Abstract
Purpose
To investigate the temporal effects of single or fractionated radiotherapy on subcutaneous RIF-1 tumor pO2 and determine the therapeutic outcomes when the timing of fractionations is guided by tumor pO2.
Methods
The time course of the tumor pO2 changes was followed by multi-site electron paramagnetic resonance (EPR) oximetry. The tumors were treated with a single 10 Gy, 20 Gy, and 10 Gy × 2 doses and the tumor pO2 was measured repeatedly for six consecutive days. In the 10 Gy × 2 group, the second dose of 10 Gy was delivered at a time when the tumors were either relatively oxygenated or hypoxic. The changes in tumor volumes were followed for nine days to determine the therapeutic outcomes.
Results
A significant increase in tumor pO2 was observed at 24 hr post 10 Gy, while 20 Gy resulted in a significant increase in tumor pO2 at 72 to 120 hr post irradiation. The tumors irradiated with a second dose of 10 Gy at 24 hr, when the tumors were oxygenated, had a significant increase in tumor doubling times (DT), as compared to tumors treated at 48 hr when they were hypoxic (p<0.01).
Conclusion
Results indicate that the time of tumor oxygenation depends on the irradiation doses, and radiotherapeutic efficacy could be optimized if irradiations are scheduled at times of increased tumor oxygenation. In vivo multi-site EPR oximetry could be potentially used to monitor tumor pO2 repeatedly during fractionated schemes to optimize radiotherapeutic outcome. This technique could also be used to identify responsive and non-responsive tumors, which will facilitate the design of other therapeutic approaches for non-responsive tumors at an early time points during the course of therapy.
Keywords: EPR oximetry, pO2, Oxygenation, Radiotherapy, RIF-1
INTRODUCTION
The level of oxygen in tumors has a profound effect on radiotherapeutic efficacy, with a decrease in responsiveness of up to a factor of three when the tumor pO2 (partial pressure of oxygen) decreases from radiobiologically oxic levels (greater than 15 –20 mm Hg) to profound hypoxia (1). Tumor pO2 also can change during the course of fractionated radiotherapy. In experimental tumors, single small radiation doses often lead to increased oxygenation, whereas decreased oxygenation has been observed at higher doses (2–9)
Given the fact that changes in tumor oxygenation occur during fractionated radiotherapy, an appropriate choice of the intervals between fractions based on tumor oxygen status should enhance therapeutic outcomes. However, this requires an appropriate technique for repeated pO2 measurements to determine the time-course of tissue pO2 changes of the tumors undergoing therapy. This is vital because tumor oxygenation levels can be affected by several factors such as tumor growth, radiation, and changes in tumor vasculature, which will result in a very unpredictable variation in tumor pO2 over time.
Tumor re-oxygenation after single-dose or during fractionated radiotherapy has been investigated previously using assays for hypoxic fractions, but little is known about the time-course of the actual changes in tumor oxygen during treatment. Using the comet assay, Oliver et al. reported a decrease in the hypoxic fraction of murine SCC VII tumors from 10% in untreated animals to 6% at 6 hr after a single dose of 10 Gy (10). An increase in the hypoxic fraction (6% – 80%) of SCC VII tumors immediately after a single dose of 10 Gy was observed, and this decreased to approximately 2% at 6 hr post irradiation (11). Some very useful information has been obtained in human tumors by using oxygen electrodes (12, 13), but repeated measurements have not been possible so far due to lack of techniques that can provide repeated pO2 measurements. The oxygen electrodes involve a significant degree of invasiveness, have limited sensitivity, and most importantly, cannot be used for repeated measurements (14). Repeated measure of tumor pO2 during fractionated radiotherapy will provide vital pO2 information that could be exploited to optimize therapy by scheduling subsequent doses at times of optimal tumor pO2.
EPR oximetry is a relatively new technique that has been used extensively for pO2 measurements in animal models (2, 15, 16) and is now being developed for clinical use (17). One particular advantage of EPR oximetry is its ability to provide detailed pO2 information repeatedly from the same tumor, which makes it ideal for measurements over a course of radiation treatment (18, 19). The recent development of multi-site EPR oximetry has further expanded its in vivo application by allowing simultaneous pO2 measurements at multiple sites in a tissue of interest (15, 16). We have investigated the temporal effects of single or fractionated radiotherapy on tumor oxygenation of subcutaneous RIF-1 tumors using repeated multi-site in vivo EPR oximetry. This information could potentially be used to optimize therapeutic outcome and to also identify responding and/or non-responding tumors. We have tested this hypothesis by determining the radiotherapeutic outcome when the timing of fractionations is guided by tumor pO2. The prognostic capability of EPR oximetry is investigated by identifying responding and non-responding tumors based on their tumor pO2 and their radiotherapeutic outcome. The results indicate a significant increase in tumor pO2 post irradiation and a significant increase in doubling time when the tumors are irradiated at times of increased tumor pO2.
MATERIALS AND METHODS
Animals and Tumor Models
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Dartmouth Medical School. The radiation-induced fibrosarcoma tumor (RIF-1) cells were a gift from Dr. J. B. Mitchell’s laboratory at the National Cancer Institute. The cells were cultured in vitro in RPMI 1640 media supplemented with 10% FBS, glutamine, and antibiotics. The procedure for tumor inoculation has been described previously (15, 16). Briefly, the subcutaneous RIF-1 tumors with an average diameter of 6 – 8 mm were obtained by injecting 50 μl of 5 × 105 RIF-1 cells in the left posterior flank of female C3H mice (18–20g, Charles River Lab, Wilmington, MA).
Paramagnetic Probe
LiPc (lithium phathalocyanine) crystals were synthesized in our laboratory; the physico-chemical properties of LiPc crystals have been described previously (20). The LiPc crystals have a single sharp EPR line whose width is highly sensitive to pO2. The EPR spectra reflect the average partial pressure of oxygen on the surface of the crystals and allow measurements of pO2 in the tumor tissue using one or more crystals with a total diameter of ~ 200 μm. The procedure for LiPc injection has been described in earlier publications (15, 16). Briefly, the mice were anesthetized using 1.5% isoflurane with 30% FiO2 (fraction of inspired oxygen), and two aggregates of the LiPc crystals (30–50 μg each) were implanted at a depth of 2 mm and at a distance of 4 mm into each tumor using 25-gauge needles. One day after the LiPc implantation, multi-site EPR oximetry was used to measure tissue pO2 of the subcutaneous tumors (baseline pO2).
Electron Paramagnetic Resonance (EPR) Oximetry
The EPR oximetry was performed on an L-band (1.2 GHz) EPR spectrometer utilizing a microwave bridge and an external loop resonator specially designed for in vivo experiments (21). A set of coils capable of generating a magnetic field gradient in the Z- direction with a magnitude up to 3.0 G/cm was used to separate the EPR spectra of the two implants in each tumor (15, 16, 22). The spectrometer parameters were: incident microwave power, 2 mW; magnetic field center, 425 gauss; scan range, 2 gauss; modulation frequency, 24 kHz; modulation amplitude was one-third of the EPR line width with scan time of 10 seconds. We averaged 5 – 6 scans to enhance the signal to noise ratio. The EPR line widths were converted to pO2 using a calibration curve determined for the LiPc crystals used in this study (15, 16, 20).
Experiment Protocol
Mice were assigned randomly into 5 groups: (A) Control (sham-irradiated tumor on day 0, n = 9), (B) tumors irradiated with a single dose of 10 Gy (10 Gy on day 0, n = 16), (C) single dose of 20 Gy (20 Gy on day 0, n = 9). Based on the changes in tumor pO2 observed in these groups, we designed two more groups: (D) 10 Gy × 2 oxygenated (10 Gy on day 0 and 10 Gy at 24 hr post first dose, n = 24), and (E) 10 Gy × 2 hypoxic (10 Gy on day 0 and 10 Gy at 48 hr post first dose, n=10). n is the total number of LiPc implants in each group i.e. total number of pO2 measurements in each group at each time point.
For tumor pO2 measurements, the mice were anesthetized (isoflurane 1.5%, 30% FiO2) and positioned in the EPR magnet. The rectal temperature of the animal was maintained at 37 °C by a heated water pad and warm air blower. EPR oximetry measurements were carried out for 30 minutes in each mouse to determine the baseline tumor pO2. The animals were then moved to the irradiator bed [Varian Linear Accelerator (Clinac 2100C); Energy: 6 Mev; Applicator: 6 cm×6 cm], and the beam was focused on the tumor. Appropriate lead shields were used to prevent irradiation to the normal tissue of the mice. The tumor pO2 measurements post irradiations were repeated at 4 hr, 24 hr, 48 hr, 96 hr and 120 hr post irradiation. The animals of groups D and E received a second dose of 10 Gy after 24 hr and 48 hr respectively. The tumor volume was measured by a standard procedure (volume = π/6 × length × width2) for 9 consecutive days. Tumor doubling time (DT) is determined by using a statistical modeling approach described below. Multi-site EPR oximetry procedure requires a minimal distance of 4–5 mm between implants, so the initial diameter of the tumor was 6–10 mm in these studies. After 5 days of experiments, the tumor size reached the maximum tumor load limit per individual mouse as per the guidelines of IACUC at Dartmouth (should not be more than 10% of total body weight). This limited our experimental procedures for carrying out growth delay measurements therefore we used the modeling approach to determine DT.
Statistical Analysis
A paired t-test was used to determine statistical significance of changes in pO2 and tumor volume within the group and an unpaired t-test was used to determine the statistical significance between groups. The paired comparison reduces the animal to animal heterogeneity and eliminates differences of the baseline pO2. The tumor growth delay was modeled using an exponential mixed model (23) on the log scale, which was estimated by function lme in the statistical package S-Plus 6.1 (Insightful Inc., Seattle, WA). Assuming an exponential growth, DT was computed as DT = ln2/a, where “a” is the rate of tumor volume growth obtained from lme. The standard error for DT was estimated using the delta-method as described by Rice (24). The tests were two-sided, and a change with a p value < 0.05 was considered statistically significant. This approach has been used previously to estimate DT (25–27). All data are expressed as Mean ± SE and n is the total number of LiPc implants (i.e. pO2 measurements at each time point) in each group and N is the total number of animals in each group.
RESULTS
Measurements of the time course of tumor oxygenation before and after single radiation doses
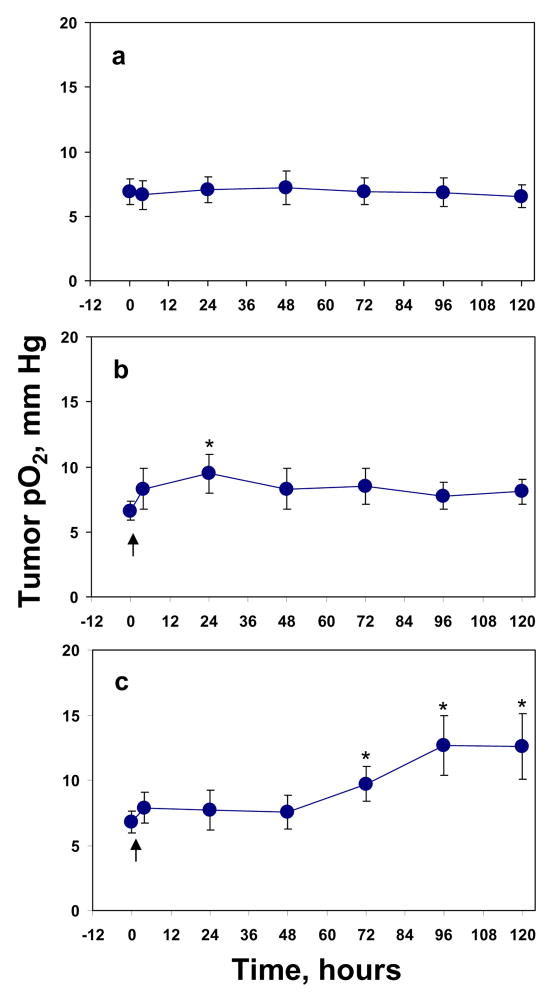
First we investigated the effect of single doses of 10 Gy and 20 Gy on RIF-1 tumor pO2 and tumor growth. Figure 1 summarizes the average tumor pO2 during six days of repeated measurements. There was no significant difference in the baseline pO2 between the two LiPc deposits of each tumor; therefore, the pO2 reported by the two LiPc deposits were pooled in each group. No significant difference was observed between the mean baseline tumor pO2 of the control (6.9 ±1.0 mm Hg) and the treatment groups; 6.6 ± 0.7 mm Hg in 10 Gy group, and 6.8 ± 0.8 mm Hg in 20 Gy group. A significant increase in tumor pO2 was observed at 24 hr post 10 Gy (p < 0.01) Figure 1b, while no such change in tumor pO2 was observed at this time point in tumors treated with 20 Gy (Figure 1c). However, a significant increase in tumor pO2 was observed at 72 to 120 hr post 20 Gy irradiation (p < 0.05).
Figure 1.
The change in average RIF-1 tumor pO2 before and after a single or fractionated radiation. (a) 0 Gy, n = 18, (b) 10 Gy, n = 24–32, (c) 20 Gy, n = 18. n is the total number of EPR probes in the tumors. Arrows indicate the time of irradiation. * p < 0.05, compared with baseline tumor pO2 in the same group.
Measurements of the time course of tumor pO2 before and after split dose radiation
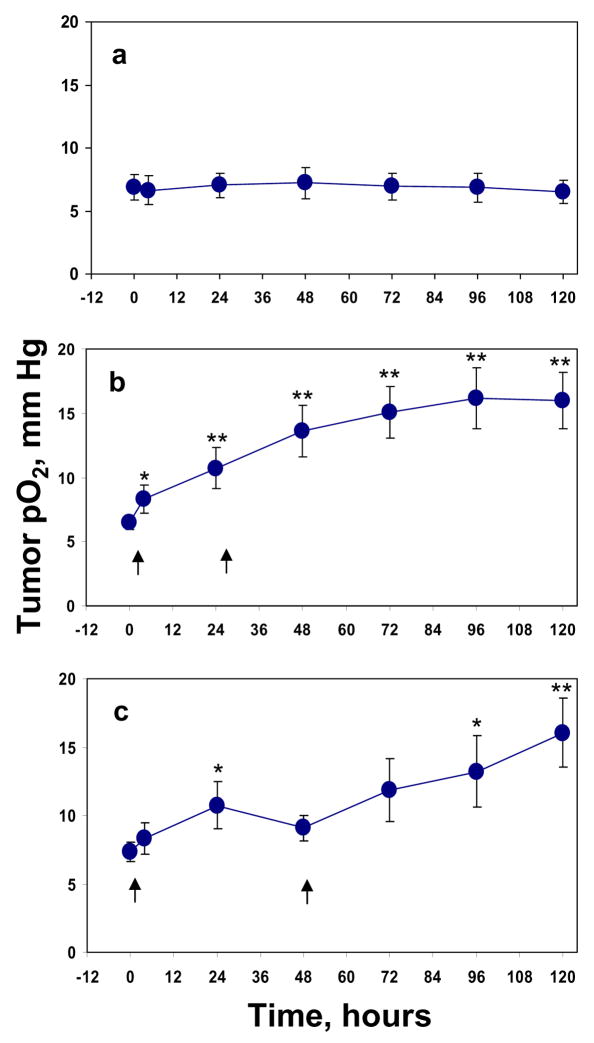
Based on the results described above, the tumors were irradiated with 10 Gy on day 0 and at 24 hr when a significant increase in tumor pO2 after first 10 Gy was observed (group D). Another group was irradiated with 10 Gy on day 0 and at 48 hr when the tumor pO2 returned to baseline (group E). A significant increase in tumor pO2 was observed at 4 hr (p < 0.05) and from 24 hr to 120 hr (p <0.01) time points in group D, Figure 2b. A significant increase in tumor pO2 at 24 hr (p < 0.05) and at the 96 hr and 120 hr time points (p < 0.05 and p < 0.01) was observed in group E, Figure 2c.
Figure 2.
The changes in average RIF-1 tumor pO2 before and after split dose radiation. (a) 0 Gy, n = 18, (b) 10 Gy +10 Gy at 0/24 hr, n = 42, (c) 10 Gy +10 Gy at 0/48 hr, n = 19. Arrows indicate the time of irradiation. * p< 0.05, ** p < 0.01, compared with baseline tumor pO2 in the same group (paired t-test).
Effect of single or fractionated doses on tumor growth
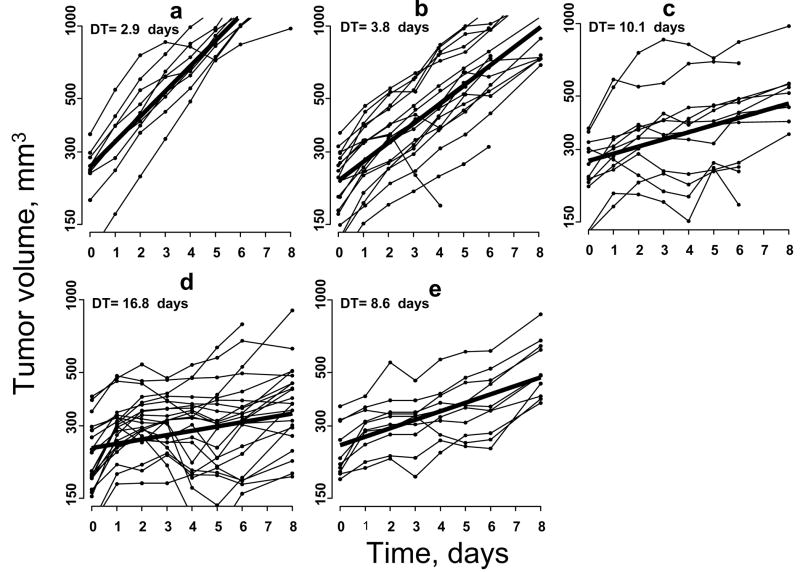
To test our hypothesis that tumor pO2 could be used as a prognostic marker to optimize fractionated radiotherapy by scheduling doses at times of increased tumor oxygenation, we investigated the growth inhibition of a control group (group A), tumors irradiated with a single dose of 10 Gy (group B), with 20 Gy (group C) or 10 Gy × 2 separated by a delay of 24 hr, at a time when oxygen is expected to be relatively higher (group D) or 10 Gy × 2 Gy separated by 48 hr, at a time when oxygen is expected to be relatively lower (group E). Table 1 shows the relative changes of RIF-1 tumor volume before and after irradiation of these groups. No significant differences in the initial tumor volume (prior to irradiation) of the control and treatment groups were observed. There was a significant decrease in the tumor volume from the baseline of the treatment groups from day 2 to day 8 (p < 0.01) as compared to the control group. There also were significant differences between group D and group E on day 8 (p < 0.05). The mean DT was 2.9 ± 0.1 days for the control group, 3.8 ± 0.1 and 10.1 ± 1.0 days for groups B and C, respectively, Figure 3. The DT further increased significantly in the group irradiated with 10 Gy at day 0 and 24 hr time points (group D) to 16.8 ± 1.8 days (p < 0.01 compared with group E). On the other hand, the DT of the tumors irradiated with 10 Gy on 0 and 48 hr time points was only 8.6 ± 0.6 days (group E).
Table 1.
Effect of radiation on RIF-1 tumor volume over days in control and irradiated groups
| G | N | Time, hours | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | 120 | 144 | 192 | |||
| Tumor volume, mm3 | A | 9 | 250±23 | 95±15 | 216±31 | 341±33 | 457±39 | 628±47 | 752±146 | 1290±245 |
| B | 16 | 214±18 | 75±8 | 134.±14A | 185±22A | 277±39A | 400±40A | 494±44A | 785±245A | |
| C | 9 | 239±25 | 53±23 | 75±22A | 85±31A | 98±40A | 135±36A | 149±41A | 245±38A | |
| D | 24 | 220±26 | 75±7 | 91±11A,b | 89±14Aa,b | 76±21Aa,b | 82±24Aa,b | 115±27A,ab | 144±31A,ab,E | |
| E | 10 | 245±21 | 52±9 | 62±16A | 73±10A | 87±17A | 108±23A | 125±24A | 276±36A | |
Abbreviations: G = groups. A, control group (sham-irradiated on day 0); B, single dose of 10 Gy radiation group (10 Gy on day 0); C, single dose of 20 Gy radiation group (20 Gy on day 0); D, 20 Gy oxygenated group (10Gy on day 0 and 10 Gy at 24 hr post first dose; E, 20 Gy hypoxic group (10Gy on day 0 and 10 Gy at 48 hr post first dose). N = numbers of animals. The values for time 0 are mean baseline absolute tumor volumes prior to the irradiation, while the values at other time points are relative mean changes in tumor volumes post irradiation. Differences between groups at the different time points were evaluated with unpaired t-test.
p < 0.05,
p < 0.01, compared with group A;
p < 0.01, compared with group B;
p < 0.05, compared with group E.
Figure 3.
The change in RIF-1 tumor volume in (a) 0 Gy, N = 9, (b) 10 Gy, N = 16, (c) 20 Gy, N = 9, (d) 10 Gy + 10 Gy at 0/24 hr, N = 24, (e) 10 Gy +10 Gy at 0/48 hr, N = 10. N is the number of animals with RIF-1 tumors in each group. Bold line indicates the mean tumor volume. Exponential mixed modeling is used to determine the DT (23, 24).
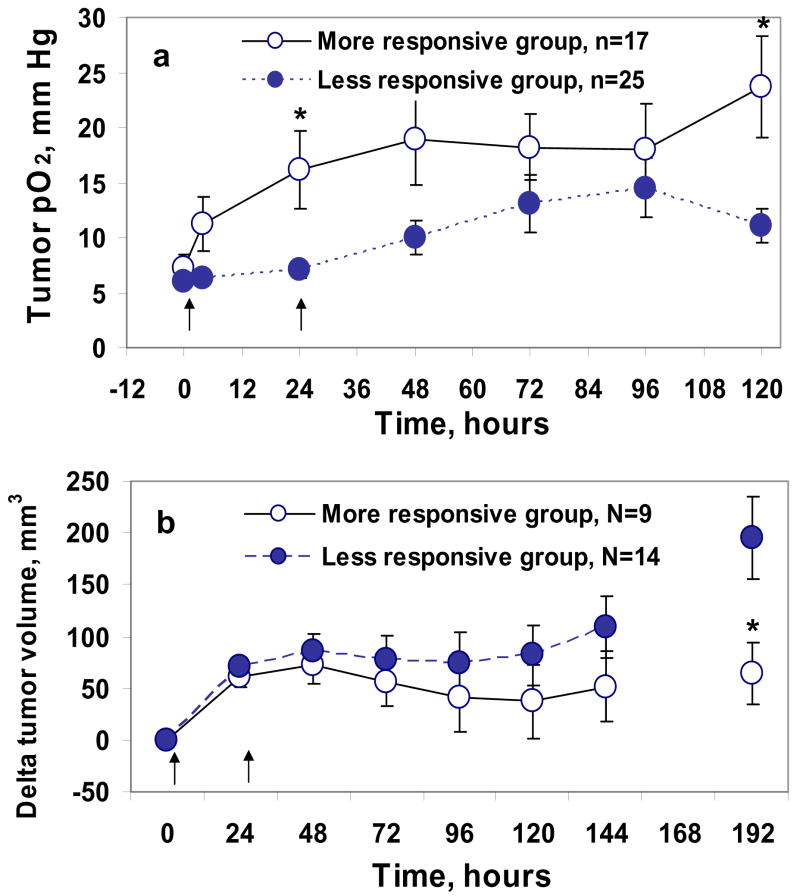
We further investigated if the tumor pO2 observed post first 10 Gy dose could be used as a prognostic indicator to predict therapeutic response during fractionated radiotherapy. The tumors of group D (10 Gy +10 Gy at day 0 and 24 hr) were divided into two sub-groups, based on their tumor pO2 at 24 hr; D1, tumors with increased pO2 <50 % of baseline pO2, (less responsive group) and D2 tumors with increased pO2 ≥ 50 % of baseline pO2 (more responsive group), and the changes in tumor volume in these two sub groups were compared. A significant difference in tumor pO2 on day 1 (p < 0.05) and in changes from the baseline tumor volume on day 8 (p < 0.05) was observed between these two groups, Figure 4. These results indicate that within a group of tumors that received the same treatment, the tumor response (i.e. therapeutic outcome) to the subsequent dose could be predicted by the response of the tumor pO2 to the first dose.
Figure 4.
Tumor pO2 (a) and tumor growth inhibition (b) of RIF-1 tumors before and after 10 Gy × 2 radiations. The interval between the doses is determined by the time course of changes in mean tumor pO2 as described in the results section. Treatment groups were: (•) less responsive group (tumor pO2 < 50 % of baseline), (○) more responsive group (tumor pO2 ≥ 50 % of baseline). * p < 0.05, compared with less responsive group (unpaired t-test). n is the number of LiPc implants in each group, N is the number of animals in each group.
Morphologic examination of the location of LiPc in the tumor
At the end of the experiment, gross and microscopic examination (H & E staining) of the tissue around the LiPc deposits confirmed that the deposits were in the interstitial compartment of the tumor tissue with no evidence of edema or infiltration of inflammatory cells (data not shown). Some accumulation of red blood cells and some necrotic cells around the LiPc deposits were observed in some samples, but these were not caused by the LiPc implants; rather, it reflects the normal histological pattern of the tumor. This is consistent with the previous report on the histological appearance of LiPc implants in RIF-1 tumors (28).
DISCUSSION
The data reported here are the continuation of our systematic studies to characterize the changes in tumor pO2 following irradiations. We have previously reported the tumor pO2 changes following the administration of a synthetic allosteric modifier of hemoglobin (efaproxiral), and during carbogen or hyperbaric oxygen therapy, with the goal to use these interventions to enhance tumor oxygenation to improve radiotherapeutic efficacy (2,15,16, 29). With the recent development of multi-site EPR oximetry, it is now possible to do simultaneous pO2 measurements at multiple sites in a tissue (15, 16). Given the heterogeneity in a tumor, the multi-site EPR oximetry approach is likely to provide a better assessment of average tumor pO2 and its dynamics during various therapies. These results provide detailed information on the changes in tumor pO2 during single and multiple irradiations by repeated pO2 measurements using EPR oximetry for five consecutive days. The knowledge of the changes in tumor pO2 during radiotherapy is crucial for the optimization of fractionated radiotherapy as demonstrated here.
The baseline tumor pO2 observed in these experiments is consistent with our previous reports (15, 16). A statistically significant increase in tumor pO2 at 24hr post 10 Gy was reproducible and consistently observed in all the irradiated groups, Figures 1 and 2. These results are in agreement with Znati et al. who reported an increase in tumor oxygenation when total doses of more than 10 Gy were used (3). Fuji et al. observed an increase in SCC VII murine tumors pO2 at 6 hr post 10, 15 or 20 Gy irradiation, and the pO2 continued to increase till 24 hr followed by a gradual decline (4). Using 2 fractions of 10 Gy each, O’Hara et al. assessed tumor pO2 from a single ink implant (20 μl volume) and observed an initial decrease followed by an increase (re-oxygenation) of tumor pO2 to the baseline level (pre-irradiation pO2) (2). No significant difference in DT was observed by O’Hara et al. in tumors with initial volume of 230 mm3. Sonveaux et al. reported that low doses of ionizing irradiation (6 Gy) induced a marked increase in mouse hepatocarcinoma (TLT) tumor blood flow and oxygenation (30). Results indicated a nitric oxide mediated effects of irradiation on the tumor vasculature and the irradiation of endothelial cells in a tumor is a key determinant of the effectiveness of radiotherapy. Crokart et al. used EPR and Oxilyte to investigate tumor oxygenation after a 2 Gy fraction in fibrosarcoma type II (FSaII) tumor models (31). An increase in pO2 as early as 3 – 4 hr post irradiation due to decrease in oxygen consumption and an increase in oxygen supply was observed. The increase in oxygen delivery was found to be due to a decrease in interstitial fluid pressure (IFP) and radiation-induced acute inflammation. Several possible mechanism have been suggested for re-oxygenation such as reduced oxygen consumption by radiation-damaged cells (32), cell loss leading to tumor shrinkage (33), migration of hypoxic cells to oxygenated state (34), and improved microcirculation (35). Clinical studies of advanced carcinoma of the cervix have shown a better local control by radiotherapy in patients with tumor pO2 greater than 10 mm Hg, as compared to patients with tumor pO2 less than 10 mm Hg (36). Patients receiving radiotherapy for soft-tissue sarcoma showed a 70% frequency of distant metastases in tumors with pO2 less than 10 mm Hg versus 35% in those with tumor pO2 greater than 10 mm Hg (37). These findings indicate that the magnitude of the observed increase of oxygenation in the tumors has significant implications for enhancing the effectiveness of fractionated radiotherapy. This also appears to affect the probability of distant metastases.
The observed increase in tumor pO2 on day 4, and day 5 after single 20 Gy or split 10 Gy × 2 is consistent with others reports (2, 5–7). Goda et al. observed a decrease in tumor pO2 initially (maximum decrease at 24 hr) followed by a gradual increase reaching a peak value at 72 hr after a single 20 Gy or split 10 Gy doses (8). An increase in tumor pO2 as late as day 3 in NFSa fibrosarcomas treated with 25.5 Gy x-ray irradiation was observed by Fukawa et al. (9). However, only a few reports describe tumor oxygenation status during fractionated radiotherapy and how this information could be used to optimize fractionated radiotherapy.
Our results indicate a significant difference in DT of 10 Gy, 10 Gy × 2 and 20 Gy groups as compared to controls (Figure 3, Table 1). A significant increase in tumor pO2 after 10 Gy and a significant increase in therapeutic efficacy was observed when the second dose of 10 Gy was delivered at time of increased tumor pO2 (24 hr) as compared to the tumors irradiated at 48 hr time point (hypoxic). Furthermore, in 10 Gy × 2 group (D), the tumors with an increase in pO2 of more then 50% from the baseline pO2 had a significant increase in DT as compared to the group with pO2 < 50 % of the baseline pO2. These results indicate that the changes in tumor pO2 could be used as a prognostic indicator of tumor response to subsequent doses during the course of radiotherapy. This type of repeated pO2 measurements might have a significant clinical application in identifying responsive and non-responsive tumors, and this vital information could than be used for an early intervention using other approaches for non-responsive tumors. We did not see any correlation between the changes in tumor pO2 and tumor volume over days in sham control group (data not shown). This is in agreement with observations in transplanted rat tumors (35), and our previous studies with the RIF-1 tumors in mice (15, 16).
In conclusion, our results indicate a significant increase in tumor doubling time when fractionated radiations were scheduled at times of increased tumor oxygenation. The changes in tumor pO2 post first dose during a fractionated regimen could also be used to identify less and more responsive tumors that received the same treatment. These results highlight the potential value of tumor pO2 assessment during fractionated radiotherapy and provide evidence that tumor pO2 could be potentially used to optimize and predict the therapeutic response. In vivo multi-site EPR oximetry was sufficiently sensitive to monitor the effects of single or fractionated radiation on tumor oxygenation. These results illustrate a unique and useful capability of in vivo multi-site EPR oximetry: obtaining repetitive and non-invasive measurements of tumor oxygenation from the same tumors during the course of therapy. This technique is currently being used to assess tissue pO2 of superficial tumors in patients undergoing radiotherapy and/or chemotherapy (29).
Acknowledgments
We thank Harriet St. Laurent and Kerry A. Tillson of Radiation Oncology, DHMC for assistance in the use of the radiation facility. This work was supported by NIH grants CA118069, CA120919, and P01EB2180.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall EJ. Radiobiology for the radiologist. 6. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 85–105. [Google Scholar]
- 2.O’Hara JA, Goda F, Demidenko E, Swartz HM. Effect on regrowth delay in a murine tumor of scheduling split-dose irradiation based on direct pO2 measurements by electron paramagnetic resonance oximetry. Radiat Res. 1998;150:549–556. [PubMed] [Google Scholar]
- 3.Znati CA, Rosenstein M, Boucher Y, Epperly MW, Bloomer WD, Jain RK. Effect of radiation on interstitial fluid pressure and oxygenation in a human tumor xenograft. Cancer Res. 1996;56:964–968. [PubMed] [Google Scholar]
- 4.Fujii H, Sakata KI, Katsumata Y, et al. Tissue oxygenation in a murine SCC VII tumor after X-ray irradiation as determined by EPR spectroscopy. Radiother Oncol. 2008;86:354–360. doi: 10.1016/j.radonc.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaupel P, Frinak S, O’Hara M. Direct measurement of reoxygenation in malignant mammary tumors after a single large dose of irradiation. Adv Exp Med Biol. 1984;180:773–782. doi: 10.1007/978-1-4684-4895-5_76. [DOI] [PubMed] [Google Scholar]
- 6.Koutcher JA, Alfieri AA, Devitt ML, et al. Quantitative changes in tumor metabolism, partial pressure of oxygen, and radiobiological oxygenation status postradiation. Cancer Res. 1992;52:4620–4627. [PubMed] [Google Scholar]
- 7.Ando K, Koike S, Ohira C, et al. Accelerated reoxygenation of a murine fibrosarcoma after carbon-ion radiation. Int J Radiat Biol. 1999;75:505–512. doi: 10.1080/095530099140438. [DOI] [PubMed] [Google Scholar]
- 8.Goda F, O’Hara JA, Rhodes ES, et al. Changes of oxygen tension in experimental tumors after a single dose of X-ray irradiation. Cancer Res. 1995;55:2249–2252. [PubMed] [Google Scholar]
- 9.Fukawa T, Takematsu K, Oka K, et al. Differences in pO2 peaks of a murine fibrosarcoma between carbon-ion and X-ray irradiation. J Radiat Res (Tokyo) 2004;45:303–308. doi: 10.1269/jrr.45.303. [DOI] [PubMed] [Google Scholar]
- 10.Olive PL, Vikse CM, Durand RE. Hypoxic fractions measured in murine tumors and normal tissues using the comet assay. Int J Radiat Oncol Biol Phys. 1994;29:487–491. doi: 10.1016/0360-3016(94)90443-x. [DOI] [PubMed] [Google Scholar]
- 11.Kim IH, Brown JM. Reoxygenation and rehypoxiation in the SCCVII mouse tumor. Int J Radiat Oncol Biol Phys. 1994;29:493–497. doi: 10.1016/0360-3016(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 12.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 13.Vaupel P, Schlenger K, Knoop C, Höckel M. Oxygenation of human tumors: evaluation of tissue distribution in breast cancer by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- 14.Raleigh JA, Dewhirst MW, Thrall DE. Measuring tumor hypoxia. Semin Radiat Oncol. 1996;6:37–45. doi: 10.1053/SRAO0060037. [DOI] [PubMed] [Google Scholar]
- 15.Hou H, Khan N, O’Hara JA, et al. Effect of the allosteric hemoglobin modifier RSR-13 on oxygenation in murine tumors: an in vivo EPR oximetry and BOLD MRI study. Int J Radiat Onc Biol Phys. 2004;59:834–843. doi: 10.1016/j.ijrobp.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 16.Hou H, Khan N, Grinberg OY, et al. The effects of Efaproxyn (efaproxiral) on subcutaneous RIF-1 tumor oxygenation and enhancement of radiotherapy-mediated inhibition of tumor growth in mice. Radiat Res. 2007;168:218–225. doi: 10.1667/RR0962.1. [DOI] [PubMed] [Google Scholar]
- 17.Swartz HM, Khan N, Buckey J, et al. Clinical Applications of EPR: Overview and Perspectives. NMR Biomed. 2004;17:335–351. doi: 10.1002/nbm.911. [DOI] [PubMed] [Google Scholar]
- 18.Bratasz A, Pandian RP, Deng Y, et al. In vivo imaging of changes in tumor oxygenation during growth and after treatment. Magn Reson Med. 2007;57:950–959. doi: 10.1002/mrm.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elas M, Bell R, Hleihel D, et al. Electron paramagnetic resonance oxygen image hypoxic fraction plus radiation dose strongly correlates with tumor cure in FSa fibrosarcomas. Int J Radiat Oncol Biol Phys. 2008;71:542–549. doi: 10.1016/j.ijrobp.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu KJ, Gast P, Moussavi M, et al. Lithium phthalocyanine: A probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci USA. 1993;90:5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swartz HM, Walczak T. Developing in vivo EPR oximetry for clinical use. Adv Exp Med Bio. 1998;454:243–252. doi: 10.1007/978-1-4615-4863-8_29. [DOI] [PubMed] [Google Scholar]
- 22.Smirnov AI, Norby SW, Clarkson RB. Simultaneous multi-site EPR spectroscopy in vivo. Magn Reson Med. 1993;30:213–220. doi: 10.1002/mrm.1910300210. [DOI] [PubMed] [Google Scholar]
- 23.Demidenko E. Mixed Models: Theory and Applications. Wiley; New York: 2004. [Google Scholar]
- 24.Rice J. Mathematical Statistics and Data Analysis. Duxbury Press; Belmont, CA: 1995. [Google Scholar]
- 25.Begg AC. Principles and practices of the tumor growth delay assay. In: Kallman RF, editor. Rodent Tumor Models in Experimental Cancer Therapy. McGraw-Hill; New York: 1987. pp. 114–121. [Google Scholar]
- 26.Tubiana M, Dutreix J, Wambersie A. Introduction to Radiology. London: Taylor & Francis; 1990. [Google Scholar]
- 27.Pogue BW, O’Hara JA, Demidenko E, et al. Photodynamic therapy with verteporfin in the radiation-induced fibrosarcoma-1 tumor causes enhanced radiation sensitivity. Cancer Res. 2003;63:1025–33. [PubMed] [Google Scholar]
- 28.Ilangovan G, Li H, Zweier JL, et al. In vivo measurement of regional oxygenation and imaging of redox status in RIF-1 murine tumor: Effect of carbogen-breathing. Magn Reson Med. 2002;48:723–730. doi: 10.1002/mrm.10254. [DOI] [PubMed] [Google Scholar]
- 29.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9:1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonveaux P, Dessy C, Brouet A, et al. Modulation of the tumor vasculature functionality by ionizing radiation accounts for tumor radiosensitization and promotes gene delivery. FASEB J. 2002;16:1979–81. doi: 10.1096/fj.02-0487fje. [DOI] [PubMed] [Google Scholar]
- 31.Crokart N, Jordan BF, Baudelet C, et al. Early reoxygenation in tumors after irradiation: determining factors and consequences for radiotherapy regimens using daily multiple fractions. Int J Radiat Oncol Biol Phys. 2005;63:901–10. doi: 10.1016/j.ijrobp.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 32.Zhao D, Constantinescu A, Chang CH, et al. Correlation of tumor oxygen dynamics with radiation response of the dunning prostate R3327-HI tumor. Radiat Res. 2003;159:621–631. doi: 10.1667/0033-7587(2003)159[0621:cotodw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Kallman RF. The phenomenon of reoxygenation and its implications for fractionated radiotherapy. Radiology. 1972;105:135–142. doi: 10.1148/105.1.135. [DOI] [PubMed] [Google Scholar]
- 34.Brown JM. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979;52:650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- 35.Yeh KA, Biade S, Lanciano RM, et al. Polarographic needle electrode measurements of oxygen in rat prostate carcinomas: accuracy and reproducibility. Int J Radiat Oncol Biol Phys. 1995;33:111–118. doi: 10.1016/0360-3016(95)00036-x. [DOI] [PubMed] [Google Scholar]
- 36.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 37.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–943. [PubMed] [Google Scholar]