Abstract
Dibenzo[a,l]pyrene (DBP) is among the most potent carcinogenic polycyclic aromatic hydrocarbons (PAHs). Previously, we showed that DBP administration to pregnant mice resulted in high mortality of offspring from an aggressive T-cell lymphoma. All mice that survive to 10 months of age exhibit lung tumors with high multiplicity. Recombinant cytochrome P450 (cyp) 1b1 from mice and the homolog 1B1 in humans exhibit high activity toward the metabolic activation of DBP. Targeted disruption of the cyp1b1 gene protects against most DBP-dependent cancers. Mice heterozygous (het) for the disrupted cyp1b1 allele were used to examine the effect of cyp1b1 gene dosage on DBP transplacental carcinogenesis. Dams were treated with 1 or 15 mg/kg of DBP or 50 mg/kg of benzo[a]pyrene (BP). Cyp1b1-null offspring did not develop lymphoma, whereas, wild-type and heterozygous siblings, born to dams given the high dose of DBP, exhibited significant mortalities between 10–30 weeks of age. At 10 months, all groups had lung adenomas or carcinomas (9.5, 40.3, 25.6 and 100% incidences for controls, BP, 1 and 15 mg/kg DBP, respectively). Cyp1b1 status did not alter BP-dependent carcinogenesis. At 1 mg/kg DBP, cyp1b1 status altered the incidence of lung tumors (19.0, 27.8 and 28.6% for nulls, heterozygous and wild-type, respectively). At 15 mg/kg, tumor multiplicities in cyp1b1 wild-type (9.3) and heterozygous (9.5) offspring were nearly twice that of cyp1b1-null siblings (5.0). These data confirm that cyp1b1 bioactivation of DBP occurs in fetal target tissues, following transplacental exposure, with the thymus and lung as primary and secondary targets, respectively.
Keywords: Cytochrome P4501B1, transplacental cancer, Polycyclic Aromatic Hydrocarbons, Lymphoma, Lung Cancer
INTRODUCTION
The fetus and infant are at increased risk, relative to adults, upon exposure to many environmental chemicals. Yet, only exposures to ionizing radiation and diethylstilbesterol to pregnant women have been sufficiently well documented as causing cancer in their children [1,2]. However, an increasing number of carcinogens have been demonstrated to be effective transplacentally in animal models including arsenic [3], 4-(methynitrosamino)-1-(3-pyridyl)-1-butanone [4], 3’-azido-3’-deoxythymidine [5] and cooked food mutagens such as 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine [6] and polycyclic aromatic hydrocarbons (PAH; ref. 7). These data, in addition to the fact that human epidemiological studies correlate maternal chemical exposure with cancer in children [1,2], highlight the importance of understanding the mechanism(s) of transplacental carcinogenesis.
We recently developed a mouse model of transplacental carcinogenesis in which maternal exposure to the potent PAH, DBP, resulted in high mortality in offspring at a relatively young age from a T-cell lymphoma. At 10 months, the offspring exhibited a 100% incidence of lung adenomas and carcinomas and most of the males had liver lesions as well [8–10].
Recombinant mouse cyp1b1 and human CYP1B1 have the highest activity toward conversion of DBP to DBPDE, a “fjord” region diol-epoxide thought to be responsible for the high mutagenic and carcinogenic potency of DBP [11,12]. In vivo evidence also shows that disruption of the cyp1b1 gene protected adult mice from DBP cancer at most sites [13,14].
We tested the hypothesis that DBP is transplacentally available to the fetus and is bioactivated by cyp1b1 in thymus and lung. Cyp1b1 is expressed at relatively high levels in both fetal thymus and lung [15,16]. The finding that fetal thymus in humans exhibits the highest CYP1B1 expression of any tissue [16] highlights the relevance of this model for human exposures. We bred cyp1b1 heterozygotes (cyp1b1+/−) so that all litters should have a 1:2:1 ratio of wild-type/heterozygote/null in order to assess fetal cyp1b1 gene dosage on DBP transplacental carcinogenesis. Cyp1b1 knockouts were completely resistant to DBP transplacental lymphoma mortality. In lung, cyp1b1 genotype influenced tumor multiplicity only at the high dose of DBP, whereas tumor burden was unaffected by genotype in all other groups.
These results confirm that DBP transplacental lymphoma in mouse is due to cyp1b1 bioactivation in fetal thymus. This finding gains further significance in human fetal risk assessment considering that, in humans, CYP1B1 expression is greater in thymus than in mouse during late stages of ontogeny.
METHODS
Mouse husbandry, carcinogen treatment, necropsy and pathology
Cyp1b1 null mice on both a B6129F1 and a 129 genetic background were obtained from the National Cancer Institute and bred to produce female heterozygote on a B6129F1 background and male heterozygote on a completely 129 genetic background. All colonies were housed in the Laboratory Animal Resource Center at Oregon State University at 20 ± 1° C and 50 ± 10% humidity and a light/dark cycle of 12 hr in micro-isolator cages with CareFRESH bedding (Absorption Corp., Bellingham, WA). During breeding, gestation and lactation, mice were fed powdered AIN93G diet (Research Diets, New Brunswick, NJ) ad libitum. When these mice were 8 weeks-old we bred the B6129F1 cyp1b1+/− females to 129 cyp1b1+/− males. Gestation day 0 was established by appearance of the vaginal plug. Offspring were fed pelleted AIN93G diet for the first three months and then AIN93M diet (Research Diets) ad lib until euthanized. On the 17th day of gestation, pregnant mice were treated with vehicle (corn oil, 5 ml/kg b.w.), 1 or 15 mg/kg DBP or 50 mg/kg benzo[a]pyrene (Midwest Research Inst., Kansas City, MO) in corn oil by gavage. To monitor the health status of the mice, sentinels were housed in the colony and tested for viral or bacterial pathogens and parasites. Upon signs of morbidity, pain or distress the mice were euthanized with an overdose of CO2, exsanguinated, and necropsied. At 10 months of age, any surviving mice were euthanized and necropsied. All procedures for treatment, housing and euthanasia of the mice used in this study have been approved by the Oregon State University Institutional Animal Care and Use Committee and successfully carried out by the investigators [8–10]. Tissues were fixed in 10% formalin, stained with H&E and analyzed by light microscopy. We previously identified the lymphoma to be a T-cell lymphoblastic lymphoma and the lung tumors as hyperplasia, adenoma, adenoma with progression, and carcinoma; the liver tumors as foci and adenoma [8].
Genotyping of mice for the Cyp1b1 allele
At necropsy, an 8 mm ear-punch was collected and lysed overnight at 55°C in 100 µl of DirectPCR Lysis Reagent containing proteinase K (Viagen Biotech Inc., Los Angeles, CA), followed by 45 min at 85°C. The lysis reaction was centrifuged for 10 sec and used directly in a PCR reaction with allele-specific primers (Table 1) to permit one-tube genotyping for the wild-type cyp1b1 allele. A 25 µl PCR reaction contained 1× GeneAmp buffer and 0.25 units AmpliTaq Gold polymerase (Promega Co., Madison, WI), 0.2 mM of each dNTP, 0.2 µM of each primer and 2 µl DNA. PCR cycling conditions were an initial 5 min 95°C enzyme activation step, followed by 35 cycles of: 30 sec at 95°C to denature the DNA; 30 sec at 55°C for primer annealing; 45 sec at 72°C for extension. A final cycle with a further 10 min extension at 72°C concluded the reaction. PCR products were separated on Novex® 8% TBE gels (Invitrogen Technologies, Carlsbad, CA). Cyp1b1 heterozygotes yielded two PCR products of 365-bp and 460-bp (NEO), respectively. Wild-type cyp1b1 mice had a single product of 365-bp. A molecular weight ladder of MspI cut pBR322 DNA (New England Biolabs, Ipswich, MA) and ethidium bromide (Sigma Chemical Co., St. Louis, MO) was used to stain the DNA, followed by UV visualization.
Table 1.
Primer Sequences for cyp1b1 and Neomycin Selection Marker
| Marker | Primer | Product Size | |
|---|---|---|---|
| Cyp1b1a | 1b1-3 | 5′-ttt gcc tgt cac cat tcc ac-3′ | |
| 1b1-3R | 5′-acg act tgg gct taa tgg tc-3′ | 365 bp | |
| Neomycin NEO-1 | NEO-2 | 5′-tga atg aac tgc agg acg ag-3′ | |
| NEO-2 | 5′-cca cag tcg atg aat cca ga-3′ | 460 bp |
Wild-type mice (Cyp1b1+/+) have a single band at 365 bp, knockout mice (Cyp1b1−/−) a single band at 460 bp and the hets (Cyp1b1+/−) both bands.
Statistical Analysis
Litter sizes per dam were compared between treatments using the exact Kruskal-Wallis test (SAS 9.1.3 Npar1way procedure). Because the experimental unit is the pregnant female, litters were accounted for in modeling of individual offspring data. Birth weights were compared between treatments using linear mixed models with litters as a random factor (SAS mixed procedure). Survival curves were compared between cyp1b1 groups using a robust score test in cox proportional-hazard regression with litters as clusters in the model (refs. 8, 17; using S-plus 7.0).
Lung tumor incidences in the three treatment groups (benzo[a]pyrene, DBP1 and DBP15) were each compared to the incidence in the control group using quasilikelihood logistic regression where the observed variation between litters is used to account for overdispersion (SAS genmod procedure). For comparing tumor incidences between the cyp1b1 gene dosages within the DBP1 treatment group, both logistic regression ignoring litters and quasilikelihood logistic regression for grouped binomial data (accounting for litters) were used and both gave very similar results (SAS genmod procedure). Offspring tumor multiplicity was compared between cyp1b1 gene dosages within the DBP15 treatment group with a linear mixed model fit to the log transformed tumor count for each offspring. Because there was large variability in the cyp1b1 group differences from litter to litter (p=0.0011) the mixed model included a random litter-by-cyp1b1 interaction (SAS Mixed procedure). Residuals (either simple or deviance residuals) were examined and found reasonable for each linear model and generalized linear model fit.
RESULTS
Treatments of dams with DBP did not result in any significant maternal or fetal toxicities. The PAHs had no significant effect on litter size (p=0.43, Kruskal-Wallis test) or birth weight (p>0.5) (data not shown). These results are consistent with previous studies in which no toxicity was observed at the 15 mg/Kg DBP maternal dose [8–10].
As previously observed, offspring born to mothers treated with 15 mg/kg DBP exhibited lymphoma-dependent mortality between 10–30 weeks of age (Figure 1A). In support of our hypothesis concerning the role of fetal cyp1b1 in DBP transplacental carcinogenesis, we observed a clear pattern of decreasing survival times with increasing cyb1b1 gene dosage (Figure 1B). None of the cyp1b1-null mice succumbed to the DBP transplacental lymphoma mortality. Siblings with both wild-type alleles exhibited sensitivity comparable to what was previously observed, whereas, mice heterozygous for wild-type cyp1b1 allele exhibited a survival curve almost exactly intermediate between the nulls and the wild-type siblings. There is strong evidence of a trend consisting of decreasing probability of survival with increasing cyp1b1 gene dosage (p=0.0002 score test ignoring litters and p=0.018 robust score test with litters as clusters). The number of litters and offspring, as well as their genotype ratio, is shown in Table 2.
Figure 1. DBP-Dependent Lymphoma Mortality.
A) Offspring, born to dams treated on the 17th day of gestation with corn oil (5 ml/Kg) (squares), benzo[a]pyrene (50 mg/Kg) (triangles), DBP (1 mg/Kg) (inverted triangles) or DBP (15 mg/Kg) (diamonds). B) Offspring of null (square), heterozygous (diamond), and wild-type (triangle) cyp1b1 genotype, born to dams given 15 mg/kg DBP. All mice were euthanized upon signs of morbidity as described in Methods. The full genotype composition is given in Table 2.
Table 2.
Cyp1b1 Genotype Composition
| Genotype Ratio | |||
|---|---|---|---|
| Group | Dams | Offspring | ( 1b1−/− : 1b1+/− : 1b1+/+ ) |
| Control | 7 | 42 | 1.24 : 2.20 : 0.56a |
| Benzo[a]pyrene | 11 | 77 | 0.88 : 1.92 : 1.20 |
| DBP (1 mg/kg) | 11 | 78 | 1.08 : 1.84 : 1.08 |
| DBP (15 mg/kg) | 11 | 79 | 0.88 : 2.04 : 1.16 |
This is not significantly different from expectations (1:2:1) based on a X2 test with a two-tailed P value.
We included a lower dose of DBP in case, at the high dose, cyp1b1 is saturated and cyp1a1 and cyp1a2 could contribute. Again, this is doubtful as previous studies have shown cyp1a enzyme to be expressed at very low levels in fetal tissue during the final trimester [5,16,18], although it is still capable of responding to induction. The benzo[a]pyrene group was included as a negative control. Benzo[a]pyrene, like DBP, is a potent carcinogenic PAH, but is bioactivated primarily by cyp1a1 and cyp1a2, rather than by cyp1b1 [12,19,20]. For this reason, we expected to see few, if any, lymphomas and no significant difference between siblings of different cyp1b1 genotypes with respect to benzo[a]pyrene-dependent transplacental carcinogenesis.
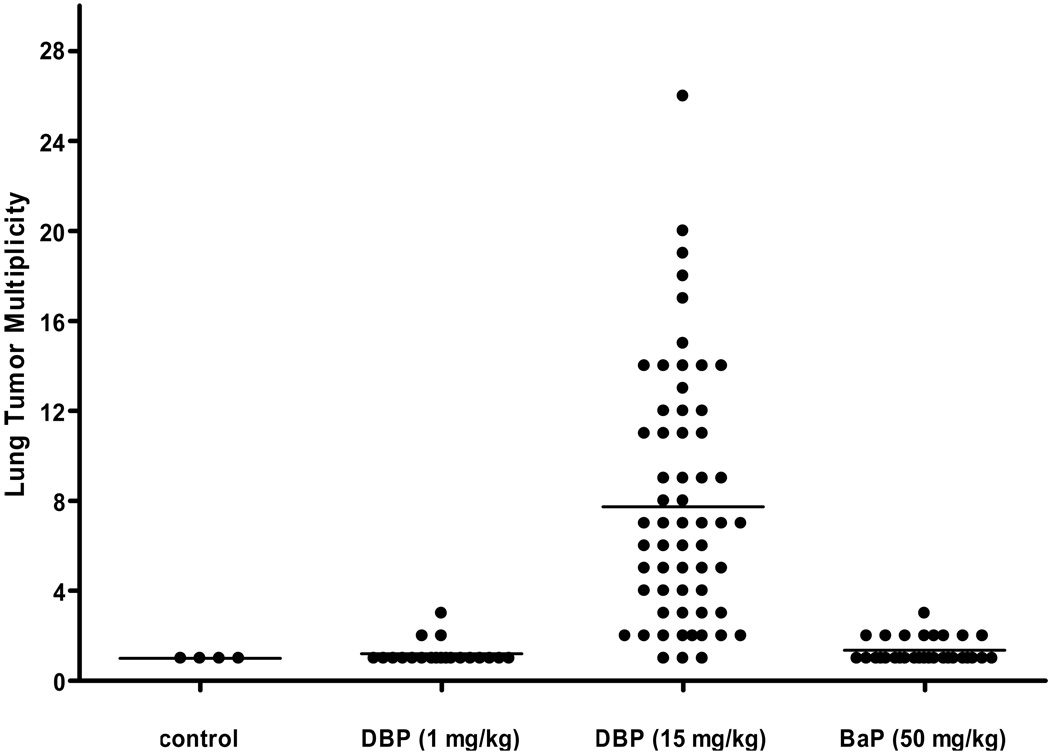
Neither benzo[a]pyrene nor the low dose of DBP produced any lymphoma-dependent mortality over the time-course examined (Figure 1A). All treatments produced higher incidence of lung adenomas and carcinomas than the spontaneous rate in controls and the rates in the BP (p=0.01, quasilikelihood F-test) and DBP15 (p<0.0001) groups were significantly higher than the incidence in the controls at 10 months of age (Table 3). Due to overdispersion between litters, DBP1 is not significant (p=0.11) compared to controls. The observed incidences are consistent with there being an impact of genotype, but the differences are too small to be statistically significant. Interestingly, all mice born to mothers receiving 15 mg/kg DBP had 100% incidence of lung tumors at the end of the study, irrespective of genotype. However, only at the high dose of DBP was there a cyp1b1 genotype-dependent impact lung tumor multiplicity (Figure 2; Table 3). Mice of the wild-type and heterozygous genotype had nearly double the numbers of tumors per mouse compared to the null offspring (9.3, 9.5, and 5.0; Table 3). After adjusting for litter variation (random litter-by-cyp1b1 effect), there was some evidence of a cyp1b1 effect (p=0.08).
Table 3.
10 Month Lung Tumor Multiplicity Sorted by cyp1b1 Genotype
| Incidence | Multiplicity | ||
|---|---|---|---|
| Controls | |||
| Wild-type | 0.0 % | (0/6) | N/A |
| Hets | 13.0 % | (3/23) | 1.00 ± 0.00 |
| Nulls | 7.7 % | (1/13) | 1.00 ± 0.00 |
| BP (50 mg/Kg) | |||
| Wild-type | 39.1 % | (9/23) | 1.33 ± 0.15 |
| Hets | 40.5 % | (15/37) | 1.33 ± 0.15 |
| Nulls | 41.2 % | (7/17) | 1.43 ± 0.17 |
| DBP (1 mg/Kg) | |||
| Wild-type | 28.6 % | (6/21) | 1.00 ± 0.00 |
| Hets | 27.8 % | (10/36) | 1.40 ± 0.20 |
| Nulls | 19.0 % | (4/21) | 1.00 ± 0.00 |
| DBP (15 mg/Kg) | |||
| Wild-type | 100.0 % | (10/10) | 9.30 ± 1.46 |
| Hets | 100.0 % | (28/28) | 9.54 ± 1.03 |
| Nulls | 100.0 % | (16/16) | 5.00 ± 0.96 |
Figure 2. Lung Tumor Multiplicity at 10 months.
Offspring were euthanized at the conclusion of the study (10 months) and lung lesions (predominantly adenomas) quantified by histopathology. Offspring born to mother administered corn oil (control), 1 mg/kg DBP, 15 mg/kg DBP, and 50 mg/kg benzo[a]pyrene (BaP). Bars, group mean; dots, individual animals.
DISCUSSION
Childhood cancer accounts for < 1% of all cancers but these 12,400 cases annually in the U.S. translate to the second leading cause of death (2,300) after accidents, in children in the U.S. [1,2]. Leukemias and lymphomas are the most common type of childhood cancer, followed by tumors of the nervous system. The etiology of 80–90% of childhood cancers is unknown [1,21–23], but considerable evidence exists for maternal exposure to environmental chemicals as contributors to development of childhood leukemias and lymphomas [24–33]. The two environmental in utero exposures that have definitively been linked with increased cancer in children and young adults are diethylstilbestrol and ionizing radiation [1]. In addition, it may well be that exposure in utero or during infancy may not result in cancer during childhood, but may predispose the individual to cancers developing later in life. Indeed, studies with DES in rodent models have shown that transplacental exposure significantly enhanced the risk of development of tumors in older animals upon exposure to chemical carcinogens [2]. In many of the rodent transplacental cancer models, in utero exposure produces cancers in middle aged adult offspring (reviewed in ref.7), that is, the significance of this research is not limited to childhood cancers.
With respect to the suitability as a model for human transplacental carcinogenesis, mice have turned out to be an excellent model in the case of DES [2] Many environmental chemicals for which there are significant human exposures, are transplacental carcinogens in rodents including the food mutagen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine [6], benzo[a]pyrene [34], and the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone [4]. The infant mouse has also been used as a cancer model with enhanced sensitivity compared to the adult for a number of chemical carcinogens such as diethylnitrosamine, PAHs and aflatoxin B1 [35–37]. The sensitivity of the infant mouse to chemical carcinogens is relevant for the present discussion as we administered a very lipophilic carcinogen during late pregnancy in our model. We have recently performed a cross-fostering experiment to estimate how much of the DBP-dependent lymphoma mortality was due to in utero exposure and how much to exposure through nursing (21 days). The results indicate a slightly greater contribution from the much shorter in utero exposure (data not shown).
Currently, over 320,000 Americans have leukemia, lymphoma or myeloma with an estimated 135,500 new cases annually and 85,580 deaths [38]. Although the etiology of the majority of such cancers is unknown, chemical exposures have been identified as one definitive risk factor (reviewed in ref. 1). The mouse can serve as a useful model for human lymphoma [39] with the recognition that certain lymphoma pathologies are species-specific and molecular markers may be distinct. A classification scheme [40] devised under the direction of the Mouse Models of Human Cancers Consortium (MMHCC) has been published and can be used for comparison to the WHO classification of lymphomas [39]. We have, for the first time in any animal model, documented that exposure of pregnant mice to a single dose of a potent PAH, DBP, results in a very aggressive T-cell lymphoma in the offspring beginning at 10 weeks of age [8–10]. Our hypothesis is that maternal transfer to the fetus results in cyp1b1-dependent bioactivation to DBP-(−)-anti-(11R,12S,13S,14R)-dihydrodiol epoxide and other reactive metabolites of DBP in the thymus producing a number of DNA-adducts. This model should recapitulate human fetal exposure to PAHs from maternal diet and airborne particles including direct and second-hand tobacco smoke as a causative factor in leukemias/lymphomas in children and young adults.
We hypothesize that cyp1b1 bioactivation of DBP occurs in fetal target tissues, primarily thymus with the secondary target of lung and that this pathway plays a greater role than other potential means of bioactivation of PAHs, such as peroxidation [41] and the aldo-keto reductase pathways [42], both of which produce oxidative damage to DNA. In previous studies, utilizing DBP or 3-methlycholanthrene in models, in which either the dam or the fetus were Ahr “non-responsive” or “responsive”, it was shown that the responsive dam reduced the risk to the fetus apparently by decreasing the bioavailability through enhanced maternal metabolism and clearance [7,8].
However, the issue of fetal versus maternal metabolism in PAH-transplacental carcinogenesis is not settled. By utilizing crosses of cyp1b1 heterozygote on the same genetic background as our DBP-transplacental model, we were able to demonstrate that DBP-dependent transplacental lymphoma mortality was dependent upon the presence of at least one wild type cyp1b1 allele and there was a very tight correlation between cyp1b1 gene dosage and mortality. In the lung, the impact of the cyp1b1 expression was apparent, but not as marked, possibly due to some contribution from CYPs in the 1a sub-family. It should be kept in mind, however, that lung tumor incidence and multiplicity determined at 10 months of age in the high dose DBP group is complicated from a statistical standpoint by the earlier lymphoma deaths. In offspring born to mothers treated with benzo[a]pyrene or low dose DBP, the statistical analysis is cleaner as we are not dealing with only a population of lymphoma survivors.
The results clearly show that cyp1b1 genotype does not influence benzo[a]pyrene-dependent lung cancer in this model. Lung cancer is the major leading cause of cancer-related deaths for both sexes in the U.S. with 173,700 new cases in 2004 and 160,400 deaths. The five-year survival rate is poor for lung cancer (15%) and any prevention approach would be beneficial. CYP1B1 has been found to be, at least in part, under epigenetic control [43,44] and it has been suggested that CYP1B1 would make an excellent target for prevention strategies [45]. In our previous studies, we demonstrated that the addition of indole-3-carbinol, green tea or caffeine to the maternal diet during pregnancy and nursing significantly reduced lung cancer multiplicity in 10-month-old offspring from mothers treated with 15 mg/kg DBP [9,10]. The results presented in this study provide further evidence that fetal cyp1b1 plays an important role in DBP transplacental carcinogenesis, primarily with lymphoma and to a lesser degree with lung tumors. We conclude that fetal cyp1b1 could be a target for cancer prevention and additional work with specific inhibitors, such as 2,3,4,5-tetramethoxystilbene may prove fruitful in that respect. In addition, as with most other CYPs in humans, 1B1 exists in the population as a number of non-synonymous allelic variants [19,46–49]. The Swiss Protein entry for human CYP1B17 () lists 21 non-synonymous gene variants, found in at least 25 allelic combinations.
The Cancer Genome Anatomy Project data for human CYP1B17) identified 4 non-synonymous SNPs with >5% frequency in a least one population. The same non-synonymous SNPs were also most common in CYP1B1 data from the NIEHS Environmental Genome Project () [50].9 If humans recapitulate the responses observed here, transplacental exposure to carcinogenic PAHs, such as DBP or benzo[a]pyrene, will lead to lymphoma or lung cancer later in life. The CYP1B1 genetic polymorphism may play a role in the target and severity of the transplacental carcinogenesis.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Abby Benninghoff and Lisbeth Siddens for their technical help; Mandy Louderback, Carianne Stearns, and Laura Magana for their excellent animal care and for their provided assistance during necropsies; and the staff of Laboratory Animal Services at Oregon State University.
The work reported here was supported by PHS grants CA90890, ES07060 and ES00210 from the NIH and by The Linus Pauling Institute at Oregon State University.
Footnotes
REFERENCES
- 1.Anderson LM. Introduction and overview. Perinatal carcinogenesis: growing a node for epidemiology, risk management, and animal studies. Toxicol Appl Pharmacol. 2004;199:85–90. doi: 10.1016/j.taap.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Anderson LM. Predictive values of traditional animal bioassay studies for human perinatal carcinogenesis risk determination. Toxicol Appl Pharmacol. 2004;199:162–174. doi: 10.1016/j.taap.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Shen J, Liu J, Xie Y, Diwan BA, Waalkes MP. Fetal onset of aberrant gene expression relevant to pulmonary carcinogenesis in lung adenocarcinoma development induced by in utero arsenic exposure. Toxicol Sci. 2007;95:313–320. doi: 10.1093/toxsci/kfl151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson LM, Hecht SS, Dixon DE, et al. Evaluation of the transplacental tumorigenicity of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in mice. Cancer Res. 1989;49:3770–3775. [PubMed] [Google Scholar]
- 5.Walker DM, Malarkey DE, Seilkop SK, et al. Transplacental carcinogenicity of 3'-azido-3'-deoxythymidine in B6C3F1 mice and F344 rats. Environ Mol Mutagen. 2007;48:283–298. doi: 10.1002/em.20297. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa R, Kimura J, Yaono M, et al. Increased risk of mammary carcinoma development following transplacental and trans-breast milk exposure to a food-derived carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), in Sprague-Dawley rats. Cancer Res. 1995;55:4333–4338. [PubMed] [Google Scholar]
- 7.Miller MS. Transplacental lung carcinogenesis: molecular mechanisms and pathogenesis. Toxicol Appl Pharmacol. 2004;198:95–110. doi: 10.1016/j.taap.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Yu Z, Loehr CV, Fischer KA, et al. In utero exposure of mice to dibenzo[a,l]pyrene produces lymphoma in the offspring: role of the aryl hydrocarbon receptor. Cancer Res. 2006;66:755–762. doi: 10.1158/0008-5472.CAN-05-3390. [DOI] [PubMed] [Google Scholar]
- 9.Yu Z, Mahadevan B, Lohr CV, et al. Indole-3-carbinol in the maternal diet provides chemoprotection for the fetus against transplacental carcinogenesis by the polycyclic aromatic hydrocarbon dibenzo[a,l]pyrene. Carcinogenesis. 2006;27:2116–2123. doi: 10.1093/carcin/bgl072. [DOI] [PubMed] [Google Scholar]
- 10.Castro DJ, Yu Z, Löhr C, et al. Chemoprevention of dibenzo[a,l]pyrene transplacental carcinogenesis in mice born to mothers administered green tea: primary role of caffeine. Carcinogenesis. in press doi: 10.1093/carcin/bgm237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luch A, Coffing SL, Tang YM, et al. Stable expression of human cytochrome P450 1B1 in V79 Chinese hamster cells and metabolically catalyzed DNA adduct formation of dibenzo[a,l]pyrene. Chem Res Toxicol. 1998;11:686–695. doi: 10.1021/tx970236p. [DOI] [PubMed] [Google Scholar]
- 12.Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004;95:1–6. doi: 10.1111/j.1349-7006.2004.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buters JT, Mahadevan B, Quintanilla-Martinez L, et al. Cytochrome P450 1B1 determines susceptibility to dibenzo[a,l]pyrene-induced tumor formation. Chem Res Toxicol. 2002;15:1127–1135. doi: 10.1021/tx020017q. [DOI] [PubMed] [Google Scholar]
- 14.Luch A, Mahadevan B, Baird WM, et al. The role of cytochrome P450 1B1 in dibenzo[a,l]pyrene-induced carcinogenesis. Polycyclic Aromatic Hydrocarbons. 2002;22:781–789. [Google Scholar]
- 15.Choudhary D, Jansson I, Schenkman JB, Sarfarazi M, Stoilov I. Comparative expression profiling of 40 mouse cytochrome P450 genes in embryonic and adult tissues. Arch Biochem Biophys. 2003;414:91–100. doi: 10.1016/s0003-9861(03)00174-7. [DOI] [PubMed] [Google Scholar]
- 16.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Expression patterns of mouse and human CYP orthologs (families 1–4) during development and in different adult tissues. Arch Biochem Biophys. 2005;436:50–61. doi: 10.1016/j.abb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Therneau TM, Grambsch PM. New York: Springer; 2000. Modeling survival data : extending the Cox model. [Google Scholar]
- 18.Xu M, Nelson GB, Moore JE, et al. Induction of Cyp1a1 and Cyp1b1 and formation of DNA adducts in C57BL/6, Balb/c, and F1 mice following in utero exposure to 3-methylcholanthrene. Toxicol Appl Pharmacol. 2005;209:28–38. doi: 10.1016/j.taap.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Shimada T, Oda Y, Gillam EM, Guengerich FP, Inoue K. Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM2009. Drug Metab Dispos. 2001;29:1176–1182. [PubMed] [Google Scholar]
- 20.Kleiner HE, Vulimiri SV, Hatten WB, et al. Role of cytochrome p4501 family members in the metabolic activation of polycyclic aromatic hydrocarbons in mouse epidermis. Chem Res Toxicol. 2004;17:1667–1674. doi: 10.1021/tx049919c. [DOI] [PubMed] [Google Scholar]
- 21.Lightfoot TJ, Roman E. Causes of childhood leukaemia and lymphoma. Toxicol Appl Pharmacol. 2004;199:104–117. doi: 10.1016/j.taap.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Bunin GR. Nongenetic causes of childhood cancers: evidence from international variation, time trends, and risk factor studies. Toxicol Appl Pharmacol. 2004;199:91–103. doi: 10.1016/j.taap.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 23.Wild CP, Kleinjans J. Children and increased susceptibility to environmental carcinogens: evidence or empathy? Cancer Epidemiol Biomarkers Prev. 2003;12:1389–1394. [PubMed] [Google Scholar]
- 24.Alexander FE, Patheal SL, Biondi A, et al. Transplacental chemical exposure and risk of infant leukemia with MLL gene fusion. Cancer Res. 2001;61:2542–2546. [PubMed] [Google Scholar]
- 25.Ma X, Buffler PA, Gunier RB, et al. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environ Health Perspect. 2002;110:955–960. doi: 10.1289/ehp.02110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perera F, Hemminki K, Jedrychowski W, et al. In utero DNA damage from environmental pollution is associated with somatic gene mutation in newborns. Cancer Epidemiol Biomarkers Prev. 2002;11:1134–1137. [PubMed] [Google Scholar]
- 27.Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A, Harnly ME. Childhood cancer and agricultural pesticide use: an ecologic study in California. Environ Health Perspect. 2002;110:319–324. doi: 10.1289/ehp.02110319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flower KB, Hoppin JA, Lynch CF, et al. Cancer risk and parental pesticide application in children of Agricultural Health Study participants. Environ Health Perspect. 2004;112:631–635. doi: 10.1289/ehp.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato I, Watanabe-Meserve H, Koenig KL, et al. Pesticide product use and risk of non-Hodgkin lymphoma in women. Environ Health Perspect. 2004;112:1275–1281. doi: 10.1289/ehp.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quintana PJ, Delfino RJ, Korrick S, et al. Adipose tissue levels of organochlorine pesticides and polychlorinated biphenyls and risk of non-Hodgkin's lymphoma. Environ Health Perspect. 2004;112:854–861. doi: 10.1289/ehp.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shu XO, Perentesis JP, Wen W, et al. Parental exposure to medications and hydrocarbons and ras mutations in children with acute lymphoblastic leukemia: a report from the Children's Oncology Group. Cancer Epidemiol Biomarkers Prev. 2004;13:1230–1235. [PubMed] [Google Scholar]
- 32.De Roos AJ, Hartge P, Lubin JH, et al. Persistent organochlorine chemicals in plasma and risk of non-Hodgkin's lymphoma. Cancer Res. 2005;65:11214–11226. doi: 10.1158/0008-5472.CAN-05-1755. [DOI] [PubMed] [Google Scholar]
- 33.Infante-Rivard C, Siemiatycki J, Lakhani R, Nadon L. Maternal exposure to occupational solvents and childhood leukemia. Environ Health Perspect. 2005;113:787–792. doi: 10.1289/ehp.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson LM, Ruskie S, Carter J, Pittinger S, Kovatch RM, Riggs CW. Fetal mouse susceptibility to transplacental carcinogenesis: differential influence of Ah receptor phenotype on effects of 3-methylcholanthrene, 12-dimethylbenz[a]anthracene, and denzo[a]pyrene. Pharmacogenetics. 1995;5:364–372. doi: 10.1097/00008571-199512000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Vesselinovitch SD, Koka M, Mihailovich N, Rao KV. Carcinogenicity of diethylnitrosamine in newborn, infant, and adult mice. J Cancer Res Clin Oncol. 1984;108:60–65. doi: 10.1007/BF00390974. [DOI] [PubMed] [Google Scholar]
- 36.Vesselinovitch SD, Mihailovich N, Wogan GN, Lombard LS, Rao KV. Aflatoxin B1, a hepatocarcinogen in the infant mouse. Cancer Res. 1972;32:2289–2291. [PubMed] [Google Scholar]
- 37.Rodriguez LV, Dunsford HA, Steinberg M, et al. Carcinogenicity of benzo[a]pyrene and manufactured gas plant residues in infant mice. Carcinogenesis. 1987;18:127–135. doi: 10.1093/carcin/18.1.127. [DOI] [PubMed] [Google Scholar]
- 38.Stewart SL, King JB, Thompson TD, Friedman DO, Wingo PA. Cancer Mortality Surveillance---United States, 1990–2000. MMWR Surveill Summ. 2006;53:1–108. [PubMed] [Google Scholar]
- 39.Teitell MA, Pandolfi PP. Lymphoid Malignancies. In: Holland EC, editor. Mouse Models of Human Cancer. New York: John Wiley & Sons, Inc; 2004. pp. 237–259. [Google Scholar]
- 40.Morse HC, 3rd Anver MR, Fredrickson TN, et al. Bethesda proposals for classification of lymphoid neoplasms in mice. Blood. 2002;100:246–258. doi: 10.1182/blood.v100.1.246. [DOI] [PubMed] [Google Scholar]
- 41.Cavalieri EL, Rogan EG. Radical cations in aromatic hydrocarbon carcinogenesis. Free Radic Res Commun. 1990;11:77–87. doi: 10.3109/10715769009109670. [DOI] [PubMed] [Google Scholar]
- 42.Palackal NT, Burczynski ME, Harvey RG, Penning TM. The ubiquitous aldehyde reductase (AKR1A1) oxidizes proximate carcinogen trans-dihydrodiols to o-quinones: potential role in polycyclic aromatic hydrocarbon activation. Biochemistry. 2001;40:10901–10910. doi: 10.1021/bi010872t. [DOI] [PubMed] [Google Scholar]
- 43.Tokizane T, Shiina H, Igawa M, et al. Cytochrome P450 1B1 is overexpressed and regulated by hypomethylation in prostate cancer. Clin Cancer Res. 2005;11:5793–5801. doi: 10.1158/1078-0432.CCR-04-2545. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima M, Iwanari M, Yokoi T. Effects of histone deacetylation and DNA methylation on the constitutive and TCDD-inducible expressions of the human CYP1 family in MCF-7 and HeLa cells. Toxicol Lett. 2003;144:247–256. doi: 10.1016/s0378-4274(03)00216-9. [DOI] [PubMed] [Google Scholar]
- 45.Guengerich PF, Chun YJ, Kim D, Gilliam EM, Shimada T. Cytochrome P450 1B1: a target for inhibition in anticarcinogenesis strategies. Mutat Res. 2003;523–524:173–182. doi: 10.1016/s0027-5107(02)00333-0. [DOI] [PubMed] [Google Scholar]
- 46.Roos PH, Bolt HM. Cytochrome P450 interactions in human cancers: new aspects considering CYP1B1. Expert Opin Drug Metab Toxicol. 2005;1:187–202. doi: 10.1517/17425255.1.2.187. [DOI] [PubMed] [Google Scholar]
- 47.Shimada T, Watanabe J, Inoue K, Guengerich FP, Gilliam EM. Specificity of 17beta-oestradiol and benzo[a]pyrene oxidation by polymorphic human cytochrome P4501B1 variants substituted at residues 48, 119 and 432. Xenobiotica. 2001;31:163–176. doi: 10.1080/00498250110043490. [DOI] [PubMed] [Google Scholar]
- 48.Shimada T, Watanabe J, Kawajiri K, et al. Catalytic properties of polymorphic human cytochrome P450 1B1 variants. Carcinogenesis. 1999;20:1607–1613. doi: 10.1093/carcin/20.8.1607. [DOI] [PubMed] [Google Scholar]
- 49.Wenzlaff AS, Cote ML, Bock CH, et al. CYP1A1 and CYP1B1 polymorphisms and risk of lung cancer among never smokers: a population-based study. Carcinogenesis. 2005;26:2207–2212. doi: 10.1093/carcin/bgi191. [DOI] [PubMed] [Google Scholar]
- 50.Livingston RJ, Von Niederhausem A, Jegga AG, et al. Pattern os sequence variation across 213 environmental response genes. Genome Res. 2004;14:1821–1831. doi: 10.1101/gr.2730004. [DOI] [PMC free article] [PubMed] [Google Scholar]