Abstract
Understanding and improving drug release kinetics from dendrimer-drug conjugates is a key step to improving their in vivo efficacy. N-Acetylcysteine (NAC) is an anti-inflammatory agent with significant potential for clinical use in the treatment of neuroinflammation, stroke and cerebral palsy. There is a need for delivery of NAC which can enhance its efficacy, reduce dosage and prevent it from binding plasma proteins. For this purpose, a poly(amidoamine) dendrimer-NAC conjugate that contains a disulfide linkage was synthesized and evaluated for its release kinetics in the presence of glutathione (GSH), Cysteine (Cys), and bovine serum albumin (BSA) at both physiological and lysosomal pH. The results indicate that the prepared conjugate can deliver ~60% of its NAC payload within 1 hour at intracellular GSH concentrations at physiological pH, whereas the conjugate did not release any drug at plasma GSH levels. The stability of the conjugate in the presence of bovine serum albumin at plasma concentrations was also demonstrated. The efficacy of the dendrimer-NAC conjugate was measured in activated microglial cells (target cells in vivo) using the reactive oxygen species (ROS) assay. The conjugates showed an order of magnitude increase in anti-oxidant activity compared to free drug. When combined with intrinsic and ligand-based targeting with dendrimers, these types of GSH sensitive nanodevices can lead to improved drug release profiles and in vivo efficacy.
Keywords: Dendrimers, PAMAM dendrimers, neuroinflammation, N-acetyl cysteine, intracellular drug delivery, glutathione sensitive release
1. INTRODUCTION
Dendrimers offer well-defined nanoscale architecture, multivalency, and versatility, leading to their emergence as a promising class of nanobiomaterials [1]. One class of the dendrimers that have been widely investigated is Poly(amidoamine) (PAMAM) dendrimers. PAMAM dendrimers have been utilized as drug carriers for gene and drug delivery, as antiviral agents and as in vivo imaging agents [2–4]. When PAMAM dendrimers are used as drug carriers, they can enhance the biodistribution of drugs and possibly take advantage of enhanced permeation and retention effect (EPR) for targeting tumors [5–7]. Additionally, it was demonstrated that the dendrimer surfaces can be modified with ligands to target specific tissues and tumors, thus capable of active receptor targeting [8]. For successful applications of dendrimer drug conjugates to emerge clinically, the dendritic carriers should eventually release the drugs loaded on to them in a well-defined and favorable rate. The release rates are dependent on the type of linking chemistry used between the drug and its carriers as well as the nanoscale structure of the dendrimer conjugate and steric effects.
Several dendrimers have been investigated as drug carriers for various cancer drugs [9–12]. The conjugates have shown the ability to target tumors and have led to improved in vivo efficacy [9,12]. Recent work has shown that the efficacy of anionic PAMAM dendrimer-methotrexate conjugate is better than those of cationic conjugates in drug resistant cell lines, perhaps due to differences in subcellular distribution and drug release [11]. PAMAM dendrimers were also evaluated as carriers for anti-inflammatory agents, such as 5-aminosalicylic acid [13], ibuprofen [14], naproxen [15], and methylprednisolone [16]. These ester or amide linked conjugates showed improvements over the free drug and their release profiles were over times scales of days to weeks. The intended application in this study requires faster release within hours to days based on neonatal rabbit models. Recent work on dendrimer-N-acetyl cysteine (NAC) conjugates showed significant enhancement in activity over the free drug and disulfide linkages used have great prospect for delivery of small drugs [17]. Consequently, objective of the work presented here is to determine the release mechanism and rates of PAMAM-S-S-NAC conjugates in the presence of various thiol containing species.
NAC is a potent antioxidant as well as mucolytic agent and a precursor of L-cysteine (Cys) and reduced glutathione (GSH). NAC has therapeutic value for reducing neuroinflammation, endothelial dysfunction, fibrosis, invasion, cartilage erosion, acetaminophen detoxification and transplant prolongation. [18]. In addition, NAC reduces cellular production of pro-inflammatory cytokines such as TNF-α and IL-1β [19]. NAC has a low oral bioavailability requiring high doses [20]. When administered intravenously, NAC binds to plasma proteins via covalent disulfide bonds and can also cause allergic reactions in some patients complicating its use [21–22]. By using a drug delivery vehicle such as PAMAM dendrimers, NAC can be protected from protein binding and can be targeted to specific tissues. The intrinsic ability of PAMAM dendrimers to target neuroinflammation has been shown previously [23]. Therefore, we hypothesize that PAMAM-S-S-NAC dendrimer conjugates can facilitate in vivo neuroinflammation targeting, combined with enhanced anti-inflammatory and anti-oxidant effects of NAC, and through tailored intracellular release.
A key challenge in dendritic drug delivery is release kinetics. Dendrimer drug complexes are shown to be unstable in plasma and buffers [24–25]. Conjugates with pH responsive linkages are widely investigated but the difference in pH of biological fluids is usually not very significant especially through intravenous route. Amide linkages are typically very stable, whereas ester linkages are cleaved faster compared to amides by pH dependent hydrolysis [15]. Hydrazone linkages are more sensitive to changes in pH compared to ester linkages but the drug and the carriers need appropriate functional groups to form a hydrazone linkage [26]. Enzymatic release of drugs from higher generation dendrimers has shown to be problematic due to steric effects and variable enzyme levels in tissue [1, 27]. Therefore development of systems that are stable in circulation but rapidly respond to small intracellular molecules for drug release would make dendrimer conjugates more versatile. One such candidate for initiating release of drugs from dendrimers effectively is Glutathione (GSH).
GSH is the most abundant thiol species in the cytoplasm, functioning as a natural oxidant scavenger and the major reducing agent in biochemical processes [28]. The intracellular GSH concentration (2–10 mM) is substantially higher than extracellular levels (2 μM in plasma), which provides opportunities for intracellular delivery of therapeutic agents by disulfide linked carriers [29–30]. Disulfide linkages were utilized on melamine based dendrimers to incorporate dansyl groups into dendrimer structure and investigate the disulfide exchange kinetics [31]. More recently, photosensitizer mesochlorin (Mce6) conjugates of linear N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer, linked by disulfide linkage for photodynamic therapy of cancer treatment was investigated [33]. Various thiol containing species exist (i.e. plasma proteins such as albumin, lysosomal proteins, etc) that can induce disulfide exchange reactions. For these purposes, we investigated the use of GSH as well as other thiol containing species such as albumin (BSA) and cysteine (Cys), for their kinetics of releasing disulfide linked NAC from PAMAM dendrimer conjugates.
2. MATERIALS AND METHODS
2.1 Materials
Polyamidoamine (PAMAM) dendrimers (Generation-4, -NH2 terminated, Mw = 14,219 Da) were purchased from Dendritech Inc, Michigan USA. Other reagents were obtained from assorted vendors in the highest quality available. These include dimethyl sulfoxide, (DMSO, Aldrich), dicyclohexylcarbodiimide (DCC, Aldrich, USA), N-hydroxysuccinimde (Aldrich, USA), 3-Mercaptopropanoic acid (Aldrich, USA), (2, 21 –Dipyridyldisulfide (Aldrithiol, Aldrich, USA) dimethylformamide (DMF, Aldrich, USA), Ethanol (Aldrich, USA), L-Cysteine (Aldrich, USA), N-Acetyl Cysteine (NAC, Aldrich, USA), Glutathione (GSH, Aldrich, USA), bovine serum albumin (BSA, Aldrich, USA), Acetonitrile (Aldrich, USA), phosphate buffer saline (PBS, pH, 7.4, Aldrich, USA) and dialysis membrane (MWCO 1000 Spectrum Laboratories Inc, USA). Dulbecco’s Modified Eagle Medium, fetal bovine serum, penicillin-streptomycin and 0.05% trypsin-EDTA were purchased from Invitrogen. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide and lipopolysaccharides (LPS) were purchased from Sigma. The Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit was purchased from Invitrogen.
2.2 Characterization
All 1H-NMR spectra were recorded on Varian Mercury spectrometer 400MHz using DMSO as solvent. MALDI-TOF spectra were recorded on a Bruker Ultraflex system equipped with a pulsed nitrogen laser (337 nm), operating in positive ion reflector mode, using 19 kV acceleration voltage and a matrix of 2, 5 dihydroxybenzoic acid.
2.3 Synthesis of PAMAM-S-S-NAC
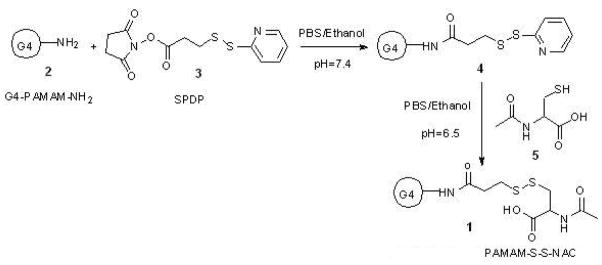
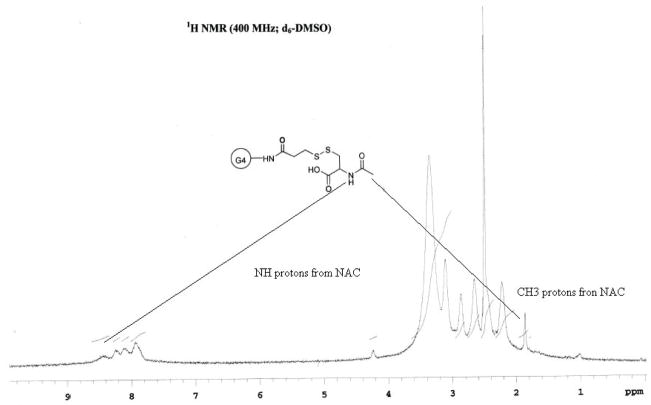
The scheme for the preparation of the conjugate is outlined in Figure 1. Briefly, a solution of SPDP in ethanol was added to a solution of PAMAM-NH2 dendrimer in PBS (pH 7.4). The reaction mixture was stirred at room temperature for 2 hours. N-Acetyl cysteine was added to this solution at once and the reaction mixture was stirred at room temperature for 4 hours. The reaction products were diluted with DMSO and dialyzed, first against DMSO followed by PBS, to remove by products and the excess of reactants. The dialysis was then repeated three times (12 hours each) with deionized water to remove any salts remaining. The final solution was lyophilized and the purified product was weighted. The overall reaction yield was 71%. The attachment of 16 copies of NAC to PAMAM-NH2-PDP dendrimers was determined by MALDI-TOF and 1H-NMR. From 1H-NMR analysis, methyl protons of N-Acetyl cysteine are used as characteristic peaks. The attachment of NAC to PAMAM-NH2-PDP dendrimers was determined by appearance of methyl protons as singlet at 1.94 ppm whereas attachment of PDP to PAMAM-NH2 was confirmed by amide protons as multiplete at 8.40–8.75 ppm. The payload of NAC was calculated by proton integration method using the amide protons in PAMAM-NH2 and methyl protons in PAMAM-S-S-NAC (Figure 2). The conjugate payload was confirmed further by the MALDI peak at 18.3 kDa (Figure 3), that agrees well with molecular mass calculated by 1H-NMR analysis
Figure 1.
Synthesis of PAMAM-S-S-NAC, (1)
Figure 2.
1H-NMR Spectrum of PAMAM-S-S—NAC
Figure 3.
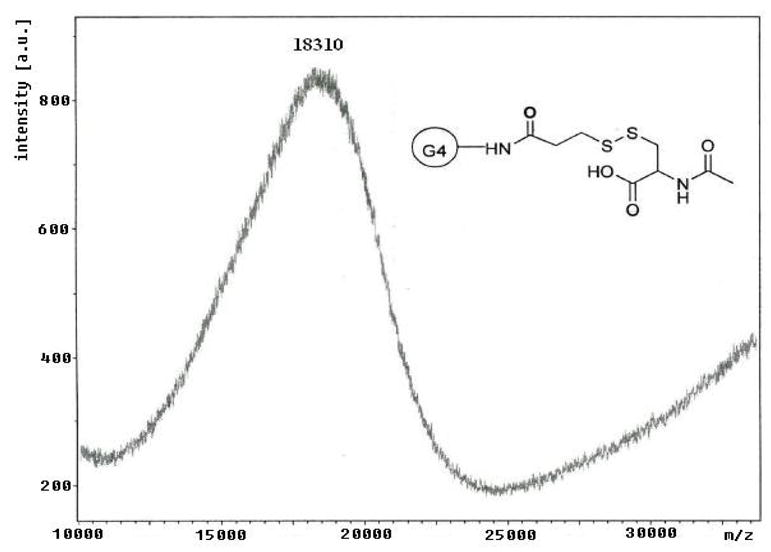
MALDI-TOF Spectrum of PAMAM-S-S-NAC
2.4 HPLC Characterization
The purity and stability of the conjugate was characterized by RP-HPLC. HPLC characterization was carried out with Waters HPLC instrument equipped with two pumps, an autosampler and dual UV detector interfaced to Breeze software. The mobile phase used was Acetonitrile/Water (0.14% TFA) and water phase had a pH of 2.5. Mobile phases were freshly prepared, filtered and degassed prior to use. Symmetry300 C4 RP-HPLC Column (5 μm particle size, 25 cm × 4.6 mm length × I.D.) equipped with a guard column (5 μm, 3.9 × 20 mm) was used for characterization of the conjugate as well as for release studies. Gradient method used for analysis was (100:0) (water:acetonitrile) to (90:10) at 10th minute to (25:75) in 20th minute followed by returning to initial conditions at 30th minute. The flow rate used was 1 ml/min. The U.V. absorbance was measured at 210 nm. The summary of HPLC chromatograms of each molecule studied is tabulated in Table 1. The analysis included oxidized forms of NAC, GSH (NAC-NAC and GSSG respectively) and GSH-NAC, since they are possible disulfide linked molecules that can be formed during release studies. The calibration for each of the analytes was prepared with the same conditions as the release studies; i.e: known concentrations of NAC were analyzed together with GSH, cysteine (Cys) or bovine serum albumin (BSA) concentrations used in the release studies in order to maintain accuracy. Additionally this approach was intended to prevent the NAC binding of BSA that may alter the release study results.
Table 1.
HPLC Analysis Summary
| RP-HPLC retention time summary of analytes (minutes) | |||||
|---|---|---|---|---|---|
| GSH | GSSG | NAC | GSH-NAC | NAC-NAC | PAMAM-S-S-NAC |
| 3.8 | 3.9 | 4.7 | 5.3 | 8.2 | 17.4 |
2.5 Drug Release Studies
Appropriate amounts of PAMAM-S-S-NAC conjugate were dissolved in release media (Citrate or PBS buffers) to form a solution of 1 mg/ml PAMAM-S-S-NAC. One of the thiol containing molecules (GSH, Cys or BSA) was added to the conjugates to form 10 mM, 2 mM, 0.5 mM 0.1 mM or 2 μM overall thiol group concentrations and to initiate the release of NAC. All samples were run as triplicates for statistical analysis. As control samples, conjugates were analyzed in both release media in the absence of reducing agents. The solutions were kept at 37 °C and stirred continuously. At predetermined time intervals, 10 μL of samples were withdrawn and immediately analyzed with RP-HPLC and the concentrations of analytes were determined by using appropriate calibrations prepared under same conditions.
2.6 In vitro Cytotoxicity Studies
In vitro cytotoxicity of the conjugate, dendrimer and NAC, at conditions similar to that used in the efficacy assays, was investigated by MTT assay. Mouse microglial cell line (BV-2) was obtained from Children’s Hospital of Michigan Cell Culture Facility. These cells were used because the eventual in vivo applications seek to target microglial cells that become activated as a result of neuroinflammation [33]. To investigate the cytotoxicity of the compounds, the cells were treated with the active compound (free drug, dendrimer, or the conjugate) for 3 hours. The lipopolysaccharide (LPS) was used to activate the cells for the ROS assay. For the cytotoxicity study, the LPS treatment was continuous for 24 hours. For both groups, the LPS concentration used was 100 ng/ml. Three concentrations of NAC were studied; 0.5 mM, 2 mM and 8 mM. For the conjugate assays, the concentrations used corresponded to equivalent NAC doses of the free drug treatment groups. Similarly, the dendrimer assays were run by using dendrimer concentrations that were equivalent to conjugate treatments. Control groups included cells receiving only LPS and no other treatment and cells with no LPS or other treatment. The proportion of viable cells in the treated group was compared to that of negative control. The cell viability is expressed as mean ± SD of three samples per group, and assessed by t test.
2.7 Cellular Uptake Dynamics by Flow Cytometry Analysis
The cellular uptake and dynamics of conjugates was evidenced by using flow cytometry analysis using FITC-labeled PAMAM-NH2 dendrimers, the same dendrimers used in the NAC conjugates. BV-2 cells were grown overnight in 6-well plate using DMEM cell culture medium supplemented with 5% FBS and 1% penicillin-streptomycin. When the cells were 70% confluent, they were treated with FITC-labeled PAMAM-NH2 dendrimers for 15, 60,120 and 240 minutes. The cells were washed with phosphate buffered saline (PBS, pH = 7.4), trypsinized and centrifuged at 1500 rpm for 5 min to obtain a cell pellet. The cells were then rinsed with PBS buffer, spun down three times, and resuspended in 1% formaldehyde, and analyzed using a flow cytometer (FACS caliber, Becton Dickinson) by counting 10,000 events. The mean fluorescence intensity of cells was calculated using the histogram plot.
2.8 Reactive Oxygen Species (ROS) Assay
Cells were treated with lipopolysaccharide (LPS) to induce the production of ROS. The cells were treated with LPS (100 ng/mL) and either NAC, PAMAM-S-S–NAC, or free PAMAM-NH2 dendrimers at appropriate concentrations to study the efficacy of the conjugates for reducing the ROS concentrations. In order to quantify the efficacy of conjugates, H2O2 released from BV-2 cells (ROS) was measured using the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit using previously established procedures [34]. The data is presented as percent reduction in H2O2 concentrations in cells treated with the active compound, compared to cells stimulated by LPS but did not receive any treatment. To investigate the effect of treatment time on the efficacy of the conjugates, two sets of experiments were performed: (1) In Group #1, the LPS and the active compounds (free drug, dendrimer, or the conjugate) were added to the cells at t=0, and the efficacy was followed after 24 and 72 hours; (2) In Group #2, the cells were treated with the active compound (free drug, dendrimer, or the conjugate) for just three hours, whereas the LPS treatment was continuous for 24 or 72 hours.
3. RESULTS AND DISCUSSIONS
3.1 Synthesis of Conjugates
To facilitate the linking of NAC to dendrimers via disulfide bond a spacer group 3-(2-Pyridyldithio)-propanoic acid (PDP) was used. To introduce sulfhydryl-reactive groups, PAMAM-NH2 dendrimers were reacted with the heterobifunctional cross-linker SPDP. The N-succinimidyl activated ester of SPDP couples to the PAMAM terminal primary amines to yield amide-linked 2-pyridyldithiopropanoyl (PDP) groups. The PAMAM-NH-PDP synthesized was than reacted with water soluble NAC to get desired conjugate PAMAM-S-S-NAC. The attachment of NAC to PAMAM-NH-PDP was determined by appearance of methyl protons as singlet at 1.94 ppm and amide protons as multiplete at 8.40–8.75 in 1H-NMR. The attachment of multiple copies of NAC to PAMAM-NH-PDP dendrimers was further determined by MALDI-TOF. MALDI-TOF analysis of the unmodified PAMAM-G4 dendrimers gave a broad peak at 14.1 kDa, which closely agrees to the theoretical molecular mass of the dendrimers 14.2 kDa. Conjugation of the PAMAM-G4 terminal amine groups to NAC by the linker resulted in a shift in the mass peak to 18.3 kDa. Each thiopropanoyl-NAC group has a molecular mass of 250 Da. Therefore, the MALDI data indicate an average of 16 NAC molecules per dendrimer molecule.
3.2 Release Studies
Dendrimer NAC conjugates were analyzed for their drug release mechanism and kinetics in the presence of GSH, Cys and BSA. Buffer solutions with pH 5 (Citrate Buffer) and pH 7.4 (Phosphate Buffered Saline) containing various concentrations of these thiol containing moieties were used in release studies.
3.2.1 GSH Triggered Release Mechanism and Rates
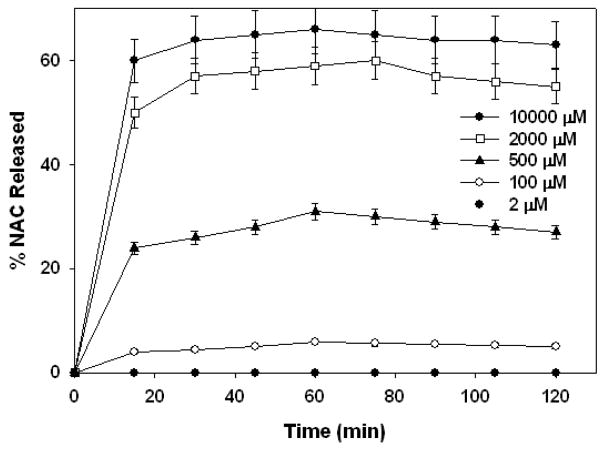
All GSH concentrations studied were between average plasma (2 μM) and intracellular (2–10 mM) GSH levels. Various GSH concentrations were used in order to determine the GSH dependent release kinetics of the conjugate. The conjugate solutions contained 730 μM NAC in the conjugated form (1 mg/ml PAMAM-S-S-NAC) at the beginning of the release studies. GSH concentration in the release media was compared to conjugated NAC concentrations in the release solutions for analysis of the release kinetics and mechanism. PBS buffer (pH=7.4) was used in order to demonstrate the GSH dependent release kinetics in intracellular environment and in blood. NAC release profile of the conjugate is shown in Figure 4. In the absence of GSH, the conjugate was stable, and did not release any NAC within 3 days.
Figure 4.
Percent total NAC released at pH 7.4 at various GSH concentrations shown on the graph legend.
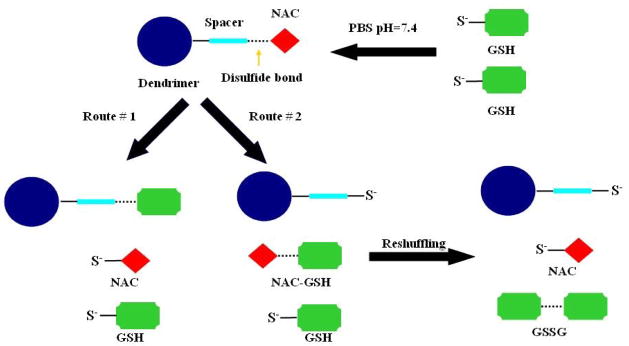
GSH can reduce the disulfide linkage in the conjugates in two possible ways. The conjugates may release NAC in free form while a GSH attaches onto the dendrimers forming the disulfide bond. The other pathway may release NAC-GSH, leaving the dendrimer with a free thiol group. The NAC-GSH released can be exchanged again with another GSH molecule and liberate NAC while forming a dimer of glutathione (GSSG). Even though disulfide exchange reactions only transfer the disulfide bond from the conjugate to its dimer form GSSG, slow oxidation reactions can also take place forming new disulfide bonds over longer periods of time. For this reason, NAC-GSH and NAC-NAC was also monitored during the release studies.
The results show that the conjugates released significant amounts of NAC within 1 hour at intracellular GSH concentrations. The release of NAC from the conjugate in the presence of GSH was fast and near completion within 1 hour. PAMAM-S-S-NAC conjugate released 47% of NAC payload in free form and 19% NAC payload in NAC-GSH form, at 1 hour. At high intracellular GSH concentrations NAC-GSH was gradually reduced to free NAC form at longer times but the overall percentage of NAC released did change notably. Similar trends were also observed in lower GSH concentrations. The amount of NAC released from the conjugate did not change significantly during the time period of two hours up to 17 hours (data not shown). This was in agreement with the expected fast disulfide exchange release mechanism. Release mechanism for PAMAM-S-S-NAC in the presence of GSH is shown in Figure 5. When the GSH-induced exchange reaction cleaves the disulfide bond on the dendrimer conjugate, NAC can be released in the free form, with the dendrimer binding the GSH (Route #1). Alternately, NAC can bind GSH to form NAC-GSH (Route 2). Eventually, the presence of the excess GSH allows for the free NAC to be released, through subsequent reshuffling reactions.
Figure 5.
NAC Release Mechanism of PAMAM-S-S-NAC in the presence of excess GSH
At intracellular GSH concentrations, the conjugate released 66% and 60% (at 10000 μM and 2000 μM GSH respectively) of its payload. The extent of release at 10000 μM GSH solution was only 6% more than the release at 2000 μM. The amount of GSH at 10000 μM and 2000 μM solutions exceeds the amount of NAC (730 μM) in conjugated form in the release media; therefore the amount released was not affected significantly. On the other hand, when the amount of GSH was limiting (at 500 μM and 100 μM GSH), the NAC release was reduced and was proportional to the GSH available; 31% and6% respectively. When the release studies were carried out at 2 μM GSH solution, there was no detectable level of NAC released. The results of release studies at pH 7.4 indicate that the conjugates prepared can release their payload in a very rapid manner in the presence of GSH. The extent of NAC release will depend on the amount of GSH available, compared to the number of disulfide linkages.
Reducing activity of GSH is attributable to its thiol group. GSH thiol group has a pKa of ~8.8 and its thiolate form is more reactive than the thiol form [35]. Therefore pH is an important parameter for reducing activity of GSH. In order to study the effect of pH on the reducing activity of GSH, we repeated the release studies at pH 5 (Citrate Buffer). The release at pH 5 was expected to be much slower due to the difference in thiolate/thiol ratios. The release studies at pH 5 are also relevant since the dendrimer conjugates are significantly taken up by the cells via endocytosis mechanism and reach the lysosomes where pH is 5. While GSH is not existent in the lysosomes and disulfide exchange reactions are disputed in lysosomes it is believed that other thiol containing molecules can carry on the task and take part in disulfide exchange reactions [32].
The results of release studies with GSH at pH 5 are shown in Figure 6. The same GSH concentrations were used as the studies at pH 7.4. The release profiles clearly indicate that the disulfide exchange reaction was significantly slowed due to reduced pH. The conjugates released their NAC payload for extended periods of time up to 20 hours. When the GSH was at intracellular concentrations and in excess of NAC in conjugated form, 95% of NAC payload was released within 20 hours. The release rates were slightly faster (nearly completed within 7 hours) at 10000 μM GSH concentration compared to 2000 μM GSH concentration (nearly completed within 20 hours). The completion of NAC release was apparent from the flattening of the curve on released graph (Figure 6). The release rates were significantly faster at these intracellular concentrations compared to lower GSH concentrations studied. At limiting GSH concentrations of 500 μM and 100 μM, the amounts released over 20 hours were 58% and 15% respectively. At 2 μM GSH concentration no significant amount NAC was released within 20 hours. The conjugate solutions containing no GSH did not release any NAC within the time period studied. Release studies at pH 5 indicate that, even though the disulfide exchange reactions are significantly slowed, the conjugates prepared can provide sustained release of their payload in the presence of GSH over a period of 20 hours.
Figure 6.
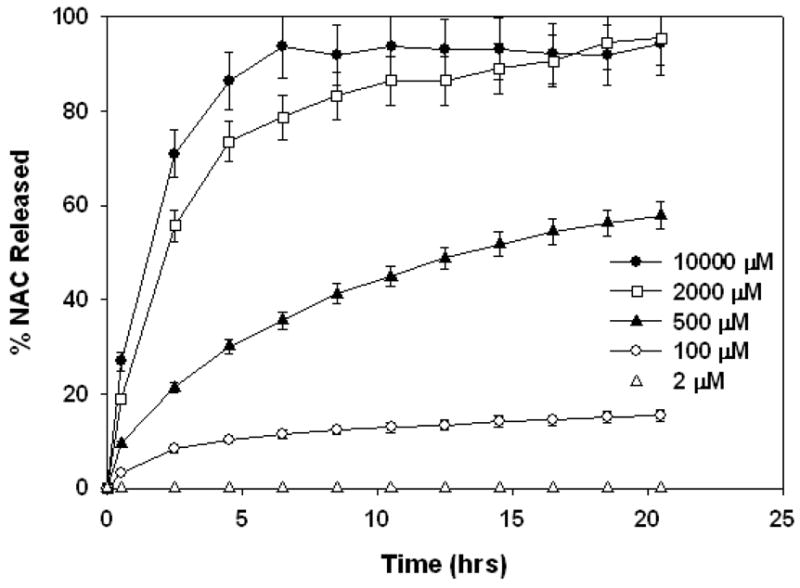
NAC Release profile at pH=5 and various GSH concentrations shown on the graph legend.
It should be noted that the maximum extent of release achieved at intracellular GSH concentrations for the two different pH buffers studied were slightly different. The conjugate released about 90% of its payload at pH 5.0 whereas approximately 65% at pH 7.4. This difference may be caused by free NAC possibly attaching back to its carrier via an oxidation reaction. The oxidation reaction is faster at pH 7.4 compared to pH 5, therefore possible NAC reattachment may be more significant at pH 7.4. This can limit the equilibrium NAC concentrations to a lower value within the release media compared to pH 5. On the other hand, this should not be an issue inside the cell, since the reductive environment is constantly replenished by glutathione reductase enzyme which should shift the equilibrium conditions to complete the release process.
3.2.2 Cysteine Triggered Release
The release studies with GSH were repeated with Cys to investigate the ability of the amino acid to reduce the conjugate and to compare the rates of release to GSH. The studies were carried out at same thiol group concentrations and in the same two buffers for investigation of pH effects, discussed earlier for GSH. The results of NAC release form the conjugate at pH=7.4 and in the presence of Cys is shown in Figure 7. The results indicate that Cys was able to reduce the conjugate and release NAC at slightly faster rates compared to GSH. The slightly faster release rates can be explained by lower pKa value of Cys (pKa =8.3) compared to GSH (pKa =8.8).
Figure 7.
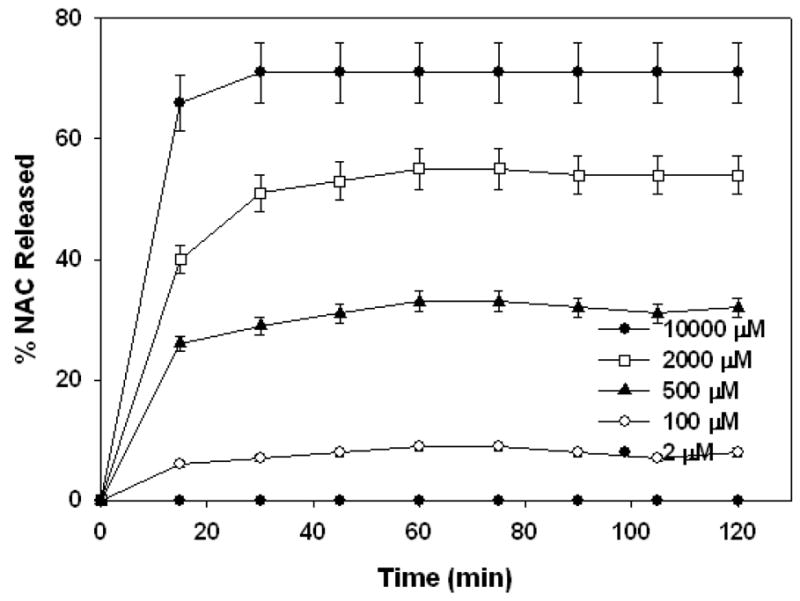
Percent total NAC released at pH 7.4 at various Cys concentrations shown on the on the graph legend.
The extent of release at all Cys concentrations was very similar to the extent of release of the corresponding GSH release studies. The similarity in extent of release combined with the release rates suggests that GSH and Cys are not affected by steric hindrance at the dendrimer surface when cleaving the drug from the dendrimer conjugate. While this should be obvious when the small size of Cys is considered, it is a quantitative proof that even though GSH is significantly larger compared to Cys, it is as effective in reducing the conjugate. Thus, GSH can be used as a drug-releasing agent from PAMAM dendrimer conjugates without steric problems most enzymes can face.
The release profile of the conjugate was also determined at pH 5 using Cys as releasing agent as shown in Figure 8. The rate of release was significantly reduced at this pH compared to pH 7.4, consistent with the result of GSH release studies. The extent of release was limited by the amount of available Cys for reducing the conjugate. The maximum extent of NAC release achieved with Cys for the two pH buffers studied agreed very well with GSH release studies. At pH 7.4, the release rate was much faster but the extent of release was less than the extent of release at pH 5. This suggests that pH of the media not only has direct implications on the rate of drug release, but also on its extent by governing the equilibrium concentrations of the thiol species. Cysteine is the most abundant thiol containing moiety in the body and also a part of GSH structure. The release studies with cysteine thus confirm that Cys can also function as a reducing agent for PAMAM-S-S-NAC as well as GSH.
Figure 8.
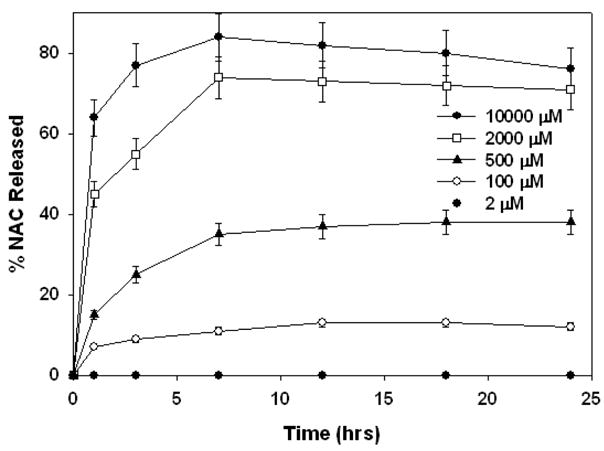
Percent total NAC released at pH 5 at various Cys concentrations shown on the on the graph legend.
3.2.3 Stability of Conjugates in Bovine Serum Albumin (BSA) Solution
Since the cysteine thiols are active reducing agents like GSH, and they do release the drug from the conjugate, it may be possible that cysteine residues on proteins can reduce the disulfide linkages on the conjugates if they are not sterically blocked. To investigate the reducing activity of Cys in protein structures, bovine serum albumin (BSA) was chosen for release studies since it is the most abundant protein in plasma. Additionally, for intravenous administration of conjugates, it is important for the drug to stay intact with the carrier until the conjugate reaches its final destination within the body. Thus the release characteristic in the presence of albumin is crucial for intravenous applications. The release studies in the presence of BSA were carried out at pH 7.4 and pH 5 as well. The BSA concentrations used for the studies were adjusted so that the overall Cys concentrations in BSA release media were the same as the GSH and Cys release studies, discussed earlier. In addition to the five thiol concentrations studied, we also analyzed the stability of the conjugate at plasma BSA concentration.
All of the release studies performed with BSA resulted in no NAC being released from the conjugates over 24 hours. BSA, with ~67 kDa molecular weight, is much bigger than the conjugate (~18 kDa). It was evident that BSA was not effective in reducing the disulfide linkages on the conjugates in any of the concentrations studied, most probably due to steric effects. This is not surprising since it was previously demonstrated that large proteins may have problems as releasing agents for dendrimer conjugates. On the other hand, the stability of PAMAM-S-S-NAC in the presence of BSA solution suggests that the conjugate can protect its payload from premature release while in blood circulation.
3.3 In vitro Cytotoxicity
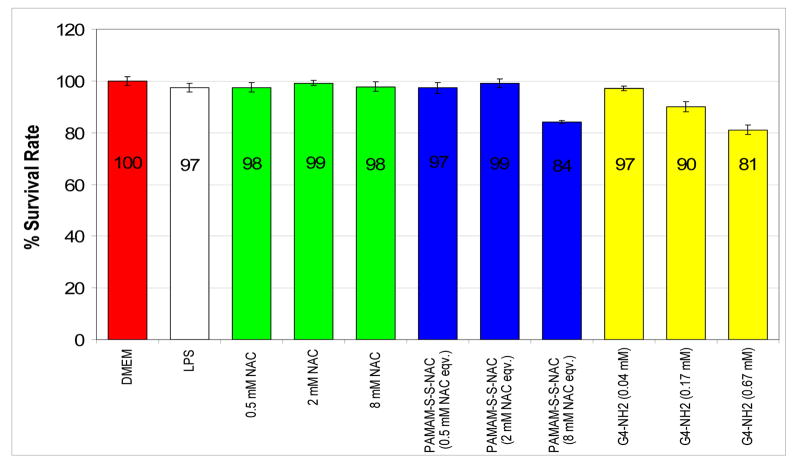
The induction of reactive oxygen species (ROS) production by LPS did not have significant cytotoxicity compared to control group that did not receive LPS treatment. The results indicate that there was no cytotoxicity associated with treatment by NAC in any of the MTT assays. When the cells were treated with the mentioned doses of free dendrimer or the PAMAM-SS-NAC conjugate, the microglial cell viability was better than 80% at all doses of the dendrimer and the conjugate. The cells that received 24 hr continuous treatment with dendrimers or the conjugates showed some cytotoxicity at the highest dose, whereas the lower doses did not produce significant cytotoxicity (data not shown). For this reason, only the lowest concentration treatment was considered for continuous treatment efficacy studies. The results of MTT assay when the cells were treated for 3 hours are shown in Figure 9. The conjugate was not cytotoxic at the two lower concentrations whereas the highest dose generated some cytotoxicity with 84% cell survival rate. Similarly, some cytotoxicity associated with free dendrimer treatment at higher concentrations was observed. The cytotoxicity of the PAMAM-NH2 dendrimers could be associated to their cationic polyvalent structure [4, 6, 9, 11]. On the other hand, it was apparent that the cytotoxicity of the free dendrimers was reduced upon conjugation with NAC. This is probably due to occupation of the charged surface groups of the dendrimer by NAC. It should be pointed out that the efficacy of the conjugate is evaluated only at the lowest concentration of the conjugate, where the cell survival rate was greater than 95%.
Figure 9.
Cytotoxicity assay, BV-2 microglial cells were stimulated with 100 ng/mL of LPS for 24 hrs. The cells were treated for 3 hrs with NAC, PAMAM-S-S-NAC conjugates and PAMAM dendrimers. Cell viability was assessed by MTT method. The proportion of viable cells in the treated group was compared to that of negative control. The cell viability is expressed as mean ± SD of three samples per group, and assessed by t test.
3.4 Cellular Uptake by Flow Cytometry
When the microglial cells were incubated with FITC-labeled dendrimers, the fluorescence intensity has increased by up to two orders of magnitude during the first two hours (Figure 10). The significant increase in fluorescence intensity observed within 15 minutes of incubation (an order of magnitude) suggests that the uptake of the dendrimer is rapid. The fluorescence intensity increased another order of magnitude from 15 minutes to 2 hours, which demonstrates that the microglial cells taken up the dendrimer rapidly, presumably by fluid-phase endocytosis, as seen in other cells [4,8,36,37]. The results suggest that treatment with dendrimer conjugates can effectively deliver very high extent of their payloads effectively into the cytoplasm within couple of hours. Therefore, in vitro efficacy of conjugates should be high even at shorter treatment time periods, since the high GSH levels in the cells should promote rapid release of the drug.
Figure 10.
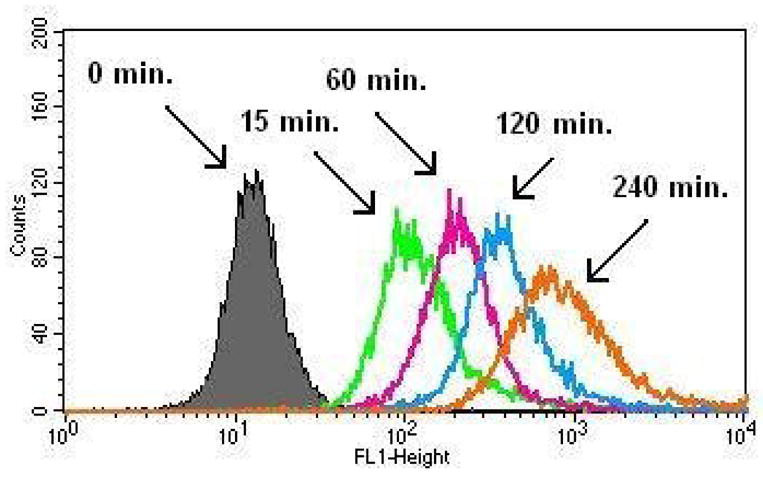
Cellular uptake of PAMAM-NH2-FITC by BV-2 microglial cells. Legend: Black: 0min, Green: 15min, Pink: 60min, Blue: 120min, Orange: 240min. The log of FITC absorption intensity (FL1-H on x-axis) is plotted against the number of cells (counts on y-axis).
3.5 Efficacy assay (ROS) in activated microglial cells
Reactive oxygen species (ROS) are important oxidative stress markers, and the oxidative stress is usually assessed by measuring ROS levels. Lipopolysaccharide (LPS) treatment is commonly used for activating microglia, resulting in ROS production [39]. NAC reduces the ROS levels due to its ability to interact with ROS and also its ability to stimulate endogenous GSH synthesis [39]. Suppression of ROS has been used widely to assess the in vivo efficacy of NAC in tissues undergoing neuroinflammatory processes [34, 40]. The reactive oxygen species formed by hydrogen peroxide (H2O2) are major contributors to oxidative damage of neuronal cells and oligodendrocytes in the brain leading to cell death and brain injury caused by activated microglial cells. Therefore the ability of the PAMAM-S-S-NAC conjugate to reduce H2O2 levels indicates the efficacy of conjugates in the activated microglial cells, which are the eventual target cells in vivo. Additionally, since the antioxidant effect of NAC is associated with its thiol group, which is occupied when in conjugated form, the conjugate would have to release the NAC to have antioxidant effects. The efficacy of the conjugates is dependent on entry of conjugates into cells and the subsequent release of free NAC.
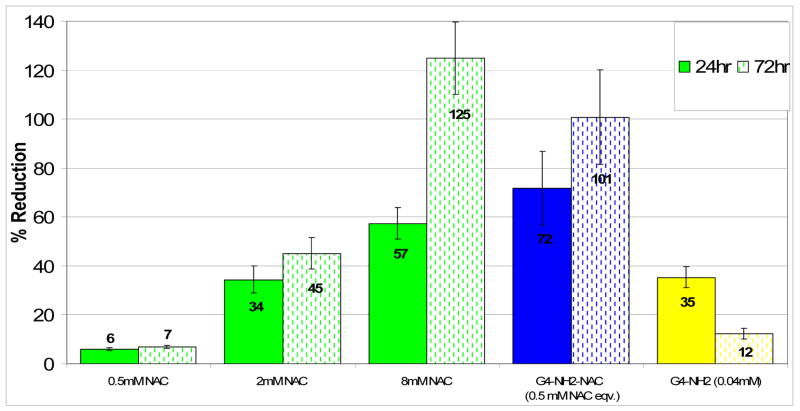
BV-2 microglial cells were treated with LPS to stimulate ROS production and increase H2O2 concentration. The LPS exposed cells were co-treated with saline, NAC, dendrimer or the conjugate simultaneously for 24 hours. The results of reduction in H2O2 levels when compared to the untreated control are shown in Figure 11. NAC treatment resulted in a dose dependant reduction in H2O2 concentrations with the lowest dose of 0.5 mM showing only 6% reduction in 24 hours and 7% percent in 72 hours. The highest dose of 8mM resulted in 57% reduction in 24 hours and 125% reduction in 72 hours. When the cells were treated with free dendrimer at concentrations corresponding to that in the conjugates, there was a small reduction in H2O2 levels, but this effect seemed to diminish over time as suggested by 35% reduction in 24 hours which decreased to just 12% at 72 hours. It is possible that this reduction in H2O2 by the free dendrimer may be due to the cationic amine groups on the surface that may interact with the peroxide free radicals. The detailed mechanism by which cationic dendrimers interact with intracellular free radicals is not clear, and is beyond the scope of the paper. The ‘short term’ nature of the effect suggests that the charge-balancing mechanism within the cell may reduce this effect eventually, or it may be possible that at longer time there is not enough dendrimer to ‘reduce’ intracellular H2O2 which is being produced continuously by the cells exposed to LPS. On the other hand, by conjugation of the linker and the drug, this cationic nature of the dendrimer is altered; therefore this effect should be even less significant for the conjugated form of the dendrimer.
Figure 11.
ROS Assay, Percent Reduction in H2O2 levels with 24 hr NAC, PAMAM-S-S-NAC or dendrimer treatment with simultaneous induction by LPS stimulation. 100% reduction denotes H2O2 concentration of cells with no induction by LPS (control group). The amount of ROS released into the media was measured using Amplex Red. Data are mean ± SD of three samples per group, and assessed by t test. For the free dendrimer, equivalent concentrations of the dendrimer that correspond to the amount present in the conjugate at the given NAC concentration are shown in bracket.
When treated with the lowest dose of NAC in the form of a dendrimer conjugate, the efficacy was increased by more than an order of magnitude compared to free NAC treatment, with 72% reduction in 24 hours and 101% reduction in 72 hours. The efficacy of 0.5 mM NAC equivalent of conjugate was comparable in efficacy to 8mM free NAC treatment, which suggests that the effective dose of NAC is reduced by about 16 times by administration in PAMAM-S-S-NAC form. At the 72-hour time point, the corresponding combined doses of free drug and free dendrimer have a significantly lower efficacy than the conjugate.
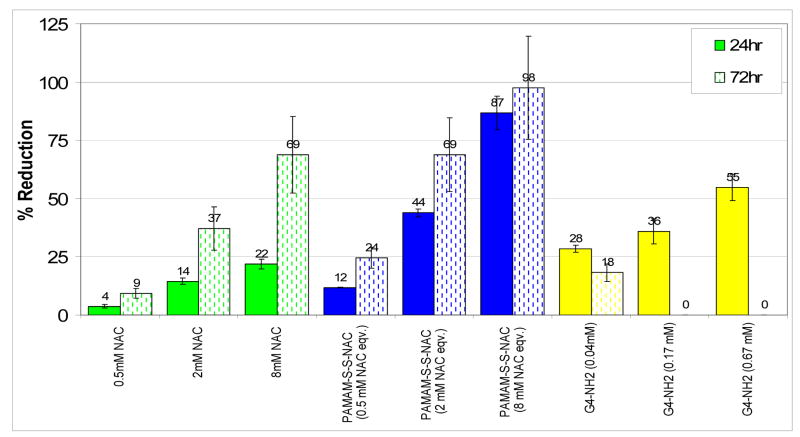
In order to understand the kinetics of dendrimer uptake and the subsequent intracellular drug release from the conjugates, the LPS treated BV-2 microglial cells were co-treated with NAC, dendrimer or conjugate for only three hours, followed by removal and refreshment of cell media containing LPS. The cells were then monitored for H2O2 concentrations for another 72 hours. The reduction of H2O2 levels at 24 hours and 72 hours after treatment is shown in Figure 12. Dose dependent efficacy of NAC is observed for the concentration range studied (0.5 mM–8 mM). When the cells were treated with equivalent concentrations of NAC in conjugated form, there was a significant enhancement in efficacy both at 24 hours and at 72 hours. Conjugation of NAC to G4-NH2 dendrimer through a fast releasing disulfide linkage has clearly enhanced the cellular entry and activity of NAC. The reduction in effective dose by conjugate treatment is as much as 4-fold at lower drug doses (0.5 mM and 2 mM), even with only three hours of treatment. While the treatment with free dendrimers showed some reduction in H2O2 levels at 24 hours, this effect faded in 72 hours similar to the earlier ROS assay with continuous treatment (Figure 11). Therefore effective intracellular delivery of NAC by conjugation to G4-NH2 PAMAM dendrimer is responsible for the enhancement in efficacy, especially in the longer time scales.
Figure 12.
ROS Assay, Percent Reduction in H2O2 levels with 3 hr of NAC, PAMAM-S-S-NAC or dendrimer treatment followed by LPS stimulation. 100% reduction denotes H2O2 concentration of cells with no induction by LPS (control group). The amount of ROS released into the media was measured using Amplex Red. Data are mean ± SD of three samples per group, and assessed by t test. The solid bars are the efficacy data for 24 hours, whereas the patterned bars denote the response after 72 hours. For free dendrimers, equivalent concentrations of the dendrimers that correspond to the amount present in the conjugate at the given NAC concentration are shown in brackets.
For both 3-hour and 24-hour treatments, the dendrimer-conjugated NAC shows superior efficacy compared to free NAC. The high efficacy of conjugates even at the lowest dose indicates that the conjugate is able to release significant amount of its payload intracellularly. This could be explained by the fact that the dendrimer may be transporting more of the NAC inside the cells, and that the dendrimer conjugate enables a sustained delivery of NAC into the activated microglial cells effectively over several days. After the conjugates are taken up by the cells via endocytosis, they will reside in the lysosomes for a period of time where the release of NAC may be relatively slow, because of the lower thiol content in the lysosomes [32,36]. As the conjugate escapes the lysosomal compartment, more NAC will be released at rates determined in the release studies. The combination of slower intracellular release, and higher NAC uptake enabled by the dendrimer, may be producing a longer pharmacodynamic effect inside the cells. Therefore, the lysosomal residence times of the conjugates may also play a role in the determining the time period that the conjugate treatment will have efficacy.
For the free drug and the free dendrimer, at the three doses studied, there is a relatively minor difference in the efficacy between 24-hour continuous treatment and three hour treatment. However, there is a significant difference in the efficacy of the 0.5 mM conjugate between continuous and three-hour treatment. This may be explained by an increase in endocytotic uptake due to the ‘activation’ of the microglial cells by sustained LPS treatment. Therefore, as more treatment time is allowed, more conjugate is transported inside the cell, perhaps releasing a factor of 5 or 6 times more drug intracellularly and providing even higher efficacy.
4. CONCLUSIONS
A PAMAM dendrimer NAC conjugate which uses a glutathione-sensitive disulfide linker for intracellular delivery of NAC in neuroinflammation treatment has been described. The conjugate prepared was characterized by NMR, MALDI, and HPLC analysis and a high NAC payload was determined. Drug release characteristics and mechanism of the conjugate in the presence of Cys, GSH and BSA in a concentration and pH dependent manner has been investigated. The conjugate released significant amounts of NAC within one hour when present at intracellular GSH concentrations and pH. At lysosomal pH, drug release was sustained for about 8 hours. At both pH buffers, the extent of release was directly proportional to amount of free thiol present. The stability of conjugates against release by large proteins such as albumin has been demonstrated, which has implications for intravenous conjugate therapies. The cytotoxicity, cellular uptake, and efficacy of the delivery system were investigated in activated microglial cells. The cellular uptake of the dendrimers was relatively rapid, with significant uptake in the first four hours. The conjugate showed up to an order of magnitude improvement in efficacy of NAC, in vitro. The significant improvement in efficacy demonstrates that NAC is being effectively transported into the cells and released from its dendritic carrier in agreement with the release kinetics determined. PAMAM dendrimer NAC conjugate reported here is a promising delivery vehicle for NAC, especially when inherent characteristic of PAMAM dendrimers to target neruoinflammation as well as their active and passive targeting capabilities are considered. The results of this study can pave the way for designing disulfide linked PAMAM dendrimer conjugates of other therapeutic agents that require highly responsive intracellular delivery. This study establishes that PAMAM dendrimers can release high drug payloads in a short time intracellularly, through the use of a ‘small’, natural biomolecule GSH.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee CC, MacKay JA, Fréchet JMJ, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–26. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 2.Svenson S, Tomalia DA. Dendrimers in biomedical applications & reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–29. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6:427–36. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 4.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–37. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Sato N, Kobayashi H, Hiraga A, Saga T, Togashi K, Konishi J, et al. Pharmacokinetics and Enhancement Patterns of Macromolecular MR Contrast Agents With Various Sizes of Polyamidoamine Dendrimer Cores. Magn Reson Med. 2001;46:1169–73. doi: 10.1002/mrm.1314. [DOI] [PubMed] [Google Scholar]
- 6.Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, et al. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65:133–48. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 7.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumour agent. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
- 8.Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP, et al. Nanoparticle Targeting of Anticancer Drug Improves Therapeutic Response in Animal Model of Human Epithelial Cancer. Cancer Res. 2005;65:5317–24. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 9.Malik N, Evagorou EG, Duncan R. Dendrimer-platinate: a novel approach to cancer chemotherapy. Anticancer Drugs. 1999;10:767–76. [PubMed] [Google Scholar]
- 10.Zhuo RX, Du B, Lu ZR. In vitro release of 5-fluorouracil with cyclic core dendritic polymer. J Controlled Release. 1999;57:249–57. doi: 10.1016/s0168-3659(98)00120-5. [DOI] [PubMed] [Google Scholar]
- 11.Gurdag S, Khandare J, Stapels S, Matherly LH, Kannan RM. Activity of Dendrimer-Methotrexate Conjugates on Methotrexate-Sensitive and -Resistant Cell Lines. Bioconjug Chem. 2006;17:275–83. doi: 10.1021/bc0501855. [DOI] [PubMed] [Google Scholar]
- 12.Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Fréchet JMJ, Dy EE, et al. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc Natl Acad Sci U S A. 2006;103:16649–56. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiwattanapatapee R, Lomlim L, Saramunee K. Dendrimers conjugates for colonic delivery of 5-aminosalicylic acid. J Controlled Release. 2003;88:1–9. doi: 10.1016/s0168-3659(02)00461-3. [DOI] [PubMed] [Google Scholar]
- 14.Kolhe P, Khandare J, Pillai O, Kannan S, Lieh-Lai M, Kannan RM. Preparation, cellular transport, and activity of polyamidoamine-based dendritic nanodevices with a high drug payload. Biomaterials. 2006;27:660–9. doi: 10.1016/j.biomaterials.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Najlah M, Freeman S, Attwood D, D’Emanuele A. Synthesis, characterization and stability of dendrimer prodrugs. Int J Pharm. 2006;308:175–82. doi: 10.1016/j.ijpharm.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Khandare J, Kolhe P, Pillai O, Kannan S, Lieh-Lai M, Kannan RM. Synthesis, Cellular Transport, and Activity of Polyamidoamine Dendrimer-Methylprednisolone Conjugates. Bioconjug Chem. 2005;16:330–7. doi: 10.1021/bc0498018. [DOI] [PubMed] [Google Scholar]
- 17.Navath RS, Kurtoglu YE, Wang B, Kannan S, Romero R, Kannan RM. Dendrimers-drug conjugates for tailored intracellular drug release based on glutathione levels. Bioconjug Chem. 2008 doi: 10.1021/bc800342d. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, et al. Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res. 2004;4:519–27. doi: 10.1002/jnr.20087. [DOI] [PubMed] [Google Scholar]
- 20.Dekhuijzen PNR. Antioxidant properties of N-Acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004;23:629–36. doi: 10.1183/09031936.04.00016804. [DOI] [PubMed] [Google Scholar]
- 21.Harada D, Naito S, Otagiri M. Kinetic Analysis of Covalent Binding between N-Acetyl-L-Cysteine and Albumin through the Formation of Mixed Disulfides in Human and Rat Serum in Vitro. Pharm Res. 2002;19:1648–54. doi: 10.1023/a:1020749211745. [DOI] [PubMed] [Google Scholar]
- 22.Yip L, Dart RC, Hurlbut KM. Intravenous administration of oral N-acetylcysteine. Crit Care Med. 1998;26:40–3. doi: 10.1097/00003246-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Kannan RM, Iezzi R, Rajaguru B, Kannan S. Dendrimer-containing particles for sustained release of compounds. US provisional patent, filed. 2007 November; [Google Scholar]
- 24.Kolhe P, Misra E, Kannan RM, Kannan S, Lieh-Lai M. Drug complexation, in vitro release and cellular entry of dendrimers and hyperbranched polymers. Int J Pharm. 2003;259:143–60. doi: 10.1016/s0378-5173(03)00225-4. [DOI] [PubMed] [Google Scholar]
- 25.Patri AK, Kukowska-Latallo JF, Baker JR., Jr Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv Rev. 2005;57:2203–14. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 26.De Jesus OLP, Ihre HR, Gagne L, Frechet JMJ, Szoka FC., Jr Polyester Dendritic Systems for Drug Delivery Applications: In Vitro and In Vivo Evaluation. Bioconjug Chem. 2002;13:453–61. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- 27.Bracci L, Falciani C, Lelli B, Lozzi L, Runci Y, Pini A, et al. Synthetic Peptides in the Form of Dendrimers Become Resistant to Protease Activity. J Biol Chem. 2003;278:46590–95. doi: 10.1074/jbc.M308615200. [DOI] [PubMed] [Google Scholar]
- 28.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 29.Arrick BA, Nathan CF. Glutathione Metabolism as a Determinant of Therapeutic Efficacy: A Review. Cancer Res. 1984;44:4224–32. [PubMed] [Google Scholar]
- 30.Saito G, Swanson JA, Lee K. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Tichy SE, Perez LM, Maria GC, Lindahl PA, Simanek EE. Evaluation of Multivalent Dendrimers Based on Melamine: Kinetics of Thiol-Disulfide Exchange Depends on the Structure of the Dendrimer. J Am Chem Soc. 2003;25:5086–94. doi: 10.1021/ja0210906. [DOI] [PubMed] [Google Scholar]
- 32.Cuchelkar V, Kopeckova P, Kopecek J. Synthesis and Biological Evaluation of Disulfide-Linked HPMA Copolymer- Mesochlorin e6 Conjugates. Macromol Biosci. 2008;8:375–83. doi: 10.1002/mabi.200700240. [DOI] [PubMed] [Google Scholar]
- 33.Saadani-Makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, et al. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am J Obstet Gynecol. 2008 doi: 10.1016/j.ajog.2008.06.090. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy A, Jana A, Yatich K, Freidt MB, Fung YK, Martinson JA, et al. Reactive oxygen species up-regulate CD11b in microglia via nitric oxide: Implications for neurodegenerative diseases. Free Radic Biol Med. 2008;45:686–99. doi: 10.1016/j.freeradbiomed.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winterbourn CC, Metodiewa D. Reactivity of Biologically Important Thiol Compounds with Superoxide and Hydrogen Peroxide. Free Radic Biol Med. 1999;27:322–28. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 36.Perumal OP, Inapagolla R, Kannan S, Kannan RM. The effect of surface functionality on cellular trafficking of dendrimers. Biomaterials. 2008;29:3469–76. doi: 10.1016/j.biomaterials.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 37.Kitchens KM, Foraker AB, Kolhatkar RB, Swaan PW, Eddington ND, Ghandehari H. Endocytosis and interaction of poly(amidoamine) dendrimers with Caco-2 cells. Pharm Res. 2007;24:2138–45. doi: 10.1007/s11095-007-9415-0. [DOI] [PubMed] [Google Scholar]
- 38.Min KJ, Jou I, Joe E. Plasminogen-induced IL-1beta and TNF-alpha production in microglia is regulated by reactive oxygen species. Biochem Biophys Res Commun. 2003;312:969–74. doi: 10.1016/j.bbrc.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Flora SJ, Poande M, Kannan GM, Mehta A. Lead induced oxidative stress and its recovery following co-administration of melatonin or N-acetylcysteine during chelation with succimer in male rats. Cell Mol Biol. 2004;50:543–5. [PubMed] [Google Scholar]
- 40.Paintlia MK, Paintlia AS, Contreras MA, Singh I, Singh AK. Lipopolysaccharide-induced peroxisomal dysfunction exacerbates cerebral white matter injury: Attenuation by N-acetyl cysteine. Exp Neurol. 2008;210:560–76. doi: 10.1016/j.expneurol.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]