Abstract
Despite recommendations for annual vaccination against influenza, more than half of patients with chronic obstructive pulmonary disease (COPD) in developed countries do not receive this vaccine. Influenza is characterized by its potentially of causing epidemics and by excess morbidity and mortality in patients with COPD and other chronic medical conditions. Good evidence of the efficacy, effectiveness, and cost-effectiveness of influenza vaccination underlines the recommendation of use in patients with COPD. Influenza vaccination could reduce influenza-related complications and exacerbations in patients with COPD, therefore reducing hospitalizations and deaths. Each year, all persons with COPD should be vaccinated with the inactivated trivalent influenza vaccine containing the most frequent two influenza A viral strains and one influenza B viral strain detected in the influenza season of the previous year. To achieve a 100% vaccination rate in patients with COPD, all patients with COPD registered in health insurance companies and attended in health centers and specialized clinics should be vaccinated during the immunization period (October–December). Antiviral therapies could be used as an adjunct to vaccination and to reduce influenza transmission in outbreaks. Antiviral therapies could reduce the duration and complications of influenza when administered within two days of the onset of illness. Research is necessary for new antiviral therapies that could prevent influenza with cost-effectiveness similar to the influenza vaccine.
Keywords: influenza vaccination, chronic obstructive pulmonary disease, vaccination program
Introduction
Influenza is an acute respiratory disease that causes epidemics and pandemics in the human populations from temperate regions. Influenza epidemics occur every year during winter months, affecting 10%–20% of the population (Cox and Subbarao 1999). Influenza viruses also can cause pandemics, during which rates of illness and death from influenza-related complications increase worldwide. Incidence rates of influenza infection vary with the nature of the virus strains and the population affected. Rates of serious illness are highest among persons aged ≥65 years, children aged <2 years, and persons with high-risk conditions for influenza-related complications, such as chronic obstructive pulmonary disease (COPD) (Barker 1986; Thompson et al 2003; Glezen et al 2000).
Influenza vaccination is the primary method of prevention and control of influenza in persons with COPD. It can reduce influenza-related respiratory illness, physician visits, hospitalization, and death (OTA 1981; Patriarca et al 1986; Heikkinen et al 1991; Mullooly et al 1994; Gross et al 1995; Bridges 2000; Anthonisen 2002). Influenza vaccination levels increased during the 1990s, but they are lower than 50% in high-risk patients in the US and other developed countries (Harper et al 2005). The objectives of this article are to review the evidence supporting the influenza vaccination in patients with COPD and to present effective strategies to increase vaccination levels in patients with COPD.
Virology, epidemiology, and pathophysiology of influenza
The influenza virus (80–120 nm) is a member of the family Orthomyxoviridae, including three serotypes: A, B, and C (Cox et al 2000). Influenza A and influenza B are the most significant pathogens in terms of morbidity and mortality. Type A is responsible for epidemics. Influenza C rarely produces clinical disease and has not been responsible for epidemics.
The influenza virus is a negative-strand RNA virus with a genome consisting on seven (influenza C) or eight (influenza A and B) segments of RNA. Each segment of RNA is encapsided by the viral nucleoprotein (NP) and is associated with a polymerase complex. The particle consisting of the NP and the RNA polymerase complex is called ribonucleoprotein (RNP) particle. The RNP particles are enclosed by a protein shell called capside, formed from the assembly of helical proteins called matrix protein (M1). The capside is surrounded by a lipoprotein envelope derived from the plasma membrane of infected cells, which contain two types of spikes (8–14 nm). One spike is a box-shaped protein called neuraminidase (N) and the other spike is a protein called hemagglutinin (H). The lipid envelope contains also a tetrameric ion channel protein called M2.
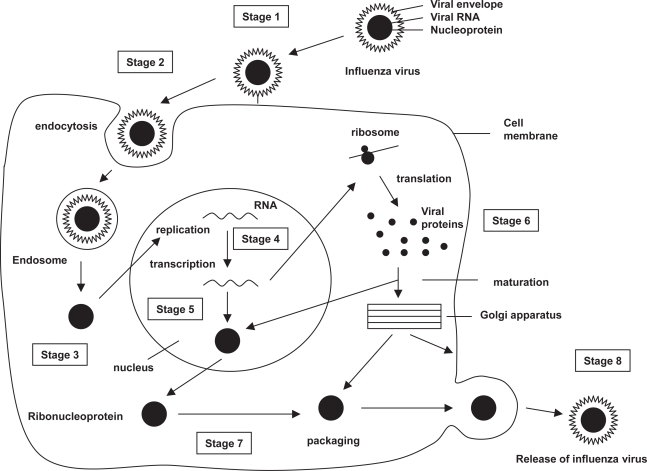
The influenza virus cycle can be divided into eight stages (Figure 1). Infection is initiated with the attachment of virions to susceptible host cells by reactions between the hemagglutinin particle and specific receptors on the respiratory epithelium (White and Wilson 1987) (stage 1). Receptor-bound viruses are taken into the cell by endocytosis (stage 2). Subviral particles formed by viral ribonucleoprotein (RBN) and protein M are released into the cell cytoplasm and transported into the nucleus (stage 3). In the cell nucleus, new viral RNA is formed by primary transcription (stage 4), and replication and secondary transcription of the viral RNA (stage 5). Viral proteins are formed by translation of messenger viral RNA (mRNA) by cell’s ribosomes (stage 6) and post-translational modification (stage 7). The same enzyme that makes mRNA is responsible for the secondary transcription, thus replicating the viral genome. In the nucleus, M1 proteins bind to newly synthesized (-) strand viral RNAs forming RNP particles. New viral particles are formed when RNP is encased by the capside and transported to the cell surface, where they are released surrounded by a cell-derived lipid membrane envelope containing hemagglutinin and neuraminidase proteins (stage 8).
Figure 1.
Influenza virus cycle.
Abbreviations: RNA, ribonucleic acid.
The hemagglutinin is responsible for both virus attachment and RNP release into the cell cytoplasm (White and Wilson 1987). The hemagglutinin is the major glycoprotein component of the viral envelope. It is homotrimeric and is composed of two polypeptide segments, designated HA1 and HA2. The HA1 segments form sialic acid-binding sites and mediate HA attachment to the host cell surface. The HA2 segment forms the membrane-spanning anchor, and its amino-terminal region, which is called fusion peptide, appears to be directly involved in the fusion reaction mechanism.
Influenza A viruses are classified into different subtypes based on their surface glycoproteins hemagglutinin (H) and neuraminidase (N). Fifteen subtypes of hemagglutinine (H1–H15) and nine subtypes of neuraminidase (N1–N9) have been identified. All of these serotypes have been found in aquatic birds. Different species of wild birds form the primary natural reservoir of the influenza A virus (Webster et al 1992). In humans, however, only three hemagglutinin proteins (H1, H2, and H3) and two neuraminidase proteins (N1 and N2) have been detected in epidemics and pandemics since 1900.
Since the discovery of the influenza virus, new antigenic variants of influenza A and B have continually emerged. The influenza virus changes continually by means of two mechanisms called antigenic drift and antigenic shift (Palese and Young 1982; Kilbourne 1987). The antigenic shift is responsible for the major antigenic changes registered in human influenza A virus. The antigenic shift is produced by the reassortment of genetic components from human and nonhuman influenza viruses. The antigenic shift produces new human influenza viruses with antigenic components from the nonhuman viruses. For example, the genetic reassortment of the human influenza virus H2N2 and the avian influenza virus H3N8 can produce a new human influenza virus H3N2. Influenza pandemics are produced by the lack of a specific immune response against the new human viruses in the whole population of the world. Persons infected by one influenza virus develop a specific immune response against the HN strain that is not able to recognize the new viral strain.
The antigenic drift is responsible for the minor antigenic changes registered in the human influenza viruses A and B. The antigenic drift is produced by point mutations in the genetic material of existing human viruses. Persons infected by one influenza virus develop a strain-specific immune response that could not be so efficient against a slightly different virus, despite belonging to the same HN strain. For this reason, people can get flu more than once and it is necessary to adapt the influenza vaccine to the minor changes detected during the influenza season in the influenza viruses.
Influenza occurs worldwide. In temperate climates it appears in the winter, but it can occur any time during pandemics. In the tropics influenza is endemic. Influenza caused by influenza A viruses are more frequent than those caused by type B possibly because of the slower rate of antigenic variation in influenza B. Influenza has a death-to-case rate of 1–2 deaths per 2000 cases (Harper et al 2005).
Influenza viruses are spread from person to person through the coughing and sneezing of infected persons. The incubation period for influenza is 1 to 4 days, with an average of 2 days. Infected individuals are infectious from the day before symptoms begin until approximately 5 days after onset of illness.
Primary influenza illness is characterized by the abrupt onset of respiratory and constitutional symptoms, including fever, headache, severe tiredness, dry cough, sore throat, muscle aches, and runny or stuffy nose. Stomach symptoms such as nausea, vomiting, and diarrhea can occur, but they are more common in children than adults. Fever usually ranges between 38ºC and 40ºC. Fever is often higher in children than in adults, while it may be absent in the elderly. Influenza illness typically resolves after 3 to 5 days, although cough and tiredness can persist for more than two weeks.
Complications of the acute influenza infection include: 1) primary viral pneumonia; 2) secondary bacterial pneumonia; 3) other secondary bacterial infections, including bronchitis, sinusitis, and otitis; 4) exacerbation of a COPD; and 5) exacerbation of an underlying heart disease.
The characteristic and uniformly observed lesion of influenza is necrosis of the cilial epithelium in the upper and middle portions of the respiratory tract. Pathological findings of complicated pneumonia are those characteristics of their causative agent (Cox and Subbarao 1999). In patients with primary influenza pneumonia, the lungs are airless, heavy with sanguineous edema, and mottled by subpleural hemorrhages. Bilateral interstitial infiltrates can be seen on chest radiography, but influenza virus infection can cause radiological changes similar to those of other causes of pneumonia. Primary viral pneumonia beguines within 24 hours of the onset of febrile illness with a dry cough that later produces bloody sputum accompanied by tachypnea, progressive cyanosis, and respiratory failure. Patients deteriorate despite antibiotic therapy, and pneumonia is associated with a high mortality rate.
Secondary pneumonia is characterized by the appearance of a new fever and productive cough during early convalescence. Clinical signs of lobar consolidation can be confirmed by radiology. Streptococcus pneumoniae, Staphylococcus aureus, and Haemophilus influenzae are the bacterial pathogens most commonly identified. Patients with secondary bacterial pneumonia respond to antibiotic therapy. A combined viral and bacterial pneumonia can present with any combination of signs and symptoms.
The influenza virus could produce the exacerbations of the underlying COPD due to the primary viral infection and secondary bacterial infections. Exacerbation of the underlying COPD is characterized by increased breathlessness, which is often accompanied by wheezing, chest tightness, and increased cough and sputum (Connors et al 1996; Anthonisen 2002). Exacerbation can also be accompanied by nonspecific symptoms, including malaise, insomnia, fatigue, depression, and confusion. The risk of dying from a COPD depends on the development of respiratory acidosis, the presence of significant comorbidities, and the need for ventilatory support (Anthonisen 1989, 2002).
The epidemiology of influenza has two dominant characteristics: 1) potentiality for causing epidemics and pandemics, 2) epidemic-associated excess mortality in high-risk persons. Epidemics and pandemics depend on the genetic variability of influenza viruses and susceptibility of the population. Influenza epidemics involve susceptible children and adolescents, who spread influenza to adults in their homes and in the community. During epidemics, influenza is associated with a death-to-case ratio of 30% in patients with high-risk conditions (Glezen 1982). In epidemics, a characteristic time relationship exists between peak occurrence of influenza cases and deaths. In the influenza pandemic of 1957 in the US, respiratory illnesses reached peak 3 weeks before mortality from influenza and pneumonia (Kilbourne 1987). This interval represents the spread from the young to the high-risk persons and elderly.
Impact of influenza in patients with COPD
Influenza can have a profound social impact, ranging from an increased demand for medical services to disruption of community services and national economy. The impact of influenza in patients with COPD can be important due to the higher incidence of influenza-related complications in patients with COPD (Barker and Mullooly 1980; Glezen et al 1987; Izurieta et al 2000; Neuzil, Mellen, et al 2000; Neuzil, Wright, et al 2000).
Influenza is the most common cause of lower respiratory tract infections in developed countries. In the US, 48 million cases occur each year, being responsible for 3.9 million hospitalizations and 36,000 deaths (NCHS 1998; Thomson 2004). Physician visits and hospitalizations account for the majority of US$3 billion in direct costs. Indirect costs due to costs related to work have been estimated in US$15 billion per year (Harper et al 2005). During influenza epidemics from 1979–80 to 2000–1, the estimated overall number of influenza-associated hospitalizations ranged from 54,000 to 400,000 per epidemic period (Simonsen et al 2000). An average of 220,000 influenza-related excess hospitalizations occurred per year, with 63% of all hospitalizations occurring among persons aged >64 years (Thomson 2004). Estimated rates for influenza-related cardiopulmonary problems are 0.4–0.6 per 100,000 in persons aged 0–49 years, 7.5 per 100,000 in those aged 50–64 years and 98.3 per 100,000 in those aged >64 years (Harper et al 2005).
Influenza is associated with a death-to-case ratio of 1–2 per 2000 cases. In the US, the number of deaths attributed to influenza-associated cardiopulmonary problems increased from 19,000 in the period 1976–90 to 36,000 in the period 1990–99 (Thomson et al 2003). This increase can be explained by the higher number of influenza infections due to the influenza A H3N2 serotype in the period 1990–99 (CDC 2004). During this period, 90% of detected viruses were from the H3N2 serotype, compared with 57% during the period 1976–90 (Thompson et al 2003). During epidemics the total number of deaths from influenza can be high due to the large number of cases. An epidemic affecting 20% of the population of the US, Canada, and Europe could result in 70,000–140,000 deaths.
In developed countries, 5% of persons aged 0–15 years and 5%–10% of adults aged 15 or more years have chronic bronchitis, emphysema, or unremitting asthma, and more than 52 million people suffer from COPD around the world (Hurd 2000; Pauwels et al 2001). In the US, over 1.5 million new cases are diagnosed from bronchitis, emphysema, or COPD each year. In 2001, the total number of persons aged 25 or more years with COPD was 12 million, of whom 2 million had emphysema, 9.2 million had bronchitis, and 0.9 million had both emphysema and chronic bronchitis (NHLBI 2003). A 10%–20% infection rate in patients with COPD can therefore generate an important increase in the demand for health services.
In the adult population, hospitalization rates can be 2 to 30 times higher among high-risk adults (Table 1). Glezen and colleagues (1987) determined the frequency of high-risk of patients hospitalized with acute respiratory disease (ARD). The risk for ARD hospitalization was 19.7 per ten thousand for persons with high-risk conditions and only 9.3 for persons without. Chronic pulmonary disorders were the most common underlying conditions identified, and persons with pulmonary conditions had the greatest risk for acute respiratory disease hospitalization. The highest rate occurred among persons older than 65 years of age with pulmonary conditions (87.5 per ten thousand), and the rate was 27.5 for persons 45 to 64 years of age. Cardiac conditions were the second most frequent group of underlying disorders of patients hospitalized with ARD. Only for persons younger than 20 years of age was the risk of ARD hospitalization greater for persons with cardiac conditions than for those with pulmonary conditions (22.9 and 14.9 per ten thousand, respectively).
Table 1.
Hospitalization rates for acute respiratory disease in patients with and without chronic conditions
| Population | Year of study | Age | Hospitalizations per 100,000 persons with high-risk conditions | Hospitalizations per 100,000 persons without high-risk conditions |
|---|---|---|---|---|
| HealthMaintenance Organizations (Barker et al 1980) | 1968–1969, 1970–1971, 1972–1973 | 15–44 years | 56–110 | 23–25 |
| 45–64 years | 392–635 | 13–23 | ||
| ≥65 years | 399–518 | - | ||
| TennesseeMedicaid (Neuzil et al 2000) | 1973–1993 | 0–11 months | 1900 | 496–1038 |
| 2–4 years | 800 | 196 | ||
| 5–17 years | 320 | 86 | ||
| 92 | 41 | |||
| Kaiser Permanente (Izurieta et al 2000) | 1992–1997 | 0–1 years | 1181 | 231 |
| 2–4 years | 713 | 53 | ||
| 5–17 years | 386 | 19 | ||
| Group Health Cooperative (Izurieta et al 2000) | 1992–1997 | 0–1 years | 772 | 193 |
| 2–4 years | 458 | 21 | ||
| 5–17 years | 216 | 16 | ||
| Population of Houston (Glezen et al 1987) | 1978–1981 | All ages | 197 | 93 |
Hospitalization rates can be 5 to 22 times higher in children who have medical conditions who put them at increase risk for influenza-related complications than in healthy individuals (Table 1). Izurieta and colleagues (2000) studied hospitalization rates for acute respiratory-disease among infants and children aged less than 17 years during the period from 1992 to 1997, and observed that hospitalization rates were 5–20 times higher among high-risk individuals at Northern California Kaiser sites and 4–22 times higher at Group Health Cooperative sites (Table 1). Hospitalization rates among children aged <2 years at high risk for complications of influenza were 60 and 48 times higher at Northern California Kaiser and Group Health Cooperative sites, respectively, than among healthy individuals aged 5–17 years.
Deaths from influenza are more common among persons of any age with chronic pulmonary disease and other chronic conditions (Simonsen et al 1997). Case fatality with influenza A epidemics in patients with COPD can be 30% or more, while in healthy persons the case-fatality rate can be 1–2 per 2000 cases. Combined pulmonary and cardiovascular disease can increase the risk of death by 800-fold (Barker and Mullooly 1982). Reports of 153 laboratory-confirmed influenza-related pediatric deaths from 40 states during the 2003–2004 influenza season indicated that 40% were aged <2 years and 30% of those aged 2–17 years have an underlying medical condition traditionally considered to place a person at risk for influenza-related complications (Harper et al 2005).
Influenza vaccination
The primary strategy for reducing the effect of influenza in persons with COPD is vaccinating these patients and their contacts each year before seasonal increases in influenza virus circulation. Influenza vaccination should be recommended in patients with COPD for two reasons. Firstly, vaccination can reduce the risk of influenza-related complications and exacerbations. Secondly, when vaccine and epidemic strains are well-matched, a higher vaccination rate can reduce the risk for influenza outbreaks due to herd immunity. Influenza vaccination is recommended in the following population groups (ACP 1996; USPSTF 1996; WHO 2002; Fukuda et al 2004; Harper et al 2005; CDC 2006):
Persons aged more than 64 years
- Persons aged <64 years with one or more of the following high-risk conditions for influenza-related complications:
- o Chronic pulmonary disease
- o Chronic heart disease
- o Diabetes
- o Renal dysfunction
- o Hemoglobinopathies
- o Cancer
- o Human immunodeficiency virus infection
- o Immunosuppressant treatment
- o Pregnant women beyond first trimester during influenza season
- o Persons aged 6 months to 18 years treated with aspirin for a long period
- o Children aged 6–23 months
Persons who can transmit influenza to high-risk patients
Two influenza vaccines are available: the inactivated trivalent influenza vaccine (TIV), produced by Sanofi-Pasteur, Chiron Corporation, and GlaxoSmithKline, and the live attenuated influenza vaccine (LAIV), produced by Med Immune Vaccines. Both inactivated influenza vaccine and LAIV are available to reduce the risk for influenza infection and illness.
The differences between TIV and LAIV vaccines are presented on Table 2. Inactivated influenza vaccine contains killed viruses, while LAIV contains live, attenuated viruses still capable of replication. LAIV is administered by intranasal spray, while inactivated influenza vaccine is administered intramuscularly by injection. Inactivated influenza vaccine is approved for use in healthy and high-risk persons aged ≥6 months, while LAIV is approved for use in healthy persons aged 5–49 years.
Table 2.
Inactivated influenza vaccine compared with live attenuated influenza vaccine
| Inactivated vaccine (TIV) | Live attenuated vaccine(LAIV) | |
|---|---|---|
| Type of vaccine | Killed virus | Live virus |
| Route of administration | Intramuscular injection | Intranasal spray |
| Vaccine strains updated | Annually | Annually |
| Number of strains included | 2 influenza A | 2 influenza A |
| 1 influenza B | 1 influenza B | |
| Influenza A strains in 2005 vaccine | California/7/2004 (H3N2) | California/7/2004 (H3N2) |
| New Caledonia/20/99 (H1N1) | New Caledonia/20/99 (H1N1) | |
| Influenza B strain in 2005 vaccine | Shanghai/361/2002 | Shanghai/361/2002 |
| Approved age | ≥6 months | 5–49 years |
| Approved for persons with chronic obstructive pulmonary disease | Yes | No |
| Approved for other high-risk persons | Yes | No |
| Can be administered to family members or close contacts of high-risk persons not severely immunosuppressed | Yes | Yes |
| Can be administered to family members or close contacts of immunosuppressed persons not requiring a protected environment | Yes | Yes |
| Can be administered to family members or close contacts of immunosuppressed persons requiring a protected environment | Yes | Inactivated vaccinepreferred |
| Can be administered simultaneously with other vaccines | Yes | Yes |
| Can be administered within 4 weeks of another inactivated vaccines | Yes | Yes |
| Can be administered within 4 weeks of another live vaccines | Yes | Yes, but prudent to space 4 weeks apart |
Since 1976, influenza vaccines contain one three viral strains: influenza A (H3N2) virus, one A (H1N1) virus, and one B virus. Recommended composition of the influenza vaccine for use in the 2006–07 influenza season include the following viral strains: A/New Caledonia/20/99 (H1N1), A/Wisconsin/67/2005 (H3N2), B/Malaysia/2506/2004 (WHO 2006). Both vaccines stimulate host response to the antigenic determinants on the viral surface. Each year, one or more virus strains might be changed on the basis of global surveillance for influenza viruses and the emergence and spread of new strains. Vaccines should be administered annually to provide optimal protection against influenza because influenza antigens change progressively with time, so that vaccination against one strain could provide little or no protection against new strains.
Inactivated and live attenuated influenza vaccine should not be administered to persons known to have anaphylactic hypersensitivity to eggs or to other components of the vaccine without first consulting a physician. Prophylactic use of antiviral agents is an option for preventing influenza among such persons. Persons who have a history of anaphylactic hypersensitivity to vaccine components but who are also at high risk for complications from influenza can benefit from vaccine after appropriate allergy evaluation and desensitization. Persons with acute febrile illness usually should not be vaccinated until their symptoms have abated. However, minor illnesses with or without fever do not contraindicate use of influenza vaccine.
The most frequent side effect of inactivated influenza vaccination is soreness at the injection site that lasts 1–2 days. Immediate reactions (eg, hives, angioedema, allergic asthma, and systemic anaphylaxis) rarely occur after influenza vaccination. These reactions probably result from hypersensitivity to certain vaccine components; the majority of reactions probably are caused by residual egg protein. Fever, tiredness, muscle aches, and other systemic symptoms can occur after vaccination with inactivated vaccine and most often affect persons who have had no previous exposure to the influenza virus antigens in the vaccine. Local reactions typically are mild and rarely interfere with the person’s ability to conduct usual daily activities. One blinded, randomized, cross-over study among 1952 adults and children with asthma, demonstrated that only body aches were reported more frequently after inactivated influenza vaccine (25.1%) than placebo-injection (20.8%) (ALAACRC 2001). One study reported 20%–28% of children with asthma aged 9 months–18 years with local pain and swelling (Park et al 1996). Wright and colleagues (1977) reported no difference in local reactions among 53 children aged 6 months–6 years with high-risk medical conditions or among 305 healthy children aged 3–12 years in a placebo-controlled trial of inactivated influenza vaccine. In a study of 12 children aged 5–32 months, no substantial local or systemic reactions were noted (Piedra et al 1993).
The use of a swine influenza inactivated vaccine in 1976 was associated with an increased frequency of Guillain-Barré syndrome (Schonberger et al 1979). The rate of persons affected by this medical problem was 1 per 120,000 vaccines. Nevertheless, this side-effect has not been observed with influenza vaccines administered after 1976 (Kaplan et al 1982).
Efficacy and effectiveness of the influenza vaccine
Efficacy and effectiveness of influenza vaccination have been demonstrated in children, adults, elderly, and patients with high-risk conditions. In health persons the vaccine is 70%–80% effective (Harper et al 2005; Fukuda et al 2004; CDC 2006). The US Preventive Services Task Force (1996) has strongly recommended that clinicians provide influenza vaccination to patients with COPD, based on the good evidence that improved health outcomes and benefits substantially outweigh harms.
The vaccine is effective in preventing secondary complications and reducing the risk for influenza-related hospitalization and death among adults aged ≥65 years with and without high-risk medical conditions. One study assessing vaccine effectiveness in different high-risk subpopulations showed that in patients aged more than 64 years with cardio-pulmonary disease (Nichol et al 1998), influenza vaccination reduced influenza-related hospitalizations by 29% and deaths from all-cause by 49%. In patients with other chronic conditions such as diabetes, renal disease, rheumatologic disease, and stroke, influenza vaccination reduced hospitalizations by 32% and deaths from all-cause by 64%. In the healthy persons, influenza vaccination reduced hospitalizations by 49% and deaths from all-cause by 55%.
Nonrandomized studies in individuals aged >64 years with or without chronic medical conditions have reported that vaccine reduces pneumonia and influenza hospitalizations by 18% to 52% and deaths from all causes by 27% to 70% (Gross et al 1995; Nichol et al 1998, 1999; Fedson et al 1992; Nordin et al 2001). One meta-analysis estimated that influenza vaccine reduced influenza-like illness by 33% (95% confidence interval [CI]: 27%–38%), hospitalizations from pneumonia and influenza by 33% (95% CI: 27%–38%), and mortality from all causes by 50% (95% CI: 46%–56%) in community-dwelling elderly (Vu et al 2002). A second meta-analysis including cohort studies in both nursing homes and noninstitutionalized elderly population estimated that influenza vaccine reduced respiratory illness by 56% (95% CI: 39%–68%), hospitalizations by 50% (95% CI: 28%–65%), and mortality by 68% (95% CI: 56%–76%) (Mullooly et al 1994).
Several case-control studies have shown the effectiveness of influenza vaccination to prevent hospitalizations. One study carried out in the UK estimated that vaccination reduced influenza, pneumonia, bronchitis, and emphysema by 63% (Ahmed et al 1997). Another study carried out in Spain showed that vaccination reduced serologically confirmed pneumonia by 79% (Ohmitt and Monto 1995).
One major factor influencing on the vaccine effectiveness is the antigenic similarity between vaccine viral strains and circulating strains (Edwards et al 1994; Nichol et al 1995; Grotto et al 1998; Pyhala et al 2001). When vaccine and circulating viruses are well matched, vaccine can reduce laboratory-confirmed influenza illness in adults aged <65 years by 70%–90% (Demichelei et al 2000), but in one study carried out when vaccine and circulating strains were not well matched, laboratory-confirmed influenza illness was reduced by 52% in healthy persons and by 38% in patients with high-risk conditions (CDC 2006). In another study in persons with high-risk conditions, vaccine effectiveness ranged from 43% to 56% when antigens from vaccine and circulating strains were similar and from 21% to 42% when they were not well matched (Nichol et al 1998).
In working population, influenza vaccination can reduce upper respiratory illness by 25%, laboratory-confirmed influenza illness by 86%–88% and febrile respiratory illness by 29%–34%. Physician visits and illness-related work absenteeism can be reduced by 42% and 32%–52%, respectively (Patriarca et al 1986; Mullooly et al 1994; Demicheli et al 2000).
Children aged ≥6 months can develop protective levels of anti-influenza antibody against specific influenza virus strains after influenza vaccination. One study in children with asthma aged 2–6 years and 7–14 years obtained a vaccine efficacy of 22%–54% and 60%–78%, respectively (Sugaya et al 1994). A 2-year randomized study of children aged 6–24 months determined that ≥89% of children seroconverted to all three vaccine strains during both years (Hoberman et al 2003). Other studies report that inactivated TIV decreases the incidence of influenza-associated otitis media among young children by approximately 30% (Heikkinen et al 1991; Clements et al 1995).
Cost-effectiveness of influenza vaccine
All evaluative studies assessing the cost-effectiveness of influenza vaccination obtained favorable cost-effectiveness ratios or cost savings (Nichol et al 2003; Rothberg 2005). In one 6-year study of elderly, members of a managed care organization obtained a net cost saving of US$73 per vaccinated person (Nichol et al 1998). An evaluative study carried out in The Netherlands obtained a cost-effectiveness ratio of €1820 per life year gained in elderly persons and cost savings in chronically ill elderly persons (Postma et al 1999). In one study assessing the cost quality adjusted life year gained (QALY) that included all age groups, cost savings were obtained in persons aged ≥65 years, while in younger individuals the cost-effectiveness ratio ranged from US$23 to US$256 per QALY (Bridges et al 2000). Cost-effectiveness of influenza vaccination is lower in healthy individuals aged <64 years because healthcare costs from influenza are lower in younger individuals.
Influenza vaccination can reduce healthcare costs with influenza illness. Economic studies of influenza vaccination of persons aged ≥65 years conducted in the US have reported overall societal cost savings and substantial reductions in hospitalization and death (Harper et al 2005). Studies of adults aged <65 years have reported that vaccination can reduce both direct medical costs and indirect costs from work absenteeism (Patriarca et al 1986; Mullooly et al 1994; Gross et al 1995; Bridges et al 2000; Nichol 2001). Reductions of 13%–44% in healthcare provider visits, 18%–45% in lost workdays, 18%–28% in days working with reduced effectiveness, and 25% in antibiotic use for influenza-associated illnesses have been reported (Patriarca et al 1986; Mullooly et al 1994; Demicheli et al 2000; Nichol et al 2003, 2004).
The cost-effectiveness of influenza vaccination depends on the following factors: 1) efficacy and effectiveness of the vaccine, 2) incidence of influenza, 3) vaccination costs, and 4) healthcare costs from influenza. A higher influenza vaccine efficacy and effectiveness is associated with a higher reduction in healthcare costs and a higher cost-effectiveness. For this reason, in evaluative studies taking into account results obtained in clinical trails and cohort studies, the cost-effectiveness is higher when vaccine and circulating viral strains are well matched. Vaccine-related reduction in healthcare costs from influenza is higher when attack rate and healthcare costs per case are higher. One cost-effectiveness analysis estimated a cost of US$60 to US$4000 per illness averted among healthy persons aged 18–64 years, depending on influenza attack rates and vaccine effectiveness (Demicheli et al 2000). Cost-effectiveness of influenza vaccination depends also on vaccination costs. In one evaluative study assessing the cost-effectiveness of vaccinating elderly people in a low cost vaccination centre, a net cost saving of US$117 per individual was obtained (Nichol et al 1998).
Antiviral treatment and chemoprophylaxis
Although influenza vaccination is the cornerstone for the control and treatment of influenza, antiviral medications can be used as an adjunct to vaccination. Four antiviral drugs are available for the prevention and treatment of influenza infections (Table 3). Oseltamivir and zanamivir are neuraminidase inhibitors, acting against both influenza A and B infections. Rimantadine and amantadine block the M2 channel of influenza A virus. Dosage recommendations vary by age group and medical conditions (Table 3).
Table 3.
Comparison of antiviral drugs for the treatment and prophylaxis of influenza
| Drug | Indication | Dosage in individuals aged >12 years | Effective against influenza | Precautions in patients with: | |||
|---|---|---|---|---|---|---|---|
| Side effects % | COPD | Renal problems | Hepatic problems | ||||
| Amantadine | Treatment | 100 mg bid* | A | 0–70 | No | Yes | Yes |
| Prophylaxis | 100 mg bid* | ||||||
| Rimantadine | Treatment | 100 mg bid* | A | 0–2 | No | Yes | Yes |
| Prophylaxis | 100 mg bid* | ||||||
| Oseltamivir | Treatment | 75 mg bid | A and B | 5–10 | No | Yes | No |
| Prophylaxis | 75 mg/day | ||||||
| Zanamivir | Treatment | 10 mg bid | A and B | <1 | Yes | No | No |
| Prophylaxis | not approved | ||||||
Abbreviations: bid, twice per day; COPD, chronic obstructive pulmonary disease.
Notes: *100 mg/day in the elderly.
When administered within two days of illness onset to healthy adults, amantadine and rimantadine can reduce the duration of uncomplicated influenza A illness, and zanamivir and oseltamivir can reduce the duration of uncomplicated influenza A and B illness by approximately 1 day, compared with placebo (Harper et al 2005). Antiviral therapies could possibly reduce influenza duration and complications in patients with COPD and other medical conditions, although information concerning the effectiveness of amantadine, rimantadine, zanamivir, and oseltamivir in patients with chronic medical conditions are limited (Hayden et al 1997; Cooper et al 2003; Jefferson et al 2000).
Resistance against rimantadine and amantadine can appear rapidly in approximately one third of patients receiving these therapies (Hayden et al 1992; Saito et al 2002). During the course of amantadine or rimantadine therapy, resistant influenza strains can replace susceptible strains within 2–3 days of starting therapy. Resistant viruses have been isolated from persons who live at home or in an institution where other residents are taking or have recently taken amantadine or rimantadine as therapy. Amantadine- and rimantadine-resistant viruses are not more virulent or transmissible than susceptible viruses (Hayden et al 1992). The screening of epidemic strains of influenza A has rarely detected amantadine- and rimantadine-resistant viruses (Ziegler et al 1999). Development of viral resistance to zanamivir and oseltamivir during treatment has been identified but does not appear to be frequent (Jackson et al 2000). To reduce the emergence of antiviral drug-resistant viruses, amantadine or rimantadine therapy for persons with influenza A illness should be discontinued as soon as clinically warranted, typically after 3–5 days of treatment or within 24–48 hours after the disappearance of signs and symptoms. The recommended duration of treatment with either zanamivir or oseltamivir is 5 days.
Chemoprophylactic drugs can be used as adjuncts to vaccination in preventing and controlling influenza. Both amantadine and rimantadine are indicated for chemoprophylaxis of influenza A infection, but not influenza B (Table 3). Both drugs are approximately 60%–90% effective in preventing illness from influenza A infection (Wintermeyer and Nahata 1995; Demicheli et al 2000). Amantadine and rimantadine have been studied extensively among nursing home populations as a component of influenza outbreak-control programs, which can limit the spread of influenza within chronic-care institutions (Guay 1994; Wintermeyer and Nahata 1995).
Antiviral therapy can be used to prevent influenza in patients with COPD and other chronic conditions in the following situations:
When they have not been vaccinated.
When influenza vaccine has a lower efficacy and effectiveness due to viral antigenic variation.
When influenza vaccine is contraindicated.
When they are known to have experienced Guillain-Barré syndrome within 6 weeks after a previous influenza vaccination.
Antiviral therapy can be used also in unvaccinated persons who can transmit influenza to high-risk patients, including healthcare workers, residents of nursing homes or chronic care facilities, close contacts of high-risk patients, and household member of high-risk persons.
Antiviral therapy can be used as an adjunct to vaccination in controlling outbreaks of influenza among patients with COPD and other high-risk conditions in long-term facilities and hospitals. The majority of published reports showing the efficacy and effectiveness of antiviral agents to control influenza outbreaks are based, however, on studies carried out in nursing homes where amantadine and rimantadine were used to prevent influenza A infections (Patriarca et al 1987; Guay 1994; Wintermeyer and Nahata 1995). The prophylactic use of zanamivir and oseltamivir in nursing homes and among patients with chronic medical conditions is more limited, but their efficacy and effectiveness could be similar to that for amantadates (Bowles et al 2002; Schilling et al 1998; Lee et al 2000; Parker et al 2001).
Persons at high risk for complications of influenza still can be vaccinated after an outbreak of influenza has begun in a community. Medication should be taken, in this situation, for two weeks in order to provide early protection until vaccine-induced antibodies appear. When influenza vaccine is administered while influenza viruses are circulating, chemoprophylaxis should be considered for persons at high risk during the time from vaccination until immunity has developed. Children aged <9 years who receive influenza vaccine for the first time can require six weeks of prophylaxis (ie, prophylaxis for four weeks after the first dose of vaccine and an additional two weeks of prophylaxis after the second dose).
When determining the timing and duration for administering influenza antiviral medications for prophylaxis, factors related to cost, compliance, and potential side effects should be considered. To be maximally effective as prophylaxis, the drug must be taken each day for the duration of influenza activity in the community. However, to be most cost-effective, one study of amantadine or rimantadine prophylaxis reported that the drugs should be taken only during the period of peak influenza activity in a community (Patriarca et al 1987).
When outbreaks occur in institutions, chemoprophylaxis should be administered to all residents, regardless of whether they received influenza vaccinations during the previous fall and should continue for a minimum of two weeks. If surveillance indicates that new cases continue to occur, chemoprophylaxis should be continued until approximately one week after the end of the outbreak. The dosage for each resident should be determined individually. Chemoprophylaxis can be offered to the unvaccinated staff providing healthcare to persons at high risk. Prophylaxis should be considered for all employees, regardless of their vaccination status, if the outbreak is caused by a variant strain of influenza that is not well-matched by the vaccine.
To limit the potential transmission of drug-resistant virus during outbreaks in institutions, whether in chronic or acute-care settings or other closed settings, measures should be taken to reduce contact as much as possible between persons taking antiviral drugs for treatment and other persons, including those taking chemoprophylaxis, and therapy with amantadine and rimantadine for persons with influenza A illness should be discontinued as soon as clinically warranted, typically after 3–5 days of treatment or within 24–48 hours after the disappearance of signs and symptoms.
Vaccination strategies in patients with COPD
Successful vaccination programs in persons with COPD should combine a plan for identifying persons with COPD, use of reminder/recall systems, assessment of practice-level vaccination rates with feedback to staff, and efforts to remove administrative and financial barriers that prevent persons from receiving the vaccine (Lawson et al 2000). All patients with COPD should be vaccinated each year with the best available influenza vaccine, and monitoring influenza disease and influenza vaccine efficacy. The following strategies could be developed to achieve higher vaccination levels:
Vaccinating patients with COPD in different healthcare facilities during the immunization period (October to December in the Northern hemisphere and April to June in the Southern hemisphere).
Developing specific influenza vaccination programs in primary healthcare centers and outpatient facilities attending patients with COPD.
The immunization period begins in October (Northern hemisphere). October and November is the best time to get vaccinated, but receiving vaccination in December or later can still be beneficial. The flu season can begin as early as October and last as late as May. The first vaccination strategy can be developed in different healthcare settings, including 1) outpatient facilities providing ongoing care, 2) outpatient facilities providing episodic or acute care, 3) nursing homes and other residential long-term care facilities, 4) acute-care hospitals, 5) visiting nurses and other providing home care to patients with COPD, and 6) workplace healthcare facilities.
Hospitals are important sites for immunization because persons at increased risk for subsequent hospitalization can easily be identified and vaccinated. In fact, each year, a percentage of patients hospitalized for influenza had been discharged during the immunization period. In one study, 39%–46% of adult patients hospitalized during the winter with influenza-related diagnoses had been hospitalized during the preceding autumn (Fedson et al 1992). The CDC (2006) recommends that persons of all ages with high-risk conditions who are hospitalized at any time during September–March should be offered and strongly encouraged to receive influenza vaccine before they are discharged. The Global Initiative for COPD considers that influenza vaccination should be given to patients with COPD in hospitals to prevent future exacerbations (Pauwels et al 2001). Thus, the hospital serves as a setting in which the Advisory Committee on immunization Practices (ACIP) of the United States recommends that vaccine providers focus their vaccination efforts in October and earlier primarily on persons aged ≥50 years, persons aged <50 years at increased risk for influenza-related complications (including children aged 6–23 months), household contacts of persons at high risk (including out-of-home caregivers and household contacts of children aged 0–23 months), and healthcare workers. Vaccination of children aged <9 years who are receiving vaccine for the first time should also begin in October or earlier because those persons need a booster dose 1 month after the initial dose. Efforts to vaccinate other persons who wish to decrease their risk for influenza infection should begin in November.
The second vaccination strategy is based on the use of reminder/recall systems to recommend and vaccinate all patients with COPD attended in primary healthcare centers and outpatient clinics. This strategy is therefore defined as a target-based strategy (ACP 1996). In this strategy, physicians responsible for patients with COPD view them as a target population for whom influenza vaccination should be provided each year: This vaccination strategy can be divide into the following steps:
Enumerate patients attending the healthcare facility by name, address, age, and principal diagnosis.
Identify patients with COPD.
Develop a recall system to immunize all patients with COPD, and not only those who have a medical visit scheduled during the immunization period.
Evaluate vaccination coverage each week, dividing the cumulative number of vaccinated patients by the target population.
Staff in facilities providing ongoing medical care to persons with COPD should identify and label the medical records of patients who should receive vaccination. Vaccine should be offered during visits beginning in September and throughout the influenza season. Persons and institutions planning substantial organized vaccination campaigns should consider scheduling these events after mid-October because the availability of vaccine in any location cannot be ensured consistently in early autumn. Scheduling campaigns after mid-October will minimize the need for cancellations because vaccine is unavailable. Campaigns conducted before November using inactivated vaccine should focus efforts on vaccination of persons aged ≥50 years, persons aged <50 years at increased risk for influenza-related complications (including children aged 6–23 months and pregnant women), healthcare workers, and household contacts of persons at high-risk (including children aged 0–23 months) to the extent feasible.
During October and November each year, vaccination should be routinely provided to all residents of chronic-care facilities with the concurrence of attending physicians. Consent for vaccination should be obtained from the resident or a family member at the time of admission to the facility or anytime afterwards. All residents should be vaccinated at one time, preceding influenza season. Residents admitted through March after completion of the vaccination program at the facility should be vaccinated at the time of admission.
Using standing orders programs is recommended for long-term-care facilities, hospitals, and home health agencies to ensure the administration of influenza vaccines for adults (CMMS 2002). Standing orders programs for influenza vaccination should be conducted under the supervision of a licensed practitioner according to a physician-approved facility or agency policy by healthcare workers trained to screen patients for contraindications to vaccination, administer vaccine, and monitor for adverse events. In the US, the Centers for Medicare and Medicaid Services (CMMS) has removed the physician signature requirement for the administration of influenza vaccines to Medicare and Medicaid patients in hospitals, long-term-care facilities, and home health agencies (CMMS 2002).
Conclusion
Despite recommendations for annual vaccination against influenza in developed countries, more than half of patients with COPD and other chronic medical conditions do not receive a vaccine. In patients with COPD, influenza is associated with complications, such as bacterial pneumonia, and worsening of pulmonary disease. Each year, all persons with COPD should be vaccinated with the inactivated TIV, containing the most frequent two influenza A viral strains and one influenza B viral strain detected in the previous influenza season. To achieve a 100% vaccination rate in patients with COPD, all patients with COPD registered in health insurance companies and attended in health centers and specialized clinics should be vaccinated during the immunization period (October–December in Northern hemisphere and April–May in Southern hemisphere). Antiviral therapies could be used as an adjunct to vaccination, since they could reduce the duration and complications of influenza when administered within two days of illness onset. Research is necessary for new antiviral therapies that could prevent influenza with cost-effectiveness similar to the influenza vaccine.
References
- Ahmed AH, Nicholson KG, Nguyen-van Tam JS, et al. Effectiveness of influenza vaccine in reducing hospital admissions during the 1989–90 epidemic. Epidemiol Infect. 1997;118:27–33. doi: 10.1017/s0950268896007121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [ACP] American College of Physicians . Guide for adult immunization. Philadelphia: ACP; 1996. [Google Scholar]
- [ALAACRC] American Lung Association Asthma Clinical Research Centers The safety of inactivated influenza vaccine in adults and children with asthma. N Engl J Med. 2001;345:1529–36. doi: 10.1056/NEJMoa011961. [DOI] [PubMed] [Google Scholar]
- Anthonisen NR. Prognosis in chronic obstructive pulmonary disease: results from multicenter clinical trials. Am Rev Respir Dis. 1989;140:S95–S99. doi: 10.1164/ajrccm/140.3_Pt_2.S95. [DOI] [PubMed] [Google Scholar]
- Anthonisen NR. Bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:526–7. doi: 10.1056/NEJMe020075. [DOI] [PubMed] [Google Scholar]
- Barker WH. Excess pneumonia and influenza associated hospitalization during influenza epidemics in the United States, 1970–78. Am J Public Health. 1986;76:761–5. doi: 10.2105/ajph.76.7.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker WH, Mullooly JP. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol. 1980;112:798–811. doi: 10.1093/oxfordjournals.aje.a113052. [DOI] [PubMed] [Google Scholar]
- Barker WH, Mullooly JP. Pneumonia and influenza deaths during epidemics: implications for prevention. Arch Intern Med. 1982;142:85–9. [PubMed] [Google Scholar]
- Bowles SK, Lee W, Simor AE, et al. Use of oseltamivir during influenza outbreaks in Ontario nursing homes, 1999–2000. J Am Geriatr Soc. 2002;50:608–16. doi: 10.1046/j.1532-5415.2002.50153.x. [DOI] [PubMed] [Google Scholar]
- Bridges CB, Thompson WW, Meltzer MI, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: a randomized controlled trial. JAMA. 2000;284:1655–60. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention Assessment of the effectiveness of the 2003–04 influenza vaccine among children and adults-Colorado, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:707–710. [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention Prevention and control of influenza. MMWR Morb Mortal Wkly Rep. 2006;55(RR-10):1–42. [Google Scholar]
- [CMMS] Centers for Medicare and Medicaid Services Medicare and Medicaid programs; conditions of participation: immunization standards for hospitals, long-term care facilities, and home health agencies. Final rule with comment period. Federal Register. 2002;67:61808–14. [PubMed] [Google Scholar]
- Clements DA, Langdon L, Bland C, et al. Influenza A vaccine decreases the incidence of otitis media in 6- to 30-month-old children in day care. Arch Pediatr Adolesc Med. 1995;149:1113–17. doi: 10.1001/archpedi.1995.02170230067009. [DOI] [PubMed] [Google Scholar]
- Connors AP, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. Am J Resp Crit Med. 1996;154:959–67. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- Cooper NJ, Sutton AJ, Abrams KR, et al. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ. 2003;326:1235–41. doi: 10.1136/bmj.326.7401.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox NJ, Subbarao K. Influenza. Lancet. 1999;354:1277–82. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- Cox NJ, Fuller F, Kaverin, et al. Orthomyxoviridae. In: van Regenmortel MHV, Fauquet CM, Bishop DHL, et al., editors. Virus taxonomy: Classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. San Diego: Academic Press; 2000. pp. 585–97. [Google Scholar]
- Demicheli V, Jefferson T, Rivetti D, et al. Prevention and early treatment of influenza in healthy adults. Vaccine. 2000;18:957–1030. doi: 10.1016/s0264-410x(99)00332-1. [DOI] [PubMed] [Google Scholar]
- Edwards KM, Dupont WD, Westrich MK, et al. A randomized controlled trial of COLD-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994;169:68–76. doi: 10.1093/infdis/169.1.68. [DOI] [PubMed] [Google Scholar]
- Fedson DS, Wajda A, Nicol JP, et al. Disparity between influenza vaccination rates and risks for influenza-associated hospital discharge and death in Manitoba in 1982–1983. Ann Intern Med. 1992;116:550–5. doi: 10.7326/0003-4819-116-7-550. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Levandowski RA, Bridges CB, et al. Inactivated influenza vaccines. In: Plotkin SA, Orenstein WA, editors. Vaccines. Philadelphia: Saunders; 2004. pp. 339–65. [Google Scholar]
- Glezen WP. Serious morbidity and mortality associated with influenza epidemics. Epidemiol Rev. 1982;4:25–40. doi: 10.1093/oxfordjournals.epirev.a036250. [DOI] [PubMed] [Google Scholar]
- Glezen WP, Decker M, Perrotta DM. Survey of underlying conditions of persons hospitalized with acute respiratory disease during influenza epidemics in Houston, 1978–1981. Am Rev Respir Dis. 1987;136:550–5. doi: 10.1164/ajrccm/136.3.550. [DOI] [PubMed] [Google Scholar]
- Glezen WP, Greenberg SB, Atmar RL, et al. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- Gross PA, Hermogenes AW, Sacks HS, et al. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123:518–27. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- Grotto I, Mandel Y, Green MS, et al. Influenza vaccine efficacy in young, healthy adults. Clin Infect Dis. 1998;26:913–17. doi: 10.1086/513934. [DOI] [PubMed] [Google Scholar]
- Guay DR. Amantadine and rimantadine prophylaxis of influenza A in nursing homes. A tolerability perspective. Drugs Aging. 1994;5:8–19. doi: 10.2165/00002512-199405010-00002. [DOI] [PubMed] [Google Scholar]
- Harper SA, Fukuda K, Uyeki TM, et al. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54:1–40. (No. RR-8) [PubMed] [Google Scholar]
- Hayden FG, Hay AJ. Emergence and transmission of influenza A viruses resistant to amantadine and rimantadine. Curr Top Microbiol Immunol. 1992;176:119–30. doi: 10.1007/978-3-642-77011-1_8. [DOI] [PubMed] [Google Scholar]
- Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. GG167 Influenza Study Group. N Engl J Med. 1997;337:874–80. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Ruuskanen O, Waris M, et al. Influenza vaccination in the prevention of acute otitis media in children. Am J Dis Child. 1991;145:445–48. doi: 10.1001/archpedi.1991.02160040103017. [DOI] [PubMed] [Google Scholar]
- Hoberman A, Greenberg DP, Paradise JL, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA. 2003;290:1608–16. doi: 10.1001/jama.290.12.1608. [DOI] [PubMed] [Google Scholar]
- Hurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest. 2000;117(suppl 2):1S–4S. doi: 10.1378/chest.117.2_suppl.1s. [DOI] [PubMed] [Google Scholar]
- Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342:232–9. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- Jackson HC, Roberts N, Wang ZM, et al. Management of influenza: use of new antivirals and resistance in perspective. Clin Drug Invest. 2000;20:447–54. [Google Scholar]
- Jefferson T, Demicheli V, Deeks J, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev. 2000;2:CD001265. doi: 10.1002/14651858.CD001265. [DOI] [PubMed] [Google Scholar]
- Kaplan JE, Katona P, Hurwitz ES, et al. Guillain-Barre syndrome in the United States, 1979–1980 and 1980–1981. Lack of an association with influenza vaccination. JAMA. 1982;248:698–700. [PubMed] [Google Scholar]
- Kilbourne E. Influenza. New York, NY: Plenum Medical Book Company; 1987. [Google Scholar]
- Lawson F, Baker V, Au D, et al. Standing orders for influenza vaccination increased vaccination rates in inpatient settings compared with community rates. J Gerontol A Biol Sci Med Sci. 2000;55:M522–M526. doi: 10.1093/gerona/55.9.m522. [DOI] [PubMed] [Google Scholar]
- Lee C, Loeb M, Phillips A, et al. Zanamivir use during transmission of amantadine-resistant influenza A in a nursing home. Infect Control Hosp Epidemiol. 2000;21:700–4. doi: 10.1086/501727. [DOI] [PubMed] [Google Scholar]
- Mullooly JP, Bennett MD, Hornbrook MC, et al. Influenza vaccination programs for elderly persons: cost-effectiveness in a health maintenance organization. Ann Intern Med. 1994;121:947–52. doi: 10.7326/0003-4819-121-12-199412150-00008. [DOI] [PubMed] [Google Scholar]
- [NCHS] National Center for Health Statistics . Health, United States. Hyattsville, MD: US Department of Health and Human Services; 1998. [Google Scholar]
- [NHLBI] National Heart, Lung and Blood Institute . Chronic obstructive pulmonary disease. Bethesda: NHLBI, US Department of Health and Human Services Publication No. 03–5229; 2003. [Google Scholar]
- Nichol KL, Lind A, Margolis KL, et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med. 1995;333:889–93. doi: 10.1056/NEJM199510053331401. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Wuorenma J, von Sternberg T. Benefits of influenza vaccination for low-, intermediate, and high-risk senior citizens. Arch Intern Med. 1998;158:1769–76. doi: 10.1001/archinte.158.16.1769. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Baken L, Nelson A. Relation between influenza vaccination and outpatient visits, hospitalization, and mortality in elderly persons with chronic lung disease. Ann Intern Med. 1999;130:397–403. doi: 10.7326/0003-4819-130-5-199903020-00003. [DOI] [PubMed] [Google Scholar]
- Nichol KL. Cost-benefit analysis of a strategy to vaccinate healthy working adults against influenza. Arch Intern Med. 2001;161:749–59. doi: 10.1001/archinte.161.5.749. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Mallon KP, Mendelman PM. Cost benefit of influenza vaccination in healthy, working adults: an economic analysis based on the results of a clinical trial of trivalent live attenuated influenza virus vaccine. Vaccine. 2003;21:2207–17. doi: 10.1016/s0264-410x(03)00029-x. [DOI] [PubMed] [Google Scholar]
- Neuzil KM, Wright PF, Mitchel EF, et al. The burden of influenza illness in children with asthma and other chronic medical conditions. J Pediatr. 2000a;137:856–64. doi: 10.1067/mpd.2000.110445. [DOI] [PubMed] [Google Scholar]
- Neuzil KM, Mellen BG, Wright PF, et al. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000b;342:225–31. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- Nordin J, Mullooly J, Poblete S, et al. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. J Infect Dis. 2001;184:665–70. doi: 10.1086/323085. [DOI] [PubMed] [Google Scholar]
- Ohmitt SE, Monto AS. Influenza vaccine effectiveness in preventing hospitalization among elderly during influenza A and type B seasons. Int J Epidemiol. 1995;24:1240–8. doi: 10.1093/ije/24.6.1240. [DOI] [PubMed] [Google Scholar]
- [OTA] Office of Technology Assessment . Washington, DC: Office of Technology Assessment; 1981. Cost effectiveness of influenza vaccination. Annual report to US Congress. [Google Scholar]
- Palese P, Young JF. Variation of influenza A, B and C viruses. Science. 1982;92:1468–74. doi: 10.1126/science.7038875. [DOI] [PubMed] [Google Scholar]
- Park CL, Frank AL, Sullivan M, et al. Influenza vaccination of children during acute asthma exacerbation and concurrent prednisone therapy. Pediatrics. 1996;98:196–200. [PubMed] [Google Scholar]
- Parker R, Loewen N, Skowronski D. Experience with oseltamivir in the control of a nursing home influenza B outbreak. Can Commun Dis Rep. 2001;27:37–40. [PubMed] [Google Scholar]
- Patriarca PA, Arden NH, Koplan JP, et al. Prevention and control of type A influenza infections in nursing homes. Benefits and costs of four approaches using vaccination and amantadine. Ann Intern Med. 1987;107:732–40. doi: 10.7326/0003-4819-107-5-732. [DOI] [PubMed] [Google Scholar]
- Patriarca PA, Weber JA, Parker RA, et al. Risk factors for outbreaks of influenza in nursing homes. A case-control study. Am J Epidemiol. 1986;124:114–19. doi: 10.1093/oxfordjournals.aje.a114355. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Buist AS, Calverley PM, et al. COLD Scientific Committee Global strategy for the diagnosis, management and prevention of COPD. NHLBI/WHO Global initiative for chronic obstructive lung disease (GOLD) Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- Piedra PA, Glezen WP, Mbawuike I, et al. Studies on reactogenicity and immunogenicity of attenuated bivalent recombinant influenza type A (CRA) and inactivated trivalent influenza virus (TI) vaccines in infants and young children. Vaccine. 1993;11:718–24. doi: 10.1016/0264-410x(93)90255-v. [DOI] [PubMed] [Google Scholar]
- Postma MJ, Bos JM, van Gennep M, et al. Economic evaluation of influenza vaccination. Assessment for The Netherlands. Pharmacoeconomics. 1999;16(suppl 1):33–40. doi: 10.2165/00019053-199916001-00005. [DOI] [PubMed] [Google Scholar]
- Pyhala R, Haanpaa M, Kleemola M, et al. Acceptable protective efficacy of influenza vaccination in young military conscripts under circumstances of incomplete antigenic and genetic match. Vaccine. 2001;19:3253–60. doi: 10.1016/s0264-410x(01)00010-x. [DOI] [PubMed] [Google Scholar]
- Rothberg MB. Cost-effective approaches to influenza prevention and treatment. Expert Rev Pharmacoeconomics Outcomes Res. 2005;5:141–52. doi: 10.1586/14737167.5.2.141. [DOI] [PubMed] [Google Scholar]
- Saito R, Oshitani H, Masuda H, et al. Detection of amantadine-resistant influenza A virus strains in nursing homes by PCR-restriction fragment length polymorphism analysis with nasopharyngeal swabs. J Clin Microbiol. 2002;40:84–8. doi: 10.1128/JCM.40.1.84-88.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling M, Povinelli L, Krause P, et al. Efficacy of zanamivir for chemoprophylaxis of nursing home influenza outbreaks. Vaccine. 1998;16:1771–4. doi: 10.1016/s0264-410x(98)00141-8. [DOI] [PubMed] [Google Scholar]
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol. 1979;110:105–23. doi: 10.1093/oxfordjournals.aje.a112795. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Clarke MJ, Williamson GD, et al. The impact of influenza epidemics on mortality: introducing a severity index. Am J Public Health. 1997;87:1944–50. doi: 10.2105/ajph.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen L, Fukuda K, Schonberger LB, et al. The impact of influenza epidemics on hospitalizations. J Infect Dis. 2000;181:831–7. doi: 10.1086/315320. [DOI] [PubMed] [Google Scholar]
- Sugaya N, Nerome K, Ishida M, et al. Efficacy of inactivated vaccine in preventing antigenically drifted influenza type A and well-matched type B. JAMA. 1994;272:1122–6. [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- [USPSTF] US Preventive Services Task Force . Guide to clinical preventive services. Washington, DC: US Department of Health and Human Services; 1996. [Google Scholar]
- Vu T, Farish S, Jenkins M, et al. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–6. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, et al. Evolution and ecology of influenza viruses. Microbiol Rev. 1992;56:152–79. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Wilson IA. Anti-peptide antibodies detect steps in protein conformational change: low-pH activation of the influenza virus hemag-glutinin. J Cell Biol. 1987;105:2887–96. doi: 10.1083/jcb.105.6.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermeyer SM, Nahata MC. Rimantadine: a clinical perspective. Ann Pharmacother. 1995;29:299–310. doi: 10.1177/106002809502900312. [DOI] [PubMed] [Google Scholar]
- Wright PF, Thompson J, Vaughn WK, et al. Trials of influenza A/New Jersey/76 virus vaccine in normal children: an overview of age-related antigenicity and reactogenicity. J Infect Dis. 1977;136(Suppl):S731–41. doi: 10.1093/infdis/136.supplement_3.s731. [DOI] [PubMed] [Google Scholar]
- [WHO] World Health Organization Influenza vaccines. Wkly Epidemiol Res. 2002;77:229–40. [Google Scholar]
- [WHO] World Health Organization Recommended composition of influenza virus vaccines for use in the 2006–2007 influenza season [online] Accessed on 10/7/06 URL: http://www.who.int/csr/disease/influenza/2007northreport.pdf.
- Ziegler T, Hemphill ML, Ziegler ML, et al. Low incidence of rimantadine resistance in field isolates of influenza A viruses. J Infect Dis. 1999;180:935–9. doi: 10.1086/314994. [DOI] [PubMed] [Google Scholar]