Abstract
Background:
Although several risk factors for cognitive decline have been identified, much less is known about factors that predict maintenance of cognitive function in advanced age.
Methods:
We studied 2,509 well-functioning black and white elders enrolled in a prospective study. Cognitive function was measured using the Modified Mini-Mental State Examination at baseline and years 3, 5, and 8. Random effects models were used to classify participants as cognitive maintainers (cognitive change slope ≥0), minor decliners (slope <0 and >1 SD below mean), or major decliners (slope ≤1 SD below mean). Logistic regression was used to identify domain-specific factors associated with being a maintainer vs a minor decliner.
Results:
Over 8 years, 30% of the participants maintained cognitive function, 53% showed minor decline, and 16% had major cognitive decline. In the multivariate model, baseline variables significantly associated with being a maintainer vs a minor decliner were age (odds ratio [OR] = 0.65, 95% confidence interval [CI] 0.55–0.77 per 5 years), white race (OR = 1.72, 95% CI 1.30–2.28), high school education level or greater (OR = 2.75, 95% CI 1.78–4.26), ninth grade literacy level or greater (OR = 4.85, 95% CI 3.00–7.87), weekly moderate/vigorous exercise (OR = 1.31, 95% CI 1.06–1.62), and not smoking (OR = 1.84, 95% CI 1.14–2.97). Variables associated with major cognitive decline compared to minor cognitive decline are reported.
Conclusion:
Elders who maintain cognitive function have a unique profile that differentiates them from those with minor decline. Importantly, some of these factors are modifiable and thus may be implemented in prevention programs to promote successful cognitive aging. Further, factors associated with maintenance may differ from factors associated with major cognitive decline, which may impact prevention vs treatment strategies.
GLOSSARY
- 3MS
= Modified Mini-Mental State Examination;
- BMI
= body mass index;
- CES-D
= Center for Epidemiologic Studies–Depression Scale score;
- CI
= confidence interval;
- CRP
= C-reactive protein;
- Health ABC
= Health, Aging and Body Composition;
- IL
= interleukin;
- MI
= myocardial infarction;
- OR
= odds ratio;
- REALM
= Rapid Estimate of Adult Literacy in Medicine;
- TNF
= tumor necrosis factor.
The concept of successful aging was first introduced to explore the hypothesis that aging is not necessarily accompanied by typical age-associated declines in function.1 Over the past few decades, several lines of research have attempted to define and predict successful aging.2 However, many of these studies have been limited with respect to measures of CNS function, including cognitive function. Previous studies of successful aging have commonly focused on physical well-being without considering cognitive abilities.2 The few studies that have incorporated cognitive function into the conceptual framework of successful aging have suggested definitions that exclude clinical impairment such as dementia, but fail to identify factors that determine optimal cognitive aging.3,4
We recently reported three distinct categories of cognitive trajectories over a 15-year period in a cohort of elderly community-dwelling women: major decliners with significant clinical decline, minor decliners with more modest or “typical” decline, and maintainers who exhibited no decline on global cognitive function over time.5 We found that compared to minor decliners, cognitive maintainers were less likely to report comorbid medical conditions such as diabetes and hypertension, more likely to engage in healthy lifestyle behaviors, less likely to report difficulties with daily living activities, and less likely to report low social integration.5
These findings not only support the hypothesis of successful cognitive aging, but further suggest that individuals who maintain cognitive capabilities have a psychosocial and behavioral profile that differs from older adults who exhibit more typical age-associated cognitive decline. How these individuals differ with regard to other variables including biologic factors that may be related to cognitive aging, such as measures of inflammation,4 lipoprotein metabolism,6 and glucose regulation,7 remains unknown. Furthermore, since the sample for the previous study consisted primarily of white women, the results may not apply to the general population.
The goals of the present study are to determine, as part of an ongoing prospective study in a biracial cohort of men and women, the proportion of elders who maintain cognitive function and examine the comprehensive psychosocial, health, and biologic factors that predict maintenance of cognitive function over several years. Identification of determinants of successful cognitive aging may not only help shape the definition of successful aging, but also influence prevention strategies to ensure optimal health and to prevent or delay cognitive impairment in old age.
METHODS
Study population.
Participants were part of the Health, Aging and Body Composition (Health ABC) study, a prospective cohort study of 3,075 community-dwelling black and white men and women living in Memphis, TN, or Pittsburgh, PA, and aged 70–79 years at recruitment in 1997. To identify potential participants, a random sample of white and all black Medicare-eligible elders, within designated zip code areas, were contacted. To be eligible, participants had to report no difficulty with activities of daily living, walking a quarter of a mile, or climbing 10 steps without resting. They also had to be free of life-threatening cancer diagnoses and have no plans to move out of the study area for at least 3 years. Overall, 22,999 potential participants were identified, of which 8,695 could not be contacted, 897 were institutionalized, dead, or had moved out of the area, 7,250 declined to participate, 3,082 were ineligible, and 3,075 were eligible. All eligible participants signed a written informed consent, approved by the institutional review boards at the clinical sites. This study was approved by the institutional review boards of the clinical sites and the coordinating center (University of California, San Francisco). A detailed discussion of the Health ABC sample has been described previously.8
Measurements.
Cognitive tests.
The Modified Mini-Mental State Examination (3MS) was administered to participants at baseline (year 1) and after 2, 4, and 7 years of follow-up (years 3, 5, and 8). The 3MS is a brief, general cognitive battery with components for orientation, concentration, language, praxis, and immediate and delayed memory.9 Possible scores range from 0 to 100, with higher scores indicating better cognitive function.
Baseline characteristics.
Baseline demographic characteristics included age, sex, and race. Psychosocial characteristics included self-reported education (categorized as < or ≥ high school), whether the participant was working or volunteering, providing care for a spouse or child, visiting with family or friends at least once a week, whether they felt the need for more social support, and whether they lived alone or with someone. The Rapid Estimate of Adult Literacy in Medicine test,10 a word recognition test of 66 terms, was also administered to determine whether literacy was at a ninth grade level (corresponding to a score of 60) or better.
Baseline health characteristics included self-rated health (categorized as good, very good, or excellent vs fair or poor), current smoking, current drinking, and weekly moderate/vigorous exercise. Weekly moderate/vigorous exercise was defined as engagement in moderate to vigorous exercise and activity (e.g., aerobics, weight training, or brisk walking) at least once a week. Depressive symptoms were assessed with the 20-item Center for Epidemiologic Studies–Depression Scale (CES-D)11 with participants scoring above 15 categorized as having high depressive symptoms. Body mass index (BMI) (kg/m2) was calculated from direct height and weight measurements. Hypertension was defined by self-report of a diagnosis, use of an antihypertensive medication, or measured systolic blood pressure exceeding 140 mm Hg or diastolic blood pressure exceeding 90 mm Hg. Diabetes was defined by self-report of diabetes diagnosis, use of diabetes drug, or fasting plasma glucose >126 mg/dL or 2-hour post-challenge glucose >200 mg/dL. History of myocardial infarction, stroke, or TIA was determined from self-report of physician diagnoses.
Baseline biologic characteristics measured included APOE genotype, determined using standard single nucleotide polymorphism analyses (Bioserve.com, Laurel, MD) and analyzed as presence or absence of the ɛ2 and ɛ4 alleles. The cytokines, C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor–α, were measured by ELISA (R&D Systems, Minneapolis, MN). Fasting plasma glucose, total cholesterol, and triglycerides were also measured (Johnson & Johnson, Vitros chemical methodology). The cytokines, glucose, and triglyceride measurements were log-transformed for statistical analyses in order to meet model assumptions and analyzed per SD.
Statistical analyses.
We defined three cognitive groups based on estimated rates of change in 3MS scores from baseline to the year 8 visit. In order to determine the maintenance of cognitive function slope, only those participants with a baseline score of at least 80 (without clinical impairment9) and one additional cognitive assessment were included. We excluded the 14 participants who were missing 3MS scores at baseline, 313 with only one cognitive test score, and 239 with baseline 3MS score below 80, leaving 2,509 participants in the analytic cohort. Excluded participants were significantly older, more likely to be male, more likely to be black, more likely have comorbidities, and less likely to have a high school education and a ninth grade literacy level.
The participant-specific slopes of 3MS scores were estimated by best linear unbiased predictions using a linear mixed model with random intercepts and slopes. Participants with predicted slopes of 0 or greater (indicating no change or improvement in cognitive scores over time) were classified as maintainers. Those with predicted slopes less than 0 (decline in cognitive score over time) but no more than one SD below the mean of the slopes were classified as minor decliners. Those with predicted slopes more than 1 SD below the mean were classified as major decliners.
No interactions between race and predictors of cognitive group were found; therefore, all subsequent analyses were performed combining black and white participants. Characteristics were compared across cognitive groups in bivariate analyses using analysis of variance for continuous variables and χ2 tests for categorical measures. Odds ratios (OR) and 95% confidence intervals (CI) were estimated for belonging to the cognitive maintainer group or the major decliner group vs the minor decliner group using a series of multinomial logistic regression models. This model was then adjusted for all baseline characteristics that were significant at the p < 0.05 level in the bivariate analyses. All analyses were conducted in Stata (Stata Corp, College Station, TX).
RESULTS
Of the 2,509 elders, 758 (30%) were maintainers, 1,340 (53%) minor decliners, and 411 (16%) major decliners. For each group, the raw mean change over 7 years on the 3MS was 1.0 (SE 0.02) points for maintainers, −2.2 (SE 0.16) points for minor decliners, and −9.0 (SE 0.30) points for major decliners (figure).
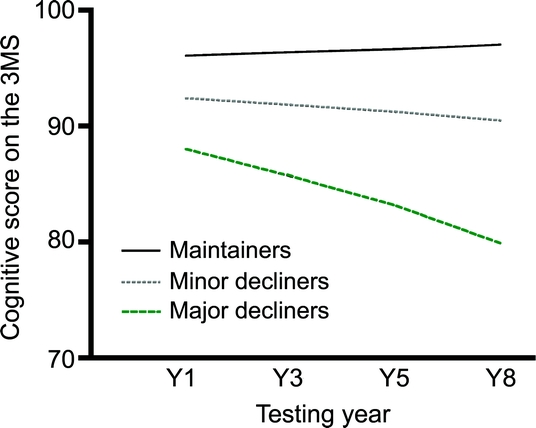
Figure Cognitive score over time by cognitive group
Cognitive group was determined by change in score on the Modified Mini-Mental State Examination (3MS) over 7 years, resulting in the classification of participants as cognitive maintainers (cognitive change slope ≥0), minor decliners (slope <0 and >1 SD below mean), or major decliners (slope ≤1 SD below mean).
In comparing cognitive maintainers to minor decliners, younger and white participants were more likely to maintain cognitive function over years (table 1). In the psychosocial domain, those with high school education or greater and at least a ninth grade literacy level were more likely to be maintainers. Those who worked or volunteered, were not taking care of a spouse or child, were receiving enough social support, and living with someone at baseline were also more likely to be cognitive maintainers than decliners.
Table 1 Predictors of cognitive group among 2,509 participants
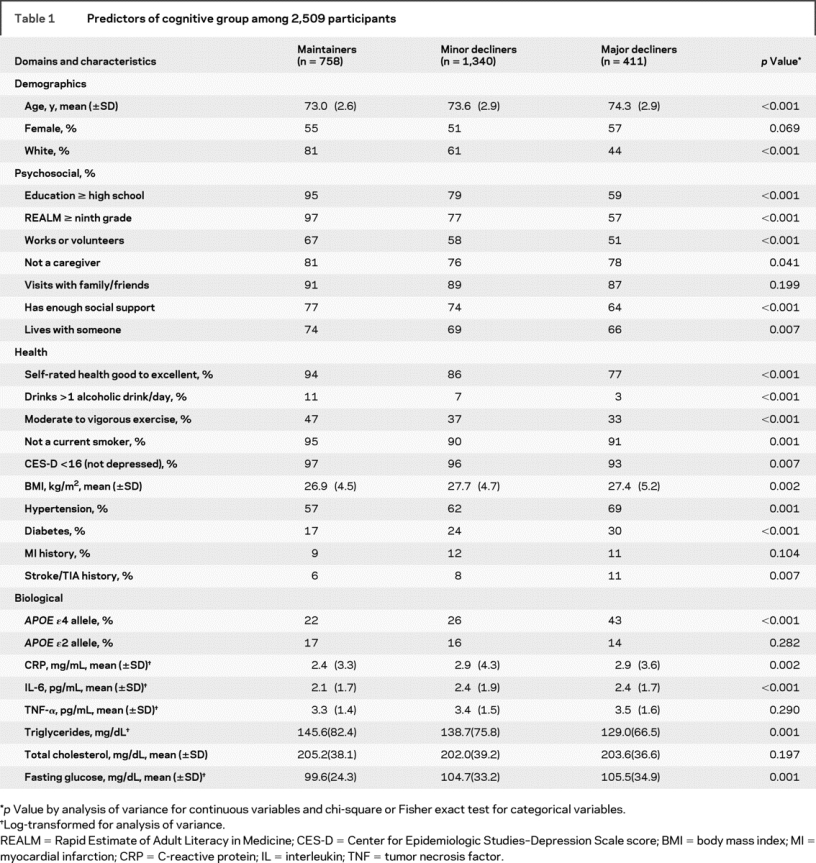
In the health domain, participants who rated their health as good, very good, or excellent (vs fair or poor) were more likely to be maintainers than decliners. So were those who drank more than one alcoholic drink per day, engaged in moderate to vigorous weekly exercise, and did not smoke at baseline. Those with lower BMI and those without hypertension, diabetes, and stroke were also more likely to be cognitive maintainers (table 1).
In the biologic domain, participants without the APOE ɛ4 allele were more likely to be maintainers than decliners. Those with lower levels of CRP, IL-6, fasting glucose, and higher levels of triglycerides were also more likely to be cognitive maintainers (table 1).
In the bivariate model, major decliners were more likely to be older, to be black, to have less than a high school education and ninth grade literacy level, to be less likely to work/volunteer, and to be less likely to have enough social support. In the health domain, major decliners were less likely to report health as good to excellent, reported the consumption of fewer drinks per day, were less likely to engage in moderate to vigorous exercise, and were more likely to report symptoms of depression. Baseline comorbidities including hypertension, diabetes, and stroke were higher in the major decliner group. Finally, major decliners were more likely to carry the APOE ɛ4 gene and tended to have higher triglyceride levels and fasting glucose levels.
In the final multivariate-adjusted model (table 2), characteristics that remained significant predictors of being a maintainer vs a minor decliner were age, white race, having a high school education level or greater and a ninth grade literacy level or greater, engaging in weekly moderate to vigorous exercise, and not smoking at baseline. At the statistical trend level (p < 0.10), cognitive maintenance was associated with working or volunteering, living with someone, and absence of the APOE ɛ4 gene.
Table 2 The likelihood of being a cognitive maintainer or major decliner: Multivariate results
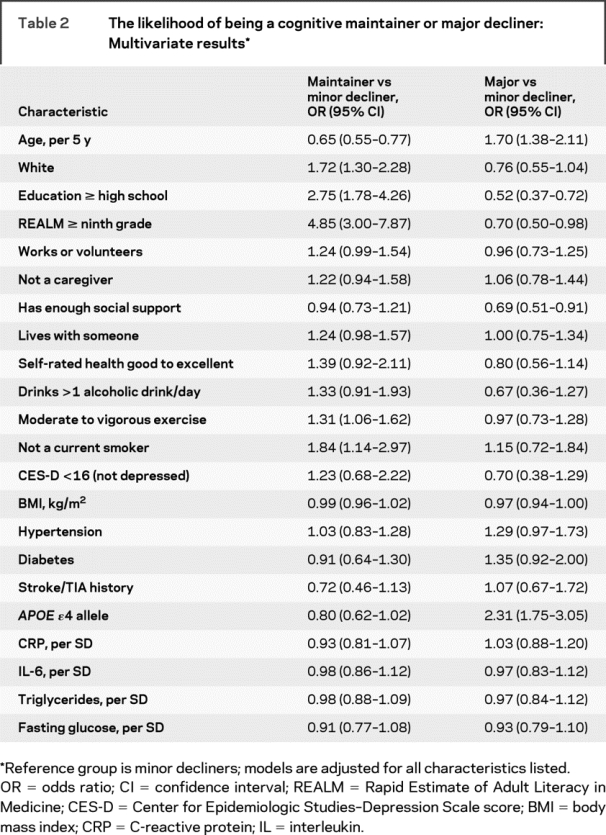
In the final multivariate model, baseline factors that significantly predicted major cognitive decline vs a minor decline included being older, having less than a high school education and lower than a ninth grade literacy level, having a lower level of social support, and presence of the APOE ɛ4 gene (table 2). At the statistical trend level (p < 0.10), major cognitive decline was associated with race, higher BMI, diabetes, and hypertension.
DISCUSSION
We identified three distinct groups based on global cognitive function trajectories over 8 years: cognitive maintainers, minor cognitive decliners, and major cognitive decliners. In contrast to the majority of past research, this study systematically assessed factors that predict maintenance or successful cognitive aging by examining these trajectories of cognitive function in a biracial cohort of elderly men and women.
A valuable outcome is the identification of several predictive factors of successful cognitive aging that are potentially modifiable. Behavioral factors including exercise, smoking, and volunteering are modifiable factors that may be used in strategies to ensure optimal cognitive function in old age. Volunteerism in the elderly, which was higher among cognitive maintainers, has been shown to reduce mortality12 and increase one's sense of well-being.13 It is suggested that benefits of volunteering stem from increased social networks, available recourses, and emotional gratification.14 Cognitive maintainers in the present study were also less likely to live alone, emphasizing the potential importance of social interaction in old age.
Maintainers were more likely to engage in moderate to vigorous exercise compared to minor cognitive decliners. In terms of cognitive function, aerobic exercise training is shown to increase brain volume in once sedentary older adults between the ages of 60 and 79 years.15 Furthermore, prospective and case-control studies report enhancing effects of physical fitness on cognitive function in late life.16–18 These findings, along with the present study, emphasize the importance of physical function on cognitive maintenance in later years.
It is hypothesized that the brain may experience accelerated aging due to metabolic dysregulation including chronic inflammation, oxidative stress, and glucose dysregulation.19–21 Interestingly, prevention programs that incorporate lifestyle changes including cessation of smoking, exercise, and proper nutrition may modify biologic factors,22 which in turn may maintain cognitive function in late life.23,24 Surprisingly, biologic markers did not significantly differentiate cognitive maintainers from minor decliners. The impact of biologic markers may have been diminished due to the nature of the two cognitive groups or may have been overpowered by other factors that were investigated such as weekly moderate/vigorous exercise.
In addition to lifestyle changes, prevention programs have recently focused on building one's cognitive reserve capacity. Cognitive reserve denotes resilience to the clinical presentation of age-related neurodegeneration due to the maximization of alternative neuronal networks or cognitive strategies.25 Cognitive reserve is reportedly determined by innate intellectual ability and by external experiences, including educational attainment,26 which was found to be higher in the cognitive maintainer group. Indeed, previous research shows that lower levels of education and literacy are associated with greater cognitive impairment and increased risk of dementia.27,28 It is important to note that education and literacy level independently predicted cognitive maintenance in the final adjusted model, indicating separate roles of these two factors on maintenance of cognitive function. Effects of education and literacy may explain the present association between race and cognitive maintenance. Although education and literacy were statistically controlled for in the present study, we cannot eliminate the residual confounding that may also contribute to cognitive reserve. Importantly, however, studies show that one can enhance cognitive reserve in old age.29,30 Cognitive training not only improves cognitive function31,32 but may also result in more efficient neuronal activation.33 Also, such cognitive training appears to produce long-term effects and not simply transient stimulus-response associations.34,35
It is interesting to note that predictors of major decline may slightly differ from predictors of cognitive maintenance. For example, behavioral factors such as working/volunteering, engagement in moderate to vigorous exercise, and not smoking seem to be more predictive of maintenance than of major cognitive decline. These differences in predictive value have important implication in defining prevention vs treatment programs in the context of cognitive aging.
An important strength of this study was the ability to define cognitive groups based on participants' cognitive trajectory over several years. In contrast to past cross-sectional definitions largely suggesting an absence of clinical cognitive impairment, this method defines successful aging based on longitudinal performance. While our primary goal was to examine factors that differentiate cognitive maintenance from typical age-related (or minor) cognitive decline, we were able to confirm factors that determine major cognitive decline, such as presence of the APOE ɛ4 gene. Also, the Health ABC study provided access to a wide range of baseline characteristics, enabling examination of factors from various domains. Finally, since the cohort included black and white men and women, the findings are generalizable to the general population of well-functioning elders. A potential limitation of the present investigation is selection bias. Because the cohort roughly represents relatively well-functioning persons aged 70 to 79 years, the proportion of cognitive maintainers may be higher than what would be found in a less well-functioning sample. Another limitation is that we only focused on global cognitive function in defining our three cognitive groups. It is well established that not all cognitive functions decline at similar rates with age. Thus, future studies should use a combination of cognitive tests when defining cognitive trajectories in an elderly population.
Overall, these results suggest that a subset of the aging population may be characterized as successful agers based on maintenance of cognitive function over several years. With an anticipated exponential expansion of the population that will develop dementia and the resulting costs to society,36 it is increasingly important to investigate and understand the mechanisms that promote optimal cognitive function in old age. The present study demonstrates that cognitive maintainers display a unique profile consisting of factors, some of which are modifiable, that differentiate them from the majority of elderly who experience age-related typical cognitive decline.
Further research is needed to replicate the present findings and expand on the potential predictors of successful cognitive aging. Also, future studies should incorporate imaging techniques to assess the correlation between cognitive maintenance and brain function/morphology over time. Elucidating predictors of optimal cognitive function will enable the development of strategies that may prevent onset or slow the progression of dementia in late life. Such models will not only reduce the projected costs to society, but will further ensure increased quality of life in later years.
AUTHOR CONTRIBUTIONS
Study design and concept by K.Y. Manuscript prepared by A.J.F. and revised by K.Y. All analyses performed by K.L. and interpreted by A.J.F. and K.Y. Final approval of submitted version by all co-authors.
Supplementary Material
Address correspondence and reprint requests to Dr. A.J. Fiocco, Department of Psychiatry, School of Medicine, University of California, San Francisco, 4150 Clement St., San Francisco, CA 94117 jazzfiocco@hotmail.com
Funded by N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, and AG021918. Supported in part by the Intramural Research Program of the NIH, National Institute on Aging. K.Y. is supported in part by AG 031155 and an anonymous foundation. A.J.F. is supported by the CIHR Institute of Aging Fellowship Award.
Disclosure: The authors report no disclosures.
Received November 21, 2008. Accepted in final form March 11, 2009.
REFERENCES
- 1.Rowe JW, Kahn RL. Human aging: usual and successful. Science 1987;237:143–149. [DOI] [PubMed] [Google Scholar]
- 2.Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry 2006;14:6–20. [DOI] [PubMed] [Google Scholar]
- 3.Habib R, Nyberg L, Nilsson LG. Cognitive and non-cognitive factors contributing to the longitudinal identification of successful older adults in the Betula Study. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2007;14:257–273. [DOI] [PubMed] [Google Scholar]
- 4.Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: The Rotterdam Study. Arch Neurol 2004;61:668–672. [DOI] [PubMed] [Google Scholar]
- 5.Barnes DE, Cauley JA, Lui LY, et al. Women who maintain optimal cognitive function into old age. J Am Geriatr Soc 2007;55:259–264. [DOI] [PubMed] [Google Scholar]
- 6.Yaffe K, Barrett-Connor E, Lin F, Grady D. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol 2002;59:378–384. [DOI] [PubMed] [Google Scholar]
- 7.Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K. Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the Rancho Bernardo study cohort. Arch Intern Med 2004;164:1327–1333. [DOI] [PubMed] [Google Scholar]
- 8.Rooks RN, Simonsick EM, Miles T, et al. The association of race and socioeconomic status with cardiovascular disease indicators among older adults in the health, aging, and body composition study. J Gerontol 2002;57:S247–256. [DOI] [PubMed] [Google Scholar]
- 9.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–318. [PubMed] [Google Scholar]
- 10.Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med 1993;25:391–395. [PubMed] [Google Scholar]
- 11.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 12.Harris AH, Thoresen CE. Volunteering is associated with delayed mortality in older people: analysis of the longitudinal study of aging. J Health Psychol 2005;10:739–752. [DOI] [PubMed] [Google Scholar]
- 13.Piliavin JA, Siegl E. Health benefits of volunteering in the Wisconsin longitudinal study. J Health Soc Behav 2007;48:450–464. [DOI] [PubMed] [Google Scholar]
- 14.Moen P, Dempster-McClain D, Williams R. Successful aging: a life-course perspective on women's multiple roles and health. Am J Sociol 1992;97:1612–1638. [Google Scholar]
- 15.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 2006;61:1166–1170. [DOI] [PubMed] [Google Scholar]
- 16.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med 2001;161:1703–1708. [DOI] [PubMed] [Google Scholar]
- 17.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol 2006;101:1237–1242. [DOI] [PubMed] [Google Scholar]
- 18.Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc 2003;51:459–465. [DOI] [PubMed] [Google Scholar]
- 19.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 2004;63:658–663. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol 2002;52:168–174. [DOI] [PubMed] [Google Scholar]
- 21.Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol 2006;65:631–641. [DOI] [PubMed] [Google Scholar]
- 22.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara CM, Goldberg AP, Ortmeyer HK, Ryan AS. Effects of aerobic and resistive exercise training on glucose disposal and skeletal muscle metabolism in older men. J Gerontol A Biol Sci Med Sci 2006;61:480–487. [DOI] [PubMed] [Google Scholar]
- 24.Meneilly GS, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol 1993;48:M117–121. [DOI] [PubMed] [Google Scholar]
- 25.Stern Y. The concept of cognitive reserve: a catalyst for research. J Clin Exp Neuropsychol 2003;25:589–593. [DOI] [PubMed] [Google Scholar]
- 26.Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychol Aging 1999;14:245–263. [DOI] [PubMed] [Google Scholar]
- 27.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994;271:1004–1010. [PubMed] [Google Scholar]
- 28.Manly JJ, Touradji P, Tang MX, Stern Y. Literacy and memory decline among ethnically diverse elders. J Clin Exp Neuropsychol 2003;25:680–690. [DOI] [PubMed] [Google Scholar]
- 29.Shihabuddin LS, Palmer TD, Gage FH. The search for neural progenitor cells: prospects for the therapy of neurodegenerative disease. Mol Med Today 1999;5:474–480. [DOI] [PubMed] [Google Scholar]
- 30.Gage FH. Neurogenesis in the adult brain. J Neurosci 2002;22:612–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E. Testing the limits of cognitive plasticity in older adults: application to attentional control. Acta Psychol 2006;123:261–278. [DOI] [PubMed] [Google Scholar]
- 32.Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E. Training effects on dual-task performance: are there age-related differences in plasticity of attentional control? Psychol Aging 2005;20:695–709. [DOI] [PubMed] [Google Scholar]
- 33.Erickson KI, Colcombe SJ, Wadhwa R, et al. Training-induced plasticity in older adults: effects of training on hemispheric asymmetry. Neurobiol Aging 2007;28:272–283. [DOI] [PubMed] [Google Scholar]
- 34.Willis SL, Nesselroades CS. Long-term effects of fluid ability training in old-old age. Developmental Psychology 1990;26:905–910. [Google Scholar]
- 35.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA 2002;288:2271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health 1998;88:1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


