We report the synthesis of a water-soluble, dextran-conjugated fluorescent K+ sensor, TAC-Limedex, whose green fluorescence strongly increases with [K+], and demonstrate its utility for assay of cellular K+ transport. K+ channels and K+-coupled ion transporters represent an important group of targets for drug discovery.1-3 K+ channels are involved in cardiac and neuronal excitability, epithelial fluid transport, extracellular and intracellular ionic homeostasis, and cell proliferation.2 K+-coupled ion transporters are involved in transepithelial fluid secretion and absorption, and in cell volume regulation and ionic homeostasis. Patch-clamp is the gold standard for assay of K+ channel function, though technically tedious for high-throughput measurements. Radioactive Rb+ uptake is generally used to assay electrically silent K+-coupled transporters such as the K+/Cl− symporter. Membrane voltage-sensing probes have also been used to assess K+ channels. There is need for a robust fluorescence assay of K+ transport for screening applications as an alternative to patch-clamp and radioactive Rb+.
We previously introduced the long-wavelength, K+-sensitive fluorescent indicator, TAC-Red, consisting of a K+-binding triazacryptand ionophore (TAC) coupled to a red fluorescing xanthylium chromophore.4 The K+ sensing mechanism of TAC-Red, and that of a newer K+ indicator TAC-Crimson,5 involves charge-transfer quenching in which K+-triazacryptand binding prevents electron-transfer-type chromophore quenching. These dyes have bright fluorescence, excellent K+-selectivity, and millisecond response kinetics to changes in [K+].4,5 However, they partition significantly into many cell types, limiting their utility as an extracellular K+ sensor.
After testing many chromophores and conjugation strategies, we devised a synthetic route to generate the K+ sensor, TAC-Limedex. TAC-Limedex consists of a triazacryptand K+ ionophore in direct conjugation with a green fluorescent chromophore, connected through an amide linkage to amino dextran via succinimidyl ester chemistry (Figure 1a). The synthesis involved conversion of TACCHO 1 to the TAC-Lime (Bodipy dye) methyl ester 2 by reaction of aldehyde 1 with methyl 3-(2,4-dimethyl-1H-pyrrol-3-yl) propanoate. Oxidation with 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) followed by treatment with boron trifluoride (BF3) yielded the triazacryptand bodipy dye, TAC-Lime ester 2. Hydrolysis of the methyl ester and conversion of the free acids to the disuccinimidyl ester gave TAC-Lime-DiSE 3, which was reacted with amino dextran to give TAC-Limedex.
Figure 1.
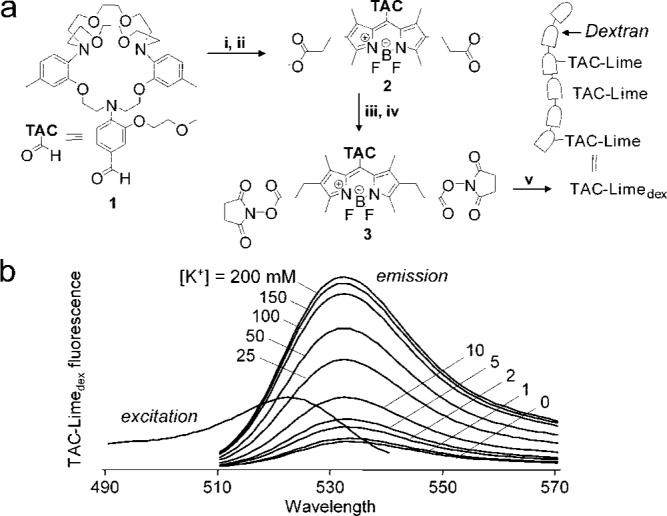
TAC-Limedex synthesis and K+ sensitivity. (a) Synthesis procedures: (i) Methyl-3-(2,4-dimethyl-1H-pyrrol-3-yl) propanoate, TFA, DCM, rt, 18 h followed by addition of DDQ, 4 h; (ii) BF3 Et2O, DIEA, DCM, 0 °C, 2 h; (iii) 0.2 M aq NaOH, 100 °C, 2 h; (iv) O-(N-succinimidyl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate, DIEA, DMF; (v) amino-dextan, TEA, H2O/DMSO. (b) Fluorescence spectra of TAC-Limedex.K+ titrations done at 500 nm excitation wavelength (for emission spectra) and 510 nm emission wavelength (for excitation spectrum). Solutions contained 5 μM TAC-Limedex (5 mM HEPES, pH 7) in balanced KCl/NaCl to maintain constant ionic strength at 200 mM.
TAC-Limedex green fluorescence was strongly K+-sensitive, increasing by 50% with increasing [K+] from 0 to 2 mM (Figure 1b). As found for TAC-Red, TAC-Limedex fluorescence was not sensitive to pH in the biologically relevant range of 5 to 9 or to anions or other cations, with the exception of the K+ analogues Cs+ and Rb+. TAC-Limedex was stable in saline solution at room temperature for 14 days.
The principle of the method to assay cellular K+ transport is diagrammed in Figure 2a. TAC-Limedex is used as an extracellular K+ sensor to detect cellular K+ efflux. TAC-Limedex is membrane impermeant (<3% cell-associated fluorescence after 1 h). With appropriate solution ionic composition and electrochemical gradients, increasing TAC-Limedex fluorescence provides a quantitative measure of K+ channel/transporter function. Figure 2b shows a representative single measurement in which HT-29 cells, after K+ channel activation by ATP, were exposed to a K+-free solution containing TAC-Limedex.K+ efflux produced a time course of increasing TAC-Limedex fluorescence, from which [K+] and K+ efflux rates, d[K+]/dt, are deduced.
Figure 2.
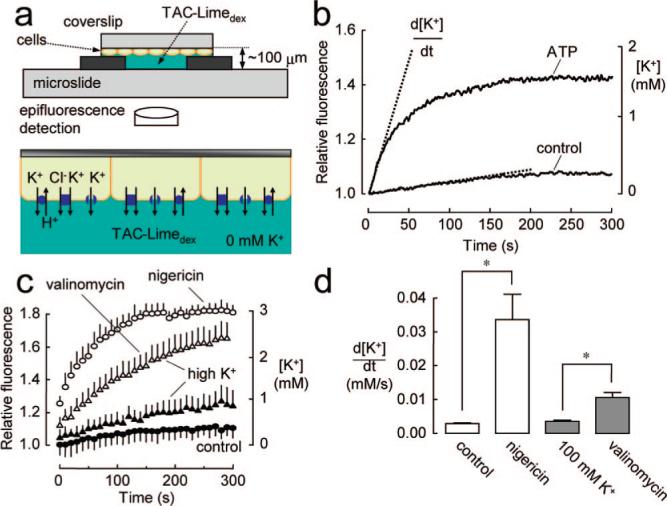
Principle of cell-based assay of K+ transport. (a) K+ efflux measurement method showing accumulation of K+ in an initially K+-free extracellular solution resulting from K+ efflux from cells. (b) Single measurement of ATP-induced K+ efflux in HT-29 cells (cell density 6.3 × 105 cells/cm2, cell/bath volume ratio 0.27). (c) K+ efflux in HT-29 cells under control conditions, and after incubations with a K+ ionophore (valinomycin) or K+/H+ antiporter (nigericin). Cells were preincubated in high K+ buffer where indicated. Each data point is mean ±SE (n ) 3). (d) Deduced K+ efflux rates, d[K+]/dt, from data in (c). *P < 0.05.
Control validation studies are shown in Figure 2c, with K+ efflux data summarized in Figure 2d. K+ efflux in HT-29 cells was relatively slow under control conditions and greatly increased by incubation with the K+/H+ ionophore nigericin, which provides a rapid pathway for electroneutral K+ efflux. Preincubation with a K+-selective ionophore, valinomycin, also increased K+ efflux, indicating that K+ conductance is rate-limiting. The valinomycin preincubation was done in a high K+, cytoplasmic-like solution to prevent cellular K+ depletion.
The electroneutral K+/Cl− cotransporter (KCC) is involved in ionic and osmotic homeostasis in many cell types and in cell growth and tumor invasion. KCC function has been measured previously by radioactive Rb+ uptake.6,7 Figure 3a shows TAC-Limedex assay of KCC function in SiHa cells, a human cervical cancer cell line with hypotonicity-stimulated KCC activity.7 K+ efflux was increased 3-fold following hypotonic challenge (200 mosm/L), with the increase in K+ efflux inhibited by the KCC inhibitor (R)-(+)-[(dihydroindenyl)oxy]alkanoic acid (DIOA). The results demonstrate utility of the TAC-Limedex assay in measuring electroneutral K+ transport.
Figure 3.

Application of TAC-Limedex for assay of K+-ion-coupled transporters and K+ channels. (a) KCC (K+/Cl−) cotransport in SiHa cells was activated by hypotonicity (200 mosm/L) in the absence or presence of 100 μM DIOA (SE, n ) 4). Fluorescence data (top) and summary of K+ efflux rates (bottom). *P < 0.05. (b) Calcium-activated K+ channels in HT-29 cells were activated by 100 μM ATP and inhibited by 10 mM TEA or 50 μM BAPTA (SE, n = 3). (c) Fluorescence platereader assay of ATP-stimulated K+ efflux for the cell system studied in (b).
Figure 3b demonstrates the utility of the TAC-Limedex assay in measuring K+ channel activity in HT-29 cells, which express a Ca2+-activated K+ channel responsive to ATP, carbachol, and Ca2+ ionophores.8 ATP treatment greatly increased K+ efflux, which was inhibited by the K+ channel blocker tetraethylammonium (TEA) or by pretreatment with the cytoplasmic Ca2+ chelator, 1,2-bis(oaminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA-AM). With 3 mM K+ in the outside solution, the signal was 54% of that at 0 K+.
Last, the measurement in Figure 3b was repeated using a commercial fluorescence platereader, in which the K+-free, TAC-Limedex-containing solution was delivered by syringe pump to freshly washed cells (with K+-free buffer) in a 96-well plate. Inclusion of ATP increased the fluorescence response, which was blocked by TEA (Figure 3c). Initial curve slopes from multiwell measurements were (fluorescence units/s ± SD): 0.17 ± 0.01 (control), 0.33 ± 0.03 (ATP), and 0.22 ± 0.02 (+TEA).
Our results establish a simple cell-based fluorescence assay for plasma membrane K+ transport. The assay takes advantage of the strong fluorescence enhancement of TAC-Limedex to small increases in [K+]. Using TAC-Limedex as an extracellular K+ sensor, the kinetics of increasing TAC-Limedex fluorescence provides a quantitative readout of K+ accumulation into an initially K+-free, extracellular solution. The TAC-Limedex signal is sufficiently bright and robust for measurements using commercial fluorescence platereaders. As such, the assay should be amenable to high-throughput screening applications for discovery of modulators of plasma membrane K+ transporters. Because the readout is K+ efflux rather than membrane potential or electrical current, both electro-genic and electrically silent K+-coupled transporters can be assayed.
For assay of K+ channels, certain limitations apply because K+ efflux into a K+-free extracellular solution is measured. Cell membrane potential is generally hyperpolarized under this condition. The K+ conductance to be assayed should be sufficiently high and sustained with an interior-negative membrane potential. Also, counterion conductance should be sufficiently high such that plasma membrane K+ conductance is rate-limiting under assay conditions.
Acknowledgment
Supported by NIH Grants EB00415, HL73856, DK72517, HL59198, DK35124, and EY13574, and grants from the Cystic Fibrosis Foundation.
Footnotes
Supporting Information Available: Experimental methods. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M. Pharmacol. Rev. 2000;52:557–594. [PubMed] [Google Scholar]
- 2.Wickenden AD. Pharmacol. Ther. 2002;94:157–182. doi: 10.1016/s0163-7258(02)00201-2. [DOI] [PubMed] [Google Scholar]
- 3.Villalonga N, Ferreres JC, Argilés JM, Condom E, Felipe A. Recent Patents Anticancer Drug Discovery. 2007;2:212–223. doi: 10.2174/157489207782497181. [DOI] [PubMed] [Google Scholar]
- 4.Padmawar P, Yao X, Bloch O, Manley GT, Verkman AS. Nat. Methods. 2005;2:825–827. doi: 10.1038/nmeth801. [DOI] [PubMed] [Google Scholar]
- 5.Magzoub M, Padmawar P, Dix JA, Verkman AS. J. Phys. Chem. B. 2006;110:21216–21221. doi: 10.1021/jp0633392. [DOI] [PubMed] [Google Scholar]
- 6.Gillen CM, Brill S, Payne JA, Forbush B., III J. Biol. Chem. 1996;271:16237–16244. doi: 10.1074/jbc.271.27.16237. [DOI] [PubMed] [Google Scholar]
- 7.Shen MR, Chou CY, Hsu KF, Hsu YM, Chiu WT, Tang MJ, Alper SL, Ellory JC. J. Biol. Chem. 2003;278:39941–39950. doi: 10.1074/jbc.M308232200. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Franklin CC, Kim HD, Turner JT. Am. J. Physiol. 1991;260:C35–C42. doi: 10.1152/ajpcell.1991.260.1.C35. [DOI] [PubMed] [Google Scholar]


