Abstract
Background
Cycle length (CL) increases as ventricular fibrillation (VF) progresses.
Objectives
We tested the hypotheses that increased CL is due to increased diastolic interval (DI), not increased action potential duration (APD), and that the DI increase is not solely due to increased post-repolarization refractoriness.
Methods
In 10 swine, VF was recorded for 20 min with a floating microelectrode (FME) through a hole in a 504 electrode epicardial plaque. Mean APD, DI, AP amplitude (APA), maximum change in voltage during the AP upstroke (, and CL were calculated from the FME recordings each minute of VF. The refractory period was estimated from the minimum DI (DImin). In 2 animals, rapid pacing was performed to gauge refractoriness.
Results
As VF progressed, CL, DI and DImin increased (p<0.05), while APD, and APA decreased (p<0.05). At 20 min, DImin was not different from mean DI at VF onset. Pacing captured, but 53% of paced wavefronts blocked within the plaque.
Conclusion
Increasing CL in VF is due to increased DI, not APD, which shortens. The increase in DImin over time is much less than the increase in mean DI, indicating that the myocardium is excitable during much of the DI. This finding, along with the ability to pace at a CL shorter than the native VF CL and the poor paced wavefront propagation, suggests that the increase in DI is not only due to increased post-repolarization refractoriness but also to poor wavefront propagation because of decreased APA and, secondary to global ischemia caused by VF.
Keywords: ventricular fibrillation, refractoriness, microelectrode
Introduction
Sudden cardiac death remains a major source of mortality.1 Studies have demonstrated an inverse relationship between time to defibrillation and survival from sudden cardiac death, with a grim prognosis for patients who do not receive shocks within 10 min.2 While certain communities achieve quick response times,3 in many cases emergency medical services responders reach patients more than 10 min after a witnessed arrest.4 Yet, the majority of animal studies of ventricular fibrillation (VF) focus on the first minute of VF.5
A prior study demonstrated increasing cycle length (CL) and diastolic interval (DI) but decreasing action potential duration (APD) during VF lasting more than 1 minute (long duration VF [LDVF]).6 That study examined the first 5 min of VF — shorter than the time to defibrillation in many communities. Additionally, the study used monophasic action potential recordings in isolated hearts whose autonomic inputs were severed. We examined the changes in epicardial cellular electrical activity over 20 min of VF in the open-chest pig using a hand-held floating microelectrode (FME) within a plaque of surface extracellular electrodes. We tested the hypothesis that CL, DI, and APD change monotonically as VF continued. Prior studies have indicated a decrease in action potential amplitude (APA) as well as an increase in post-repolarization refractoriness in ischemic myocardium.7 Therefore, we tested the hypothesis that the increase in DI in LDVF occurs both because of decreased APA causing poor wavefront conduction and because of increased post-repolarization refractoriness due to the global cardiac ischemia during VF.
Methods
Animal Preparation
Ten mixed breed swine weighing 36±4.7 kg (mean± standard deviation) were anesthetized with intramuscular telezol (4.4 mg/kg), xylazine (4.4 mg/kg) and atropine (0.04 mg/kg), followed by maintenance with 1.5-2% isoflurane in 100% oxygen via an endotracheal tube. Animals were ventilated with tidal volumes of 12 ml/kg at a rate of 10-15 cycles/min. Animals were instrumented for ECG leads II and III, femoral arterial blood pressure (ABP) and temperature measurements. Through the right internal jugular vein, a lead (model 6942, Sprint, Medtronic) with electrodes in the right ventricle and superior vena cava was inserted for defibrillation. Body temperature was maintained at 37°±1° C. Femoral ABP, blood gases, pH, and electrolytes were monitored every 30 min and maintained within acceptable physiologic ranges (ABP = 70-125 mmHg during sinus rhythm, PCO2=35-60 mmHg and pH=7.35-7.45) by giving intravenous sodium bicarbonate or by adjusting ventilation parameters. Throughout the experiment, lactated ringers solution was administered at a rate of 2-5 ml/kg/hr through the left internal jugular vein.
The chest was opened via a median sternotomy. The left hemiazygous vein was ligated and the pericardium was opened. Isotonic saline warmed to 37° C was dripped over the exposed epicardium throughout the study. At the end of the protocol, the heart was excised and fixed in formalin. All pre-operative and operative care of the animals complied with the Animal Welfare Act8 and the “Guide for the Care and Use of Animals”, National Institutes of Health Publication #85-27.9
Experimental Protocol
Electrical recordings were obtained simultaneously from 503 unipolar extracellular electrodes in an epicardial plaque and a handheld intracellular floating microelectrode (FME). The 503 plaque electrodes were 2 mm apart in a 21×14 array with 1 electrode missing in the center where a 4 mm diameter hole was present (Figure 1A). The plaque was placed on the anterior left ventricle. The FME was inserted through the center hole and impaled into an epicardial cell. Once a stable FME recording of sinus rhythm was obtained, VF was induced in the majority of animals with a 9V battery applied to the epicardium. In a few animals with small hearts in which the mapping plaque covered almost all of the exposed epicardium, VF was induced by rapid pacing via the defibrillation electrode in the right ventricle. If the FME impalement was lost within the first 3 min of VF, the animal was defibrillated. The animal was allowed to recover until hemodynamic and metabolic factors had returned to baseline; at that time, VF was reinduced and another attempt was made to record from the FME. If the FME impalement was lost after more than 3 min of VF, VF was allowed to continue, and a new FME impalement was obtained as quickly as possible.
Figure 1.
Panel A: Diagram of the epicardial plaque containing 503 extracellular electrodes shown as dots and a 4 mm diameter hole in the center for insertion of the FME. In 2 animals, pacing was performed during VF from a bipolar electrode next to the FME site. Panel B: Examples of the variables determined during VF. For each activation n, the APD was the time between the onset of the AP and the time when the transmembrane potential returns 60% of the way back to the take-off potential before the AP began, represented here by APDn. The DIn was the interval between the time that the APA returns 60% of the way back to the previous take-off potential (APA) and the initiation of the next AP. Cycle length is the sum of the APD plus the following DI.
In 2 animals, bipolar pacing at 3 to 5 times diastolic threshold was performed from a site 2 mm away from the FME for short time periods between 5 and 22 min of VF. Cycle length was varied until 1:1 capture was observed in the FME recording. LDVF was recorded for at least 20 min. The signals from the plaque electrodes and the FME were recorded with a 528 channel mapping system with 14 bits accuracy at a sampling rate of 2000 samples/s. In 7 of the 10 animals, the signals from the FME and the 24 surrounding epicardial electrodes were recorded at a sampling rate of 16,000 samples/s to provide higher temporal resolution for calculating, the maximum change in voltage during the upstroke of the action potential. The signals from the FME were recorded with a high-impedance, capacitance compensation preamplifier with DC coupling (World Precision Instruments Duo 773) prior to routing them to the mapping systems. Data were transferred to tape and hard drive and analyzed offline using PV-Wave® and Microsoft® Excel.
Microelectrode Signal Analysis During LDVF
During each minute of LDVF, a 3 to 5 s segment of the FME recording was analyzed. The first segment was taken as soon as possible after VF induction; segments from later points in VF were selected from a time window within 10 s of each integer minute time point (e.g. VF data for 5 min were obtained sometime between 4:50 and 5:10 of VF) to allow for selection of a segment with a stable baseline.
Activations from the FME recording were identified automatically using an algorithm that detected upstrokes in the electrical signal, based on a 5-point derivative. Automated selections were reviewed manually. If a detected activation did not repolarize at least 60% of the APA, it and the following DI were excluded from further analysis. For the remaining activations, APA was calculated as the change in voltage from the point at which the upstroke slope became more positive than 0.5 V/s to the peak of the action potential. was defined as the maximum change in voltage during the upstroke of an action potential, on the basis of a 5-point derivative applied to the 16,000 samples/s data. Mean values of both and APA were calculated for each 3 to 5 s segment. Detected activations that were >2 standard deviations smaller than the mean APA or were excluded from further analysis, as they were thought not to represent an actual new activation potential.
For each activation, we also calculated the action potential duration (APD), cycle length (CL) and diastolic interval (DI). APD was measured at the point at which the amplitude had repolarized 60 percent of the way from the peak of the AP to the takeoff potential. The DI was defined as the time interval between the time that the APA returned to 60% of the take-off potential and the time of the onset of the next AP. CL was defined as the time interval between consecutive takeoff potentials (Figure 1B). The minimum DI (DImin) for each data segment was identified as an estimate of the post-repolarization refractory period.
The mean values of the APA, , APD, DI and CL as well as DImin were determined for each time segment for each animal. In addition, the pooled means and standard deviations for all animals of the mean CL, APA, , APD, DI, and DImin for each animal were calculated for each min of VF from 0 to 6 min. As an uninterrupted FME impalement could not be maintained in all animals for 20 min, data from every 2 min of LDVF were combined, so that a minimum of data from 6 animals was present for each time period. At each time point, pooled means of the percentage of the CL spent in activation (APD%) and in the DI (DI%) were calculated.
Plaque Data Analysis
Data recorded from the epicardial plaque were analyzed with an automated algorithm. Wavefront parameters were quantified with software that grouped activations recorded by the plaque electrodes into wavefronts, as described previously.10-12 Electrograms with a minimum slope of -0.5 V/s were considered to represent activation. During each minute of VF, mean values of wavefront conduction velocity (CV), the epicardial area swept out by each wavefront and the minimum dV/dt of each activation were calculated. The data after 6 min of VF were pooled in the same manner as the FME data.
Microelectrode and Plaque Data Analysis During Pacing in LDVF
During pacing, segments of 1:1 capture in the FME recording were identified. A 2 to 4 s episode of 1:1 capture was analyzed, as well as 2 to 4 s segments of native VF immediately prior to and after pacing. Activations, APD, , APA and CL were detected as described above. Activation fronts that arose from the pacing site were visualized using a computer animation program. The percentage of paced activation fronts that propagated off the plaque without totally blocking in the mapped region was determined.
Statistical Analysis
Means ± standard deviations are given. Results were considered significant at p<0.05. Pooled mean values of FME data (CL, mean , mean APA, mean APD, APD%, mean DI, DI%, and DImin) and of plaque data (CV, area swept out by wavefronts, dV/dt) at the beginning of VF and at 20 min of LDVF were compared by Student’s t-test. For each pacing episode, the mean CL for each pre-pacing segment and the following pacing segment were compared using Student’s t-test. The mean percent change in CL for all 6 pacing episodes was calculated and compared to zero using a paired Student’s t-test.
To test whether the duration of VF affected CL, mean , mean APA, mean APD, APD%, mean DI, and DI%, a repeated measures analysis of variance was performed in which the within-subject factor was VF duration. If there was a significant duration effect, a post-hoc test was performed to determine whether the variables changed after the first 3 min of VF. The mean values of the variables at 0, 1, 2, and 3 min were compared to the mean values during the remainder of the VF episode. The mean value of DI for min 4-11 was compared to that of min 12-20 to test whether the variable reached a plateau. These analyses were performed without combining the results for every 2 min of VF for min 7-20.
Results
Electrophysiologic Changes During the First 20 min of VF
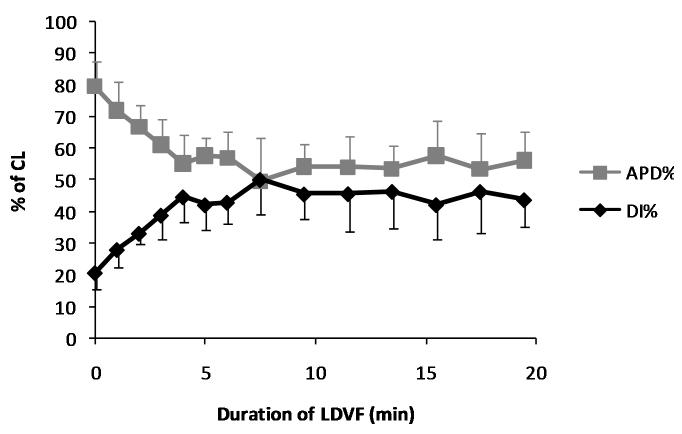
Over the course of LDVF, there were substantial changes in the morphology of the FME recordings (Figure 2). Mean CL increased from 121.5±13.3 ms at VF onset to 148.9±16.4 ms at 20 min of LDVF (p<0.05, Figure 3). APD decreased from 96.5±9.9 ms at VF onset to 83.8±13.2 ms at 20 min (p<0.05). DI increased from 25.1±6.0ms to 65.2±12.3 ms (p<0.05, Figure 3). When APD and DI were expressed as a percentage of the CL (APD% and DI%), similar results were observed. APD% decreased from 80±3.6% to 56±6.9% of the CL (p<0.05), while DI% increased from 20±3.5% to 44±6.9% of the CL (p<0.05, Figure 4). decreased as VF continued, from 17.5 ±8.1 V/s at VF onset to 5.17 ±2.27 V/s at 20 min of LDVF (p<0.05, Figure 5A). APA also declined from 41.1±15.0 mV at VF onset to 25.2±8.1 mV at 20 min of LDVF (p<0.05, Figure 5B).
Figure 2.

Recordings of 2 s segments from the FME in a single animal at 0, 2, 5, 10 and 15 min after the induction of VF. The time in minutes of the start of recording is shown to the left of each trace. All signals are autoscaled to the same height, but no correction is made for baseline drift. Mean APA for each segment is listed below each tracing.
Figure 3.
Mean with standard deviation bars of DI, APD, CL and DImin for the first 20 min of LDVF in all animals. All 4 variables are significantly different at 20 min compared with VF onset.
Figure 4.
Mean with standard deviation bars of APD and DI as a percentage of the CL (APD% and DI%) during the first 20 min of LDVF in all animals. Both variables are significantly different at 20 min compared to VF onset.
Figure 5.

Mean (A) and mean APA (B) over the first 20 min of LDVF, with standard deviation bars. Both values are significantly different at 20 min compared with VF onset. Mean APA and in sinus rhythm were 80.1 ±15.8 mV and 79.6 ± 34.9 V/s.
All variables were affected by VF duration (p<0.05). When a Bonferroni correction was performed to correct for multiple comparisons by multiplying the p-values by 8 (the number of variables), significance (p<0.05) was retained for all variables except APD. For all variables, the mean values during the first 3 min of VF were different from those during the rest of the LDVF episode, even after correction for multiple comparisons (p<0.05). The mean value of DI for min 4-11 of VF was not significantly different from that for min 12-20, indicating that DI reached a plateau.
Analysis of the epicardial plaque data revealed significant changes in CV, area swept out by the wavefronts and dV/dt as LDVF continued. CV dropped from 0.51±0.32 M/s at VF onset to 0.23±0.13 M/s (p<0.05) at 20 min. Area swept out by the wavefronts dropped from 246±61.6 mm2 at VF onset to 168±42.2 mm2 (p<0.05). DV/dt changed from -1.34 ± 0.70 V/s to -1.01±0.25 V/s (p<0.05) (Figure 7).
Figure 7.

Mean CV with standard deviation bars of dV/dt (A), CV (B) and area swept out by each wavefront (C) over the first 20 min of LVDF. All three values are significantly different (p<0.05) at 20 min compared to VF onset.
Refractoriness
DImin increased as VF continued (p<0.05, Figure 3). At VF onset, DImin was 12.9±3.3 ms; at 20 min of VF it increased to 28.6 ±11.2 ms (p<0.05). The mean DImin after 20 min of LDVF was not significantly different than the mean DI at the onset of VF (25.1±5.95 ms).
During the 6 episodes of pacing analyzed, the paced CL was 16 ± 5% (23.9±11.8 ms) faster (p<0.05) than the LDVF CL just before pacing (Figure 6). This decrease in CL by pacing was significantly less that the DImin just before pacing (33.1±13.6 ms), consistent with the assumption that the DImin is a period of post-repolarization refractoriness. After pacing terminated, the native VF returned to a slower CL that was 33 ± 8% longer (p<0.05) than the paced CL. The CL of VF after termination of pacing was not significantly different from the CL of native VF immediately before pacing (p=0.31). Of the 132 paced activation fronts, 62 (47%) propagated off the edge of the epicardial plaque. The remaining activation fronts were abolished by collision with other activation fronts or totally blocked spontaneously within the mapped region (Figure 6C).
Figure 6.
(A) Mean with standard deviation bars of CL during native and paced LDVF for the 6 pacing episodes. Asterisks indicate p<0.05 compared to paced LDVF. (B) FME recording of native VF, which was then paced at a CL of 150 ms with 1:1 capture. Pacing artifacts are present beginning after the seventh activation. (C) Activation sequence during a paced cycle. Each colored square represents the activation time after the pacing stimulus according to the color code to the right. Electrode sites that are gray indicate that conduction blocked before reaching them. Arrows indicate direction of wavefront propagation, and lines of block are indicated in black. The 2 Xs indicate the site of bipolar pacing, while the O indicates the FME recording site.
Discussion
During the first 20 min of LDVF in open chest pigs, there is an increase in CL (p<0.05). As previously reported for the first 5 min of LDVF in isolated rabbit hearts with a MAP recording,6 the increase in CL in our study was due to an increase in DI (p<0.05), despite a decrease in the APD (p<0.05). The similarity of the changes observed in the first 5 min of VF in intact and isolated models suggests that the denervation of hearts in isolated models is not the major cause of the observed electrophysiologic changes.
The changes in APD and DI observed in our study do not progress linearly as VF persists. Instead, the majority of the change in APD and DI occurs during the first 3 min of LDVF. After this, the rate of change is much less, but the changes persist for at least the first 20 min of LDVF. The initial rapid changes in DI and APD followed by much slower change raises the possibility that the biochemical processes responsible for these effects follow a similar trajectory. One potential cause for the observed changes is the disruption of potassium homeostasis and acid-base equilibrium that occurs in VF. Prior studies in ischemic models during sinus rhythm have demonstrated that extracellular potassium accumulates during periods of myocardial ischemia.13-17 Most of these studies show extracellular potassium rising over the first 5-10 min of ischemia, before reaching a plateau that persists until 20-30 min of ischemia after which extracellular potassium begins to rise again.13-15, 17 During times of ischemic extracellular hyperkalemia, there is a shortening of APD16 and slowed conduction velocity.15, 17 In perfused hearts, similar changes in APD and conduction velocity can be produced by using a perfusate with low pH and high potassium.16 Although we are aware of no study examining these changes in LDVF, these changes have been shown to develop more quickly at a faster heart rate,17 raising the possibility that the initial increase in extracellular potassium may occur within the first 3 min rather than the first 5-10 min of VF because the extremely rapid VF activation rate may accelerate the ischemic changes during VF.
In our study, both and APA decrease (p<0.05) during LDVF. This may have been caused by an increase in the takeoff potential secondary to the hyperkalemia due to the global ischemia during LDVF.18 Because we could not measure the absolute takeoff potential, we could not test this hypothesis with our current data.
We used the minimum DI as an estimate of post-repolarization refractoriness of the tissue at the FME, because the tissue could be excited with DIs longer than this value. As LDVF progresses, there is an increase in the minimum DI (p<0.05). While this increase suggests that there is some increase in post-repolarization refractoriness, the magnitude of the increase in DImin is not large enough to account for the total increase in mean DI. We estimated the percentage of the CL in which the tissue was excitable but not excited by subtracting the DImin from the mean DI and then dividing the result by the mean CL (Figure 3). At VF onset, the tissue was excitable but unexcited only 10% of the time. By 20 min of LDVF, this value had increased to 25% of the CL. This calculation suggests that the tissue, if properly stimulated, has the capacity to activate faster than the native VF activation rate.
This conclusion is bolstered by the finding that during episodes of rapid pacing, the activation rate of the tissue could be accelerated, even late during LDVF. This supports the idea that an increase in post-repolarization refractoriness is not the sole cause of the increase in CL and DI. While the tissue can be paced, the wavefronts have difficulty propagating very far, as only 47% are able to propagate the approximate 2 cm distance to the edge of the plaque. The poor wavefront propagation may play a role in the increase in DI and could be due to a number of factors. The significant drop in APA and probably reflects the waning ability of cells to generate a stimulus sufficient to excite neighboring cells. Wavefront propagation could also be impeded by the effects of ischemia, including electrolyte changes and acidosis.15, 16 Another consideration is the possible role of the uncoupling of the gap junctions as an additional etiology of poor wavefront propagation, as gap junctions have been shown to uncouple in sinus rhythm during periods of ischemia19 and acidosis,20 causing slowed conduction velocity.21 The net effect of these changes is poor wavefront propagation not only during pacing but during native LDVF as indicated by the decreased minimum dV/dt of the electrode recordings and the decreased CV and areas swept out by the wavefronts (Figure 7).
Limitations
A limitation of this study is that the distribution of ion channels and specialized conduction tissue in the porcine model is not an exact replica of the human myocardium. While our model was in the intact animal, anesthesia may alter the neurohormonal input to the heart during VF. Additionally, exposure of the heart to air may provide a degree of electrical insulation that could alter epicardial recordings.
Another limitation is the possibility of selection bias in the FME recordings. As the longest uninterrupted recording in any animal without FME dislodgement was 11 min, it is possible that the cells which are still electrically active late in VF may have different baseline characteristics than the rest of the myocardium. If this were the case, the data recorded from new FME impalements late in LDVF could represent recordings from a particular subset of cells. Another consideration is whether or not prolonged impalement with an FME alters the electrophysiologic properties of a myocardial cell, and the changes observed are produced by the impalement, not an intrinsic change during VF. The similarity of the FME recordings during the first 5 min of VF with those obtained by Tovar and Jones with MAP recordings6 suggest that the impact of the FME impalement is likely insignificant.
An additional limitation of using an FME is the difficulty of maintaining a constant impalement. As multiple impalements were performed in each animal, it was not possible to know the absolute resting potential during the recordings. While APA could be calculated, we were unable to measure the increase in takeoff potential during LDVF.
A limitation of the data regarding pacing in VF is the small number of animals. Due to the technical challenge of maintaining FME impalement for 20 min, we did not attempt pacing in all animals. While our pacing findings raise interesting questions, further studies in additional animals will be helpful in understanding these findings. The relatively high pacing output during VF (3-5 times diastolic threshold before VF induction) may have underestimated post-repolarization refractoriness. Because the diastolic threshold is increased after several minutes of ischemia,22 the pacing output we used may not have been 3-5 times threshold during the global ischemia of VF.
Conclusion
CL increases over the course of LDVF because of an increase in DI that is large enough to more than offset a decrease in APD. APA and decrease during LDVF and probably contribute to the increase in DI by making cells less able to excite their neighboring cells. While an increase in post-repolarization refractoriness also may contribute to the increase in DI, it does not explain all of the increase, as evidenced by the finding that cells can be paced faster than the native VF, though the propagation of the paced wavefronts is often poor, consistent with the decrease in APA and .
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute grant HL28429, HL66256, and HL85370.
Abbreviation Glossary
- CL
cycle length
- AP
action potential
- APD
action potential duration
- DI
diastolic interval
- VF
ventricular fibrillation
- LDVF
long duration ventricular fibrillation
maximum change in voltage during AP upstroke
- FME
floating microelectrode
- APA
action potential amplitude
Footnotes
DISCLOSURES: None
References
- 1.Zheng ZJ, Croft JB, Giles WH, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–63. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.Valenzuela TD, Roe DJ, Cretin S, et al. Estimating effectiveness of cardiac arrest intervention: a logistic regression survival model. Circulation. 1997;96:3308–3313. doi: 10.1161/01.cir.96.10.3308. [DOI] [PubMed] [Google Scholar]
- 3.Myerburg RJ, Fenster J, Velez M, et al. Impact of community-wide police car deployment of automated external defibrillators on survival from out-of-hospital cardiac arrest. Circulation. 2002;106:1058–64. doi: 10.1161/01.cir.0000028147.92190.a7. [DOI] [PubMed] [Google Scholar]
- 4.Stiell IG, Walker RG, Nesbitt LP, et al. BIPHASIC Trial: a randomized comparison of fixed lower versus escalating higher energy levels for defibrillation in out-of-hospital cardiac arrest. Circulation. 2007;115:1511–7. doi: 10.1161/CIRCULATIONAHA.106.648204. [DOI] [PubMed] [Google Scholar]
- 5.Ideker RE. Ventricular fibrillation: how do we put the genie back in the bottle? Heart Rhythm. 2007;4:665–74. doi: 10.1016/j.hrthm.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Tovar OH, Jones JL. Electrophysiological deterioration during long-duration ventricular fibrillation. Circulation. 2000;102:2886–91. doi: 10.1161/01.cir.102.23.2886. [DOI] [PubMed] [Google Scholar]
- 7.Kimura S, Bassett AL, Kohya T, et al. Simultaneous recording of action potentials from endocardium and epicardium during ischemia in the isolated cat ventricle: relation of temporal electrophysiologic heterogeneities to arrhythmias. Circulation. 1986;74:401–9. doi: 10.1161/01.cir.74.2.401. [DOI] [PubMed] [Google Scholar]
- 8.The Animal Welfare Information Center . Animal Welfare Act: US Department of Agriculture. Agricultural Research Center; Beltsville, MD: 1990. [Google Scholar]
- 9.US Govt Printing Office; Guide for the Care and Use of Laboratory Animals: National Research Council, Institute of Laboratory Animal Research, Committee on the Care and Use of Laboratory Animals. 1996
- 10.Rogers JM, Usui M, KenKnight BH, et al. A quantitative framework for analyzing epicardial activation patterns during ventricular fibrillation. Ann Biomed Eng. 1997;25:749–60. doi: 10.1007/BF02684159. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Rogers JM, Kenknight BH, et al. Evolution of the organization of epicardial activation patterns during ventricular fibrillation. J Cardiovasc Electrophysiol. 1998;9:1291–1304. doi: 10.1111/j.1540-8167.1998.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Walcott GP, Killingsworth CR, et al. Quantification of activation patterns during ventricular fibrillation in open-chest porcine left ventricle and septum. Heart Rhythm. 2005;2:720–8. doi: 10.1016/j.hrthm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Hill JL, Gettes LS. Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation. 1980;61:768–78. doi: 10.1161/01.cir.61.4.768. [DOI] [PubMed] [Google Scholar]
- 14.Kleber AG. Extracellular potassium accumulation in acute myocardial ischemia. J Mol Cell Cardiol. 1984;16:389–94. doi: 10.1016/s0022-2828(84)80610-0. [DOI] [PubMed] [Google Scholar]
- 15.Kleber AG, Janse MJ, Wilms-Schopmann FJ, et al. Changes in conduction velocity during acute ischemia in ventricular myocardium of the isolated porcine heart. Circulation. 1986;73:189–98. doi: 10.1161/01.cir.73.1.189. [DOI] [PubMed] [Google Scholar]
- 16.Weiss J, Shine KI. Extracellular K+ accumulation during myocardial ischemia in isolated rabbit heart. Am J Physiol. 1982;242:H619–28. doi: 10.1152/ajpheart.1982.242.4.H619. [DOI] [PubMed] [Google Scholar]
- 17.Weiss J, Shine KI. [K+]o accumulation and electrophysiological alterations during early myocardial ischemia. Am J Physiol. 1982;243:H318–27. doi: 10.1152/ajpheart.1982.243.2.H318. [DOI] [PubMed] [Google Scholar]
- 18.Kleber AG. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res. 1983;52:442–50. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- 19.Tansey EE, Kwaku KF, Hammer PE, et al. Reduction and redistribution of gap and adherens junction proteins after ischemia and reperfusion. Ann Thorac Surg. 2006;82:1472–9. doi: 10.1016/j.athoracsur.2006.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eloff BC, Gilat E, Wan X, et al. Pharmacological modulation of cardiac gap junctions to enhance cardiac conduction: evidence supporting a novel target for antiarrhythmic therapy. Circulation. 2003;108:3157–63. doi: 10.1161/01.CIR.0000101926.43759.10. [DOI] [PubMed] [Google Scholar]
- 21.Cascio WE, Yang H, Muller-Borer BJ, et al. Ischemia-induced arrhythmia: the role of connexins, gap junctions, and attendant changes in impulse propagation. J Electrocardiol. 2005;38:55–9. doi: 10.1016/j.jelectrocard.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Elharrar V, Foster PR, Jirak TL, et al. Alterations in canine myocardial excitability during ischemia. Circ Res. 1977;40:98–105. doi: 10.1161/01.res.40.1.98. [DOI] [PubMed] [Google Scholar]