Abstract
Cognitive control involves adjustments in behavior to conflicting information, develops throughout childhood and declines in aging. Accordingly, developmental and age-related changes in cognitive control and response-conflict detection were assessed in a response-compatibility task. We recorded performance measures, pre-RT activity and medial frontal negativity (MFN), sequentially-occurring, putative ERP indices, respectively, of cognitive control and response-conflict detection. When response conflict reached the highest levels by requiring incompatible responses on post-error trials, children and older adults showed the greatest performance decrements. ERPs indicated that young adults implemented control (pre-RT) and detected the increased conflict (MFN) only when that conflict was at the highest levels, whereas children and older adults did so at lower levels (e.g., post-error, compatible responses). Consequently, the developmental and age-related performance decrements observed here may be due to the undifferentiated and inefficient manner in which children and older adults recruited the processes associated with cognitive-control and response-conflict detection.
INTRODUCTION
Executive processing is a term used to describe a variety of cognitive functions that work separately and in concert to control and coordinate the selection and execution of willed actions. They are a vital aspect of one's ability to adapt quickly and accurately to changing environmental circumstances. As summarized in the conflict-monitoring theory (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Botvinick, Cohen, & Carter, 2004; but see Holroyd & Coles, 2002), it is thought that these executive processes control actions by providing the means to (1) monitor and detect response conflicts and (2) upregulate cognitive control whenever interference arises from competing information streams, such as when a prepotent response must be inhibited and overridden (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Johnson, Barnhardt, & Zhu, 2004).
A wide variety of evidence, including neuroanatomical (Huttenlocher & Dabholkar, 1997), event-related potential (ERP; Friedman, Nessler, Johnson, Ritter, & Bersick, 2008), structural magnetic resonance imaging (MRI; Raz, Gunning-Dixon, Head, Dupuis, & Acker, 1998) and functional MRI (fMRI; van Veen & Carter, 2006) indicates that these kinds of control processes depend critically upon the prefrontal cortex and its interconnections. Specifically, in the conflict-monitoring model, the anterior cingulate cortex (ACC) monitors and detects the conflict, while regions such as the dorsolateral prefrontal cortex (Kerns et al., 2004) are responsible for implementing top-down control (Botvinick et al., 2004). Relative to other brain regions, the prefrontal cortex appears to undergo a more protracted developmental course through adolescence or early adulthood (Sowell et al., 2003), and shows greater alteration than other brain areas as individuals age (Raz, 2000). Therefore, the goal of the current study was to assess lifespan changes in the monitoring and detection of response conflict and the upregulation of cognitive control using performance indices and ERP components that putatively emanate from prefrontal cortex.
In the current experiment, response conflict was manipulated via the creation of compatible- and incompatible-response conditions. In the latter, participants must respond in the direction opposite that indicated by a central arrow (Ridderinkhof et al., 1997), requiring what Botvinick and coworkers have termed “response override” (Botvinick et al., 2004). The requirement to generate incompatible relative to compatible responses leads to RT slowing and increased error rates (Ridderinkhof et al., 1997; see also Nessler, Friedman, Johnson, & Bersick, 2007). Hence, there is a necessity to upregulate control to reduce the ensuing response conflict through inhibition of the incorrect, but prepotent response tendency. In the only extant developmental (Ridderinkhof et al., 1997) and age-related (Nessler, Friedman et al., 2007) studies, relative to young adults, similar-magnitude RT slowing on incompatible- compared to compatible-response trials was found for 10-11 year-old children (Ridderinkhof et al., 1997) and older adults (Nessler, Friedman et al., 2007). These data suggest that children and older adults can deal with the conflict associated with incompatible responses.
Nonetheless, the presence or absence of developmental and/or age-related changes in the processing engendered by incompatible responses may depend upon whether the response on the previous trial was correct or erroneous. Indeed, in addition to “response override,” the commission of an error heightens response conflict. In this case, the conflict-monitoring hypothesis (Botvinick et al., 2001) posits that errors create conflict because they differ from the correct, appropriate response. Correct RTs on trials that follow the error are slower than those after a correct response (i.e., post-error slowing). Although this phenomenon could simply reflect the increased conflict on post-error trials, some authors have suggested that post-error slowing reflects the upregulation of cognitive control in order to rectify the circumstance that initially enabled the error (Gehring & Fencsik, 2001; Hajcak, McDonald, & Simons, 2003; Ladouceur, Dahl, & Carter, 2007; Rabbitt, 1966; Scheffers, Humphrey, Stanny, Kramer, & Coles, 1999; see Ullsperger, 2006 for other interpretations).
Prior investigations suggest that children and older adults are able to effectively handle post-error conflict, provided it is not combined with incompatible-response requirements. For example, Davies et al. (2004) and Santesso et al. (2006) reported that post-error relative to post-correct trials led to similarly prolonged RTs in children and young adults. These limited findings imply that, to the extent that an aspect of post-error slowing reflects control processes, children can exert a measure of cognitive control in this situation. Similarly, in an age-related investigation, Nessler, Friedman et al. (2007) found that older, relative to, young adults showed similar RT slowing and error rates on post-error compatible-response trials. Conversely, when post-error and incompatible-response conflicts were present, thereby augmenting conflict to a higher level, older adults showed increased error rates and a nonsignificant trend for greater RT slowing. These findings suggest that older adults exhibit deficiencies when post-error and incompatible-response conflicts are combined. To our knowledge, however, no comparable data have been collected from children.
Nonetheless, it is difficult to determine unambiguously, on the basis of the RT findings in these few investigations, which of the processes, the upregulation of control or conflict detection, is in place or still developing or maintained or less efficient with aging. The temporal precision of the ERP technique is advantageous here because, as described below, two, sequentially-occurring ERP components have been associated putatively with cognitive-control and response-conflict detection processes.
Notably, an RT-locked positivity peaking at around 50-100 ms before RT is reduced (i.e., more negative-going) under conditions that require greater amounts of cognitive control, suggesting that an overlapping negative component underlies this phenomenon (Johnson et al., 2004; Johnson, Henkell, Simon, & Zhu, 2008; Nessler, Johnson, Bersick, & Friedman, 2007). This negative-going activity onsets a few hundred ms before RT and is prominent over medial frontal scalp locations. Hereafter, we use the label “pre-RT activity” to refer to it. For example, Johnson et al. (2008) asked participants to make truthful and directed lie (i.e., press in the direction opposite of the truth) responses about their attitudes. Negative-going, pre-RT activity was greater in the directed-lie compared to the truthful condition, supporting the idea that successful deception requires the use of control processes to ensure that the responses selected conformed to the person's overall goal of lying about firmly-held attitudes. Extrapolating from these results, pre-RT activity should be more negative-going on post-error than post-correct trials because an error on trial N should engender the upregulation of cognitive control to reduce error propensity on trial N+1. This effect might be greater when incompatible compared to compatible responses are required. To test this hypothesis directly, post-error and post-correct trials were compared.
Closely following the pre-RT activity is a post-RT negativity (peaking within the first 100 ms after RT) that appears to reflect the amount of response conflict that is present at the time the response is generated (Botvinick et al., 2004; Hogan, Vargha-Khadem, Kirkham, & Baldeweg, 2005; Johnson et al., 2004; Nessler, Friedman et al., 2007; West, 2004). This activity has been labeled the medial frontal negativity or MFN (Gehring & Willoughby, 2002) to distinguish it from the error negativity (Ne; Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991) or error-related negativity (ERN; Gehring, Goss, Coles, Meyer, & Donchin, 1993) recorded on error trials.
Developmental and age-related investigations suggest that the MFN and ERN reflect at least partly distinct processes, although this is currently controversial (Bartholow et al., 2005; Gehring & Fencsik, 2001; Johnson et al., 2004; Masaki, Falkenstein, Sturmer, Pinkpank, & Sommer, 2007; Nessler, Friedman et al., 2007; Taylor, Stern, & Gehring, 2007). The ERN is typically found to be reduced, relative to young adults, in children (Davies et al., 2004; Hogan et al., 2005; Segalowitz & Davies, 2004) and older adults (Falkenstein et al., 2001; West, 2004), suggesting alterations in the error-monitoring system. In addition, the study by Nessler, Friedman et al. (2007) demonstrated a dissociation between error monitoring and response conflict detection -- respectively, the ERN was smaller in older than young adults whereas, in accord with the increased error rate, the MFN was larger in older than young adults in the post-error, incompatible-response condition. Hence, older adults appeared to detect the heightened response conflict (MFN), leading to the conclusion that the implementation of cognitive control prior to the decision may have been altered (Nessler, Friedman et al., 2007).
To further investigate these phenomena, the current study's aims were to assess developmental and age-related change in the executive processes reflected by the ERN (error monitoring), pre-RT activity (upregulation of control) and the MFN (response conflict detection) to single and combined sources of conflict. In accord with prior studies indicating a less efficient error-monitoring system in children and older adults, we expected that ERN magnitude would be smaller in these two age groups (Falkenstein et al., 2001; Segalowitz & Davies, 2004). Based on ERP (e.g., Friedman et al., 2008; Nessler, Friedman et al., 2007) and hemodynamic (Lustig, Head, Janes, & Buckner, 2006; Milham et al., 2002; Reuter-Lorenz & Mikels, 2006; Velanova, Lustig, Jacoby, & Buckner, 2007) data indicating that control processes may be recruited in an undifferentiated manner with aging, we predicted that older adults would show an increment in control processes (pre-RT activity) even on relatively low-conflict trials when only a single source of conflict was present (i.e., post-error, compatible- response; post-correct, incompatible-response -- compared to post-correct, compatible). By contrast, young adults were expected to recruit these processes only on highest-demand trials, when two sources of conflict were combined (i.e., post-error, incompatible response). We could not predict with any confidence whether an “undifferentiated” pattern of control processing would also hold for children.
Based on the behavioral data indicating that children and older adults can effectively deal with a single source of conflict, it was expected that low-conflict trials would engender similar amounts of response conflict as indicated by equivalent MFN magnitudes across the age span. However, when post-error and incompatible-response conflicts were combined, children and older, relative to, young adults were expected to show compromised behavioral performance. In addition, it was predicted that the performance decrement in older adults would be associated with heightened response conflict (enhanced MFN activity; Nessler, Friedman et al., 2007). No clear-cut prediction for the MFN could be made for children due to the lack of prior data.
METHOD
Participants
Twenty children, 16 young and 19 older adults were recruited for the study. Four children and three older adults were eliminated for failure to perform the task. An additional two older adults and one child were removed from the data set because they failed to provide a sufficient number of artifact-free trials (N≥9) in one or more of the critical conditions analyzed below. The demographic and neuropsychological data for the 15 children (M age = 10.2; 7 female), 16 young (M age = 24.7 ±4.0; 12 female) and 14 older adults (M age = 72.5; 6 female) retained for study are presented in Table 1. Young and older volunteers were administered the Modified Mini-Mental Status examination (mMMS; Mayeux, Stern, Rosen, & Leventhal, 1981) and achieved a score within the normal range. IQ for the children was obtained from the Wechsler Intelligence Scale for Children (WISC-III; Wechsler, 1991). IQ for young adults was estimated from the Vocabulary and Block design subtests of the Wechsler Adult Intelligence Scale-III (Wechsler, 1997). Older volunteers were administered the full WAIS-III. All participants obtained IQ scores within the average to above average range. All signed informed consent or assent forms according to the criteria of the New York State Psychiatric Institute's Institutional Review Board and were paid for their participation.
Table 1.
Demographic characteristics and neuropsychological measures for the Young and Older Adults and Children.
| Young Adults (N=16) |
Older Adults (N=14) |
Children (N=15) |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age | 24.0 | 3.0 | 72.6 | 5.8 | 10.2 | 0.7 |
| SES £ | 54.3 | 13.3 | 40.9 | 15.7 | 40.4¶ | 19.7 |
| Years of Education | 16.0 | 1.7 | 17.2 | 1.6 | 4.5 | 0.7 |
| mMMS § | 54.9 | 2.1 | 54.7 | 1.9 | NA | |
| Digits Forward 1 | 7.7 | 1.0 | 6.5 | 0.8 | 9.8 | 2.0 |
| Digits Backward 1 | 6.3 | 1.4 | 5.3 | 0.9 | 6.3 | 2.2 |
| LQ | 82.8 | 25.2 | 88.8 | 20.2 | 61.8 | 31.9 |
| Verbal IQ 1 | 126.2 | 24.7 | 131.7 | 13.4 | 122.1 | 36.1 |
| Performance IQ 1 | 111.9 | 24.9 | 118.8 | 11.4 | 113.6 | 35.6 |
| Depression 2 | NA | 1.30 | 0.9 | NA | ||
| Dementia 2 | NA | 0.07 | 0.3 | NA | ||
Notes. NA = not applicable; MMS = modified mini-mental status exam; SES = socioeconomic status (higher score = lower SES; (Watt, 1976); LQ = laterality quotient (Oldfield, 1971);
WAIS-III (David Wechsler, 1997) for young and older adults; WISC-III for children;
From the Short Care (Gurland et al, 1984); cutoff is 6 for depression and 7 for dementia.
Modified Mini-Mental Status Exam (Mayeux et al., 1981); maximum score = 57.
Based on a score averaged across the SES values for the child's mother and father.
Higher score = lower SES.
In addition, older-adult volunteers underwent a semi-structured interview (SHORT CARE; Gurland, Golden, Teresi, & Challop, 1984) to ensure that they were free from dementia, depression, and not limited in the activities of daily living (see Table 1), as well as a medical and neurological examination. This examination was conducted by a board-certified neurologist in order to assess prospective volunteers for the presence of neurodegenerative disorders, clinically detectable neurovascular disease, disturbances in gait, visual acuity, and visual fields, and the presence of tremors or rheumatological disorders.
Stimuli and Experimental Procedures
Participants were seated comfortably in a sound-damped and electrically-shielded room. They sat facing a 17″ computer monitor about 100 cm from the screen and held a small response box on their laps. The target stimuli were arrows (200-ms duration) pointing either to the left or the right presented centrally on the computer monitor with a randomly-jittered inter-trial-interval of 900-1200 ms. Participants made choice button-press responses with their left and right index finger depending on the direction of a central arrow. In the Incompatible condition, participants pressed the button corresponding to the direction opposite that in which the arrow pointed. In the Compatible condition, participants pressed the button corresponding to the same direction in which the central arrow pointed. Participants were asked to respond as quickly as possible. Each Response-Compatibility condition was comprised of three blocks of 120 stimuli1. The condition with which participants began and the order of conditions were counterbalanced across participants within each age group.
Electroencephalographic (EEG) Recording
EEG was recorded from 62 scalp sites (sintered Ag/AgCl) in accord with the extended ten-twenty system (Sharbrough et al., 1990) using an Electro cap (Neuromedical Supplies). All electrodes, including the mastoids, were referred to the nosetip. Horizontal and vertical electrooculogram (EOG) were recorded bipolarly with electrodes placed, respectively, at the outer canthi of both eyes and above and below the left eye. EOG and EEG were recorded continuously with Synamp amplifiers (Neuromedical Supplies; DC; 100 Hz low-pass filter; 500 Hz digitization rate). Eye-movement artifacts were corrected off-line (Semlitsch, Anderer, Schuster, & Presslich, 1986), and remaining artifacts (e.g., muscle activity) were manually rejected.
Data Analyses
For both the behavioral and ERP analyses, the SPSS Version 14 for Windows (repeated-measures ANOVA) program was used. The Greenhouse-Geisser epsilon (ε) correction (Jennings & Wood, 1976) was used when appropriate. Uncorrected degrees of freedom are reported along with the epsilon value; the P values reflect the epsilon correction. Significant main and interaction effects were followed-up with subsidiary ANOVAs and/or post-hoc analyses using the Tukey Honestly Significant Difference test (HSD). Because we had predicted that older adults and children might show the greatest deficiencies in upregulating control when post-error and response-incompatibility conflicts were present, planned comparisons (subsidiary ANOVAs) were performed separately for each age group when interactions in the predicted direction were marginally significant. Partial η2 is presented as an estimate of main and interaction effect sizes. The structure of the ANOVAs is described in the corresponding Results section.
Behavioral Analyses
Only trials with RTs between 100 and 900 ms were analyzed. The mean percentage of trials that fell outside this criterion and the mean percentage of timeouts were, respectively (±SE), for young adults, 0.5 (±.2) and 1.5 (±.4), for older adults, 1.3 (±.4) and 2.9 (±.8), and for children, 3.4 (±.8) and 5.4 (±1.3). Percent correct responses and the corresponding RTs based on these criteria are considered below.
ERP Analyses
As noted, we were interested in both error-related processing (ERN) and response-conflict monitoring and detection (MFN). Therefore, response-locked ERP data associated with correct and incorrect responses were computed. The data were epoched into 900-ms lengths (450 ms prior to and following mean RT). Because we were also interested in control processes that might precede the RT response, a baseline immediately before the response (e.g., the 100 ms preceding RT) could not be used. A baseline placed several hundred ms before the response might overcome this problem (Nessler, Friedman et al., 2007). However, RTs could vary across age groups and conflict conditions and, therefore, a baseline of this type could confound response-with stimulus-related effects. In an attempt to counteract some of these problems, each single trial was baseline corrected using the 100 ms preceding the stimulus prior to computing RT-locked averages (for a similar procedure see Fiehler, Ullsperger, & von Cramon, 2005; Johnson, Barnhardt, & Zhu, 2003; Johnson et al., 2004).
Determination of Latencies for the Components under Investigation
Error-Related Negativity (ERN/Ne)
Based on prior investigations (Falkenstein et al., 2001; Morris, Yee, & Nuechterlein, 2006; Nessler, Friedman et al., 2007) and the data from this experiment (Figure 1), the ERN was measured at midline locations Fz and FCz. We first determined the peak latencies of the ERN at these sites for erroneous, incompatible-response trials (where we expected the largest amplitude) as the most negative time point between −50 ms pre- and 100 ms post-RT for each age group. This analysis indicated that older adults, young adults and children had mean latencies of, respectively, 40, 26, and 14 ms post RT. The latencies were rounded to the nearest 10 ms and an 80 ms window surrounding the mean value was used to capture ERN magnitude (shaded regions in Figure 1; young = −10 to 70; older = 0 to 80; children = −30 to 50 ms).
Figure 1.
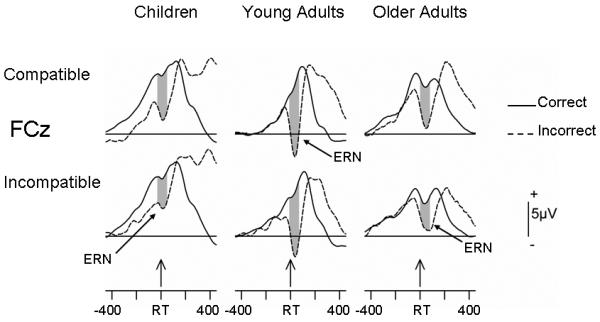
Grand mean RT-locked, post-correct ERP waveforms on correct- and erroneous-response trials averaged across the participants within each Age Group (N=16 young; 14 older adults and 15 children). The waveforms have been averaged separately for compatible- and incompatible-response conditions. The large-amplitude, negative component associated with erroneous trials is the error-related negativity, ERN, or Ne. Shaded region indicates the 80-ms window for measuring ERN magnitude.
Medial Frontal Negativity (MFN)
Based on previous studies (Johnson et al., 2004; Nessler, Friedman et al., 2007) and the scalp distribution of the MFN in the current data (Figure 2), the data at electrodes AF3, AFz, AF4, F3, Fz, F4, FC3, FCz, and FC4 were chosen for statistical evaluation. Using these 9 sites, the time interval for measuring MFN magnitude was determined (−50 pre- to 100 ms post-RT) in the condition that showed the largest MFN (post-error, incompatible-response trials; Figure 3). The analysis revealed that older adults, young adults and children had mean latencies of, respectively, 41, 3 (both peaking post RT), and −10 ms (pre RT). To capture MFN magnitude, these latencies were used as the center of an averaged voltage of 80-ms length (older adults = 0–80 ms post RT). Because young adults' and children's peaks lay close to mean RT, this point was chosen as the center of their measurement window (−40 to +40 ms).
Figure 2.

Surface Potential (SP) and Current source density (CSD) scalp topographies for the three age groups computed on the waveforms associated with post-error trials on which an incompatible response was called for. The maps were computed at the time point for which the MFN showed maximal peak amplitude. Dots represent the 62 scalp locations. The maps were computed by calculating contours using the spherical spline method (Perrin, Pernier, Bertrand, & Echallier, 1989) and the data from all 62 electrode sites.
Figure 3.
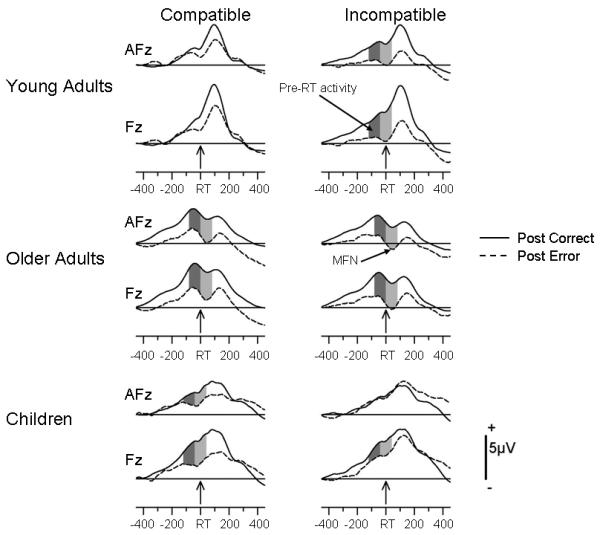
Grand mean RT-locked ERP waveforms averaged across the 16 young adults, 14 older adults and 15 children. The data are superimposed for Post-Correct and Post-Error trials. The ERPs associated with Compatible and Incompatible responses are depicted, respectively, in the left and right columns. The waveforms are illustrated for scalp locations AFz and Fz. Dark and light shading indicate reliable differences between post-error and post-correct waveforms for, respectively, pre-RT and MFN activities.
Pre-RT Activity
For older adults, the peak latency of a pre-RT positive peak on post-correct, compatible trials (its amplitude was most positive in this condition; Figure 3) was computed at the 9 sites listed above as the most positive peak between −200 ms and RT. The mean peak latency occurred 45 ms prior to RT, which was rounded to 40 ms. Hence, the averaged voltage extended from −80 ms pre RT to 0 ms (at RT). As can be observed in Figure 3, children and young adults did not show a clear, pre-RT positivity. Therefore, averaged voltages for the 80 ms preceding the onset of the MFN measurement window (−120 to −40 ms) were used for these groups.
RESULTS
Behavioral Data - Post-Correct vs. Post-Error Trials
To determine whether differential age-group changes in accuracy and RT occurred on trials on which two sources of conflict were combined (post-error, incompatible responses) compared to those with a single source of conflict (post-correct, incompatible responses; post-error, compatible responses), between-Age-Group ANOVAs assessed the within-subjects factors of Correctness and Response Compatibility on the accuracy and RT data. These data are presented in Table 2.
Table 2.
Mean RT and percent correct (±SE) on post-correct and post-error trials under the compatible-and incompatible-response conditions.
| Compatible | Incompatible | Grand Mean | |
|---|---|---|---|
| Young Adults (N=16) | |||
| Percent Correct | |||
| Post Correct | 86.3 (2.1) | 81.4 (2.4) | 83.8 (2.0) |
| Post Error | 85.8 (3.2) | 77.2 (4.3) | 81.5 (3.1) |
| Grand Mean | 86.1 (2.3) | 79.3 (2.9) | |
| RT | |||
| Post Correct | 375 (16) | 400 (17) | 387 (16) |
| Post Error | 390 (20) | 426 (17) | 408 (17) |
| Grand Mean | 382 (17) | 413 (16) | |
|
| |||
| Older Adults (N=14) | |||
| Percent Correct | |||
| Post Correct | 90.3 (2.2) | 85.2 (2.6) | 87.7 (2.1) |
| Post Error | 91.1 (3.5) | 72.0 (4.5) | 81.6 (3.3) |
| Grand Mean | 90.7 (2.5) | 78.6 (3.2) | |
| RT | |||
| Post Correct | 453 (17) | 513 (18) | 483 (17) |
| Post Error | 511 (21) | 561 (18) | 536 (18) |
| Grand Mean | 482 (19) | 537 (17) | |
|
| |||
| Children (N=15) | |||
| Percent Correct | |||
| Post Correct | 79.5 (2.1) | 76.3 (2.5) | 77.9 (2.0) |
| Post Error | 71.3 (3.3) | 62.7 (4.4) | 67.0 (3.2) |
| Grand Mean | 75.4 (2.4) | 69.5 (3.1) | |
| RT | |||
| Post Correct | 486 (17) | 510 (17) | 498 (16) |
| Post Error | 509 (20) | 503 (17) | 506 (17) |
| Grand Mean | 497 (18) | 506 (17) | |
Percent Correct
The main effect of Correctness (F(1,42) = 16.37, P <0.0001; partial η2 = .28) revealed that, relative to post-correct trials (M = 83), post-error trials (M = 77) engendered lower accuracy. Similarly, the main effect of Response Compatibility (F(1,42) = 23.17, P <0.0001; partial η2 = .36) indicated that incompatible (M = 76) relative to compatible responses (M = 84) led to reduced accuracy. Importantly, Correctness interacted marginally with Age Group (F(2,42) = 2.50, P <0.09; partial η2 = .11), as did Age Group, Correctness and Response Compatibility (F(2,42) = 2.30, P <0.10). Planned comparisons in the form of subsidiary ANOVAs were performed to explain the triple interaction.
Young adults were less accurate on incompatible- than compatible-response trials (main effect of Response Compatibility F(1,15) = 9.95, P <0.007; partial η2 = .40) on both post-correct and post-error trials. The main effect of Correctness was not significant (F<1), but the Correctness X Response Compatibility interaction was marginally reliable (F(1,15) = 3.06, P <0.10; partial η2 = .17). Post-hoc testing of the interaction revealed that, while there was no reduction in accuracy on post-error relative to post-correct trials in the compatible-response condition, there was a reliable decrease when two sources of conflict were present in the incompatible-response condition.
For older adults, the main effects of Correctness (F(1,13) = 5.14, P <0.04; partial η2 = .28) and Response Compatibility (F(1,13) = 9.16, P <0.01; partial η2 = .41) were significant, but were modulated by the Correctness X Response Compatibility interaction (F(1,13) = 8.23, P <0.01; partial η2 = .38). Post-hoc testing of the interaction indicated that the accuracy reduction was greatest and larger than that found for the young (see Table 2), when two sources of conflict were combined (post-error, incompatible responses). Compared to this highest conflict condition, the accuracy decrease was smaller when a single source of conflict was associated with incompatible responses on post-correct trials, and absent when induced solely by post-error conflict (post-error, compatible responses).
For the children, the Correctness (F(1,14) = 18.28, P <0.001; partial η2 = .57) and Response Compatibility (F(1,14) = 4.78, P <0.05; partial η2 = .25) main effects indicated accuracy reductions, respectively, on post-error and incompatible-response trials. The Response Compatibility X Correctness interaction (F(1,14) = 2.42, P >0.14; partial η2 = .15) was not significant. Although the interaction was not reliable, the trend indicated the greatest accuracy decrement when both post-error and incompatible-response conflicts occurred together (Table 2).
RT
The main effect of Correctness (F(1,42) = 49.42, P <0.0001; partial η2 = .54) indicated that RTs on post-error (M = 483 ms) relative to post-correct (M = 456 ms) trials were slowed (Table 2). The main effect of Response Compatibility (F(1,42) = 22.52, P <0.0001; partial η2 = .35) revealed that incompatible (M = 485 ms) relative to compatible (M = 454 ms) responses were prolonged. However, the interactions of Age Group and Correctness (F(2,42) = 11.46, P <0.0001; partial η2 = .35) and Age Group and Response Compatibility (F(1,42) = 3.86, P <0.03; partial η2 = .16) indicated that, as verified by post-hoc testing, the slowing in each case was greatest for the older adults (i.e., post-error vs. post correct and incompatible vs. compatible; see Table 2). Although the three-way interaction of Age Group, Correctness and Response Compatibility was only marginally significant (F(1,42) = 2.90, P <0.07; partial η2 = .12), planned comparisons (subsidiary ANOVAs) were performed to examine more closely the source of the differential slowing.
For young adults the main effects of Correctness (M difference = 21 ms; F(1,15) = 23.37, P <0.0001; partial η2 = .65) and Response Compatibility (M difference = 31 ms; F(1,15) = 8.55, P <0.01; partial η2 = .36) were significant, but the interaction was not (F=1.30). Similarly, for older adults the Correctness (M difference = 53 ms; F(1,13) = 30.94, P <0.0001; partial η2 = .70) and Response Compatibility (M difference = 55 ms; F(1,13) = 14.87, P <0.002; partial η2 = .53) main effects were reliable, but the interaction was not (F<1).
By contrast, for children, neither the main effect of Correctness (M difference = 8 ms; F(1,14) = 1.67, P > 0.21; partial η2 = .11) nor Response Compatibility (M difference = 9 ms; F<1) was reliable. Importantly, however, the Response Compatibility X Correctness interaction F(1,13) = 6.56, P <0.02; partial η2 = .32) was significant. As verified by post-hoc tests, RT slowing was significant when only a single source of conflict was present (i.e., post-error, compatible-response trials; M difference = 23 ms; post-correct, incompatible-response trials, M difference = 24 ms), but no further slowing was evident when the two sources of conflict were combined (i.e., post-error, incompatible-response trials; see Table 2).
In sum, the performance data indicated that children did not differ from young adults when only a single source of incompatible-response conflict was present, while older adults evinced greater RT-slowing. Relative to young adults, older adults also showed greater RT slowing (associated with preserved accuracy) when only a single source of post-error conflict was present. By contrast, children produced similar-magnitude RT slowing to young adults when only post-error conflict was present, but the amount of slowing did not prevent an accuracy decrease. Although older adults showed prolonged RTs on combined-conflict (post-error, incompatible-response) trials, it too did not avert an accuracy decrement. Children did not demonstrate RT slowing on these highest-conflict trials, resulting in a trend for the greatest accuracy decrease in this condition.
ERP Data
In the between-group and subsidiary ANOVAs detailed below, main and interaction effects of Electrode Location are not reported unless they interacted with Age Group (children, young adults, older adults) and one or more of the following factors, Response Compatibility (compatible, incompatible), and/or Correctness on the previous trial (post correct, post error) as, by themselves, they are not of interest with respect to the hypotheses under investigation.
Error Trials - ERN Magnitude2
For these analyses, the post-correct data were averaged separately for correct- and incorrect-response trials. Because some previous studies have reported larger ERN amplitudes under conditions of greater response conflict (Bartholow et al., 2005; Nessler, Friedman et al., 2007), averages were created for compatible- and incompatible-response trials (Figure 1 depicts the 4 resulting averages for the three age groups). The averaged voltages based on the data in Figure 1 were subjected to an Age Group X Correct/Incorrect X Response Compatibility X Electrode Location (Fz, FCz) ANOVA. This analysis revealed only a main effect of Correct/Incorrect (F(1,42) = 54.07, P <0.0001, partial η2 = .56), indicating that error trials elicited greater negative-going activity than correct trials, i.e., robust ERNs in all three age groups. Despite the impression obtained from Figure 1 of larger differences between incorrect and correct trials in young relative to older adults and children, the interaction of Age Group and Correctness was not reliable (F(2,42) = 1.66, P >0.20, partial η2 = .07). However, the Age Group X Response Compatibility interaction was marginally significant (F(2,42) = 2.95, P <0.06, partial η2 = .12).
To determine the source of the interaction, subsidiary ANOVAs were performed. As expected from the overall analysis, all three age groups showed significant main effects of Correct/Incorrect (Fs > 8.51, Ps < 0.01 or less; partial η2s >0.39). However, only older adults also exhibited a main effect of Response Compatibility (F(1,13) = 12.08, P <0.004, partial η2 = .48), indicating that, on both correct and erroneous trials, incompatible- relative to compatible-responses elicited greater negative-going activity (Correct/Incorrect X Response Compatibility interaction F <1). The Correct/Incorrect X Response Compatibility interaction was also not reliable for children or young adults (Fs < 2.28, Ps > 0.15).
Post-Correct Versus Post-Error Trials3
Figure 3 depicts the grand-averaged, response-locked data for children, young and older adults. The ERPs are comprised of two prominent components. The first, a pre-RT positivity, is reduced as a result of increased conflict (as described below), suggesting, as noted in the introduction, that an overlapping negative component is producing this effect (Johnson et al., 2008). The second component, a post-RT negativity, is the MFN, which increases with increments in response conflict. To determine developmental and/or age-related differences in the upregulation of cognitive control and/or response conflict monitoring and detection, which were expected to be greatest when two sources of conflict were combined (post-error, incompatible-responses), the pre-RT activity and MFN data depicted in Figure 3 were subjected to between-Age-Group ANOVAs with the within-subjects factors of Response Compatibility, Correctness, and Electrode Location (9 scalp sites).
Pre-RT Activity
The main effect of Correctness (F(1,42) = 31.26, P <0.0001, partial η2 = .43) indicated that, as predicted, trials following an error (M = 1.61 μV) produced more negative-going activity than those following a correct response (M = 3.88 μV). The main effect of Response Compatibility (F(1,42) = 7.84, P <0.008, partial η2 = .16) revealed that, relative to compatible responses (M = 3.88 μV), incompatible responses (M = 1.61 μV) were more negative going. However Age Group interacted with Correctness (F(2,42) = 3.95, P <0.03, partial η2 = .16) as did Age Group and Response Compatibility (F(2,42) = 5.32, P <0.009, partial η2 = .20; see Table 3). To parse these interactions, subsidiary ANOVAs were performed.
Table 3.
Mean (±SE) Pre-RT amplitudes collapsed across the 9 sites from the ANOVA for post-correct and post-error trials under compatible- and incompatible-response conditions.
| Compatible | Incompatible | Grand Mean | |
|---|---|---|---|
| Young Adults (N=16) | |||
| Post Correct | 2.2 (0.8) | 2.6 (0.7) | 2.4 (0.8) |
| Post Error | 2.0 (0.9) | 1.0 (0.9) | 1.5 (0.8) |
| Grand Mean | 2.1 (0.7) | 1.8 (0.7) | |
|
| |||
| Older Adults (N=14) | |||
| Post Correct | 6.4 (0.9) | 4.9 (0.8) | 5.7 (0.8) |
| Post Error | 3.3 (0.9) | 0.7 (0.9) | 1.9 (0.8) |
| Grand Mean | 4.9 (0.8) | 2.8 (0.8) | |
|
| |||
| Children (N=15) | |||
| Post Correct | 3.6 (0.8) | 3.5 (0.8) | 3.5 (0.8) |
| Post Error | 1.2 (0.9) | 1.5 (0.9) | 1.4 (0.8) |
| Grand Mean | 2.4 (0.7) | 2.5 (0.7) | |
For the young, the ANOVA only revealed a significant Response Compatibility X Correctness interaction (F(2,42) = 6.28, P <0.02, partial η2 = .29). As indicated by post-hoc tests, reliable pre-RT negativity was not observed when only a single source of either post-error or incompatible-response conflict was present. However, when the two sources of conflict were combined on post-error, incompatible-response trials, more negative-going pre-RT activity was observed (Figure 3 top 2 rows; Table 3).
For older adults, the main effect of Correctness showed that, relative to post-correct trials, post-error trials engendered greater negative-going activity (F(1,13) = 34.84, P <0.0001; partial η2 = .73). The main effect of Response Compatibility (F(1,13) = 20.70, P <0.001; partial η2 = .61) indicated that incompatible relative to compatible responses produced greater negative-going activity. However, the Response Compatibility X Correctness interaction was marginally significant F(1,13) = 3.17, P <0.10). Post-hoc testing indicated that, by contrast with the young, the pre-RT activity was present on trials with only one source of conflict while, like the young, it was largest when two sources of conflict were combined (Figure 3 middle two rows; Table 3).
For children, the main effect of Correctness (F(1,14) = 5.79, P <0.03, partial η2 = .29) revealed that, relative to post-correct trials, post-error trials produced greater pre-RT negative-going activity. By contrast with older adults, incompatible compared to compatible responses did not elicit increased pre-RT negativity (Response Compatibility main effect F<1). The interaction of Response Compatibility and Correctness was also not significant (F<1), suggesting, by contrast with young adults, that pre-RT activity did not differ when two sources relative to a single source of conflict were present.
MFN
The main effect of Correctness was reliable (F(1,42) = 45.03, P <0.0001, partial η2 = .52), indicating that trials following an error (M = 1.46 μV) generated more negative-going MFNs than those after a correct response (M = 4.10 μV). The main effect of Response Compatibility was significant (F(1,42) = 10.92, P <0.002, partial η2 = .21), showing that, relative to compatible responses (M = 3.31 μV), incompatible responses (M = 2.27 μV) were more negative going. However, Age Group interacted marginally with Correctness (F(2,42) = 2.40, P <0.10, partial η2 = .10), as did Age Group and Response Compatibility (F(2,42) = 2.95, P <0.06, partial η2 = .12). To determine the source of these interactions, planned comparisons, in the form of subsidiary ANOVAs, were performed.
For the young, post-error relative to post-correct trials were significantly more negative-going (F(1,15) = 8.86, P <0.009, partial η2 = .37). The main effect of Response Compatibility was marginally significant (F(1,15) = 3.82, P <0.07, partial η2 = .20). Importantly however, the Correctness X Response Compatibility interaction (F(1,15) = 7.77, P <0.01, partial η2 = .34) was reliable. As verified by post-hoc tests, the interaction indicated that, compared to post-correct, compatible-response trials, the increase in MFN magnitude was reliable when two sources of conflict were combined (post-error, incompatible-response trials) but not when only a single source of conflict was present (post-correct, incompatible responses; post-error, compatible responses; Table 4).
Table 4.
Mean (±SE) MFN amplitudes collapsed across the 9 sites from the ANOVA for post-correct and post-error trials under compatible- and incompatible-response conditions.
| Compatible | Incompatible | Grand Mean | |
|---|---|---|---|
| Young Adults (N=16) | |||
| Post Correct | 3.5 (0.9) | 3.4 (0.8) | 3.4 (0.8) |
| Post Error | 2.4 (0.8) | 0.7 (0.9) | 1.6 (0.7) |
| Grand Mean | 2.9 (0.8) | 2.0 (0.8) | |
|
| |||
| Older Adults (N=14) | |||
| Post Correct | 5.2 (0.9) | 3.6 (0.8) | 4.4 (0.9) |
| Post Error | 1.7 (0.9) | −0.8 (1.0) | 0.5 (0.8) |
| Grand Mean | 3.5 (0.8) | 1.4 (0.8) | |
|
| |||
| Children (N=15) | |||
| Post Correct | 4.7 (0.9) | 4.5 (0.8) | 4.6 (0.8) |
| Post Error | 2.4 (0.9) | 2.3 (1.0) | 2.4 (0.8) |
| Grand Mean | 3.5 (0.8) | 3.4 (0.8) | |
For older adults, the main effects of Correctness (F(1,13) = 39.78, P <0.0001, partial η2 = .75) and Response Compatibility (F(1,13) = 14.56, P <0.002, partial η2 = .53) indicated, respectively, greater negative-going activity on post-error relative to post-correct trials, and incompatible- compared to compatible-response trials. The Response Compatibility X Correctness interaction (F=1.38) was not reliable, suggesting that, unlike young adults, MFN magnitude was not differentially enlarged on dual- compared to single-conflict trials (Figure 3; Table 4).
For children, by contrast with older adults but similar to young adults, the main effect of Response Compatibility was not significant (F<1), suggesting that children did not show reliable MFNs when only a single source of response-incompatibility conflict was present. However, the main effect of Correctness (F(1,14) = 7.90, P <0.01, partial η2 = .36) indicated increased MFNs on post-error relative to post-correct trials, an effect that was of similar magnitude for lower- (compatible-response) and highest-conflict (incompatible-response) trials (Response Compatibility X Correctness interaction F<1).
To summarize, young adults showed more negative-going pre-RT and MFN components only when two sources of conflict were combined (post-error incompatible responses) but not when a single source of conflict was present. By contrast, only older adults showed reliable increases in pre-RT and MFN magnitudes when the only source of conflict was response incompatibility on post-correct trials. However, if the single source of conflict was post-error, then both older adults and children exhibited increased pre-RT and MFN amplitudes. Older adults and children also produced more negative-going pre-RT and MFN activities when post-error and response-incompatibility conflicts were combined. However, except for the pre-RT activity in older adults, these effects did not differ quantitatively from those observed for single-source conflict trials suggesting, compared to young adults, a less differentiated pattern of ERP activity in these groups.
DISCUSSION
The primary aim of the current investigation was to determine whether developmental and age-related differences in the upregulation of cognitive control and the detection of response conflict would occur when engendered by “response override” (i.e., incompatible responses) and post-error induced forms of conflict. Indeed, developmental and age-related behavioral differences were most prominent when participants had to simultaneously recover from an error and deal with incompatible-response conflict on the current trial, i.e., when conflict was augmented to the highest level (see also Nessler, Friedman et al., 2007). Consistent with the behavioral data, the cognitive control processes reflected by pre-RT activity and response conflict detection indicated by the MFN showed clear developmental and age-related alterations, as discussed below.
Children and young adults showed similar-magnitude performance decrements when response incompatibility was the only source of conflict. However, in stark contrast to the behavioral results of two earlier studies (Davies et al., 2004; Santesso et al., 2006), children showed an accuracy decrease, relative to young and older adults, on compatible trials when only a single, post-error source of conflict was present. Moreover, when confronted with a second, incompatible-response source of conflict on post-error trials, children, relative to young adults, not only demonstrated decreased accuracy but also failed to slow their RTs. Hence, while the executive processes that deal with situations in which “response override” is necessary appear to be relatively mature in 9-10 year old children, those employed to overcome post-error conflict appear to be still developing.
The behavioral results for older adults also indicated deficiencies in the executive processes recruited to manage conflict, but differed from those found in the children. Independent of the type of conflict, when only a single source of conflict was present (post-correct, incompatible responses; post-error, compatible responses), relative to young adults, older adults showed greater RT-slowing. An aspect of the slowing may have reflected increased recruitment of control, which appeared to successfully avert a decrease in accuracy. However, the magnitude of RT slowing on incompatible, post-error trials (when a second source of incompatible-response conflict was present), was not sufficient to avoid a larger accuracy decrement in comparison to the young (see also Nessler, Friedman et al., 2007). Thus, while older adults appeared able to deal with post-error conflict on compatible-response trials and incompatible-response conflict on post-correct trials, they showed clear deficiencies when post-error and incompatible-response conflicts were combined.
The performance decrements in children and older, relative to, young adults were accompanied by differences in pre-RT and MFN components. For young adults, there was no reliable difference in upregulating control (pre-RT activity) or response conflict detection (MFN) when a single source of conflict (post-correct, incompatible; post-error, compatible) was compared to a condition with presumably little or no conflict (post-correct, compatible). Then again, when demand was heightened by the combination of two sources of conflict (post-error, incompatible), the upregulation of control (pre-RT activity) and the amount of conflict detected (MFN) were increased. By contrast, children appeared to upregulate control (pre-RT activity) and detect increased amounts of conflict (MFN) when only a single source of conflict was present (post-error, compatible-response trials). In addition to showing increments in upregulating control and conflict detection on these single-conflict trials, older adults also demonstrated increases in these processes in the other single-conflict condition, i.e., post-correct, incompatible responses4. In other words, both children and older adults showed relatively undifferentiated executive processes on low- as well as high-demand trials, whereas only young adults appeared to respond differentially depending upon the level of cognitive demand. However, older adults did produce evidence (at least at a trend level) of increments in the upregulation of control (pre-RT) when two sources of conflict, relative to a single source, were combined.
Nonetheless, the relatively undifferentiated pattern of upregulating cognitive control and response-conflict detection in older adults may be interpretable within the recently formulated framework that distinguishes between proactive and reactive cognitive control (Braver, Gray, & Burgess, 2007; Paxton, Barch, Racine, & Braver, 2008; see also Velanova et al., 2007). The distinction refers to findings in situations characterized by increased cognitive demand that older adults do not appear to maintain a tonic, proactive task set (e.g., procedural rules for dealing with a given set of stimulus-response contingencies). Maintaining a proactive set would serve to prepare older adults for processing in a fast and efficient manner to inhibit and override prepotent responses (DePisapia & Braver, 2006). Moreover, a proactive strategy would be beneficial in a situation where the rules for responding are constant over an entire block of trials (as in the present study). Specifically, for compatible blocks, young adults appeared able to utilize a proactive-control strategy, which was maintained even following an error, as suggested by the lack of pre-RT and MFN differences between post-error and post-correct trials under the compatible-response condition (Figure 3, two top-left rows). Similarly, a proactive-control strategy appeared to be used successfully during incompatible-response blocks, as long as post-error conflict was not present. By contrast, in the incompatible-response condition, on post-error relative to post-correct trials, young adults showed more negative-going pre-RT activity onsetting well before the behavioral response. Because an error on the previous trial presumably triggers an increase in cognitive control on the next trial, neural activity associated with such upregulation could have begun quite early. In line with a decrease in accuracy, young adults also produced greater MFN magnitudes on these trials (Tables 3 and 4; Figure 3). These data suggest that, when incompatible responses were required, proactive control was not sufficient to overcome the heightened conflict associated with an error on the previous trial, and reactive control (in the form of negative-going pre-RT activity) became necessary.
Based on this reasoning, it would appear that both children and older adults engaged a more reactive-control strategy, even at relatively low levels of response conflict, as evinced by their more negative-going pre-RT activity on post-error compatible-response trials (children, older adults) and post-correct, incompatible-response trials (older adults). This type of reactive strategy may be less efficient than a proactive one (Paxton et al., 2008), as attested to here by the performance deficits observed in children and older adults. Moreover, these results are consistent with interpretations made in earlier studies (Friedman et al., 2008; Lustig et al., 2006; Milham et al., 2002; Reuter-Lorenz & Mikels, 2006; Velanova et al., 2007) that older, relative to young adults, appear to recruit executive processes at lower levels of cognitive demand (i.e., in an undifferentiated manner). Older adults experience detrimental alterations in prefrontal neurocognitive mechanisms (West, 1996; Raz & Rodrigue, 2006). This may necessitate the recruitment of control processes even at low levels of cognitive demand in these participants (Friedman et al., 2008; Reuter-Lorenz & Mikels, 2006).
By contrast with older adults, the prefrontal networks of 9-10 year-old children are most likely still developing (Bunge & Zelazo, 2006; Dempster, 1992; Rubia et al., 2006). Based on the current results, what appears to be maturing is the ability to selectively recruit and use efficiently putative prefrontal control processes under post-error conflict conditions (see also Bunge et al., 2002). Committing an error on the previous trial compared to response-override conditions might create a situation that engenders greater response conflict. Therefore, compared to young adults, 9-10 year-old children might need to engage greater amounts of cognitive control when post-error processing provides the only source of conflict. Although there are no previous ERP or fMRI studies that speak to this point, a recent behavioral investigation appears to support the undifferentiated recruitment of executive processes in children observed here (Davidson, Amso, Anderson, & Diamond, 2006). The authors used a task-switching paradigm in which low-demand pure blocks of the same task were compared with high-demand mixed blocks, in which participants had to switch between two tasks. They observed that the RT increment and accuracy decrement on a more difficult task (e.g., press right when the stimulus is on the left) relative to an easier task (e.g., press right when the stimulus is on the right) in pure blocks was large and reliable in children as old as 13 years of age but not in young adults. In young adults, this difference was only found in mixed blocks of trials, for which cognitive demand was at the highest level. Here again, while young adults appeared to titrate their control in line with cognitive demand, children did not appear to do so.
A second aim of the current study was to assess developmental and age-related alterations in the error-monitoring system as reflected by ERN amplitude. Inconsistent with prior research, the error-monitoring system appeared to be relatively mature in the children and maintained in older adults (Davies et al., 2004; Falkenstein et al., 2001; Ladouceur et al., 2007; Mathalon et al., 2003; Santesso et al., 2006; West, 2004). In accord with our results, Eppinger, Kray, Mock, & Mecklinger (2008) have very recently reported the absence of age-related changes in ERN amplitude when performance differences between young and older adults have been controlled. Interestingly, Eppinger and colleagues also measured ERN activity using a pre-stimulus baseline highly similar to that used here. On the other hand, Eppinger et al. (2008) were the first to observe age-equivalent ERN magnitudes among many previous reports of age inequality in ERN magnitude. Additionally, the visual impressions gleaned from current Figure 1 suggest ERN reductions (though not statistically significant), relative to young adults, in both children and older adults. Thus, it is perhaps premature, at this time, to attempt to assign functional significance to this infrequently-reported result.
Nonetheless, according to one theoretical stance, error monitoring and detection are specific instances of the operation of a general conflict-monitoring system (Botvinick et al., 2001; Botvinick et al., 2004). To the extent that this is true, ERN amplitude might be modulated by variables, such as response compatibility, analogously to the MFN. Indeed, for older adults only, both the ERN on post-correct, erroneous trials and the MFN on post-correct, correct trials were more negative-going when incompatible- compared to compatible-responses were required (Figure 1). This finding confirms the results of earlier investigations (Bartholow et al., 2005; Nessler, Friedman et al., 2007) suggesting that, in addition to error processing, the ERN may also be influenced by response conflict.
To conclude, the current study is unique in examining the upregulation of cognitive control and response conflict detection under response-override and post-error conditions in children, young and older adults. For children, the executive processes that upregulate control and detect conflict following an error do not yet appear to have reached young-adult levels. For older adults, although the deficits in cognitive-control processes observed on post-error trials were less marked than those for children, they also showed deficiencies in managing incompatible-response conflict. Older adults and children appeared to revert to reactive-control strategies even under these presumably low-conflict conditions, while young adults appeared to utilize a more efficient proactive-control strategy. On these bases, the efficiency with which cognitive control is applied and conflict detected under the highest-demand conditions are still developing in 9-10 year-old children and may be altered in older adults.
ACKNOWLEDGMENTS
The research reported in this paper was supported by grants from the NIA (AG05213) and NICHD (HD14959), and by the New York State Department of Mental Hygiene. We are grateful to Mr. Charles L. Brown, III for computer programming and technical assistance, and Ms. Efrat Schori, Ms. Rebecca Edelblum and Ms. Brenda Malcolm for their assistance in the recruiting and screening of participants. We also acknowledge the helpful discussions of these data with Drs. Ray Johnson, Jr., Marianne de Chastelaine, and Daniela Czernochowski. We also thank all volunteers for participating in this experiment.
Footnotes
Two flankers were presented to the left and two to the right of the central arrow, which participants were instructed to ignore. The flankers were congruent (i.e., pointed in same direction), incongruent (i.e., pointed in the opposite direction -- a representation of the incorrect response) or neutral (diamond shapes) with respect to the direction of the central arrow. In each block, the probability of each type of flanker was 33 percent. Although we included Flanker Type in formal analyses of the ERP data, this variable did not produce developmental or age-related effects and did not influence the magnitude of the ERP components under investigation. Therefore, for clarity of presentation, we focus only on Response-Compatibility effects associated with post-correct and post-error trials.
Post-correct trials were used because there were too few errors following an erroneous response. The mean number (±SD) of trials entering the averages for compatible-correct, compatible-incorrect, incompatible-correct, and incompatible-incorrect were, respectively, for young adults, 263 (±46), 37 (±20), 229 (±52), 44 (±22); for older adults, 288 (±34), 25 (±12), 263 (±56), 25 (±16); for the children, 226 (±51), 37 (±18), 197 (±44), 41 (±14).
The mean number (±SD) of trials entering the 4 post-correct and post-error averages (post-correct compatible, post-correct incompatible, post-error compatible, post-error incompatible) were, respectively, for the young: 264 (±46.0), 229.3 (±52.0), 36.0 (±19.8), 44.0 (±22.0); for the older adults these values were: 289.6 (±35.0), 260.4 (±53.0), 25.1 (±13.0), 27.0 (±15.7); for the children these values were: 220.9 (±48.0), 195.9 (±45.6), 39.8 (±16.6), 41.8 (±13.4).
It is important to note that the age differences occurring prior to RT would not have been observable had a pre-RT rather than a pre-stimulus baseline been used.
REFERENCES
- Bartholow BD, Pearson MA, Dickter CL, Sher KJ, Fabiani M, Gratton G. Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology. 2005;42(1):33–42. doi: 10.1111/j.1469-8986.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Science. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In: Comway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation in Working Memory. Oxford University Press; New York: 2007. pp. 76–106. [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Zelazo PD. A Brain-Based Account of the Development of Rule Use in Childhood. Current Directions in Psychological Science. 2006;15:118–121. [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44(11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPs in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25(3):355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Developmental Review. 1992;12(1):45–75. [Google Scholar]
- DePisapia N, Braver TS. A model of dual control mechanisms through anterior cingulate and prefrontal cortex interactions. Neurocomputing. 2006;69:1322–1326. [Google Scholar]
- Eppinger B, Kray J, Mock B, Mecklinger A. Better or worse than expected? Aging, learning, and the ERN. Neuropsychologia. 2008;46(2):521–539. doi: 10.1016/j.neuropsychologia.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78(6):447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Changes of error-related ERPs with age. Experimental Brain Research. 2001;138(2):258–262. doi: 10.1007/s002210100712. [DOI] [PubMed] [Google Scholar]
- Fiehler K, Ullsperger M, von Cramon DY. Electrophysiological correlates of error correction. Psychophysiology. 2005;42(1):72–82. doi: 10.1111/j.1469-8986.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Friedman D, Nessler D, Johnson R, Jr., Ritter W, Bersick M. Age-related changes in executive function: an event-related potential (ERP) investigation of task-switching. Aging, Neuropsychology and Cognition. 2008;15(1):95–128. doi: 10.1080/13825580701533769. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Fencsik DE. Functions of the medial frontal cortex in the processing of conflict and errors. Journal of Neuroscience. 2001;21(23):9430–9437. doi: 10.1523/JNEUROSCI.21-23-09430.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Willoughby. AR. The Medial Frontal Cortex and the Rapid Processing of Monetary Gains and Losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gurland B, Golden RR, Teresi JA, Challop J. The SHORT CARE: An efficient instrument for the assessment of depression, dementia and disability. Journal of Gerontology. 1984;39:166–169. doi: 10.1093/geronj/39.2.166. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003;40(6):895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hogan AM, Vargha-Khadem F, Kirkham FJ, Baldeweg T. Maturation of action monitoring from adolescence to adulthood: an ERP study. Developmental Science. 2005;8(6):525–534. doi: 10.1111/j.1467-7687.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Developmental anatomy of prefrontal cortex. In: Krasnegor NA, Lyon GR, Goldman-Rakic PS, editors. Development of the Prefrontal cortex: Evolution, Neurobiology, and Behavior. Paul H. Brookes Publishing Company; Baltimore: 1997. pp. 69–83. [Google Scholar]
- Jennings JR, Wood CC. The ε-adjustment procedure for repeated measures analyses of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Johnson R, Jr., Barnhardt J, Zhu J. The deceptive response: effects of response conflict and strategic monitoring on the late positive component and episodic memory-related brain activity. Biol Psychol. 2003;64(3):217–253. doi: 10.1016/j.biopsycho.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Johnson R, Jr., Barnhardt J, Zhu J. The contribution of executive processes to deceptive responding. Neuropsychologia. 2004;42(7):878–901. doi: 10.1016/j.neuropsychologia.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Johnson R, Jr., Henkell H, Simon E, Zhu J. The self in conflict: the role of executive processes during truthful and deceptive responses about attitudes. Neuroimage. 2008;39(1):469–482. doi: 10.1016/j.neuroimage.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, III, M. AW, Cho RY, Stenger VA, Carter CS. Anterior Cingulate Conflict Monitoring and Adjustments in Control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Carter CS. Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev Sci. 2007;10(6):874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Lustig C, Head D, Janes W, Buckner RL. Frontal-parietal responses to increased demand across the lifespan: Evidence from task switching; Paper presented at the Cognitive Aging Conference.2006. [Google Scholar]
- Masaki H, Falkenstein M, Sturmer B, Pinkpank T, Sommer W. Does the error negativity reflect response conflict strength? Evidence from a Simon task. Psychophysiology. 2007;44(4):579–585. doi: 10.1111/j.1469-8986.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Bennett A, Askari N, Gray M, Rosenbloom MJ, Ford JM. Response-monitoring dysfunction in aging and Alzheimer's disease: an event-related potential study. Neurobiology of Aging. 2003;24:675–685. doi: 10.1016/s0197-4580(02)00154-9. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Stern Y, Rosen J, Leventhal J. Depression, intellectual impairment, and Parkinson disease. Neurology. 1981;31(6):645–650. doi: 10.1212/wnl.31.6.645. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, et al. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain and Cognition. 2002;49(3):277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Morris SE, Yee CM, Nuechterlein KH. Electrophysiological analysis of error monitoring in schizophrenia. Journal of Abnormal Psychology. 2006;115(2):239–250. doi: 10.1037/0021-843X.115.2.239. [DOI] [PubMed] [Google Scholar]
- Nessler D, Friedman D, Johnson R, J., Bersick M. ERPs suggest that age affects cognitive control but not response conflict detection. Neurobiology of Aging. 2007;28(11):1769–1782. doi: 10.1016/j.neurobiolaging.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Nessler D, Johnson R, J., Bersick M, Friedman D. The temporal trajectory of age-related decreases in executive function: ERP and behavioral evidence. Journal of Cognitive Neuroscience. 2007;19(Supplement):235. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex. 2008;18(5):1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM. Errors and error correction in choice-response tasks. JournaL of Experimental Psychology. 1966;71(2):264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition - II. Vol. 2. Erlbaum; Marwah, NJ: 2000. pp. 1–90. [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Mikels JA. The aging mind and brain: Implications of enduring plasticity for behavioral and cultural change. In: Baltes PB, Reuter-Lorenz PA, Rossler F, editors. Lifespan Development and Brain: The Perspective of Biocultural Co-constructivism. Cambridge University Press; Cambridge: 2006. pp. 255–276. [Google Scholar]
- Ridderinkhof KR, van der Molen MW, Band GP, Bashore TR. Sources of interference from irrelevant information: a developmental study. Journal of Experimental Child Psychology. 1997;65(3):315–341. doi: 10.1006/jecp.1997.2367. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses in 10-year-old children and young adults. Developmental Science. 2006;9(5):473–481. doi: 10.1111/j.1467-7687.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Scheffers MK, Humphrey DG, Stanny RR, Kramer AF, Coles MG. Error-related processing during a period of extended wakefulness. Psychophysiology. 1999;36(2):149–157. [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: an electrophysiological strategy. Brain and Cognition. 2004;55(1):116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sharbrough F, Chatrian GE, Lsser RP, Luders H, Nuwer M, Picton TW. Guidelines for Standard Electrode Position Nomenclature. American EEG Society; Bloomfield: 1990. [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13(2):160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Ullsperger M. Performance monitoring in neurological and psychiatric patients. International Journal of Psychophysiology. 2006;59(1):59–69. doi: 10.1016/j.ijpsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- van Veen C, Carter CS. Conflict and Cognitive Control in the Brain. Current Directions in Psychological Science. 2006;15:237–240. [Google Scholar]
- Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cerebral Cortex. 2007;17(5):1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- Watt NF. Two factor index of social position: Amherst modification (An adaptation of Hollingshead and Redlich, 1958) 1976 Unpublished manuscript. [Google Scholar]
- Wechsler D. The Wechsler Intelligence Scale for Children - Third Edition. Harcourt - The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale Third Edition. Harcourt; New York: 1997. [Google Scholar]
- West R. The effects of aging on controlled attention and conflict processing in the Stroop task. Journal of Cognitive Neuroscience. 2004;16(1):103–113. doi: 10.1162/089892904322755593. [DOI] [PubMed] [Google Scholar]
- West R. An application of prefrontal cortex theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]


