Renal Function and Blood Pressure in Diabetes: Early versus Late
Perhaps the most obvious, and least contentious, examples of hypertension caused by the kidneys are renal artery stenosis and diabetic nephropathy, because there are readily-identifiable, physical limitations in the ability of the kidneys to excrete sodium in each case. The former is characterized by a global restriction in renal perfusion, and the latter is characterized more specifically by glomerular injury and reduced GFR, but in each case there must be an increase in arterial pressure in order to maintain salt and water balance 1–3. Thus, in longstanding diabetes, Types I and II, a progressive decline in GFR is matched by a reciprocal increase in arterial pressure, which enables maintenance of sodium balance and body fluid volume homeostasis at the expense of deleterious side-effects of chronic hypertension 4.
In this framework, decreased GFR in diabetic nephropathy is responsible for imparting a chronic sodium-retaining influence on the kidneys, and hypertension is the counterbalancing natriuretic influence required to maintain sodium balance and sustain life. It therefore becomes curious that the early stages of diabetes are characterized by increased GFR and sodium balance, yet blood pressure is normal rather than low. In order for sodium balance to be maintained at normal blood pressure in the face of the chronic natriuretic influence of elevated GFR, there must be a concurrent sodium-retaining influence. If not, then the natriuretic effect of increased GFR would act unopposed and result in the maintenance of sodium balance at a lower blood pressure, similar to the effect of a diuretic. However, the presence of an underlying salt-retaining influence has been difficult to realize conceptually, because of the increase in absolute sodium excretion in diabetes and because normal blood pressure typically does not spur research interest. This review focuses on how sodium balance is maintained at the onset of diabetes with normal blood pressure and increased GFR, and how disruption of the mechanisms that sustain that balance influence blood pressure.
Sodium-Retaining Influence Early in Diabetes: Role of the Renin-Angiotensin System
The renin-angiotensin system is our most powerful mechanism for regulating sodium balance in response to variations in sodium intake and/or excretion 5, 6. It is essential protection against decreases in blood pressure due to decreased extracellular fluid volume, and in diabetes the primary threat is glucose-induced osmotic natriuresis and diuresis. We 7–9 and others 10 have shown that plasma renin activity (PRA) increases early during the first week of streptozotocin (STZ)-induced Type I diabetes in rats, and the increase in PRA occurs even when diabetes is induced in rats with reduced kidney mass and very low baseline PRA 11 and in rats on high and low salt intakes 9. Miller et al. 12 have reported that young diabetic patients under poor glycemic control have a significantly greater drop in arterial pressure following acute losartan administration compared to the response when under good glycemic control, suggesting that angiotensin II (AngII) is playing a more important role supporting blood pressure under those conditions. Similarly, we have shown that induction of diabetes in rats with chronic angiotensin converting enzyme (ACE) inhibition 11 (Figure 1, open squares), or in rats with AngII levels chronically clamped at normal levels 13, causes arterial pressure to decrease. These data suggest that stimulation of the renin-angiotensin system is compensation for the glucose-induced natriuresis and diuresis at the onset of hyperglylcemia and is essential to prevent blood pressure from decreasing.
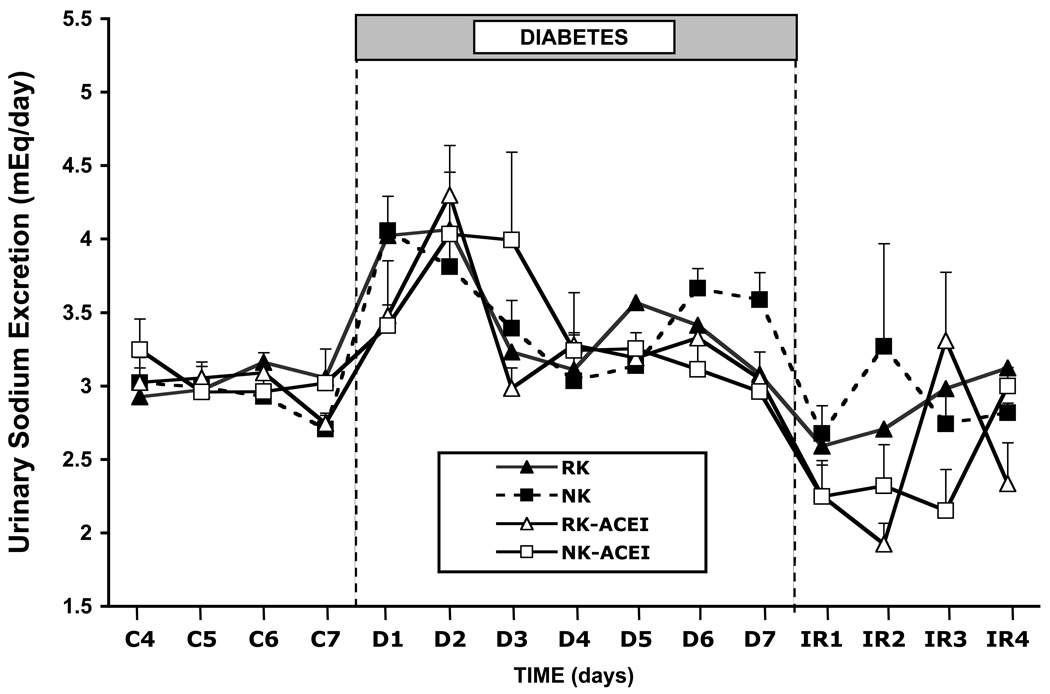
Figure 1.
Mean arterial pressure in rats with normal kidneys or a 70% reduction in kidney mass, with or without chronic ACE inhibition, during baseline conditions, a 7-day diabetic period, and a recovery period 11.
Postulating a role for AngII in renal pathophysiology in diabetes has been a challenge historically, because PRA typically is not elevated in sustained diabetes 14–17. Even studies that show early increases in PRA have reported a return to normal levels by the second week of diabetes 7, 10. However, there now is considerable evidence for significant activation of an intrarenal renin-angiotensin system in chronic diabetes, independent of any measurable increase in circulating renin or angiotensin II 18–21. This provides one explanation for a continued sodium-retaining influence even after circulating AngII levels have normalized. Another intriguing mechanism for promoting sodium retention in diabetes is tubular glucose itself. Hyperglycemia in diabetes increases proximal tubular sodium reabsorption more than can be accounted for by glomerulotubular balance alone, suggesting that it is a primary event 22. Thus, there is evidence that AngII, and perhaps glucose itself, impart a sustained sodium-retaining influence on the kidneys in diabetes.
Then Why isn’t Blood Pressure Increased?
It is important to return to the initiating event as well as the initial question. Hyperglycemia is the initial event, and this causes significant natriuresis and diuresis even in animals with reduced kidney mass that do not have an increase in GFR 11. Thus, the body is threatened with volume loss and decreased blood pressure. Stimulation of the renin-angiotensin system is a compensatory response to prevent blood pressure from decreasing and to help restore sodium balance. With sustained diabetes, intrarenal AngII generation continues that action. The initial question was: Why isn’t blood pressure decreased in the face of chronically elevated GFR? And our hypothesis is that AngII provides a counterbalancing antinatriuretic influence, such that sodium balance is maintained at normal blood pressure. However, if that is correct, then induction of diabetes, but without an increase in GFR, should result in hypertension due to unopposed actions of AngII.
Role of increased GFR in Maintaining Sodium Balance
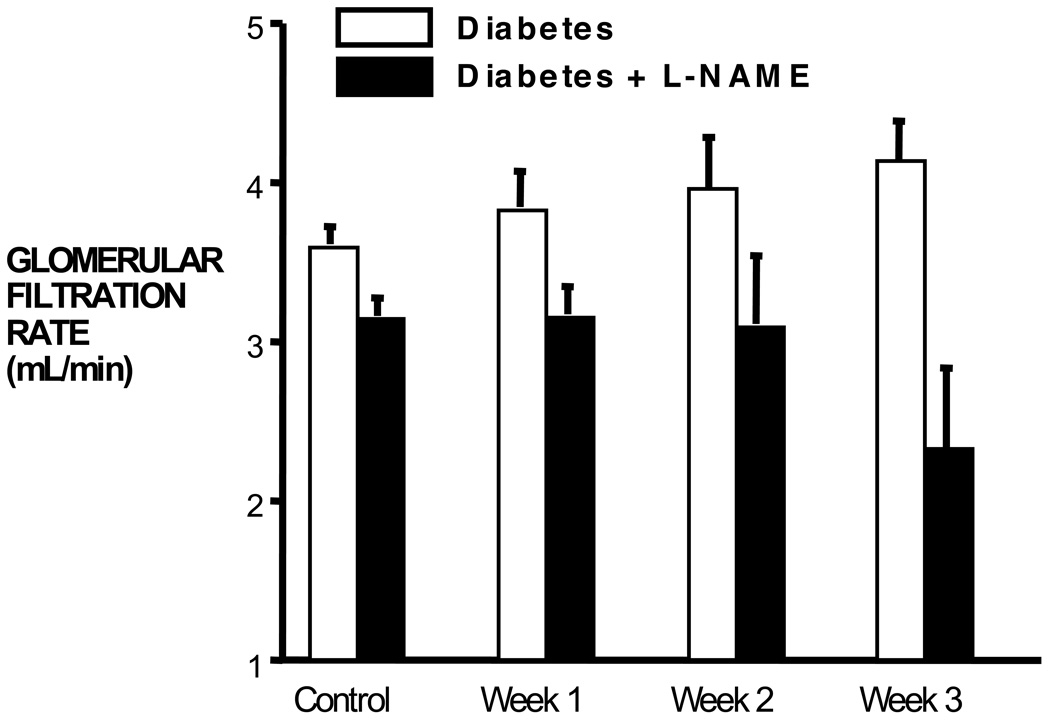
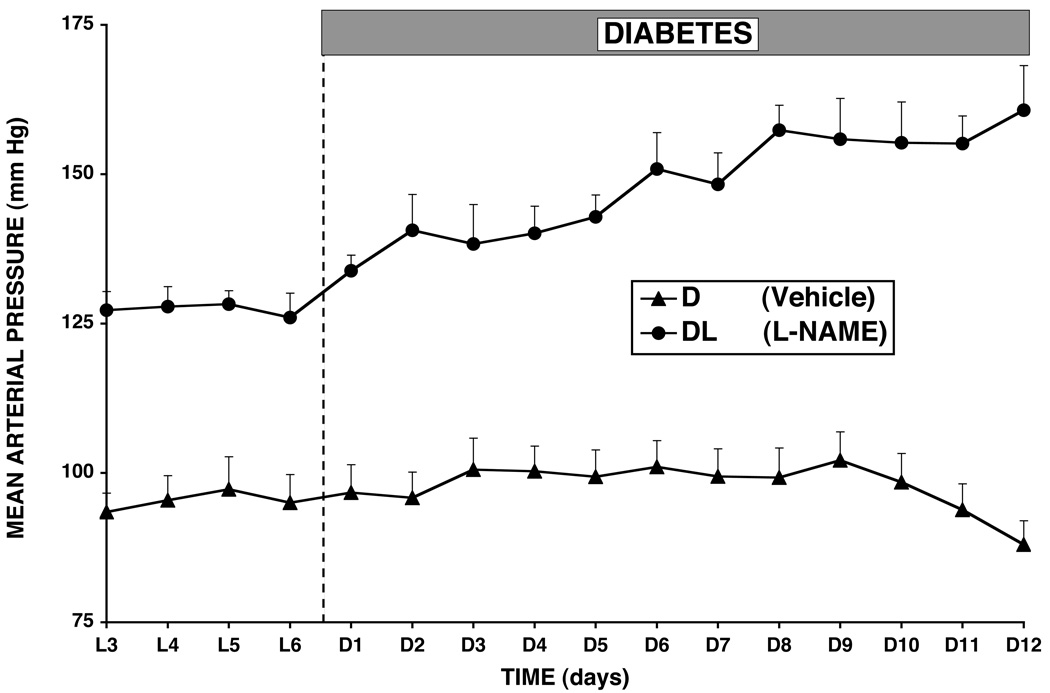
It is important to realize that the natriuretic and diuretic effect of hyperglycemia is due primarily to its effect to decrease tubular reabsorption. This is evident clearly by the measurement of similar increases in urinary sodium excretion in rats with normal kidneys compared with rats with 70% reduction in kidney mass, in which the latter had a much lower baseline GFR and no increase in GFR during diabetes 11 (Figure 2). Thus, the initial perturbation against fluid volume homeostasis in diabetes is the osmotic diuresis. Increases in renin-angiotensin system activity and GFR follow, and sodium balance is maintained at normal blood pressure. However, while studying the effect of nitric oxide on blood flow and blood pressure early in diabetes, we observed that, without nitric oxide, there was no increase in GFR 7, 9, 23 (Figure 3). Moreover, there was hypertension 7, 9, 13, 23 that was AngII dependent 13. Thus, when diabetes was induced in rats treated chronically with L-NAME, GFR did not increase and mean arterial pressure increased approximately 20 mm Hg above the L-NAME baseline blood pressure (Figure 4). When we used tempol to block superoxide chronically in L-NAME-treated rats, we found that the increase in GFR during diabetes was restored and the hypertension was prevented 23. These studies suggested that, without the increase in GFR, the sodium-retaining actions of AngII predominated and required an increase in arterial pressure to maintain sodium balance.
Figure 2.
Figure 3.
Glomerular filtration rate in rats during a control period and once per week over a 3-week diabetic period. The Diabetes + L-NAME rats received L-NAME continuously, iv, throughout the experiment 7.
Figure 4.
Mean arterial pressure during baseline conditions and 12 days of diabetes in normal rats and rats treated continuously with L-NAME 13.
However, that hypothesis was based on correlating changes in GFR and blood pressure in rats infused chronically with L-NAME, and nitric oxide obviously does much more in the body than influence GFR. To test the role of GFR in diabetes without blocking nitric oxide synthesis, we used surgical reduction of nephron mass to provide a mechanical limitation to GFR 11. It is critical to know the distinction between this model and the 5/6 nephrectomy model used most often in rat studies. The 5/6 model most often employed is an infarction model created by removing one kidney and tying off branches of the contralateral renal artery. It is known to have increased renin secretion for up to 4 weeks post infarct, and be highly linked to induction of renal inflammatory and immune system cascades that have been shown to induce and mediate sustained increases in blood pressure 24–27. Surgical reduction of renal mass, on the other hand, which is what we used 11, was compared with the 5/6 infarction model by Griffin et al 28 and shown to be protected from the injury-mediated hypertension. This is a low-renin model that is normotensive on low-salt intake. We reported that GFR did not increase in those rats upon induction of diabetes and, similar to earlier reports 29, they became hypertensive over the 7-day diabetic period (Figure 1, closed triangles), with blood pressures returning to control levels when intravenous insulin was used to restore normoglycemia 11. Moreover, despite low baseline renin, PRA increased significantly during diabetes, and chronic ACE inhibition prevented the hypertension 11 (Figure 1, open triangles).
Link between GFR, AngII, Sodium Balance and Arterial Pressure Early in Diabetes
Sustained hyperglycemia induces natriuresis and decreases sodium balance. Our data in normal and reduced kidney mass rats, with or without increased GFR or AngII, show that induction of diabetes increases urinary sodium excretion similarly in each case and that daily sodium balance is restored within several days in each case (Figure 2), regardless of which response to diabetes is missing or blocked 11. The difference is the arterial pressure at which sodium balance is restored. Thus, if GFR and AngII both increase, as occurs normally, mean arterial pressure remains normal. Likewise, if neither increases, as when diabetes is induced in reduced kidney mass rats with chronic ACE inhibition, arterial pressure also does not change. However, if GFR increases, but AngII does not, then mean arterial pressure falls, and the decrease in arterial pressure provides the antinatriuretic influence that opposes increased GFR and restores sodium balance. If AngII increases, but GFR does not, as occurred in the rats with 70% reduction in kidney mass, then hypertension ensues, and the increased arterial pressure is required to offset the sodium-retaining actions of AngII and enable restoration and maintenance of sodium balance. That response is similar to what we measured when diabetes was induced in L-NAME-treated rats 7, 9, 13, 23, but in that case we hypothesized that the failure of GFR to increase in that model was due, at least in part, to AngII-mediated constriction of the afferent arteriole in the absence of nitric oxide.
Mechanism for the Increase in GFR In Diabetes
There is no consensus on the mechanism for the increase in GFR, but there is good evidence that nitric oxide may be involved 7, 13, 23, 30–36. Nitric oxide derived from neuronal nitric oxide synthase (nNOS) has been implicated in particular, and indeed blockade of nNOS has been shown to attenuate the increase in GFR in diabetes 33, 34, 36, 37. There also is evidence that nitric oxide is not increased in diabetes 38–41, but issues of experimental model, the level of oxidative stress, and the time after induction of diabetes play a major role in that assessment, and our results with chronic L-NAME treatment and measurements in conscious, undisturbed animals 7, 9, 13, 23 corroborate the nNOS blockade findings, even though they do not distinguish between NOS isoforms.
However, the role of nitric oxide to increase GFR is not as straightforward as having simply a direct vasodilatory action on the afferent arteriole explain the phenomenon. Although the potential contribution of that mechanism is not discounted, whether it is through endothelial NOS (eNOS) or nNOS derived nitric oxide, the constitutive expression of nNOS in macula densa cells 42, 43 raises the possibility that nitric oxide may mediate afferent arteriolar vasodilation through its effect to attenuate sodium chloride transport at the macula densa 43–47. That would lead to vasodilation, at least in large part, by signaling a reduction in the tubuloglomerular feedback (TGF) vasoconstrictor signal. However, that effect of nitric oxide could be due either to: a) actions that are similar to the effect of furosemide, in which afferent arteriolar resistance decreases as a function of decreased macula densa transport primarily along the normal TGF curve (which would mean moving leftward along the control TGF line in Figure 5, because blocking macula densa transport essentially is sensed similar to a decrease in tubular flow), or b) an effect to blunt the sensitivity of TGF, which means that the TGF curve is shifted (as shown by the upper dashed line in Figure 5). Nitric oxide has been shown to blunt TGF sensitivity 45, 46, 48–53, and the consequence of that action is that, for any given level of sodium chloride delivery to the macula densa, there is a lesser TGF signal for constriction of the afferent arteriole.
Figure 5.
Tubuloglomerular feedback as determined by measuring single nephron GFR, or an index, in response to changes in tubular flow, or perfusion rate. Bottom dashed line represents the resetting and increased sensitivity measured in response to NO synthesis inhibition 52,53, and the top dashed line shows the effect of increased NO levels as estimated by the response to administration of NO donors 50, 51 Comparing point B to point A shows that with increased NO levels and decreased TGF sensitivity, there will be a greater GFR for any given level of tubular flow rate.
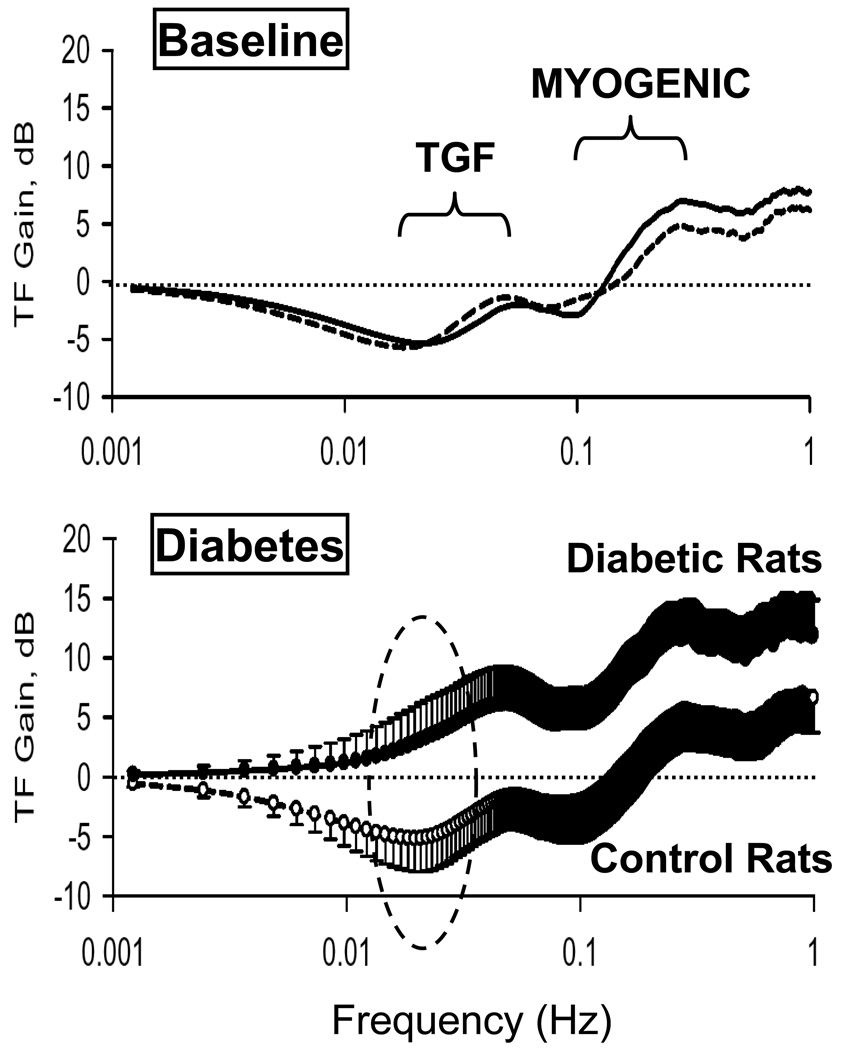
Decreased TGF sensitivity, therefore, can increase GFR. In an effort to shed light on how TGF and renal autoregulation in general may contribute to renal vasodilation early in diabetes, we used transfer function analysis 54–57 of our 18 hour daily renal blood flow and arterial pressure records collected from chronically-instrumented rats during baseline conditions and through the onset and maintenance of diabetes in those same animals 58. We showed that the onset of diabetes increased transfer function gain, i.e. increased transmission of arterial pressure power to renal blood flow power, throughout the frequency range analyzed, suggesting that there was a generalized change in the renal vasculature upon induction of diabetes 58 (Figure 6). However, there was evidence in particular of blunted TGF, as shown by the changes in transfer function gain within the dashed oval in Figure 6. Moreover, our follow-up study showed that chronic L-NAME treatment, which prevented the increase in GFR, also completely prevented the diabetes-induced shifts in transfer function gain 59. Although the role of eNOS 31, 60–63 versus nNOS 33, 34, 36, 37, cannot be determined from those studies, these results, using unique, 24 hr/day methods for chronic renal blood flow measurement, strongly implicate nitric oxide in diabetes-induced renal vasodilation and increased GFR. In addition, they suggest that an effect to blunt the sensitivity of the TGF mechanism may play a role.
Figure 6.
Transfer function (TF) gain for dynamic renal blood flow autoregulation in control rats diabetic rats, during the baseline period (top panel) and the diabetic period (bottom panel). The “TGF” and “Myogenic” labels denote the approximate frequency ranges at which the respective autoregulatory mechanisms operate. The oval circle illustrates the impairment in TGF in the diabetic rats.
Another hypothesis for increased GFR in diabetes is that it is a consequence of decreased sodium chloride concentration at the macula densa, due to a combination of glucose-stimulated proximal tubular sodium reabsorption and the osmotic diuretic effect of tubular glucose 64, 65. Thus, despite increased total sodium chloride delivery, as reflected simply by the natriuresis, the macula densa senses a decrease in tubular sodium chloride concentration 64, 65. In other words, it behaves as if one were moving leftward along the control TGF curve in Figure 5, and the macula densa might be viewed anthropomorphically in this sense as being “tricked” by those tubular conditions. In that scheme, therefore, the TGF mechanism is functioning normally, and would be mediating dilation of the afferent arteriole through withdrawal of the TGF vasoconstrictor signal in response to decreased tubular sodium chloride concentration 64, 65. We have invoked this possibility previously 11, and these osmotic diuretic 66 and sodium transport 67 effects of hyperglycemia also may contribute to the stimulation of renin secretion early in diabetes.
This evaluation of GFR control essentially has tried to explain why, in the face of increased distal tubular flow and total sodium chloride delivery in diabetes, there is not the predicted TGF signal to reduce GFR. Some reports have suggested that TGF is doing exactly that in diabetes 68, 69, but the weight of evidence that supports a role for nitric oxide argues that the TGF vasoconstrictor signal is diminished in diabetes. The effect of nitric oxide to impair macula densa transport could accomplish this by moving leftward along the normal TGF curve or by blunting TGF sensitivity. Both actions may occur and have additive or potentiating vasodilator effects, and if there is a decrease in distal tubular sodium chloride concentration due to the proximal tubular actions of glucose, then that also would decrease the TGF vasoconstrictor signal and contribute to the vasodilation. Our data suggest that TGF may be blunted early in diabetes, but that does not exclude the other 2 mechanisms or the possibility that all are involved simultaneously. Finally, our observations that the failure of GFR to increase in L-NAME-treated diabetic rats is AngII and superoxide dependent, suggest that nitric oxide also may influence GFR by protecting against AngII- and/or superoxide-mediated afferent arteriolar constriction early in diabetes 70, 71. Thus, if stimulation of the renin-angiotensin system is a normal response to counteract sodium and volume loss early in diabetes, the protective action of nitric oxide against AngII-mediated afferent arteriolar vasoconstriction would be important.
Implications Beyond Type I Diabetes
The hypothesis we have developed is that a balance between the natriuretic effect of increased GFR and the antinatriuretic effect of AngII is required for sodium balance to be maintained at normal arterial pressure at the earliest stages of Type I diabetes. The increase in GFR is strongly nitric oxide-dependent, but whether that is due to independent or combined effects of nitric oxide to blunt TGF or protect against AngII-mediated afferent arteriolar constriction is not known. However, if there is an increase in AngII and no increase in GFR, then there is an increase in arterial pressure. In the pre-Type II diabetic, obese state most generally described as metabolic syndrome 72–74, there also is AngII-dependent hypertension 75–79. However, GFR typically is elevated, which would tend to argue against extrapolating our hypothesis to this condition. On the other hand, impairment of endothelial function also is a characteristic of the obese, metabolic syndrome state 80–84, and if eNOS plays a role in the increased GFR in this pre-Type II diabetic condition, then it is possible that GFR is not increased as much as it should be. Thus, the gradual evolution of hypertension during the progression of metabolic syndrome towards overt Type II diabetes would be compensation for increased renin-angiotensin system activity that would help maintain sodium balance in the face of a gradual impairment in renal vasodilatory capability. This also is a hypothesis, and with little or no direct experimental evidence, but our work in Type I diabetes suggests that such a mechanism has the potential to contribute to the development of hypertension in metabolic syndrome.
ACKNOWLEDGEMENTS
Cited work from our laboratory depended on fundamental contributions by these key coauthors: Drs. Tracy D. Bell, Gerald F. DiBona, Sharyn M. Fitzgerald, and Modesto Rojas.
SOURCES OF FUNDING
Our work was supported by National Heart Lung and Blood Institute grants HL56259 and HL75625.
Footnotes
Disclaimer: The manuscript and its contents are confidential, intended for journal review purposes only, and not to be further disclosed.
Author Disclosures
Michael W. Brands:
Research Grant: HL56259 HL75625, Amount: >= $10,000
Hicham Labazi: No disclosures
DISCLOSURES
None.
References
- 1.Guyton AC, Coleman TG. Quantitative analysis of the pathophysiology of hypertension. Circ Res. 1969;24:1–19. [PubMed] [Google Scholar]
- 2.Borst JGG, Borst-De Geus A. Hypertension explained by Starling's theory of circulatory homeostasis. The Lancet. 1963;1:677–682. doi: 10.1016/s0140-6736(63)91443-0. [DOI] [PubMed] [Google Scholar]
- 3.Hall JE, Guyton AC, Brands MW. Pressure-volume regulation in hypertension. Kidney Int. 1996;49(Suppl 55):S35–S41. [PubMed] [Google Scholar]
- 4.Poulsen PL, Juhl B, Ebbehoj E, Klein F, Christiansen C, Mogensen CE. Elevated ambulatory blood pressure in microalbuminuric IDDM patients is inversely associated with renal plasma flow. A compensatory mechanism? Diabetes Care. 1997;20:429–432. doi: 10.2337/diacare.20.3.429. [DOI] [PubMed] [Google Scholar]
- 5.Hall JE, Guyton AC, Smith MJ., Jr Coleman TG. Blood pressure and renal function during chronic changes in sodium intake: role of angiotensin. Am J Physiol. 1980;239:F271–F280. doi: 10.1152/ajprenal.1980.239.3.F271. [DOI] [PubMed] [Google Scholar]
- 6.Hall JE, Mizelle HL, Brands MW, Hildebrandt DA. Pressure natriuresis and angiotensin II in reduced kidney mass, salt-induced hypertension. Am J Physiol. 1992;262:R61–R71. doi: 10.1152/ajpregu.1992.262.1.R61. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald SM, Brands MW. Nitric oxide may be required to prevent hypertension at the onset of diabetes. Am J Physiol Endocrinol Metab. 2000;279:E762–E768. doi: 10.1152/ajpendo.2000.279.4.E762. [DOI] [PubMed] [Google Scholar]
- 8.Brands MW, Fitzgerald SM, Hewitt WH, Hailman AE. Decreased cardiac output at the onset of diabetes: renal mechanisms and peripheral vasoconstriction. Am J Physiol. 2000;278:E917–E924. doi: 10.1152/ajpendo.2000.278.5.E917. [DOI] [PubMed] [Google Scholar]
- 9.Brands MW, Fleming C, Bell TD, Sturgis LC, Janardhanan R, Labazi H. Lack of blood pressure salt-sensitivity supports afferent arteriolar action of nitric oxide in Type I diabetes. Clin. Expr. Pharm. Physiol. 2007;34:475–479. doi: 10.1111/j.1440-1681.2007.04597.x. [DOI] [PubMed] [Google Scholar]
- 10.Kikkawa R, Kitamura E, Fujiwara Y, Haneda M, Shigeta Y. Biphasic alteration of renin-angiotensin-aldosterone system in streptozotocin-diabetic rats. Ren Physiol. 1986;9:187–192. doi: 10.1159/000173083. [DOI] [PubMed] [Google Scholar]
- 11.Rojas M, Bell TD, Sturgis LC, Janardhanan R, Fleming C, Brands MW. Blood pressure control early in diabetes requires a balance between glomerular filtration rate and the renin-angiotensin system. Am. J. Hypertens. 2006;19:1249–1255. doi: 10.1016/j.amjhyper.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Miller JA, Floras JS, Zinman B, Skorecki KL, Logan AG. Effect of hyperglycemia on arterial pressure, plasma renin activity, and renal function in early diabetes. Clinical Science. 1996;90:189–195. doi: 10.1042/cs0900189. [DOI] [PubMed] [Google Scholar]
- 13.Brands MW, Cloud LJ. Control of arterial pressure by angiotensin II and nitric oxide at the onset of diabetes. Am J Hypertens. 2003;16:600–603. doi: 10.1016/s0895-7061(03)00902-6. [DOI] [PubMed] [Google Scholar]
- 14.Cassis LA. Downregulation of the renin-angiotensin system in streptozotocin-diabetic rats. Am J Physiol. 1992;262:E105–E109. doi: 10.1152/ajpendo.1992.262.1.E105. [DOI] [PubMed] [Google Scholar]
- 15.Kalinyak JE, Sechi LA, Griffin CA, Don BR, Tavangar K, Kraemer FB, Hoffman AR, Schambelan M. The renin-angiotensin system in streptozotocin-induced diabetes mellitus in the rat. J Am Soc Nephrol. 1993;4:1337–1345. doi: 10.1681/ASN.V461337. [DOI] [PubMed] [Google Scholar]
- 16.Price DA, Porter LE, Gordon M, Fisher ND, De'Oliveira JM, Laffel LM, Passan DR, Williams GH, Hollenberg NK. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol. 1999;10:2382–2391. doi: 10.1681/ASN.V10112382. [DOI] [PubMed] [Google Scholar]
- 17.Vallon V, Wead LM, Blantz RC. Renal hemodynamics and plasma and kidney angiotensin II in established diabetes mellitus in rats: effect of sodium and salt restriction. J Am Soc Nephrol. 1995;5:1761–1767. doi: 10.1681/ASN.V5101761. [DOI] [PubMed] [Google Scholar]
- 18.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The Collecting Duct Is the Major Source of Prorenin in Diabetes. Hypertension. 2008;51:1567–1604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh R, Singh AK, Leehey DJ. A novel mechanism for angiotensin II formation in streptozotocin-diabetic rat glomeruli. Am J Physiol Renal Physiol. 2005;288:F1183–F1190. doi: 10.1152/ajprenal.00159.2003. [DOI] [PubMed] [Google Scholar]
- 20.Carey RM, Siragy HM. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14:274–281. doi: 10.1016/s1043-2760(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 21.Hollenberg NK, Price DA, Fisher ND, Lansang MC, Perkins B, Gordon MS, Williams GH, Laffel LM. Glomerular hemodynamics and the renin-angiotensin system in patients with type 1 diabetes mellitus. Kidney Int. 2003;63:172–178. doi: 10.1046/j.1523-1755.2003.00701.x. [DOI] [PubMed] [Google Scholar]
- 22.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest. 2001;107:217–224. doi: 10.1172/JCI10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brands MW, Bell TD, Gibson B. Nitric oxide may prevent hypertension early in diabetes by counteracting renal actions of superoxide. Hypertension. 2004;43:57–63. doi: 10.1161/01.HYP.0000104524.25807.EE. [DOI] [PubMed] [Google Scholar]
- 24.Dzielak DJ. The immune system and hypertension. Hypertension. 1992;19:I36–I44. doi: 10.1161/01.hyp.19.1_suppl.i36. [DOI] [PubMed] [Google Scholar]
- 25.Norman RA, Jr, Galloway PG, Dzielak DJ, Huang M. Mechanisms of partial renal infarct hypertension. J Hypertens. 1988;6:397–403. [PubMed] [Google Scholar]
- 26.Goncalves AR, Fujihara CK, Mattar AL, Malheiros DM, Noronha Ide L, de Nucci G, Zatz R. Renal expression of COX-2, ANG II, and AT1 receptor in remnant kidney: strong renoprotection by therapy with losartan and a nonsteroidal anti-inflammatory. Am J Physiol Renal Physiol. 2004;286:F945–F954. doi: 10.1152/ajprenal.00238.2003. [DOI] [PubMed] [Google Scholar]
- 27.Fujihara CK, Antunes GR, Mattar AL, Andreoli N, Malheiros DM, Noronha IL, Zatz R. Cyclooxygenase-2 (COX-2) inhibition limits abnormal COX-2 expression and progressive injury in the remnant kidney. Kidney Int. 2003;64:2172–2181. doi: 10.1046/j.1523-1755.2003.00319.x. [DOI] [PubMed] [Google Scholar]
- 28.Griffin KA, Picken M, Bidani AK. Method of renal mass reduction is a critical modulator of subsequent hypertension and glomerular injury. J Am Soc Nephrol. 1994;4:2023–2031. doi: 10.1681/ASN.V4122023. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, Yuan C, Schooley JF, Jr, Haddy FJ, Pamnani MB. A consistent model of insulin-dependent diabetes mellitus hypertension. Am J Hypertens. 1992;5:671–680. doi: 10.1093/ajh/5.10.671. [DOI] [PubMed] [Google Scholar]
- 30.Mattar AL, Fujihara CK, Ribeiro MO, De Nucci G, Zatz R. Renal effects of acute and chronic nitric oxide inhibition in experimental diabetes. Nephron. 1996;74:136–143. doi: 10.1159/000189293. [DOI] [PubMed] [Google Scholar]
- 31.Veelken R, Hilgers KF, Hartner A, Haas A, Bohmer KP, Sterzel RB. Nitric oxide synthase isoforms and glomerular hyperfiltration in early diabetic nephropathy. J Am Soc Nephrol. 2000;11:71–79. doi: 10.1681/ASN.V11171. [DOI] [PubMed] [Google Scholar]
- 32.Tolins JP, Shultz PJ, Raij L, Brown DM, Mauer SM. Abnormal renal hemodynamic response to reduced renal perfusion pressure in diabetic rats: role of NO. Am J Physiol. 1993;265:F886–F895. doi: 10.1152/ajprenal.1993.265.6.F886. [DOI] [PubMed] [Google Scholar]
- 33.Komers R, Lindsley JN, Oyama TT, Allison KM, Anderson S. Role of neuronal nitric oxide synthase (NOS1) in the pathogenesis of renal hemodynamic changes in diabetes. Am J Physiol Renal Physiol. 2000;279:F573–F583. doi: 10.1152/ajprenal.2000.279.3.F573. [DOI] [PubMed] [Google Scholar]
- 34.Ito A, Uriu K, Inada Y, Qie YL, Takagi I, Ikeda M, Hashimoto O, Suzuka K, Eto S, Tanaka Y, Kaizu K. Inhibition of neuronal nitric oxide synthase ameliorates renal hyperfiltration in streptozotocin-induced diabetic rat. J Lab Clin Med. 2001;138:177–185. doi: 10.1067/mlc.2001.116843. [DOI] [PubMed] [Google Scholar]
- 35.Choi KC, Lee SC, Kim SW, Kim NH, Lee JU, Kang YJ. Role of nitric oxide in the pathogenesis of diabetic nephropathy in streptozotocin-induced diabetic rats. Korean J Intern Med. 1999;14:32–41. doi: 10.3904/kjim.1999.14.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson SC, Deng A, Komine N, Hammes JS, Blantz RC, Gabbai FB. Early diabetes as a model for testing the regulation of juxtaglomerular NOS I. Am J Physiol Renal Physiol. 2004;287:F732–F738. doi: 10.1152/ajprenal.00340.2003. [DOI] [PubMed] [Google Scholar]
- 37.Komers R, Lindsley JN, Oyama TT, Anderson S. Effects of long-term inhibition of neuronal nitric oxide synthase (NOS1) in uninephrectomized diabetic rats. Nitric Oxide. 2004;11:147–155. doi: 10.1016/j.niox.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes. Hypertension. 1998;31:1047–1060. doi: 10.1161/01.hyp.31.5.1047. [DOI] [PubMed] [Google Scholar]
- 39.Keynan S, Hirshberg B, Levin-Iaina N, Wexler ID, Dahan R, Reinhartz E, Ovadia H, Wollman Y, Chernihovskey T, Iaina A, Raz I. Renal nitric oxide production during the early phase of experimental diabetes mellitus. Kidney Int. 2000;58:740–747. doi: 10.1046/j.1523-1755.2000.00220.x. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz D, Schwartz IF, Blantz RC. An analysis of renal nitric oxide contribution to hyperfiltration in diabetic rats. J Lab Clin Med. 2001;137:107–114. doi: 10.1067/mlc.2001.112691. [DOI] [PubMed] [Google Scholar]
- 41.Ishii N, Patel KP, Lane PH, Taylor T, Bian K, Murad F, Pollock JS, Carmines PK. Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. J Am Soc Nephrol. 2001;12:1630–1639. doi: 10.1681/ASN.V1281630. [DOI] [PubMed] [Google Scholar]
- 42.Welch WJ, Tojo A, Lee J-U, Kang DG, Schnackenberg CG, Wilcox CS. Nitric oxide synthase in the JGA of the SHR: expression and role in tubuloglomerular feedback. Am J Physiol. 1999;277:F130–F138. doi: 10.1152/ajprenal.1999.277.1.F130. [DOI] [PubMed] [Google Scholar]
- 43.Ollerstam A, Persson AE. Macula densa neuronal nitric oxide synthase. Cardiovasc Res. 2002;56:189–196. doi: 10.1016/s0008-6363(02)00536-9. [DOI] [PubMed] [Google Scholar]
- 44.Kovacs G, Komlosi P, Fuson A, Peti-Peterdi J, Rosivall L, Bell PD. Neuronal nitric oxide synthase: its role and regulation in macula densa cells. J Am Soc Nephrol. 2003;14:2475–2483. doi: 10.1097/01.asn.0000088737.05283.2b. [DOI] [PubMed] [Google Scholar]
- 45.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol. 2003;65:501–529. doi: 10.1146/annurev.physiol.65.050102.085738. [DOI] [PubMed] [Google Scholar]
- 46.Thorup C, Persson AEG. Macula densa derived nitric oxide in regulation of glomerular capillary pressure. Kidney Int. 1996;49:430–436. doi: 10.1038/ki.1996.62. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Carretero OA, Garvin JL. Nitric oxide produced by THAL nitric oxide synthase inhibits TGF. Hypertension. 2002;39:662–666. doi: 10.1161/hy0202.103470. [DOI] [PubMed] [Google Scholar]
- 48.Braam B, Turkstra E, Koomans HA. Concerted actions of renal endothelial and macula densa NO systems in the maintenance of extracellular fluid volume. Acta Physiol Scand. 2000;168:125–132. doi: 10.1046/j.1365-201x.2000.00659.x. [DOI] [PubMed] [Google Scholar]
- 49.Ichihara A, Navar LG. Neuronal NOS contributes to biphasic autoregulatory response during enhanced TGF activity. Am J Physiol. 1999;277:F113–F120. doi: 10.1152/ajprenal.1999.277.1.F113. [DOI] [PubMed] [Google Scholar]
- 50.Thomson SC, Deng A. Cyclic GMP mediates influence of macula densa nitric oxide over tubuloglomerular feedback. Kidney Blood Press Res. 2003;26:10–18. doi: 10.1159/000069766. [DOI] [PubMed] [Google Scholar]
- 51.Wilcox CS, Welch WJ. Interaction between nitric oxide and oxygen radicals in regulation of tubuloglomerular feedback. Acta Physiol Scand. 2000;168:119–124. doi: 10.1046/j.1365-201x.2000.00668.x. [DOI] [PubMed] [Google Scholar]
- 52.Blantz RC, Deng A, Lortie M, Munger K, Vallon V, Gabbai FB, Thomson SC. The complex role of nitric oxide in the regulation of glomerular ultrafiltration. Kidney Int. 2002;61:782–785. doi: 10.1046/j.1523-1755.2002.00220.x. [DOI] [PubMed] [Google Scholar]
- 53.Thorup C, Persson AE. Inhibition of locally produced nitric oxide resets tubuloglomerular feedback mechanism. Am J Physiol. 1994;267:F606–F611. doi: 10.1152/ajprenal.1994.267.4.F606. [DOI] [PubMed] [Google Scholar]
- 54.Bidani AK, Hacioglu R, Abu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. “Step” vs. “dynamic” autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol Renal Physiol. 2003;285:F113–F120. doi: 10.1152/ajprenal.00012.2003. [DOI] [PubMed] [Google Scholar]
- 55.DiBona GF, Sawin LL. Effect of renal denervation on dynamic autoregulation of renal blood flow. Am J Physiol Renal Physiol. 2004;286:F1209–F1218. doi: 10.1152/ajprenal.00010.2004. [DOI] [PubMed] [Google Scholar]
- 56.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1153–R1167. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Cupples WA. Interaction between nitric oxide and renal myogenic autoregulation in normotensive and hypertensive rats. Can J Physiol Pharmacol. 2001;79:238–245. [PubMed] [Google Scholar]
- 58.Bell TD, DiBona GF, Wang Y, Brands MW. Mechanisms for renal blood flow control early in diabetes as revealed by chronic flow measurement and transfer function analysis. J Am Soc Nephrol. 2006;17:2184–2192. doi: 10.1681/ASN.2006030216. [DOI] [PubMed] [Google Scholar]
- 59.Bell TD, Brands MW. Role of nitric oxide in renal blood flow control during diabetes as revealed by continuous measurement. Hypertension. 2006;48:e60. Abstract P79. [Google Scholar]
- 60.Sugimoto HS, K./Matsuda, M./Kushiro, M./Hayashi, Y./Hiragushi, K./Wada, J./Makino H. Increased expression of endothelial cell nitric oxide synthase (ecNOS) in afferent and glomerular endothelial cells is involved in glomerular hyperfiltration of diabetic nephropathy. Diabetologia. 1998;41:1426–1434. doi: 10.1007/s001250051088. [DOI] [PubMed] [Google Scholar]
- 61.De Vriese AS, Stoenoiu MS, Elger M, Devuyst O, Vanholder R, Kriz W, Lameire NH. Diabetes-induced microvascular dysfunction in the hydronephrotic kidney: role of nitric oxide. Kidney Int. 2001;60:202–210. doi: 10.1046/j.1523-1755.2001.00787.x. [DOI] [PubMed] [Google Scholar]
- 62.Hiragushi K, Sugimoto H, Shikata K, Yamashita T, Miyatake N, Shikata Y, Wada J, Kumagai I, Fukushima M, Makino H. Nitric oxide system is involved in glomerular hyperfiltration in Japanese normo-and micro-albuminuric patients with type 2 diabetes. Diabetes Res Clin Pract. 2001;53:149–159. doi: 10.1016/s0168-8227(01)00260-1. [DOI] [PubMed] [Google Scholar]
- 63.Zhao HJ, Wang S, Cheng H, Zhang MZ, Takahashi T, Fogo AB, Breyer MD, Harris RC. Endothelial nitric oxide synthase deficiency produces accelerated nephropathy in diabetic mice. J Am Soc Nephrol. 2006;17:2664–2669. doi: 10.1681/ASN.2006070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–2576. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- 65.Thomson SC, Vallon V, Blantz RC. Kidney function in early diabetes: the tubular hypothesis of glomerular filtration. Am J Physiol Renal Physiol. 2004;286:F8–F15. doi: 10.1152/ajprenal.00208.2003. [DOI] [PubMed] [Google Scholar]
- 66.Leyssac PP, Holstein-Rathlou NH, Skott O. Renal blood flow, early distal sodium, and plasma renin concentrations during osmotic diuresis. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1268–R1276. doi: 10.1152/ajpregu.2000.279.4.R1268. [DOI] [PubMed] [Google Scholar]
- 67.Woods LL, Mizelle HL, Hall JE. Control of renal hemodynamics in hyperglycemia: possible role of tubuloglomerular feedback. Am J Physiol. 1987;252:F65–F71. doi: 10.1152/ajprenal.1987.252.1.F65. [DOI] [PubMed] [Google Scholar]
- 68.Faulhaber-Walter R, Chen L, Oppermann M, Kim SM, Huang Y, Hiramatsu N, Mizel D, Kajiyama H, Zerfas P, Briggs JP, Kopp JB, Schnermann J. Lack of A1 adenosine receptors augments diabetic hyperfiltration and glomerular injury. J Am Soc Nephrol. 2008;19:722–730. doi: 10.1681/ASN.2007060721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pollock CA, Lawrence JR, Field MJ. Tubular sodium handling and tubuloglomerular feedback in experimental diabetes mellitus. Am J Physiol. 1991;260:F946–F952. doi: 10.1152/ajprenal.1991.260.6.F946. [DOI] [PubMed] [Google Scholar]
- 70.Navar LG, Ichihara A, Chin SY, Imig JD. Nitric oxide-angiotensin II interactions in angiotensin II-dependent hypertension. Acta Physiol Scand. 2000;168:139–147. doi: 10.1046/j.1365-201x.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 71.Ito S, Arima S, Ren YL, Juncos LA, Carretero OA. Endothelium-derived relaxing factor/nitric oxide modulates angiotensin II action in the isolated microperfused rabbit afferent but not efferent arteriole. J Clin Invest. 1993;91:2012–2019. doi: 10.1172/JCI116423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 73.Haffner SM. Risk constellations in patients with the metabolic syndrome: epidemiology, diagnosis, and treatment patterns. Am J Med. 2006;119:S3–S9. doi: 10.1016/j.amjmed.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 74.Lastra G, Manrique C, Sowers JR. Obesity, cardiometabolic syndrome, and chronic kidney disease: the weight of the evidence. Adv Chronic Kidney Dis. 2006;13:365–373. doi: 10.1053/j.ackd.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 75.Hall JE, Brands MW, Dixon WN, Smith MJ., Jr Obesity-induced hypertension. Renal function and systemic hemodynamics. Hypertension. 1993;22:292–299. doi: 10.1161/01.hyp.22.3.292. [DOI] [PubMed] [Google Scholar]
- 76.Kirpichnikov D, Sowers JR. Diabetes mellitus and diabetes-associated vascular disease. Trends Endocrinol Metab. 2001;12:225–230. doi: 10.1016/s1043-2760(01)00391-5. [DOI] [PubMed] [Google Scholar]
- 77.Izzo JL., Jr Prehypertension: demographics, pathophysiology, and treatment. Curr Hypertens Rep. 2007;9:264–268. doi: 10.1007/s11906-007-0049-8. [DOI] [PubMed] [Google Scholar]
- 78.Deedwania PC, Schmieder R. Angiotensin receptor blockers: Cardiovascular protection in the metabolic syndrome. J Renin Angiotensin Aldosterone Syst. 2006;7 Suppl 1:S12–S18. doi: 10.3317/jraas.2006.018. [DOI] [PubMed] [Google Scholar]
- 79.Sharma AM. Is there a rationale for angiotensin blockade in the management of obesity hypertension? Hypertension. 2004;44:12–19. doi: 10.1161/01.HYP.0000132568.71409.a2. [DOI] [PubMed] [Google Scholar]
- 80.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dobrian AD, Davies MJ, Schriver SD, Lauterio TJ, Prewitt RL. Oxidative stress in a rat model of obesity-induced hypertension. Hypertension. 2001;37:554–560. doi: 10.1161/01.hyp.37.2.554. [DOI] [PubMed] [Google Scholar]
- 82.Whaley-Connell A, Pavey BS, Afroze A, Bakris GL. Obesity and insulin resistance as risk factors for chronic kidney disease. J Cardiometab Syndr. 2006;1:209–214. doi: 10.1111/j.1559-4564.2006.05631.x. [DOI] [PubMed] [Google Scholar]
- 83.Sharma V, McNeill JH. The etiology of hypertension in the metabolic syndrome part three: the regulation and dysregulation of blood pressure. Curr Vasc Pharmacol. 2006;4:321–348. doi: 10.2174/157016106778521643. [DOI] [PubMed] [Google Scholar]
- 84.Bigazzi R, Bianchi S. Insulin resistance, metabolic syndrome and endothelial dysfunction. J Nephrol. 2007;20:10–14. [PubMed] [Google Scholar]