Abstract
Objective
The diagnosis and treatment of youth with severe nonepisodic irritability and hyperarousal, a syndrome defined as severe mood dysregulation (SMD) by Leibenluft, has been the focus of increasing concern. We conducted the first randomized double-blind, placebo-controlled trial in SMD youth, choosing lithium on the basis of its potential in treating irritability and aggression and neuro-metabolic effects.
Methods
SMD youths 7–17 years were tapered off their medications. Those who continued to meet SMD criteria after a 2-week, single-blind, placebo run-in were randomized to a 6-week double-blind trial of either lithium (n = 14) or placebo (n = 11). Clinical outcome measures were: (1) Clinical Global Impressions–Improvement (CGI-I) score less than 4 at trial's end and (2) the Positive and Negative Syndrome Scale (PANSS) factor 4 score. Magnetic resonance spectroscopy (MRS) outcome measures were myoinositol (mI), N-acetyl-aspartate (NAA), and combined glutamate/glutamine (GLX), all referenced to creatine (Cr).
Results
In all, 45% (n = 20/45) of SMD youths were not randomized due to significant clinical improvement during the placebo run-in. Among randomized patients, there were no significant between-group differences in either clinical or MRS outcome measures.
Conclusion
Our study suggests that although lithium may not result in significant clinical or neurometabolic alterations in SMD youths, further SMD treatment trials are warranted given its prevalence.
Introduction
Recently, more attention has focused on the diagnosis and treatment of youth with severe nonepisodic irritability and hyperarousal, a syndrome defined as severe mood dysregulation (SMD) Leibenluft et al. 2003). This increased focus prompts questions regarding the degree to which this presentation reflects a developmental variant of mania. Youths with SMD do not fit well into our current psychiatric nosology, although recent data indicate that this is a common symptom cluster, with a prevalence of 3.2% in the community (Brotman et al. 2006). Such children do not meet criteria for bipolar disorder (BD) due to the nonepisodic nature of their mood symptoms (Pogge et al. 2001; Leibenluft et al. 2003; Reich et al. 2005). While they commonly meet criteria for attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD), these diagnoses do not fully capture the complex, severe levels of their mood and behavioral symptoms. Not surprisingly, such diagnostic dilemmas have hindered efforts to study how to treat youth with SMD, who are presenting for psychiatric care with increasing frequency.
Lithium is one agent whose efficacy for irritability and aggression has been studied in children. Three out of four double-blind, randomized, controlled trials (RCTs) of youths with conduct disorder (CD) found that lithium, compared to placebo, resulted in significantly reduced aggression and behavior problems (Campbell et al. 1984; Campbell et al. 1995; Rifkin et al. 1997; Malone et al. 2000). Data supporting lithium's efficacy in treating irritability associated with pediatric mood disorders is less robust. One of two double-blind, placebo-controlled RCTs showed significant global improvement (without specific reference to irritability) in youths with either BD or high risk for BD and secondary substance abuse (Geller et al. 1998a). However, the other failed to show benefit in prepubertal youth with major depressive disorder (MDD) and elevated risk for BD, including BD type I in first- or second-degree relatives or a multigenerational/loaded MDD family history (Geller et al. 1998b). The only other placebo-controlled RCT of lithium in BD youths, a 2-week discontinuation study, did not show significant benefit for continued lithium over placebo substitution in preventing mania relapse, again without specific reference to irritability (Kafantaris et al. 2004). Taken as a whole, these data provide some, albeit mixed, indication that lithium may be an effective treatment for youth with severe nonepisodic irritability and hyperarousal.
Lithium's primary mechanism of action is thought to result from depletion of intracellular second messengers, including myoinositol (mI), causing inhibition of downstream serotonergic, glutamatergic, and cholinergic neurotransmission (Berridge and Irvine 1989). Magnetic resonance spectroscopy (MRS) studies indicate that manic or depressed BD adults may have increased mI (Gould et al. 2004; Silverstone et al. 2005). At therapeutic levels, lithium results in significantly decreased frontal mI, with a corresponding trend in the hippocampus (Moore et al. 1999). Similar studies have yielded mixed results in BD youths, with one study showing significantly decreased frontal mI (Davanzo et al. 2001), while another did not (Patel et al. 2006). Lithium is also thought to increase N-acetyl aspartate (NAA), an intraneuronal marker of neural health as well as a putative indicator of energetics within neuronal mitochondria (Tsai and Coyle 1995; Moore et al. 2000a; Moore et al. 2000b; Stork and Renshaw 2005).
On the basis of what is known about the neuro-metabolic effects of lithium, as well as its possible role in treating irritability and aggression, we conducted the first double-blind, placebo-controlled RCT of lithium in youths with severe nonepisodic irritable mood and hyperarousal, while also performing MRS pre- and posttreatment. To capture the relevant patient population, we used Leibenluft et al.'s criteria for SMD because, unlike any Diagnostic and Statistical Manual of Mental Disorders (DSM) (American Psychiatric Association 2000) diagnosis, these criteria offer a precise definition of irritability, i.e., markedly increased reactivity to negative emotional stimuli manifest verbally or behaviorally at least three times weekly. SMD criteria also require abnormal mood (anger or sadness), present at least half of the day most days, and hyperarousal (≥3 of insomnia, agitation, distractibility, racing thoughts or flight of ideas, pressured speech, intrusiveness) (Leibenluft et al. 2003). We hypothesized that lithium would result in significantly greater clinical improvement than placebo, as defined by reduction in scores on the Clinical Global Impressions–Improvement Scale (CGI-I) (Spearing et al. 1997) and on the excitement subscale of the Positive and Negative Syndrome Scale (PANSS) (sum of the excitement [hyperactivity], hostility, uncooperativeness, and poor impulse control scores) (Kay et al. 1989). The PANSS excitement subscale has been shown to explain 54.9% of the total variance in manic-like excitement and have good correlation with changes in the Young Mania Rating Scale (YMRS) (Lindenmayer et al. 2004).
Furthermore, on the basis of the above-mentioned MRS studies in BD adults and children, we hypothesized that SMD youths treated with lithium would have significant neurometabolic alterations. We modeled our MRS methodology on pioneering studies by Manji and Moore that paired lithium treatment with MRS in BD adults. These studies were conducted with spectra acquired in four regions of interest (see MRS methods section, below) (Moore et al. 1999; Moore et al. 2000a). On the basis of those studies, we hypothesized that lithium treatment would be associated with significantly reduced mI and increased NAA across all regions, but especially in frontal and temporal regions. Additionally, on the basis of nontreatment studies of youths with either ADHD or depression, adult normal volunteers, and animal models (Courvoisie et al. 2004; Rosenberg et al. 2005; Moore et al. 2006; Shaltiel et al. 2008; Shibuya-Tayoshi et al. 2008), we hypothesized that lithium treatment in SMD youths would be associated with significantly reduced glutamate and glutamine (GLX). The latter is a marker of the major excitatory neurotransmitter glutamate, whose resonances can not fully be resolved at magnetic fields less than 4 Tesla (Zarate et al. 2002; Stork and Renshaw 2005).
Methods
Subjects
This study was conducted at the National Institute of Mental Health Division of Intramural Research Programs (NIMH DIRP) from August, 2002, until December, 2007, and was approved by the National Institute of Health's Combined NeuroScience Institutional Review Board. After the studies were explained, and prior to participation, parents gave written informed consent, and children gave written assent. Subjects (ages 7–17 years) were recruited through advertisements placed in local parenting magazines, on support groups' websites, and distributed to psychiatrists nationwide.
Screening
SMD inclusion criteria were: (1) irritability as defined by markedly increased reactivity to negative emotional stimuli manifest verbally or behaviorally at least three times weekly; (2) abnormal mood (anger or sadness), present at least half of the day most days; (3) hyperarousal (≥3 of insomnia, agitation, distractibility, racing thoughts or flight of ideas, pressured speech, intrusiveness); (4) symptoms cause severe impairment in at least one setting (home, school, or peers) and at least mild impairment in a second setting; (5) SMD symptom onset must be before age 12 and must be currently present for at least 12 months without symptom-free periods greater than 2 months (Leibenluft et al. 2003).
SMD exclusion criteria were: (1) presence of cardinal bipolar symptoms, including elevated/expansive mood, grandiosity/inflated self-esteem, or episodically decreased need for sleep (Geller et al. 1998c); (2) distinct episodes of manic symptoms greater than 1 day; (3) pervasive developmental disorder; (4) psychosis; (5) substance abuse within 3 months; (6) medical illness that is unstable or could cause SMD symptoms; (7) intelligence quotient [IQ] <70; and (8) pregnancy.
Following a telephone interview to screen for relevant inclusion/exclusion criteria, record review, and consultation with the child's treating clinician, potential SMD subjects were invited to the NIMH DIRP (n = 196). On-site screening included the Child Schedule for Affective Disorders Present and Lifetime Version (K-SADS-PL) with an additional SMD supplement, designed in collaboration with Joan Kaufman, Ph.D., to ascertain whether children met criteria for this syndrome. All diagnostic measures and were administered to parent and child individually by trained graduate level clinicians with established interrater reliability (κ ≥ 0.9, including distinguishing SMD subjects from those with narrow phenotype BD—i.e., distinct manic episodes involving elevated, expansive mood (Kaufman et al. 1997; Leibenluft et al. 2003). Diagnoses were based on best-estimate procedures (Leckman et al. 1982) generated in a consensus conference led by two psychiatrists with extensive experience evaluating children with bipolar-spectrum illness. Of note, SMD is not a DSM diagnosis, but rather, SMD criteria were designed to capture youth with nonepisodic irritability and hyperarousal who are frequently diagnosed as having bipolar disorder in clinical settings. Thus, for SMD subjects, all other forms of psychopathology are considered to be co-morbid, such as ADHD or anxiety disorders, because these other disorders are, in essence, dependent rather than independent variables. Full-scale intelligence quotient (FSIQ) was measured by the Wechsler Abbreviated Scale of Intelligence (WASI) for all subjects and controls (Wechsler 2005).
Enrollment
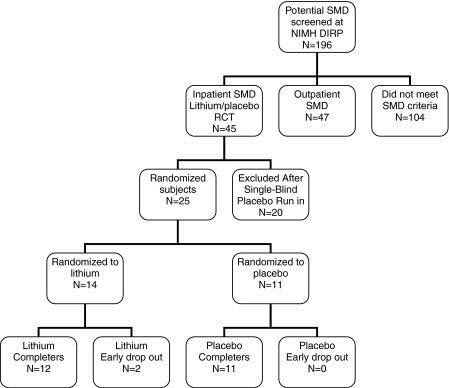
Those subjects who met SMD criteria after on-site screening, had not previously been treated with lithium, and were not functioning well on their current medications were offered enrollment in our lithium RCT (n = 45). To maximize safety and observational data, this study was conducted on the inpatient child psychiatric unit of the NIMH DIRP. Subjects were admitted to the hospital (baseline 1) and gradually weaned off all of their outpatient psychiatric medications for four drug half-lives by the attending child/adolescent psychiatrist (K.E.T.), with close observation for deterioration in functioning and side effects. Medications with longer drug half-lives (e.g., atypical neuroleptics, antidepressants) were tapered prior to those with short half-lives (e.g., psychostimulants). Then, they completed a 2-week single-blind placebo run-in, at the end of which (baseline 2) only those who continued to meet SMD criteria (n = 25) were randomized to either lithium or placebo for a 6-week double-blind RCT. Those no longer meeting SMD criteria (n = 20) received medication stabilization, according to the clinical judgment of the treating board-certified child and adolescent psychiatrist (K.E.T.), and were discharged. During the trial, lorazepam was available as a PRN medication; however, no SMD subject received lorazepam during the treatment trial (Fig. 1).
FIG. 1.
Disposition of SMD subjects in randomized, placebo-controlled trial of lithium.
Assessments
Our primary categorical clinical outcome measure was a CGI-I score less than 4 at the trial's end (i.e., children who received scores of 3 [improved], 2 [much improved], or 1 [symptom free]) (Spearing et al. 1997). Our primary continuous clinical outcome measure was the PANSS factor 4 score (sum of excitement, hostility, uncooperativeness, and poor impulse control; continuous outcome measure) (Kay et al. 1989).
Graduate-level clinicians with established interrater reliability (κ ≥ 0.9) rated participants weekly on the CGI-I and PANSS, as well as the following measures: YMRS (Young et al. 1978), Children's Depression Rating Scale (CDRS) (Emslie et al. 1990), and Children's Global Assessment–Severity (CGA-S) (Shaffer et al. 1983). Of note, because their illness is not episodic and therefore SMD subjects do not meet Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) criteria for mania, the patients' YMRS scores should not be interpreted as a measure of mania severity per se, but rather as a measure of the severity of the criteria “B” symptoms. Our inpatient unit teachers completed the 39-item Conners' teacher report of ADHD symptoms (Werry et al. 1975). Unit staff completed the Overt Aggression Scale (OAS) whenever a subject was aggressive either verbally or physically toward objects, others, or self (Silver and Yudofsky 1991; Yudofsky et al. 1997).
Medication
During the single-blind and double-blind phases, all subjects received pills twice daily and had blood draws weekly. During the first week of the double-blind phase, those randomly assigned to lithium received lithium carbonate 150 mg twice daily for 2 days and then 300 mg twice daily for 5 days. An unblinded psychiatrist at the NIMH DIRP reviewed lab results and clinical measures to ensure subject safety and to adjust lithium carbonate dose. The target steady state therapeutic level was between 0.8 and 1.2 mmol/L. All subjects received either lithium monotherapy or placebo without additional psychotropic agents. Any patient who showed clinical worsening, defined as a CGI-I score of 5 (“worse”) or greater for 2 consecutive weeks, or a clinically significant adverse event was assessed by an independent clinician and removed from the treatment trial.
MRS protocol
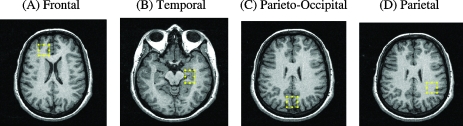
Subjects completed MRS scans while medication-free during the single-blind phase and again at the end of the treatment trial. All scans were performed without sedation on a 1.5 Tesla General Electric Signa scanner using the standard GE Point Resolved SpectroScopy (PRESS) sequence with the following parameters: echo time 30 milliseconds (msec), repetition time 2000 msec, number of excitations (NEX) = 8, water suppression on, and total acquisition time 5 minutes. On the basis of prior MRS studies in BD adults, spectra were acquired in four 8-mL regions of interest (ROIs), although as with these prior studies, a priori hypotheses were focused on the frontal and temporal ROIs (Moore et al. 1999; Moore et al. 2000a). MRI technologists, who were blind to participant group, placed each ROI to maximize gray matter content according to landmarks in: (1) the right frontal cortex (orbitofrontal cortex [OFC]; lateral to the falx cerebri, anterior to the frontal horn of the lateral ventricle, and superior to the orbits); (2) left temporal cortex centered on left hippocampus (slice inferior to frontal voxel slice that maximally captures the hippocampus); (3) central parieto-occipital lobe (centered on posterior falx cerebri, posterior to cingulate gyrus, and including midline precuneus), and (4) left parietal lobe predominantly white matter (same slice as parieto-occipital voxel centered on left corona radiata and lateral to posterior horn of lateral ventricle) (Figs. 2 and 3).
FIG. 2.
Magnetic resonance spectroscopy voxel placement. (A) Frontal: Placed in the right orbitofrontal cortex (OFC). (B) Temporal: Placed in the left hippocampus. (C) Central parieto-occipital: Placed in the vicinity of the precuneus. (D) Parietal: Placed in the white matter of the corona radiata. Voxel locations are based upon Moore et al. (1999) and Moore et al. (2000a).
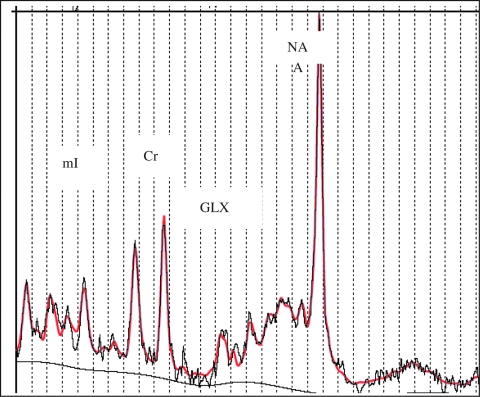
FIG. 3.
Sample LC model processed spectra. The black line represents acquired spectra; the red line represents fitted spectra from same subject. Horizontal axis is chemical shift in parts per million (ppm). LC model: version 6.1–0, S.W. Provencher, Ontario, Canada. mI = Myoinositol; Cr = creatine; GLX = combined glutamate/glutamine; NA = N-acetyl-aspartate.
Quantitative MRS processing
All MRS scans were analyzed with Linear Combinations Model (LCModel, v6.1-0, Steven Provencher, Ontario, Canada). As described elsewhere (Provencher 1993), LCModel is an automated program for in vivo MRS analysis that fits acquired MRS data based on standard reference information supplied by the manufacturer. Although the full reference set was used for spectral fitting, our a priori hypotheses were focused on the MRS intensities of: (1) glutamate/glutamine (GLX, which cannot be fully resolved at 1.5 Tesla) at a chemical shift of 2.1–2.5 parts per million (ppm); (2) NAA at 2.02 ppm; and (3) mI at 3.5 ppm. These were assayed relative to the creatine/phosphocreatine (Cr) resonance at 3.02 ppm, which served as an internal signal reference to avoid partial volume effects—i.e., having some cerebrospinal fluid (CSF) present in a primarily gray matter ROI—because CSF does not contain neurometabolites (mI, NAA, GLX) and thus would alter our results (Komoroski et al. 2004; Hancu et al. 2005). We used Cramer–Rao bounds of 20% or less for each metabolite as a cutoff to ensure data quality. Previously, we have published data comparing unmedicated (baseline II) SMD subjects (both subsequently randomized and not) versus age/sex matched typically developing controls (Dickstein et al. 2008).
Data analysis
Clinical outcome measures were examined with an intent-to-treat analysis, with last observation carried forward, implemented in SAS (v. 9.1) (SAS Institute, Cary, North Carolina). Because repeated measures within the same individual do not represent independent observations, we used the general linear model (SAS GENMOD procedure) for the categorical outcome variable of CGI-I <4 at week 6, and we used a mixed model (SAS PROCMIXED procedure) for continuous outcomes (PANSS-4), with treatment group and study week as fixed effects and subject as a random effect. Efficacy was tested via interactions between treatment group and study week. Demographic factors comparing those randomized versus not and those treated with lithium versus placebo were analyzed with independent t-tests for continuous variables or Pearson chi-squared for categorical variables implemented in Statistical Package for Social Sciences (SPSS, v14.0, Chicago, IL). Between-group differences in pre-and posttreatment MRS neurometabolites were analyzed with a repeated measures analysis of variance (ANOVA) implemented in SPSS.
Results
Subject characteristics: randomized versus nonrandomized
Of 196 patients screened on-site at the NIMH DIRP, 45 subjects entered the lithium RCT (baseline 1) (see Fig. 1). Of those 45, 25 subjects were randomized to lithium (n = 14) or placebo (n = 11), and 20 subjects were excluded prior to randomization at the end of the single-blind placebo run-in (baseline 2), due to improvement in their status to the point where lithium treatment was no longer indicated.
There were no differences in baseline 1 characteristics between those SMD subjects who were randomized (randomized [R], n = 25) versus nonrandomized (NR, n = 20). Specifically, there were no significant differences in age (mean age in years: R 11.45 ± 2.11, NR 12.14 ± 2.02, t = −1.11, degrees of freedom [df ] = 43, p = 0.28), full-scale IQ (R 105.2 ± 14.9, NR 96.9 ± 14.9, t = 1.74, df = 39, p = 0.09), or sex (% male: R 75%, NR 55%, χ2 = 2.21 p = 0.14). There were also no significant differences in clinical ratings at study entry, including YMRS (R 14.6 ± 4.7, NR 13.8 ± 6.0, t = 0.47, df = 41, p = 0.64), CDRS (R 29.8 ± 5.1, NR 30.5 ± 7.6, t = −0.35, df = 41, p = 0.73), CGI-S (R 4.9 ± 0.8, NR 4.7 ± 0.9, t = 0.81, df = 40, p = 0.42), or Children's Global Assessment Scale (CGAS) (R 44.7 ± 5.3, NR 44.1 ± 5.6, t = 0.40, df = 42, p = 0.69). Last, there were no significant between group differences in current K-SADS psychiatric diagnoses, including no differences in rates of current MDD (R 20%, NR 20%, χ2 = 0.00, p = 1.00), current ADHD (R 92%, NR 85%, χ2 = 0.55, p = 0.46), and current ODD (R 88%, NR 85%, χ2 = 0.09, p = 0.77). There was a trend toward significantly more current separation anxiety in those NR (R 12%, NR 35%, χ2 = 3.40, p = 0.07).
Of those 20 nonrandomized subjects, 14 subjects were excluded for not meeting SMD criteria at baseline 2 (i.e., after medication withdrawal and a 2-week placebo run-in), 4 withdrew their assent (including refusal to have weekly blood draws or homesickness), 1 was excluded for diagnostic exclusion criteria (pervasive developmental disorder [PDD]), and 1 was excluded for being unmanageable on the unit due to antisocial behavior. Among those who no longer met SMD criteria, 78.6% (n = 11/14) 91.7% (n = 11/12) no longer had markedly excessive reactivity to negative emotional stimuli, and 71.4% (n = 10/14) no longer had anger, sadness, or irritability present most days and noticeable to others. Most of these 14 also no longer had hyperarousal symptoms (percent who no longer had: insomnia 85.7% [n = 12/14], agitation 64.3% [n = 9/14], distractibility 50% [n = 7/14], racing thoughts 100% [n = 14/14], pressured speech 92.9% [n = 13/14], intrusiveness 71.4% [n = 10/14]).
Subject characteristics: randomized to lithium versus placebo
As shown in Table 1, there were no significant differences between those randomized to lithium (SMD-Li, n = 14) and those randomized to placebo (SMD-Plac; n = 11) at study entry with respect to age or full-scale IQ. However, there was a significant difference in sex, with a greater percentage of male subjects in those randomized to lithium than to placebo.
Table 1.
Baseline Demographic and Clinical Characteristics of SMD Youths Randomized to Lithium (n = 14, SMD-Lithium) versus Placebo (n = 11, SMD-Placebo)
| Characteristic | SMD-lithium (n = 14)* | SMD-placebo (n = 11) | Statistics | ||
|---|---|---|---|---|---|
| Age | 10.85 ± 1.99 | 12.18 ± 2.13 | t = ±1.60, df = 23, p = 0.12 | ||
| Full-scale IQ | 108.9 ± 15.7 | 100.0 ± 12.7 | t = 1.47, df = 22, p = 0.16 | ||
| Baseline II | |||||
| YMRS | 14.9 ± 4.0 | 14.3 ± 4.8 | t = 0.28, df = 21, p = 0.78 | ||
| CDRS | 29.9 ± 6.4 | 26.7 ± 5.0 | t = 1.33, df = 22, p = 0.20 | ||
| PANSS factor 4 | 17.6 ± 3.3 | 16.5 ± 2.0 | t = 0.92, df = 22, p = 0.37 | ||
| CGI-Severity | 4.8 ± 0.9 | 4.4 ± 0.7 | t = 1.03, df = 19, p = 0.32 | ||
| CGAS | 43.3 ± 8.4 | 45.3 ± 3.2 | t = ±0.72, df = 22, p = 0.48 | ||
| n | % | n | % | ||
| Sex | |||||
| Male | 13 | 93% | 6 | 55% | χ2 = 4.96, |
| Female | 1 | 7% | 5 | 45% | p = 0.03 |
| DSM-IV-TR diagnoses | |||||
| MDD | 3 | 21% | 2 | 18% | χ2 = 0.03, p = 0.84 |
| ADHD | 14 | 100% | 9 | 82% | χ2 = 2.77, p = 0.10 |
| ODD | 12 | 86% | 10 | 91% | χ2 = 0.16, p = 0.69 |
| CD | 0 | 0% | 0 | 0% | N/A |
| Any anxiety disorder | |||||
| GAD | 2 | 14% | 5 | 45% | χ2 = 2.97, p = 0.09 |
| Separation anxiety disorder | 2 | 14% | 1 | 9% | χ2 = 0.16, p = 0.69 |
| Specific phobia | 4 | 29% | 1 | 9% | χ2 = 1.46, p = 0.23 |
| Social phobia | 1 | 7% | 1 | 9% | χ2 = 0.03, p = 0.86 |
| Panic disorder | 0 | 0% | 0 | 0% | N/A |
| PTSD | 1 | 7% | 0 | 0% | χ2 = 0.82, p = 0.37 |
| Enuresis | 4 | 29% | 1 | 9% | χ2 = 1.22, p = 0.27 |
Lithium group includes 2 subjects who were withdrawn after week 3. FSIQ data not obtained in 1 placebo completer.
Abbreviations: SMD = Severe mood dysregulation; df = degrees of freedom; IQ = intelligence quotient; YMRS = Young Mania Rating Scale; CDRS = Children's Depression Rating Scale; PANSS-4 = Positive and Negative Symptom Scale for Schizophrenia factor 4; CGI-S = Clinical Global Impressions–Severity; CGAS = Children's Global Assessment Scale; DSM-IV-TR = Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision; MDD = major depressive disorder; ADHD = attention-deficity/hyperactivity disorder; ODD = oppositional defiant disorder; CD = conduct disorder; GAD = generalized anxiety disorder; N/A = not available; PTSD = posttraumatic stress disorder.
There were no significant differences in clinical ratings at study entry between those randomized to lithium and those randomized to placebo, including YMRS (Li 14.9 ± 4.0, Plac 14.3 ± 4.8, t = 0.28, df = 21, p = 0.78), CDRS (Li 29.9 ± 6.4, Plac 26.7 ± 5.0, t = 1.33, df = 22, p = 0.20), CGI-S (Li 4.8 ± 0.9, Plac 4.4 ± 0.7, t = 1.03, df = 19, p = 0.32), or CGAS (Li 43.3 ± 8.4, Plac 45.3 ± 3.2, t = −0.72, df = 22, p = 0.48). Last, there were no significant between group differences in current K-SADS psychiatric diagnoses, including no differences in rates of current MDD (Li 21%, Plac 18%, χ2 = 0.03, p = 0.84), current ADHD (Li 100%, Plac 82%, χ2 = 2.77, p = 0.10), current ODD (Li 86%, Plac 91%, χ2 = 0.16, p = 0.69), or current SAD (Li 14%, Plac 9%, χ2 = 0.16, p = 0.69).
Primary clinical outcome measures
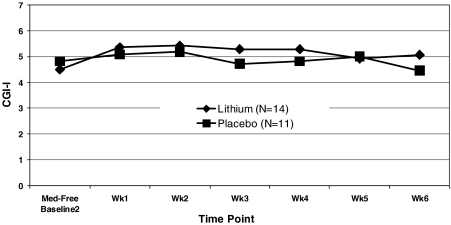
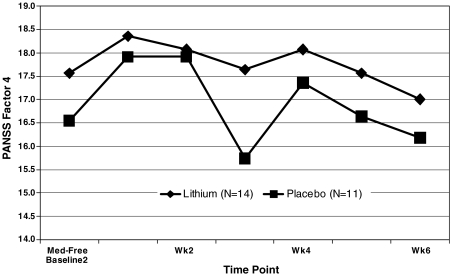
We did not find a significant between-group difference in our primary categorical clinical outcome measure, the CGI-I less than 4 (improved) by trial's end at week 6 (LogOR = 1.00, standard error [SE] = 1.23, χ2 = 0.66, p = 0.41, Cohen d effect size = 0.23] (Fig. 4). Further exploration showed that 3/14 lithium subjects, all of whom were completers, and only 1/11 placebo subjects achieved a CGI-I less than 4 at trial's end. We also did not find a significant between-group difference in our primary continuous outcome measures, the PANSS-4. Specifically, we did not find a significant effect of treatment group (F[1,25] = 0.88, p = 0.36), time [(F[6,87] = 1.96, p = 0.08), or group × time interaction (F[6,87] = 0.44, p = 0.85) (Cohen d effect size = 0.50).
FIG. 4.
Clinical Global Impressions–Improvement (CGI-I) weekly results in severe mood disregulation (SMD) youths randomized to lithium versus placebo.
FIG. 5.
Weekly Positive and Negative Symptom Scale for Schizophrenia-4 (PANSS-4) results in SMD youths randomized to lithium versus placebo. PANSS-4 = Sum of excitement (hyperactivity), hostility, uncooperativeness, and poor impulse control.
Secondary clinical outcome measures
Exploratory analyses of other continuous clinical outcome measures failed to reveal significant group or group × time effects. These include the: (1) YMRS: group (F[1,25] = 0.86, p = 0.36), time (F[6,90] = 0.70, p = 0.64), group × time interaction (F[6,90] = 1.60, p = 0.16; (2) CDRS: group (F[1,25] = 1.44, p = 0.24), time (F[6,85] = 0.41, p = 0.87), or group × time interaction (F[6,85] = 1.47, p = 0.19); (3) Conners 39-item hyperactivity subscale: group (F[1,25] = 0.33, p = 0.57], time (F[6,84] = 2.03, p = 0.07), or group × time interaction (F[6,84]= 0.93, p = 0.47); (4) Conners 39-item conduct subscale: group (F[1,25] = 0.43, p = 0.51), time (F[6,75] = 1.58, p = 0.16), group × time interaction (F[6,75] = 0.36, p = 0.90); or (5) number of OAS per week: group (F[1,25] = 1.67, p = 0.21), time (F[6,82] = 1.13, p = 0.35), or group × time interaction (F[6,82] = 0.70, p = 0.65).
Dosage and lithium levels
For those randomized to lithium, the mean serum lithium level by week 6 was 0.82 ± 0.28 mmol/L. The mean number of weeks with a therapeutic level (0.8–1.2 mmol/L) was 3.4 ± 1.7.
Adverse events
Two subjects randomized to lithium discontinued from the study prematurely, both after week 3, one due to clinical worsening and the other due to homesickness. No subject was withdrawn from study due to adverse physical effect in either the lithium or placebo group. Additionally, there was not a significant thyroid stimulating hormone (TSH) × time interaction comparing TSH at prerandomization (baseline 2) and after week 6 of the medication trial using a repeated measures ANOVA (baseline 2 mean TSH level: SMD-Li+ [n = 10] 2.01 ± 0.88, SMD-Plac [n = 7] 2.05 ± 0.87; med trial week 6 mean TSH level: SMD-Li + 4.11 ± 3.88, SMD-Plac 3.02 ± 1.51] main effect of TSH F[1,15] = 3.94, p = 0.07, TSH × group interaction F[1,15] = 0.54, p = 0.48).
MRS results
MRS data did not show a significant group × time interaction for any metabolite in any region of interest, with the exception of parieto-occipital GLX/Cr (Group: F[1,12]= 0.003, p = 0.96, ηp2 = 0.00; Group × time: F[1,12] = 8.31 p = 0.01, ηp2 = 0.41), which increased in the lithium group and decreased in the placebo group (Table 2).
Table 2.
MRS Results in Youth with Severe Mood Dysregulation (SMD) Pre- and Posttreatment with Lithium versus Placebo
| |
|
|
mI/Cr |
NAA/Cr |
GLX/Cr |
Cr |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | Group | Time | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n |
| Frontal | SMD-Li+ | Pre | 0.85 | 0.18 | 9 | 1.42 | 0.27 | 8 | 2.15 | 0.32 | 5 | 4.30 | 0.44 | 9 |
| Post | 1.01 | 0.20 | 9 | 1.39 | 0.29 | 8 | 2.56 | 1.19 | 5 | 4.17 | 1.27 | 9 | ||
| SMD-Plac | Pre | 1.02 | 0.31 | 8 | 1.43 | 0.23 | 8 | 2.41 | 0.86 | 6 | 4.28 | 0.81 | 10 | |
| Post | 0.98 | 0.24 | 8 | 1.38 | 0.15 | 8 | 2.21 | 0.26 | 6 | 3.84 | 1.04 | 10 | ||
| Time | F(1,15) = 0.74, p = 0.40, ηp2 = 0.05 | F(1,14) = 0.45, p = 0.45, ηp2 = 0.03 | F(1,9) = 0.12, p = 0.73, ηp2 = 0.01 | F(1,17) = 1.12 p = 0.30, ηp2 = 0.06 | ||||||||||
| Time × group | F(1,15) = 1.71 p = 0.21, ηp2 = 0.10 | F(1,17) = 0.02, p = 0.90, ηp2 = 0.00 | F(1,9) = 1.00, p = 0.34, ηp2 = 0.10 | F(1,17) = 0.35, p = 0.56, ηp2 = 0.02 | ||||||||||
| Temporal | SMD-Li+ | Pre | 1.03 | 0.12 | 8 | 1.23 | 0.21 | 8 | 2.44 | 0.38 | 6 | 4.31 | 0.73 | 8 |
| Post | 0.94 | 0.21 | 8 | 1.14 | 0.14 | 8 | 2.37 | 0.70 | 6 | 4.08 | 0.94 | 8 | ||
| SMD-Plac | Pre | 0.98 | 0.26 | 9 | 1.13 | 0.15 | 8 | 2.33 | 0.27 | 9 | 4.64 | 0.74 | 9 | |
| Post | 0.88 | 0.14 | 9 | 1.23 | 0.20 | 8 | 2.21 | 0.36 | 9 | 4.43 | 0.99 | 9 | ||
| Time | F(1,15) = 2.25, p = 0.15, ηp2 = 0.13 | F(1,14) = 0.003, p = 0.96, ηp2 = 0.00 | F(1,13) = 0.31, p = 0.59, ηp2 = 0.02 | F(1,15) = 0.72, p = 0.41, ηp2 = 0.05 | ||||||||||
| Time × group | F(1,15) = 0.00, p = 1.00, ηp2 = 0.00 | F(1,14) = 2.41, p = 0.14, ηp2 = 0.15 | F(1,13) = 0.02, p = 0.88, ηp2 = 0.00 | F(1,15) = 0.003, p = 0.96, ηp2 = 0.00 | ||||||||||
| Parietal | SMD-Li+ | Pre | 0.93 | 0.45 | 7 | 1.52 | 0.30 | 7 | 1.75 | 0.32 | 3 | 4.68 | 0.49 | 7 |
| Post | 0.94 | 0.24 | 7 | 1.51 | 0.21 | 7 | 2.23 | 1.01 | 3 | 4.43 | 0.97 | 7 | ||
| SMD-Plac | Pre | 0.95 | 0.32 | 9 | 1.59 | 0.23 | 9 | 2.49 | 1.01 | 7 | 4.35 | 0.80 | 9 | |
| Post | 0.87 | 0.19 | 9 | 1.44 | 0.21 | 9 | 2.23 | 1.01 | 3 | 4.63 | 0.68 | 9 | ||
| Time | F(1,14) = 0.11, p = 0.75, ηp2 = 0.01 | F(1,14) = 1.19, p = 0.30, ηp2 = 0.08 | F(1,8) = 0.01, p = 0.94, ηp2 = 0.00 | F(1,14) = 0.003, p = 0.96, ηp2 = 0.00 | ||||||||||
| Time × group | F(1,14) = 0.15, p = 0.70, ηp2 = 0.01 | F(1,14) = 0.78, p = 0.39, ηp2 = 0.05 | F(1,8) = 1.55, p = 0.25, ηp2 = 0.16 | F(1,14) = 0.97, p = 0.34, ηp2 = 0.07 | ||||||||||
| Parieto-occipito | SMD-Li+ | Pre | 0.85 | 0.13 | 7 | 1.34 | 0.19 | 7 | 2.19 | 0.56 | 6 | 5.25 | 0.49 | 7 |
| Post | 0.83 | 0.08 | 7 | 1.24 | 0.09 | 7 | 2.44 | 0.44 | 6 | 5.22 | 0.61 | 7 | ||
| SMD-Plac | Pre | 0.83 | 0.13 | 10 | 1.40 | 0.16 | 10 | 2.41 | 0.46 | 8 | 4.92 | 0.45 | 10 | |
| Post | 0.79 | 0.15 | 10 | 1.24 | 0.13 | 10 | 2.15 | 0.40 | 8 | 4.87 | 0.45 | 10 | ||
| Time | F(1,15) = 0.46, p = 0.51, ηp2 = 0.03 | F(1,15) = 0.77, p = 0.39, ηp2 = 0.05 | F(1,12) = 0.003, p = 0.96, ηp2 = 0.00 | F(1,15) = 0.07, p = 0.79, ηp2 = 0.01, F(1,15) = 0.003, p = 0.96, ηp2 = 0.00 |
||||||||||
| Time × group | F(1,15) = 0.04, p = 0.84, ηp2 = 0.00 | F(1,15) = 3.31, p = 0.09, ηp2 = 0.09 | F(1,12) = 8.31 p = 0.01, ηp2 = 0.41 | |||||||||||
All data meeting Cramer–Rao lower bounds of 20% or less were included for analysis.
Abbreviations: n (%) = Number and (percent) of total spectra acquired that were included in each analysis after meeting the above Cramer-Rao cutoff for data quality; ηp2 = partial eta squared; ROI = regions of interest; mI = myoinositol; Cr = creatine; NAA = N-acetyl aspartate; GLX = glutamate and glutamine; SD = standard deviation; Li = lithium; Plac = placebo.
Discussion
Our results suggest that lithium treatment is not associated with significant clinical or neurometabolic alterations in SMD youth suffering from severe, functionally impairing, nonepisodic irritability and ADHD symptoms. Although our study is strengthened by the double-blind, placebo-controlled design combined with MRS, important limitations include significantly lower percentage of female SMD subjects randomized to lithium versus placebo, small sample size, the number of enrolled subjects who were not randomized, and short duration of treatment. Nevertheless, ours is the first RCT in SMD youth, highlighting the critical need for treatment studies (both pharmacologic and psychotherapeutic) in this common syndrome (Brotman et al. 2006; Leibenluft et al. 2006).
An important and unexpected finding is that 45% of the SMD youth made significant clinical improvement between admission to our inpatient child psychiatric research unit (baseline 1) and the end of the single-blind placebo run in (baseline 2). These children were not randomized because their clinical status improved throughout the medication withdrawal and placebo run-in phases to the point where they no longer met criteria for SMD. Prior studies of youth with irritability and CD with a similar design—i.e., randomization of only those who continued to meet criteria after inpatient admission and single blind placebo-run in—had randomization rates of 62% of initially admitted subjects (Campbell et al. 1995; Cueva et al.1996; Malone et al. 2000). Thus, our study's finding that a significant number of irritable youth improve with inpatient hospitalization aligns with previous trials of similar design. Because, by definition, the SMD youths had a chronic course of functionally impairing irritability, it is possible that those SMD youths who exhibited significant symptom improvement after admission were experiencing a protracted “honeymoon” phase that would have not persisted with time. However, our data may also demonstrate the powerful therapeutic impact of admission to a psychiatric research unit with highly skilled staff and teachers, working as a team to provide round-the-clock care and a structured milieu. Perhaps another reason for the SMD improvement following admission is a child's removal from environmental triggers, including parents or family members, teachers, or peers. Obviously, it would be important to identify the therapeutic factors that resulted in such clinical improvement prerandomization to guide future treatment of SMD symptoms, including irritability, in children and adolescents.
Importantly, specific psychotherapeutic interventions were not employed in our current study. Among the interventions potentially worth studying in SMD is creative problem solving (CPS), which has shown benefit in treating ODD, including significantly reduced numbers of restraints, seclusions, and patient/staff injuries on an inpatient unit after CPS was implemented versus before (Greene et al. 2003; Greene et al. 2004). Further study is also warranted to determine the impact of standardized daily routines and family therapy in treating the functionally disabling irritability of SMD youth, given prior studies showing such interventions benefit children and adults with mood disorders (Keitner et al. 1995; Frank et al. 1997; Miklowitz et al. 2000; Fristad et al. 2003; Pavuluri et al. 2004; Miklowitz et al. 2003; Rea et al. 2003).
The MRS data did not indicate significant differences between SMD youth treated with lithium and those treated with placebo. In BD adults, lithium treatment results in significantly decreased frontal mI and increased NAA (Moore et al.1999; Moore et al. 2000a; Silverstone et al. 2002; Silverstone et al. 2003). However, data in pediatric BD samples treated with lithium have been mixed. Davanzo et al. found that lithium treatment in BD youth was associated with decreased mI/Cr, but did not impact significantly on NAA/Cr or GLX/Cr in the anterior cingulate cortex (ACC) (Davanzo et al. 2001). Patel et al. (2006) failed to show differences in mI, GLX, or Cr in the prefrontal cortex after lithium treatment (Patel et al. 2006), whereas Patel et al. (2008) found that lithium treatment in depressed BD adolescents was associated with decreased medial prefrontal cortex, but not left or right lateral prefrontal cortex, NAA (Patel et al. 2008). One interpretation of our negative MRS findings is that the neurochemistry of SMD differs from that of BD. Indeed, whereas data indicate that BD youths have increased ACC mI/Cr and mI over those with intermittent explosive disorder or healthy controls (Davanzo et al. 2003), we recently found that, compared to healthy controls (n = 43), medication-free SMD youth (n = 36) had decreased, rather than increased, temporal mI/Cr (Dickstein et al. 2008). Moreover, while four of five studies of pediatric BD subjects, both medicated and medication-free, have demonstrated decreased frontal cortex NAA versus controls (Castillo et al. 2000; Cecil et al. 2003; Chang et al. 2003; Sassi et al. 2005; Olvera et al. 2007), our study of medication-free SMD youth showed no significant differences versus controls in frontal NAA/Cr, mI/Cr, Glx/Cr, or Cr.
Because of the small sample size, these data should be considered preliminary. Nonetheless, they do not support the use of lithium in SMD youth. Not only did the current study fail to detect lithium-placebo treatment differences, but the overall rate of improvement in the lithium group was relatively small, given prior findings on pharmacological responses in a range of pediatric mental syndromes. Thus, unfortunately, when considering other agents besides lithium, the prior literature that would guide treatment in these patients is very limited. Clinical trials to address the irritability and hyperarousal captured by the SMD criteria are hampered by the fact that the syndrome does not map well onto any DSM diagnosis.
Atypical antipsychotic medications may merit future study in SMD youth. In particular, risperidone is the first medication to receive a Food and Drug Administration (FDA) indication for the treatment of the core symptom of SMD, namely irritability. Specifically, risperidone now has a FDA indication for the treatment of irritability in PDD-spectrum illness based on two recent RCTs (McCracken et al. 2002; Shea et al. 2004), as well as an FDA indication for the treatment of pediatric BD. Other atypical antipsychotic medications have also shown promise in pediatric BD. Indeed, in a study by DelBello et al. demonstrating that BD adolescents whose acute mania remitted with olanzapine, this treatment was associated with significantly increased ventral prefrontal cortex NAA (Barzman et al. 2006; DelBello et al. 2002; DelBello et al. 2006). However, consideration of atypical antipsychotics for clinical or research purposes will need to incorporate measures of known side effects, such as metabolic syndrome (Correll et al. 2006).
Other antimanic agents, including antiepileptic drugs (AEDs), may also merit study in SMD youth. Divalproex has been shown to result in clinical improvement in youth with explosive temper and ODD or conduct disorder (Donovan et al. 1997; Donovan et al. 2000) as well as to reduce aggression in youth at high risk for BD (Saxena et al. 2006). Thus, divalproex may warrant further study in SMD youth. Carbamazepine is another AED that has been studied in treating irritability in children, adolescents, and adults (Mattes et al. 1984; Foster et al. 1989; Kowatch et al. 2000). However, in studies of children and adolescents, carbamazepine has not shown significant superiority to placebo in treating aggressive youth (Cueva et al. 1996), and a recent, large double-blind randomized placebo controlled trial of BD youth failed to demonstrate significant benefit from oxcarbazepine (Wagner et al. 2006).
Beyond antimanic agents, selective serotonin reuptake inhibitors (SSRIs) merit further study in SMD youths, given their role in treating disorders characterized by irritability, including MDD, anxiety disorders, premenstrual dysphoric disorder, and PDD (Fatemi et al. 1998; Birmaher et al. 2003; March et al. 2004; Owley et al. 2005; Ryan 2005). While concern regarding SSRI-induced increases in suicidal thinking precipitated the introduction of an FDA black-box warning, a recent meta-analysis of all pediatric trials of fluoxetine, sponsored by its manufacturer, showed no differences in aggression or hostility between those treated with drug versus placebo (Gibbons et al. 2006; Tauscher-Wisniewski et al. 2007).
Psychostimulants are a third important medication category that bear consideration in the treatment of SMD youth. Given that the “hyperarousal” symptoms of SMD consist of those symptoms common to ADHD and the “B” criteria of mania, there is necessarily a high rate of ADHD in youth with SMD. In addition, several studies suggest that stimulants may reduce aggression and hostility in patients with ADHD (Carlson et al. 2000; Connor et al. 2002; Sinzig et al. 2007). In sum, while a number of current medications may result in clinical improvement in children and adolescents suffering from psychiatric disorders involving irritability, our study of lithium is the first to study medication use specifically in SMD youths, and no significant difference in clinical outcome was noted.
As noted above, our study has several important limitations. Although it is possible that our sample size may contribute to a type II error, the very small effect size suggests that a very large study would be needed to detect a medication effect. Moreover, the overall low response rate to lithium suggests that this is not a promising treatment approach. While significantly fewer SMD girls than boys were randomized to lithium versus to placebo, few gender differences in lithium response have been identified in BD adults (Viguera et al. 2000; Viguera et al. 2001). It is also possible that SMD youth might have needed higher doses of lithium, longer duration of exposure, or both. Studies indicate that children have shorter elimination half-lives and greater lithium clearance than adults (Vitiello et al. 1988). Moreover, one study using lithium-7 MRS (rather than 1H-proton MRS) demonstrated that children (n = 9) had lower brain-to-serum lithium ratios than adults (n = 18) (Moore et al. 2002), suggesting that children may require higher serum levels of lithium to achieve the same brain concentrations. With respect to limitations of our MRS methodology, our study was modeled on prior work in BD adults, and thus evaluated neurometabolites in four ROIs, referenced to Cr to control for partial volume effects (Moore et al. 1999; Moore et al. 2000c). However, different MRS methodologies might yield different results; such methodologies might include using different ROI locations (e.g., anterior cingulate cortex), a whole-brain approach, or evaluating absolute concentrations with a higher strength magnetic field (Moore et al. 2000x; Davanzo et al. 2001). Resolution of these possibilities will require additional studies of lithium in SMD youth, maximizing their safe exposure to lithium while collecting clinical and neurometabolic data.
Conclusions
Our data suggest that lithium may not result in significant clinical or neurometabolite alterations in youths with chronic irritability and hyperarousal. However, further study is warranted in larger samples to fully assess brain and behavioral alterations associated with lithium treatment in these SMD youths.
Disclosures
Industry funding was not provided for any aspect of this study or for the authors. Additionally, Dr. Dickstein's extramural position at E.P. Bradley Hospital does not have any industry funding. Also, as acknowledged/cited in the Methods and Discussion section, we have recently published MRS data from SMD subjects (regardless of randomized or not) while medication free versus typically developing controls. Drs. Dickstein, Towbin, Van Der Veen, Rich, Pine, and Leibenluft, and Ms. Brotman, Ms. Knopf, and Ms. Onelio have no other conflicts of interest or financial ties to report.
Footnotes
This work was conducted at, approved by, and supported entirely by the National Institute of Mental Health Division of Intramural Research Programs (NIMH DIRP) Bethesda Maryland.
Dr. Dickstein is currently funded by NIMH K22 MH074249, a Narsad Young Investigator Award, and E.P. Bradley Hospital.
Acknowledgments
We gratefully acknowledge the energy and dedication of the NIMH DIRP Section on Bipolar Spectrum Disorders, Inpatient Child Psychiatric Unit, and MRI facility. We also thank the children and families who participated and made this research possible.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington (DC): American Psychiatric Association; 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- Barzman DH. DelBello MP. Adler CM. Stanford KE. Strakowski SM. The efficacy and tolerability of quetiapine versus divalproex for the treatment of impulsivity and reactive aggression in adolescents with co-occurring bipolar disorder and disruptive behavior disorder(s) J Child Adolesc Psychopharmacol. 2006;16:665–670. doi: 10.1089/cap.2006.16.665. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Birmaher B. Axelson DA. Monk K. Kalas C. Clark DB. Ehmann M. Bridge J. Heo J. Brent DA. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42:415–423. doi: 10.1097/01.CHI.0000037049.04952.9F. [DOI] [PubMed] [Google Scholar]
- Brotman MA. Schmajuk M. Rich BA. Dickstein DP. Guyer AE. Costello EJ. Egger HL. Angold A. Pine DS. Leibenluft E. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006;60:991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Campbell M. Small AM. Green WH. Jennings SJ. Perry R. Bennett WG. Anderson L. Behavioral efficacy of haloperidol and lithium carbonate. A comparison in hospitalized aggressive children with conduct disorder. Arch Gen Psychiatry. 1984;41:650–656. doi: 10.1001/archpsyc.1984.01790180020002. [DOI] [PubMed] [Google Scholar]
- Campbell M. Adams PB. Small AM. Kafantaris V. Silva RR. Shell J. Perry R. Overall JE. Lithium in hospitalized aggressive children with conduct disorder: A double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1995;34:445–453. [PubMed] [Google Scholar]
- Carlson GA. Loney J. Salisbury H. Kramer JR. Arthur C. Stimulant treatment in young boys with symptoms suggesting childhood mania: A report from a longitudinal study. J Child Adolesc Psychopharmacol. 2000;10:175–184. doi: 10.1089/10445460050167287. [DOI] [PubMed] [Google Scholar]
- Castillo M. Kwock L. Courvoisie H. Hooper S. Proton MR spectroscopy in children with bipolar affective disorder: preliminary observations. AJNR Am J Neuroradiol. 2000;21:832–838. [PMC free article] [PubMed] [Google Scholar]
- Cecil KM. DelBello MP. Sellars MC. Strakowski SM. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J Child Adolesc Psychopharmacol. 2003;13:545–555. doi: 10.1089/104454603322724931. [DOI] [PubMed] [Google Scholar]
- Chang K. Adleman N. Dienes K. Barnea-Goraly N. Reiss A. Ketter T. Decreased N-Acetylaspartate in children with familial bipolar disorder. Biol Psychiatry. 2003;53:1059–1065. doi: 10.1016/s0006-3223(02)01744-4. [DOI] [PubMed] [Google Scholar]
- Connor DF. Glatt SJ. Lopez ID. Jackson D. Melloni RH. Psychopharmacology and aggression. I: A meta-analysis of stimulant effects on overt/covert aggression-related behaviors in ADHD. J Am Acad Child Adolesc Psychiatry. 2002;41:253–261. doi: 10.1097/00004583-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Correll CU. Penzner JB. Parikh UH. Mughal T. Javed T. Carbon M. Malhotra AK. Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2006;15:177–206. doi: 10.1016/j.chc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Courvoisie H. Hooper SR. Fine C. Kwock L. Castillo M. Neurometabolic functioning and neuropsychological correlates in children with ADHD-H: Preliminary findings. J Neuropsychiatry Clin Neurosci. 2004;16:63–69. doi: 10.1176/jnp.16.1.63. [DOI] [PubMed] [Google Scholar]
- Cueva JE. Overall JE. Small AM. Armenteros JL. Perry R. Campbell M. Carbamazepine in aggressive children with conduct disorder: A double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 1996;35:480–490. doi: 10.1097/00004583-199604000-00014. [DOI] [PubMed] [Google Scholar]
- Davanzo P. Thomas MA. Yue K. Oshiro T. Belin T. Strober M. McCracken J. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology. 2001;24:359–369. doi: 10.1016/S0893-133X(00)00207-4. [DOI] [PubMed] [Google Scholar]
- Davanzo P. Yue K. Thomas MA. Belin T. Mintz J. Venkatraman TN. Santoro E. Barnett S. McCracken J. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am J Psychiatry. 2003;160:1442–1452. doi: 10.1176/appi.ajp.160.8.1442. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Schwiers ML. Rosenberg HL. Strakowski SM. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad.Child Adolesc Psychiatry. 2002;41:1216–1223. doi: 10.1097/00004583-200210000-00011. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Kowatch RA. Adler CM. Stanford KE. Welge JA. Barzman DH. Nelson E. Strakowski SM. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry. 2006;45:305–313. doi: 10.1097/01.chi.0000194567.63289.97. [DOI] [PubMed] [Google Scholar]
- Dickstein DP. van der Veen JW. Knopf L. Towbin KE. Pine DS. Leibenluft E. Proton magnetic resonance spectroscopy in youth with severe mood dysregulation. Psychiatry Res. 2008;163:30–39. doi: 10.1016/j.pscychresns.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Donovan SJ. Susser ES. Nunes EV. Stewart JW. Quitkin FM. Klein DF. Divalproex treatment of disruptive adolescents: A report of 10 cases. J Clin Psychiatry. 1997;58:12–15. doi: 10.4088/jcp.v58n0102. [DOI] [PubMed] [Google Scholar]
- Donovan SJ. Stewart JW. Nunes EV. Quitkin FM. Parides M. Daniel W. Susser E. Klein DF. Divalproex treatment for youth with explosive temper and mood lability: A double-blind, placebo-controlled crossover design. Am J Psychiatry. 2000;157:818–820. doi: 10.1176/appi.ajp.157.5.818. [DOI] [PubMed] [Google Scholar]
- Emslie GJ. Weinberg WA. Rush AJ. Adams RM. Rintelmann JW. Depressive symptoms by self-report in adolescence: Phase I of the development of a questionnaire for depression by self-report. J Child Neurol. 1990;5:114–121. doi: 10.1177/088307389000500208. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. Realmuto GM. Khan L. Thuras P. Fluoxetine in treatment of adolescent patients with autism: A longitudinal open trial. J Autism Dev Disord. 1998;28:303–307. doi: 10.1023/a:1026008602540. [DOI] [PubMed] [Google Scholar]
- Foster HG. Hillbrand M. Chi CC. Efficacy of carbamazepine in assaultive patients with frontal lobe dysfunction. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:865–874. doi: 10.1016/0278-5846(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Frank E. Hlastala S. Ritenour A. Houck P. Tu XM. Monk TH. Mallinger AG. Kupfer DJ. Inducing lifestyle regularity in recovering bipolar disorder patients: Results from the maintenance therapies in bipolar disorder protocol. Biol Psychiatry. 1997;41:1165–1173. doi: 10.1016/s0006-3223(96)00241-7. [DOI] [PubMed] [Google Scholar]
- Fristad MA. Gavazzi SM. Mackinaw-Koons B. Family psychoeducation. An adjunctive intervention for children with bipolar disorder. Biol Psychiatry. 2003;53:1000–1008. doi: 10.1016/s0006-3223(03)00186-0. [DOI] [PubMed] [Google Scholar]
- Geller B. Cooper TB. Sun K. Zimerman B. Frazier J. Williams M. Heath J. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998a;37:171–178. doi: 10.1097/00004583-199802000-00009. [DOI] [PubMed] [Google Scholar]
- Geller B. Cooper TB. Zimerman B. Frazier J. Williams M. Heath J. Warner K. Lithium for prepubertal depressed children with family history predictors of future bipolarity: A double-blind, placebo-controlled study. J Affect Disord. 1998b;51:165–175. doi: 10.1016/s0165-0327(98)00178-5. [DOI] [PubMed] [Google Scholar]
- Geller B. Williams M. Zimerman B. Frazier J. Beringer L. Warner KL. Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultra-rapid or ultradian cycling. J Affect Disord. 1998c;51:81–91. doi: 10.1016/s0165-0327(98)00175-x. [DOI] [PubMed] [Google Scholar]
- Gibbons RD. Hur K. Bhaumik DK. Mann JJ. The relationship between antidepressant prescription rates and rate of early adolescent suicide. Am J Psychiatry. 2006;163:1898–1904. doi: 10.1176/ajp.2006.163.11.1898. [DOI] [PubMed] [Google Scholar]
- Gould TD. Quiroz JA. Singh J. Zarate CA. Manji HK. Emerging experimental therapeutics for bipolar disorder: Insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry. 2004;9:734–755. doi: 10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- Greene RW. Ablon JS. Goring JC. A transactional model of oppositional behavior: Underpinnings of the Collaborative Problem Solving approach. J Psychosom Res. 2003;55:67–75. doi: 10.1016/s0022-3999(02)00585-8. [DOI] [PubMed] [Google Scholar]
- Greene RW. Ablon JS. Goring JC. Raezer-Blakely L. Markey J. Monuteaux MC. Henin A. Edwards G. Rabbitt S. Effectiveness of collaborative problem solving in affectively dysregulated children with oppositional-defiant disorder: Initial findings. J Consult Clin Psychol. 2004;72:1157–1164. doi: 10.1037/0022-006X.72.6.1157. [DOI] [PubMed] [Google Scholar]
- Hancu I. Blezek DJ. Dumoulin MC. Automatic repositioning of single voxels in longitudinal 1H MRS studies. NMR Biomed. 2005;18:352–361. doi: 10.1002/nbm.965. [DOI] [PubMed] [Google Scholar]
- Kafantaris V. Coletti DJ. Dicker R. Padula G. Pleak RR. Alvir JM. Lithium treatment of acute mania in adolescents: A placebo-controlled discontinuation study. J Am Acad Child Adolesc Psychiatry. 2004;43:984–993. doi: 10.1097/01.chi.0000129223.89433.74. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kay SR. Opler LA. Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): Rationale, standardisation. Br J Psychiatry Suppl. 1989:59–67. , [PubMed] [Google Scholar]
- Keitner GI. Ryan CE. Miller IW. Kohn R. Bishop DS. Epstein NB. Role of the family in recovery and major depression. Am J Psychiatry. 1995;152:1002–1008. doi: 10.1176/ajp.152.7.1002. [DOI] [PubMed] [Google Scholar]
- Komoroski RA. Kotrla KJ. Lemen L. Lindquist D. Diaz P. Foundas A. Brain metabolite concentration ratios in vivo: Multisite reproducibility by single-voxel 1H MR spectroscopy. Magn Reson Imaging. 2004;22:721–725. doi: 10.1016/j.mri.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kowatch RA. Suppes T. Carmody TJ. Bucci JP. Hume JH. Kromelis M. Emslie GJ. Weinberg WA. Rush AJ. Effect size of lithium, divalproex sodium, and carbamazepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:713–720. doi: 10.1097/00004583-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Leckman JF. Sholomskas D. Thompson WD. Belanger A. Weissman MM. Best estimate of lifetime psychiatric diagnosis: A methodological study. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Leibenluft E. Charney DS. Towbin KE. Bhangoo RK. Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- Leibenluft E. Cohen P. Gorrindo T. Brook JS. Pine DS. Chronic versus episodic irritability in youth: A community-based, longitudinal study of clinical and diagnostic associations. J Child Adolesc Psychopharmacol. 2006;16:456–466. doi: 10.1089/cap.2006.16.456. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP. Brown E. Baker RW. Schuh LM. Shao L. Tohen M. Ahmed S. Stauffer VL. An excitement subscale of the Positive and Negative Syndrome Scale. Schizophr Res. 2004;68:331–337. doi: 10.1016/S0920-9964(03)00087-2. [DOI] [PubMed] [Google Scholar]
- Malone RP. Delaney MA. Luebbert JF. Cater J. Campbell M. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry. 2000;57:649–654. doi: 10.1001/archpsyc.57.7.649. [DOI] [PubMed] [Google Scholar]
- March J. Silva S. Petrycki S. Curry J. Wells K. Fairbank J. Burns B. Domino M. McNulty S. Vitiello B. Severe J. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- Mattes JA. Rosenberg J. Mayes D. Carbamazepine vs propranolol in patients with uncontrolled rage outbursts: A random assignment study. Psychopharmacol Bull. 1984;20:98–100. [PubMed] [Google Scholar]
- McCracken JT. McGough J. Shah B. Cronin P. Hong D. Aman MG. Arnold LE. Lindsay R. Nash P. Hollway J. McDougle CJ. Posey D. Swiezy N. Kohn A. Scahill L. Martin A. Koenig K. Volkmar F. Carroll D. Lancor A. Tierney E. Ghuman J. Gonzalez NM. Grados M. Vitiello B. Ritz L. Davies M. Robinson J. McMahon D. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ. Simoneau TL. George EL. Richards JA. Kalbag A. Sachs-Ericsson N. Suddath R. Family-focused treatment of bipolar disorder: 1-year effects of a psychoeducational program in conjunction with pharmacotherapy. Biol Psychiatry. 2000;48:582–592. doi: 10.1016/s0006-3223(00)00931-8. 4. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ. George EL. Richards JA. Simoneau TL. Suddath RL. A randomized study of family-focused psychoeducation and pharmacotherapy in the outpatient management of bipolar disorder. Arch Gen Psychiatry. 2003;60:904–912. doi: 10.1001/archpsyc.60.9.904. [DOI] [PubMed] [Google Scholar]
- Moore GJ. Bebchuk JM. Parrish JK. Faulk MW. Arfken CL. Strahl-Bevacqua J. Manji HK. Temporal dissociation between lithium-induced changes in frontal lobe myo-inositol and clinical response in manic-depressive illness. Am J Psychiatry. 1999;156:1902–1908. doi: 10.1176/ajp.156.12.1902. [DOI] [PubMed] [Google Scholar]
- Moore GJ. Bebchuk JM. Hasanat K. Chen G. Seraji-Bozorgzad N. Wilds IB. Faulk MW. Koch S. Glitz DA. Jolkovsky L. Manji HK. Lithium increases N-acetyl-aspartate in the human brain: In vivo evidence in support of bcl-2′s neurotrophic effects? Biol Psychiatry. 2000a;48:1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- Moore GJ. Bebchuk JM. Wilds IB. Chen G. Manji HK. Menji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000b;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Moore CM. Breeze JL. Gruber SA. Babb SM. Frederick BB. Villafuerte RA. Stoll AL. Hennen J. Yurgelun-Todd DA. Cohen BM. Renshaw PF. Choline, myo-inositol and mood in bipolar disorder: A proton magnetic resonance spectroscopic imaging study of the anterior cingulate cortex. Bipolar Disord. 2000c;2:207–216. doi: 10.1034/j.1399-5618.2000.20302.x. [DOI] [PubMed] [Google Scholar]
- Moore CM. Demopulos CM. Henry ME. Steingard RJ. Zamvil L. Katic A. Breeze JL. Moore JC. Cohen BM. Renshaw PF. Brain-to-serum lithium ratio and age: An in vivo magnetic resonance spectroscopy study. Am J Psychiatry. 2002;159:1240–1242. doi: 10.1176/appi.ajp.159.7.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CM. Biederman J. Wozniak J. Mick E. Aleardi M. Wardrop M. Dougherty M. Harpold T. Hammerness P. Randall E. Renshaw PF. Differences in brain chemistry in children and adolescents with attention deficit hyperactivity disorder with and without comorbid bipolar disorder: A proton magnetic resonance spectroscopy study. Am J Psychiatry. 2006;163:316–318. doi: 10.1176/appi.ajp.163.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera RL. Caetano SC. Fonseca M. Nicoletti M. Stanley JA. Chen HH. Hatch JP. Hunter K. Pliszka SR. Soares JC. Low levels of N-acetyl aspartate in the left dorsolateral prefrontal cortex of pediatric bipolar patients. J Child Adolesc Psychopharmacol. 2007;17:461–473. doi: 10.1089/cap.2007.0102. [DOI] [PubMed] [Google Scholar]
- Owley T. Walton L. Salt J. Guter SJ., Jr Winnega M. Leventhal BL. Cook EH., Jr An open-label trial of escitalopram in pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry. 2005;44:343–348. doi: 10.1097/01.chi.0000153229.80215.a0. [DOI] [PubMed] [Google Scholar]
- Patel NC. DelBello MP. Cecil KM. Adler CM. Bryan HS. Stanford KE. Strakowski SM. Lithium treatment effects on myo-inositol in adolescents with bipolar depression. Biol Psychiatry. 2006;60:998–1004. doi: 10.1016/j.biopsych.2006.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NC. DelBello MP. Cecil KM. Stanford KE. Adler CM. Strakowski SM. Temporal change in N-acetyl-aspartate concentrations in adolescents with bipolar depression treated with lithium. J Child Adolesc Psychopharmacol. 2008;18:132–139. doi: 10.1089/cap.2007.0088. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. Graczyk PA. Henry DB. Carbray JA. Heidenreich J. Miklowitz DJ. Child- and family-focused cognitive-behavioral therapy for pediatric bipolar disorder: development and preliminary results. J Am Acad Child Adolesc Psychiatry. 2004;43:528–537. doi: 10.1097/00004583-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Pogge DL. Wayland-Smith D. Zaccario M. Borgaro S. Stokes J. Harvey PD. Diagnosis of manic episodes in adolescent inpatients: Structured diagnostic procedures compared to clinical chart diagnoses. Psychiatry Res. 2001;101:47–54. doi: 10.1016/s0165-1781(00)00248-1. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rea MM. Tompson MC. Miklowitz DJ. Goldstein MJ. Hwang S. Mintz J. Family-focused treatment versus individual treatment for bipolar disorder: Results of a randomized clinical trial. J Consult Clin Psychol. 2003;71:482–492. doi: 10.1037/0022-006x.71.3.482. [DOI] [PubMed] [Google Scholar]
- Reich W. Neuman RJ. Volk HE. Joyner CA. Todd RD. Comorbidity between ADHD and symptoms of bipolar disorder in a community sample of children and adolescents. Twin Res Hum Genet. 2005;8:459–466. doi: 10.1375/183242705774310105. [DOI] [PubMed] [Google Scholar]
- Rifkin A. Karajgi B. Dicker R. Perl E. Boppana V. Hasan N. Pollack S. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry. 1997;154:554–555. doi: 10.1176/ajp.154.4.554. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR. MacMaster FP. Mirza Y. Smith JM. Easter PC. Banerjee SP. Bhandari R. Boyd C. Lynch M. Rose M. Ivey J. Villafuerte RA. Moore GJ. Renshaw P. Reduced anterior cingulate glutamate in pediatric major depression: A magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:700–704. doi: 10.1016/j.biopsych.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Ryan ND. Treatment of depression in children and adolescents. Lancet. 2005;366:933–940. doi: 10.1016/S0140-6736(05)67321-7. [DOI] [PubMed] [Google Scholar]
- Sassi RB. Stanley JA. Axelson D. Brambilla P. Nicoletti MA. Keshavan MS. Ramos RT. Ryan N. Birmaher B. Soares JC. Reduced NAA levels in the dorsolateral prefrontal cortex of young bipolar patients. Am J Psychiatry. 2005;162:2109–2115. doi: 10.1176/appi.ajp.162.11.2109. [DOI] [PubMed] [Google Scholar]
- Saxena K. Howe M. Simeonova DI. Steiner H. Chang KD. Divalproex sodium reduces overall aggression in youth at high risk for bipolar disorder. J Child Adolesc Psychopharmacol. 2006;16:252–259. doi: 10.1089/cap.2006.16.252. [DOI] [PubMed] [Google Scholar]
- Shaffer D. Gould MS. Brasic J. Ambrosini P. Fisher P. Bird H. Aluwahlia S. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Shaltiel G. Maeng S. Malkesman O. Pearson B. Schloesser RJ. Tragon T. Rogawski M. Gasior M. Luckenbaugh D. Chen G. Manji HK. Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry. 2008;13:858–872. doi: 10.1038/mp.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea S. Turgay A. Carroll A. Schulz M. Orlik H. Smith I. Dunbar F. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114:e634–e641. doi: 10.1542/peds.2003-0264-F. [DOI] [PubMed] [Google Scholar]
- Shibuya-Tayoshi S. Tayoshi S. Sumitani S. Ueno S. Harada M. Ohmori T. Lithium effects on brain glutamatergic and GABAergic systems of healthy volunteers as measured by proton magnetic resonance spectroscopy. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:249–256. doi: 10.1016/j.pnpbp.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Silver JM. Yudofsky SC. The Overt Aggression Scale: Overview and guiding principles. J Neuropsychiatry Clin Neurosci. 1991;3:S22–S29. [PubMed] [Google Scholar]
- Silverstone PH. Wu RH. O'Donnell T. Ulrich M. Asghar SJ. Hanstock CC. Chronic treatment with both lithium and sodium valproate may normalize phosphoinositol cycle activity in bipolar patients. Hum Psychopharmacol. 2002;17:321–327. doi: 10.1002/hup.420. [DOI] [PubMed] [Google Scholar]
- Silverstone PH. Wu RH. O'Donnell T. Ulrich M. Asghar SJ. Hanstock CC. Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patients. Int Clin Psychopharmacol. 2003;18:73–79. doi: 10.1097/00004850-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Silverstone PH. McGrath BM. Kim H. Bipolar disorder and myo-inositol: A review of the magnetic resonance spectroscopy findings. Bipolar Disord. 2005;7:1–10. doi: 10.1111/j.1399-5618.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Sinzig J. Dopfner M. Lehmkuhl G. Uebel H. Schmeck K. Poustka F. Gerber WD. Günter M. Knölker U. Gehrke M. Hässler F. Resch F. Brünger M. Ose C. Fischer R. Long-acting methylphenidate has an effect on aggressive behavior in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17:421–432. doi: 10.1089/cap.2007.0011. [DOI] [PubMed] [Google Scholar]
- Spearing MK. Post RM. Leverich GS. Brandt D. Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): The CGI-BP. Psychiatry Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Stork C. Renshaw PF. Mitochondrial dysfunction in bipolar disorder: Evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Tauscher-Wisniewski S. Nilsson M. Caldwell C. Plewes J. Allen AJ. Meta-analysis of aggression and/or hostility-related events in children and adolescents treated with fluoxetine compared with placebo. J Child Adolesc Psychopharmacol. 2007;17:713–718. doi: 10.1089/cap.2006.0138. [DOI] [PubMed] [Google Scholar]
- Tsai G. Coyle JT. N-acetylaspartate in neuropsychiatric disorders. Prog Neurobiol. 1995;46:531–540. doi: 10.1016/0301-0082(95)00014-m. [DOI] [PubMed] [Google Scholar]
- Viguera AC. Tondo L. Baldessarini RJ. Sex differences in response to lithium treatment. Am J Psychiatry. 2000;157:1509–1511. doi: 10.1176/appi.ajp.157.9.1509. [DOI] [PubMed] [Google Scholar]
- Viguera AC. Baldessarini RJ. Tondo L. Response to lithium maintenance treatment in bipolar disorders: Comparison of women and men. Bipolar Disord. 2001;3:245–252. doi: 10.1034/j.1399-5618.2001.30503.x. [DOI] [PubMed] [Google Scholar]
- Vitiello B. Behar D. Malone R. Delaney MA. Ryan PJ. Simpson GM. Pharmacokinetics of lithium carbonate in children. J Clin Psychopharmacol. 1988;8:355–359. [PubMed] [Google Scholar]
- Wagner KD. Kowatch RA. Emslie GJ. Findling RL. Wilens TE. McCague K. D'Souza J. Wamil A. Lehman RB. Berv D. Linden D. A double-blind, randomized, placebo-controlled trial of oxcarbazepine in the treatment of bipolar disorder in children and adolescents. Am J Psychiatry. 2006;163:1179–1186. doi: 10.1176/ajp.2006.163.7.1179. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio (Texas): The Psychological Corporation; 2005. [Google Scholar]
- Werry JS. Sprague RL. Cohen MN. Conners' Teacher Rating Scale for use in drug studies with children—an empirical study. J Abnorm Child Psychol. 1975;3:217–229. doi: 10.1007/BF00916752. [DOI] [PubMed] [Google Scholar]
- Young RC. Biggs JT. Ziegler VE. Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yudofsky SC. Kopecky HJ. Kunik M. Silver JM. Endicott J. The Overt Agitation Severity Scale for the objective rating of agitation. J Neuropsychiatry Clin Neurosci. 1997;9:541–548. doi: 10.1176/jnp.9.4.541. [DOI] [PubMed] [Google Scholar]
- Zarate CA. Quiroz J. Payne J. Manji HK. Modulators of the glutamatergic system: Implications for the development of improved therapeutics in mood disorders. Psychopharmacol Bull. 2002;36:35–83. [PubMed] [Google Scholar]