Abstract
After a review of Arthur Benton’s conceptual and methodological contributions to the understanding of normal and pathological development, we discuss agenesis of the corpus callosum (CC), criteria for potential neuroanatomical compensatory mechanisms in CC agenesis, and the results of an examination of magnetic resonance imaging (MRI) data of the CC in 193 children with spina bifida meningomyelocele (SBM). There were 26 CC regional patterns. Although complete agenesis did not occur, partial agenesis was observed in 102 children and within 15 CC regional patterns. Only 4.1% had a normal CC. Quantitative assessment of the area of the CC in 26 NC children and 68 children with SBM revealed that all subgroups with CC anomalies had smaller areas than did a subgroup with a normal CC. Areas were especially small in rostral/splenial agenesis and splenial agenesis but larger with rostral agenesis. Subgroups with normal/hypoplastic regions or complete hypoplasia also had CC areas that were smaller than normal but larger than the areas for the splenial agenesis groups. The relative rarity of anterior commissure enlargement (3.1%) and longitudinal bundles of Probst (0.1%) suggest that these particular fiber tract anomalies are unlikely candidates for structural compensatory mechanisms. The hippocampal commissure, enlarged in 13%, may be a more promising candidate. Overall, however, the functionality of anomalous fiber tracts and commissures in SBM is yet to be determined.
Keywords: Spinal dysraphism, Magnetic resonance imaging, Anterior commissure, Neural tube defect, Plasticity
This paper pays brief tribute to Arthur Benton’s contributions to understanding both normal and pathological development of neurocognitive functions. It then develops some of the themes and methods in his work into an investigation of a contemporary developmental question: options for neuroanatomical functionality after congenital absence (partial agenesis) or thinning (hypoplasia) of regions of the great cerebral commissure, the corpus callosum (CC).
BENTON’S CONTRIBUTIONS TO NORMAL AND PATHOLOGICAL DEVELOPMENT
Arthur Benton’s career was unique in a number of ways, not the least of which was his broad view of normal and pathological development. The biographical notes for his American Psychological Association Distinguished Professional Contribution Award for 1978 (American Psychological Association, 1979) indicate that he studied adults with brain injury at the Neurological Institute of New York (1935) and children with cerebral palsy for the Kentucky Crippled Children’s Commission (1946). Review of his publications reveals papers on typical child function (e.g., normal children and the Visual Retention Test; Benton & Collins, 1949); typical adult function (e.g., young adults and the Block Design Test; Benton, 1941); adult brain pathologies (reaction time in adults with cerebral lesions; Benton, 1958); childhood developmental pathologies (developmental aphasia; Benton, 1964); and adult function in survivors of childhood brain disorders (adult effects of early bilateral frontal lesions; Ackerly & Benton, 1947).
Benton did not directly interest himself in potential compensatory mechanisms in the immature brain, in all likelihood because the imaging capabilities that make the current study possible were not available until the later years of his life. Nevertheless, he had a broad and open-minded approach to children’s problems that presaged not only some of the questions we currently study in child neuropsychology, but also how we study them. Four of his insights have influenced career trajectories for us, and for many others: (a) the theoretical value of comparing brain-damaged children and adults on a task on which performances of normal children and adults have also been analyzed (and, more recently, imaged); (b) the particular value in studying neurocognitive function in children with explicit brain damage but preserved intellect; (c) functional representations in children’s brains that might be associated with abnormal behavior; and (d) the methodological value of analyzing intragroup outcome variability in relation to variability in the medical manifestations of brain injury (e.g., Dennis et al., 2005; Fletcher et al., 2005; Hannay, 2000). We briefly discuss each of these four insights and how they have shaped contemporary investigations in child neuropsychology.
Benton believed that significant insights into the nature of a particular psychological function might emerge from a direct comparison of normal children, atypical children, normal adults, and atypical adults on the same paradigms. For example, he conducted parallel studies of right—left discrimination and finger localization in normal children (Benton, 1955a), children with mental retardation (Benton, 1955b), and abnormal and brain-injured adults (Benton & Cohen, 1955).
In an era in which a key comparison was between children with mental deficiency deemed to have no brain damage and typically developing groups, Benton argued that this was the wrong comparison and questioned the assumption that children with mental retardation had intact brains. He focused his attention on the particular value of studying specific neurocognitive deficits exhibited by children with explicit brain damage who had intact intelligence (Benton, 1962).
In an era in which the concept of minimal brain damage was popularly applied to diverse groups of children with heterogeneous behavioral problems, Benton asked such penetrating questions as why children with the most severe quantum of cortical brain damage, those with cerebral hemispherectomy, displayed no signs of minimal brain damage and whether some forms of brain damage that produced what was then termed hyperkinetic impulse disorder in children might arise from midbrain rather than cortical damage (Benton, 1962). He asked wide-ranging questions about functional brain representation in children and was interested in why and how some children escaped deficits despite significant brain insult.
While contemporary investigations of children with brain damage were usually concerned with descriptions of individual groups, Benton proposed the novel idea that intragroup variability could be used as an investigative tool, and he sought to understand how medical variability within the same etiology might be related to variability in cognitive and behavioral outcome. In his extensive review of studies of premature birth, for example, he correlated intellectual outcome with the type and severity of medical compromise within the group, finding that both low birth weight and the presence of intracranial hemorrhage were risk factors for poor cognitive outcome (Benton, 1940).
CORPUS CALLOSUM AGENESIS
The last question, how intragroup variability may reveal neurocognitive function, is the primary context for the current study. By investigating within-group variability, we approach the general question of how the immature brain might be reorganized so as to compensate in the face of significant brain insult. A productive forum for discussion of this question has been the CC and one of its signature deficits, impairment of interhemispheric transfer (IHT; Hannay, 2000; Hannay et al., in press). Acquired insult to the CC through vascular or neoplastic damage or section of the CC produces striking IHT impairments in various modalities (e.g., Geschwind & Kaplan, 1962; Pollmann, Maertens, von Cramon, Lepsien, & Hugdahl, 2002). In contrast, neither partial nor complete agenesis of the CC in children produces equally severe IHT deficits on comparable tasks (e.g., Hannay, 2000; Lehmann & Lampe, 1970).
Criteria for structural compensatory mechanisms
The concept of compensatory mechanisms has been invoked to explain why children with congenital CC agenesis differ so dramatically in IHT from children and adults with acquired CC damage or section. If a brain mechanism is compensatory, we would expect that it should be evident in more than a small proportion of a large sample with agenesis of the CC and have some relation with the nature of the CC anomaly. If these criteria hold, then the structure in question is a candidate for a compensatory mechanism. Whether it is actually compensatory would require an additional demonstration that its presence enhances functionality and that its absence reduces functionality.
We do not know which changes in the brains of children with CC agenesis are related to compensatory adaptation and which are associated with functional deficits. The first step to this end, and the topic of this paper, is to identify and briefly review evidence of candidates for compensatory adaptation previously mentioned in the literature.
Candidates for compensatory mechanisms in CC agenesis
A number of candidates have been proposed as possible structural compensatory mechanisms in children with CC agenesis. From the human and placental mammal literature, these include: enlargement of the anterior commissure (AC; Livy et al., 1997; Martin, 1985; Risse, LeDoux, Springer, Wilson, & Gazzaniga, 1978); development of longitudinal bundles of Probst (Barkovich, 2005); enlargement of the hippocampal commissure (HC; Ozaki & Wahlsten, 1993); and strengthening of ipsilateral and/or subcortical pathways (Dennis, 1976; Gazzaniga, Holtzman, & Smylie, 1987).
The appearance of some of these compensatory mechanisms may depend on the temporal sequence of development of the CC and other cerebral commissures, all of which develop fairly closely in time. Pioneer axons of the AC begin to cross in the lamina reuniens at 10 weeks of gestation, and by Week 11 the anterior commissure is present. Similarly, pioneer HC axons cross the midline through another site that develops in the lamina reuniens, the massa commissuralis, at 10 weeks following the AC axons, with the HC being essentially complete by 11–12 weeks (Barkovich, 2005; Barkovich et al., 1992; Rakic & Yakovlev, 1968; Raybaud & Girard, 2005). The earlier development of these pathways presumably could provide alternate routes for potential CC fibers to cross interhemispherically when the callosal bed does not develop, assuming the AC and HC are sufficiently developed at the time (Barkovich, 2005). The first CC fibers cross at about 13–14 weeks in the massa commissuralis (Hewitt, 1962; Rakic & Yakovlev, 1968; Raybaud & Girard, 2005; Ren et al., 2006), and all sections of the CC are present by about 18–20 weeks of fetal development, with the developmental sequence basically being genu, body, splenium, and rostrum. When there is callosal agenesis, the AC and HC may be absent, small, or occasionally enlarged (Barkovich, 2005; Paul et al., 2007). In the next section, we consider anatomical evidence for each proposed compensatory mechanism.
Anterior commissure
In a normally developing human, the AC can be found in the anterior wall of the third ventricle. There is evidence that the AC connects inferior temporal and occipital lobes, occipital convexity, and perhaps the prefrontal convexity and central fissure (Di Virgilio, Clarke, Pizzolato, & Schaffner, 1999; Risse et al., 1978; Rocha-Miranda, Bender, Gross, & Mishkin, 1975). The AC is related to the amygdaloid complex, anterior-inferior insula, posterior-medial orbito-frontal cortex and some entorhinal-perirhinal cortex (Raybaud & Girard, 2005). The connections through the AC are not entirely clear in humans and appear to carry axons from wider regions than those for other primates and form homotopic and heterotopic connections (Di Virgilio et al., 1999). Some have suggested that fibers usually connecting visual and auditory cortices (posterior body and splenium) may cross in the AC of humans if there is complete agenesis of the CC, in which case the AC would appear enlarged and might be functionally adaptive (Fischer et al., 1992; Paul et al., 2007), at least for some behavioral tasks (Barr & Corballis, 2002).
To illustrate, Fischer et al. (1992) studied two boys with complete agenesis of the CC who showed mild abnormalities on dichotic listening (Kimura, 1961a, 1961b) that were suggestive of enhanced left-ear perception of the stimuli. The child with an absent or small AC showed an exaggerated right visual field (RVF) superiority for object and word naming, which correlated with a reduced ability to name left visual field (LVF) stimuli; the child with an enlarged AC had a slight RVF superiority on the same task. While his enlarged AC might have transferred verbal information from the right to left hemisphere, it is possible that, as a left-hander, his language was more bilaterally represented (Barr & Corballis, 2002; Hannay, 2000).
Barr and Corballis (2002) studied visual interhemispheric transfer in two right-handed women with CC agenesis. The woman with an enlarged AC had no more difficulty in matching colors and letters between than within visual fields, in contrast to the woman with a normal AC, who did display interhemispheric matching problems. Two of the latter woman’s 5 children, both girls, also had complete CC agenesis and a normal AC and, like their mother, showed integration of words between visual fields but difficulty with colors (Corballis & Finlay, 2000), suggesting that a normal AC was sufficient to integrate form but not color.
With agenesis of the CC, AC enlargement (hyperplasia) is relatively uncommon. For example, a recent study of complete agenesis reported that the AC was absent in 60% of cases and enlarged in 10% (Hetts, Sherr, Gobuty, Chao, & Barkovich, 2006). Based on autopsies of 12 children, 10 with complete agenesis and 2 with partial agenesis of the CC, Loeser and Alvord (1968) reported that the AC was enlarged in 1 (11.1%) child, normal in 8 (66.7%) children, absent in 2 (22.2%), and unknown in 1 (11.1%) of their cases. The child with the enlarged AC had complete CC agenesis. Meyer, Roricht, and Niehaus (1998) reported no enlargement of the AC in individuals with complete CC agenesis, the AC being smaller than normal in 4 and normal in 2. Because of these kinds of findings, Rauch and Jinkins (1994) had suggested that enlargement could serve as a compensatory mechanism in only a small proportion of children.
Longitudinal bundles of Probst
Longitudinal bundles of Probst are known to develop in cases of complete CC agenesis (Barkovich, 1996; Barkovich & Norman, 1988; Pirola et al., 1998; Rakic & Yakovlev, 1968). Indeed, Probst’s bundles were present on both sides in 60% of Loeser and Alvord’s (1968) 10 autopsy cases of complete CC agenesis. One of these cases had a Probst bundle only on the left. In the two cases of partial CC agenesis, Probst’s bundles were present, but only where the CC was absent. Probst’s bundles are thought to contain fibers intended for the CC that then run parallel to the interhemispheric fissure. Associated with Probst’s bundles is an everted (unrotated) cingulate gyrus such that the cingulate sulcus remains unformed. These abnormalities are accompanied by crescent-shaped lateral ventricles and are more easily seen on coronal magnetic resonance imaging (MRI; Barkovich, 2005; Rauch & Jinkins, 1994).
In acallosal mice some fibers have been shown to leave Probst’s bundles and form ipsilateral cortico-cortical connections (Ozaki, Iwahashi, & Shimada, 1989; Ozaki, Murakami, Toyoshima, & Shimada, 1987) while others cross over on the dorsal HC (Ozaki & Shimada, 1988). The one child with complete agenesis of the CC and spina bifida meningomyelocele (SBM) in the Loeser and Alvord (1968) study did not have Probst’s bundles, and her AC was absent. Rauch and Jinkins (1994) suggested that Probst’s bundles are evident with partial CC agenesis but that they are frequently smaller and form segmental bundles in the region of agenesis because fewer fibers would need an alternative pathway (see also Paul et al., 2007, and Tovar-Moll et al., 2007).
Hippocampal commissure
The HC, which is part of the fornix, crosses under the caudal body and rostral splenium of the CC and connects the hippocampi. It has been suggested that enlargement of the HC might be an indicator of CC fibers using the HC as an interhemispheric conduit. The CC agenesis literature provides little support for this. As Rauch and Jinkins (1994) noted, the HC is quite small and is usually difficult to visualize on MRI. Although they do not provide data on the status of the HC from their autopsied children, Loeser and Alvord (1968) suggest that the HC in CC agenesis is usually absent, occasionally present, but never enlarged. The CC carries fibers from neocortical areas of the brain, and the HC carries fibers from the hippocampus, so Rauch and Jinkins (1994) also considered it unlikely that the HC would be enlarged by aberrant CC fibers. Several studies of mice with congenital CC agenesis, however, report that fibers in Probst’s bundle fibers traverse the midline by growing across the dorsal surface of the hippocampal commissure when it is large enough (Ozaki et al., 1987; Ozaki & Wahlsten, 1993). In fact, an enlarged hippocampal commissure may be mistaken for the splenium of the corpus callosum in humans on a sagittal view, but can be seen to connect the fornices on the coronal view (Barkovich, 2005).
Ipsilateral and/or subcortical pathways
Strengthening of the ipsilateral and/or subcortical pathways has been suggested as a compensatory mechanism (Risse et al., 1978), and there is some evidence for subcortical transfer from callosotomy studies (Gazzaniga et al., 1987; Gazzaniga, Kutas, Van Patten, & Fendrich, 1989). Prior to the use of MRI, however, it was not possible to rule out transfer by spared callosal fibers. However, Funnell, Corballis, and Gazzaniga (2000) argued that even since the advent of MRI, few studies of these patients have checked for callosal sparing in their patients so there currently is little evidence for subcortical transfer.
Compensatory mechanisms in spina bifida meningomyelocele
The evaluation of the aforementioned CC compensatory mechanisms has been complicated not only by the relative paucity of individuals with congenital complete CC agenesis as their primary disorder, but also by the inability to visualize both the CC and putatively compensatory commissures. With the discovery of a population with homogeneous etiology and variable patterns of partial CC agenesis whose brains can be imaged in vivo with MRI procedures, it is possible to revisit the issue in a systematic manner.
Spina bifida meningomyelocele
Spina bifida meningomyelocele (SBM) is a neural tube defect that is the product of a complex pattern of gene—environment interactions and is associated with distinctive physical, neural, and cognitive manifestations (Fletcher et al., 2005). It is a common, severely disabling birth defect, with current prevalence levels in North America of 0.3–0.5 per 1,000 births (postdietary fortification data; Williams, Rasmussen, Flores, Kirby, & Edmonds, 2005). Children with SBM sustain a disruption of neuroembryogenesis that initiates a cascade of brain events that includes primary central nervous system (CNS) malformations such as CC agenesis and a secondary destructive process (hydrocephalus) that can produce, among other things, CC hypoplasia and changes in brain microstructure (Detrait et al., 2005).
The regions of the CC that do or do not develop give some evidence of the nature and timing of the insult during gestation and, to some degree, the type of insult (Barkovich, 2005). The sequence of CC development may be suggested by the pattern of partial agenesis. If the genu and body are present while the splenium and rostrum are absent, partial agenesis from a congenital genetic disorder may be inferred; if the rostrum and splenium are present while the genu and body are hypoplastic or absent, a destructive lesion due to secondary hydrocephalus is more likely (Barkovich, 2005). However, the basis of CC abnormalities is not always clear in children with SBM, who may have a combination of congenital and acquired effects on the cerebral white matter (Miller, Widjaja, Blaser, Dennis, & Raybaud, 2008).
We have studied a very large sample of children with a variety of patterns of partial agenesis of the CC that come from a single congenital etiology, SBM. This permits us to examine the likelihood of potential structural compensatory mechanisms. Although these children rarely show complete agenesis of the CC, Fletcher et al. (2005) reported in a large sample of children with SBM that only 4% had a normal CC, 52% had partial agenesis, and 44% had only hypoplasia of the CC. In this sample, we can determine the likelihood of specific potential compensatory mechanisms involving the enlargement of the AC, the development of Probst’s bundles, and the enlargement of the HC in relation to partial CC agenesis to determine whether any of them meet the proposed criteria for potential compensatory mechanisms. Our imaging technology does not permit evaluation of the strengthening of ipsilateral and/or subcortical pathways. The hypotheses are as follows:
The AC should be normal in typically developing children. In children with SBM, AC enlargement should occur more frequently with partial agenesis of the CC. The greater the partial agenesis, the more frequent AC enlargement should be, especially if the agenesis involves the splenium and body, because the AC is known to carry fibers connecting occipital and temporal lobe cortices.
While longitudinal bundles of Probst been reported with partial agenesis of the CC, they may not be present with even complete agenesis of the CC, so we expected to find very few cases with our population.
The HC should be normal in our typically developing children. The prediction about the relation between HC status and partial CC agenesis was less clear. A possible prediction is that HC enlargement should occur more frequently with more extensive partial agenesis of the CC in children with SBM. However, it was not clear whether HC enlargement would occur in the absence of Probst’s bundles, and we were not expecting to find many cases with Probst’s bundles.
An area measurement of the CC will demonstrate differences in subgroups defined by the pattern of dysmorphology, such that area measures will be largest in controls, next largest in children with hypoplasia of the CC, and then in those with agenesis of the CC, in particular the rostrum, splenium, and rostrum/splenium. The difference hypothesized between hypoplastic and agenesis subgroups reflects the congenital nature of agenesis, which leads to failure of the CC to fully develop and is also usually associated with some secondary hydrocephalus and hypoplasia. In addition, showing these predicted quantitative differences would essentially test the validity of the regional qualitative coding by the radiologists and also would validate our implicit hypothesis that children identified with agenesis on this coding have more extensive loss of the CC.
METHOD
Participants
The data for this research were collected in compliance with regulations of the University of Houston, the University of Texas—Health Science Center at Houston, and The Hospital for Sick Children in Toronto and their committees for the protection of human subjects. Typically developing controls (NC; n = 46) and school-aged children with SBM and shunted hydrocephalus (n = 193) underwent an MRI study. Children in the NC group were volunteers who responded to local advertising. Children with SBM were medically stable, recruited from clinics serving children in Houston (n = 126) and The Hospital for Sick Children in Toronto (n = 67). Exclusion criteria were neurological disorders unrelated to SBM, severe psychiatric disorder, uncontrolled seizure disorder, and an inability to control the upper limbs.
NC children were just over a year older (M = 12.8 years; SD = 3.33) than children with SBM (M = 11.5 years; SD = 3.22), F(1, 237) = 5.87, p = .016. The groups did not differ in sex, with females representing 43.5% of the NC group and 46.5% of the group with SBM. The chi-square for ethnicity could not be computed because of small samples of Blacks (NC = 3; SBM = 16) and Asians/other ethnicities (NC = 3; SBM = 7). Only one of the 46 NC children was Hispanic. In the children with SBM, the ratio of Hispanics (n = 17) to non-Hispanics (n = 176) was much higher (1:9.7). Hispanics have a higher incidence of SBM than do other ethnicities and also tend to show more severe neural phenotypes (Fletcher et al., 2005) and differences in heritability (Volcik et al., 2001), so this difference is not surprising. Handedness could not be established on 4 children with SBM. As expected, the children with SBM had a significantly higher rate of left handedness, χ2(1) = 5.63, p < .018. Nonright-handedness is associated with variations in interhemispheric transfer in typically developing people (Cherbuin & Brinkman, 2006) and in SBM (Hannay et al., in press), as well as variations in the neural phenotype of SBM (Fletcher et al., 2005).
MRI acquisition procedures
Each child received a MRI scan of the brain on comparable magnets (General Electric, Milwaukee, WI) in Houston and Toronto. After a sagittal localizer, two whole brain coronal sequences were completed. One series involved 3D fast spin-echo T2-weighted images, field of view (FOV) 24 cm, time to repetition (TR) 4,000 ms, echo time (TE) 102 ms, echo train length (ETL) 16, 256 × 256 matrix, 1 repetition with contiguous 1.7 mm coronal images. The other series was a 3D-spoiled gradient-echo with contiguous 1.7-mm coronal images, FOV 24 cm, TR 18 ms, TE 3 ms, flip angle 25 degrees, 124 locations, 256 × 256 matrix, 1 repetition. Separate T1 and T2 acquisitions were necessary to ensure adequate visualization of cerebrospinal fluid (CSF) versus gray and white matter.
Each scan was coded by radiologists in Houston and Toronto blinded to etiology. The coding scheme was broad, including coding for the nature and severity of hydrocephalus, the range of abnormalities associated with the characteristic Chiari II malformation, other specific abnormalities, and the status of the CC, AC, HC, and Probst bundles. The research team developed conventions and a detailed coding form that was applied to each scan. Each MRI film was displayed simultaneously in axial, coronal, and sagittal planes in a large viewing room. The radiologist walked around the viewing screens, identifying the different codes from the form upon prompting by a research assistant, who recorded the findings. The radiologists, who were blinded to patient histories and behavioral data, agreed on conventions and coding criteria based on independent dual coding of MRIs early in the grant.
In addition to the qualitative coding, an area measurement of the CC was calculated from the midsagittal slice depicting the CC (Fletcher et al., 1996). A region-filling algorithm was used to count the total number of pixels enclosed within the irregularly shaped boundaries. The total area of the slice (excluding the CC) was computed, so that the CC measure is a ratio of the CC area/whole brain slice area. This ratio permits control of individual differences in brain size due to variations in age and the effects of the disorder. Although the regions of the corpus callosum can be quantified based on anatomical subdivisions proposed, for example, by Witelson and Goldschmidt (1991), landmarks that might be used for such a regional quantification are not reliably visualized on a malformed CC. Moreover, it does not seem meaningful to estimate the area of the rostrum, for example, when it is absent. The value would be expressed as 0, which violates assumptions of parametric statistics. We find it most productive to measure the entire area and to qualitatively code regions of the corpus callosum. This measure was available on 68 children with SBM and 26 controls. Problems related to shunt artifacts, movement, and difficulties with scan transfer reduced the number of available scans suitable for quantification.
RESULTS
Patterns of agenesis of the CC
The CC was normal in all but 1 of the 46 NC children; 1 had a completely hypoplastic CC for no apparent reason. There were 26 patterns of CC development in children with SBM (Table 1). Regions that are absent (or not visualized) are printed in italics. Patterns in which the splenium and rostrum are absent (n = 42; 21.8%), the rostrum is absent (n = 40; 20.7%), and then the splenium is absent (n = 19; 9.8%) are given first. These patterns (1–14) likely involve congenital absence of a region (n = 101; 52.3%) although this cannot be known for certain. In only 3 of these children were the genu and body both normal so the rate of some form of acquired dysmorphology of the body and genu in combination with congenital partial agenesis was high (98/101). The genu was absent in 6 (3.1%) children with SBM while the body was absent in 3 (1.6%). Only 8 (4.1%) children with SBM had a completely normal CC.
TABLE 1.
Patterns of dysmorphology of the corpus callosum in children with SBM
| Rostrum | Genu | Body | Splenium | N | % | |
|---|---|---|---|---|---|---|
| 1 | Absent | Absent | Hypoplastic | Absent | 3 | 1.6 |
| 2 | Absent | Hypoplastic | Absent | Absent | 1 | 0.5 |
| 3 | Absent | Normal | Absent | Absent | 1 | 0.5 |
| 4 | Absent | Hypoplastic | Hypoplastic | Absent | 30 | 15.5 |
| 5 | Absent | Normal | Hypoplastic | Absent | 7 | 3.6 |
| 6 | Absent | Absent | Hypoplastic | Hypoplastic | 2 | 1.0 |
| 7 | Absent | Normal | Hypoplastic | Hypoplastic | 11 | 5.7 |
| 8 | Absent | Hypoplastic | Hypoplastic | Hypoplastic | 24 | 12.4 |
| 9 | Absent | Normal | Normal | Normal | 3 | 1.6 |
| 10 | Normal | Absent | Hypoplastic | Absent | 1 | 0.5 |
| 11 | Hypoplastic | Hypoplastic | Hypoplastic | Absent | 10 | 5.2 |
| 12 | Hypoplastic | Normal | Hypoplastic | Absent | 1 | 0.5 |
| 13 | Normal | Hypoplastic | Hypoplastic | Absent | 1 | 0.5 |
| 14 | Normal | Normal | Hypoplastic | Absent | 6 | 3.1 |
| 15 | Normal | Hypoplastic | Absent | Hypoplastic | 1 | .5 |
| 16 | Hypoplastic | Hypoplastic | Hypoplastic | Hypoplastic | 39 | 20.2 |
| 17 | Hypoplastic | Normal | Hypoplastic | Hypoplastic | 9 | 4.7 |
| 18 | Hypoplastic | Hypoplastic | Normal | Hypoplastic | 1 | 0.5 |
| 19 | Hypoplastic | Normal | Normal | Hypoplastic | 1 | 0.5 |
| 20 | Hypoplastic | Hypoplastic | Hypoplastic | Normal | 1 | 0.5 |
| 21 | Hypoplastic | Normal | Normal | Normal | 1 | 0.5 |
| 22 | Normal | Hypoplastic | Hypoplastic | Hypoplastic | 5 | 2.6 |
| 23 | Normal | Normal | Hypoplastic | Hypoplastic | 16 | 8.3 |
| 24 | Normal | Normal | Normal | Hypoplastic | 3 | 1.6 |
| 25 | Normal | Normal | Hypoplastic | Normal | 7 | 3.6 |
| 26 | Normal | Normal | Normal | Normal | 8 | 4.1 |
Note. Regions that are absent (or not visualized) are printed in italics. SBM = spina bifida meningomyelocele.
Anterior commissure
The AC was normal in all of the NC children. It was enlarged in only 3.1% (n = 6) of the children with SBM and then only when the splenium of the CC was absent or hypoplastic (Table 2). Among the 6 children with an enlarged AC, 3 had a completely hypoplastic CC. One had a CC that was hypoplastic but was missing the splenium. One child was missing the rostrum, but had a normal genu and hypoplasia of the body and splenium. The remaining child was missing the rostrum and splenium and had a normal genu and hypoplasia of the body.
TABLE 2.
The relation between the status of the splenium and status of the anterior commissure in children with SBM
| Splenium |
||||||
|---|---|---|---|---|---|---|
| Absent |
Hypoplastic |
Normal |
||||
| Anterior commissure | n | % | n | % | n | % |
| Enlarged | 2 | 3.3 | 4 | 3.6 | 0 | 0 |
| Normal | 30 | 49.2 | 71 | 63.4 | 14 | 70.0 |
| Hypoplastic | 26 | 42.6 | 35 | 31.3 | 6 | 30 |
| Absent or not visualized | 3 | 4.9 | 2 | 1.8 | 0 | 0 |
| Total | 61 | 100 | 112 | 100 | 20 | 100 |
Note. SBM = spina bifida meningomyelocele.
Probst’s bundles
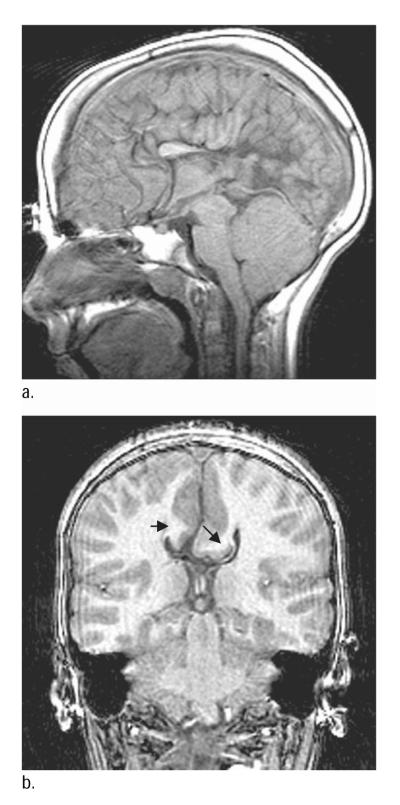
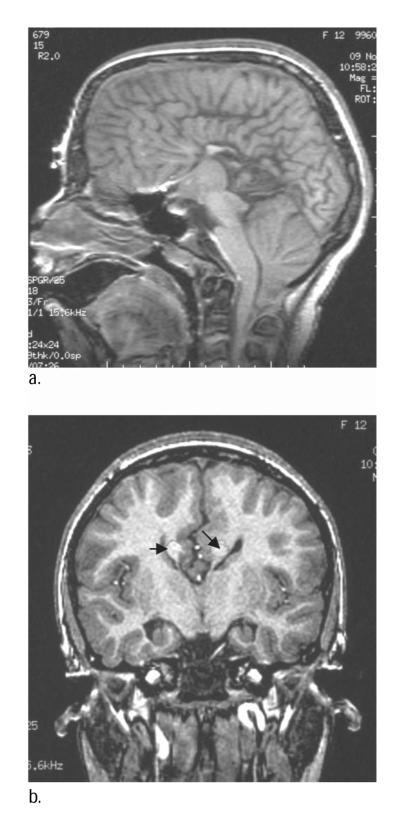
None of the NC children showed Probst’s bundles. No child with SBM had complete agenesis of the CC. Probst’s bundles were evident in 2 children with SBM (0.1%) who were missing three regions of the CC (Table 3). Child 1 had a normal genu, but was missing the rest of the CC, and the AC and HC were absent or too small to be visualized (Figure 1). Child 2 had hypoplasia of the body and was missing the other regions of the CC, although the AC and HC were normal (Figure 2). A total of 3 other children with SBM were missing all but one part of the CC (Table 3; Children 3–5); 1 had hypoplasia of the genu, and 2 had hypoplasia of the body. Their other cerebral commissures were either both hypoplastic (n = 2) or all normal enlarged (n = 1).
TABLE 3.
Status of several cerebral commissures and the occurrence of Probst’s bundles in children with SBM and almost complete agenesis of the corpus callosum
| Child 1 | Child 2 | Child 3 | Child 4 | Child 5 | ||
|---|---|---|---|---|---|---|
| Corpus callosum | Rostrum | absent | absent | absent | absent | absent |
| Genu | normal | absent | hypo | absent | absent | |
| Body | absent | hypo | absent | hypo | hypo | |
| Splenium | absent | absent | absent | absent | absent | |
| Probst’s bundles | yes | yes | no | no | no | |
| Other cerebral commissures | Anterior | absent | normal | hypo | normal | hypo |
| Hippocampal | absent | normal | hypo | enlarged | hypo |
Note. hypo = hypoplastic. SBM = spina bifida meningomyelocele.
Figure 1.
a. Midsagittal magnetic resonance imaging (MRI) scan of the brain of Child 1 with spina bifida meningomyelocele (SBM) and partial agenesis of the corpus callosum (only genu present, anterior and hippocampal commissures absent). b. Probst’s bundles indicated by black arrows on coronal MRI of brain.
Figure 2.
a. Midsagittal magnetic resonance imaging (MRI) scan of the brain of Child 2 with spina bifida meningomyelocele (SBM) and partial agenesis of the corpus callosum (only body present, anterior and hippocampal commissures present). b. Probst’s bundles indicated by black arrows on coronal MRI of brain.
Hippocampal commissure
The HC was normal in 69.5% (n = 32) of the NC children and absent/not visualized in the remainder 30.5% (n = 12). The HC was enlarged in 13% (n = 25) of the children with SBM, hypoplastic in 27.5% (n = 53), absent or too small to be visualized in 52.3% (n = 101), and normal in 0.02% (n = 4). The status of the HC was significantly related to the frequency of partial CC agenesis, χ2(3) = 26.45, p < .001, with SBM (Table 4). Partial agenesis was most frequent when the HC was enlarged (18/25; 72%), less frequent with an absent/not-visualized HC (n = 55; 54.4%) or a hypoplastic HC (26/53; 49.1%), and least frequent (3/14; 21.4%) with a normal HC. Probst’s bundles were not associated with enlargement of the HC.
TABLE 4.
The relation between status of the splenium and status of the hippocampal commissure in children with SBM
| Splenium |
||||||
|---|---|---|---|---|---|---|
| Hippocampal commissure |
Absent |
Hypoplastic |
Normal |
|||
| n | % | n | % | n | % | |
| Enlarged | 15 | 24.6 | 10 | 8.9 | 0 | 0 |
| Normal | 1 | 1.6 | 6 | 5.4 | 7 | 35.0 |
| Hypoplastic | 15 | 24.6 | 31 | 27.7 | 7 | 35.0 |
| Absent or not visualized | 30 | 49.2 | 65 | 58.0 | 6 | 30.0 |
| Total | 61 | 100 | 112 | 100 | 20 | 100 |
Note. SBM = spina bifida meningomyelocele.
Area measurement of the CC
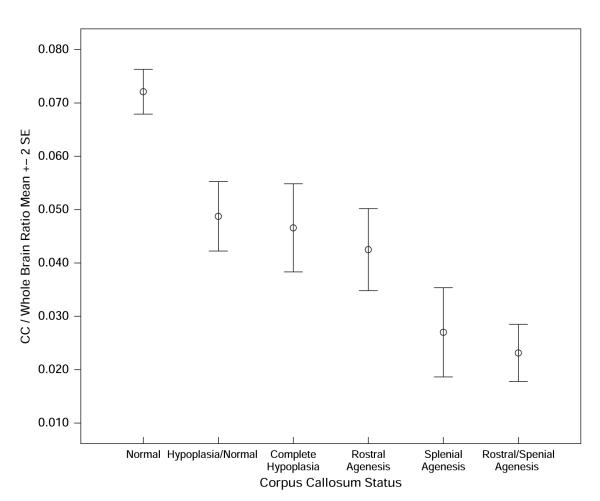
Based on the qualitative coding of the MRI, six subgroups were created based on the primary pattern of CC anomaly, with some participants in the agenesis subgroups also demonstrating hypoplasia: normal CC (n = 31), including 26 NC and 5 SBM; complete hypoplasia of the CC with no agenesis (n = 14), all SBM; hypoplastic/normal CC (n = 16), all SBM; agenesis of the rostrum, all SBM (n = 9); agenesis of the rostrum and splenium, all SBM (n = 14); and agenesis primarily of the splenium, all SBM (n = 10). In a one-way analysis of variance (ANOVA), the main effect of the CC/whole brain ratio was significant, F(5, 88) = 39.90, p < .0001 (Figure 3). Follow-up comparisons using Tukey’s LSD procedure to control the overall alpha level at p < .05 showed that children with a normal CC had significantly larger CC/whole brain ratios than did all other groups: normal CC, hypoplasia/normal, complete hypoplasia, agenesis of the rostrum, agenesis of the splenium, and agenesis of rostrum and splenium. The hypoplasia/normal, complete hypoplasia, and rostrum agenesis groups did not differ significantly, nor did the rostrum/splenium and splenium agenesis groups differ reliably from each other. However, the hypoplasia/normal, complete hypoplasia, and rostrum agenesis groups had significantly larger CC/whole brain areas than did the groups with rostral/splenial and splenial agenesis.
Figure 3.
Ratio of the area measurement of the corpus callosum (CC): whole brain in children with normal CC, hypoplasia/normal regions, complete hypoplasia, rostrum agenesis, splenium agenesis, and rostrum and splenium agenesis.
DISCUSSION
In children with SBM, complete agenesis of the CC is rarely present, in agreement with recent viewpoints that CC agenesis in conditions like SBM differs in important ways from classical callosal agenesis (Miller et al., 2008). Two processes are operative in the CC anomalies of children with SBM. The first is a disorder involving prolonged disruption of neuroembryogenesis that results in partial agenesis and hypoplasia of the CC, primarily in the rostrum and splenium (Barkovich, 2005). In some forms of callosal agenesis, termed partial segmental agenesis of the corpus callosum, a two-stage mechanism has been identified that, while a developmental defect, produces an absent body but present splenium (Raybaud & Girard, 2005). The second is a destructive process, involving secondary hydrocephalus that tends to produce hypoplasia, but not absence, of the genu and body, in agreement with earlier evidence from the SBM population (Fletcher et al., 2005).
The new information contributed by the present study is the identification of 14 CC patterns indicative of congenital partial CC agenesis in just over half of the children that involved the absence of the rostrum and/or the splenium. These children also showed the effects of secondary hydrocephalus. Most of the other children had 1 of 12 patterns of dysmorphology of the CC that might be related to the effects of secondary hydrocephalus involving hypoplasia of one or more regions of the CC and absence of the body in one case. Very few children had a completely normal CC. In addition, the subdivisions of the CC completed through qualitative coding of the MRI were supported by area measurements of the CC in a subset of this population, helping to validate the radiological qualitative coding. As hypothesized, Figure 3 shows that children identified with a normal CC have larger area measurements than all five groups with an abnormal CC. Children with SBM and rostrum/splenial or splenial agenesis, which often involves some degree of the posterior body, also had smaller area measurements than did children with hypoplasia/normal regions of the CC and complete hypoplasia. These are the regions of the CC that develop last (splenium and then rostrum) and sustain a disruption of neuroembryogenesis. It is, perhaps, not surprising that agenesis of the rostrum was not associated with as small a CC/whole brain ratio as the splenial and rostrum/splenial agenesis groups since the rostrum by itself is not a very large region of the CC. The children with hypoplasia/normal region CCs and completely hypoplastic CCs had similar area ratios probably because 50% of the former group had three regions of hypoplasia while an additional 31.3% had two regions of hypoplasia.
Given the high rate of partial agenesis of the CC in children with SBM, it is important to evaluate the likelihood of various proposed neuroanatomical compensatory mechanisms with this disorder. It has been proposed that enlargement of other commissural pathways, specifically the AC and HC, may provide compensatory pathways for interhemispheric transfer of some auditory and visual information (Martin, 1985; Ozaki & Walsten, 1993; Risse et al., 1978). The splenium was absent in one third of the children with SBM, so IHT abnormalities in this population, such as those observed by Hannay et al. (in press), are not unexpected.
On the basis of the current MRI findings, we can rule out enlargement of the AC as a major compensatory mechanism for partial agenesis of the CC involving the splenium, at least in SBM, because only a small percentage had an enlarged AC. Few of those were missing the splenium. Indeed, no particular pattern of CC partial agenesis was related to enlargement of the AC. Absence of the body was rare, so any compensatory mechanisms must involve hypoplasia.
Because it occurred in more children, enlargement of the HC cannot be ruled out as a potential structural compensatory mechanism for partial CC agenesis. It is possible that secondary hydrocephalus destroys not only central regions of the CC, but also the AC and HC. However, the rate of enlargement of the AC has always been rather small even with complete agenesis produced by other disorders (Loeser & Alvord, 1968; Meyer et al., 1998; Rauch & Jenkins, 1994).
Probst’s bundles were clearly evident in 2 individuals with partial CC agenesis. In Case 1, it seemed reasonable that Probst’s bundles might develop because the genu of the CC was the only region present, and the other commissures derived from the same plate (AC and HC) were missing (or not large enough to be visualized). No such rationale could be applied to the finding of Probst’s bundles for Case 2 because the AC and HC appeared to be normal. Case 2 is also difficult to explain because 3 other children who have what appear to be similar commissural development—namely, three regions of the CC absent along with a hypoplastic, normal, or enlarged AC and HC—did not develop Probst’s bundles. Probst’s bundles were reported by Rauch and Jinkins (1994) in 60% of their sample of 10 children with complete agenesis of the CC and in both of their children with partial agenesis of the CC. Tovar-Moll et al. (2007) also identified structures as Probst bundles in cases of complete and partial agenesis and, additionally, identified a commissural tract within Probst bundles connecting the left frontal and right occipital cortices. In contrast, using diffusion tensor imaging (DTI), Lee et al. (2004) reported Probst’s bundles with 2 cases of complete CC agenesis but no bundles with a case of partial agenesis of the CC in which fibers from the parieto-occipital lobe and the frontal lobe crossed in the remaining genu. It is difficult to explain these apparent inconsistencies. These studies had small samples in which agenesis was associated with a variety of etiologies. It would be interesting to reexamine our 5 cases of almost complete regional CC agenesis with DTI to determine which cortical regions are connected through their remaining CC and how this relates to the status of their AC and HC and the presence of Probst’s bundles. Our rate of HC enlargement was much higher than the rate of Probst’s bundles. This may occur because some potential CC axons are crossing the midline on the dorsal surface of the HC in the absence of distinct Probst’s bundles.
As forebrain neuroanatomical anomalies are investigated in more detail in children with SBM, other structures may emerge as candidates for functional compensatory mechanisms. Miller et al. (2008) reported structural abnormalities that might be potentially relevant to IHT function in individuals with meningomyelocele and Chiari II malformation. In half of their large sample, they identified an abnormal gray matter structure that they termed hypothalamic adhesion crossing the anterior-inferior portion of the third ventricle and unrelated to the AC. An abnormal bundle of white matter forming a callosal ridge was noted on the dorsal callosal surface in two thirds of the cases, which was believed to be an aberrant crossing cingular bundle that represented dysplasia and not the effects of hydrocephalus. The relation between this bundle and Probst’s bundles remains to be established. In the normally developing brain, the first axons crossing the commissural midline appear to arise from the cingulate gyri and are followed by neocortical commissural axons.
Future directions
In neurodevelopmental disorders, it cannot be assumed that a larger brain area is functionally compensatory. In some instances, it is maladaptive; for example, in autism, increased white matter volume predicts poorer motor function (Mostofsky, Burgess, & Larson, 2007). Typical development involves calibration of relative thickness of gray and white matter with age, so that thinner gray matter in the frontal polar cortex and other frontal regions is actually normal and adaptive (O’Donnell, Noseworthy, Levine, & Dennis, 2005). The brains of children and adults with SBM show a complex pattern of reorganization. Some of this involves missing brain, some involves present but dysmorphic brain, and some involves anomalous fiber tracts (Miller et al., 2008; Tovar-Moll et al., 2007). An important area for future investigation is identification of which brain changes in SBM promote, and which impair, functionality. Fibers in anomalous tracts such as Probst’s bundle may be functional electrically (Lefkowitz, Durand, Smith, & Silver, 1991) but that does not necessarily mean that the tracts promote behavioral, cognitive, and motor function.
The early studies on CC agenesis focused on comparison with adult CC section and emphasized the benefits of a congenital, rather than acquired, loss of the CC. Subsequent studies considered that whatever brain reorganization occurs in CC agenesis might have costs as well as benefits. For example, although CC agenesis permits the transfer of information from one hand to the other, it prevents the normal development of fine motor control within each hand, so that synkinesias (associated or overflow movements in one hand while the other hand is being moved) persist into the teen years when they would normally have disappeared by age 7 (Dennis, 1976). In children with SBM, CC partial agenesis and/or hypoplasia are associated with anomalous lateral asymmetries. For instance, these children differ from age peers in accuracy and variability of line bisection; they attend more to left or inferior hemispace and show more intrasubject variability (Dennis et al., 2005). Of interest, CC agenesis or hypoplasia both result in an exaggerated, abnormal attentional bias to left hemispace (pseudoneglect). Hypoplasia of the splenium is associated with a right-ear advantage for perception of consonant—vowel (CV) syllables while agenesis of the splenium is not (Hannay et al., in press). An important area for future studies is the delineation of both costs and benefits of congenital CC agenesis in relation to patterns of presence and absence of the other cerebral commissures. In particular, it would be useful to examine visual and auditory IHT in children with SBM without these particular dysmorphologies during functional imaging techniques such as functional magnetic resonance imaging (fMRI) and magnetic source imaging (MSI; Papanicolaou, 1998).
FINAL THOUGHTS ABOUT OUR MENTOR, COLLEAGUE, AND FRIEND
Benton had an implicit methodology in many of his studies of children that was unusual for its day, in that he considered that individual differences inform outcome, rather than obscure it. We believe he would have approved our method of analyzing intragroup outcome variability in relation to variability in normal and aberrant brain patterns of development. Benton found value in analyzing neurocognitive function in children with explicit brain damage but preserved intellect. Assuming that this mindset would have continued, he would have found compatible our strategy of looking at neurodevelopmental disorders in terms of specific neurocognitive deficits in children with overall preserved cognitive development. In continuing to choose tasks of basic functions for which performances of typically developing children and adults were available, he would have found himself in harmony with recent approaches to neuroimaging. fMRI studies, for example, demand well-specified tasks of basic functions, rather than measures such as IQ, that represent the final common path of gene, environment, and experiential and learning history.
We do not know how Arthur Benton would have approached the problem that we address in this paper, had his active research career been extended into the current era of sophisticated neuroimaging. On the basis of his earlier interests in pediatric issues, however, we can infer at least some of the directions he might have taken to address the issue explored in this paper.
We would like to believe that Benton would have read the present study with a critical but approving eye and then planned a functional neuroimaging experiment of some basic aspect of interhemispheric transfer that compared the subgroups of compensatory patterns of CC agenesis. That he is not our current collaborator in that endeavor is our loss.
Acknowledgments
This research was funded by National Institutes of Child Health and Human Development Grant PO1 HD35946, “Spina Bifida: Cognitive and Neurobehavioral Variability.” We would also like to acknowledge the work of Amy Hampson and Andrea Martin with data collection, and Irene Townsend and Sue Inwood with recruitment.
REFERENCES
- Ackerly SS, Benton AL. Report of a case of bilateral frontal lobe defect. Research Publications: Association for Research in Nervous and Mental Disease. 1947;27:479–504. [PubMed] [Google Scholar]
- American Psychological Association Distinguished Professional Contribution Award for 1978. American Psychologist. 1979 January;34:56–64. [PubMed] [Google Scholar]
- Barkovich AJ. Analyzing the corpus callosum. AJNR American Journal of Neuroradiology. 1996;17:1643–1645. [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ. Pediatric neuroimaging. 4th ed Lippincott, Williams, & Wilkin; Philadelphia: 2005. [Google Scholar]
- Barkovich AJ, Lyon G, Evard P. Formation, maturation, and disorders of white matter. AJNR American Journal of Neuroradiology. 1992;13:447–461. [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Norman D. Anomalies of the corpus callosum: Correlation with further anomalies of the brain. AJR American Journal of Roentgenology. 1988;151:171–179. doi: 10.2214/ajr.151.1.171. [DOI] [PubMed] [Google Scholar]
- Barr MS, Corballis MC. The role of the anterior commissure in callosal agenesis. Neuropsychology. 2002;16:459–471. [PubMed] [Google Scholar]
- Benton AL. Mental development of prematurely born children. American Journal of Orthopsychiatry. 1940;10:719–746. [Google Scholar]
- Benton AL. A study of the performances of young adults on the Kohs Block Designs Test. Journal of Applied Psychology. 1941;25:426–427. [Google Scholar]
- Benton AL. Development of finger-localization capacity in school children. Child Development. 1955a;26:225–230. [PubMed] [Google Scholar]
- Benton AL. Right-left discrimination and finger-localization in defective children. Archives of Neurology and Psychiatry. 1955b;74:383–389. doi: 10.1001/archneurpsyc.1955.02330180001001. [DOI] [PubMed] [Google Scholar]
- Benton AL. Le temps de réaction chez les maladies présentant des lesions cérébrales. Revue de Psychologie Appliquée. 1958;8:103–119. [Google Scholar]
- Benton AL. Behavioral indices of brain injury in school children. Child Development. 1962;33:199–208. [Google Scholar]
- Benton AL. Developmental aphasia and brain damage. Cortex. 1964;1:40–52. [Google Scholar]
- Benton AL, Cohen BD. Right-left discrimination and finger-localization in normal and brain-injured subjects. Proceedings of the Iowa Academy of Science. 1955;62:447–451. [Google Scholar]
- Benton AL, Collins NT. Visual Retention Test performance in children: Normative and clinical observations. Archives of Neurology and Psychiatry. 1949;62:610–617. doi: 10.1001/archneurpsyc.1949.02310170085006. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Brinkman C. Hemispheric interactions are different in left-handed individuals. Neuropsychology. 2006;20:700–707. doi: 10.1037/0894-4105.20.6.700. [DOI] [PubMed] [Google Scholar]
- Corballis MC, Finlay DC. Interhemispheric visual integration in three cases of familial callosal agenesis. Neuropsychology. 2000;14:60–70. [PubMed] [Google Scholar]
- Dennis M. Impaired sensory and motor differentiation with corpus callosum agenesis: A lack of callosal inhibition during ontogeny? Neuropsychologia. 1976;14:455–469. doi: 10.1016/0028-3932(76)90074-9. [DOI] [PubMed] [Google Scholar]
- Dennis M, Edelstein K, Frederick J, Copeland K, Francis D, Blaser SE, et al. Peripersonal spatial attention in children with spina bifida: Associations between horizontal and vertical line bisection and congenital malformations of the corpus callosum, midbrain, and posterior cortex. Neuropsychologia. 2005;43:2000–2010. doi: 10.1016/j.neuropsychologia.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: Developmental biology, epidemiology, and genetics. Neurotoxicology and Teratology. 2005;27:515–524. doi: 10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio G, Clarke S, Pizzolato G, Schaffner T. Cortical regions contributing to the anterior commissure in man. Experimental Brain Research. 1999;124:1–7. doi: 10.1007/s002210050593. [DOI] [PubMed] [Google Scholar]
- Fischer M, Ryan SB, Dobyns WB. Mechanisms of interhemispheric transfer and patterns of cognitive function in acallosal patients of normal intelligence. Archives of Neurology. 1992;49:271–277. doi: 10.1001/archneur.1992.00530270085023. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Bohan TP, Brandt ME, Kramer LA, Brookshire BL, Thorstad K, et al. Morphometric evaluation of the hydrocephalic brain: Relationships with cognitive abilities. Child’s Nervous System. 1996;12:192–199. doi: 10.1007/BF00301250. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Copeland K, Frederick J, Blaser SE, Kramer LA, Northrup H, et al. Spinal lesion level in spina bifida meningomyelocele: A source of neural and cognitive heterogeneity. Journal of Neurosurgery. 2005;102:268–279. doi: 10.3171/ped.2005.102.3.0268. [DOI] [PubMed] [Google Scholar]
- Funnell MG, Corballis PM, Gazzaniga MS. Cortical and subcortical interactions following partial and complete callosotomy. Archives of Neurology. 2000;57:185–189. doi: 10.1001/archneur.57.2.185. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Holtzman JD, Smylie CS. Speech without conscious awareness. Neurology. 1987;37:682–685. doi: 10.1212/wnl.37.4.682. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS, Kutas M, Van Patten C, Fendrich R. Human callosal function: MRI verified neuropsychological functions. Neurology. 1989;39:942–946. doi: 10.1212/wnl.39.7.942. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Kaplan E. A human cerebral disconnection syndrome. Neurology. 1962;12:675–685. doi: 10.1212/wnl.12.10.675. [DOI] [PubMed] [Google Scholar]
- Hannay HJ. Functioning of the corpus callosum in children with early hydrocephalus. Journal of the International Neuropsychological Society. 2000;6:351–361. doi: 10.1017/s1355617700633106. [DOI] [PubMed] [Google Scholar]
- Hannay HJ, Walker A, Dennis M, Kramer L, Blaser S, Fletcher JM. Auditory interhemispheric transfer in relation to patterns of partial dysgenesis and hypoplasia of the corpus callosum in spina bifida meningomyelocele. Journal of the International Neuropsychological Society. doi: 10.1017/S1355617708080958. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetts SW, Sherr EH, Gobuty S, Chao S, Barkovich AJ. Anomalies of the corpus callosum: MR analysis of the phenotypic spectrum. American Journal of Roentgenology. 2006;187:1343–1348. doi: 10.2214/AJR.05.0146. [DOI] [PubMed] [Google Scholar]
- Hewitt W. The development of the human corpus callosum. Journal of Anatomy. 1962;96:355–358. [PMC free article] [PubMed] [Google Scholar]
- Kimura D. Cerebral dominance and the perception of verbal stimuli. Canadian Journal of Psychology. 1961a;15:166–171. [Google Scholar]
- Kimura D. Some effects of temporal lobe-damage on auditory perception. Canadian Journal of Psychology. 1961b;15:156–165. doi: 10.1037/h0083218. [DOI] [PubMed] [Google Scholar]
- Lee S-K, Mori S, Kim DJ, Kim SY, Kim SY, Kim DI. Diffusion tensor MR imaging visualizes the altered hemispheric fiber connection in callosal dysgenesis. AJNR American Journal of Neuroradiology. 2004;25:25–28. [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz M, Durand D, Smith G, Silver J. Electrical properties of axons within Probst’s bundles of acallosal mice and callosi that have been reformed upon glial-coated polymer implants. Experimental Neurology. 1991;113:306–314. doi: 10.1016/0014-4886(91)90020-d. [DOI] [PubMed] [Google Scholar]
- Lehmann HJ, Lampe H. Observations on the interhemispheric transmission of information in 9 patients with corpus callosum defect. European Neurology. 1970;4:129–147. doi: 10.1159/000114016. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Schalomon PM, Roy M, Zacharias MC, Pimenta SJ, Lent SR, et al. Increased axon number in the anterior commissure of mice lacking a corpus callosum. Experimental Neurology. 1997;146:491–501. doi: 10.1006/exnr.1997.6564. [DOI] [PubMed] [Google Scholar]
- Loeser JD, Alvord EC. Clinicopathological correlations in agenesis of the corpus callosum. Neurology. 1968;18:745–756. doi: 10.1212/wnl.18.8.745. [DOI] [PubMed] [Google Scholar]
- Martin A. A qualitative limitation on visual transfer via the anterior commissure. Evidence from a case of callosal agenesis. Brain. 1985;108:43–63. doi: 10.1093/brain/108.1.43. [DOI] [PubMed] [Google Scholar]
- Meyer B-U, Roricht S, Niehaus L. Morphology of acallosal brains as assessed by MRI in six patients leading a normal daily life. Journal of Neurology. 1998;245:106–110. doi: 10.1007/s004150050187. [DOI] [PubMed] [Google Scholar]
- Miller E, Widjaja E, Blaser S, Dennis M, Raybaud C. The old and the new: Supratentorial MR findings in Chiari II malformation. Child’s Nervous System. 2008;24:563–575. doi: 10.1007/s00381-007-0528-x. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Burgess MP, Larson JCG. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130:2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- O’Donnell S, Noseworthy MD, Levine B, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. NeuroImage. 2005;24:948–954. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Ozaki HS, Iwahashi K, Shimada M. Ipsilateral corticocortical projections of fibers which course within Probst’s longitudinal bundle seen in the brains of mice with congenital absence of the corpus callosum: A study with the horseradish peroxidase technique. Brain Research. 1989;493:66–73. doi: 10.1016/0006-8993(89)91000-7. [DOI] [PubMed] [Google Scholar]
- Ozaki HS, Murakami TH, Toyoshima T, Shimada M. The fibers which leave Probst’s longitudinal bundle seen in the brain of an acallosal mouse: A study with the horse radish peroxidase technique. Brain Research. 1987;400:239–246. doi: 10.1016/0006-8993(87)90623-8. [DOI] [PubMed] [Google Scholar]
- Ozaki HS, Shimada M. The fibers which course within Probst’s longitudinal bundles seen in the brain of a congenitally acallosal mouse: A study with horseradish peroxidase technique. Brain Research. 1988;441:5–14. doi: 10.1016/0006-8993(88)91377-7. [DOI] [PubMed] [Google Scholar]
- Ozaki HS, Wahlsten D. Cortical axon trajectories and growth cone morphologies in fetuses of acallosal mouse strains. Journal of Comparative Neurology. 1993;33:595–604. doi: 10.1002/cne.903360411. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC. Fundamentals of functional brain imaging. Swets & Zeitlinger; Lisse, The Netherlands: 1998. [Google Scholar]
- Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, et al. Agenesis of the corpus callosum: Genetic, developmental and functional aspects of connectivity. Nature Reviews. Neuroscience. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- Pirola B, Bortotto L, Giglio S, Piovan E, Janes A, Guerrini R, et al. Agenesis of the corpus callosum with Probst’s bundles owing to haploinsufficiency for a gene in an 8 cM region of 6q25. Journal of Medical Genetics. 1998;35:1031–1033. doi: 10.1136/jmg.35.12.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann S, Maertens M, von Cramon DY, Lepsien J, Hugdahl K. Dichotic listening in patients with splenial and nonsplenial callosal lesions. Neuropsychology. 2002;16:56–64. doi: 10.1037//0894-4105.16.1.56. [DOI] [PubMed] [Google Scholar]
- Rakic P, Yakovlev PI. Development of the corpus callosum and cavum septi in man. Journal of Comparative Neurology. 1968;132:45–77. doi: 10.1002/cne.901320103. [DOI] [PubMed] [Google Scholar]
- Rauch RA, Jinkins JR. Magnetic resonance imaging of corpus callosum dysgenesis. In: Lassonde M, Jeeves MA, editors. Callosal agenesis: A natural split brain. Plenum; New York: 1994. [Google Scholar]
- Raybaud C, Girard N. Malformations of the telencephalic commissures. In: Tortori-Donati P, editor. Pediatric neuroradiology. Springer; Berlin: 2005. [Google Scholar]
- Ren T, Anderson A, Shen W-B, Huang H, Plachez C, Zhang J, et al. Imaging, anatomical, and molecular analysis of callosal formation in the developing human fetal brain. The Anatomical Record Part A. 2006;288A:191–204. doi: 10.1002/ar.a.20282. [DOI] [PubMed] [Google Scholar]
- Risse GL, LeDoux J, Springer SP, Wilson DH, Gazzaniga MS. The anterior commissure in man: Functional variation in a multisensory system. Neuropsychologia. 1978;16:23–31. doi: 10.1016/0028-3932(78)90039-8. [DOI] [PubMed] [Google Scholar]
- Rocha-Miranda CE, Bender DB, Gross CG, Mishkin M. Visual activation of neurons in inferotemporal cortex depends on striate cortex and forebrain commissures. Journal of Neurophysiology. 1975;38:475–491. doi: 10.1152/jn.1975.38.3.475. [DOI] [PubMed] [Google Scholar]
- Tovar-Moll F, Moll J, de Oliveira-Souza R, Bramati I, Andreiulo PA, Lent R. Neuroplasticity in human callosal dysgenesis: A diffusion tensor imaging study. Cerebral Cortex. 2007;17:531–541. doi: 10.1093/cercor/bhj178. [DOI] [PubMed] [Google Scholar]
- Volcik KA, Blanton SH, Tyerman GH, Jong ST, Rott EJ, Page TZ, et al. Methylenetetrahydrofolate reductase and spina bifida: Evaluation of level of defect and maternal genotypic risk in Hispanics. American Journal of Medical Genetics. 2001;95:21–27. [PubMed] [Google Scholar]
- Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995–2002. Pediatrics. 2005;116:580–586. doi: 10.1542/peds.2005-0592. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Goldsmith CH. The relationship of hand preference to anatomy of the corpus callosum in men. Brain Research. 1991;545:175–182. doi: 10.1016/0006-8993(91)91284-8. [DOI] [PubMed] [Google Scholar]