIand/or Kit-induced cytokine release in mast cells.
HuMCs were pre-treated with hypothemycin (Hypo; 10 μM) (A, B) or imatinib (10 μM) (C, D) for 15 min and then stimulated with streptavidin (SA) (100 ng/ml) and/or SCF (30 ng/ml) for 15 hours. The supernatants were measured for IL-8 (A, C) and GM-CSF (B, D). BMMCs were pre-treated with hypothemycin (Hypo; 10 μM) (E, F) or imatinib (10 μM) (G, H) for 15 min and then stimulated with DNP-HSA (DNP; 10 ng/ml) and/or SCF (30 ng/ml) for 9 hours. The supernatants were measured for IL-6 (E, G) and TNF-α (F, H). Each cytokine value is the means +/- S.E.M. of n=3 experiments. Where indicated, the values obtained in the presence of hypothemycin were statistically different from those in the absence of hypothemycin (*: 0.01 < p < 0.05, **: 0.001 < p < 0.01, ***: p < 0.001). DMSO had a minimal effect on human mast cell IL-8 release and the BMMC IL-6 release.
Hypothemycin and its intracellular targets
Since hypothemycin clearly inhibits the Kit response by acting on Kit kinase but inhibits the FcεRI response by an unknown mechanism, we investigated the target for hypothemycin in the FcεRI signaling pathway. To facilitate these studies, we examined these responses in BMMCs in view of the identical effects of hypothemycin in human and mouse mast cells. Where examined, imatinib inhibited all responses induced by SCF but failed to influence responses mediated by antigen (data not shown).
Early signaling events
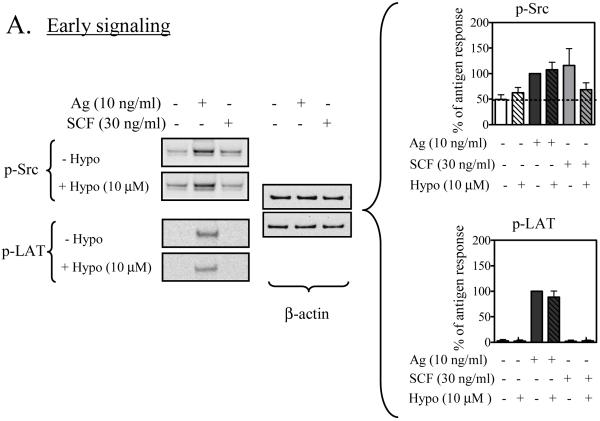
We initially examined the effects of hypothemycin on early signaling events which regulate both degranulation and cytokine production: namely phosphorylation of Src kinases, which plays a role in both the Kit and FcεRI response (Blank and Rivera, 2004; Gilfillan and Tkaczyk, 2006; Rivera and Gilfillan, 2006), and of the adaptor molecule LAT, which plays a major role in the FcεRI response (Tkaczyk et al., 2004). We have previously demonstrated that the anti-phospho-Src antibody utilized in these studies primarily recognizes phosphorylation of the Src kinase, Lyn (Iwaki et al., 2005). As shown in Figure 5A, and as previously demonstrated (Tkaczyk et al., 2004), antigen (lane 2) induced an increase in the phosphorylation of Lyn and LAT. These responses were not inhibited by an optimal concentration of hypothemycin (10 μM). SCF only slightly increased the phosphorylation of Lyn (lane 3), which, however, was reduced by hypothemycin. These data suggest that hypothemycin does not affect FcεRI signaling at the level of these responses and, by inference, upstream events including FcεRI aggregation / phosphorylation and Syk activation which lead to the phosphorylation of LAT.
Figure 5. The effect of hypothemycin on early and intermediate FcεRI- and/or Kit-mediated signaling events in BMMCs.
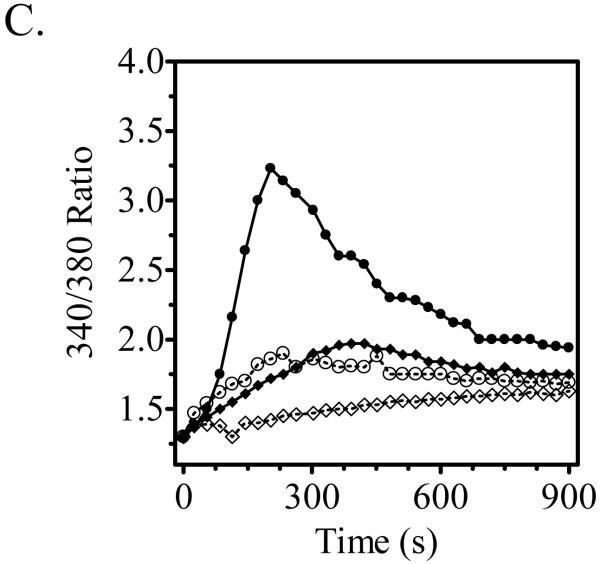
Sensitized BMMCs were pre-treated with 10 μM hypothemycin (Hypo) or buffer for 15 min and then stimulated for 2 min with antigen (Ag; 10 ng/ml DNP-HSA) or SCF (30 ng/ml). The extracted proteins were probed for phospho (p)-proteins; (A) p-Src and p-LAT, (B) p-AKT, p-Btk and p-PLCγ. DMSO in a concentration equal to 10 μM hypothemycin did not alter the phosphorylation. β-actin was used as loading control. The histograms, which represent the means +/- S.E.M. of n=3 experiments, were generated by scanning of blots and then normalized to the antigen response. The dashed line represents the constitutive phosphorylation in the cells. Where indicated, the values obtained in the presence of hypothemycin were statistically different from those in the absence of hypothemycin (*: 0.01 < p < 0.05, **: 0.001 < p < 0.01, ***: p < 0.001). BMMCs were analyzed for calcium mobilization (C). Sensitized cells were loaded with Fura-2 AM ester, pre-incubated with 10 μM hypothemycin for 15 min, and then stimulated with DNP-HSA (10 ng/ml) and/or SCF (30 ng/ml). The result is a representative of 3 experiments. ●: DNP-HSA,: ◆: SCF, ○: DNP-HSA + 10 μM hypothemycin, ◇: SCF + 10 μM hypothemycin.
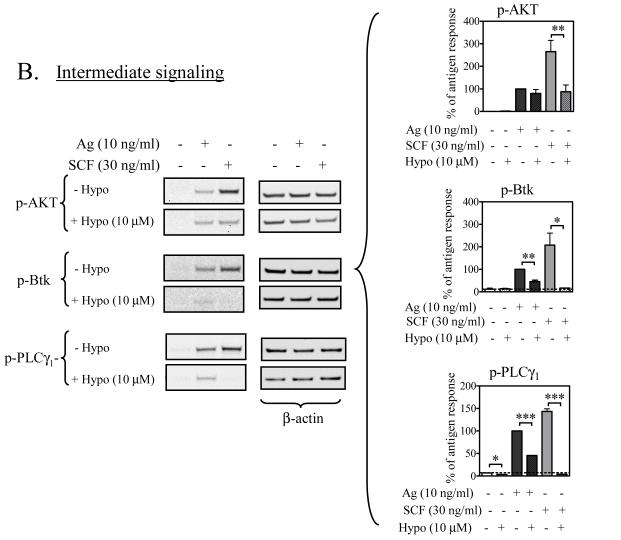
Intermediate signaling events
We next examined downstream events, namely: PI3K activation, as monitored by the surrogate marker, AKT phosphorylation (Tkaczyk et al., 2003), Btk activation as monitored by its phosphorylation (Iwaki et al., 2005), and PLCγ1 activation as monitored by its phosphorylation and calcium mobilization (Tkaczyk et al., 2003) (Figure 5B,C). We have previously demonstrated that the latter two responses may play a role in the integration of the signals produced by FcεRI and Kit for the synergistic enhancement of mast cell degranulation (Hundley et al., 2004; Iwaki et al., 2005). As previously demonstrated (Hundley et al., 2004; Tkaczyk et al., 2004; Iwaki et al., 2005), both antigen and SCF induced the phosphorylation of AKT, Btk, and PLCγ1. As with the upstream events, hypothemycin failed to inhibit the phosphorylation of AKT in response to antigen (Figure 5B). However, consistent with the notion that hypothemycin inhibits Kit directly and FcεRI-mediated signaling downstream of PI3K, SCF-induced AKT phosphorylation was substantially reduced. These data, thus, suggested that PI3K was not the target for hypothemycin in antigen-stimulated cells. In contrast to these results, however, Btk phosphorylation induced by either antigen or SCF was markedly inhibited. Furthermore, PLCγ1 phosphorylation in response to antigen was also substantially reduced under these conditions (Figure 5B). Thus, hypothemycin appears to be mediating its effects at the level of Btk phosphorylation/activation with consequential downstream inhibition of PLCγ1.
To further confirm this conclusion, we examined the ability of hypothemycin to block intracellular calcium mobilization in response to antigen or SCF (Figure 5C). As reported (Hundley et al., 2004), both antigen and SCF induced an increase in calcium mobilization, with the response to SCF being markedly lower and slower than that observed by antigen. Degranulation and critical signaling events leading to this response occur within 5 minutes of challenge. When examined over this time frame, antigen- and SCF-induced calcium mobilization was substantially inhibited by an optimal concentration of hypothemycin (10 μM) (Figure 5C). In total, the data suggest that hypothemycin blocks degranulation by its ability to inhibit at the level of Btk, and hence, PLCγ1 activation and calcium mobilization.
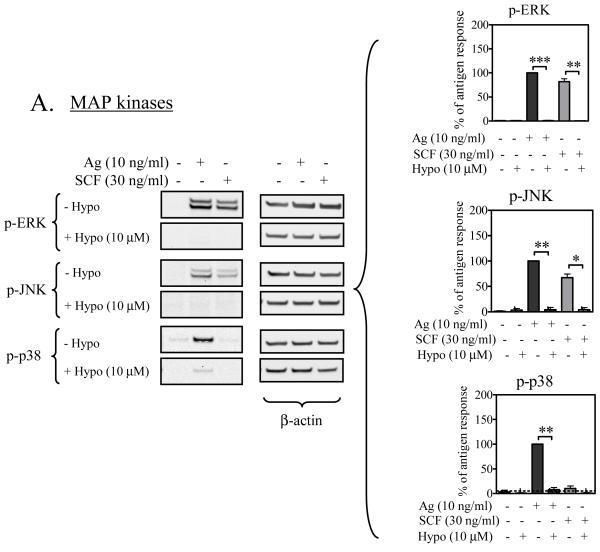
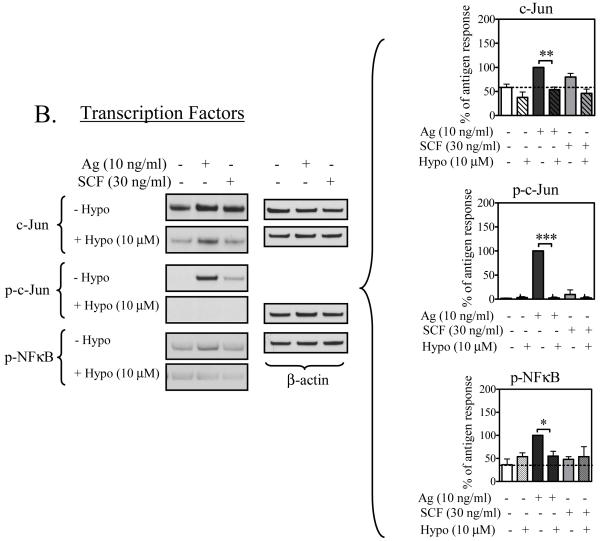
MAP Kinases and transcription factors
Cytokine production downstream of Btk appears to occur via activation of the MAP kinase cascades (Gilfillan and Tkaczyk, 2006). We thus next examined whether or not hypothemycin inhibits antigen- and SCF-mediated cytokine production through an inhibition of these events and, as a consequence, the expression/phosphorylation of downstream transcription factors that are known to promote cytokine production in mast cells (Hundley et al., 2004; Qiao et al., 2006). As seen in Figure 6A, the increase in the phosphorylation of the MAP kinases ERK1/2, JNK, and p38 induced by antigen, or ERK1/2 and JNK, enhanced by SCF, were virtually abrogated by an optimal concentration of hypothemycin (10 μM). Similarly, the induction of the AP1 transcription complex component c-Jun and its phosphorylation was also markedly inhibited by hypothemycin (Figure 6B). Furthermore, the antigen-induced phosphorylation of NFκB was also inhibited by hypothemycin (Figure 6B). Thus, these data support the conclusion that hypothemycin inhibits cytokine production in response to antigen and SCF as a consequence of the inhibition of the activation of MAP kinases and downstream transcription factors.
Figure 6. The effect of hypothemycin on FcεRI- and or Kit-mediated MAP kinase activation and phosphorylation of transcription factors in BMMCs.
Sensitized BMMCs were pre-treated with 10 μM hypothemycin (Hypo) or buffer for 15 min (106 cells per sample) and then stimulated for 2 min (MAP kinases) or 30 min (transcription factors) with antigen (Ag; 10 ng/ml DNP-HSA) or SCF (30 ng/ml). The extracted proteins were probed for phospho (p)-proteins; (A) p-ERK, p-p38 and p-JNK; (B) c-Jun, p-c-Jun and p-NFκB. DMSO in a concentration equal to 10 μM hypothemycin did not alter the phosphorylation. β-actin was used as loading control. The histograms, which represent the means +/- S.E.M. of n=3 experiments, were generated by scanning of blots and then normalized to the antigen response. The dashed line represents the constitutive phosphorylation in the cells. Where indicated, the values obtained in the presence of hypothemycin were statistically different from those in the absence of hypothemycin (*: 0.01 < p < 0.05, **: 0.001 < p < 0.01, ***: p < 0.001).
The effect of hypothemycin on mast cell degranulation in vivo using passive cutaneous anaphylaxis
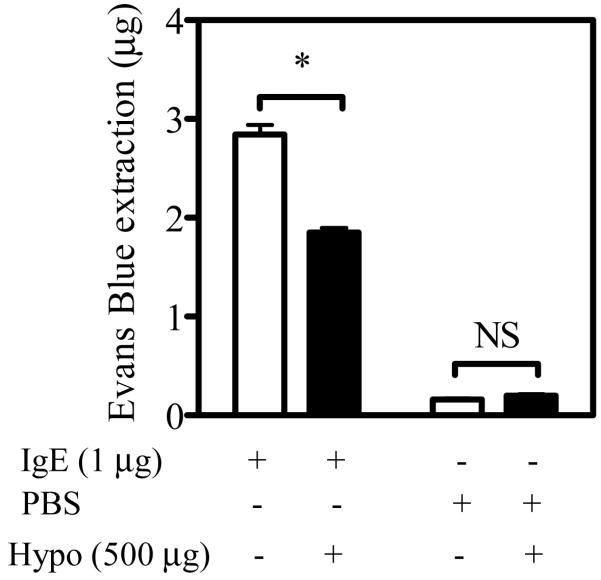
Finally, to determine whether or not the inhibitory effects of hypothemycin in mast cells in culture extended to mast cells in vivo, we examined the ability of hypothemycin to reverse passive cutaneous anaphylaxis in the ears of mice. This is primarily an FcεRI/mast cell-dependent allergic reaction reflecting a mast cell mediator-induced increase in vascular permeability (Strait et al., 2002). As seen in Figure 7, mice treated with hypothemycin had a significant reduction in the anaphylactic response in IgE-treated ears compared to ears treated with PBS. The degree of inhibition observed is similar to that reported in mice deficient/defective in the critical signaling molecules Gab2 and P110δ (Gu et al., 2001; Ali et al., 2004). Our data thus demonstrate that mast cell responses in vivo could indeed be reduced by co-inhibiting FcεRI and Kit responses.
Figure 7. The effect of hypothemycin on in vivo passive cutaneous anaphylaxis.
Balb/c mice (∼30 g) sensitized with IgE in the left ear and PBS in the right ear were treated for 8 hours with 500 μg hypothemycin (Hypo) or its solvent β-cyclodextran. Antigen challenge was done by tail vein injections with 500 μg/ml DNP-HSA in 0.5% Evans blue solution. Thirty min after injection, the mice were sacrificed, the ears were removed and Evans blue dye was extracted, and quantitated by absorption. The figure shows the means +/- S.E.M. of n=3 experiments (each containing 3 control mice and 3 hypothemycin-treated mice). Where indicated, the values obtained in the presence of hypothemycin were statistically different from those in the absence of hypothemycin (*: 0.01 < p < 0.05).
Discussion
In this study, we have sought to examine the concept that concurrent inhibition of Kit- and FcεRI-mediated signaling may provide a coordinated suppression of mast cell activation. Of the known Kit inhibitors, the most widely investigated is imatinib. This agent has been described as a relatively selective inhibitor that, besides Kit, affects the activity of Abelson cytoplasmic tyrosine kinase and the platelet-derived growth factor receptor (Heinrich et al., 2000; Scheinfeld, 2006). It has been reported that imatinib decreases allergic peribronchial eosinophil accumulation, airway hyperreactivity, and cytokine levels in vivo (Berlin and Lukacs, 2005; Berlin et al., 2006), although in mast cell explant in culture anti-IgE-mediated histamine release was unaffected (Beck et al., 2004). However, as with other compounds which target Kit, imatinib has not been reported to inhibit any of the kinases involved in the FcεRI signaling pathway. In contrast, as reported here, hypothemycin not only blocks Kit- but also FcεRI-mediated signaling and therefore appeared to be an attractive compound to test the efficacy of co-inhibition of Kit- and FcεRI-mediated responses.
The ability of hypothemycin to inhibit Kit kinase activation was confirmed by the inhibition of the SCF-induced autophosphorylation of critical tyrosine residues within the cytosolic domain of Kit (Figure 1). As these serve as docking sites for SH2 domain-containing signaling molecules which mediate the downstream signaling of Kit, SCF-mediated signaling events including Btk phosphorylation, AKT phosphorylation, and PLCγ1 phosphorylation leading to calcium mobilization were also inhbited (Figure 5). The inhibition of these signalling events account for the ability of hypothemycin to effectively inhibit SCF-mediated mast cell adhesion (Figure 2), chemotaxis (data not shown) and, as with imatinib, the ability of SCF to potentiate antigen-mediated degranulation (Figure 3) and cytokine production (Figure 4). Unlike imatinib, however, hypothemycin also blocked the ability of antigen to induce both degranulation and cytokine production, thus demonstrating the ability of hypothemycin to inhibit the FcεRI-, as well in addition to the Kit-mediated signaling pathway. As hypothemycin was relatively ineffective at inhibiting Src kinases and Syk when screened against a panel of kinases (Schirmer et al., 2006), these data, and our observations that hypothemycin does not block antigen-mediated phosphorylation of Src kinases and LAT (Figure 5), indicated that the target of hypothemycin in the FcεRI-dependent signaling cascade was downstream from these initial signaling events and, by inference, FcεRI aggregation and phosphorylation. Moreover, as hypothemycin failed to inhibit AKT phosphorylation (Figure 5), it appears that the target is also downstream of PI3K.
As antigen-mediated Btk phosphorylation, as well as downstream signalling events, were markedly suppressed (Figure 5), hypothemycin most likely inhibits FcεRI-mediated degranulation at the level of Btk activation. However, resting Btk kinase activity was not blocked in an in vitro kinase assay (Schirmer et al., 2006), indicating that Btk is not directly inhibited by hypothemycin. Instead, hypothemycin may inhibit a subsidiary cryptic event associated with the phosphorylation and activation of Btk downstream of the activation of Lyn, Syk, and PI3K. Regardless, the data clearly indicate that Btk phosphorylation is the first recognizable step in the FcεRI-mediated signaling cascade inhibited by hypothemycin (Figure 8).
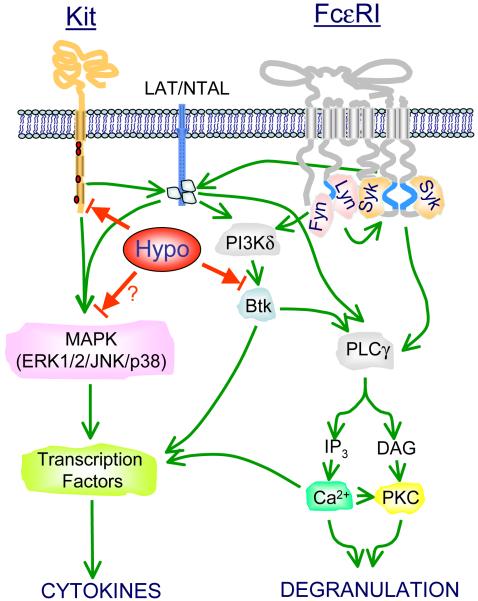
Figure 8. Proposed sites of action of hypothemycin on SCF- and antigen-mediated mast cell activation.
For clarity, many of the intermediate signaling events have been omitted. The readers are referred to (Gilfillan and Tkaczyk, 2006)) for further details. Hypothemycin (Hypo) inhibits Kit-mediated signaling and the potentiation of antigen-mediated degranulation and cytokine production by directly inhibiting Kit kinase activity. In contrast, hypothemycin does not inhibit early FcεRI-mediated responses including activation of Src kinases, phosphorylation of the transmembrane adaptor molecules LAT and NTAL, and the activation of PI3K. The ability of hypothemycin to inhibit FcεRI-mediated mast cell activation appears to be due to the inhibition of the phosphorylation and thus activation of Btk. This, in turn, results in the consequential inhibition of PLCγ1-dependent calcium mobilization, a critical signal for both degranulation and activation of specific transcription factors. It may also be possible, however, that inhibition of the MAPK cascade may also contribute to the downregulation of cytokine production. The green lines represent positive pathways and the red lines, negative pathways.
Data obtained with mast cells derived from Btk-/- mice have demonstrated that Btk is a critical enzyme for optimal phosphorylation of PLCγ1 calcium mobilization, degranulation, and cytokine production in mast cells (Hata et al., 1998; Iwaki et al., 2005). We have, furthermore, previously demonstrated that Btk is also important for the ability of SCF to potentiate antigen-dependent mast cell degranulation (Iwaki et al., 2005). This may be partially explained by Btk providing a mechanism for amplification of the PLCγ1-dependent calcium signal (Iwaki et al., 2005). Thus, the observed ability of hypothemycin to attenuate the antigen induced increase in PLCγ1 phosphorylation, calcium mobilization, and subsequently, degranulation, is entirely consistent with our conclusion that hypothemycin in blocking the FcεRI-mediated signaling cascade at the level of Btk. The suppression of cytokine production by hypothemycin could also be attributable to inhibition of Btk. Indeed, it has been previously demonstrated that antigen-mediated cytokine production is defective in Btk-/- BMMCs (Hata et al., 1998). However it is possible that hypothemycin may also be blocking cytokine production by inhibiting the activation of MAP kinases. Regardless, the net outcome of either or both of these mechanisms would account for the observed inhibition of elevated phosphorylation of transcription factors in response to antigen and SCF (Figure 6).
The ability of hypothemycin to inhibit the synergistic release of mast cell activation in culture translated into a significant inhibition of the antigen-induced passive cutaneous anaphylaxis response in mice (Figure 7), demonstrating its efficacy against a mast cell-driven allergic reaction in vivo. The residual response observed in these studies, may however reflect the need to optimize the bioavailability and dosage regimen of potential inhibitory molecules such as hypothemycin. Future studies to expand the therapeutic repertoire for a coordinated approach targeting Kit and FcεRI-mediated signaling would require testing of hypothemycin in a more complex model, for example in a mouse asthma mouse of allergic airway inflammation. This would provide information on how hypothemycin would affect a more complex allergic reaction involving multiple cell types. However, the further properties of hypothemycin to inhibit SCF dependent mast cell adhesion and chemotaxis would suggest that, in addition to inhibiting mast cell degranulation, hypothemycin may also prevent mast cell infiltration into sites of inflammation.
In summary, the results presented in this study provide proof of principle for the concept of concurrent inhibition of Kit- and FcεRI-mediated signaling as an approach for inhibition of mast cell-driven allergic reactions. To test this concept, we have utilized a molecule, hypothemycin, which inhibits both responses, with the aim of providing a basis for further investigation of this concept and potentially the development and optimization of novel compounds which may have a similar mode of action. Although hypothemycin irreversibly inhibits a specific subset of protein kinases, these properties may have inherent therapeutic advantages especially when used topically in disorders such as atopic asthma and dermatitis. As noted here, the ability to suppress both Kit and FcεRI-mediated signals at multiple levels would allow maximum suppression of antigen-induced release of inflammatory mediators and possibly other physiological functions such as migration and maturation of mast cells. An alternative approach however could be the concurrent administration of separate compounds targeting Kit- and FcεRI-specific signaling cascades. This latter approach may help to minimize potential issues with specificity of targeting.
Acknowledgements
The authors would like to thank Dr. Sumati Murli and others at Kosan Biosciences Incorporated for the supply of hypothemycin and advice regarding this study, and Dr. Elizabeth Greiner, NIH for supplying purified imatinib mesylate.
This work was supported by the NIAID and NHLBI Intramural Programs of the National Institutes of Health.
Abbreviations
- Ag
antigen
- β-hex
β-hexosaminidase
- BMMC
mouse bone marrow-derived mast cell
- BSA
bovine serum albumin
- DNP
dinitrophenyl
- FcεRI
the high affinity receptor for IgE
- GM-CSF
granulocyte macrophage colony stimulating factor
- HSA
human serum albumin
- HuMC
human mast cell
- Hypo
hypothemycin
- IgE
immunoglobulin E
- IL
interleukin
- LAT
linker for activation of T cells
- MAP
mitogen-activated protein
- PBS
phosphate buffered saline
- PI3K
phosphoinositide 3-kinase
- PLC
phospholipase C
- SCF
stem cell factor
- S.E.M.
standard error of mean
- TNF
tumor necrosis factor
Footnotes
The authors have no financial relationships with a biotechnology or pharmaceutical manufacturer that has an interest in the materials or subject matter discussed.
References
- Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110δ phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell FcεRI expression and function. J Allergy Clin Immunol. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Berlin AA, Hogaboam CM, Lukacs NW. Inhibition of SCF attenuates peribronchial remodeling in chronic cockroach allergen-induced asthma. Lab Invest. 2006;86:557–565. doi: 10.1038/labinvest.3700419. [DOI] [PubMed] [Google Scholar]
- Berlin AA, Lukacs NW. Treatment of cockroach allergen asthma model with imatinib attenuates airway responses. Am J Respir Crit Care Med. 2005;171:35–39. doi: 10.1164/rccm.200403-385OC. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Dahinden CA. c-kit ligand: a unique potentiator of mediator release by human lung mast cells. J Exp Med. 1992;175:237–244. doi: 10.1084/jem.175.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Brown JM, Swindle EJ, Kushnir-Sukhov NM, Holian A, Metcalfe DD. Silica-directed mast cell activation is enhanced by scavenger receptors. Am J Respir Cell Mol Biol. 2007;36:43–52. doi: 10.1165/rcmb.2006-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SG. Anaphylaxis: clinical concepts and research priorities. Emerg Med Australas. 2006;18:155–169. doi: 10.1111/j.1742-6723.2006.00831.x. [DOI] [PubMed] [Google Scholar]
- Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven MA, Gilfillan AM, Rivera J. Cutting Edge: Lentiviral short hairpin RNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- Gleixner KV, Mayerhofer M, Aichberger KJ, Derdak S, Sonneck K, Bohm A, Gruze A, Samorapoompichit P, Manley PW, Fabbro D, Pickl WF, Sillaber C, Valent P. PKC412 inhibits in vitro growth of neoplastic human mast cells expressing the D816V-mutated variant of KIT: comparison with AMN107, imatinib, and cladribine (2CdA) and evaluation of cooperative drug effects. Blood. 2006;107:752–759. doi: 10.1182/blood-2005-07-3022. [DOI] [PubMed] [Google Scholar]
- Growney JD, Clark JJ, Adelsperger J, Stone R, Fabbro D, Griffin JD, Gilliland DG. Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412. Blood. 2005;106:721–724. doi: 10.1182/blood-2004-12-4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Saito K, Klaman LD, Shen J, Fleming T, Wang Y, Pratt JC, Lin G, Lim B, Kinet JP, Neel BG. Essential role for Gab2 in the allergic response. Nature. 2001;412:186–190. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- Hata D, Kawakami Y, Inagaki N, Lantz CS, Kitamura T, Khan WN, Maeda-Yamamoto M, Miura T, Han W, Hartman SE, Yao L, Nagai H, Goldfeld AE, Alt FW, Galli SJ, Witte ON, Kawakami T. Involvement of Bruton’s tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–932. [PubMed] [Google Scholar]
- Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcεRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven MA, Metcalfe DD, Gilfillan AM. Btk plays a crucial role in the amplification of FcεRI-mediated mast cell activation by Kit. J Biol Chem. 2005;280:40261–40270. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
- Jensen BM, Metcalfe DD, Gilfillan AM. Targeting Kit activation: A potential therapeutic approach in the treatment of allergic inflammation. Inflamm Allergy Drug Targets. 2007;6:57–62. doi: 10.2174/187152807780077255. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int J Biochem Cell Biol. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- MacGlashan DW, Jr., Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, Sterbinsky SA, Hamilton RG, Lichtenstein LM. Down-regulation of FcεRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- Mekori YA. The mastocyte: the “other” inflammatory cell in immunopathogenesis. J Allergy Clin Immunol. 2004;114:52–57. doi: 10.1016/j.jaci.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Okayama Y, Hagaman DD, Metcalfe DD. A comparison of mediators released or generated by IFN-γ-treated human mast cells following aggregation of FcγRI or FcεRI. J Immunol. 2001;166:4705–4712. doi: 10.4049/jimmunol.166.7.4705. [DOI] [PubMed] [Google Scholar]
- Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Quintas-Cardama A, Kantarjian HM, Akin C, Manshouri T, Lamb P, Cortes JE, Tefferi A, Giles FJ, Verstovsek S. EXEL-0862, a novel tyrosine kinase inhibitor, induces apoptosis in vitro and ex vivo in human mast cells expressing the KIT D816V mutation. Blood. 2007;109:315–322. doi: 10.1182/blood-2006-04-013805. [DOI] [PubMed] [Google Scholar]
- Petti F, Thelemann A, Kahler J, McCormack S, Castaldo L, Hunt T, Nuwaysir L, Zeiske L, Haack H, Sullivan L, Garton A, Haley JD. Temporal quantitation of mutant Kit tyrosine kinase signaling attenuated by a novel thiophene kinase inhibitor OSI-930. Mol Cancer Ther. 2005;4:1186–1197. doi: 10.1158/1535-7163.MCT-05-0114. [DOI] [PubMed] [Google Scholar]
- Potapova O, Laird AD, Nannini MA, Barone A, Li G, Moss KG, Cherrington JM, Mendel DB. Contribution of individual targets to the antitumor efficacy of the multitargeted receptor tyrosine kinase inhibitor SU11248. Mol Cancer Ther. 2006;5:1280–1289. doi: 10.1158/1535-7163.MCT-03-0156. [DOI] [PubMed] [Google Scholar]
- Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcεR1 and Toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol. 2006;533:327–340. doi: 10.1016/j.ejphar.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr. Signaling by Kit protein-tyrosine kinase - the stem cell factor receptor. Biochem Biophys Res Commun. 2005a;337:1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Roskoski R., Jr. Structure and regulation of Kit protein-tyrosine kinase - the stem cell factor receptor. Biochem Biophys Res Commun. 2005b;338:1307–1315. doi: 10.1016/j.bbrc.2005.09.150. [DOI] [PubMed] [Google Scholar]
- Saini SS, MacGlashan DW, Jr., Sterbinsky SA, Togias A, Adelman DC, Lichtenstein LM, Bochner BS. Down-regulation of human basophil IgE and FCεRI alpha surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J Immunol. 1999;162:5624–5630. [PubMed] [Google Scholar]
- Scheinfeld N. A comprehensive review of imatinib mesylate (Gleevec) for dermatological diseases. J Drugs Dermatol. 2006;5:117–122. [PubMed] [Google Scholar]
- Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of Abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Kennedy J, Murli S, Reid R, Santi DV. Targeted covalent inactivation of protein kinases by resorcylic acid lactone polyketides. Proc Natl Acad Sci U S A. 2006;103:4234–4239. doi: 10.1073/pnas.0600445103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittenhelm MM, Shiraga S, Schroeder A, Corbin AS, Griffith D, Lee FY, Bokemeyer C, Deininger MW, Druker BJ, Heinrich MC. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC, Dunbar CE, Wiestner A. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105:2473–2479. doi: 10.1182/blood-2004-07-2527. [DOI] [PubMed] [Google Scholar]
- Shah NP, Lee FY, Luo R, Jiang Y, Donker M, Akin C. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood. 2006;108:286–291. doi: 10.1182/blood-2005-10-3969. [DOI] [PubMed] [Google Scholar]
- Strait RT, Morris SC, Yang M, Qu XW, Finkelman FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–668. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]
- Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM. The phospholipase Cγ1-dependent pathway of FcεRI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J Biol Chem. 2003;278:48474–48484. doi: 10.1074/jbc.M301350200. [DOI] [PubMed] [Google Scholar]
- Tkaczyk C, Horejsi V, Iwaki S, Draber P, Samelson LE, Satterthwaite AB, Nahm DH, Metcalfe DD, Gilfillan AM. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and FcεRI aggregation. Blood. 2004;104:207–214. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- Tkaczyk C, Metcalfe DD, Gilfillan AM. Determination of protein phosphorylation in FcεRI-activated human mast cells by immunoblot analysis requires protein extraction under denaturing conditions. J Immunol Methods. 2002;268:239–243. doi: 10.1016/s0022-1759(02)00210-7. [DOI] [PubMed] [Google Scholar]
- Verstovsek S, Akin C, Manshouri T, Quintas-Cardama A, Huynh L, Manley P, Tefferi A, Cortes J, Giles FJ, Kantarjian H. Effects of AMN107, a novel aminopyrimidine tyrosine kinase inhibitor, on human mast cells bearing wild-type or mutated codon 816 c-kit. Leuk Res. 2006;30:1365–1370. doi: 10.1016/j.leukres.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Winssinger N, Barluenga S. Chemistry and biology of resorcylic acid lactones. Chem Commun (Camb) 2007:22–36. doi: 10.1039/b610344h. [DOI] [PubMed] [Google Scholar]
- Zermati Y, De Sepulveda P, Feger F, Letard S, Kersual J, Casteran N, Gorochov G, Dy M, Ribadeau Dumas A, Dorgham K, Parizot C, Bieche Y, Vidaud M, Lortholary O, Arock M, Hermine O, Dubreuil P. Effect of tyrosine kinase inhibitor STI571 on the kinase activity of wild-type and various mutated c-kit receptors found in mast cell neoplasms. Oncogene. 2003;22:660–664. doi: 10.1038/sj.onc.1206120. [DOI] [PubMed] [Google Scholar]