Abstract
Purpose
To examine the prevalence of astigmatism (≥ 1.00 diopter (D)) and high astigmatism (≥ 2.00 D) at 6 and 9 months post-term and 2 and 3 years postnatal, in preterm children with birth weight < 1251g who developed high-risk prethreshold retinopathy of prematurity (ROP) and participated in the Early Treatment for ROP (ETROP) Study.
Design
Randomized controlled clinical trial
Participants
401 infants who developed prethreshold ROP in one or both eyes and were randomized after they were determined to have a high risk (≥ 15%) of poor structural outcome without treatment, using the RM-ROP2 risk management program. Refractive error was measured by cycloplegic retinoscopy. Eyes with additional retinal, glaucoma, or cataract surgery were excluded.
Intervention
Eyes were randomized to receive laser photocoagulation at high-risk prethreshold ROP (early treated (ET)) or to be conventionally managed (CM), receiving treatment only if threshold ROP developed.
Main Outcome Measures
Astigmatism and high astigmatism at each visit. Astigmatism was classified as “with-the rule” (75° – 105° (WTR)), “against-the-rule” (0° – 15° and 165° – 180° (ATR), or “oblique” (16° – 74° and 106° – 164° (OBL)).
Results
The prevalence of astigmatism in ET and CM eyes was similar at each test age. For both groups, there was an increase in prevalence of astigmatism from approximately 32% at 6 months to approximately 42% by 3 years, mostly occurring between 6 and 9 months. Among eyes that could be refracted, astigmatism was not influenced by zone of acute-phase ROP, presence of plus disease, or retinal residua of ROP. Eyes with astigmatism and high astigmatism most often had WTR astigmatism.
Conclusions
By age 3 years, nearly 43% of eyes treated at high-risk prethreshold ROP developed astigmatism ≥ 1.00 D and nearly 20% had astigmatism ≥ 2.00 D. Presence of astigmatism was not influenced by timing of treatment of acute-phase ROP, characteristics of acute-phase or cicatricial ROP. These findings reinforce the need for follow-up eye examinations in infants with high risk prethreshold ROP.
Keywords: astigmatism, refractive error, prematurity
Introduction
Several studies of preterm infants have shown a high prevalence of astigmatism in infants refracted at less than one year of age.1–4 In an early study of 146 infants with gestational age (GA) of 36 weeks or less, who were refracted by eight weeks of age and who showed no evidence of retinopathy of prematurity (ROP), investigators found astigmatism ≥ 1.00 diopter (D) in 71% of right eyes and 66% of left eyes, and in 83% of cases, the astigmatism was against-the-rule (ATR).1 More recently, measurement of refractive error at six months corrected age in 247 preterm infants with birth weight ≤1500 g showed astigmatism ≥1.00 D in 52% of infants and astigmatism ≥2.00 D in 18%.2 Finally, two studies have reported refractive error measurements in both preterm and full-term infants. In the first, the prevalence of astigmatism ≥1.00 D at six months corrected age was similar in a group of 27 preterm infants with GA of 28 to 35 weeks and no ROP (48.2%) and a group of 19 full-term infants.3 A second study of infants with and without ROP reported a similar prevalence of astigmatism > 1.00 D in 652 infants with GA <37 weeks (51.5%) and in 551 infants with GA of 37 to 43 weeks (59.7%), with all refractions during the first week of life.4
Some studies have documented a decreasing prevalence of astigmatism between infancy and childhood in preterm infants.2,3,5,6 In a study of 228 infants with birth weight ≤ 1500 g, prevalence of astigmatism ≥ 1.00 D decreased from 52% at six months corrected age to 28% at 30 months corrected age, and prevalence of astigmatism ≥ 2.00 D decreased from 18% to 5%.2 Over this time course, astigmatism that was initially with-the-rule (WTR) disappeared more often than did ATR or oblique-axis (OBL) astigmatism.2 Follow-up testing in a group of 27 preterm infants with GA of 28 to 35 weeks and no ROP also showed a decrease in prevalence of astigmatism from 48% at six months corrected age to 27% at 12 months corrected age, to 8% at 4 years corrected age.3 Another study, in which refractive error was measured over a ten-year period in 198 children with birth weight ≤ 1500 g and GA of 24 to 35 weeks, reported that prevalence of astigmatism ≥ 1.00 D declined from 54% at 6 months to 27% at 30 months, and to 21% by 10 years.5 In contrast, a recent study, in which 293 low birth weight (< 1701 g) children were refracted at six months corrected age and again at age 10 to 12 years, reported little change in astigmatism power or axis over this long period, with 75% of children showing a change in cylinder power of no more than 0.75 D.6
One factor that appears to be associated with both prevalence and amount of astigmatism in preterm infants and children is presence of ROP.2,5,7–10 Holmström et al., in their study of 247 preterm infants with birth weight ≤ 1500 g, reported astigmatism ≥ 1.00 D in 63% of right eyes and 65% of left eyes with ROP, compared 45% of right eyes and 44% of left eyes with no ROP.2 Astigmatism ≥2.00 D occurred in 24% of right eyes and 20% of left eyes with ROP, compared to 14% of right eyes and 17% of left eyes without ROP. Axis of astigmatism was most frequently ATR, with the exception that OBL astigmatism was most common in eyes that had undergone cryotherapy.2 Studies of young children also indicate that both prevalence and amount of astigmatism increase as severity of ROP increases,8–11 with the highest risk of astigmatism occurring in eyes that had undergone peripheral retinal ablation.5,8,10,11
The purpose of the present paper is to examine the prevalence, amount, and axis of astigmatism at 6 and 9 months corrected age and at 2 and 3 years postnatal age in a group of preterm children with birth weight < 1251 g who developed prethreshold ROP that was classified as “high risk” and participated in the Early Treatment for ROP (ETROP) Study.12,13 Comparison of prevalence and amount of astigmatism in eyes randomized to early treatment (ET) versus eyes randomized to conventional management (CM), with treatment only if threshold ROP developed, will indicate whether treatment protocol influences the development of astigmatism in this group of children with ROP. In addition, detailed data from fundus examinations conducted on all study participants allow examination of the relation between characteristics of acute-phase and cicatricial ROP and the development of astigmatism. Finally, the present results will provide the first data on prevalence of astigmatism and its change over the first three years of life in low birth weight infants whose eyes developed “high risk” prethreshold ROP.
Participants and Methods
401 infants with birth weight ≤ 1251g were enrolled in the ETROP Study at one of 26 participating centers in the U.S. between October 1, 2000 and September 30, 2002.13 All infants had developed prethreshold ROP in one or both eyes that, using the RM-ROP2 risk management program, put the eyes at high risk (≥ 15%) of a poor structural outcome at three months post term.12 In the 317 infants who developed high-risk prethreshold ROP in both eyes, one eye was randomly assigned to treatment within 48 hours (early treatment (ET)) and the fellow eye was observed until regression occurred or until threshold ROP developed and was treated (conventional management (CM)). Of the 84 infants who developed high-risk prethreshold ROP in only one eye, 44 eyes were randomized to ET while 40 eyes were randomized to CM.
The Review Boards of all participating institutions approved the study protocol. Parents or guardians of all participants gave informed consent prior to enrollment in the randomized trial and upon entry into the follow-up phase of the Study. Study design details, describing standardized eye examinations and definitions of prethreshold and threshold ROP have been previously published.13–15 As previously described, study-certified examiners performed cycloplegic retinoscopy when infants reached 6 and 9 months post term and 2 and 3 years postnatal age.16 The cycloplegic agent was 1% cyclopentolate hydrochloride, unless there was a medical contraindication, in which case either 0.5% cyclopentolate or 1% tropicamide was used.
In keeping with refractive error categories from previous studies, astigmatism and high astigmatism were defined as plus cylinder of ≥ 1.00 D and ≥ 2.00 D, respectively.2–5,8,17–20 Astigmatism was further classified as WTR (75° – 105°), ATR (0° – 15° and 165° – 180°), and OBL (16° – 74° and 106° – 164°). Prevalence rates were computed by eyes with ≥ 1.00 D and ≥ 2.00 D cylinder divided by eyes with valid refraction.
Results
Early-Treated and Conventionally-Managed Eyes
This analysis includes data from refractive assessment of 283 ET and 272 CM eyes at 6 months, 304 ET treated and 280 CM eyes at 9 months, 281 ET and 253 CM eyes at 2 years, and 268 ET and 244 CM eyes at 3 years.
Not included in the analysis are data from participants who died prior to the examination (15 at 6 months, an additional 7 at 9 months, an additional 5 at 2 years, and an additional 1 at 3 years), participants who did not attend the study examination (20 at 6 months, 7 at 9 months, 35 at 2 years, and 48 at 3 years), eyes that were unable to be refracted due to retinal detachment, media opacity, pupillary miosis, or other difficulty in refracting (46 ET and 50 CM eyes at 6 months, 15 ET and 22 CM eyes at 9 months, 12 ET and 21 CM eyes at 2 years, and 13 ET and 23 CM eyes at 3 years), or subsequent to the time of an operative procedure, i.e. eyes that underwent vitrectomy, scleral buckling procedures, iridectomy, glaucoma procedures, or cataract surgery (1 ET and 4 CM eyes at 6 months, 17 ET and 27 CM eyes at 9 months, 16 ET and 25 CM eyes at 2 years, and 16 ET and 21 CM eyes at 3 years).
Prevalence of Astigmatism and High Astigmatism
As shown in Table 1, the prevalence of astigmatism at each test age was similar in ET and CM groups. For both ET and CM eyes, there was a small increase in prevalence of astigmatism over time, most of which occurred between 6 and 9 months. For the group of ET eyes, prevalence of high astigmatism doubled between the 6-month and 3-year examinations, and showed an increase at each successive examination age between 6 months and 3 years. The CM group of eyes showed an increase in prevalence of high astigmatism between the 6 and 9 month examinations, with little change thereafter.
Table 1.
Percentage of eyes with astigmatism and high astigmatism at 6 and 9 months corrected age and at 2 and 3 years postnatal age.
| Refractive status | Treatment at high-risk prethreshold n/N* (%) | Conventionally-managed high-risk eyes | |||
|---|---|---|---|---|---|
| Total conventionall y-managed high-risk eyes n/N* (%) | Treatment at threshold n/N (%) | Regressed no treatment n/N (%) | |||
| Astigmatism greater than or equal to 1.00 Diopters | 6 months | 92/283 (32.5%) | 87/272 (32.0%) | 49/172 (28.5%) | 37/99 (37.4%) |
| 9 months | 117/304 (38.5%) | 113/280 (40.4%) | 66/172 (38.4%) | 47/107 (43.9%) | |
| 2 years | 107/281 (38.1%) | 93/253 (36.8%) | 59/155 (38.1%) | 34/98 (34.7%) | |
| 3 years | 116/268 (43.3%) | 99/244 (40.6%) | 65/150 (43.3%) | 33/93 (35.5%) | |
| Astigmatism greater than or equal to 2.00 Diopters | 6 months | 26/283 (9.2%) | 30/272 (11.0%) | 16/172 (9.3%) | 14/99 (14.1%) |
| 9 months | 41/304 (13.5%) | 44/280 (15.7%) | 28/172 (16.3%) | 16/107 (15.0%) | |
| 2 years | 45/281 (16.0%) | 31/253 (12.3%) | 22/155 (14.2%) | 9/98 (9.2%) | |
| 3 years | 53/268 (19.8%) | 31/244 (12.7%) | 23/150 (15.3%) | 8/93 (8.6%) | |
See Results Section for early treatment (ET) and conventional management (CM) eyes with unavailable refractive error data.
Within the group of CM eyes (rightmost two columns of Table 1), the prevalence of astigmatism and high astigmatism was similar in eyes that reached threshold and underwent peripheral retinal ablation, compared to eyes in which the ROP regressed without reaching threshold.
Astigmatism, High Astigmatism, and Location of Acute-Phase ROP
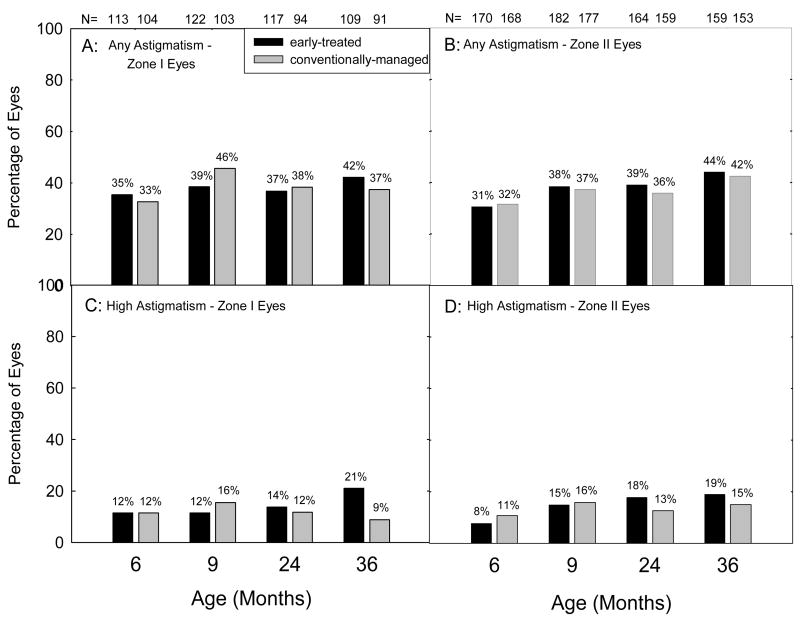
Figure 1 presents the prevalence of astigmatism (Figures 1A and 1B) and high astigmatism (Figures 1C and 1D) at 6 and 9 months and 2 and 3 years of age, stratified by location (zone 1 vs. zone II) of the acute-phase retinopathy. Not included in the data are eyes that could not be refracted at each age (Table 2). At each study age, a higher percentage of eyes with ROP in zone I than eyes with ROP in zone II were excluded due to lack of refractive error information.
Figure 1.
Prevalence of astigmatism (≥ 1.00 diopter (D), Figures 1A and 1B) and high astigmatism (≥ 2.00 D, Figures 1C and 1D) at 6 and 9 months corrected and at 2 and 3 years postnatal age as related to location (zone) of the most severe acute-phase ROP in each eye. Numbers above each bar indicate the number of eyes that provided data at each age.
Table 2.
Comparison of the percentage of eyes with zone I versus zone II Retinopathy of Prematurity (ROP) and the percentage of eyes with versus without plus disease for which refractive error data were not available due to inability to be refracted or to operative procedures expected to interfere with refractive error development.*
| Group | Age | Zone of ROP | Presence of Plus Disease | ||
|---|---|---|---|---|---|
| Zone I n/N (%) | Zone II n/N (%) | Plus n/N (%) | No Plus n/N (%) | ||
| Treated at high-risk prethreshold ROP | 6 mo | 25/138 (18.1) | 22/192 (11.5) | 31/218 (14.2) | 16/112 (14.3) |
| 9 mo | 15/137 (10.9) | 17/199 (8.5) | 25/226 (11.1) | 7/110 (6.4) | |
| 2 yr | 12/129 (9.3) | 16/180 (8.9) | 23/206 (11.2) | 5/103 (4.9) | |
| 3 yr | 13/122 (10.7) | 16/175 (9.1) | 24/201 (11.9) | 5/96 (5.2) | |
| Conventionally managed, with treatment at threshold, if reached | 6 mo | 30/134 (22.4) | 24/192 (12.5) | 37/222 (16.7) | 17/104 (16.3) |
| 9 mo | 29/132 (22.0) | 20/197 (10.2) | 36/227 (15.9) | 13/102 (12.7) | |
| 2 yr | 27/121 (22.3) | 19/178 (10.7) | 34/205 (16.6) | 12/94 (12.8) | |
| 3 yr | 25/116 (21.6) | 19/171 (11.1) | 33/199 (16.6) | 11/88 (12.5) | |
vitrectomy, scleral buckling procedures, iridectomy, glaucoma procedures, or cataract surgery
As shown in Figure 1, among the eyes that could be refracted, prevalence of astigmatism was similar in eyes with zone I ROP and eyes with zone II ROP, as was prevalence of high astigmatism. Furthermore, eyes in the ET group and eyes in the CM group showed similar prevalence of astigmatism and high astigmatism.
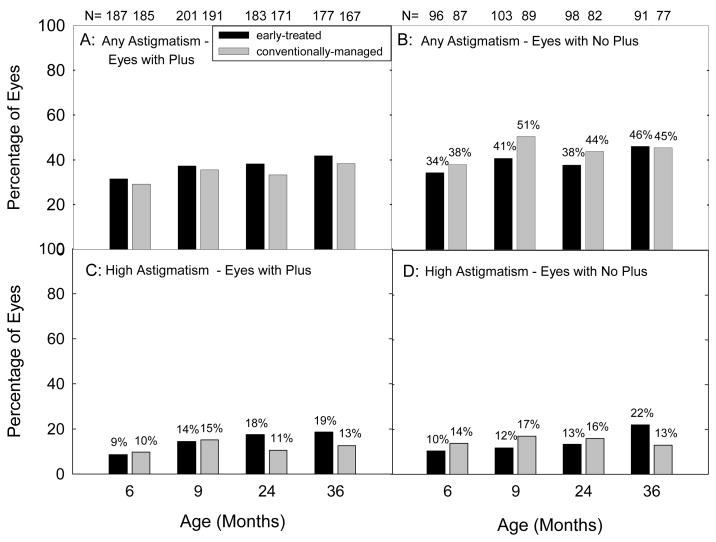
Astigmatism, High Astigmatism, and Presence of Plus Disease
Figure 2 presents the prevalence of astigmatism (Figures 2A and 2B) and high astigmatism (Figures 2C and 2D) at 6 and 9 months and 2 and 3 years of age, stratified by the presence or absence of plus disease. Not included in this data are eyes that could not be refracted at each age (Table 2). With the exception of the 6 month examination, a higher percentage of eyes with plus disease than eyes without plus disease could not be included in the analysis.
Figure 2.
Prevalence of astigmatism (≥ 1.00 diopter (D), Figures 2A and 2B) and high astigmatism (≥ 2.00 D, Figures 2C and 2D) at 6 and 9 months corrected and at 2 and 3 years postnatal age as related as related to the presence or absence of plus disease at the time of the most severe acute-phase ROP in each eye. Numbers above each bar indicate the number of eyes that provided data at each age.
As shown in Figure 2, prevalence of astigmatism and prevalence of high astigmatism were similar in ET and CM eyes with and without plus disease, for the eyes that could be refracted.
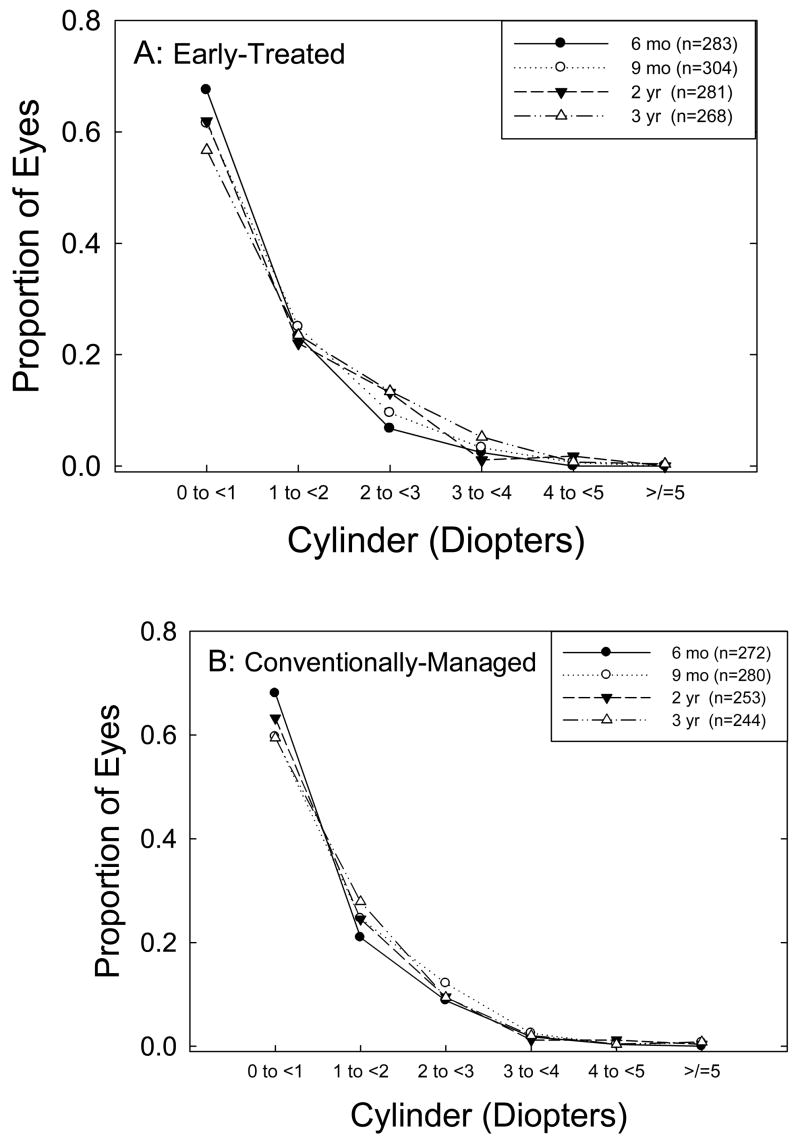
Distribution of Astigmatic Refractive Errors
Figure 3 provides the overall distribution of astigmatic refractive errors at each of the four test ages for ET eyes (3A) and for CM eyes (3B). The distributions are similar for ET and CM eyes, with both groups showing a decrease in prevalence of low astigmatism (< 1.00 D) between 6 months and 3 years.
Figure 3.
Distribution of astigmatic refractive errors at four ages in (A) eyes treated at high-risk prethreshold ROP, and (B) eyes with high-risk prethreshold ROP that were managed conventionally.
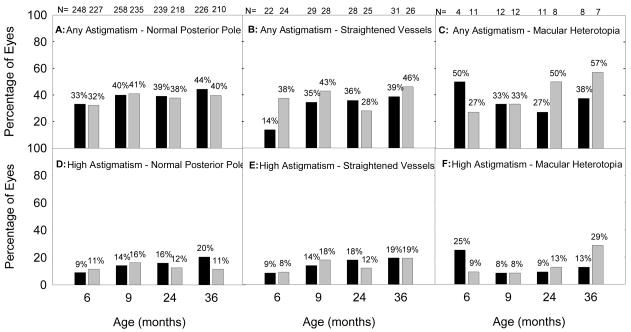
Astigmatism, High Astigmatism, and Retinal Residua of ROP
Figure 4 presents the prevalence of astigmatism (4A, 4B, and 4C) and high astigmatism (4D, 4E, and 4F) at 6 and 9 months and 2 and 3 years of age, stratified by severity of ROP residua. Prevalence of astigmatism is similar for the three categories of retinal residua of ROP, as is prevalence of high astigmatism, although this conclusion is limited by the small number of eyes with macular heterotopia at each age.
Figure 4.
Prevalence of astigmatism (≥ 1.00 diopter (D), Figures 4A, 4B and 4C) and high astigmatism (≥ 2.00 D, Figures 4D, 4F, and 4G) at 6 and 9 months corrected and at 2 and 3 years postnatal age as related to severity of retinal residua of ROP in each eye. Black bars indicate prevalence in eyes that underwent treatment at high risk prethreshold ROP; gray bars indicate prevalence in eyes with high risk prethreshold ROP that were managed conventionally. Numbers above each bar indicate the number of eyes that provided data at each age.
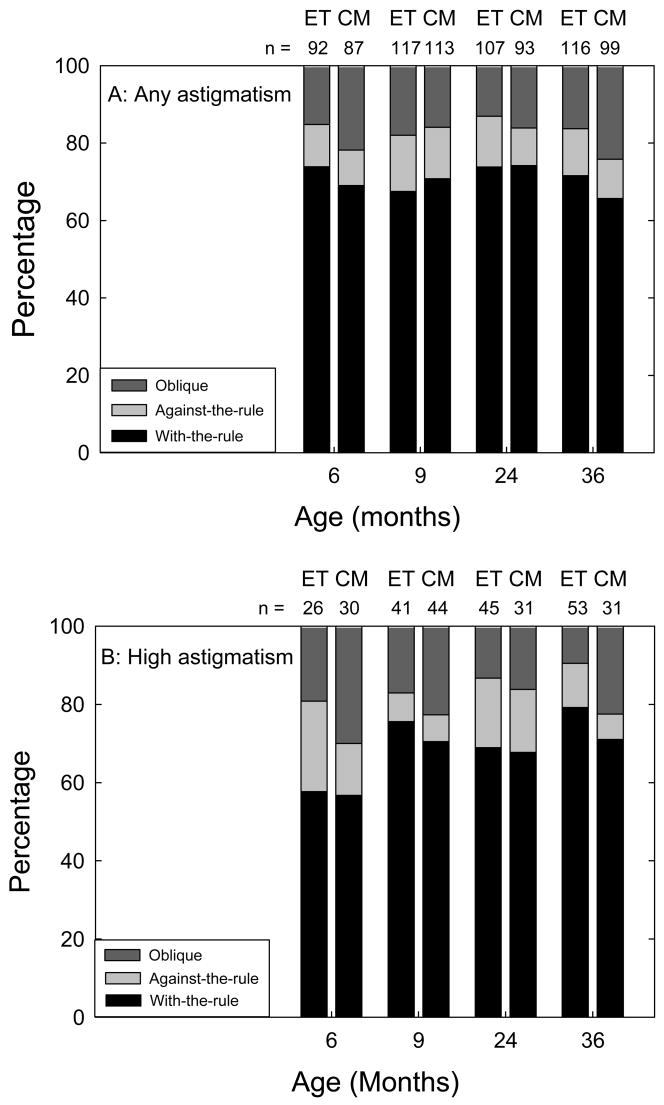
Astigmatism, High Astigmatism, and Axis of Astigmatism
At all examinations, eyes with astigmatism ≥ 1.00 D were much more likely to have WTR astigmatism than ATR or OBL astigmatism (Figure 5). The same was found with eyes having high astigmatism, although the proportion of eyes that were WTR was less at 6 months (57–58%) than at older ages (71% or higher). Little difference in the type of axis of astigmatism was seen between ET and CM eyes.
Figure 5.
Axis of astigmatism in eyes with any astigmatism (≥ 1.00 diopter (D), Figure 5A) and in eyes with high astigmatism (≥ 2.00 D, Figure 5B) at 6 and 9 months corrected and at 2 and 3 years postnatal age. Numbers above each bar indicate the number of eyes that provided data at each age. Early Treatment (ET); Conventional Management (CM)
Discussion
The results of the ETROP Study indicated that early treatment of eyes with high-risk prethreshold ROP provided better visual function and retinal structural outcomes, compared to conventional management, in which eyes were treated if they reached threshold severity.13 Significant refractive errors are a concerning and frequently associated finding in premature eyes, with or without ROP. In a previous paper, we reported no difference in the prevalence of myopia or high myopia during infancy in ET versus CM eyes with high-risk prethreshold ROP in the ETROP Study.14 A follow-up report on ETROP Study eyes indicated that approximately 70% of eyes that had high-risk prethreshold ROP during the neonatal period were myopic in early childhood, and that the proportion with high myopia increased steadily between 6 months and 3 years.16 Prevalence of myopia and high myopia were unrelated to severity of acute-phase ROP, as indicated by zone of disease and presence versus absence of plus disease, and unrelated to timing of treatment of high-risk prethreshold ROP. However, prevalence of myopia increased with increasing severity of cicatricial ROP.
The results of the present study extend the examination of refractive error in the ETROP Study population to that of astigmatism. As in the previous studies of myopia in this group of eyes,14,16 prevalence of astigmatism increased with age (Table 1 and Figure 3), but was not related to zone of ROP (Figure 1), to presence versus absence of plus disease (Figure 2), nor to timing of treatment of high-risk prethreshold disease (Table 1, Figures 1–5). However, in contrast to results for myopia, prevalence of astigmatism was unrelated to severity of cicatricial disease (Figure 4).
A comparison of our findings at 6 months (a nearly 32% prevalence of astigmatism ≥ 1.00 D and 10% prevalence of astigmatism ≥ 2.00 D) with previously published data on astigmatism in preterm infants2–5,7,8,11,17–21 is complicated by the fact that infants in the present study represent a limited subset of preterm infants, as well as a limited subset of infants with ROP. However, the finding of an increase of approximately 30% in prevalence of astigmatism ≥ 1.00 D in both ET and CM eyes between 6 months post-term and 3 years of age (Table 1) differs from the decrease in prevalence of astigmatism reported over a similar time period in three previous studies of preterm infants,2,3,5 and is more consistent with the trend reported by Theng et al. who found an increase in astigmatism during the first 3 years of life in preterm children.7 While the present results indicating a prevalence of WTR astigmatism (Figure 5) support previous reports that a majority of preterm infants have WTR astigmatism,3,4,9 it also differs from a previous report of a predominance of ATR astigmatism in infants with birth weight ≤1500 g, and a predominance of OBL astigmatism in eyes that underwent cryotherapy.2 Prevalence of OBL astigmatism in the present study was approximately 20% at all ages (Figure 5) and was similar in ET eyes (all of which received peripheral retinal ablation) and in CM eyes (only two-thirds of which received peripheral retinal ablation).
The question has also been raised whether treatment affects astigmatism. Quinn et al. compared cryotreated eyes with untreated eyes with threshold ROP and found a tendency for a greater proportion of treated than control eyes to have astigmatism of ≥ 1.00 D, at the age of 10 years.22 Based on their comparison of the distribution of refractive errors in treated eyes versus control eyes, Quinn et al. concluded that the differences were likely due to cryotherapy’s effectiveness in preserving eyes with significant refractive errors that otherwise would have been lost. With the exception of one CM eye that underwent cryotherapy, all other eyes receiving treatment in our study were treated with laser photocoagulation. Several studies looking at comparative refractive outcomes have shown that there is no difference in the prevalence of astigmatism between cryotherapy and laser photocoagulation groups.23–25 The only difference that has been found is that patients receiving cryotherapy are more likely than those receiving laser photocoagulation to have ATR astigmatism.25
This study has several limitations. First, our results must be seen in the context that eyes randomized into the ETROP Study were a very specific group of eyes, included only if prethreshold ROP was present and if the eyes were judged to be at high risk of an unfavorable outcome based on the RM-ROP2 risk management program which takes into account several variables including race, birth weight, gestational age, location and timing of onset of ROP, speed of progression and severity of acute-phase ROP.12 Therefore, an analysis of astigmatism in eyes with zone I ROP versus zone II ROP or with or without plus disease is not independent of these other variables. Also, more eyes having zone I ROP or plus disease were excluded from the study because of lack of available refractive error data. Had such data been available, the results may have demonstrated a higher prevalence of astigmatism in eyes with more severe ROP (zone I or plus disease). Lastly, although all eyes were subject to two independent examinations by study-certified ophthalmologists prior to randomization, distinguishing zone I from zone II and determining the presence of plus disease remain subjective tasks. Any redundancy between groups (e.g., zone I versus zone II; plus versus no plus) could mask differences in prevalence that might be more clearly seen if the analysis had been limited to eyes that were unquestionably in one group or another.
In summary, the results of the present study indicate that, by three years of age, over 40% of eyes with high-risk prethreshold ROP are likely to develop astigmatism ≥ 1.00 D, and nearly 10 to 20% of such eyes will have high astigmatism (≥ 2.00 D). There was no evidence that earlier treatment of eyes with high-risk prethreshold ROP influenced development of astigmatism, nor was there evidence that zone I ROP or the presence of plus disease, both indicators of severe acute-phase ROP, significantly affect astigmatism development. In contrast to myopia, which is more prevalent in eyes with cicatricial ROP than in those without,16 no significant difference was found in prevalence of astigmatism or high astigmatism between eyes with normal appearing posterior poles, those with straightened vessels, and those with macular heterotopia. The majority of astigmatic eyes had WTR astigmatic axis, and axis of astigmatism was oblique in approximately 20% of eyes, with little change over time. These findings regarding astigmatism, along with previously published data on the development of myopia in infants with high-risk prethreshold ROP, emphasize the importance of follow-up eye exams for infants who have been diagnosed with high risk prethreshold ROP. Early identification and treatment of high astigmatism and/or astigmatism at oblique axis in these patients may lessen the risk of subsequent amblyopia development.
Acknowledgments
This study was supported by cooperative agreements 5U10EY12471 and 5U10EY12472 with the National Eye Institute of the National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland.
Footnotes
No authors have proprietary interest in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dobson V, Fulton AB, Manning K, et al. Cycloplegic refractions of premature infants. Am J Ophthalmol. 1981;91:490–5. doi: 10.1016/0002-9394(81)90238-5. [DOI] [PubMed] [Google Scholar]
- 2.Holmström M, el Azazi M, Kugelberg U. Ophthalmological long-term follow up of preterm infants: a population based, prospective study of the refraction and its development. Br J Ophthalmol. 1998;82:1265–71. doi: 10.1136/bjo.82.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saunders KJ, McCulloch DL, Shepherd AJ, Wilkinson AG. Emmetropisation following preterm birth. Br J Ophthalmol. 2002;86:1035–40. doi: 10.1136/bjo.86.9.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varughese S, Varghese RM, Gupta N, et al. Refractive error at birth and its relation to gestational age. Curr Eye Res. 2005;30:423–8. doi: 10.1080/02713680590959295. [DOI] [PubMed] [Google Scholar]
- 5.Larsson EK, Holmström GE. Development of astigmatism and anisometropia in preterm children during the first 10 years of life: a population-based study. Arch Ophthalmol. 2006;124:1608–14. doi: 10.1001/archopht.124.11.1608. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor AR, Stephenson TJ, Johnson A, et al. Change of refractive state and eye size in children of birth weight less than 1701g. Br J Ophthalmol. 2006;90:456–60. doi: 10.1136/bjo.2005.083535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theng JTS, Wong TY, Ling Y. Refractive errors and strabismus in premature Asian infants with and without retinopathy of prematurity. Singapore Med J. 2000;41:393–7. [PubMed] [Google Scholar]
- 8.Larsson EK, Rydberg AC, Holmström GE. A population-based study of the refractive outcome in 10-year-old preterm and full-term children. Arch Ophthalmol. 2003;121:1430–6. doi: 10.1001/archopht.121.10.1430. [DOI] [PubMed] [Google Scholar]
- 9.Laws D, Shaw DE, Robinson J, et al. Retinopathy of prematurity: a prospective study. Review at six months Eye. 1992;6:477–83. doi: 10.1038/eye.1992.101. [DOI] [PubMed] [Google Scholar]
- 10.Kent D, Pennie F, Laws D, et al. The influence of retinopathy of prematurity on ocular growth. Eye. 2000;14:23–9. doi: 10.1038/eye.2000.6. [DOI] [PubMed] [Google Scholar]
- 11.Pennefather PM, Tin W, Strong NP, et al. Refractive errors in children born before 32 weeks gestation. Eye. 1997;11:736–43. doi: 10.1038/eye.1997.188. [DOI] [PubMed] [Google Scholar]
- 12.Hardy RJ, Palmer EA, Dobson V, et al. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Risk analysis of prethreshold retinopathy of prematurity. Arch Ophthalmol. 2003;121:1697–701. doi: 10.1001/archopht.121.12.1697. [DOI] [PubMed] [Google Scholar]
- 13.Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–96. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 14.Davitt BV, Dobson V, Good WV, et al. Early Treatment for Retinopathy of Prematurity Cooperative Group. Prevalence of myopia at 9 months in infants with high-risk prethreshold retinopathy of prematurity. Ophthalmology. 2005;112:1564–8. doi: 10.1016/j.ophtha.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Good WV, Hardy RJ. The multicenter study of Early Treatment for Retinopathy of Prematurity (ETROP) Ophthalmology. 2001;108:1013–4. doi: 10.1016/s0161-6420(01)00540-1. [DOI] [PubMed] [Google Scholar]
- 16.Quinn GE, Dobson V, Davitt BV, et al. Early Treatment for Retinopathy of Prematurity Cooperative Group. Progression of myopia and high myopia in the Early Treatment for Retinopathy of Prematurity Study: findings to 3 years of age. Ophthalmology. 2008;115:1058–64.e1. doi: 10.1016/j.ophtha.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 17.Snir M, Nissenkorn I, Sherf I, et al. Visual acuity, strabismus, and amblyopia in premature babies with and without retinopathy of prematurity. Ann Ophthalmol. 1988;20:256–8. [PubMed] [Google Scholar]
- 18.Page JM, Schneeweiss S, Whyte HEA, Harvey P. Ocular sequelae in premature infants. Pediatrics. 1993;92:787–90. [PubMed] [Google Scholar]
- 19.Darlow BA, Clemett RS, Horwood LJ, Mogridge N. Prospective study of New Zealand infants with birth weight less than 1500 g and screened for retinopathy of prematurity: visual outcome at age 7–8 years. Br J Ophthalmol. 1997;81:935–40. doi: 10.1136/bjo.81.11.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saw SM, Chew SJ. Myopia in children born premature or with low birth weight. Acta Ophthalmol Scand. 1997;75:548–50. doi: 10.1111/j.1600-0420.1997.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 21.Snir M, Friling R, Weinberger D, et al. Refraction and keratometry in 40 week old premature (corrected age) and term infants. Br J Ophthalmol. 2004;88:900–4. doi: 10.1136/bjo.2003.037499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn GE, Dobson V, Siatkowski RM, et al. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Does cryotherapy affect refractive error? Results from treated versus control eyes in the cryotherapy for retinopathy of prematurity trial. Ophthalmology. 2001;108:343–7. doi: 10.1016/s0161-6420(00)00527-3. [DOI] [PubMed] [Google Scholar]
- 23.Algawi K, Goggin M, O’Keefe M. Refractive outcome following diode laser versus cryotherapy for eyes with retinopathy of prematurity. Br J Ophthalmol. 1994;78:612–4. doi: 10.1136/bjo.78.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knight-Nanan DM, O’Keefe M. Refractive outcome in eyes with retinopathy of prematurity treated with cryotherapy or diode laser: 3 year follow up. Br J Ophthalmol. 1996;80:998–1001. doi: 10.1136/bjo.80.11.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Ghamdi A, Albiani DA, Hodge WG, Clarke WN. Myopia and astigmatism in retinopathy of prematurity after treatment with cryotherapy or laser photocoagulation. Can J Ophthalmol. 2004;39:521–5. doi: 10.1016/s0008-4182(04)80142-x. [DOI] [PubMed] [Google Scholar]