Abstract
The purpose of this study was to determine whether a dietary supplement consisting of L-selenomethionine, vitamin C, vitamin E succinate, α-lipoic acid and N-acetyl cysteine could improve the survival of mice after total-body irradiation. Antioxidants significantly increased the 30-day survival of mice after exposure to a potentially lethal dose of X rays when given prior to or after animal irradiation. Pretreatment of animals with antioxidants resulted in significantly higher total white blood cell and neutrophil counts in peripheral blood at 4 and 24 h after 1 Gy and 8 Gy. Antioxidants were effective in preventing peripheral lymphopenia only after low-dose irradiation. Antioxidant supplementation was also associated with increased bone marrow cell counts after irradiation. Supplementation with antioxidants was associated with increased Bcl2 and decreased Bax, caspase 9 and TGF-β1 mRNA expression in the bone marrow after irradiation. Maintenance of the antioxidant diet was associated with improved recovery of the bone marrow after sublethal or potentially lethal irradiation. Taken together, oral supplementation with antioxidants appears to be an effective approach for radioprotection of hematopoietic cells and improvement of animal survival, and modulation of apoptosis is implicated as a mechanism for the radioprotection of the hematopoietic system by antioxidants.
INTRODUCTION
Exposure to ionizing radiation results in a deposition of energy in tissues that can damage cellular structures, including DNA (1). The biological effects of ionizing radiation are mediated in part by free radicals. During radiation exposure, three parameters determine radiation toxicity: the total dose absorbed, the rate at which the dose is delivered and the quality of the radiation (1, 2). Total-body irradiation (TBI) may result in a hematopoietic, gastrointestinal (GI) or cerebrovascular syndrome as well as multiple organ dysfunction depending on the total dose absorbed (1–4). Consequently, medical management of the acute radiation syndrome must address the associated risk of infection and hemorrhage (4–7).
Despite the devastating sequelae of TBI, there is currently a lack of prophylactic (radioprotective) or therapeutic agents that are safe, effective and approved for patient care (2, 5). This medical concern is heightened due to the risk of radiological terrorism or radiation accidents (1, 3, 6, 8). Protection or reconstitution of the hematopoietic and immune systems is a critical area of research in the development of radioprotectors and therapeutic agents (2, 5–7). Nutraceuticals including vitamin C, vitamin E succinate, selenium, α-lipoic acid and N-acetyl cysteine (NAC), in addition to hematopoietic growth factors and cytokines such as stem cell factor (SCF), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 3 (IL3) are among agents that show hematopoietic radioprotective effects after TBI (2, 9–12). These various radioprotectors have commonly been evaluated in animal models as injection formulations.
A major consideration with injectable formulations of hematopoietic growth factors and cytokines is that they usually require trained personnel for administration before or soon after radiation exposure; this may not be feasible in many instances of radiation exposure. In addition, the ability to stockpile the hematopoietic growth factors and cytokines may be a limiting factor in the event of a massive radiological terrorist attack or radiation accident (4). Furthermore, the prolonged administration of hematopoietic cytokines and growth factors is a concern for pro-inflammatory and immunogenic effects in addition to exhaustion of the hematopoietic stem and progenitor cell reservoir (4, 11, 13).
Antioxidant vitamins offer several advantages over other potential radioprotective agents. They are readily available and have low toxicity over a wide dose range; they can be administered orally; they are effective in attenuating numerous radiation-induced phenomena such as chromosomal DNA damage, mutagenesis, transformation and the formation of clastogenic factors (14–17). It was shown that the bone marrow of mice is depleted of endogenous vitamins C and E after TBI at doses as low as 50 cGy within 24 h (18). Depletion of vitamins C and E coincided with increased markers of oxidative cellular damage in the bone marrow (18, 19). Irradiation with 3 Gy resulted in repletion of endogenous vitamin C and E levels in the bone marrow 1 week after exposure; however, no recovery was observed after 6 Gy TBI (18). Therefore, repletion of antioxidant vitamins in the bone marrow, particularly in the myelosuppressive dose range, may be a putative therapeutic target for radioprotection or recovery of the hematopoietic system after exposure to ionizing radiation.
Hematopoietic recovery after TBI is dependent on the presence of spared hematopoietic stem and progenitor cells in the bone marrow (4). The hematopoietic stem cell niche components in the bone marrow, including osteoblasts, regulate stem cell cycling and are important targets in effecting bone marrow recovery after radiation-induced myelosuppression (11, 20). Therefore, protection of various cell types exposed to radiation through antioxidant supplementation is an approach that could potentially increase hematopoietic cell survival or recovery after TBI.
Our previous studies have shown that exposure of human epithelial cell lines in vitro to various types of ionizing radiation [γrays, X rays, protons and high-atomic number and high-energy (HZE) particles] induces oxidative stress which is abolished by an antioxidant combination containing L-selenomethionine (SeM), vitamin C, vitamin E succinate, α-lipoic acid and NAC (21, 22). Likewise, we reported that TBI of mice and rats with γ rays, protons or HZE particles decreased the serum total antioxidant capacity within 4 h, which was prevented by dietary supplementation with antioxidants (23, 24). The antioxidant combination was formulated to provide the equivalent of 2000 mg/day, 1000 mg/day and 400 μg/day, which represent the upper limits of the RDA for vitamin C, vitamin E succinate and selenium, respectively (25). Although there is no published RDA for NAC or α-lipoic acid, these thiol supplements were formulated according to effective doses determined previously for humans, 2400 mg/day and 1200 mg/day, respectively (26, 27).
The purpose of this study was to determine the efficacy of the previously characterized antioxidant supplement in improving the survival of mice irradiated with a potentially lethal dose of X rays. We hypothesized that dietary anti-oxidants could protect peripheral leukocytes and bone marrow cells from radiation-induced depletion as well as promote recovery of the bone marrow of irradiated mice. The results demonstrate that dietary antioxidants prevent peripheral neutropenia and bone marrow cell depletion after low- and high-dose TBI. Prophylactic and therapeutic oral administration of the antioxidant supplement significantly increased survival of animals exposed to a potentially lethal dose of radiation and had a protective effect on cells of hematopoietic origin.
MATERIALS AND METHODS
Animals
Male ICR mice aged 4–5 weeks were purchased from Taconic Farms Inc. (Germantown, NY). Animals were acclimated for 7 days in the University of Pennsylvania animal facility. Five animals were housed per cage with ad libitum access to water and food pellets. The animal care and treatment procedures were approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania and the Brook-haven National Laboratory.
Survival Experiment
Upon acclimation, the animals were randomly assigned to the AIN-93G rodent (Control) diet or AIN-93G diet supplemented with SeM (0.06 μg/g diet), α-lipoic acid (85.7 μg/g diet), NAC (171.4 μg/g diet), sodium ascorbate (142.8 μg/g diet), and vitamin E succinate (71.4 μg/g diet); the diets were obtained from Bio-Serv (Frenchtown, NJ). After 7 days, the animals were exposed to total-body X rays. Animals were maintained on their respective diets until they were killed humanely except where noted. One group of irradiated animals (AO → Control) was changed from the antioxidant diet to the control diet 4 h prior to irradiation and maintained on the control diet for the remainder of the experiment. Another group of irradiated animals (Control → AO) was changed from the control diet to the antioxidant diet 2 h after irradiation and maintained on this diet for the remainder of the experiment. Animals were evaluated twice daily after irradiation. Moribund animals were euthanized.
Irradiation
Total-body irradiation of animals was performed with an orthovoltage Philips RT-250 irradiator, 225 kVp X-ray source (University of Pennsylvania) operating at 15 mA and filtered with 0.2 cm copper. The animals were restrained in holders and exposed to TBI at a dose rate of 3.4 Gy/min for total doses of 1, 6 and 8 Gy. For determinations of bone marrow cellularity, animals were exposed to 1 Gy total-body γ radiation at a dose rate of 5 cGy/min or 7 Gy at a dose rate of 35 cGy/min using a cesium-137 source (Controlled Environment Radiation Facility, Brookhaven National Laboratory, Upton, NY). Sham-irradiated animals were restrained similarly. All animals were weighed prior to irradiation. After irradiation animals were returned to the animal facility.
Peripheral Hematopoietic Evaluations
Some animals were killed 4 h and 24 h after irradiation for peripheral complete blood cell (CBC) analyses. Some moribund animals that received 8 Gy X rays in the 30-day survival experiment underwent CBC analysis when they were euthanized. Animals were killed by CO2 asphyxiation followed by cardiac puncture under sterile conditions. Blood was collected in Eppendorf tubes containing 20 U of heparin and kept at ambient temperature. A 50-μl whole blood aliquot per animal was diluted with 200 μl 5% BSA in PBS and analyzed with an ADVIA 2120 Hematology System (Bayern Diagnostic, Dublin, Ireland).
Blood Culture
Moribund animals were euthanized by CO2 asphyxiation followed by cardiac puncture under sterile conditions. The blood specimens were immediately transferred aseptically into 20-ml bottles of BBL Septi-Chek medium with trypticase soy broth or brain heart infusion (Becton Dickinson, Cockeysville, MD). The bottles were kept at ambient temperature in the dark until submission to Antech Diagnostics (Lake Success, NY) for analysis.
Bone Marrow Cell Isolation, Quantification, RNA Isolation, and cDNA Synthesis
Tibiae and femurs were removed and the ends of the bones were cut bluntly. The bone marrow cavity was flushed with PBS using a sterile 22-gauge needle. The bone marrow was carefully resuspended to obtain a single cell suspension, aliquots were taken for cell counts, and the remaining cells were centrifuged at 1000 rpm for 10 min at 4°C, resuspended in RNAlater stabilization solution (Qiagen, Valencia, CA), and stored at −80°C. Bone marrow cellularity was determined in aliquots using a Coulter Counter. Total RNA was isolated using RNeasy Mini Kit (Qiagen). cDNA was synthesized using the Superscript II First Strand cDNA Synthesis System (Invitrogen, Carlsbad, CA) using 2 μg Total RNA, then was diluted and frozen in aliquots and stored at −20°C.
Quantitative Real-Time PCR
Primers were designed using the default parameters of the Primer Express® software v2.1 (PE Applied Biosystems). Primer sets were designed based on the relevant murine nucleotide sequences as deposited on GenBank and supplied by IDT (Coralville, IA). The primer sequences were as follows: BAX forward primer 5′-AGACACCTGAGCTGACCT TGGA-3′and reverse primer 5′-GAGACACTCGCTCAGCTTCTTG-3′; BCL2 forward primer 5′-TGCCACCTGTGGTCCATCT-3′and reverse primer 5′-GTGCAGCTGACTGGACATCTCT-3′; caspase 9 forward primer 5′-TCACGGCTTTGATGGAGATG-3′and reverse primer 5′-GAGGATGACCACCACAAAGCA-3′; caspase 7 forward primer 5′-ACAGCAACTCGGCCTGCTT-3′and reverse primer 5′-TTCCCGT AAATCAGGTCCTCTTC-3′; caspase 8 forward primer 5′-AAGGCAA TCTGTCTTTCCTGAAA-3′and reverse primer 5′-TCGGTTGCAGTC TAGGAAGTTG-3′; TGF-beta 1 forward primer 5′-TGGAGCAACAT GTGGAACTC-3′and reverse primer 5′-GTCAGCAGCCGGTTAC CA-3′; and HPRT forward primer 5′-GAAAGACTTGCTCGAGATGT CATG-3′and reverse primer 5′-CACACAGAGGGCCACAATGT-3′.
cDNA generated was amplified in Sybr Advantage qPCR Premix (Clontech, Mountain View, CA), consisting of a full-length Taq™ poly-merase with a hot-start Taq antibody and SYBR Green I, by using an ABI 7500 Fast Real Time PCR System (PE Applied Biosystems). A sample volume of 20 μl was used for all assays and contained a 1× final concentration of SYBR Advantage Premix, 250 nM gene-specific primers, and 2 μl cDNA template. Assays were carried out using the following protocol: stage 1, 95°C for 20 s, stage 2, 95°C for 3 s, 58–64°C for 34 s (depending on optimal annealing temperature), and stage 2 repeated for 40 cycles followed by the dissociation stage. A cycle threshold (Ct) was assigned at the beginning of the logarithmic phase of PCR amplification, and the difference between the Ct values of the control and experimental samples were used to determine the relative expression of the gene in each sample. Relative expression levels were normalized to the constitutively expressed housekeeping gene hypoxanthinephophoribosyltransferase (HPRT).
Statistical Analysis
The CBC counts were compared between control and antioxidant treatment groups by a Student’s t test. The Kaplan-Meier 30-day survival curves were compared using a log-rank test. The proportions of positive blood cultures between days 7–10 and days 11–19 after irradiation were compared by a χ2 test. qPCR data were analyzed with one-way ANOVA followed by Bonferroni’s post-hoc test or Student’s t test. The statistical analyses were performed using Prism Version 2.01 statistical software (GraphPad Software, San Diego, CA). P < 0.05 was accepted as statistically significant.
RESULTS
Thirty-Day Survival of Mice after Total-Body Irradiation
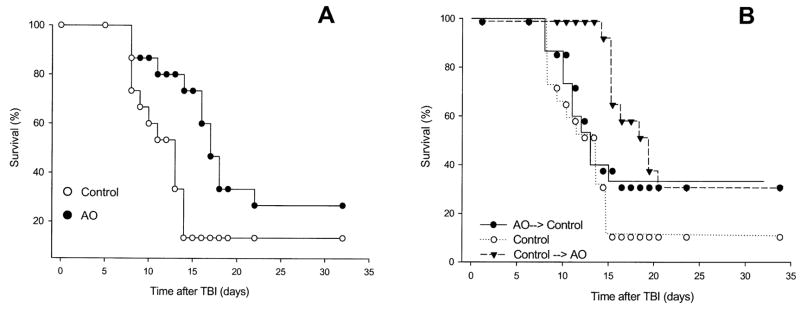
The effects of dietary antioxidant supplementation on survival were determined in mice irradiated with X rays at a total-body dose of 8 Gy. Antioxidant supplementation initiated 7 days prior to TBI and maintained for the duration of the observation period was effective in improving survival (Fig. 1A, P = 0.034). The antioxidant treatment resulted in a hazard ratio of 0.46 (0.15–0.93) compared to irradiated animals maintained on the control diet. The Control → AO treatment exhibited a significant survival benefit (Fig. 1B, P = 0.0057) with a hazard ratio of 0.37 (0.096–0.67). The AO → Control treatment also increased the survival of the irradiated animals compared to irradiated animals fed the control diet, although the increase was not statistically significant (Fig. 1B, P = 0.29). The 30-day survivals for the animals fed antioxidant-supplemented diets starting 7 days prior to TBI or 2 h after the irradiation were not significantly different (P = 0.70). We observed that 6 Gy TBI with X rays resulted in 0/20 and 1/20 animal deaths in mice fed the control or antioxidant diet for 7 days prior to irradiation and maintained on these diets for the subsequent 30 days (data not shown).
FIG. 1.
Effect of antioxidants (AO) on mouse survival after 8 Gy TBI. Panel A: Male ICR mice were fed a control diet (n = 15) or the diet supplemented with antioxidants (n = 15) for 7 days prior to TBI. The animals were maintained on their respective diets and observed for 30 days after TBI. Panel B: The survival of mice fed the control diet was compared to that of animals fed the antioxidant-supplemented diet for 7 days and transferred to the control diet 4 h prior to TBI (AO → Control, n = 15) as well as that of animals fed the control diet for 7 days and transferred to the antioxidant-supplemented diet 2 h after TBI (Control → AO, n = 15). The animals were maintained on the latter diets for the remainder of the 30-day observation period. Assessment of survival in irradiated animals fed the control diet was carried out twice; the results of only one of these experiments are shown.
Peripheral White Blood Cell Count 4 and 24 h after TBI
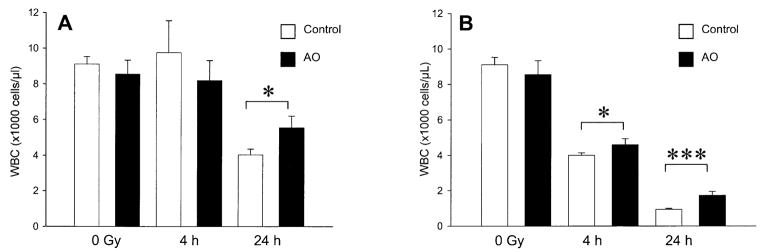
There was no significant change in total peripheral white blood cell count 4 h after 1 Gy TBI in mice fed either control or antioxidant-supplemented diets (Fig. 2). At 24 h after 1 Gy TBI, animals fed the antioxidant-supplemented diet had significantly higher total white blood cell counts than the animals fed the control diet (Fig. 2A, P = 0.022), although both groups had lower counts than unirradiated animals. Irradiation with 8 Gy resulted in decreased peripheral white blood cell counts at 4 h and 24 h after TBI in both the control mice and those fed the antioxidant diets. Dietary antioxidant treatment was associated with higher total white blood cell counts at 4 (P = 0.024) and 24 (P = 0.0004) h after 8 Gy compared to the control diet (Fig. 2B).
FIG. 2.
Effect of prophylactic dietary antioxidant (AO) supplementation on peripheral white blood cell counts after low- and high-dose TBI. Panel A: Male ICR mice were fed a control diet or the diet supplemented with antioxidants for 7 days prior to TBI with 1 Gy. The animals were killed 4 h after TBI (control, n = 4 and antioxidant, n = 4) or 24 h after TBI (control, n = 3 and antioxidant, n = 3). Unirradiated control diet (n = 4) and unirradiated antioxidant diet (n = 4) groups were also analyzed. Panel B: Mice were irradiated with 8 Gy and killed at 4 h after TBI (control, n = 4 and antioxidant, n = 3) or 24 h after TBI (control, n = 4 and antioxidant, n = 4). Unirradiated control diet (n = 4) and unirradiated antioxidant diet (n = 4) groups were also analyzed. The bars are means ± SD. Significant difference at *P < 0.05 or ***P < 0.0001 as analyzed by unpaired two-tailed Student’s t test.
Peripheral Polymorphonuclear Cell Count 4 and 24 h after TBI
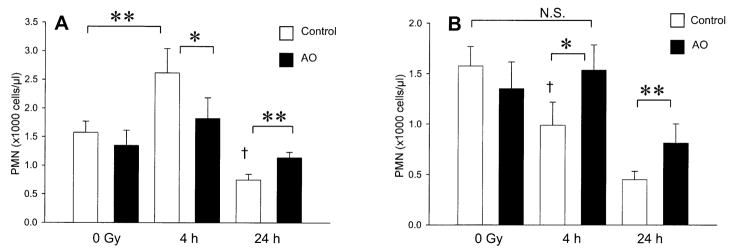
Irradiation with a dose of 1 Gy resulted in a significant increase in peripheral polymorphonuclear cell counts 4 h after TBI in animals fed the control diet (Fig. 3A, P = 0.0068). No increase in peripheral polymorphonuclear cells was observed 4 h after TBI in mice fed the antioxidant diets (Fig. 3A, P = 0.26). At 24 h after 1 Gy, a significant decrease in peripheral polymorphonuclear cell counts was observed in animals fed the control and antioxidant diets; however, mice fed the antioxidant diets had significantly more peripheral polymorphonuclear cells (Fig. 3A, P = 0.0033). Exposure to 8 Gy resulted in a significant decrease in peripheral polymorphonuclear cell counts in animals fed the control diet at 4 h (Fig. 3B, P = 0.0077), and antioxidant supplementation completely prevented this decrease (Fig. 3B, P = 0.81). At 24 h, dietary antioxidants significantly attenuated the decrease in peripheral polymorphonuclear cells induced by 8 Gy (Fig. 3B, P = 0.01). At 24 h after 8 Gy, the absolute neutrophil count was 450 ± 80 cells/μl in control animals and 800 ± 200 cells/μl in the animals fed the antioxidant diet. Unirradiated animals fed the control diet and antioxidant diet had absolute neutrophil counts of 1600 ± 200 and 1400 ± 300 cells/μl, respectively.
FIG. 3.
Effect of prophylactic dietary antioxidant (AO) supplementation on peripheral neutrophil (PMN) counts after low- and high-dose TBI. Panel A: Male ICR mice were fed a control diet or the diet supplemented with antioxidants for 7 days prior to TBI with 1 Gy. The animals were killed 4 h after TBI (control, n = 3 and antioxidant, n = 3) or 24 h after TBI (control, n = 3 and antioxidant, n = 4). Unirradiated control diet (n = 4) and unirradiated antioxidant diet (n = 4) groups were also analyzed. Panel B: Mice were irradiated with 8 Gy and killed 4 h after TBI (control, n = 3 and antioxidant, n = 3) or 24 h after TBI (control, n = 4 and antioxidant, n = 4). Unirradiated control diet (n = 4) and unirradiated antioxidant diet (n = 4) groups were also analyzed. Bars show means ± SD. Significant difference at *P < 0.05 or **P < 0.01 as analyzed by unpaired two-tailed Student’s t test. †Significant difference at P < 0.01 compared to unirradiated control diet-fed group; corresponding irradiated antioxidant-fed group showed no significant (N.S.) difference from respective unirradiated antioxidant and control diet-fed group.
Peripheral Lymphocyte Count 4 and 24 h after TBI
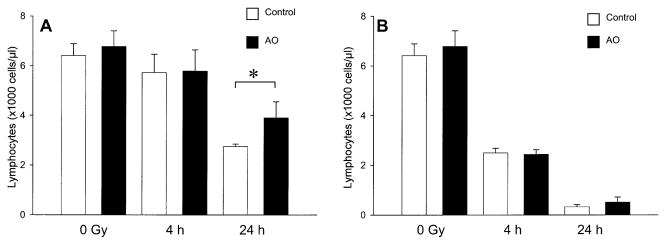
There was no change in peripheral lymphocyte counts at 4 h after 1 Gy in mice fed control or antioxidant diets (Fig. 4A). At 24 h after 1 Gy, antioxidant supplementation was associated with significant attenuation of radiation-induced lymphocyte depletion (Fig. 4A, P = 0.039). Antioxidant supplementation did not significantly attenuate radiation-induced lymphopenia at 4 or 24 h after 8 Gy TBI (Fig. 4B, P = 0.69 and P = 0.14, respectively).
FIG. 4.
Effect of prophylactic dietary antioxidant (AO) supplementation on peripheral lymphocyte cell counts after low- and high-dose TBI. Panel A: Male ICR mice were fed a control diet or a diet supplemented with anti-oxidants for 7 days prior to TBI with 1 Gy. The animals were killed 4 h after TBI (control, n = 4 and antioxidant, n = 4), or 24 h after TBI (control, n = 4 and antioxidant, n = 3). Unirradiated control diet (n = 4) and unirradiated antioxidant diet (n = 4) groups were also analyzed. Panel B: Mice were irradiated with 8 Gy and killed 4 h after TBI (control, n = 5 and antioxidant, n = 4), or 24 h after TBI (control, n = 4 and antioxidant, n = 4). Unirradiated control diet (n = 4) and unirradiated antioxidant diet (n = 4) groups were also analyzed. Bars are means ± SD. *Significant difference at P < 0.05 as analyzed by unpaired two-tailed Student’s t test.
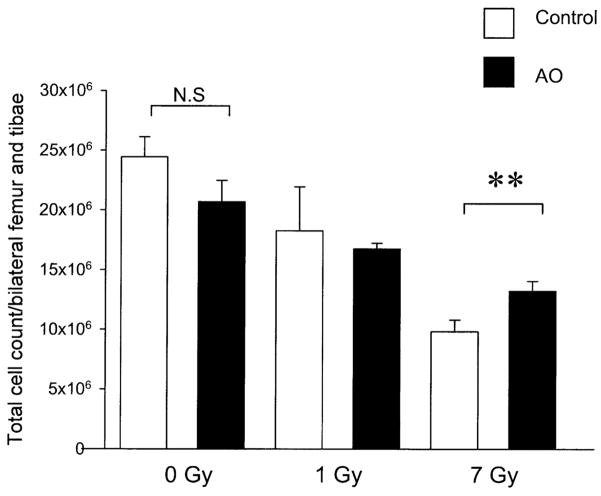
Effect of Antioxidant Dietary Supplements on Radiation-Induced Bone Marrow Cell Depletion
Unirradiated animals fed the control or antioxidant-supplemented diets had comparable total numbers of bone marrow cells harvested from the tibiae and femurs (Fig. 5, P = 0.057). At 4 h after 1 Gy TBI, the total numbers of bone marrow cells harvested from the tibiae and femurs were similar in animals fed the control and antioxidant-supplemented diets (Fig. 5). At 4 h after 7 Gy, the difference in the total number of bone marrow cells for animals fed the control diet and for antioxidant-fed animals was statistically significant (Fig. 5, P = 0.01).
FIG. 5.
Effect of dietary antioxidant (AO) supplementation on bone marrow cell depletion after TBI with γ rays. Male ICR mice were fed a control diet or diet supplemented with antioxidants for 7 days prior to TBI with 1 Gy or 7 Gy γ rays from a cesium-137 source. Four hours after exposure, animals were killed. Both femurs and tibiae were flushed with PBS and marrow cellularity was determined with a Coulter Counter. n = 3 animals in each group. **Significant difference at P < 0.01 by Student’s t test.
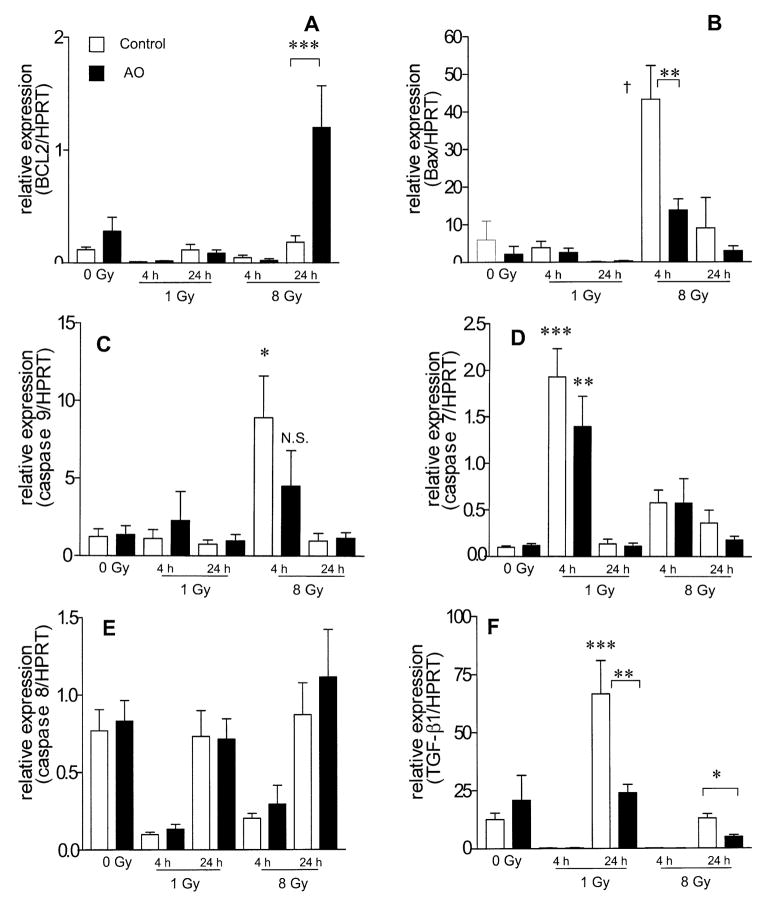
Gene Expression in Bone Marrow after TBI
Four hours after 1 and 8 Gy TBI, Bcl2 mRNA expression levels were decreased in control and antioxidant-fed animals compared to unirradiated controls (Fig. 6A). Twenty-four hours after 8 Gy TBI, Bcl2 mRNA expression in mice fed the control diet returned to the level observed in unirradiated controls (Fig. 6A); however, in animals fed the antioxidant diet, Bcl2 expression significantly exceeded unirradiated control levels by tenfold (Fig. 6A, P < 0.001). Four hours after 8 Gy, Bax and caspase 9 mRNA expression were up-regulated sevenfold compared to control levels. Antioxidant supplementation significantly attenuated both Bax and caspase 9 mRNA overexpression at 4 h after 8 Gy TBI by twofold (P < 0.01) and fourfold, respectively (Fig. 6B and C). At 24 h after 8 Gy TBI, Bax and caspase 9 mRNA expression returned to the levels in unirradiated animals in both control mice and those fed the antioxidant diet (Fig. 6B and C). Four hours after 1 Gy, caspase 7 mRNA expression increased 20-fold in control mice and 14-fold in those fed the antioxidant diet (Fig. 6D). Twenty-four hours after 1 Gy, caspase 7 mRNA expression levels returned to the level observed in unirradiated controls in both control mice and those fed the antioxidant diet (Fig. 6D). At 4 h after 8 Gy, caspase 7 mRNA expression increased sixfold in control and antioxidant-fed animals compared to unirradiated controls (Fig. 6D). Caspase 8 expression was decreased at 4 h after exposure to 1 and 8 Gy in control diet and antioxidant-supplemented animals compared to unirradiated controls (Fig. 6E). At 24 h, caspase 8 expression returned to the level in unirradiated controls, with no difference between control and antioxidant-supplemented animals (Fig. 6E). TGF-β1 mRNA expression was significantly decreased by 98–99.5% at 4 h after 1 and 8 Gy in both control and antioxidant-fed animals (Fig. 6F). At 24 h after 1 Gy, TGF-β1 mRNA expression increased significantly by fivefold in mice fed the control diet, and this increase was abrogated by antioxidant supplementation to a level that was not significantly different from that in unirradiated controls (Fig. 6F). At 24 h after 8 Gy TBI, TGF-β1 mRNA expression in mice fed the control diet was significantly greater than that in mice fed the antioxidant diet (P = 0.015, Fig. 6F).
FIG. 6.
Effect of prophylactic dietary antioxidant (AO) supplementation on mRNA levels of apoptosis genes and TGF-β1 in bone marrow cells after TBI. Male ICR mice were fed a control diet or a diet supplemented with antioxidants for 7 days prior to X-ray exposure. mRNA transcription levels at 4 and 24 h after TBI were analyzed. All expression levels are normalized to HPRT and to the sham-irradiated control mice. Columns represent means ± SEM (n = 3). Panel A: ***P < 0.001; panel B: **P < 0.01 and †P < 0.001 compared to unirradiated control; panel C: *P < 0.05 compared to unirradiated control; N.S., nonsignificant difference compared to unirradiated control; panel D: **P < 0.01 and ***P < 0.001 compared to unirradiated control; panel E: *P < 0.05 by Student’s t test, **P < 0.01 and ***P < 0.001 compared to unirradiated control by one-way ANOVA.
Peripheral Blood Counts and Blood Culture of Moribund Lethally Irradiated Animals
Moribund animals in both the control and antioxidant-fed groups that were killed 2 weeks after 8 Gy TBI exhibited pancytopenia (Table 1). Peripheral blood cultures were positive for enteric gram negative bacteria in 62% (13/21) of the animals examined (Tables 2). There were significantly more positive blood cultures (80%; 12/15) between 11 and 18 days after TBI than between 7 and 10 days after TBI (16%; 1/6) (P = 0.0069).
TABLE 1.
Peripheral Blood Cell Counts in Moribund Animals after 8 Gy TBI
| Diet | WBC (×103 cells/μl) | Lymphocytes (×103 cells/μl) | PMN cells (×103 cells/μl) | RBC (×106 cells/μl) | Hemoglobin (g/dl) | Platelets (×103 cells/μl) |
|---|---|---|---|---|---|---|
| Control | 0.6 ± 0.5 | 0.3 ± 0.3 | 0.10 ± 0.09 | 4 ± 3 | 5 ± 4 | 80 ± 90 |
| Antioxidants | 0.5 ± 0.2 | 0.3 ± 0.2 | 0.08 ± 0.06 | 4 ± 2 | 5 ± 1 | 50 ± 60 |
Note. The data are means ± SD with n = 3.
TABLE 2.
Blood Culture Results in Moribund Animals after 8 Gy TBI
| Diet | Pathogen isolated | Time after irradiation (days) |
|---|---|---|
| Control | E. coli | 13 |
| Control | Proteus vulgaris | 14 |
| Control | Klebsiella pneumoniae | 14 |
| Antioxidants | E. coli | 8 |
| Antioxidants | Enterobacter cloacae | 11 |
| Antioxidants | Enterobacter cloacae and Proteus vulgaris | 16 |
| Antioxidants→Control | E. coli | 12 |
| Antioxidants→Control | E. coli | 13 |
| Antioxidants→Control | E. coli | 13 |
| Control→Antioxidants | E. coli | 13 |
| Control→Antioxidants | E. coli | 14 |
| Control→Antioxidants | E. coli | 17 |
Peripheral Blood Count and Bone Marrow Cell Recovery after Sublethal TBI
Peripheral blood counts
At 4 weeks after 6 Gy TBI, leukocyte counts were significantly higher in animals that received dietary antioxidants compared to those on the control diet. Total white blood cell counts were higher by 230% in animals supplemented with antioxidants than in animals fed the control diet (P = 0.0052; Table 3). Antioxidant supplementation was also associated with 255% higher lymphocyte counts (P = 0.048; Table 3), 257% higher peripheral polymorphonuclear cell counts (P = 0.043; Table 3), and 159% higher platelet counts (P = 0.0018; Table 3) compared to the animals fed the control diet. Red blood cell counts and hemoglobin content were similar between animals fed the antioxidant and control diets 4 weeks after 6 Gy TBI (Table 3). At 8 weeks after 6 Gy, total white blood cell counts remained higher (by 175%) in antioxidant-supplemented animals compared to those fed the control diet (P = 0.0078; Table 3). Lymphocyte counts were similar in mice fed the control and antioxidant diets at 8 weeks after irradiation. Peripheral neutrophil counts remained significantly higher in antioxidant-fed animals, by 264%, compared to those for mice fed the control diet (P = 0.0011; Table 3), as were platelets, which were 200% of those of mice fed the control diet (P = 0.0068; Table 3). Unlike the findings at 4 weeks after TBI, at 8 weeks after irradiation, red blood cell and hemoglobin concentrations were significantly higher in the mice fed the antioxidant diet (113% and 116% of those of mice fed the control diet, respectively; P = 0.0021 and P = 0.0047; Table 3).
TABLE 3.
Peripheral Blood Cell Count Recovery after 6 Gy TBI
| Diet | WBC (×103 cells/μl) | Lymphocytes (×103 cells/μl) | PMN (×103 cells/μl) | RBC (×106 cells/μl) | Hemoglobin (g/dl) | Platelets (×103/μl) |
|---|---|---|---|---|---|---|
| Control (4 weeks) | 3.6 ± 0.4 | 1.8 ± 0.4 | 1.4 ± 0.2 | 9.8 ± 0.1 | 16.6 ± 0.4 | 705 ± 49 |
| Antioxidants (4 weeks) | 8.3 ± 1.2** | 4.6 ± 1.4* | 3.6 ± 1.0* | 9.8 ± 0.3 | 15.8 ± 0.8 | 1119 ± 74** |
| Control (8 weeks) | 3.7 ± 0.9 | 2.0 ± 0.6 | 1.4 ± 0.3 | 9.9 ± 0.4 | 15.6 ± 0.8 | 584 ± 130 |
| Antioxidants (8 weeks) | 6.5 ± 1.0** | 2.1 ± 0.5 | 3.7 ± 1.0** | 11.2 ± 0.2** | 18.2 ± 0.6** | 1168 ± 260** |
Notes. The data are means ± SD with n = 3–5 animals.
Significant difference at P < 0.05 and
P < 0.01 compared to age-matched irradiated controls by Student’s t test.
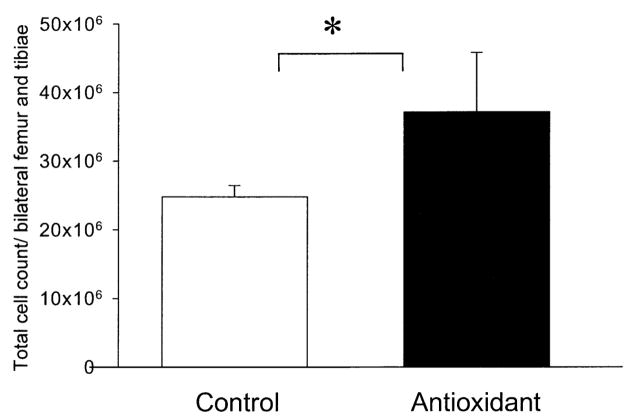
Bone marrow counts
Four weeks after 6 Gy, animals fed the antioxidant-supplemented diet had significantly higher numbers of bone marrow cells than animals fed the control diet (P = 0.016; Fig. 7).
FIG 7.
Effect of dietary antioxidant supplementation on bone marrow cell recovery after 6 Gy TBI with X-rays. Animals were fed a control diet (n = 5) or a diet supplemented with antioxidants (n = 4) from 7 days prior to TBI until they were killed. Four weeks after exposure both femurs and tibiae were harvested and flushed with PBS. Bone marrow cellularity was quantified with a Coulter Counter. The data are means ± SD. *P < 0.05 by Student’s t test.
Recovery of Peripheral Blood Counts in Animals Surviving a Potentially Lethal Dose of TBI
In animals that survived 8 Gy TBI, peripheral blood counts were assessed 8 weeks after irradiation and compared to those for age-matched unirradiated animals fed the control or antioxidant diet over the same period. Surviving irradiated animals fed the control diet had 36% of the total peripheral white blood cell count of nonirradiated control animals, while irradiated antioxidant-supplemented animals had significantly more leukocytes, which was equivalent to 60% of the total peripheral white blood cell count of non-irradiated controls (P = 0.027; Table 4). Antioxidant supplementation in irradiated animals resulted in peripheral lymphocyte counts that were 49% of those of unirradiated controls, whereas in surviving irradiated animals fed the control diet, the peripheral lymphocyte counts were 24% of those of unirradiated control animals. The difference between the animals fed the control diet and the antioxidant-supplemented diet was again statistically significant (P = 0.00038; Table 4). By 8 weeks after TBI, peripheral polymorphonuclear cell and platelet counts were comparable in unirradiated control animals and irradiated surviving animals fed either the control or antioxidant diet (P = 0.91 and P = 0.59; Table 4).
TABLE 4.
Peripheral Blood Cell Counts 8 Weeks after 8 Gy TBI
| Diet | WBC (×103 cells/μl) | Lymphocytes (×103 cells/μl) | PMN (×103 cells/μl) | RBC (×106 cells/μl) | Hemoglobin (g/dl) | Platelets (×103/μl) |
|---|---|---|---|---|---|---|
| Control (0 Gy) | 10 ± l | 8 ± 1 | 1.4 ± 0.1 | 9.8 ± 0.2 | 15.5 ± 0.0 | 1230 ± 280 |
| Antioxidants (0 Gy) | 9 ± 1 | 6 ± 1 | 1.7 ± 0.4 | 9.9 ± 0.8 | 16.8 ± 1.0 | 1375 ± 310 |
| Control (8 Gy) | 3.6 ± 1.1 | 1.9 ± 0.3 | 1.5 ± 0.6 | 10.0 ± 0.3 | 15.9 ± 0.4 | 1160 ± 90 |
| Antioxidants (8 Gy) | 6.0 ± 0.4** | 3.9 ± 0.3** | 1.5 ± 0.4 | 10.0 ± 0.2 | 15.9 ± 0. | 1120 ± 100 |
| Control→Antioxidants (8 Gy) | 5.3 ± 1.0 | 3.1 ± 0.9* | 2.0 ± 1.2 | 9.4 ± 0.8 | 14.3 ± 2.2 | 1074 ± 80 |
Notes. The data are presented as means ± SD with n = 3–5 animals per group.
Significant difference at P < 0.05.
P < 0.01 compared to age-matched controls as analyzed by Student’s t test.
DISCUSSION
The current study was undertaken to evaluate antioxidants as countermeasures against adverse biological effects induced by ionizing radiation. Dietary antioxidant supplements increased survival when administered as a preventative measure prior to radiation exposure as well as when given as treatment after radiation exposure. The administration of dietary antioxidants prior to the radiation exposure was associated with significant protective effects against radiation-induced leukocyte depletion in peripheral blood and bone marrow, suggesting that antioxidants may improve the survival of irradiated animals by attenuating the deleterious effects of radiation on the host immune system. Analysis of the expression of selected genes involved in the stress or death receptor pathways to apoptosis as well as TGF-β1 in the bone marrow after radiation exposure demonstrated that dietary antioxidant supplementation was associated with abrogated pro-apoptosis gene (Bax and cas-pase 9) expression, increased anti-apoptosis gene (Bcl2) expression, and decreased expression of a gene (TGF-β1) that encodes a protein known to inhibit bone marrow proliferation. These results implicate apoptosis as a key process that is modulated by antioxidants to attenuate the effects of radiation on the hematopoietic system and suggest a possible mechanism for improved survival.
We have previously demonstrated the preventive effect of antioxidants against radiation-induced oxidative stress in vitro and in vivo, which was measured by radiation-induced reductions of serum or plasma total antioxidant status in animals (21–24, 28). The present study assessed the hematopoietic radioprotective efficacy of an oral formulation consisting of SeM, α-lipoic acid, NAC, vitamin C and vitamin E succinate in vivo using animal survival and immune cell counts as the end points. Previous studies by others evaluated antioxidant agents individually or as injections because parenteral administration attains systemic antioxidant levels that bypass GI uptake regulation (15). The present study demonstrated that orally administered antioxidants are also effective in protecting the hematopoietic system from the deleterious effects of ionizing radiation as well as in increasing survival.
Several studies have assessed the efficacy of hematopoietic cytokines and growth factors in affecting animal survival after TBI (4, 9–11, 29–31). Because of their wide availability and easy administration, orally administered antioxidants represent an attractive therapeutic option that may be used alone or in combination with hematopoietic cytokines, growth factors, antibiotics, fluids and supportive care in the event of a radiological disaster. A previous study found the combination of receptor-mediated signaling induced by SCF and free radical scavenging mediated by tempol to be synergistic in increasing animal survival after 9 Gy TBI (32).
Our data indicate a highly significant effect of dietary antioxidants in the prevention of neutropenia after low- and high-dose TBI. At 4 h and 24 h after a potentially lethal dose of TBI (8 Gy), severe neutropenia (absolute neutrophil count < 500 cells/μl) was observed only in irradiated animals fed the control diet. This finding suggests a mechanism by which antioxidant supplementation may affect immune function early after radiation exposure. In contrast, 4 h after 1 Gy TBI, animals fed the control diet demonstrated significant neutrophilia, consistent with past observations (33). Interestingly, antioxidant supplementation completely abolished the neutrophilic response after 1 Gy TBI. This observation is further evidence that dietary antioxidants likely attenuate the radiation-induced inflammatory state, which results in neutrophil demarginalization (33). For instance, in the thymus, neutrophils are recruited from the periphery after massive TBI-induced lymphoid apoptosis and prior to clearance of the apoptotic cells by macrophages (34, 35). Such apoptotic tissue injury initiates an immune response that leads to neutrophil demarginalization and peripheral neutrophilia prior to tissue infiltration. Taken together, the lack of a neutrophilic response after 1 Gy TBI in animals supplemented with antioxidants could represent indirect evidence of attenuated systemic cell death by apoptosis after low-dose TBI with this treatment.
Dietary antioxidants were also effective in preventing peripheral lymphopenia associated with low-dose TBI but not high-dose TBI. This finding is not surprising since peripheral and lymphoid organ lymphocytes are among the most radiation-sensitive cells (33) and radiation-induced apoptosis in lymphocytes is thought to be mediated by different mechanisms at low (e.g., below 2 Gy) and high (e.g., 10 Gy) doses (36). The observed attenuation of peripheral lymphopenia by antioxidants after low-dose TBI supports the concomitant observation of an abolished neutrophilic response with this treatment. It is likely that the radiation-induced signaling pathway leading to apoptosis in lymphocytes is not sensitive to modulation by antioxidants at the higher radiation dose.
Total-body radiation exposure is known to result in increased serum levels of cytokines including IL-1β, IL3, IL6, G-CSF and Flt-3 (37, 38). It has been shown that bone marrow recovery after TBI is mediated in large part by hematopoietic cytokines and growth factor release (20, 38–41). Several hematopoietic growth factors and cytokines have been demonstrated to generate reactive oxygen species (ROS) as part of their signaling cascade in hematopoietic cells, including stem cells (42, 43). The role of ROS generation in bone marrow cell depletion, immune function and animal survival after radiation exposure is unknown. The mechanisms involved in the efficacy of the antioxidant treatment initiated after radiation exposure remain to be elucidated, particularly in the context of ROS signaling. It was reported previously that in vitro protection against radiation-induced micronucleus formation in human lymphocytes by vitamin C, vitamin E or β-carotene was more effective when antioxidants were added after the radiation exposure than before (17). An in vivo study showed that vitamin C or vitamin E was equally as effective in reducing γ-radiation-induced bone marrow micronucleus formation and chromosomal aberrations when given to mice 2 h before or after irradiation (44). These two studies evaluated the protective effects of antioxidant vitamins only at radiation doses of 1 and 2 Gy. In the current study the protective effects of antioxidant vitamins were demonstrated for 1 and 8 Gy. Because of the unpredictable nature of a radiation accident or terrorist attack, the ability of antioxidant vitamins to be effective after radiation exposure may be of great benefit for the treatment of patients.
Since the survival of animals exposed to TBI in this study was dependent primarily on the ability of the bone marrow to recover, we assessed the effects of dietary antioxidants on peripheral blood counts at 4 and 24 h after irradiation and bone marrow gene expression changes at 4 h after irradiation. Evaluation of bone marrow cell counts was done at 4 h because peripheral blood count data indicated significant decreases in total leukocyte, neutrophil and lymphocyte counts at this time after 8 Gy TBI. It was expected that gene expression changes in cells of the bone marrow would be correlated with the observed changes in blood and bone marrow cell counts. In addition, 4 h was chosen because the onset of radiation-induced apoptosis of terminal lymphocytes and bone marrow progenitor cells is expected to occur within 4–6 h after TBI (16, 33, 45).
We analyzed the gene expression patterns of selected members of the apoptosis stress pathway, Bcl2, Bax and caspase 9 (46). We assessed gene expression for caspase 8, a member of the death receptor pathway to apoptosis, and caspase 7, an effector caspase downstream of the stress and death receptor pathways (46). Likewise, we assessed the gene expression patterns of TGF-β1, a known inhibitor of bone marrow recovery after exposure to ionizing radiation (47–49).
We found significantly increased expression of Bcl2 mRNA in bone marrow cells 24 h after 8 Gy TBI in animals supplemented with antioxidants compared to animals fed a control diet. Previous reports showed a protective effect of endogenous or transgenic Bcl2 on death of lymphopoietic cells, including mature lymphocytes, after irradiation (50). Furthermore, we observed a protective effect of dietary antioxidants on bone marrow cell depletion 4 h after 7 Gy TBI. It is possible that Bcl2 is involved in the protective effect of dietary antioxidants on radiation-induced bone marrow cell depletion.
Dietary antioxidants significantly attenuated the up-regulation of Bax and caspase 9 mRNA 4 h after 8 Gy TBI. These findings suggest that at potentially lethal doses of radiation, bone marrow cell apoptosis is a rapid phenomenon mediated by the stress pathway to apoptosis. While Bax and caspase 9 are involved in the stress pathway to apoptosis, activation of the death receptor pathway is thought to play a role in radiation-induced lymphocyte apoptosis (33, 36). Antioxidant supplementation did not affect expression of caspase 8, the effector of the death receptor pathway, in unirradiated or irradiated animals. Because of the correlation in cell depletion between peripheral leukocytes and bone marrow cells after radiation exposure, it is possible that the inability of antioxidant supplementation to affect caspase 8 gene expression may explain the differential efficacy of dietary antioxidants in protecting peripheral neutrophils and lymphocytes from low and high radiation doses. Given the observed differences in caspase 9 and caspase 8 expression at 4 and 24 h after 8 Gy TBI, the relative contributions of the stress and death receptor pathways to apoptosis to the kinetics of bone marrow cell depletion after TBI require further elucidation.
Preliminary results of protein expression studies in bone marrow lysates indicate that dietary antioxidant supplementation is associated with decreased total p53 levels and decreased poly(ADP-ribose) polymerase (PARP) cleavage at 4 h after 1 and 7 Gy TBI with γ rays compared to animals fed the control diet (data not shown). PARP is cleaved by activated caspase 3 and is therefore a marker of apoptosis, while p53 activation is required for radiation-induced apoptosis in mature lymphocytes and lymphopoietic cells and is associated with DNA damage (51–55).
These results implicate an anti-apoptosis effect in anti-oxidant-supplemented animals that was not observed in animals fed the control diet. Moreover, because lymphocytes and lymphopoietic cells are known to be very sensitive to radiation-induced apoptosis, it is likely that the observed protective effects of antioxidants on peripheral lymphocyte counts (1 Gy) and bone marrow cells (7 Gy) are partly mediated by anti-apoptosis mechanisms. These results are consistent with a prior in vitro study that demonstrated protection of lymphoblastoid cells from radiation-induced apoptosis by vitamin C, vitamin E or β-carotene (45).
We observed that TGF-β1 mRNA expression was nearly completely abolished at 4 h after 1 and 8 Gy TBI regardless of diet. However, at 24 h, dietary antioxidant supplementation significantly abrogated the increased TGF-β1 mRNA expression after 1 and 8 Gy TBI. These findings are particularly relevant because TGF-β1 is known to inhibit the ability of bone marrow progenitor cells to reconstitute the hematopoietic system after radiation- or chemotherapy-induced myelosuppression by inhibiting stem and progenitor cell cycle progression (47–49, 56, 57). It is worth noting that a previous report showed no changes in TGF-β1 mRNA expression in the bone marrow of mice 2–14 days after TBI with 7.75 Gy γ rays (58). This discrepancy may be attributed to strain and sex differences in male ICR mice and female BDF mice that affect radiation sensitivity. In male ICR mice, the LD50 after TBI is 7.55 Gy, whereas 7.75 Gy TBI is a sublethal dose in female BDF mice (58–60). Furthermore, in the current study, we assessed gene expression within 24 h of radiation exposure, as opposed to the observations at 2–14 days after TBI in the study cited above. Nonetheless, the modulation of TGF-β1 expression by antioxidants after TBI requires further study, because this cytokine is known to have effects on hematopoietic cell proliferation. While TGF-β1 is thought to inhibit the growth of early hematopoietic progenitor and stem cells, it is thought to stimulate the growth of late hematopoietic progenitor cells (47, 57).
In the present study, moribund animals that had been exposed to 8 Gy TBI exhibited a profound pancytopenia at the time of euthanasia. Necropsy revealed several pathological findings in animals fed both control and antioxidant-supplemented diets: cardiomegaly, hepatitis/cirrhosis of the liver, intestinal bleeding and subcutaneous bleeding, and severe orchitis (data not shown). Blood cultures were positive for bacterial growth in most samples analyzed, which is consistent with the expected compromised immune status of lethally irradiated animals (61, 62). Nonetheless, hematopoietic death encompasses not only depressed immunity, which leads to infection and sepsis, but also thrombocytopenia-induced spontaneous bleeding and likely other incompletely characterized sequelae. In this study, as reported previously in other studies, gram negative enteric organisms were the predominant cause of bacteremia (61, 62).
Survival of humans and animals after TBI is dependent in large part on the ability of the bone marrow to recover and overcome radiation-induced myelosuppression (1, 33, 63, 64). Four and 8 weeks after sublethal and potentially lethal TBI, we observed higher peripheral blood leukocyte, platelet and bone marrow cell counts in antioxidant-supplemented animals compared to the animals fed the control diet. These findings suggest that antioxidant supplementation resulted in protection of hematopoietic stem cells at the time of the radiation exposure, thereby leading to increased recovery of the bone marrow and subsequently the peripheral blood counts. These findings are of clinical interest not only in the setting of a radiological accident but also in patients undergoing myelosuppressive treatment regimens for cancer. Current therapies to stimulate the bone marrow for this group of patients, including G-CSF, are administered through injections, are relatively costly, and may increase the risk of developing secondary malignancies such as AML or myelodysplastic syndromes (65).
This report shows that oral administration of antioxidants is an effective countermeasure for the toxic hematopoietic effects of radiation at low and high doses. Dietary antioxidants likely attenuate the radiation-induced inflammatory response and hematopoietic cell death in the periphery and bone marrow, resulting in improved bone marrow recovery and animal survival. The molecular signals involved in these phenomena require further elucidation; however, apoptosis of hematopoietic cells may be involved.
Acknowledgments
We would like to thank Dr. Zhaozong Zhou for critical discussion of the animal experiments, Jeremiah Donahue for assistance with animal irradiation, Ms. Mary-Ann Kershaw, Ms. Kerri Bonti and the staff of the Brookhaven National Laboratory (BNL) Animal Facility for facilitating experiments done at BNL. We also would like to thank Dr. Suresh Shelat and Ms. Susan Shibutani of the Children’s Hospital of Philadelphia Hematology Laboratory for assistance with peripheral blood count measurements. This work was supported by a grant from the National Space Biomedical Research Institute (NSBRI) through NASA NCC 9–58 and NIH Training Grant 2T32CA009677.
References
- 1.Koenig KL, Goans RE, Hatchett RJ, Mettler FA, Jr, Schumacher TA, Noji EK, Jarrett DG. Medical treatment of radiological casualties: current concepts. Ann Emerg Med. 2005;45:643–652. doi: 10.1016/j.annemergmed.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, Moulder JE, Preston RJ, Seed TM, Wong RS. Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: Report of a workshop; Bethesda, Maryland. December 17–18, 2001; [DOI] [PubMed] [Google Scholar]; Radiat Res. 2003;159:812–834. doi: 10.1667/rr3021. [DOI] [PubMed] [Google Scholar]
- 3.Mettler FA, Jr, Voelz GL. Major radiation exposure—what to expect and how to respond. N Engl J Med. 2002;346:1554–1561. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 4.Herodin F, Drouet M. Cytokine-based treatment of accidentally irradiated victims and new approaches. Exp Hematol. 2005;33:1071–1080. doi: 10.1016/j.exphem.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Augustine AD, Gondre-Lewis T, McBride W, Miller L, Pellmar TC, Rockwell S. Animal models for radiation injury, protection and therapy. Radiat Res. 2005;164:100–109. doi: 10.1667/rr3388. [DOI] [PubMed] [Google Scholar]
- 6.Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–123. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 7.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Zaharevitz D. Models for evaluating agents intended for the pro-phylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop; December 3–4, 2003; [DOI] [PubMed] [Google Scholar]; Radiat Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 8.Coleman CN, Stone HB, Moulder JE, Pellmar TC. Modulation of radiation injury. Science. 2004;304:693–694. doi: 10.1126/science.1095956. [DOI] [PubMed] [Google Scholar]
- 9.Drouet M, Mourcin F, Grenier N, Leroux V, Denis J, Mayol JF, Thullier P, Lataillade JJ, Herodin F. Single administration of stem cell factor, FLT-3 ligand, megakaryocyte growth and development factor, and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: long-term follow-up of hematopoiesis. Blood. 2004;103:878–885. doi: 10.1182/blood-2003-05-1400. [DOI] [PubMed] [Google Scholar]
- 10.Farese AM, Casey DB, Smith WG, Vigneulle RM, McKearn JP, MacVittie TJ. Leridistim, a chimeric dual G-CSF and IL-3 receptor agonist, enhances multilineage hematopoietic recovery in a nonhuman primate model of radiation-induced myelosuppression: effect of schedule, dose, and route of administration. Stem Cells. 2001;19:522–553. doi: 10.1634/stemcells.19-6-522. [DOI] [PubMed] [Google Scholar]
- 11.Herodin F, Bourin P, Mayol JF, Lataillade JJ, Drouet M. Short-term injection of antiapoptotic cytokine combinations soon after lethal gamma-irradiation promotes survival. Blood. 2003;101:2609–2616. doi: 10.1182/blood-2002-06-1634. [DOI] [PubMed] [Google Scholar]
- 12.MacVittie TJ, Farese AM, Smith WG, Baum CM, Burton E, McKearn JP. Myelopoietin, an engineered chimeric IL-3 and G-CSF receptor agonist, stimulates multilineage hematopoietic recovery in a nonhuman primate model of radiation-induced myelosuppression. Blood. 2000;95:837–845. [PubMed] [Google Scholar]
- 13.Farese AM, Herodin F, McKearn JP, Baum C, Burton E, MacVittie TJ. Acceleration of hematopoietic reconstitution with a synthetic cytokine (SC-55494) after radiation-induced bone marrow aplasia. Blood. 1996;87:581–591. [PubMed] [Google Scholar]
- 14.Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann NY Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- 15.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 16.Konopacka M, Palyvoda O, Rzeszowska-Wolny J. Inhibitory effect of ascorbic acid post-treatment on radiation-induced chromosomal damage in human lymphocytes in vitro. Teratog Carcinog Mutagen. 2002;22:443–450. doi: 10.1002/tcm.10040. [DOI] [PubMed] [Google Scholar]
- 17.Konopacka M, Rzeszowska-Wolny J. Antioxidant vitamins C, E and beta-carotene reduce DNA damage before as well as after gamma-ray irradiation of human lymphocytes in vitro. Mutat Res. 2001;491:1–7. doi: 10.1016/s1383-5718(00)00133-9. [DOI] [PubMed] [Google Scholar]
- 18.Umegaki K, Aoki S, Esashi T. Whole body X-ray irradiation to mice decreases ascorbic acid concentration in bone marrow: comparison between ascorbic acid and vitamin E succinate. Free Radic Biol Med. 1995;19:493–497. doi: 10.1016/0891-5849(95)00033-t. [DOI] [PubMed] [Google Scholar]
- 19.Umegaki K, Sano M, Suzuki K, Tomita I, Esashi T. Increases in 4-hydroxynonenal and hexanal in bone marrow of rats subjected to total body X-ray irradiation: association with antioxidant vitamins. Bone Marrow Transplant. 1999;23:173–178. doi: 10.1038/sj.bmt.1701531. [DOI] [PubMed] [Google Scholar]
- 20.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Scadden DT. Osteoblastic cells regulate the hematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy AR, Ware JH, Guan J, Donahue JJ, Biaglow JE, Zhou Z, Stewart J, Vazquez M, Wan XS. Selenomethionine protects against adverse biological effects induced by space radiation. Free Radic Biol Med. 2004;36:259–266. doi: 10.1016/j.freeradbiomed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Wan XS, Bloch P, Ware JH, Zhou Z, Donahue JJ, Guan J, Stewart J, Kennedy AR. Detection of oxidative stress induced by low-and high-linear energy transfer radiation in cultured human epithelial cells. Radiat Res. 2005;163:364–368. doi: 10.1667/0033-7587(2005)163[0364:doosib]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Guan J, Stewart J, Ware JH, Zhou Z, Donahue JJ, Kennedy AR. Effects of dietary supplements on the space radiation-induced reduction in total antioxidant status in CBA mice. Radiat Res. 2006;165:373–378. doi: 10.1667/rr3523.1. [DOI] [PubMed] [Google Scholar]
- 24.Guan J, Wan XS, Zhou Z, Ware J, Donahue JJ, Biaglow JE, Kennedy AR. Effects of dietary supplements on space radiation-induced oxidative stress in Sprague-Dawley rats. Radiat Res. 2004;162:572–579. doi: 10.1667/rr3249. [DOI] [PubMed] [Google Scholar]
- 25.Prentice A, Branca F, Decsi T, Michaelsen KF, Fletcher RJ, Guesry P, Manz F, Vidailhet M, Pannemans D, Samartin S. Energy and nutrient dietary reference values for children in Europe: methodological approaches and current nutritional recommendations. Br J Nutr. 2004;92(Suppl 2):S83–S146. doi: 10.1079/bjn20041159. [DOI] [PubMed] [Google Scholar]
- 26.Aitio ML. N-acetylcysteine—passe-partout or much ado about nothing? Br J Clin Pharmacol. 2006;61:5–15. doi: 10.1111/j.1365-2125.2005.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wollin SD, Jones PJ. Alpha-lipoic acid and cardiovascular disease. J Nutr. 2003;133:3327–3330. doi: 10.1093/jn/133.11.3327. [DOI] [PubMed] [Google Scholar]
- 28.Wan XS, Ware JH, Zhou Z, Donahue JJ, Guan J, Kennedy AR. Protection against radiation-induced oxidative stress in cultured human epithelial cells by treatment with antioxidant agents. Int J Radiat Oncol Biol Phys. 2006;64:1475–1481. doi: 10.1016/j.ijrobp.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Bertho JM, Frick J, Prat M, Demarquay C, Dudoignon N, Trompier F, Gorin NC, Thierry D, Gourmelon P. Comparison of autologous cell therapy and granulocyte-colony stimulating factor (G-CSF) injection vs. G-CSF injection alone for the treatment of acute radiation syndrome in a non-human primate model. Int J Radiat Oncol Biol Phys. 2005;63:911–920. doi: 10.1016/j.ijrobp.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 30.Farese AM, Casey DB, Vigneulle RM, Siegel NR, Finn RF, Klover JA, Smith WG, McKearn JP, MacVittie TJ. A single dose of pegylated leridistim significantly improves neutrophil recovery in sublethally irradiated rhesus macaques. Stem Cells. 2001;19:514–521. doi: 10.1634/stemcells.19-6-514. [DOI] [PubMed] [Google Scholar]
- 31.MacVittie TJ, Farese AM, Herodin F, Grab LB, Baum CM, McKearn JP. Combination therapy for radiation-induced bone marrow aplasia in nonhuman primates using synthokine SC-55494 and recombinant human granulocyte colony-stimulating factor. Blood. 1996;87:4129–4135. [PubMed] [Google Scholar]
- 32.Liebmann J, DeLuca AM, Epstein A, Steinberg SM, Morstyn G, Mitchell JB. Protection from lethal irradiation by the combination of stem cell factor and tempol. Radiat Res. 1994;137:400–404. [PubMed] [Google Scholar]
- 33.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–528. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- 34.Iyoda T, Nagata K, Akashi M, Kobayashi Y. Neutrophils accelerate macrophage-mediated digestion of apoptotic cells in vivo as well as. in vitro J Immunol. 2005;175:3475–3483. doi: 10.4049/jimmunol.175.6.3475. [DOI] [PubMed] [Google Scholar]
- 35.Uchimura E, Watanabe N, Niwa O, Muto M, Kobayashi Y. Transient infiltration of neutrophils into the thymus in association with apoptosis induced by whole-body X-irradiation. J Leukoc Biol. 2000;67:780–784. doi: 10.1002/jlb.67.6.780. [DOI] [PubMed] [Google Scholar]
- 36.Albanese J, Dainiak N. Regulation of TNFRSF6 (Fas) expression in ataxia telangiectasia. Radiat Res. 2000;154:616–624. doi: 10.1667/0033-7587(2000)154[0616:rotfei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Peterson VM, Adamovicz JJ, Elliott TB, Moore MM, Madonna GS, Jackson WE, 3rd, Ledney GD, Gause WC. Gene expression of hematoregulatory cytokines is elevated endogenously after sublethal gamma-irradiation and is differentially enhanced by therapeutic administration of biologic response modifiers. J Immunol. 1994;153:2321–2330. [PubMed] [Google Scholar]
- 38.Prat M, Demarquay C, Frick J, Thierry D, Gorin NC, Bertho JM. Radiation-induced increase in plasma Flt3 ligand concentration in mice: evidence for the implication of several cell types. Radiat Res. 2005;163:408–417. doi: 10.1667/rr3340. [DOI] [PubMed] [Google Scholar]
- 39.Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM, Kronenberg HM, Scadden DT. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 40.Bertho JM, Demarquay C, Frick J, Joubert C, Arenales S, Jacquet N, Sorokine-Durm I, Chau Q, Lopez M, Gourmelon P. Level of Flt3-ligand in plasma: a possible new bio-indicator for radiation-induced aplasia. Int J Radiat Biol. 2001;77:703–712. doi: 10.1080/09553000110043711. [DOI] [PubMed] [Google Scholar]
- 41.Huchet A, Belkacemi Y, Frick J, Prat M, Muresan-Kloos I, Altan D, Chapel A, Gorin NC, Gourmelon P, Bertho JM. Plasma Flt-3 ligand concentration correlated with radiation-induced bone marrow damage during local fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:508–515. doi: 10.1016/s0360-3016(03)00584-4. [DOI] [PubMed] [Google Scholar]
- 42.Iiyama M, Kakihana K, Kurosu T, Miura O. Reactive oxygen species generated by hematopoietic cytokines play roles in activation of receptor-mediated signalling and in cell cycle progression. Cell Signal. 2006;18:174–182. doi: 10.1016/j.cellsig.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Sattler M, Winkler T, Verma S, Byrne CH, Shrikhande G, Salgia R, Griffin JD. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood. 1999;93:2928–2935. [PubMed] [Google Scholar]
- 44.Sarma L, Kesavan PC. Protective effect of vitamins C and E against gamma-ray-induced chromosomal damage in mouse. Int J Radiat Biol. 1993;63:759–764. doi: 10.1080/09553009314552161. [DOI] [PubMed] [Google Scholar]
- 45.Ortmann EK, Mayerhofer T, Getoff N, Kodym R. Effect of antioxidant vitamins on radiation-induced apoptosis in cells of a human lymphoblastic cell line. Radiat Res. 2004;161:48–55. doi: 10.1667/rr3102. [DOI] [PubMed] [Google Scholar]
- 46.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fortunel NO, Hatzfeld JA, Monier MN, Hatzfeld A. Control of hematopoietic stem/progenitor cell fate by transforming growth factor-beta. Oncol Res. 2003;13:445–453. doi: 10.3727/096504003108748483. [DOI] [PubMed] [Google Scholar]
- 48.Jacobsen SE, Veiby OP, Myklebust J, Okkenhaug C, Lyman SD. Ability of flt3 ligand to stimulate the in vitro growth of primitive murine hematopoietic progenitors is potently and directly inhibited by transforming growth factor-beta and tumor necrosis factor-alpha. Blood. 1996;87:5016–5026. [PubMed] [Google Scholar]
- 49.Scandura JM, Boccuni P, Massague J, Nimer SD. Transforming growth factor beta-induced cell cycle arrest of human hematopoietic cells requires p57KIP2 up-regulation. Proc Natl Acad Sci USA. 2004;101:15231–15236. doi: 10.1073/pnas.0406771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91:2272–2282. [PubMed] [Google Scholar]
- 51.Alvarez S, Drane P, Meiller A, Bras M, Deguin-Chambon V, Bouvard V, May E. A comprehensive study of p53 transcriptional activity in thymus and spleen of gamma irradiated mouse: high sensitivity of genes involved in the two main apoptotic pathways. Int J Radiat Biol. 2006;82:761–770. doi: 10.1080/09553000600949624. [DOI] [PubMed] [Google Scholar]
- 52.Fujikawa K, Hasegawa Y, Matsuzawa S, Fukunaga A, Itoh T, Kondo S. Dose and dose-rate effects of X rays and fission neutrons on lymphocyte apoptosis in p53+/+ and p53−/− mice. J Radiat Res (Tokyo) 2000;41:113–127. doi: 10.1269/jrr.41.113. [DOI] [PubMed] [Google Scholar]
- 53.Kapasi AA, Singhal PC. Aging splenocyte and thymocyte apoptosis is associated with enhanced expression of p53, bax, and caspase-3. Mol Cell Biol Res Commun. 1999;1:78–81. doi: 10.1006/mcbr.1999.0106. [DOI] [PubMed] [Google Scholar]
- 54.Samuni AM, Kasid U, Chuang EY, Suy S, Degraff W, Krishna MC, Russo A, Mitchell JB. Effects of hypoxia on radiation-responsive stress-activated protein kinase, p53, and caspase 3 signals in TK6 human lymphoblastoid cells. Cancer Res. 2005;65:579–586. [PubMed] [Google Scholar]
- 55.Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123:641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 56.Jacobsen SE, Okkenhaug C, Myklebust J, Veiby OP, Lyman SD. The FLT3 ligand potently and directly stimulates the growth and expansion of primitive murine bone marrow progenitor cells in vitro: synergistic interactions with interleukin (IL) 11, IL-12, and other hematopoietic growth factors. J Exp Med. 1995;181:1357–1363. doi: 10.1084/jem.181.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fortunel NO, Hatzfeld A, Hatzfeld JA. Transforming growth factor-beta: pleitropic role in the regulation of hematopoiesis. Blood. 2000;96:2022–2036. [PubMed] [Google Scholar]
- 58.Chang CM, Limanni A, Baker WH, Dobson ME, Kalinich JF, Jackson W, Patchen ML. Bone marrow and splenic granulocyte-macrophage colony-stimulating factor and transforming growth factor-beta mRNA levels in irradiated mice. Blood. 1995;86:2130–2136. [PubMed] [Google Scholar]
- 59.Kurishita A, Katoh H, Uehara Y, Uchida A, Mizutani Y, Ono T, Hirose S, Okada S. Post-irradiation treatment with OK432 can prevent radiation-induced bone marrow death. Int J Radiat Biol. 1991;59:711–716. doi: 10.1080/09553009114550621. [DOI] [PubMed] [Google Scholar]
- 60.Ledney GD, Elliott TB, Landauer MR, Vigneulle RM, Henderson PL, Harding RA, Tom SP., Jr Survival of irradiated mice treated with WR-151327, synthetic trehalose dicorynomycolate, or ofloxacin. Adv Space Res. 1994;14:583–586. doi: 10.1016/0273-1177(94)90513-4. [DOI] [PubMed] [Google Scholar]
- 61.Brook I, MacVittie TJ, Walker RI. Recovery of aerobic and anaerobic bacteria from irradiated mice. Infect Immun. 1984;46:270–271. doi: 10.1128/iai.46.1.270-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nose M, Uzawa A, Ogyu T, Suzuki G. OK-432 reduces mortality and bacterial translocation in irradiated and granulocyte-colony stimulating factor (G-CSF)-treated mice. J Radiat Res (Tokyo) 2001;42:191–200. doi: 10.1269/jrr.42.191. [DOI] [PubMed] [Google Scholar]
- 63.MacVittie TJ, Farese AM, Jackson W., 3rd Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys. 2005;89:546–555. doi: 10.1097/01.hp.0000173143.69659.5b. [DOI] [PubMed] [Google Scholar]
- 64.Weiss JF. Pharmacologic approaches to protection against radiation-induced lethality and other damage. Environ Health Perspect. 1997;105(Suppl 6):1473–1478. doi: 10.1289/ehp.97105s61473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hershman D, Neugut AI, Jacobson JS, Wang J, Tsai WY, McBride R, Bennett CL, Grann VR. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]