Abstract
The R-spondin (Rspo) family of protein consists of secreted cysteine-rich proteins that can activate β-catenin signaling via the Frizzled/LRP5/6 receptor complex. Here, we report that targeted inactivation of the mouse Rspo2 gene causes developmental limb defects, especially in the hindlimb. Although the initiation of the expression of apical ectodermal ridge (AER)-specific genes including fibroblast growth factor 8 (FGF8) and FGF4, occurred normally, the maintenance of these marker expressions was significantly defective in the hindlimb of Rspo2(-/-) mice. Consistent with the ligand role of R-spondins in the Wnt/β-catenin signaling pathway, expression of Axin2 and Sp8, targets for β-catenin signaling, within AER was greatly reduced in Rspo2(-/-) embryos. Furthermore, sonic hedgehog (Shh) signaling within the hindlimbs of Rspo2(-/-) mice was also significantly decreased. Rspo2 is expressed in the AER of all limb buds, however the stunted phenotype is significantly more severe in the hindlimbs than the forelimbs, and strongly biased to the left side. Our findings strongly suggest that Rspo2 expression in the AER is required for AER maintenance likely by regulating Wnt/β-catenin signaling.
Keywords: R-spondin2, Wnt, β-catenin, Axin2, FGF8, shh, AER, limb development
Introduction
The human and mouse R-spondin (Rspo) families of the secreted proteins are composed of 4 homologous members (Rspo1-Rspo4), which are capable of promoting β-catenin signaling (Kazanskaya et al., 2004; Kim et al., 2005; Kim et al., 2006; Nam et al., 2006). Exogenous Rspo proteins induce the stabilization of β-catenin proteins and activate β-catenin-dependent gene expression in cultured cells (Kazanskaya et al., 2004; Kim et al., 2005; Kim et al., 2006; Nam et al., 2006). Ectopic expression of the human RSPO1 gene in the transgenic mouse model increases the expression of β-catenin protein and the expression of downstream target genes that accompany the enhanced cell proliferation (Kim et al., 2005). Furthermore, the mouse Rspo3 protein is shown to bind directly with the extracellular domains of LRP6 and Frizzled8, two receptor components of the canonical Wnt pathway (Nam et al., 2006). In another study, the human RSPO1 protein is demonstrated to be a high affinity ligand for the LRP6 receptor (Wei et al., 2007). These results suggest that the Rspo family of proteins represents a novel class of secreted proteins that are structurally unrelated to the Wnt ligands, but can similarly activate β-catenin-transduced signaling.
In mouse embryos and adults, the Rspo genes are expressed in dynamic and tissue-specific patterns (Kazanskaya et al., 2004; Nam et al., 2007). Recent studies in mice and humans reveal that Rspo function is critical for normal development and diseases. Mutations in the human RSPO1 gene cause XX>male sex conversion, defects in skin differentiation, and predisposition to squamous skin carcinoma (Parma et al., 2006). Mutations in the RSPO4 gene are linked to anonychia syndrome in humans (Bergmann et al., 2006; Blaydon et al., 2006). Furthermore, mice lacking the Rspo3 gene have defects of placental development, resulting in early embryonic lethality (Aoki et al., 2007).
Tetrapod limb development is regulated coordinately by interactions between multiple signals (Capdevila and Izpisua Belmonte, 2001). Two key structures, the apical ectodermal ridge (AER) and the zone of polarizing activity (ZPA), control proximal-distal outgrowth and anterior-posterior patterning, respectively (Capdevila and Izpisua Belmonte, 2001). FGFs and BMPs secreted from the AER, and Shh from the ZPA, represent major signaling cues that regulate limb outgrowth and patterning (Capdevila and Izpisua Belmonte, 2001). Wnt/β-catenin signaling also regulates many aspects of limb development, including AER development (Church and Francis-West, 2002). Misexpression of Wnt3a and β-catenin in developing limbs of chicken embryos induces ectopic expression of AER-specific genes (Kengaku et al., 1998). In mice, conditional inactivation of β-catenin in the limb surface ectoderm, including the AER, causes a reduction or inhibition of FGF8 expression in the AER and incomplete development of the limb skeleton, underscoring the crucial role of β-catenin signaling in AER formation and maintenance (Barrow et al., 2003; Soshnikova et al., 2003). Wnt3 is expressed ubiquitously throughout the limb surface ectoderm, including the AER in mice (Barrow et al., 2003). Conditional inactivation of mouse Wnt3 in the limb surface ectoderm and the AER also causes similar defects in AER formation and maintenance (Barrow et al., 2003). Mice lacking the LRP6 gene, encoding a co-receptor for Wnt/β-catenin signaling, develop severe limb defects, including AER defects (Pinson et al., 2000). In contrast, mice carrying a targeted mutation of the Dickkopf-1 (Dkk1) gene, a negative regulator of Wnt signaling, have an expanded AER and extra digits (MacDonald et al., 2004; Mukhopadhyay et al., 2001), defects that are phenotypically opposite to those observed with reduced β-catenin signaling.
Wnt3 is implicated as a specific Wnt ligand that activates β-catenin signaling in the AER (Barrow et al., 2003). However, Wnt3 expression is detected in the entire limb surface ectoderm (Barrow et al., 2003), and it raises the question of whether or not Wnt3 is the only ligand that specifically activates β-catenin signaling in the AER. The Rspo2 gene is strongly expressed in the AER of developing limbs (Kazanskaya et al., 2004; Nam et al., 2007), suggesting the possibility that Rspo2 may activate β-catenin signaling in the AER. Our initial analysis of the Rspo2 gene-targeted mutant mice indicates that Rspo2 is required for normal development of multiple tissues, including craniofacial structures, lung, kidney, and limbs. Here, we focus on the analysis of limb defects in Rspo2 gene-targeted mice. Our findings indicate that Rspo2 plays a critical role in AER development and function, and suggest a strong possibility that Rspo2 function in the AER is mediated via Wnt/β-catenin-dependent signaling.
Materials and Methods
Generation of Rspo2 gene-targeted mice
A targeting vector was designed to replace 308 bp spanning exon 3 of the mouse Rspo2 gene with an IRES-LacZ-PGKneor cassette carrying a splice acceptor site (Figure 1A). Embryonic stem cell colonies (mouse strain 129) were screened for gene targeting events by PCR or Southern blot analysis. Germline-transmitting animals were identified in the progeny of chimeric animals crossed to C57BL/6 strain mice. F2 generation animals and embryos were genotyped for wild type and targeted Rspo2 alleles by PCR analysis using primer sets specific for wild type and targeted loci (Figure 1A). The primer sequences are as follows: GS1, 5′-GAAGAGAAGGAATGCGTCAGTATGG-3′; GS2, 5′-TGGAGGGGATAACTGGTTAGTTTGC-3′; Neo, 5′-GGGCCAGCTCATTCCTCCCACTCAT-3′. RNA was isolated from brain tissue of E18.5 embryos, and RT-PCR was performed to evaluate transcription of the native Rspo2 gene and targeted gene using the following primers. Rspo2F, 5′-ATGCGTTTTTGCCTCTTCTCATTTGCC-3′; Rspo2R, 5′-TTGGTTCACTCTGTCTGTAGCGAG-3′; β-actinF, 5′-ATGGATGACGATATCGCTGCG; β-actinR, 5′-CAATAGTGATGACCTGGCCGT-3′
Figure 1.
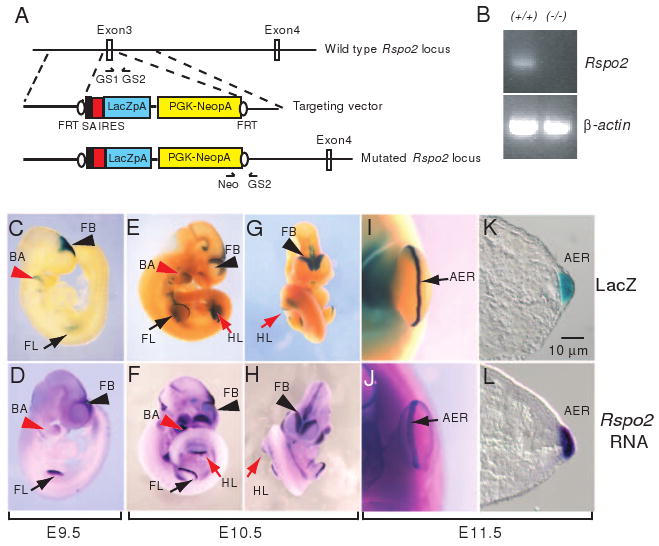
Generation of Rspo2 gene-targeted mice. (A) An IRES-LacZ-PGKneor cassette flanked by a splicing acceptor (SA) was replaced with exon 3 of the mouse Rspo2 gene. Arrows indicate the positions of GS1, GS2, and Neo primers for genotyping. Black ovals indicate Flp-recombinase target sites (FRT). (B) Rspo2 RNA expression was not detected in Rspo2(-/-) mice. PCR primers specific for exon 2 and exon 5 were used to detect Rspo2 mRNA spanning the predicted coding region. Total RNA isolated from brain tissue of P1 (postnatal day 1) mice was used to synthesize cDNA templates. β-actin RNA expression is shown as control. LacZ expression (C, E, G, I and K) in Rspo2(+/-) mouse embryo and Rspo2 RNA expression in wild type mouse embryo (D, F, H, J and L) at embryonic day 9.5 (E9.5, C and D), E10.5 (E-H) and E11.5 (I-L). Abbreviations: FB, forebrain (black arrowheads); FL, forelimb bud (black arrows); HL, hindlimb bud (red arrows); BA, branchial arch (red arrowheads); AER, apical ectodermal ridge (black arrows in I and J).
Gross examination, histology, and skeletal preparation
E9.5-17.5 embryos/fetuses were harvested and fixed in 10% neutral formalin. Gross anatomical defects of null embryos were examined and compared with wild type and heterozygote littermates. TopGAL mice were obtained from The Jackson Laboratory. β-galactosidase (LacZ) enzyme expression was examined using X-gal (Invitrogen) substrate. Skeletal preparations of E18.5 fetuses and newborn mice were prepared and stained as previously described (Yoon and Wold, 2000).
In situ hybridization and immunohistochemistry
Whole mount in situ hybridization using digoxigenin-labeled antisense RNA probes was performed as described (Yoon and Wold, 2000). The stained embryos were photographed as a whole mount and further processed as cryosections (14-15 μm) for microscopic photography. For immunohistochemical analyses, embryos were fixed in 10% neutral buffered formalin or 4% paraformaldehyde in PBS. Embryos were processed as either paraffin-embedded sections (5 μm) or cryosections (10 μm). Hematoxylin/eosin staining was performed on paraffin sections. TUNEL assays were performed using the Apoptag® Peroxidase (Qbiogene), according to the manufacturer's specifications.
Results and Discussion
Targeted disruption of the Rspo2 gene in mice
To examine the potential roles of the Rspo2 gene in mouse development, we generated Rspo2 gene-targeted mice (Figure 1A). Exon 3 of the Rspo2 gene was replaced with a SA-IRES-LacZ-PGKneor cassette. Previous biochemical assays indicated that deletion of the cysteine-rich domain abolishes the activity of the R-spondin proteins in β-catenin-activation assays and also eliminates binding to the Frizzled8 and LRP6 receptors (Kazanskaya et al., 2004; Nam et al., 2006). Thus, removal of exon 3, which encodes the first half of the cysteine-rich domain, is predicted to ablate production of normally spliced Rspo2 transcripts and functional proteins, yielding a null allele. Upon analysis of RNA from the resulting homozygous mutant mouse, we failed to detect read-through or alternatively spliced transcripts using a primer set spanning exon 2 and exon 5 covering the entire coding region of the Rspo2 gene, thereby confirming the effectiveness of targeting strategy (Figure 1B).
The β-galactosidase (β-gal) encoding LacZ gene cassette inserted into the Rspo2 locus provides a surrogate marker of Rspo2 expression. We collected Rspo2(+/-) embryos ranging from E7.5-E11.5 and compared β-gal enzyme expression to Rspo2 mRNA expression in similarly staged wild type embryos. At E9.0-E9.5, prominent β-gal enzyme expression derived from the targeted Rspo2 locus recapitulated the endogenous Rspo2 mRNA expression pattern in the ectoderm of forelimb field, branchial arches and forebrain (Figure 1C and D). In the embryos at later stages, LacZ expression was continuously colocalized with Rspo2 mRNA expression in the AER of the limb buds, branchial arches, and forebrain (Figure 1E-L), suggesting that our targeting strategy preserved the spatiotemporal regulation of the Rspo2 locus.
While Rspo2(+/-) male mice on a 129XC57BL6 hybrid background appeared to be normal and fertile, Rspo2(+/-) female mice gradually lost their fertility beginning at about four months of age (25% sterile at 4 months and 85% sterile at 5+ months). The cause of progressive sterility remains unknown. However, given the recent observations on the XX>male sex conversion in human patients carrying RSPO1 mutations (Parma et al., 2006), a more detailed assessment of the reproductive organ of Rspo2(+/-) female mice is warranted. Genotyping of the offspring from crosses between Rspo2(+/-) mice failed to detect any surviving Rspo2(-/-) pups. Subsequent analysis of embryos and fetuses ranging from E9.5 to E18.5 showed normal Mendelian distribution of the genotypes (Rspo2(+/+), 27.3%; Rspo2(-/+), 48.9%; and Rspo2(-/-), 23.8%, n=407), indicating that Rspo2(-/-) mice survived during gestation but died immediately after birth. In Rspo2(-/-) mice, obvious morphological phenotypes included defects in the limbs (Figure 2) and craniofacial structures, including a cleft palate (data not shown). In addition, the lungs of Rspo2(-/-) mice showed hypoplasia and pulmonary vascular defects (data not shown), which was likely the cause of lethality. Sporadically, we observed the absence of a discernable kidney (data not shown). This report is focused on the analysis of limb defects in Rspo2(-/-) mice, and studies of the other defects will be described elsewhere.
Figure 2.
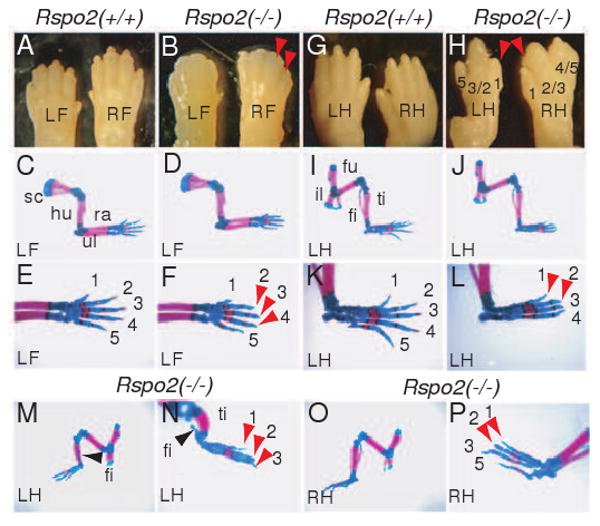
Limb skeletal defects in Rspo2(-/-) mice. (A and B) Gross morphology of the distal forelimbs of Rspo2(+/+) and Rspo2(-/-) mice at P1. The right (RF) and left (LF) feet are indicated. Red arrowheads indicate the absence of claws in Rspo2(-/-) mouse. (C-F) Whole mount forelimb skeleton of Rspo2(+/+) and Rspo2(-/-) mouse fetuses at E18.5. Skeleton of the left forelimbs are presented. A magnified view of the foot skeleton shows the absence of ossification of the most distal phalangeal bones (indicated by red arrowheads in F) in Rspo2(-/-) mouse. (G and H) Gross morphology of the distal hindlimbs of Rspo2(+/+) and Rspo2(-/-) mice at P1. The right (RH) and left (LH) feet are indicated. Red arrowheads indicate the missing claws. Fusions between digits 2 and 3, and digits 4 and 5 are shown in Rspo2(-/-) mouse. (I-P) Whole mount hindlimb skeleton of Rspo2(+/+) and Rspo2(-/-) mice at E18.5. The skeleton of the left hindlimbs (LH) of Rspo2(-/-) showed mild defects (J and L). Note the absence of digit 5 and lack of ossification of the most distal phalangeal bones were detected. Hindlimb skeleton of Rspo2(-/-) mouse with more severe defects (M-P). The left hindlimb of Rspo2(-/-) mice (M and N) showed more severe defects than the right hindlimb of the same mice (O and P). The left hindlimb was photographed from the anterior side to better visualize the defect in the fibula (black arrowheads). Red arrowheads in N and P indicate the absence of ossification of the distal phalangeal bones. Abbreviations: fi, fibula; fu, femur; hu, humerus; il, ilium; ra, radius; sc, scapula; ti, tibia; ul, ulna.
Limb defects in Rspo2(-/-) mice
In Rspo2(-/-) mice, the forelimbs appeared to be relatively normal except for the absence of claws (Figure 2A and B), whereas, in the hindlimbs, syndactyly with the absence of claws was consistently observed (Figure 2G and H). These defects were observed with a complete penetrance (n=12). We failed to detect any evidence of a disrupted dorsal-ventral patterning in Rspo2(-/-) mice. Whole mount skeletal preparations clearly showed that the more distal and posterior portion of the hindlimb, including the fibula, metatarsals, and phalanges, were severely stunted (Figure 2I-P). Generally, the fibula and digits of the left hindlimb were affected more severely than those of the right hindlimb (91.7%, n=12, Figure 2M-P). In the forelimbs of Rspo2 mutant mice, most of the skeleton was normal except for the absence of ossification of the most distal phalangeal bones (Figure 2E and F). The lack of the claws and the most distal phalangeal bones in both forelimbs and hindlimbs is phenotypically reminiscent of anonychia syndrome in humans (OMIM 206800). Interestingly, mutations in the human RSPO4 gene were recently identified in anonychia patients (Bergmann et al., 2006; Blaydon et al., 2006). The phenotype of Rspo2(-/-) mice suggests the strong possibility of Rspo2 function in nail development in humans. Whether mutations in the human RSPO2 gene also contributes to anonychia in humans remains to be determined.
Abnormal gene expression in the AER of Rspo2(-/-) mice
Robust Rspo2 expression in the AER (Nam et al., 2007)(Figure 1I-L) and skeletal defects of the distal portion of forelimbs and hindlimbs in Rspo2 mutants suggest that Rspo2 may be crucial for AER development and function during limb development. We examined FGF8 mRNA expression, because FGF8 is a key signaling molecule secreted from the AER and its expression is required for AER formation and maintenance (Lewandoski et al., 2000; Moon and Capecchi, 2000). Consistent with a relatively mild observed phenotype (claw defect) in the forelimbs of Rspo2(-/-) mice, FGF8 was expressed in the forelimbs of Rspo2(-/-) embryos at a level comparable to those of Rspo2(+/+) and Rspo2(+/-) embryos at E9.5 (data not shown, n=5), E10.5 (Figure 3A-C, n=5) and E11.5 (Figure 3M-O, n=4), suggesting that FGF8 expression in forelimbs was not significantly disrupted in Rspo2(-/-) embryos.
Figure 3.
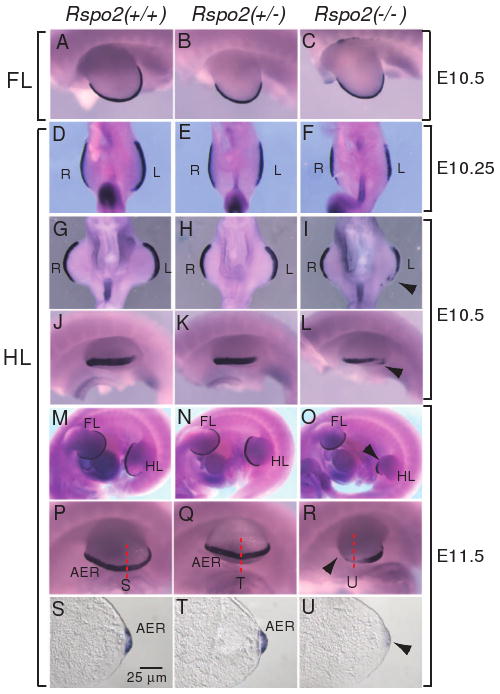
FGF8 expression in the AER of the developing limbs of Rspo2(-/-) mouse embryos. FGF8 expression was analyzed by whole mount in situ hybridization in the forelimbs (A-C) and hindlimbs (D-R) of Rspo2(+/+), Rspo2(+/-), and Rspo2(-/-) embryos at E10.25 (D-F), E10.5 (A-C and G-L) and E11.5 (M-R). The left side (A-C and J-R) and ventral side (D-I) views are presented, respectively. The right limbs (R), left limbs (L), forelimbs (FL) and hindlimbs (HL) of mouse embryos are indicated. Black arrowheads indicate the reduced FGF8 expression. The red dotted lines indicate the sectioning planes for the sections presented in S-U. (S-U) Transverse sections of the hindlimbs showed reduced expression of FGF8 expression within the AER region of Rspo2(-/-) embryos at E11.5.
In the hindlimbs of Rspo2(-/-) embryos at E10-E10.25, we detected relatively normal or slightly reduced FGF8 expression (Figure 3D-F, n=4). However, FGF8 expression in the AER of the hindlimb of Rspo2(-/-) embryos became reduced and disrupted as the hindlimbs developed further (Figure 3G-U, n=9). At E10.5, the domain and level of FGF8 expression in Rspo2(-/-) embryos was much reduced compared to those of Rspo2(+/+) and Rspo2(+/-) embryos (Figure 3G-L). This defect became more severe in Rspo2(-/-) embryos at E11.5 (Figure 3O and R). The sections of the stained limbs clearly showed the reduced FGF8 expression and the less prominent AER structure in Rspo2(-/-) embryo (Figure 3S-U). Consistent with observed skeletal defects, the reduction of FGF8 expression in the left hindlimb was more prominent than that of the right hindlimb (Figure 3I, L and R, 100%, n=9). These results indicate that the induction of FGF8 expression occurs normally in the hindlimbs of Rspo2(-/-) embryos; however, the maintenance of FGF8 expression in the AER of hindlimbs is incomplete in Rspo2(-/-) embryos.
FGF4 is another member of the FGF family of genes expressed in the AER (Niswander and Martin, 1992; Suzuki et al., 1992). Conditional inactivation of the mouse FGF4 gene in the limbs does not result in any limb defects possibly due to functional complementation by other FGFs (Moon et al., 2000; Sun et al., 2000). Several studies in chicken embryos, however, suggest that FGF4 provides mitogenic and morphogenetic signaling cues in the developing limbs (Laufer et al., 1994; Niswander et al., 1993). We examined FGF4 expression in Rspo2(-/-) embryos. Similar to FGF8 expression, FGF4 expression in the forelimbs was apparently normal (data not shown), whereas the expression in the hindlimbs was clearly reduced and disrupted in Rspo2(-/-) embryos at E10.5 and 11.5 (Figure 4F and H, n= 5). In the hindlimb of E11.5 embryo shown in Figure 4H, a portion of the recognizable AER appears to be missing in a discontinuously jagged pattern. Expression of other genes known to be expressed in AER such as BMP4 and Wnt5a was similarly reduced within the AER of the hindlimbs of Rspo2(-/-) embryos at E10.5 (data not shown).
Figure 4.
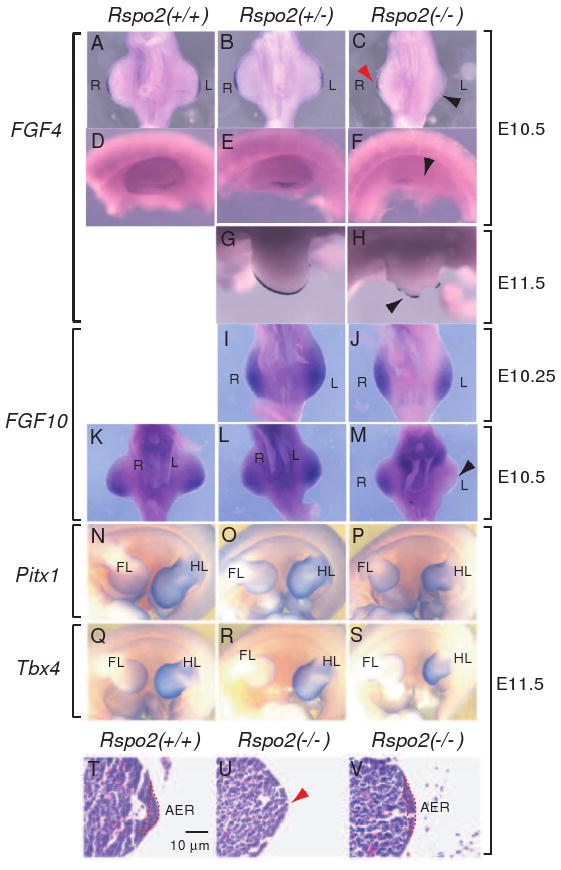
Gene expression in the developing limbs of Rspo2(-/-) mouse embryos. (A-H) FGF4 expression was analyzed by whole mount in situ hybridization in Rspo2(+/+), Rspo2(+/-), and Rspo2(-/-) embryos at E10.5 (A-F) and E11.5 (G-H). (I-M) FGF10 RNA expression was analyzed in Rspo2(+/+), Rspo2(+/-), and Rspo2(-/-) mouse embryos at E10.25 (I-J) and E10.5 (K-M), respectively. The ventral side (A-C and I-M) and left side views (D-H) of the hindlimbs are presented, respectively. The right (R) and left (L) limbs are indicated. Black and red arrowheads indicate the reduced FGF4 (C and H) and FGF10 (M) expression in the hindlimbs of Rspo2(-/-) mouse embryos. (N-S) RNA expression of the hindlimb-specific markers, Pitx1 (N-P) and Tbx4 (Q-S), in the left hindlimbs of E11.5 mouse embryos. The left side views are presented. The forelimbs (FL) and hindlimbs (HL) of mouse embryos are indicated. (T-V) Transverse paraffin sections of the hindlimbs stained with hematoxylin and eosin. The AER is outlined with a red dotted line. Red arrowhead in panel U indicates the absence of a discernable AER structure.
FGF10 is expressed in mesenchymal cells underlying the AER and is required for the activation of FGF8 expression in the AER (Min et al., 1998; Sekine et al., 1999). Conversely, the loss of FGF8 expression results in a decrease of FGF10 expression in the limb buds (Moon and Capecchi, 2000), suggesting a positive reciprocal regulation of FGF8 and FGF10 expression. FGF10 expression in the hindlimbs of Rspo2(-/-) embryos at E10-E10.25 appeared unchanged compared to wild type embryos (Figure 4J, n=3), suggesting that Rspo2 does not regulate the induction of FGF10 expression. However, Rspo2(-/-) embryos at E10.5 or later showed clearly reduced FGF10 expression especially in the left hindlimbs (Figure 4M, n=4). Reduced FGF8 expression in Rspo2(-/-) embryos at similar stages is likely to cause a failure of maintaining FGF10 expression in the hindlimbs. This result is consistent with the phenotype of FGF8 null-mice in which FGF10 expression is reduced (Moon and Capecchi, 2000).
Consistent with the reduced AER-marker gene expression, histological sections of the hindlimbs of Rspo2(-/-) embryos at E11.5 showed that the AER was either lacking (Figure 4U) or less organized with reduced epithelial cells (Figure 4V) compared to the wild type hindlimbs (Figure 4T). Taken together, our results clearly demonstrated that AER function, as represented by several AER-specific marker expressions, was obviously disrupted in the hindlimbs of Rspo2(-/-) embryos. Interestingly, induction of AER in the hindlimbs seems not to be disrupted as shown by relatively normal expression of AER markers in E10-10.25 embryos. However, at E10. 5 and later, maintenance of AER function appears to be largely affected in the hindlimbs of Rspo2(-/-) mice as manifested by the disrupted FGF signaling.
Hindlimb-specific defects in Rspo2(-/-) mice
Rspo2 is expressed in the AER and mesenchymal cells proximal to body trunk of both hindlimbs and forelimbs (Nam et al., 2007)(Figure 1E-H); yet, defects associated with loss of Rspo2 are more severe in the hindlimbs. To determine whether hindlimb-specific defects observed in Rspo2(-/-) mice are associated with a change in hindlimb-specific gene expression, we analyzed the expression of two hindlimb-specific markers, Pitx1 and Tbx4. Mice lacking these transcription factor genes display defects in hindlimb development (Lanctot et al., 1999; Naiche and Papaioannou, 2003). Interestingly, expressions of Pitx1 and Tbx4 mRNA were unaffected in Rspo2(-/-) mice (Figure 4P and S, n=3, respectively), suggesting that the cause of hindlimb-specific defects is independent of Pitx1 and Tbx4 expression.
What may be the molecular cause for the hindlimb-specific defects in Rspo2(-/-) mice? Mice lacking both distal-less class homeobox genes, Dlx5 and Dlx6, display defects in the skeletal structures of the hindlimbs, whereas the forelimbs are grossly normal, and represent a mouse model of split hand/foot malformation type I (SFHM I) (Merlo et al., 2002; Robledo et al., 2002). Interestingly, both the Dlx5 and Dlx6 genes are highly expressed in the AER of both the forelimbs and hindlimbs in mouse embryos (Robledo et al., 2002). Therefore, it is possible that Rspo2 and Dlx5/6 may function in the same regulatory pathway to regulate hindlimb development. The genetic hierarchy and the nature of any molecular interaction between Rspo2 and Dlx5/6 remain to be determined. In addition, it is also noteworthy to mention that the mouse polydactylous mutation, luxate (lx), causes an anterior shift of the anteriorposterior border in the hindlimbs (Yada et al., 2002). Although the molecular identity of the gene responsible for this mutation is currently unknown, this mutation represents another example of hindlimb-specific gene function. Whether Rspo2 and lx genetically interact each other can be an interesting topic for the future studies.
Defective Shh signaling in Rspo2(-/-) limbs
Shh is a key secreted protein expressed in the ZPA, a signaling center that controls outgrowth and anterior-posterior patterning of limb (Capdevila and Izpisua Belmonte, 2001). Shh expression in the ZPA is maintained by FGFs including FGF8 secreted from the AER (Lewandoski et al., 2000; Moon and Capecchi, 2000). The observed reduction of FGF8 expression and loss of the posterior hindlimb digits in Rspo2(-/-) mice (Figures 2 and 3) suggests that Shh expression may be disrupted in Rspo2(-/-) mice. We observed reduced Shh expression in the left hindlimbs and, to a lesser extent, in the right hindlimbs of Rspo2(-/-) embryos at E10.5 (Figure 5C, n=3), and the disruption of Shh expression became more severe at E11.5 (Figure 5F, n=4). Furthermore, the expression of Gli1, a direct target gene of Shh signaling (Lee et al., 1997), was also severely reduced in the left side of hindlimbs of Rspo2(-/-) embryos at E11.5 (Figure 5I, n=3). Therefore, the syndactyly observed in the Rspo2(-/-) limbs is consistent with a disruption of anterior and posterior patterning regulated by Shh. It is plausible that Rspo2 regulates Shh expression through FGF signaling, as FGF8 is shown to activate Shh expression (Lewandoski et al., 2000; Moon and Capecchi, 2000). Alternatively, the Rspo2 proteins secreted from the cells of the AER may directly regulate Shh expression independent of FGF8 signaling.
Figure 5.
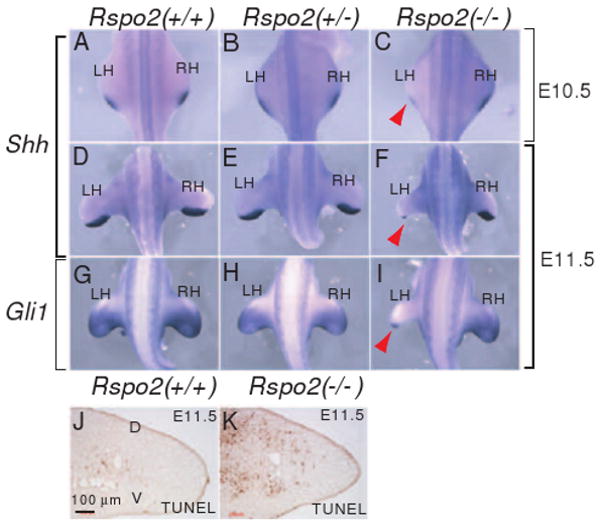
Disruption of Shh signaling in the ZPA of Rspo2(-/-) mouse embryos. (A-F) Shh RNA expression was analyzed in Rspo2(+/+), Rspo2(+/-) and Rspo2(-/-) mouse embryos at E10.5 (A-C) and E11.5 (D-F). (G-I) RNA expression of Gli1, a downstream target gene for Shh signaling. The dorsal views of embryos at the hindlimb level are presented. The left (LH) and right (RH) hindlimbs are indicated. Red arrowheads (C, F and I) indicate the reduced expression of Shh and Gli1 RNA expression in Rspo2(-/-) embryos. (J and K) Cell apoptosis was detected by TUNEL staining in the left hindlimbs of Rspo2(+/+) and Rspo2(-/-) mouse embryos at E11.5. Transverse sections along the proximal-distal axis were analyzed and the presented sections were from the posterior portion of the limbs.
Increased cell apoptosis in mesenchymal cells is observed in the limbs of Shh and FGF8-null embryos (Chiang et al., 1996; Moon and Capecchi, 2000), suggesting that Shh and FGF8 are survival factors for these cells. Consistent with this observation, we observed a significant increase in cell apoptosis in the mesenchymal cells within the posterior region of the Rspo2 mutant hindlimb compared to that of wild type mice (Figure 5J and K). Therefore, the increase in cell apoptosis in Rspo2(-/-) mice correlated with a reduction of Shh and FGF8 expression and disrupted normal limb skeletal development.
In contrast, cell proliferation, as assessed by BrdU incorporation and PCNA and phospho-histone3 protein expression, was unchanged within the developing limbs of homozygous embryos (data not shown). Interestingly, a massive proliferative effect on the intestinal epithelium of mice accompanying the activation of β-catenin signaling is seen with the injected human RSPO1 protein (Kim et al., 2005). Although little data are currently available to differentiate the binding specificity and affinity of Rspo family members to the individual Frizzled, LRP5, or LRP6 receptors, all four Rspo family members are demonstrated to activate β-catenin signaling with similar activities in vitro (Kazanskaya et al., 2004; Kim et al., 2005; Kim et al., 2006; Nam et al., 2006). However, lack of overt proliferation deficits in the AER and underlying mesenchymal cells in Rspo2(-/-) mice suggests that the biological responses induced by different Rspo family members may be context-dependent in vivo.
Reduced Wnt/β-catenin signaling in the AER of Rspo2(-/-) mice
TopGAL transgenic mice express a β-catenin signaling-responsive LacZ reporter gene (DasGupta and Fuchs, 1999). We detected a high level of LacZ expression in the AER of the TopGAL embryos at E10.5/E11.5 (Figure 6A-C), consistent with strong β-catenin signaling activity in the AER. Interestingly, Rspo2 expression highly overlapped with LacZ expression in the AER of the TopGAL mice (Figure 1I-L) (Nam et al., 2007), suggesting that Rspo2 may activate β-catenin signaling in the AER.
Figure 6.
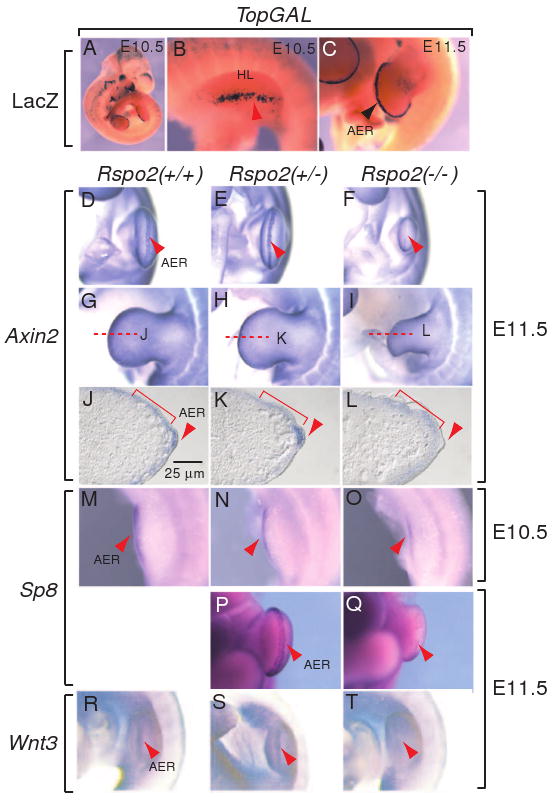
Inhibition of Wnt/β-catenin signaling in the AER of the hindlimbs of Rspo2(-/-) mouse embryos. (A-C) LacZ expression in the AER of the left hindlimbs of TopGAL transgenic mouse embryos at E10.5 (A and B) and E11.5 (C). The left hindlimds (HL) are presented in B and C. (D-L) RNA expression of Axin2, a β-catenin target gene, in the AER of the left hindlimbs of Rspo2(+/+), Rspo2(+/-), and Rspo2(-/-) mouse embryos at E11.5. The ventral side (D-F) and leftside (G-I) views of the hindlimbs are presented. The red dotted lines indicate the sectioning planes for the sections presented in J-L. (J-L) Transverse sections of the left hindlimbs of mouse Rspo2 mutant embryos stained with Axin2 riboprobe. Red brackets and arrowheads indicate Axin2 expression in mesenchymal cells and the AER, respectively. (M-Q) RNA expression of Sp8, another β-catenin downstream gene, in the left hindlimbs of Rspo2(+/+), Rspo2(+/-), and Rspo2(-/-) embryos at E10.5 (M-O) and E11.5 (P-Q). (R-T) Wnt3 RNA expression in the left hindlimbs of Rspo2(+/+), Rspo2(+/-), and Rspo2(-/-) embryos at E11.5. Red arrowheads indicate the expression of markers in the AER.
Because the LacZ gene was inserted into the Rspo2 locus and was expressed in the AER, we could not use the TopGAL expression to determine β-catenin activity in Rspo2 mutant mice. Therefore, to determine whether Rspo2 modulates β-catenin signaling in the AER, we examined expression of the Axin2 and Sp8 genes, two targets for Wnt/β-catenin signaling. The Axin2 gene is a direct downstream target of the β-catenin/TCF complex, and its expression correlates well with β-catenin signaling domains in mouse embryos (Jho et al., 2002). Strong Axin2 mRNA expression was detected in the AER of the hindlimbs of wild type and Rspo2(+/-) mice (Figure 6D, E, J and K); however, this expression was eliminated or significantly reduced in the AER of Rspo2(-/-) embryos (Figure 6F and L, n=4). Interestingly, Axin2 expression in limb mesenchymal cells underneath the surface ectoderm outside of the AER appeared to be relatively unchanged in all examined embryos (Figure 6G-I and J-L), indicating that defects associated with Rspo2 mutation are largely restricted within the AER.
The expression of Sp8 gene, encoding a zinc-finger transcription factor, is positively regulated by Wnt/β-catenin signaling in chicken embryos (Kawakami et al., 2004) and by Wnt3 in mouse embryos (Bell et al., 2003). Mice lacking the Sp8 gene showed severe truncation of both forelimbs and hindlimbs (Bell et al., 2003). Furthermore, the Sp8 transcription factor regulates FGF8 expression and limb outgrowth (Bell et al., 2003; Kawakami et al., 2004) and appears to function as an intermediate for FGF8 activation by β-catenin in the limbs. Sp8 expression was slightly reduced in the hindlimbs of Rspo2(-/-) embryos at E10.5 (Figure 6O, n=3) and was severely reduced at E11.5 (Figure 6Q, n=3). Taken together, these results are consistent with reduced or compromised β-catenin signaling in the AER of Rspo2(-/-) mice.
The Wnt3 gene is expressed in the limb surface ectoderm, including the AER, and overlaps with Rspo2 expression in the AER (Barrow et al., 2003). Conditional inactivation of the Wnt3 gene in the AER as well as in the limb surface ectoderm results in severe defects in the AER and limb skeleton (Barrow et al., 2003). Wnt3 expression in the AER was reduced or abolished in Rspo2(-/-) mice compared to littermate controls (Figure 6T, n=3). In contrast, no obvious difference in Wnt3 expression was observed in the surface ectoderm outside of the AER of wild type, heterozygous, and homozygous mutant mice (Figure 6R-T). Normal Axin2 expression within the mesenchymal cells underlying the surface ectoderm in Rspo2(-/-) embryos is likely induced by the Wnt3 protein originating from the surface ectoderm (Figures 6J-L and 7). Reduced Wnt3 expression in the AER of Rspo2(-/-) embryos suggests that Rspo2 positively regulates Wnt3 expression in the AER (Figure 7). It is plausible that this regulation is mediated through the β-catenin-dependent signaling pathway. Reciprocally, it is possible that Wnt3 can positively regulate Rspo2 expression in AER, possibly through the β-catenin dependent pathway. The existence of such a positive regulatory loop in Wnt3 and Rspo2 expression remains to be determined.
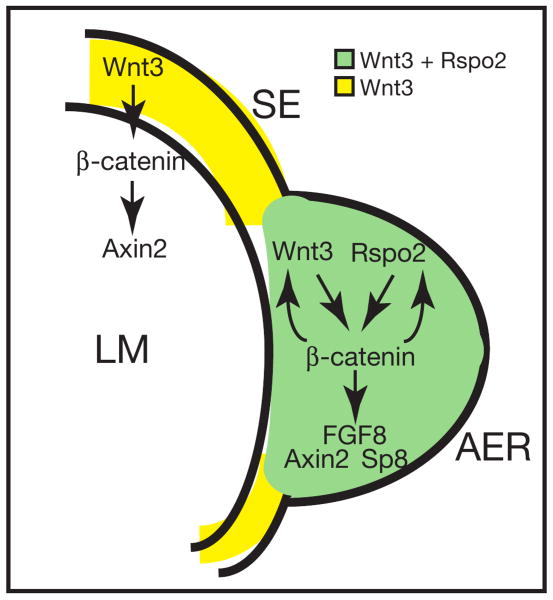
Figure 7.
An integrated model of Rspo2-induced signaling in the AER of the mouse hindlimb. In the AER, Rspo2 and Wnt3 may act together or independently to activate strong β-catenin signaling, which leads to the activation of FGF8, Sp8 and Axin2 genes. In contrast, in the surface ectoderm (SE) outside of the AER where no Rspo2 expresses, Wnt3 may activate Axin2 in limb mesenchymal (LM) cells likely via β-catenin signaling. Although Wnt3 alone may be an enough signal for Axin2 expression, Rspo2 may be essential to regulate FGF8 and Sp8 expression. Solid arrows indicate the regulatory interactions that were proposed in this study and previously determined.
It is demonstrated that β-catenin signaling is crucial for FGF8 expression in the AER (Barrow et al., 2003; Soshnikova et al., 2003). Our data and previously published results (Barrow et al., 2003) strongly suggest that the Rspo2 and Wnt3 proteins are two specific ligands that likely activate β-catenin signaling in the AER. It is possible that the Rspo2 and Wnt3 ligands activate β-catenin signaling independently (Figure 7). However, it is noteworthy to mention that the Rspo and canonical Wnt proteins such as Wnt1 activate β-catenin signaling in a synergistic manner, although mechanistic details of this synergism are presently unknown (Kim et al., 2005; Nam et al., 2006). Recent biochemical analyses of human Rspo1 and mouse Rspo3 proteins suggest that the Rspo family of proteins are high-affinity ligands for the LRP6 receptors, whereas they are relatively weak ligands for the Frizzled receptor (Nam et al., 2006; Wei et al., 2007). In contrast, Wnt ligand appears to have a high affinity to the Frizzled receptor (Hsieh et al., 1999; Rulifson et al., 2000), whereas it is a generally poor ligand for the LRP6 receptor (Wu and Nusse, 2002). Therefore, it is possible that the Wnt3 and Rspo2 proteins form a specific complex that becomes a higher affinity ligand than either Wnt3 or Rspo2 alone to both receptors. The Wnt3/Rspo2 ligand complex may enhance a formation of Frizzled/LRP6 receptor complex, which then can transmit a robust β-catenin signaling inside cells. We previously showed that Wnt1 and Rspo proteins indeed formed a complex in cultured cells (Nam et al., 2006). Therefore, the secreted Rspo2 and Wnt3 proteins may synergistically activate β-catenin signaling in the AER, which subsequently leads to the activation of gene expression of β-catenin signaling targets, FGF8, Sp8 and Axin2. High level of LacZ expression detected in the AER of TopGAL mice (Figure 6A-C) is consistent with a synergistic function between Rspo2 and Wnt3 proteins in the AER. Conversely, in the surface ectoderm outside the AER where only Wnt3 is expressed, no LacZ expression is observed. Therefore, the Rspo2 protein expressed in the AER may provide a mechanism for fine-tuning Wnt/β-catenin signaling to maintain the AER. It remains to be determined whether this is a general aspect of the Rspo family proteins in the biological contexts wherein both the Wnt and Rspo family genes are coexpressed.
Biological defects associated with the mutations of the other Rspo family genes
Among the four members of the Rspo gene family, the disruption of the Rspo2 (this study) and Rspo3 genes (Aoki et al., 2007) in mice are reported to date. The disruption of mouse Rspo3 gene results in embryonic lethality due to placental defects, which is consistent with the expression pattern of the mouse Rspo3 mRNA in the chorion and allantois (Aoki et al., 2007). Unfortunately, embryonic lethality caused by placental defects prevents further assessment of Rspo3 role in embryonic and postnatal development. Although the phenotypes of mice lacking Rspo1 and Rspo4 have not yet been reported, a recent study documents the case of XX>male sex conversion in humans with presumed RSPO1 null mutations (Parma et al., 2006). This study also shows that additional defects in skin differentiation and predisposition to squamous cell carcinoma of the skin result from the human RSPO1 mutations (Parma et al., 2006). Two recent studies describe mutations in the human RSPO4 gene that are linked to anonychia, a condition in which the nails and occasionally distal bones of phalanges are missing (Bergmann et al., 2006; Blaydon et al., 2006). Interestingly, in both the forelimbs and hindlimbs of Rspo2(-/-) mice, we observed the absence of the most distal limb structures: the distal phalangeal bones and the claws. Therefore, a causal relationship between loss of Rspo2 or RSPO4 gene function and defective claw and nail development suggests a significant role for the Rspo family of proteins in claw and nail development.
In this study, we describe a critical role for Rspo2 in AER development. Our results demonstrate that Rspo2 function in the AER is required for maintenance by regulating the expression of several key signaling molecules in the developing limb. Inactivation of the Rspo2 gene was associated with reduced FGF8 and FGF4 expression in the AER and a disruption of Shh signaling in the ZPA, further confirming regulatory interactions between the AER and the ZPA in the developing limbs. In the AER of Rspo2(-/-) limbs, Wnt/β-catenin signaling activity was markedly disrupted as shown by the decreased expression of Wnt3, Axin2 and Sp8 genes. Interestingly, ablation of the Rspo2 gene resulted in hindlimb-specific AER defects, suggesting that the regulation of AER development and function in the forelimb and hindlimbs may employ non-overlapping signaling pathways that require Rspo2 function.
Acknowledgments
We thank Drs. Lucy Liaw, Doug Spicer, Calvin Vary and Don Wojchowski for comments and suggestions. We also thank Kathleen Carrier (Histology Core at MMCRI), Winston Thomas and Shera Kash (Deltagen Inc.), and Sophie Hazell and Chip Lomas (Nuvelo Inc.) for their technical assistance. This research was partially supported by NIH P20 RR018789 grant to J.K.Y.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki M, Mieda M, Ikeda T, Hamada Y, Nakamura H, Okamoto H. R-spondin3 is required for mouse placental development. Dev Biol. 2007;301:218–226. doi: 10.1016/j.ydbio.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Waclaw RR, Campbell K, Potter SS, Scott WJ. Sp8 is crucial for limb outgrowth and neuropore closure. Proc Natl Acad Sci U S A. 2003;100:12195–200. doi: 10.1073/pnas.2134310100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C, Senderek J, Anhuf D, Thiel CT, Ekici AB, Poblete-Gutierrez P, van Steensel M, Seelow D, Nurnberg G, Schild HH, Nurnberg P, Reis A, Frank J, Zerres K. Mutations in the gene encoding the Wnt-signaling component R-spondin 4 (RSPO4) cause autosomal recessive anonychia. Am J Hum Genet. 2006;79:1105–9. doi: 10.1086/509789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon DC, Ishii Y, O'Toole EA, Unsworth HC, Teh MT, Ruschendorf F, Sinclair C, Hopsu-Havu VK, Tidman N, Moss C, Watson R, de Berker D, Wajid M, Christiano AM, Kelsell DP. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nat Genet. 2006;38:1245–7. doi: 10.1038/ng1883. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Izpisua Belmonte JC. Patterning mechanisms controlling vertebrate limb development. Annu Rev Cell Dev Biol. 2001;17:87–132. doi: 10.1146/annurev.cellbio.17.1.87. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Church VL, Francis-West P. Wnt signalling during limb development. Int J Dev Biol. 2002;46:927–36. [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–68. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci U S A. 1999;96:3546–51. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Esteban CR, Matsui T, Rodriguez-Leon J, Kato S, Belmonte JC. Sp8 and Sp9, two closely related buttonhead-like transcription factors, regulate Fgf8 expression and limb outgrowth in vertebrate embryos. Development. 2004;131:4763–74. doi: 10.1242/dev.01331. [DOI] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7:525–34. doi: 10.1016/j.devcel.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Kengaku M, Capdevila J, Rodriguez-Esteban C, De La Pena J, Johnson RL, Belmonte JC, Tabin CJ. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science. 1998;280:1274–7. doi: 10.1126/science.280.5367.1274. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–9. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- Kim KA, Zhao J, Andarmani S, Kakitani M, Oshima T, Binnerts ME, Abo A, Tomizuka K, Funk WD. R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle. 2006;5:23–6. doi: 10.4161/cc.5.1.2305. [DOI] [PubMed] [Google Scholar]
- Lanctot C, Moreau A, Chamberland M, Tremblay ML, Drouin J. Hindlimb patterning and mandible development require the Ptx1 gene. Development. 1999;126:1805–10. doi: 10.1242/dev.126.9.1805. [DOI] [PubMed] [Google Scholar]
- Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994;79:993–1003. doi: 10.1016/0092-8674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–52. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26:460–3. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Adamska M, Meisler MH. Hypomorphic expression of Dkk1 in the doubleridge mouse: dose dependence and compensatory interactions with Lrp6. Development. 2004;131:2543–52. doi: 10.1242/dev.01126. [DOI] [PubMed] [Google Scholar]
- Merlo GR, Paleari L, Mantero S, Genova F, Beverdam A, Palmisano GL, Barbieri O, Levi G. Mouse model of split hand/foot malformation type I. Genesis. 2002;33:97–101. doi: 10.1002/gene.10098. [DOI] [PubMed] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–61. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon AM, Boulet AM, Capecchi MR. Normal limb development in conditional mutants of Fgf4. Development. 2000;127:989–96. doi: 10.1242/dev.127.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon AM, Capecchi MR. Fgf8 is required for outgrowth and patterning of the limbs. Nat Genet. 2000;26:455–9. doi: 10.1038/82601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Belmonte JC, Westphal H. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–34. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- Naiche LA, Papaioannou VE. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development. 2003;130:2681–93. doi: 10.1242/dev.00504. [DOI] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Smith PF, Choi S, Yoon JK. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J Biol Chem. 2006;281:13247–57. doi: 10.1074/jbc.M508324200. [DOI] [PubMed] [Google Scholar]
- Nam JS, Turcotte TJ, Yoon JK. Dynamic expression of R-spondin family genes in mouse development. Gene Expr Patterns. 2007;7:306–12. doi: 10.1016/j.modgep.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Niswander L, Martin GR. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992;114:755–68. doi: 10.1242/dev.114.3.755. [DOI] [PubMed] [Google Scholar]
- Niswander L, Tickle C, Vogel A, Booth I, Martin GR. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 1993;75:579–87. doi: 10.1016/0092-8674(93)90391-3. [DOI] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–9. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–8. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Robledo RF, Rajan L, Li X, Lufkin T. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev. 2002;16:1089–101. doi: 10.1101/gad.988402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulifson EJ, Wu CH, Nusse R. Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless. Mol Cell. 2000;6:117–26. [PubMed] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–41. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Soshnikova N, Zechner D, Huelsken J, Mishina Y, Behringer RR, Taketo MM, Crenshaw EB, 3rd, Birchmeier W. Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes Dev. 2003;17:1963–8. doi: 10.1101/gad.263003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Lewandoski M, Meyers EN, Liu YH, Maxson RE, Jr, Martin GR. Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nat Genet. 2000;25:83–6. doi: 10.1038/75644. [DOI] [PubMed] [Google Scholar]
- Suzuki HR, Sakamoto H, Yoshida T, Sugimura T, Terada M, Solursh M. Localization of HstI transcripts to the apical ectodermal ridge in the mouse embryo. Dev Biol. 1992;150:219–22. doi: 10.1016/0012-1606(92)90020-h. [DOI] [PubMed] [Google Scholar]
- Wei Q, Yokota C, Semenov MV, Doble B, Woodgett J, He X. R-spondin1 Is a High Affinity Ligand for LRP6 and Induces LRP6 Phosphorylation and beta-Catenin Signaling. J Biol Chem. 2007;282:15903–11. doi: 10.1074/jbc.M701927200. [DOI] [PubMed] [Google Scholar]
- Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J Biol Chem. 2002;277:41762–9. doi: 10.1074/jbc.M207850200. [DOI] [PubMed] [Google Scholar]
- Yada Y, Makino S, Chigusa-Ishiwa S, Shiroishi T. The mouse polydactylous mutation, luxate (lx), causes anterior shift of the anteroposterior border in the developing hindlimb bud. Int J Dev Biol. 2002;46:975–82. [PubMed] [Google Scholar]
- Yoon JK, Wold B. The bHLH regulator pMesogenin1 is required for maturation and segmentation of paraxial mesoderm. Genes Dev. 2000;14:3204–14. doi: 10.1101/gad.850000. [DOI] [PMC free article] [PubMed] [Google Scholar]