Abstract
We examined transplant outcomes after second HLA-matched sibling transplants for primary (16%) and secondary (84%) graft failure in 166 patients with severe acquired aplastic anemia. Performance scores were < 90 in 67% of patients. Most (88%) transplantations used the same donor for both transplants and 84% of second transplants used bone marrow graft. We identified two prognostic factors: inter-transplant interval (surrogate for primary graft failure and early secondary graft failure) and performance status. Shorter inter-transplant interval (≤3 months) and poor performance score (<90) at second transplantation were associated with high mortality. The 8-year probabilities of overall survival when second transplantation was ≤ 3 and > 3 months from first transplant in patients with performance scores of 90–100% were 56% and 76%, respectively. Corresponding probabilities in patients with lower performance scores were 33% and 61%. The predominant cause of failure after second transplantation was non-engraftment (72 of 166 patients) and frequent in patients with primary or early secondary graft failure (51 of 72; 71%). Therefore, novel approaches including conditioning regimens with greater immunosuppression should be explored for these patients.
Keywords: severe aplastic anemia, second transplantation, graft failure
INTRODUCTION
HLA-matched sibling bone marrow (BM) transplantation is an effective treatment for acquired severe aplastic anemia (SAA), particularly in children and young adults (1–3). Despite significant improvements in overall survival in the last twenty years, (2, 4, 5) the rate of graft failure has not changed significantly and remains approximately 10% (5–7). Many patients with graft failure undergo a second transplant receiving grafts from their initial donor or a different donor (6, 8, 9). We report factors affecting outcome after 166 second HLA-matched sibling transplantation for primary or secondary graft failure after an initial HLA-matched sibling transplant.
PATIENTS, MATERIALS AND METHODS
Patients
Data on patients with SAA undergoing second HLA-matched sibling transplantations between 1986–2004 were obtained from the Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin. All patients received BM grafts from their HLA-matched sibling for their first transplant. Excluded were 6 patients who received peripheral blood progenitor cells (PBPC) for their first transplant. The Institutional Review Board of the Medical College of Wisconsin approved this study.
Endpoints
Neutrophil recovery was defined as achieving absolute neutrophil count (ANC) ≥0.5 × 109/L for 3 consecutive days and platelets ≥20 × 109/L unsupported for 7 days. Acute and chronic graft-versus-host disease (GVHD) were diagnosed and graded by transplant centers using standard criteria (10). Primary graft failure was defined as failure to achieve ANC ≥0.5 × 109/L for 3 consecutive days and secondary graft failure, sustained decline in neutrophil count after initial recovery. Death from any cause was considered an event and surviving patients censored at last follow-up.
Statistical methods
The probabilities of neutrophil and platelet recovery and acute and chronic graft-versus-host disease (GVHD) were calculated using the cumulative incidence estimator where death without the event was the competing event (11). The probabilities of early mortality (day-100) and overall survival were calculated using Kaplan-Meier estimator (12). The 95% confidence interval (CI) was calculated using log transformation. Regression models for neutrophil and platelet recovery and early mortality were constructed using the pseudo-value method(13) and for overall mortality, Cox regression (12). All models were constructed using a stepwise forward selection, with a p-value ≤0.05 to indicate statistical significance. Variables considered in regression models are shown in Table 1. Only variables that attained p-value ≤0.05 during model building were retained in the final model. We tested for an effect of transplant center and found none (14). All P-values are 2-sided and analyses were done using SAS software version 9.1 (SAS Institute, Cary, NC).
Table 1.
Patient, disease and transplant characteristics
| 1st transplant | 2nd transplant | 3rd transplant | |
|---|---|---|---|
| Variable | N (%) | N (%) | N (%) |
| Number of patients | 166 | 166 | 23 |
| Age at transplant, years | |||
| ≤10 | 64 (39) | 56 (34) | 11 (48) |
| 11–20 | 49 (29) | 52 (31) | 3 (13) |
| 21–30 | 37 (22) | 41 (25) | 6 (26) |
| ≥31 | 16 (10) | 17 (10) | 3 (13) |
| Male sex | 97 (58) | 97 (58) | 17 (74) |
| Karnofsky score pre-transplant | |||
| <90 | 82 (50) | 111 (67) | 15 (65) |
| ≥90 | 83 (50) | 54 (33) | 8 (35) |
| Unknown | 1 | 1 | 0 |
| Reason for second and third transplants | |||
| Primary graft failure | 26 (16) | 5 (22) | |
| Secondary graft failure | 140 (84) | 18 (78) | |
| Interval from first transplant to second transplant | N/A | N/A | |
| ≤3 months | 47 (28) | ||
| >3 months | 119 (72) | ||
| Conditioning regimen | |||
| Cy + ATG | 36 (21) | 73 (44) | 7 (30) |
| Cy + TBI/TLI/TAI ± other | 21 (12) | 31 (19) | 3 (13) |
| Cy alone | 88 (53) | 8 (5) | 0 |
| Bu + Cy | 17 (10) | 16 (10) | 2 (9) |
| Other | 4 (1) | 32 (19) | 6 (26) |
| None* | 0 | 12 (7) | 5 (22) |
| GVHD prophylaxis | |||
| Cyclosporine + methotrexate ± other | 134 (80) | 104 (63) | 0 |
| Cyclopsorine ± other | 27 (16) | 54 (32) | 6 (26) |
| MTX ± other | 3 (2) | 1 (1) | 13 (57) |
| Tacrolimus ± other | 1 (1) | 3 (2) | 1 (4) |
| None | 4 (2) | 3 (13) | |
| Nucleated cells infused, × 108/kg | |||
| < 3.0 | 42 (26) | 39 (25) | 8 (38) |
| ≥ 3.0 | 120 (74) | 114 (75) | 13 (62) |
| Unknown | 4 | 13 | 2 |
| Donor type | N/A | ||
| Same related donor as for 1st transplant | 146 (88) | 21 (91) | |
| Different related donor | 20 (12) | 2 (9) | |
| Unrelated donor | 0 | 0 | |
| Donor-recipient gender match | |||
| Male donor →Male recipient | 54 (33) | 52 (31) | 9 (41) |
| Male donor →Female recipient | 45 (27) | 46 (28) | 0 |
| Female donor →Male recipient | 43 (26) | 45 (27) | 8 (36) |
| Female donor →Female recipient | 24 (14) | 23 (14) | 5 (23) |
| Missing | 0 | 0 | 1 |
| Graft type | |||
| Bone marrow | 166 (100) | 140 (84) | 16 (70) |
| Peripheral blood progenitor cells | 26 (16) | 7 (30) | |
| Year of transplant | |||
| 1986–1989 | 47 (28) | 42 (25) | 7 (30) |
| 1990–1993 | 46 (28) | 41 (25) | 5 (22) |
| 1994–1997 | 40 (24) | 36 (22) | 1 (4) |
| 1998–2002 | 33 (20) | 41 (25) | 8 (35) |
| 2003–2004 | 0 | 6 (3) | 2 (9) |
| Median follow-up of survivors after second transplant, months | 97 (6 – 215) | 122 (19 – 188) | |
N=12 received second transplantation without conditioning. Four of 12 patients were transplanted for primary graft failure and the interval between their first and second transplantation were 0.69, 1.74, 2.60, and 6.88 months. The remaining 8 patients were transplanted for secondary graft failure and the interval between first and second transplantation were 1.25, 1.38, 2.37, 5.76, 8.78, 9.61, 18.68, and 29.90 months.
N=5 patients received a third transplant without a conditioning. Two of 5 patients were transplanted for primary graft failure and the interval between their second and third transplantation were 0.86, and 4.64 months. The remaining 3 patients were transplanted for secondary graft failure and the interval between second and third transplantation were 1.41, 4.67, and 26.74 months.
RESULTS
Patient, disease and transplant characteristics for the first and second transplantations are shown in Table 1. Characteristics are also provided for the twenty three patients who underwent a third transplant after their second transplant failed. All patients received BM grafts for their first transplant; 84% of patients received BM grafts and 16%, PBPC for their second transplant. Median time between the first and second transplant was 7 months (range 1 – 114); two-thirds of second transplantations occurred within 1 year from the first.
Hematopoietic recovery
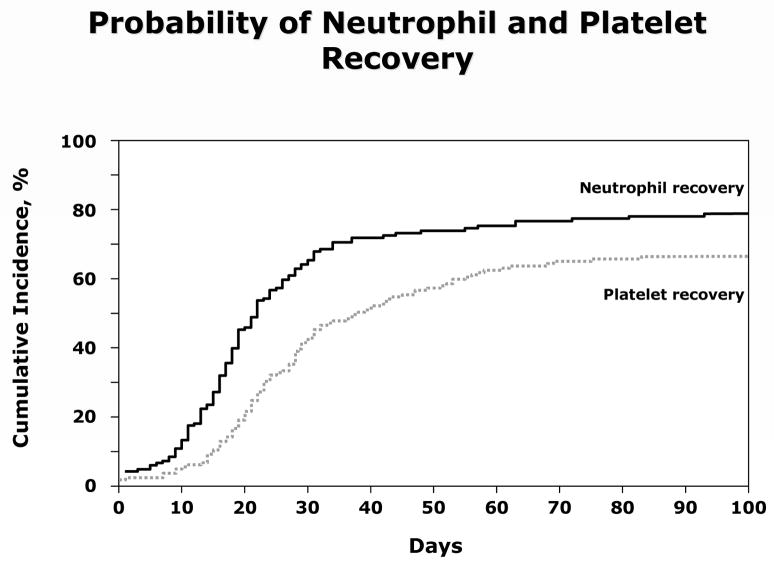
The probabilities of neutrophil recovery at day-28 and platelet recovery at day-60 after second transplantation were 63% (95% CI 55% – 70%) and 62% (95% CI 58% – 73%), respectively (Figure 1). Thirty-six patients failed to achieve neutrophil recovery and 54 patients, platelet recovery. In addition 18 patients experienced graft failure after initial hematopoietic recovery; 14 of these patients experienced graft failure <12 months after second transplantation and the remaining 4 patients, experienced graft failure between 13 and 37 months. In multivariate analysis, neutrophil recovery after second transplantation was more likely with PBPC grafts (odds ratio [OR] 12.91, 95% CI 2.65 – 62.83, p=0.002) and when the indication for transplantation was secondary graft failure after the first transplant (OR 4.39, 95% CI 1.60 –12.08, p=0.004). Neither performance score (OR 1.68, 95% CI 0.84 – 3.37, p=0.144) or conditioning regimen (cyclophoaphamide with limited field irradiation vs. cyclophophamide plus ATG, OR 0.56, 95% CI 0.24 – 1.31, p=0.178 and other regimens vs. cyclophophamide plus ATG, OR 0.99, 95% CI 0.49 – 2.03, p=0.982) were associated with neutrophil recovery. Platelet recovery was also more likely with transplantation of PBPC grafts (OR 11.25, 95% CI 2.30 – 54.99, p=0.003), when the indication for second transplant was secondary graft failure (OR 8.80, 95% CI 2.63–29.43, p<0.001) and patients with good (90–100) performance scores (OR 3.60, 95% CI 1.47–8.81, p=0.005). Platelet recovery was also associated with conditioning regimen for second transplantation. Recovery was less likely with an irradiation-containing conditioning regimen (OR 0.22, 95% CI 0.08 – 0.60, p=0.003).
Figure 1.
Cumulative incidence of neutrophil and platelet recovery after second HLA-matched sibling donor transplantation for graft failure.
Graft-versus-host disease
The probability of grades 2–4 acute GVHD by day-100 after transplantation was 9% (95% CI 5 – 14). The 8-year probability of chronic GVHD was 16% (95% CI 10 – 22). The rate of chronic GVHD was higher after transplantation with PBPC compared to BM (26% vs. 14%), but this difference did not reach statistical significance (p=0.274).
Overall survival
With median follow-ups of more than 8 years after second transplantation, 97 of 166 patients are alive. Early (day-100) mortality rates were high, 30% (49 of 166) and primary graft failure (n=24) the most frequent cause of death during this period. Fewer deaths (n=20) occurred beyond 100 days. In multivariate analysis, performance score at second transplantation and the inter-transplant interval were independent predictors of overall survival. When patients required a second transplant within three months of their first transplant for primary or early secondary graft failure, the risks of early and overall mortality were RR 3.12, 95% CI 1.47 – 6.62, p=0.003 and RR 2.15, 95% CI 1.32 – 3.51, p=0.002, respectively. Risks of early and overall mortality in patients with performance scores <90 were (RR 5.81, 95% CI 2.12 – 16.13, p<0.001) and (RR 1.88, 95% CI 1.05 – 3.38, p=0.033), respectively.
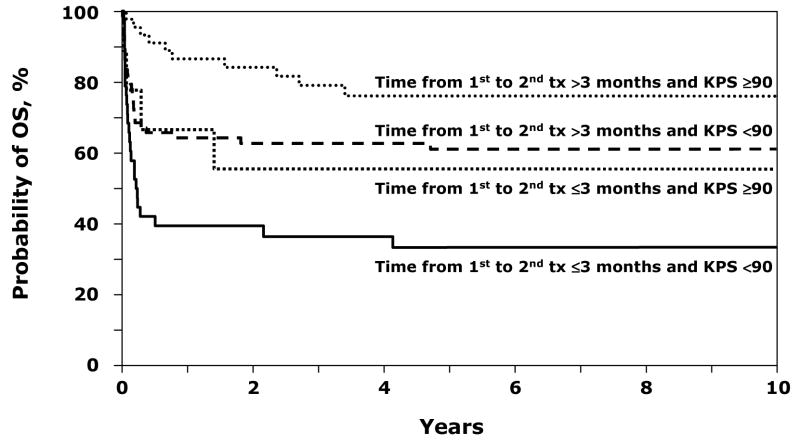
Considering the influence of inter-transplant interval and performance status together, overall survival was highest when the second transplant occurred after 3 months from the first transplant and in patients with performance scores of 90–100. In patients with performance scores of 90–100, the 8-year probabilities of overall survival when the interval between the first and second transplant was within 3 months and beyond 3 months were 56% and 76%, respectively (Figure 2). Corresponding probabilities in patients with lower performance scores were 33% and 61%. Two transplant strategies, using a different sibling donor (RR 1.22, p=0.558) and PBPC grafts, (RR 0.56, p=0.159), were not associated with overall mortality. We did not find an association between types of conditioning regimen and overall mortality (cyclophoaphamide with limited field irradiation vs. cyclophophamide plus ATG, RR 1.31, 95% CI 0.70 – 2.45, p=0.394 and other regimens vs. cyclophophamide plus ATG, RR 1.00, 95% CI 0.58 – 1.72, p=0.993. Twenty-three patients received a third transplant for secondary graft failure after their second transplant (Table 1). Sustained hematopoietic recovery was achieved for fifteen patients and 11 of 23 patients are alive at last follow-up. The causes of death for the entire cohort are shown in Table 2; graft failure and infection being the most common causes of death.
Figure 2. Overall Survival after second HLA-matched sibling donor transplantation for graft failure in severe aplastic anemia by time from first to second transplant and karnofsky performance score.
Eight-year probabilities of overall survival are as follows: a) Probability of performance scores of 90–100, when the interval between the first and second transplant was beyond 3 months was 76% (95% CI 62 – 88). b) Probability of performance scores of <90, when the interval between the first and second transplant was beyond 3 months was 61% (95% CI 50 – 72). c) Probability of performance scores of 90–100, when the interval between the first and second transplant was within 3 months was 56% (95% CI 24 – 85). d) Probability of performance scores of <90, when the interval between the first and second transplant was within 3 months was 33% (95% CI 19 – 49).
Table 2.
Causes of death
DISCUSSION
In this study, we identified two factors associated with survival after second HLA-matched sibling transplantation for SAA: inter-transplant interval of more than 3 months and good performance score (90–100) at second transplantation. Inter-transplant interval is as a surrogate for the type and rapidity of graft failure, allowing cases of primary graft failure or early secondary graft failure to be distinguished from cases of late secondary graft failure. Stratified by inter-transplant interval and performance score, three prognostic groups emerged. Patients with inter-transplant interval >3 months and good performance did well in the long-term; estimated 8-year survival of 76%, whereas patients transplanted after a short interval and poor performance score fared poorly (estimated 8-year survival of 33%). Patients with a long inter-transplant interval but sub-optimal performance or a good performance score but short inter-transplant interval had an intermediate outcome; (estimated 8-year survival rates of 61% and 56%, respectively). Our observations are limited to patients who failed their first transplant and received second transplantation. Only a third of patients with primary or secondary failure after first transplantation for SAA undergo second transplantation (Center for International Blood and Marrow Transplant Research, January 2009). The decision to offer a second transplant is at the discretion of the treating physician and the rationale for not offering a second transplant is not collected by this registry. This is a limitation when analyzing data collected by an observational database.
Our finding that a long interval is associated with survival substantiates the findings of three previous studies (8, 9, 15). Two of these studies demonstrated an association between a longer interval and survival; the other demonstrated an association between secondary graft failure and survival (most of the patients in our analysis with secondary graft failure also had a long inter-transplant interval). It is not surprising that a short interval is disadvantageous; when the second transplant occurs soon after the first, there is little time to recover from the toxicity or the myelosuppressive effects of conditioning, and, consequently, the risk for death from infection or organ injury is heightened. An alternative explanation is that the rapidity of graft failure may reflect the potency of the barrier to sustained engraftment with the conditioning regimens used during the study era. Most patients received cyclophosphamide with or without anti-thymocyte globulin and limited field irradiation. While this is effective for first transplantation, regimens with greater immunosuppressive potency may be required for sustained engraftment for patients requiring second transplantation.
The type of GVHD prophylaxis had no noticeable effect on outcome. This finding runs counter to the results of a study by Stucki and colleagues (9). In that study, which examined transplantations between 1970 and 1997, higher survival was associated with use of cyclosporine and methotrexate prophylaxis regimen (as opposed to the methotrexate alone regimen). The lack of effect of GVHD prophylaxis in our study can best be explained by the fact that virtually all patients transplanted in this more recent period received calcineurin inhibitor based GVHD prophylaxis.
We did not observe a relationship between the various conditioning regimens employed and survival. The intensity of the regimens used was fairly similar and may explain our inability to identify regimens that may have enhanced hematopoietic recovery particularly in those with primary or early secondary graft failure. The observed negative association between irradiation based conditioning regimens and platelet recovery is difficult to account for, since the use of irradiation has previously been shown to be associated with sustained engraftment (6).
Utilizing a different sibling donor for the second transplant conferred no detectable advantage in our analysis. Similarly, even though using a PBPC graft was associated with improved myeloid recovery (both neutrophils and platelets), this had no measurable effect on survival. A recent study from the CIBMTR comparing PBPC and BM grafts as first transplants for SAA demonstrated a higher rate of chronic GVHD and lower survival after PBPC transplants in younger patients (16). In the absence of a graft-versus-tumor effect for SAA, the burden of morbidity and late mortality associated with chronic GVHD must be weighed against any potential benefit derived from faster hematopoietic recovery (17, 18).
New approaches are needed for patients undergoing a second transplantation for SAA, particularly for those with primary or early secondary graft failure and have a poor performance score, since their prognosis is dismal. Efforts should focus on preventing graft failure after the first transplant by optimizing conditioning regimen (cyclophosphamide and ATG) and GVHD prophylaxis calcinuerin inhibitor and short course methotrexate. Transplantation of peripheral blood progenitor cells results in faster hematopoietic recovery but did not translate into survival advantage. About 45% of patients in this analysis received cyclosporine and anti-thymocyte globulin and the remaining patients a variety of conditioning regimens. Given the relatively small study population of 166 patients we were unable to identify an optimal regimen that may ensure sustained hematopoietic recovery. Nevertheless, cyclophosphamide and ATG, the most frequent regimen was successfully used for several patients. While conditioning regimens with greater immunosuppression or myeloablation are worthy of consideration for patients with early graft failure careful consideration must be given to the risks and benefits such regimens.
Acknowledgments
Supported by Public Health Service Grant (U24-CA76518-08) from the National Cancer Institute, the National Heart, Lung and Blood Institute and the National Institute of Allergy and Infectious Diseases; Health Resources and Services Administration (HHSH234200637015C); Office of Naval Research (N00014-06-1-0704 and N00014-08-1-0058); and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Association of Medical Microbiology and Infectious Disease Canada; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gambro BCT, Inc.; Gamida Cell, Ltd.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka Pharmaceutical Development & Commercialization, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Saladax Biomedical, Inc.; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS, Inc.; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Doney K, Leisenring W, Storb R, Appelbaum FR. Primary treatment of acquired aplastic anemia: outcomes with bone marrow transplantation and immunosuppressive therapy. Seattle Bone Marrow Transplant Team. Ann Intern Med. 1997;126:107–115. doi: 10.7326/0003-4819-126-2-199701150-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bacigalupo A, Brand R, Oneto R, et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy--The European Group for Blood and Marrow Transplantation experience. Semin Hematol. 2000;37:69–80. doi: 10.1016/s0037-1963(00)90031-3. [DOI] [PubMed] [Google Scholar]
- 3.Fuhrer M, Rampf U, Baumann I, et al. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood. 2005;106:2102–2104. doi: 10.1182/blood-2005-03-0874. [DOI] [PubMed] [Google Scholar]
- 4.Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2007;92:11–18. doi: 10.3324/haematol.10075. [DOI] [PubMed] [Google Scholar]
- 5.Passweg JR, Socie G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858–864. [PubMed] [Google Scholar]
- 6.Champlin RE, Horowitz MM, van Bekkum DW, et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood. 1989;73:606–613. [PubMed] [Google Scholar]
- 7.Champlin RE, Perez WS, Passweg JR, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109:4582–4585. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCann SR, Bacigalupo A, Gluckman E, et al. Graft rejection and second bone marrow transplants for acquired aplastic anaemia: a report from the Aplastic Anaemia Working Party of the European Bone Marrow Transplant Group. Bone Marrow Transplant. 1994;13:233–237. [PubMed] [Google Scholar]
- 9.Stucki A, Leisenring W, Sandmaier BM, Sanders J, Anasetti C, Storb R. Decreased rejection and improved survival of first and second marrow transplants for severe aplastic anemia (a 26-year retrospective analysis) Blood. 1998;92:2742–2749. [PubMed] [Google Scholar]
- 10.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 11.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Klein J, Moeschberger M. Survival Analysis: Techniques of censored and truncated data. New York, N.Y: Springer-Verlag; 2003. [Google Scholar]
- 13.Klein JP, Andersen PK. Regression modeling of competing risks data based on pseudovalues of the cumulative incidence function. Biometrics. 2005;61:223–229. doi: 10.1111/j.0006-341X.2005.031209.x. [DOI] [PubMed] [Google Scholar]
- 14.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–1500. doi: 10.1002/(sici)1097-0258(19990630)18:12<1489::aid-sim140>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.de Medeiros CR, Bitencourt MA, Medeiros BC, Ioshizumi L, Pasquini R. Second bone marrow transplantation for severe aplastic anemia: analysis of 34 cases. Bone Marrow Transplant. 2001;28:941–944. doi: 10.1038/sj.bmt.1703257. [DOI] [PubMed] [Google Scholar]
- 16.Schrezenmeier H, Passweg JR, Marsh JC, et al. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood. 2007;110:1397–1400. doi: 10.1182/blood-2007-03-081596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108:2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]