Abstract
Objective
The aim of this study was to test the effectiveness and safety of lamotrigine in maintenance of manic and depressive symptom control in pediatric bipolar disorder (PBD).
Methods
A 14-week open trial was conducted with 46 subjects presenting with mania or hypomania. Lamotrigine was slowly titrated to a therapeutic dose over an 8-week period, during which acute symptoms were stabilized using second-generation antipsychotics (SGA), followed by a 6-week lamotrigine monotherapy phase.
Results
The response rate on manic symptoms (Young Mania Rating Score [YMRS] <12) was 72%, on depressive symptoms was 82% (Children's Depression Rating Scale–Revised [CDRS-R] <40), and the remission rate was 56% at the 14-week end point, on an average end-point lamotrigine dose of 1.8 mg/lb. There was further reduction in depressive symptoms during the lamotrigine maintenance phase. Benign rash was noted in 6.4% of patients. Out of half of the subjects who were in remission at 8 week, 3 subjects (23%) relapsed by week 14.
Conclusion
Lamotrigine monotherapy appears to be effective in maintaining symptom control of manic and depressive symptoms in PBD and shows minimal adverse effects, although a future double-blind controlled trial is needed to confirm this finding. Portal of entry for lamotrigine treatment can be during acute illness and can sustain symptom control after establishing mood stabilization.
Introduction
Studies of efficacy and safety of lamotrigine in adult bipolar disorder (BD), and of safety in pediatric epilepsy, have collectively triggered interest in examining the effects of lamotrigine in pediatric bipolar disorder (PBD). Also, existing agents for mood stabilization, such as lithium and divalproex sodium, require frequent blood draws to monitor serum levels and are associated with high drop-out after 4 months either due to poor response to treatment or adverse events (Findling et al. 2005). Lamotrigine may be an attractive alternative that is indicated for maintenance treatment of adult BD in patients who have attained remission, and it has been found to be effective in preventing future depressive and manic episodes (Bowden et al. 2003; Calabrese et al. 2003). Lamotrigine was also found to be efficacious in patients with rapid cycling BD, regardless of whether they are type I or II (Calabrese et al. 2000; Goldberg et al. 2008; Suppes et al. 2008). Although some anecdotal data are available to show that lamotrigine is comparable to lithium for acute adult manic episodes (Ichim et al. 2000), others have found it to be less effective (Anand et al. 1999; Frye et al. 2000). On reviewing the major trials of lamotrigine in which it was found to be useful, patients received mood stabilizers or second-generation antipsychotics (SGAs) to treat the acute symptoms along with the lamotrigine, underlining the need to be treated with adjuvant medication in acute mania or hypomania (Goodwin et al. 2004).
Along with efficacy, safety is an important factor in choosing the appropriate medication. Lamotrigine labeling includes a black box warning for serious rash, which may discourage clinicians from prescribing it in PBD. In adult studies, rash was reported to be related to the starting dose and the rate of dose escalation (Chou and Fazzio 2006). In view of the potential risk for developing rash, it appeared appropriate to implement a slow titration regime over 8 weeks toward attaining full dose. In such slow upward titration, it is not feasible to dose lamotrigine in acutely ill patients to a therapeutic dose without rescue medications for acute symptoms.
Thus far, there is only one open trial of lamotrigine in pediatric bipolar depression (Chang et al. 2006) that examined the safety and effectiveness of this drug for primarily depressive symptoms. In a sample of 20 adolescents with bipolar spectrum disorders with current depressive episodes, lamotrigine was either used as a monotherapy or as an adjuvant to mood stabilizers (e.g., lithium or divalproex sodium) and/or SGAs to treat acute symptoms. Adding lamotrigine was found to be effective in the reduction of both depressive and manic symptoms. No serious adverse events were reported in this study.
There are no studies examining the effectiveness of lamotrigine monotherapy for maintaining symptom control in manic, mixed, and hypomanic patients with PBD. Lamotrigine may be an attractive choice for maintenance of treatment gains, particularly because of its effectiveness in depressive symptoms characterizing mixed episodes, its ability to maintain treatment gains in chronic illness, and its potential effects on rapid cycling. There is extensive literature that documented safe use of lamotrigine in pediatric epilepsy with slow upward titration of dose (Messenheimer 1998; Guberman et al. 1999).
Building on the previous work on lamotrigine use in BD and pediatric epilepsy, illustrating its usefulness in symptom control and safety with slow titration in adults, we designed the current study to test lamotrigine's effectiveness in maintaining symptom control in PBD. Our first hypothesis was that after acute symptom control was established with SGAs while titrating up lamotrigine to full dose, SGAs could be withdrawn and lamotrigine could be continued without return of symptoms. Our second hypothesis was that there would be further reduction of depressive symptoms while on a full dose of lamotrigine. This second prediction is based on preliminary evidence for its effectiveness in pediatric bipolar depression (Chang et al. 2006). Our third hypothesis was that lamotrigine would be safe and tolerable in the PBD population, with slow upward titration to full dose. In summary, the specific exploratory questions posed through this study concern introducing lamotrigine in an acute episode of manic, mixed, or hypomanic illness; its effectiveness in maintaining control of manic, hypomanic, and depressive symptoms in mixed episodes, and its safety and side-effect profile.
Methods
This was a single-site, prospective, open-label, outpatient treatment trial of lamotrigine for manic, mixed, and hypomanic episodes of PBD. The duration of the trial was 14 weeks, with an initial 8 weeks of dose titration, followed by 6 weeks of administering the full dose. This study was approved by the University of Illinois at Chicago (UIC) Institutional Review Board. Parents gave written permission and children gave assent to participate in this trial.
Subjects
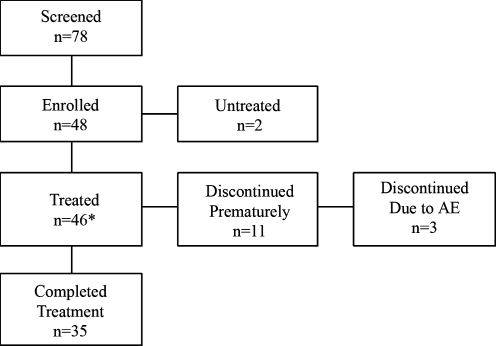
Subjects were screened at our Pediatric Mood Disorders Program to determine if they qualified for the study according to the inclusion and exclusion criteria. Inclusion criteria were: A Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994) diagnosis of BD Type I, a mixed or manic episode or BD Type II hypomanic episode, age 8–18 years old, a baseline score of >15 on the Young Mania Rating Scale (YMRS) (Young et al. 1978), and medication free or currently clinically unstable on medications justifying us to wash out the ineffective regime. To participate, subjects were required to consent to being washed out of their current medications at study entry. The washout period consisted of tapering their previous medications over 1 week prior to study entry, except for those who received aripiprazole or fluoxetine, who required a 4-week washout period. Exclusion criteria included: Active substance abuse measured through urine drug screen; serious medical problems; a history of allergy to lamotrigine; and the presence of another DSM-IV Axis I diagnosis that required psychopharmacologic treatment, including attention-deficit/hyperactivity disorder (ADHD). (In this study, onset of ADHD-like symptoms such as inattention, hyperactivity, and impulsivity after 7 years of age was not considered to be co-morbid ADHD.) A total of 78 potential subjects were screened initially. As can be seen in the CONSORT Chart (Fig. 1), a total of 46 of the initial 78 potential subjects participated in the study.
FIG. 1.
CONSORT chart of the patient flow in the lamotrigine clinical trial. AE = Adverse event; LOCF = last observation carried forward. *Included in analysis with LOCF.
Assessment procedure
All patients underwent a standard clinical assessment consisting of a diagnostic interview with the patient and family. In addition, each child and the parent or legal guardian were interviewed using the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) (Geller et al. 1998). WASH-U-KSADS interviews were completed by M.N.P. and T.M., both of whom are board-certified child psychiatrists, and a doctoral-level nurse practitioner in child psychiatry (J.A.C.). Live diagnostic interviews of 10 cases were coded by the three researchers to establish interrater reliability. Using the Cohen kappa, reliability of diagnoses was 0.94 between the raters. Clinical information from all sources was combined and further discussed to resolve any diagnostic disagreement in a weekly consensus conference involving the research team (M.N.P., J.A.C., T.M.).
Efficacy and safety measures
The primary efficacy measure was the YMRS. Secondary measures included the Clinical Global Impressions Scale for Bipolar Disorder (CGI-BP) (Spearing et al. 1997) and the Child Depression Rating Scale–Revised (CDRS-R) (Poznanski et al. 1984) to rate depressive symptoms. Three team members (J.A.C., T.M., M.M.), who have previously established interrater reliability for each of these rating scales, completed all ratings on a biweekly basis by interviewing the subject and his or her primary caregiver. For the purpose of this study, response for manic symptoms was defined as a score less than 12 on the YMRS. Response for depressive symptoms was defined as a score less than 40 on the CDRS-R. Remission was defined as CGI–Severity Overall less than or equal to 2, YMRS score less than 12, and a CDRS-R score less than 40.
Physical examinations and laboratory assessments were obtained at baseline and at the end of the study. Laboratory assessments included calcium, phosphorous, uric acid, fasting glucose, total protein, albumin, liver function tests, thyroid function tests, fasting lipid profile, creatinine kinase, electrolytes, urinalysis with drug screen, blood pregnancy test for females of child-bearing age, complete blood count (CBC), and electrocardiogram (ECG). Height, weight, blood pressure, and heart rate were also obtained. At the time of designing the study, blood testing for bioavailable androgens (free testosterone) was considered in females if one of the following features was present: Menstrual irregularities, obesity, or hyperandrogenism (i.e., hirsutism and alopecia). Adverse events were recorded using a comprehensive checklist developed by our research team (i.e., Pediatric Side Effects Checklist [P-SEC]) (Pavuluri et al. 2006). This checklist was completed every 2 weeks by a research nurse in collaboration with subjects and their primary caregivers.
Dosing of lamotrigine
It was planned to initiate lamotrigine at 12.5 mg/week. The dose was to be gradually increased at 12.5 mg/week for the first 4 weeks, 25 mg/week for the next 2 weeks, and to be titrated up to full dose (end-point dose, 150 mg if ≤30 kg body weight and 200 mg if >30 kg) during the last 2 weeks of the initial 8-week titration period.
Rationale for the initial slow titration
There are two types of helper T cells namely, T helper type-1 (Th1) and T helper type-2 (Th2). Th1 cells characteristically produce interferon-γ (IFN-γ) and immunoglobulin E (IgE), whereas Th2 cells produce interleukin-4 (IL-4), IL-5, and IL-10 and IgE. These subsets of T cells mutually suppress the responses of each other. On administration of low doses of antigen (allergen or lamotrigine in this case), desensitization and prevention of allergic responses upon future exposure to the allergen become possible. This desensitization is due to the activation of Th1 response, which can suppress the Th2 response responsible for the production of IgE antibodies involved in allergic reaction. Therefore, by introducing relatively small doses of antigen at the outset, Th1 cells, instead of Th2 cells, are activated and IgG thus produced binds to the allergen, leading to allostatic clearance of allergen through IFN-γ. The drug, then, is unlikely to induce pathogenic IgE antibody and the resultant allergic response after this initial low-dose desensitization (Kamogawa et al. 1993; Singh et al. 1999; Gor et al. 2003; Korstanje 2004).
Because this is an exploratory study to determine tolerable dosing, we adjusted dose and upward titration in the event of untoward side effects, such as nausea or vomiting. After a maximum dose was established for an individual (end-point dose, or titrated to the penultimate dose in case they reported nausea or vomiting), all patients were to be treated with that fixed dose for each individual during the last 6 weeks of monotherapy. This strategy allowed us to find an optimal and tolerable dose in this population.
Acute-phase treatment
Acute symptoms were addressed during the 8-week lamotrigine titration phase by using SGAs. This was imperative given that we recruited acutely ill unmedicated patients to this study. The order of preference for SGAs given for the first 4 weeks of acute illness was risperidone, aripiprazole, quetiapine, and ziprasidone. The order was modified according to reported previous ill effects of any SGA. For example, if patients did not respond to risperidone and were agitated on aripiprazole, they received quetiapine. The SGA was slowly withdrawn over 2–4 weeks as tolerated (i.e., between the 4- to 8-week period). An overall guideline for withdrawal of SGAs was followed with reduction at 0.25 mg of risperidone, 2.5–5 mg of aripiprazole, 25–50 mg of seroquel, or 20–40 mg of ziprasidone every other day until they were off of the SGA. All patients were on a full dose of lamotrigine monotherapy at the end of the 8-week dosing period. Benztropine was allowed on as-needed basis for extrapyramidal symptoms if on SGAs, but only during the first 4-week period.
Data analysis
Intent-to-treat analyses (Fisher et al. 1990) were carried out on scores at 8 weeks and 14 weeks. Effect sizes were calculated on subjects who remained in the study at each point by dividing the difference between baseline (week 0 or 8) and end-point (week 8 or 14) scores by the standard deviation of the appropriate baseline measure, producing a widely used measure of treatment effect (Cohen d).
Results
Demographics
As shown in the CONSORT chart, there were a total of 78 screened subjects, of which 48 were eligible; they consented and were enrolled into the study. One subject withdrew because his father withdrew consent, and another subject declined participation because of schedule conflicts. The final sample consisted of 46 subjects, 35 of whom completed 14 weeks of treatment, and 33 of which had all assessments. The urine drug screen did not show positive results for drug use in any subjects either at the study entry or the end point. Table 1 reports the demographic characteristics of the sample. The sample was approximately equally divided by gender. Ages ranged from 8 to 18 years, with a median of 13 years. Subjects presenting with manic, mixed, and hypomanic episodes were included in the sample. There were 20 (43.5%) subjects who met the cross-sectional symptom criteria for ADHD, but did not qualify for the age of onset criteria for the DSM-IV diagnosis of ADHD symptoms given that they were reported to have no ADHD-like symptoms prior to the onset of bipolar illness. Furthermore, onset of bipolar illness was reported to be earlier than 7 years of age in 8 patients, but attentional symptoms were not noted without the presence of mood instability.
Table 1.
Demographic and Clinical Characteristics of Patients with Bipolar Disorder (n = 46)
| Variable | Mean (SD) or frequency (%) |
|---|---|
| Mean age (years) | 13.3 (2.85) |
| Mean (SD) Hollingshead SES | 2.35 (0.97) |
| Gender (male) | 21 (45.7%) |
| Ethnicity | |
| African-American | 13 (28.3%) |
| Caucasian | 28 (60.9%) |
| Hispanic | 3 (6.5%) |
| Asian | 2 (4.3%) |
| Manic episode at baseline | 15 (32.6%) |
| Mixed episode at baseline | 13 (28.3%) |
| Hypomanic episode at baseline | 18 (39.1%) |
| Rapid cycling illness | 35 (76.1%) |
| Co-morbid anxiety | 6 (13.0%) |
SES = Socioeconomic status.
Dosing of lamotrigine
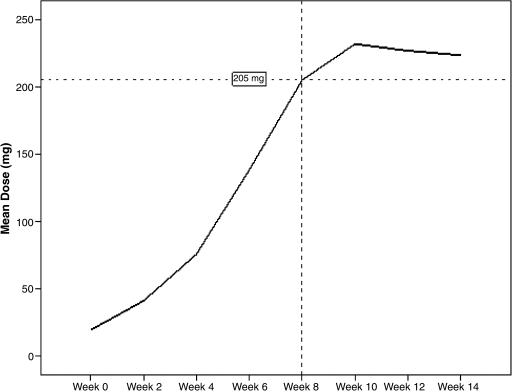
Figure 2 charts the average dose trajectory of the study. At the end of the 8-week titration period, the average dose of the sample was 205 mg. Mean end-point dose of the sample was 194.32 ± 80.31 mg. Patients were dosed at 1.8 ± 0.58 mg/lb, with no differences by age.
FIG. 2.
Dosing curve of lamotrigine over 14 weeks.
SGA use in acute phase of illness
The mean doses of SGAs were: Risperidone 1.0 ± 0.35 mg (n = 10); aripiprazole 12.5 ± 2.5 mg (n = 8); quetiapine 340 ± 52 mg (n = 9); and ziprasidone 60 ± 20 mg (n = 6). None of the subjects was on SGA or any other psychotropic medications during the 6-week trial of full-dose lamotrigine. Benzotropine was required in 6 cases with a mean dose of 1.6 ± 0.8 mg/day at the end of first 4 weeks of SGA therapy, and was weaned off subsequently, along with the SGAs.
Treatment effectiveness
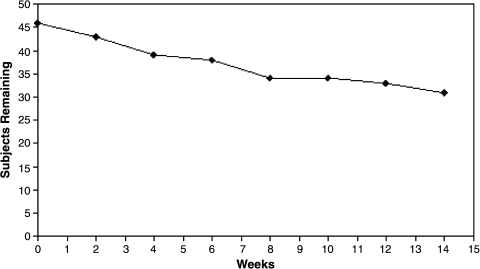
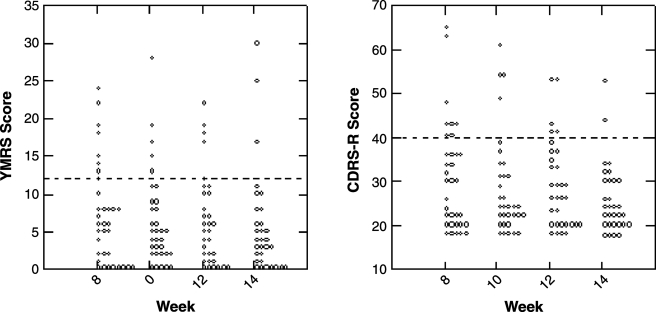
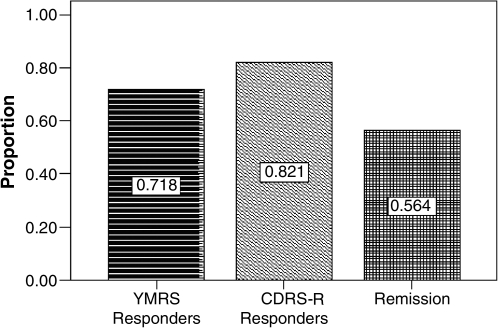
As Fig. 3 indicates, the retention rate at the end of this 14-week study was at 76.1% (n = 35). Reasons for drop out were due to adverse events (n = 3, 6.4%), inadequate response (n = 4; 8.7%), lost to follow up (n = 2; 4.4%), or protocol violation (n = 2; 4.4%). Acute symptoms subsided with acute SGA treatment in combination with the titration dose of lamotrigine in the initial 8-week period (Table 2). There is significant response rate at the end of 8 weeks on the clinician-rated YMRS at 7.06 ± 6.28 (d = 1.40, p < 0.001), and CDRS-R at 31.39 ± 12.37 (d = 1.55, p < 0.001), and parent-rated CMRS at 11.45 ± 7.04 (d = 1.05, p < 0.001). Figure 4 illustrates the pattern of response on manic and depressive symptoms over the entire 14-week study period. On the YMRS, the mania response rate was 71% at the end of 14 weeks. The depression response rate on the CDRS-R was 82% and the remission rate was 56%, also at the end of 14 weeks (Fig. 5). Out of half of the subjects who were in remission at 8 weeks, 3 subjects (23%) relapsed by week 14. Depressive symptoms continued to decline with lamotrigine monotherapy until the end point of 14 weeks, reaching a final score of 26.10 ± 7.89 (d = 0.35). Therefore, by week 14, an additional 8 subjects (34.8% of those who were not in remission at week 8) had attained remission. Furthermore, aggression and irritability as measured by the Overt Aggression Scale (OAS) significantly declined over the 8-week titration period, and this gain was maintained through the last 6 weeks of lamotrigine monotherapy. Suicidal ideation, as measured by the OAS, did not change significantly during the study period (d = 0.42, not significant [NS]).
FIG. 3.
Survival rate of patients receiving lamotrigine over 14 weeks.
Table 2.
Treatment Response to Lamotrigine Monotherapy in Manic and Hypomanic Patients
| Scale Scores | Baseline scoremean (SD) | Week 8 mean (SD) | Week 14 mean (SD) |
|---|---|---|---|
| YMRS (SD) | 19.61 (8.94) | 7.06 (6.28)** | 5.68 (7.08) |
| CDRS-R (SD) | 52.00 (13.25) | 31.39 (12.37)** | 26.10 (7.89)† |
| CGI-BP Overall | 4.32 (0.69) | 2.20 (1.19)** | 1.87 (0.92) |
| CGI-BP Mania | 3.80 (1.08) | 2.08 (1.22)** | 1.74 (0.86) |
| CGI-BP Depression score | 4.32 (1.31) | 2.36 (1.41)** | 1.87 (1.06) |
| OAS-Aggression | 9.87 (5.51) | 3.17 (4.56)** | 2.97 (3.66) |
| OAS-Irritability | 5.67 (1.95) | 2.50 (2.37)** | 1.79 (2.08) |
| OAS-Suicidality | 0.55 (1.21) | 0.03 (0.19) | 0.04 (0.19) |
| CMRS-P | 25.14 (12.98) | 11.45 (7.04)** | 10.33 (9.55) |
| Lamotrigine dose (mg/day) | 20.45 (8.98)a | 217.07 (55.46) | 194.32 (80.31) |
| Weight (lb) | 115.63 (32.37) | 118.35 (31.99) | 115.73 (32.30) |
| Fasting glucose level (mg/dl) | 78 | — | 80 |
| Cholesterol (mg/dl) | 128 | — | 120 |
| Low-density lipoprotein (mg/dL) | 54 | — | 53 |
| Thyroid-stimulating hormone (MCIU/mL) | 2 | — | 2 |
Initial dose.
p < 0.05 for baseline to week 8 comparison.
p < 0.01 for baseline to week 8 comparison.
p < 0.05 for week 8 to week 14 comparison.
SD = Standard deviation; YMRS = Young Mania Rating Scale; CDRS-R = Children's Depression Rating Scale–Revised; CGI-BP = Clinical Global Impressions Scale–Bipolar Disorder; OAS = Overt Aggression Scale; CMRS-P = Child Mania Rating Scale–Parent Version.
FIG. 4.
Clinical symptom control in patients treated with lamotrigine. YMRS = Young Mania Rating Scale; CDRS-R =Children's Depression Rating Scale-Revised.
FIG. 5.
Percentages of patients achieving response and remission with lamotrigine treatment. YMRS = Young Mania Rating Scale; CDRS-R = Children's Depression Rating Scale-Revised.
Safety and adverse events
Overall, lamotrigine was well-tolerated, and most adverse effects were mild to moderate. Side effects that were reported in more than 10% of the sample include sedation (n = 11; 23.8%), stomachache (n = 9; 19.6%), increased urination (n = 5; 10.9%), and increased appetite (n = 5; 10.9%). None of the subjects showed significant weight gain. The drop-out rate due to adverse events was 6.4% (n = 3; aged 8, 12, and 15 years), and was due to benign rash. Given the seriousness associated with potential Steven–Johnson syndrome, we considered all rashes as serious and discontinued the medication. In all instances, parents noted the rash even before it became systemic. The rash consisted of macular red blotches (erythema multiforme) spread over multiple regions of the body, manifesting initially on the trunk. There was an associated increase in temperature and systemic malaise. In 1 subject, rash appeared with a 12.5-mg dose within 3 days of starting lamotrigine. It was more extensive and overt, requiring emergency intervention on the weekend prior to seeing M.N.P. In the second subject, a rash also appeared with a 12.5-mg dose within 3 days of starting lamotrigine. It was milder when he saw M.N.P., but it progressed further over 24 h. It appeared that, even if the rash was identified early, we could not stop the trajectory unless treatment with prednisone started to show effect. Rash was treated with prednisone 10 mg/day, gradually reducing to nil over 5–7 days. In the third subject, rash manifested on a 25-mg dose early in the second week. It was minor and was treated with prednisone. It is hard to determine if prednisone contained the rash, which otherwise would have become more severe. None of the 3 subjects with rash was rechallenged with lamotrigine.
Laboratory testing indicated no significant abnormalities in CBC, liver function tests, fasting lipid profile, fasting blood glucose level, or thyroid-stimulating hormone at the study entry or the end of the study (Table 2). No significant ECG changes were noted at any point. There was no clinical indication of polycystic ovarian syndrome during the study.
Discussion
This is the first study of lamotrigine in a manic and hypomanic PBD population, and in the 8- to 18-year age range. Our main objective was to determine the effectiveness and safety of lamotrigine use in this special population. Results from the current study supported our first hypothesis that lamotrigine was effective in maintaining control of manic, hypomanic, and depressive symptoms (in mixed episodes) over 6 weeks, after acute treatment with SGAs and upward titration of lamotrigine. In addition, response on symptoms of aggression and irritability were maintained. Furthermore, there was no increase in suicidal ideation. Importantly, our findings demonstrated that SGAs could be withdrawn after reaching the full dose of lamotrigine without return of symptoms over a 6-week period. Our results also indicate that lamotrigine continued to reduce depressive symptoms during the 6-week treatment period with a full dose of this medication, similar to the findings reported by Chang et al. (2006) Finally, the proposed lamotrigine dosing regime was found to be safe and tolerable with beign rash in 6.4% of the PBD population. Lamotrigine was effective at an average dose of approximately 200 mg at the end of 8 weeks of upward titration. This dose is slightly higher than the 131.6 mg mean end dose reported by Chang et al. (2006). Adult patients required and tolerated doses from 200 mg to 500 mg (Calabrese et al. 2000; Bowden et al. 2003; Goldberg et al. 2008).
Response in manic symptoms was maintained over 6 weeks after the full dose was reached. There are no studies in pediatric mania or hypomania to draw comparisons, but adult studies have reported similar results in maintaining symptom control (Calabrese et al. 2000; Bowden et al. 2003; Goldberg et al. 2008). Our study found a similar response rate for depression to that reported by Chang et al. (2006). It is also noteworthy that there was significant improvement in depressive symptoms throughout the 14-week study. This result is similar to those of preliminary studies of lamotrigine for depressive symptoms in PBD patients (Kusumakar and Yatham 1997; Carandang et al. 2003; Chang et al. 2006). This level of response is especially attractive given the restricted repertoire of medications to treat depressive symptoms in manic, mixed, and hypomanic episodes as well as bipolar depression, with the potential risk of worsening manic symptoms and suicidal ideation with antidepressants (Biederman et al. 1996). Similar concerns may apply to lamotrigine because of its antidepressant effects. However, we found no worsening of manic symptoms or suicidal ideation during the 14-week trial period. Remission, with response on YMRS, CDRS-R, and CGI-I was similar to the rate reported by Chang et al. (2006). This level of recovery is also comparable to that seen in adult rapid cycling BD (Calabrese et al. 2000).
Ongoing work aiming to further understand the neurobiological mechanisms of lamotrigine's action as a mood stabilizer will help make sense of the response pattern in BD such as that seen in the current study. It is a use-dependent sodium channel blocker that decreases glutamate release by reducing N-methyl-d-aspartate (NMDA) glutamate receptor neurotransmission (Anand et al. 2000). Some of the earlier studies have shown that the control of glutamate release was impaired, and perhaps increased in acute episodes (Theberge et al. 2002). Lamotrigine was shown to reverse the ketamine-evoked glutamate release that caused serotonin 1A receptor activation (Martin et al. 1998; Millan et al. 1999; Deakin et al. 2008). This may help explain stabilization of mood, without worsening of agitation as is often seen with antidepressants in PBD (Biederman et al. 1996).
One of the serious concerns for using lamotrigine in PBD is the risk for rash. Although we saw widespread rash in 2 subjects and very mild rash in 1, rash resolved with treatment and with no residual effects. All 3 subjects discontinued the study due to rash. Occurrence of rash was limited to initial exposure. We adopted the strategy of not rechallenging these patients with lamotrigine given the propensity for Stevens–Johnson syndrome, known to be more common in pediatric populations (Guberman et al. 1999). There were no other major side effects. Eleven patients dropped out of the study, but only 7 withdrew due to adverse events or lack of response to treatment. There was no weight gain or development of metabolic syndrome due to lamotrigine. Given the need to treat PBD over prolonged periods, and the increased weight gain and associated increase in lipid levels observed with SGAs (Frazier et al. 2001; Pavuluri et al. 2005), lamotrigine may serve as an important alternative for attaining and maintaining mood stability.
These results must be interpreted with the caveat that this is an open trial with a small number of subjects. However, data analysis was conducted independently of the outcome ratings, which were completed by three different clinicians. Furthermore, effects of some of the antipsychotics with a longer half-life for their metabolites may have had lingering effect on patients receiving the full maintenance dose of lamotrigine. Again, the withdrawal of antipsychotics was over 4 weeks, and the clinical trial that followed was for 6 weeks period, which may have mitigated this possibility.
Conclusions
This was an exploratory study to find lamotrigine's role in mood stabilization in adolescent BD. We found it to be effective in maintaining symptom control of a broad range of manic, depressive, irritable, and aggressive symptoms in PBD. There was no increase in suicidal ideation. Slow titration resulted in a benign rash in 6.4% of patients that resolved with discontinuation of the medication or treatment with prednisone. Portal of entry for lamotrigine appeared to be feasible and advantageous in acute illness, where it can be added to SGAs to gain effective symptom control and maintenance at an average dose of 200 mg/day. There was no weight gain or related metabolic abnormalities.
Disclosures
Dr. Pavuluri's work is supported by the National Institutes of Health (NIH), National Institute of Child Health and Human Development (NICHD), NARSAD, Dana Foundation, Colbeth Foundation, Abbott Pharmaceuticals (study medication), and Janssen Research Foundation (study medication). Dr. Sweeney has received support from NIH, GlaxoSmithKline, AstraZeneca, and Eli Lilly. The other authors have no financial ties or conflicts of interests to disclose. All data analysis and writing for this manuscript were completed by the authors.
Footnotes
Dr. Henry served as the statistical expert on this manuscript.
This research was funded by NIH-MO1-RR-13987 and GlaxoSmithKline-NeuroHealth.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington (DC): American Psychiatric Association; 1994. (DMA-IV) [Google Scholar]
- Anand A. Oren DA. Berman RM. Cappiello A. Charney DS. Lamotrigine treatment of lithium failure outpatient mania—a double-blind, placebo-controlled trial. In: Soares JC, editor; Gershon S, editor. Bipolar Disorders, Abstract Book, Third International Conference on Bipolar Disorder; Pittsburgh, PA. Copenhagen: Munksgaard; 1999. p. 23. June 17–19. [Google Scholar]
- Anand A. Charney DS. Oren DA. Berman RM. Hu XS. Cappiello A. Krystal JH. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: Support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- Biederman J. Faraone S. Mick E. Wozniak J. Chen L. Ouellette C. Marrs A. Moore P. Garcia J. Mennin D. Lelon E. Attention-deficit hyperactivity disorder and juvenile mania: An overlooked comorbidity? J Am Acad Child Adolesc Psychiatry. 1996;35:997–1008. doi: 10.1097/00004583-199608000-00010. [DOI] [PubMed] [Google Scholar]
- Bowden CL. Calabrese JR. Sachs G. Yatham LN. Asghar SA. Hompland M. Montgomery P. Earl N. Smoot TM. DeVeaugh-Geiss J. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60:392–400. doi: 10.1001/archpsyc.60.4.392. [DOI] [PubMed] [Google Scholar]
- Calabrese JR. Suppes T. Bowden CL. Sachs GS. Swann AC. McElroy SL. Kusumakar V. Ascher JA. Earl NL. Greene PL. Monaghan ET. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. Lamictal 614 Study Group. J Clin Psychiatry. 2000;61:841–850. doi: 10.4088/jcp.v61n1106. [DOI] [PubMed] [Google Scholar]
- Calabrese JR. Bowden CL. Sachs G. Yatham LN. Behnke K. Mehtonen OP. Montgomery P. Ascher J. Paska W. Earl N. DeVeaugh-Geiss J. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry. 2003;64:1013–1024. doi: 10.4088/jcp.v64n0906. [DOI] [PubMed] [Google Scholar]
- Carandang CG. Maxwell DJ. Robbins DR. Oesterheld JR. Lamotrigine in adolescent mood disorders. J Am Acad Child Adolesc Psychiatry. 2003;42:750–751. doi: 10.1097/01.CHI.0000046884.27264.28. [DOI] [PubMed] [Google Scholar]
- Chang K. Saxena K. Howe M. An open-label study of lamotrigine adjunct or monotherapy for the treatment of adolescents with bipolar depression. J Am Acad Child Adolesc Psychiatry. 2006;45:298–304. doi: 10.1097/01.chi.0000194566.86160.a3. [DOI] [PubMed] [Google Scholar]
- Chou JC. Fazzio L. Maintenance treatment of bipolar disorder: Applying research to clinical practice. J Psychiatr Pract. 2006;12:283–299. doi: 10.1097/00131746-200609000-00003. [DOI] [PubMed] [Google Scholar]
- Deakin JF. Lees J. McKie S. Hallak JE. Williams SR. Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: A pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- Findling RL. McNamara NK. Youngstrom EA. Stansbrey R. Gracious BL. Reed MD. Calabrese JR. Double-blind 18-month trial of lithium versus divalproex maintenance treatment in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:409–417. doi: 10.1097/01.chi.0000155981.83865.ea. [DOI] [PubMed] [Google Scholar]
- Fisher LD. Dixon DO. Herson J. Frankowski RK. Hearon MS. Pearce KE. Intention to treat in clinical trials. In: Pearce KE, editor. Statistical Issues in Drug Research and Development. New York: Marcel Dekker; 1990. pp. 331–350. [Google Scholar]
- Frazier JA. Biederman J. Tohen M. Feldman PD. Jacobs TG. Toma V. Rater MA. Tarazi RA. Kim GS. Garfield SB. Sohma M. Gonzalez-Heydrich J. Risser RC. Nowlin ZM. A prospective open-label treatment trial of olanzapine monotherapy in children and adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2001;11:239–250. doi: 10.1089/10445460152595568. [DOI] [PubMed] [Google Scholar]
- Frye MA. Ketter TA. Kimbrell TA. Dunn RT. Speer AM. Osuch EA. Luckenbaugh DA. Cora-Ocatelli G. Leverich GS. Post RM. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607–614. doi: 10.1097/00004714-200012000-00004. [DOI] [PubMed] [Google Scholar]
- Geller B. Warner K. Williams M. Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: Assessment and validity using the WASH-U-KSADS, CBCL, and TRF. J Affect Disord. 1998;51:93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- Goldberg JF. Bowden CL. Calabrese JR. Ketter TA. Dann RS. Frye MA. Suppes T. Post RM. Six-month prospective life charting of mood symptoms with lamotrigine monotherapy versus placebo in rapid cycling bipolar disorder. Biol Psychiatry. 2008;63:125–130. doi: 10.1016/j.biopsych.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Goodwin GM. Bowden CL. Calabrese JR. Grunze H. Kasper S. White R. Greene P. Leadbetter R. A pooled analysis of 2 placebo-controlled 18-month trials of lamotrigine and lithium maintenance in bipolar I disorder. J Clin Psychiatry. 2004;65:432–441. doi: 10.4088/jcp.v65n0321. [DOI] [PubMed] [Google Scholar]
- Gor DO. Rose NR. Greenspan NS. TH1-TH2: A procrustean paradigm. Nat Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- Guberman AH. Besag FM. Brodie MJ. Dooley JM. Duchowny MS. Pellock JM. Richens A. Stern RS. Trevathan E. Lamotrigine-associated rash: Risk/benefit considerations in adults and children. Epilepsia. 1999;40:985–991. doi: 10.1111/j.1528-1157.1999.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Ichim L. Berk M. Brook S. Lamotrigine compared with lithium in mania: A double-blind randomized controlled trial. Ann Clin Psychiatry. 2000;12:5–10. doi: 10.1023/a:1009066725103. [DOI] [PubMed] [Google Scholar]
- Kamogawa Y. Minasi LA. Carding SR. Bottomly K. Flavell RA. The relationship of IL-4- and IFN gamma-producing T cells studied by lineage ablation of IL-4-producing cells. Cell. 1993;75:985–995. doi: 10.1016/0092-8674(93)90542-x. [DOI] [PubMed] [Google Scholar]
- Korstanje C. The T-helper1/T-helper2 paradigm: still valid for drug discovery but with some essential refinement. Curr Opin Investig Drugs. 2004;5:487–488. [PubMed] [Google Scholar]
- Kusumakar V. Yatham LN. An open study of lamotrigine in refractory bipolar depression. Psychiatry Res. 1997;72:145–148. doi: 10.1016/s0165-1781(97)00082-6. [DOI] [PubMed] [Google Scholar]
- Martin P. Carlsson ML. Hjorth S. Systemic PCP treatment elevates brain extracellular 5-HT: A microdialysis study in awake rats. Neuroreport. 1998;9:2985–2988. doi: 10.1097/00001756-199809140-00012. [DOI] [PubMed] [Google Scholar]
- Messenheimer JA. Rash in adult and pediatric patients treated with lamotrigine. Can J Neurol Sci. 1998;25:S14–S18. doi: 10.1017/s0317167100034910. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Brocco M. Gobert A. Joly F. Bervoets K. Rivet J. Newman-Tancredi A. Audinot V. Maurel S. Contrasting mechanisms of action and sensitivity to antipsychotics of phencyclidine versus amphetamine: Importance of nucleus accumbens 5-HT2A sites for PCP-induced locomotion in the rat. Eur J Neurosci. 1999;11:4419–4432. doi: 10.1046/j.1460-9568.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. Birmaher B. Naylor MW. Pediatric bipolar disorder: A review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. Henry DB. Carbray JA. Sampson GA. Naylor MW. Janicak PG. A one-year open-label trial of risperidone augmentation in lithium nonresponder youth with preschool-onset bipolar disorder. J Child Adolesc Psychopharmacol. 2006;16:336–350. doi: 10.1089/cap.2006.16.336. [DOI] [PubMed] [Google Scholar]
- Poznanski E. Grossman J. Buchsbaum Y. Banegas M. Freeman L. Gibbons R. Preliminary studies of the reliability and validity of the children's depression rating scale. J Am Acad Child Adolesc Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Singh VK. Mehrotra S. Agarwal SS. The paradigm of Th1 and Th2 cytokines: Its relevance to autoimmunity and allergy. Immunol Res. 1999;20:147–161. doi: 10.1007/BF02786470. [DOI] [PubMed] [Google Scholar]
- Spearing MK. Post RM. Leverich GS. Brandt D. Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): The CGI-BP. Psychiatry Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Suppes T. Marangell LB. Bernstein IH. Kelly DI. Fischer EG. Zboyan HA. Snow DE. Martinez M. Al Jurdi R. Shivakumar G. Sureddi S. Gonzalez R. A single blind comparison of lithium, lamotrigine for the treatment of bipolar II depression. J Affect Disord Epub March. 2008;19 doi: 10.1016/j.jad.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge J. Bartha R. Drost DJ. Menon RS. Malla A. Takhar J. Neufeld RW. Rogers J. Pavlosky W. Schaefer B. Densmore M. Al Semaan Y. Williamson PC. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- Young RC. Biggs JT. Ziegler VE. Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]