Abstract
Nonsurgical treatment options, such as hormonal therapy, chemotherapy, radiation, and bisphosphonate therapy, are undoubtedly improving outcomes for women with breast cancer; however, these therapies also carry significant skeletal side effects. For example, adjuvant hormonal treatments, such as aromatase inhibitors that disrupt the estrogen-skeleton axis, have the potential to cause decreased bone mineral density. Similarly, chemotherapy often induces primary ovarian failure in premenopausal women, resulting in decreased levels of circulating estrogen and subsequent osteopenia. In both cases, women receiving these therapies are at an increased risk for the development of osteoporosis and skeletal fracture. Furthermore, women undergoing radiation therapy to the upper body may have an increased incidence of rib fracture, and those receiving bisphosphonates maybe vulnerable to the development of osteonecrosis of the jaw. Therefore, women with breast cancer who are undergoing any of these therapies should be closely monitored for bone mineral loss and advised of skeletal health maintenance strategies.
Hormonal Therapy
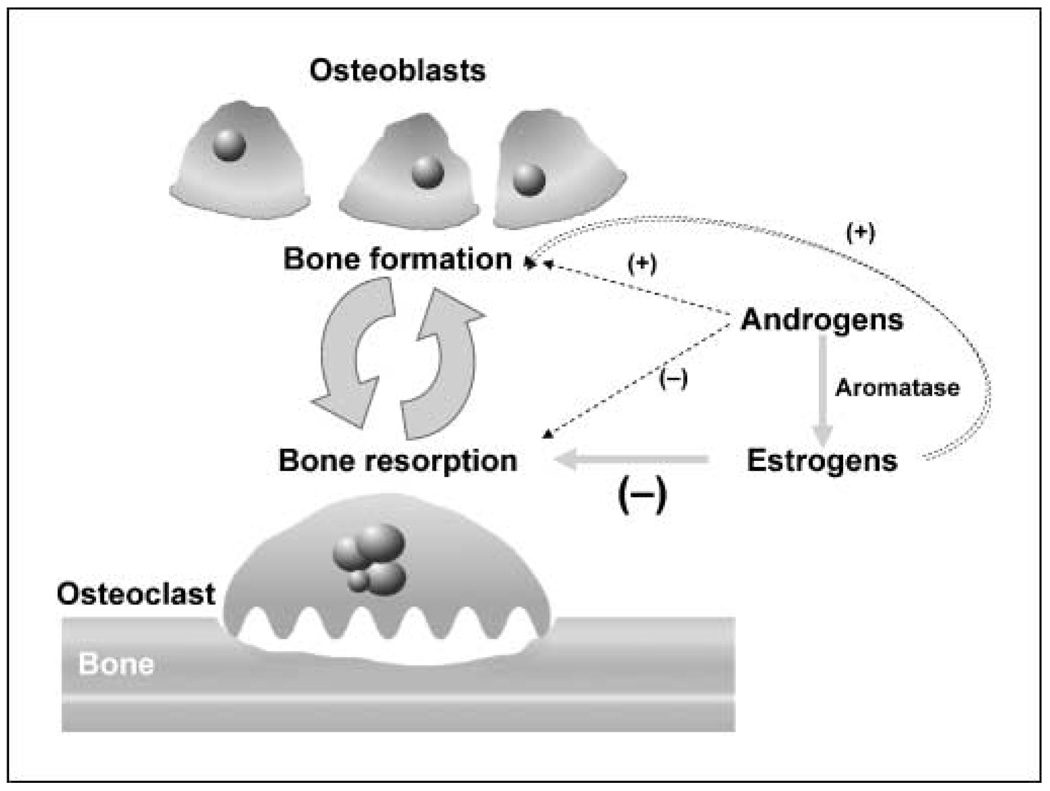
In adults, the skeleton undergoes complete turnover every 10 years. Bone mass maintenance is a balance between the activity of osteoblasts, which form bone, and osteoclasts, which resorb it. Estrogen plays a key regulatory role in this cycle of bone remodeling by mediating effects through the estrogen receptor (ER)present on several cell types in the bone. Estrogen stimulates osteoblasts to produce osteoprotegerin, a decoy receptor for the receptor of activated nuclear factor-κB (1). Osteoprotegerin blocks the binding of receptor of activated nuclear factor-κB ligand to receptor of activated nuclear factor-κB on osteoclasts, leading to impaired osteoclast activity and decreased bone resorption. Additionally, estrogen is believed to directly induce apoptosis of bone-resorbing osteoclasts (2, 3). Thus, in premenopausal women, estrogen both inhibits bone remodeling and suppresses bone resorption, contributing to bone strength (Fig. 1). As estrogen levels decline in postmenopausal women, this regulation diminishes and bone resorption increases out of proportion to bone formation, leading to a net loss in bone and weakened bony microarchitecture. Despite the persistence of low levels of circulating estrogen in the postmenopausal state (produced by the conversion of peripheral tissue androgens to estrogen by the aromatase enzyme), bone mass can decrease by as much as 3% yearly in the first 5 years after menopause (4).
Fig. 1.
Contribution of estrogens and androgens to bone remodeling. Estrogen and androgens help to maintain a balance between bone formation and bone resorption. Estrogen inhibits osteoclast activity and contributes to osteoclast apoptosis; androgens are converted to estrogen by aromatization and may also directly affect osteoblast differentiation. As such, a decrease in estrogen and/or androgens leads to increased bone resorption and an imbalance in bone remodeling, which ultimately manifests as bone loss (1–3, 73, 74). Adapted from Skeletal Complications Across the Cancer Continuum CME Lecture 2005 series with permission from the Postgraduate Institute for Medicine.
The ER is expressed by 70% of breast tumors (5), and circulating estrogen can promote the growth of ER-positive tumors. Current breast cancer therapies exploit this relationship either by decreasing circulating estrogen levels or by blocking or down-regulating the receptor itself. Although some of the estrogen-mimicking agents seem to be bone sparing, others that disrupt the estrogen-skeleton axis cause adverse effects on bone remodeling, leading to decreased bone mineral density (BMD)and an increased risk of osteoporosis and fracture.
Selective ER modulators
Tamoxifen is a selective ER modulator that binds to the ER and acts as an estrogen antagonist in breast tissue. Tamoxifen is routinely used as adjuvant therapy in patients with ER-positive breast cancers and preventive therapy in high-risk patients because it has been shown to decrease the risk of breast cancer (6, 7). In bone, tamoxifen has both positive and negative effects depending on the menopausal state; premenopausal women taking tamoxifen may experience bone loss, whereas the drug seems to have agonistic effects in postmenopausal women (8, 9).
Two placebo-controlled trials in postmenopausal women with breast cancer showed statistically significant increases in BMD in the groups receiving tamoxifen versus placebo. In a double-blind placebo-controlled trial, which included 140 postmenopausal women with axillary node-negative breast cancer, Love et al. (10)showed that tamoxifen treatment resulted in a 0.61% increase in lumbar spine BMD compared with a 1% decrease in lumbar spine BMD for placebo-treated women (P < 0.001). In a similar study of postmenopausal women with low-risk breast cancer, Kristensen et al. (11) showed an ~2% increase in BMD in the tamoxifen-treated group compared with a 5% decrease in BMD in the placebo-treated group (P = 0.00074).
The National Surgical Adjuvant Breast and Bowel Project P-1 study showed a 21% decrease in fracture risk in patients ages >50 years taking tamoxifen versus placebo for primary prevention of breast cancer, but this was not found to be statistically significant (hazard ratio, 0.79; 95% confidence interval, 0.60–1.05; ref. 6). The International Breast Cancer Intervention Study 1, a randomized breast cancer prevention trial, including both premenopausal and postmenopausal women, showed no difference in fracture incidence in the tamoxifen group versus placebo (7).
Several studies have suggested that another selective ER modulator, the fixed-ring benzothiophene derivative raloxifene, which was approved for the prevention of osteoporosis in postmenopausal women in 1997, may reduce the risk of breast cancer in postmenopausal women (12–19). The recently reported results of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene P-2 trial, a prospective, double-blind, randomized clinical trial comparing the incidence of breast cancer and other outcomes in postmenopausal women with increased breast cancer risk, showed an equivalent reduction in the risk of invasive breast cancer in women receiving either tamoxifen or raloxifene and a nonstatistically significant increase in the risk of noninvasive breast cancer in women receiving raloxifene. In terms of skeletal outcome, no difference in fracture incidence or type was observed between the two groups, although this trial did not include comparison with placebo (20). In sum, selective ER modulators do not seem to contribute to skeletal complications in postmenopausal women with or at risk of developing breast cancer.
Aromatase inhibitors
Postmenopausal women maintain a low level of circulating estrogen due to the aromatization of androgens to estrogen in tissues, such as fat and muscle, by the cytochrome P450 aromatase enzyme. Inhibitors of this enzyme are now commonly used for adjuvant endocrine therapy in postmenopausal women with breast cancer. There are two major classes of aromatase inhibitors: the nonsteroidal reversible inhibitors, such as anastrozole and letrozole, and the steroidal irreversible inhibitors, such as exemestane (21). Randomized clinical trials evaluating each of these aromatase inhibitors in the adjuvant therapy of breast cancer have shown decreased cancer recurrences and improved disease-free survival in women who received aromatase inhibitors compared with tamoxifen, although no differences in overall survival have been reported to date (22–25). Consequently, aromatase inhibitors are commonly administered to postmenopausal women with ER-positive breast cancer.
Animal studies suggest that although the steroidal inhibitor exemestane may have bone-sparing effects in ovariectomized rats, the nonsteroidal inhibitor letrozole does not. In two separate studies, Goss et al. showed that exemestane treatment prevented the bone loss that normally occurs in animals after ovariectomy, yet this effect was not observed after letrozole treatment (26, 27). Exemestane may mediate its protective effect through androgenic effects. Both exemestane and its metabolite, 17-hydroxyexemestane, are proposed to have androgenic properties (27), and androgens have been previously shown to be important for maintenance of BMD independent of their conversion to estrogen (28).
In contrast, clinical trials have indicated that both classes of aromatase inhibitors result in bone loss to some extent (Fig. 2). A recent double-blind trial by Lonning et al. (29) compared the effects of exemestane versus placebo on BMD in 147 postmenopausal women after surgical resection of early breast cancer. They observed a slight increase in the annual rate of femoral neck BMD loss in the exemestane group (2.72% versus 1.48%; P = 0.024), although there was not a significant increase in BMD loss in the lumbar spine for the exemestane group. A large (n = 5187), randomized, placebo-controlled phase III trial evaluated the nonsteroidal inhibitor letrozole in postmenopausal women with primary breast cancer who had completed 5 years of adjuvant tamoxifen therapy (24). Compared with placebo, patients receiving letrozole (2.5 mg) experienced more cases of patient-reported osteoporosis than women receiving placebo (8% versus 6%; P = 0.003). Additionally, recent updates in the Arimidex, Tamoxifen, Alone or in Combination trial indicate that there is a statistically significant increase in fracture rate for women taking the nonsteroidal inhibitor anastrozole compared with tamoxifen (22.6 fractures per woman-year compared with 15.6; P < 0.0001; ref. 23). At this time, no trials have directly compared the extent of bone loss in women taking steroidal versus nonsteroidal aromatase inhibitors.
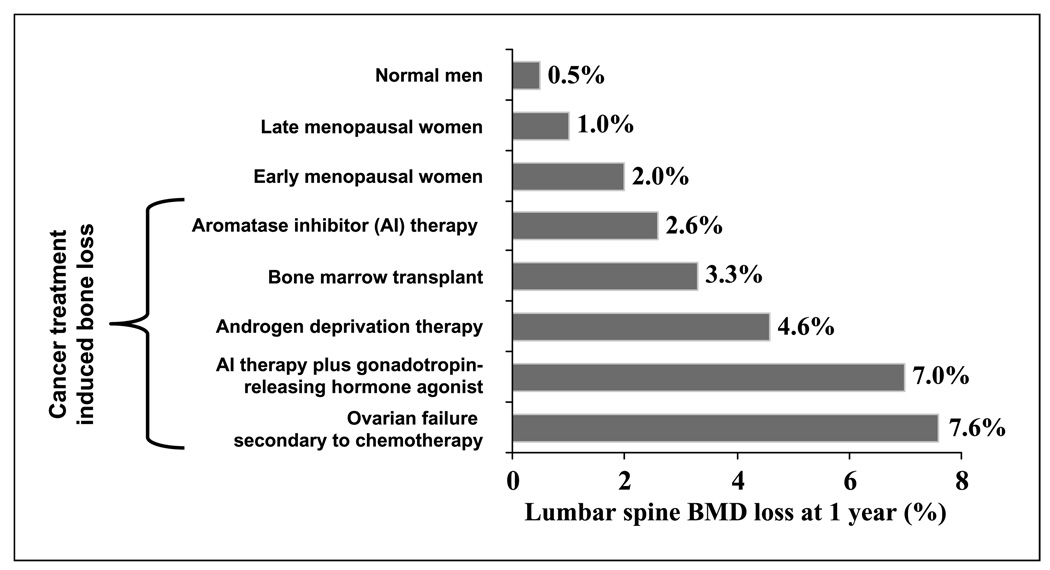
Fig. 2.
Extent of bone loss due to cancer therapy. Menopausal women lose bone at a rate of1% to 2% yearly. Cancer treatments, such as aromatase inhibitor therapy and chemotherapy, accelerate this process, leading to significant bone loss and subsequent skeletal complications (39, 75–79). Adapted from Skeletal Complications Across the Cancer Continuum CME Lecture 2005 series with permission from the Postgraduate Institute for Medicine.
In summary, clinical trials indicate that aromatase inhibitor treatment results in significant bone loss in postmenopausal women with breast cancer. As such, these women should be monitored carefully for changes in BMD and treated appropriately. Several multicenter randomized clinical trials evaluating the role of bone-targeted antiresorptive therapies to prevent bone loss associated with aromatase inhibitors are under way.
Selective ER down-regulators
Recently, a new class of endocrine agents, the selective ER down-regulators, has been introduced. Selective ER down-regulators down-regulate cellular levels of the ER and act as pure ER antagonists without any agonist effects. Selective ER down-regulators represent a potential treatment option for patients unable to tolerate the effects of selective ER modulators or who have tamoxifenresistant or aromatase inhibitor–resistant disease. Fulvestrant is currently the only selective ER down-regulator used in the clinics and is approved for second-line treatment of advanced breast cancer in postmenopausal women. The effect of fulvestrant on bone is controversial. In animal studies, fulvestrant was shown to increase bone turnover; however, the opposite results were obtained when the rats were ovariectomized (30–32). Currently, BMD is not an end point in clinical trials testing the efficacy of fulvestrant.
Chemotherapy
Chemotherapy is used as either neoadjuvant or adjuvant therapy in premenopausal and postmenopausal women diagnosed with breast cancer. Chemotherapy can have both direct and indirect effects on the bone microenvironment, ultimately leading to decreased BMD (33).
Direct effects
Animal studies by Wheeler et al. (34)showed that male rats treated with methotrexate had decreased cancellous bone volume and decreased mineralizing surface compared with saline-injected controls. Additionally, cortical cross-sectional area and periosteal mineralization rates were lower in the methotrexate group. Another study by Greep et al. (35) examined the effects of chemotherapy on BMD in postmenopausal women with early-stage breast cancer. The for-age bone density scores of postmenopausal women who received adjuvant chemotherapy were ~0.5 SD lower than women who had not received chemotherapy. These studies suggest that chemotherapy can have direct, nonhormonal effects on the skeleton.
Indirect effects
The incidence of primary ovarian failure in women receiving breast cancer chemotherapy ranges from 20% to 90% depending on age and treatment regimen (36–38). Primary ovarian failure leads to a sudden decrease in estrogen production and early menopause (33, 39). This decrease in estrogen is believed to permit increased osteoclast survival and activity (2, 3, 40). Consequently, these women often develop osteopenia and are placed at an increased risk for developing osteoporosis (Fig. 2; refs. 41, 42).
Several studies have shown a correlation between adjuvant chemotherapy and decreased BMD in women with breast cancer. One study by Bruning et al. (43) examined BMD of the lumbar spine in premenopausal women with breast cancer who received adjuvant chemotherapy compared with women with breast cancer who did not receive chemotherapy. They showed that 71% of the women who received chemotherapy experienced amenorrhea (loss of menses) at the time of BMD measurements compared with 16% of the women who did not receive chemotherapy. In addition, the BMD measurements of the amenorrheic women in the chemotherapy group were significantly lower than the premenopausal women in the nonchemotherapy group (1.17 g/cm2 compared with 1.29 g/cm2; P = 0.036). Headley et al. (44) showed that women who became permanently amenorrheic as a result of chemotherapy had a BMD 14% lower than women who maintained menses after chemotherapy (P = 0.004). These studies suggest that chemotherapy leads to an increased risk of early menopause and, subsequently, an increased risk of bone loss.
Shapiro et al. (39) showed that chemotherapy-induced ovarian failure causes significant bone loss in the spine. This study examined 49 premenopausal women with stage I/II breast cancer receiving adjuvant chemotherapy. Dual-energy X-ray absorptiometry scans and measurement of markers of skeletal turnover (i.e., osteocalcin and bone-specific alkaline phosphatase) were used to assess bone loss at baseline and 6 and 12 months after initiation of chemotherapy. Thirty-five of these women were found to have ovarian failure, defined as a negative pregnancy test result, >3 months of amenorrhea, and follicle-stimulating hormone levels >30 mIU/mL at the 12-month evaluation. Significant bone loss was observed by 6 months after initiation of chemotherapy in the amenorrheic women. These women had an ~4% decrease in BMD in the spine compared with the women who retained ovarian function (P = 0.0001). This bone loss continued at the 12-month interval.
Radiation-Induced Fractures
Breast conservation surgery combined with radiotherapy has become the standard of care for patients with early-stage breast cancer. One potential complication of this treatment is rib fracture following X-ray exposure, although few studies have investigated this phenomenon. A retrospective study by Pierce et al. (45) examined the incidence of various radiation-induced complications in 1,624 patients with early-stage breast cancer treated between 1968 and 1985. The median follow-up time for survivors was 79 months. They found that the incidence of rib fracture was between 0.4% and 2.2% depending on the type of linear accelerator used. Another retrospective study by Meric et al. (46) examined the incidence of radiation-induced complications in 294 women receiving surgery and radiotherapy treatment between 1990 and 1992. They found the risk of rib fractures to be 0.3%. These data suggest that radiotherapy for breast cancer may lead to a small risk of rib fracture.
Bisphosphonates
Bisphosphonates are osteoclast inhibitors that are now widely used in cancer therapy to inhibit bone loss resulting from treatment or bone metastases. There are three classes of bisphosphonates: (a) first-generation compounds, such as clodronate; (b) second-generation compounds, which are stronger and contain a single nitrogen atom, such as pamidronate; and (c)third-generation compounds, such as zoledronic acid, which contain one or two nitrogen atoms in a ring form and are the most potent (47). The first-generation bisphosphonates are metabolized into cytotoxic analogues of ATP, inducing osteoclast cell death. The nitrogen-containing bisphosphonates, on the other hand, function by inhibiting the activity of farnesyl diphosphate and geranylgeranyl diphosphate. Because farnesyl diphosphate and geranylgeranyl diphosphate are required for post-translational lipid modification (prenylation) of small GTPases, bisphosphonates interfere with the function of GTPases, such as Ras, Rac, and Rho. This leads to disruption of the actin cytoskeleton, altered tracking of intracellular components, and impaired integrin signaling within the osteoclast (47). Second- and third-generation bisphosphonates do not have an effect on the osteoblast in vivo; thus, the bone formation is intact (47). In addition, in vitro evidence suggests that bisphosphonates may have antiangiogenic and antitumor properties, but these data have not been confirmed in vivo (48–50).
Maintenance of BMD following cancer therapy
Several groups have shown that bisphosphonates are able to reduce bone loss associated with breast cancer chemotherapy. A study by Delmas et al. (51) examined the effects of risedronate on BMD in 53 women who were postmenopausal due to chemotherapy or radiotherapy after breast cancer surgery. The annual rate of change in lumbar BMD in the risedronate group was 0.3 ± 0.5% compared with −1.4 ± 0.5% in the placebo group (P = 0.018). Powles et al. (52)conducted a large, double-blind, randomized, two-center trial to examine BMD in 311 women with primary breast cancer who had received chemotherapy and/or tamoxifen and who were given clodronate or placebo for 2 years. They showed that the change in BMD for the lumbar spine was −0.16% at 2 years for the clodronate group compared with −1.88% for the placebo group (P = 0.04).
A study by Vehmanen et al. (53) examined bone loss in 73 premenopausal women receiving the cyclophosphamide, methotrexate, and 5-fluorouracil regimen. The patients were randomized to receive or not to receive oral clodronate daily for 3 years. This study showed that women who lost menstrual function, indicative of ovarian failure, had increased bone loss compared with women who maintained menstrual function. Further, all women in the clodronate group lost less lumbar BMD than the women in the control group (−3% compared with −7.4%; P = 0.003)at 3 years.
Osteonecrosis of the jaw
Although bisphosphonates seem to beneficially reduce bone loss associated with chemotherapy, use of these agents has also been associated with the development of a potentially harmful side effect. In the past several years, the first cases of osteonecrosis of the jaw (ONJ) have been reported in patients receiving long-term oral and i.v. bisphosphonate therapy for osteoporosis or bone metastases (54, 55). ONJ is an extremely painful condition that presents with exposure of the mandibular or maxillary bones and results in vulnerability to bone and tooth loss and oral infection (56, 57). In addition to long-term bisphosphonate therapy, ONJ has also been associated with oral fungal infections, trauma, herpes zoster, and radiation therapy (58–66). In one study of 211 myeloma patients receiving zoledronic acid, 10% developed ONJ by 36 months, whereas 4% of 413 myeloma patients receiving pamidronate developed the disease by 36 months (67).
Inhibition of the osteoclast by bisphosphonates is hypothesized to disrupt the critical balance between the osteoclast and the osteoblast. In a situation where healing of the bone is necessary, such as after chronic inflammation and infection associated with gum disease, the disruption of the dynamic and coupled processes of bone resorption and formation may contribute to the development of ONJ. The antiangiogenic effects of the bisphosphonates are also hypothesized to contribute to the process of necrosis (68). This complication is thought to become more likely if the patient is undergoing any manipulations in the oral cavity, such as tooth extractions and placement of oral implants (69).
Meticulous oral hygiene, antibiotics, and the discontinuation of bisphosphonate therapy are currently recommended for therapy of ONJ. The diagnosis of ONJ is a clinical diagnosis made by physical examination. Biopsy of the affected bone can be associated with worsening of the situation. More studies must be initiated to determine the exact mechanisms and cause of this complication and how it can be prevented and treated.
Conclusion
Chemotherapy and hormonal therapies for breast cancer have the potential to lead to significant bone loss primarily through the disruption of the bone-enhancing properties of estrogen. Current recommendations for avoiding the skeletal complications of cancer therapy include adequate intake of calcium and vitamin D, regular weight-bearing exercise, cessation of smoking, reduction in alcohol intake, and bisphosphonate therapy for osteoporotic patients (36, 70). Patients who are being treated with hormonal therapies are at increased risk of skeletal complications and should have regular BMD monitoring by dual-energy X-ray absorptiometry. The role of antiresorptive, osteoclast inhibitor therapy to prevent cancer therapy-associated bone loss is under active investigation. It is recommended that patients receive a thorough oral examination and treatment for dental infections before initiating bisphosphonate therapy (56, 57, 67, 71, 72). In addition to treating the disease, careful monitoring of bone health is now an essential component of the treatment of breast cancer.
Open Discussion
Dr. Guise: Are there differences in the different aromatase inhibitors on bone? There has been some talk that exemestane may have some androgenic effects, but the clinical data don’t seem to support that.
Dr. Weilbaecher: There were some rat data showing that exemestane could have some testosterone-like effects that would be more bone forming. However, based on the fracture data from the exemestane clinical trials, it looked like there was more fracture and more bone loss.
Dr. Coleman: We have some biochemical data that show that profound estradiol suppression is overwhelming any androgenic effect. The change in resorption markers is essentially identical to anastrozole. The only difference is that there is a slightly greater increase in bone formation markers than you would expect through the coupling process. It may be that at the end of 5 years of exemestane you’ve lost 6% of bone and with anastrazole you’ve lost 8%, but basically there is not a lot of difference between them.
Dr. Bruland: Are you aware of any data supporting the possibility that these subclinical metastases in bone marrow act as a nidus for subsequent visceral metastases?
Dr. Weilbaecher: I don’t know of any clinical data, but I wonder if we could answer that with animal models.
Dr. Suva: How far should you be looking to suppress resorption in these patients, and then is there any avenue for an anabolic regimen for the skeleton? Is there a way to separate the effects on the tumors from the effects on the skeleton? Can you rescue the skeleton after you have eradicated the tumor?
Dr. Guise: I don’t know the answer to that. The data with aromatase inhibitor clearly suggest that by just going from a postmenopausal level of estradiol to 0 is enough to have clinical effects on bone. Do we need to suppress that level of bone resorption? The clinical data suggest yes. The effects of anabolic agents in breast cancer are going to be controversial, because unlike myeloma, where the osteoblast is suppressed, I don’t believe it is in breast cancer. To use PTH in breast cancer patients is relatively contraindicated because of the data in rats that PTH causes osteosarcoma and a lot of these women get radiation to the breast. Therefore, any type of radiation is a contraindication to teriparatide, because it predisposes patients to osteosarcoma as well. There are real issues about using anabolic agents, specifically PTH, in breast cancer patients and maybe even in prostate cancer patients.
Dr. Powles: With an aromatase inhibitor, if it is powerful enough, could you reach the point where you couldn’t up-regulate the estrogen receptor to preserve bone? The evidence is that when you’ve got an aromatase inhibitor as well, the bone loss is getting less with years.
Dr. Coleman: We don’t have any 5-year data, so we don’t know that. The only evidence that we’ve got is that if you look at the fracture rates, they increase very quickly. Within the first 6 months the fracture rates had gone up by about 40% at a time when you’ve only lost 1% of your bone mineral density, so there’s something about the resorption state that is driving the fracture rate. Then, when you get to the end of 5 years, the fracture rates seem to come back together again. As soon as the drug has disappeared, it looks as though the resorption markers are settling and the fracture rates are coming back together again.
Dr. Suva: Is there cortical bone loss as well as trabecular bone loss? Do we know in which compartment bone loss is occurring in that scenario?
Dr. Coleman: We are also seeing changes at the hip.
Dr. Guise: We are looking at the effects of aromatase inhibitors on bone in mice, and we find that there’s not a big effect. We don’t see a marked effect on trabecular bone, but we do see some effect on cortical bone. In fact, in mice aromatase inhibitors reduce bone density even more than ovariectomy. We don’t know yet whether this is a rodent effect and whether it can be translated to humans.
Footnotes
Presented at the First Cambridge Conference on Advances in Treating Metastatic Bone Cancer, a symposium held in Cambridge, Massachusetts, October 28–29, 2005.
References
- 1.Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- 2.Hughes DE, Dai A, Tiffee JC, Li HH, Mundy GR, Boyce BF. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-β. Nat Med. 1996;2:1132–1136. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- 3.Kameda T, Mano H, Yuasa T, et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186:489–495. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riggs BL, Khosla S, Melton LJ., III A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 5.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 7.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 8.Cosman F. Selective estrogen-receptor modulators. Clin Geriatr Med. 2003;19:371–379. doi: 10.1016/s0749-0690(02)00114-3. [DOI] [PubMed] [Google Scholar]
- 9.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 10.Love RR, Mazess RB, Barden HS, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- 11.Kristensen B, Ejlertsen B, Dalgaard P, et al. Tamoxifen and bone metabolism in postmenopausal low-risk breast cancer patients: a randomized study. J Clin Oncol. 1994;12:992–997. doi: 10.1200/JCO.1994.12.5.992. [DOI] [PubMed] [Google Scholar]
- 12.Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometriumin post-menopausal women. N Engl J Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- 13.Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 14.Lippman ME, Krueger KA, Eckert S, et al. Indicators of lifetime estrogen exposure: effect on breast cancer incidence and interaction with raloxifene therapy in the multiple outcomes of raloxifene evaluation study participants. J Clin Oncol. 2001;19:3111–3116. doi: 10.1200/JCO.2001.19.12.3111. [DOI] [PubMed] [Google Scholar]
- 15.Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216–220. doi: 10.1001/jama.287.2.216. [DOI] [PubMed] [Google Scholar]
- 16.Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Multiple outcomes of raloxifene evaluation. Breast Cancer Res Treat. 2001;65:125–134. doi: 10.1023/a:1006478317173. [DOI] [PubMed] [Google Scholar]
- 17.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 18.Cauley JA, Lucas FL, Kuller LH, Vogt MT, Browner WS, Cummings SR Study of Osteoporotic Fractures Research Group. Bone mineral density and risk of breast cancer in older women: the study of osteoporotic fractures. JAMA. 1996;276:1404–1408. [PubMed] [Google Scholar]
- 19.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 20.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 21.Simpson ER, Dowsett M. Aromatase and its inhibitors: significance for breast cancer therapy. Recent Prog Horm Res. 2002;57:317–338. doi: 10.1210/rp.57.1.317. [DOI] [PubMed] [Google Scholar]
- 22.Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98:1802–1810. doi: 10.1002/cncr.11745. [DOI] [PubMed] [Google Scholar]
- 23.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 24.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 25.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 26.Goss PE, Qi S, Cheung AM, Hu H, Mendes M, Pritzker KP. Effects of the steroidal aromatase inhibitor exemestane and the nonsteroidal aromatase inhibitor letrozole on bone and lipid metabolism in ovariectomized rats. Clin Cancer Res. 2004;10:5717–5723. doi: 10.1158/1078-0432.CCR-04-0438. [DOI] [PubMed] [Google Scholar]
- 27.Goss PE, Qi S, Josse RG, et al. The steroidal aromatase inhibitor exemestane prevents bone loss in ovariectomized rats. Bone. 2004;34:384–392. doi: 10.1016/j.bone.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev. 2004;25:389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 29.Lonning PE, Geisler J, Krag LE, et al. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J Clin Oncol. 2005;23:5126–5137. doi: 10.1200/JCO.2005.07.097. [DOI] [PubMed] [Google Scholar]
- 30.Sibonga JD, Dobnig H, Harden RM, Turner RT. Effect of the high-affinity estrogen receptor ligand ICI 182,780 on the rat tibia. Endocrinology. 1998;139:3736–3742. doi: 10.1210/endo.139.9.6172. [DOI] [PubMed] [Google Scholar]
- 31.Turner RT, Evans GL, Dobnig H. The high-affinity estrogen receptor antagonist ICI 182,780 has no effect on bone growth in young male rats. Calcif Tissue Int. 2000;66:461–464. doi: 10.1007/s002230010092. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher A, Chambers TJ, Tobias JH. The estrogen antagonist ICI 182,780 reduces cancellous bone volume in female rats. Endocrinology. 1993;133:2787–2791. doi: 10.1210/endo.133.6.8243306. [DOI] [PubMed] [Google Scholar]
- 33.Lester J, Dodwell D, McCloskey E, Coleman R. The causes and treatment of bone loss associated with carcinoma of the breast. Cancer Treat Rev. 2005;31:115–142. doi: 10.1016/j.ctrv.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Wheeler DL, Vander Griend RA, Wronski TJ, Miller GJ, Keith EE, Graves JE. The short- and long-term effects of methotrexate on the rat skeleton. Bone. 1995;16:215–221. doi: 10.1016/8756-3282(94)00032-u. [DOI] [PubMed] [Google Scholar]
- 35.Greep NC, Giuliano AE, Hansen NM, Taketani T, Wang HJ, Singer FR. The effects of adjuvant chemotherapy on bone density in postmenopausal women with early breast cancer. Am J Med. 2003;114:653–659. doi: 10.1016/s0002-9343(03)00127-x. [DOI] [PubMed] [Google Scholar]
- 36.Ramaswamy B, Shapiro CL. Osteopenia and osteoporosis in women with breast cancer. Semin Oncol. 2003;30:763–775. doi: 10.1053/j.seminoncol.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 37.Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 38.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19:3306–3311. doi: 10.1200/JCO.2001.19.14.3306. [DOI] [PubMed] [Google Scholar]
- 40.Saad F, Olsson C, Schulman CC. Skeletal morbidity in men with prostate cancer: quality-of-life considerations throughout the continuum of care. Eur Urol. 2004;46:731–739. doi: 10.1016/j.eururo.2004.08.016. discussion 739–40. [DOI] [PubMed] [Google Scholar]
- 41.Howell SJ, Berger G, Adams JE, Shalet SM. Bone mineral density in women with cytotoxic-induced ovarian failure. Clin Endocrinol (Oxf) 1998;49:397–402. doi: 10.1046/j.1365-2265.1998.00550.x. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro CL, Phillips G, Van Poznak CH, et al. Baseline bone mineral density of the total lumbar spine may predict for chemotherapy-induced ovarian failure. Breast Cancer Res Treat. 2005;90:41–46. doi: 10.1007/s10549-004-2625-9. [DOI] [PubMed] [Google Scholar]
- 43.Bruning PF, Pit MJ, de Jong-Bakker M, van den Ende A, Hart A, van Enk A. Bone mineral density after adjuvant chemotherapy for premenopausal breast cancer. Br J Cancer. 1990;61:308–310. doi: 10.1038/bjc.1990.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Headley JA, Theriault RL, LeBlanc AD, Vassilopoulou-Sellin R, Hortobagyi GN. Pilot study of bone mineral density in breast cancer patients treated with adjuvant chemotherapy. Cancer Invest. 1998;16:6–11. doi: 10.3109/07357909809039747. [DOI] [PubMed] [Google Scholar]
- 45.Pierce SM, Recht A, Lingos TI, et al. Long-term radiation complications following conservative surgery (CS) and radiation therapy (RT) in patients with early stage breast cancer. Int J Radiat Oncol Biol Phys. 1992;23:915–923. doi: 10.1016/0360-3016(92)90895-o. [DOI] [PubMed] [Google Scholar]
- 46.Meric F, Buchholz TA, Mirza NQ, et al. Long-term complications associated with breast-conservation surgery and radiotherapy. Ann Surg Oncol. 2002;9:543–549. doi: 10.1007/BF02573889. [DOI] [PubMed] [Google Scholar]
- 47.Heymann D, Ory B, Gouin F, Green JR, Redini F. Bisphosphonates: new therapeutic agents for the treatment of bone tumors. Trends Mol Med. 2004;10:337–343. doi: 10.1016/j.molmed.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Wood J, Schnell C, Green JR. Zoledronic acid (Zometa), a potent inhibitor of bone resorption, inhibits proliferation and induces apoptosis in human endothelial cells in vitro and is anti-angiogenic in a murine growth factor implant model [abstract 2620] Proc Am Soc Clin Oncol. 2000 [Google Scholar]
- 49.Croucher P, Jagdev S, Coleman R. The anti-tumor potential of zoledronic acid. Breast. 2003;12 Suppl 2:S30–S36. doi: 10.1016/s0960-9776(03)80161-3. [DOI] [PubMed] [Google Scholar]
- 50.Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonates compound zoledronic acid. J Pharmacol Exp Ther. 2002;302:1055–1061. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- 51.Delmas PD, Balena R, Confravreux E, Hardouin C, Hardy P, Bremond A. Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: a double-blind, placebo-controlled study. J Clin Oncol. 1997;15:955–962. doi: 10.1200/JCO.1997.15.3.955. [DOI] [PubMed] [Google Scholar]
- 52.Powles TJ, McCloskey E, Paterson AH, et al. Oral clodronate and reduction in loss of bone mineral density in women with operable primary breast cancer. J Natl Cancer Inst. 1998;90:704–708. doi: 10.1093/jnci/90.9.704. [DOI] [PubMed] [Google Scholar]
- 53.Vehmanen L, Saarto T, Elomaa I, Makela P, Valimaki M, Blomqvist C. Long-term impact of chemotherapy induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. Eur J Cancer. 2001;37:2373–2378. doi: 10.1016/s0959-8049(01)00317-3. [DOI] [PubMed] [Google Scholar]
- 54.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 55.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 56.Woo SB, Hande K, Richardson PG. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353:99–102. discussion 99–102. [PubMed] [Google Scholar]
- 57.Purcell PM, Boyd IW. Bisphosphonates and osteonecrosis of the jaw. Med J Aust. 2005;182:417–418. doi: 10.5694/j.1326-5377.2005.tb06762.x. [DOI] [PubMed] [Google Scholar]
- 58.Bagan JV, Murillo J, Jimenez Y, et al. Avascular jaw osteonecrosis in association with cancer chemotherapy: series of 10 cases. J Oral Pathol Med. 2005;34:120–123. doi: 10.1111/j.1600-0714.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- 59.Buhl MR, Joseph TP, Snelling BE, Buhl L. Temporofacial zygomycosis in a pregnant woman. Infection. 1992;20:230–232. doi: 10.1007/BF02033066. [DOI] [PubMed] [Google Scholar]
- 60.Hall HD, Jacobs JS, O’Malley JP. Necrosis of maxilla in patient with herpes zoster. Report of a case. Oral Surg Oral Med Oral Pathol. 1974;37:657–662. doi: 10.1016/0030-4220(74)90128-5. [DOI] [PubMed] [Google Scholar]
- 61.Nandakumar H, Shankaramba KB. Massive sequestration of the upper jaw: a case report. Br J Oral Maxillofac Surg. 1990;28:55–56. doi: 10.1016/0266-4356(90)90014-c. [DOI] [PubMed] [Google Scholar]
- 62.Napoli JA, Donegan JO. Aspergillosis and necrosis of the maxilla: a case report. J Oral Maxillofac Surg. 1991;49:532–534. doi: 10.1016/0278-2391(91)90184-n. [DOI] [PubMed] [Google Scholar]
- 63.Owotade FJ, Ugboko VI, Kolude B. Herpes zoster infection of the maxilla: case report. J Oral Maxillofac Surg. 1999;57:1249–1251. doi: 10.1016/s0278-2391(99)90497-4. [DOI] [PubMed] [Google Scholar]
- 64.Ramon Y, Oberman M, Horowitz I, Freedman A. Extensive maxillary sequestration resulting from rhinocerebral mucormyocosis. J Oral Surg. 1977;35:989–991. [PubMed] [Google Scholar]
- 65.Shannon MT, Sclaroff A, Colm SJ. Invasive aspergillosis of the maxilla in an immunocompromised patient. Oral Surg Oral Med Oral Pathol. 1990;70:425–427. doi: 10.1016/0030-4220(90)90202-4. [DOI] [PubMed] [Google Scholar]
- 66.Wang J, Goodger NM, Pogrel MA. Osteonecrosis of the jaws associatedwith cancer chemotherapy. J Oral Maxillofac Surg. 2003;61:1104–1107. doi: 10.1016/s0278-2391(03)00328-8. [DOI] [PubMed] [Google Scholar]
- 67.Durie BG, Katz M, Crowley J. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353:99–102. doi: 10.1056/NEJM200507073530120. discussion 99–102. [DOI] [PubMed] [Google Scholar]
- 68.Woo SB, Hellstein JW, Kalmar JR. Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 69.Lugassy G, Shaham R, Nemets A, Ben-Dor D, Nahlieli O. Severe osteomyelitis of the jaw in longterm survivors of multiple myeloma: a new clinical entity. Am J Med. 2004;117:440–441. doi: 10.1016/j.amjmed.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 70.Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology. 2002;60:79–85. discussion 86. [PubMed] [Google Scholar]
- 71.Jimenez-Soriano Y, Bagan JV. Bisphosphonates, as a new cause of drug-induced jaw osteonecrosis: an update. Med Oral Patol Oral Cir Bucal. 2005;10 Suppl 2:E88–E91. [PubMed] [Google Scholar]
- 72.Migliorati CA. Bisphosphonate-associated oral osteonecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:135. doi: 10.1016/j.tripleo.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 73.Hofbauer LC, Hicok KC, Chen D, Khosla S. Regulation of osteoprotegerin production by androgens and anti-androgens in human osteoblastic lineage cells. Eur J Endocrinol. 2002;147:269–273. doi: 10.1530/eje.0.1470269. [DOI] [PubMed] [Google Scholar]
- 74.Riggs BL, Khosla S, Melton LJ., III Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 75.Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral densityi n men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–1222. [PubMed] [Google Scholar]
- 76.Kanis J. Pathogenesis of osteoporosis and fracture osteoporosis. London (England): Blackwell Healthcare Communications Ltd.; 1997. pp. 22–55. [Google Scholar]
- 77.Lee WY, Cho SW, Oh ES, et al. The effect of bone marrow transplantation on the osteoblastic differentiation of human bone marrow stromal cells. J Clin Endocrinol Metab. 2002;87:329–335. doi: 10.1210/jcem.87.1.8135. [DOI] [PubMed] [Google Scholar]
- 78.Eastell R, Hannon RA, Cuzick J, et al. Effect of anastrozole on bone densityand bone turnover: results of the “Arimidex” (anastrozole), Tamoxifen, Alone or in Combination (ATAC) study[abstract 1170] J Bone Miner Res. 2002;17:S165. doi: 10.1359/jbmr.060508. [DOI] [PubMed] [Google Scholar]
- 79.Gnant M, Hausmaninger H, Samonigg H, et al. Changes in bone mineral densityca used by anastrozole or tamoxifen in combination with goserelin (± zoledronate) as adjuvant treatment for hormone receptor-positive premenopausal breast cancer: results of a randomized multicenter trial [abstract 12].. 25th Annual San Antonio Breast Cancer Symposium; 2002 December 11; San Antonio, Texas. [Google Scholar]