Abstract
Background
Recent work demonstrated that application of peripheral nerve and cortical stimulation independently can induce modest improvements in motor performance in patients with stroke.
Objective
To test the hypothesis that combining peripheral nerve stimulation (PNS) to the paretic hand with anodal direct current stimulation (tDCS) to the ipsilesional primary motor cortex (M1) would facilitate beneficial effects of motor training more than each intervention alone or sham (tDCSSham and PNSSham).
Methods
Nine chronic stroke patients completed a blinded, cross-over designed study. In separate sessions, we investigated the effects of single applications of PNS+tDCS, PNS+tDCSSham, tDCS+PNSSham and PNSSham+tDCSSham prior to motor training on the ability to perform finger motor sequences with the paretic hand.
Results
PNS+tDCS resulted in a 41.3% improvement in the number of correct key presses relative to PNSSham+tDCSSham, 15.4% relative to PNS+tDCSSham and 22.7% relative to tDCS+PNSSham. These performance differences were maintained 1 and 6 days after the end of the training.
Conclusions
These results indicate that combining PNS with tDCS can facilitate the beneficial effects of training on motor performance beyond levels reached with each intervention alone, a finding of relevance for the neurorehabilitation of motor impairments after stroke.
Keywords: stroke, rehabilitation, transcranial direct current stimulation, nerve stimulation
Introduction
Despite recent advances1, 2 training-based customarily used neurorehabilitative treatments are insufficient to induce complete recovery of motor function in most stroke patients3. Thus, developing safe and more effective interventions to enhance training effects after stroke is a crucial need.
In recent years, different forms of non-invasive brain stimulation techniques have been explored. One of these interventions, transcranial direct current stimulation (tDCS), has generated excitement as a potential neurorehabilitative adjuvant strategy to facilitate performance of motor4-7 and language tasks 8, 9 in stroke patients. While the precise mechanisms mediating these effects are not known, it has been proposed that tDCS could influence Na+ and Ca++ channels and NMDA-receptor activity10, 11. When applied in isolation, the beneficial effects of tDCS on motor performance appear to be modest6. For instance, anodal tDCS applied over the ipsilesional primary motor cortex (M1) results in an approximately 10% improvement in performance of activities of daily living (ADL)-like tasks5, while cathodal tDCS applied over the contralesional M1 result in quantitatively similar improvements4, 7.
Peripheral nerve stimulation (PNS) has also been proposed as a possible adjuvant strategy capable of facilitating motor functions like pinch strength12, swallowing13, ADL-like tasks14 and training effects in stroke patients15-17. The mechanisms underlying the effects of PNS on motor cortical function are still under investigation, but may include modulation of corticomotor excitability18 that last beyond the period of stimulation18-20. Additionally PNS applied to one body part can modulate BOLD activity in its motor cortical representation in M1 and possibly in the dorsal premotor cortices21, 22. PNS applied in stroke patients over specific body parts (often the paretic hand) results in changes in motor cortical excitability that are somatotopically specific to the stimulated region. Mechanisms underlying PNS-induced motor effects may include modulation of GABAergic interneurons with little if any effects over NMDA receptor activity17, 18, 23.
The magnitude of facilitatory effects induced by a single session of PNS or tDCS on performance of motor tasks in stroke patients appear to be moderate and quantitatively comparable4, 5, 7, 15, 17. Here, we tested the hypothesis that combination of both tDCS and PNS would enhance the beneficial effects of motor training beyond levels reached by application of either intervention alone in patients with chronic stroke.
Material and methods
Patients
Nine single unilateral ischemic stroke patients (age range 40 - 73 year; 4 female) participated in the study (Table 1). All participants had severe motor deficits at stroke onset, as reflected by muscle strength score of 2 or less, and subsequent good recovery to the point of being able to perform the finger sequence task. The experimental protocol was approved by the Institutional Review Board of the National Institute of Neurological Disorders and Stroke and written informed consent was obtained from all patients. We excluded patients who had professionally practiced playing a keyboard musical instrument or trained as typists, patients with cerebellar or brainstem lesions, and those with severe depression, language disturbances, or serious cognitive deficits (MMSE <23/30 points).
Table 1. Patient Data Table.
| Age (years) | Sex | Time after stroke (months) | Lesion site | Handedness (EDS) | MMSE | FMS (%) | MAS | |
|---|---|---|---|---|---|---|---|---|
| Patient1 | 65 | F | 43 | R Cortical and subcortical (ant. temporal, insula and corona radiata) | Right (50/50) | 29/30 | 97 | 0 |
| Patient2 | 41 | M | 31 | L Cortical (post. Frontal and sup. temporal) | Right (50/50) | 29/30 | 87 | 1+ |
| Patient3 | 61 | F | 65 | L Cortical (fronto-parietal and insula) | Right (47/50) | 28/30 | 89 | 2 |
| Patient4 | 62 | M | 52 | R Cortical (fronto-parieto-temporal) | Left (10/50) | 25/30 | 91 | 0 |
| Patient5 | 73 | M | 41 | L Cortical and subcortical (fronto-parieto-occipital and basal ganglia) | Right (44/50) | 28/30 | 94 | 0 |
| Patient6 | 53 | M | 68 | R Cortical and subcortical (parietal operculum, post-insula and corona radiata) | Right (47/50) | 30/30 | 94 | 1+ |
| Patient7 | 46 | F | 55 | L Cortical (parieto-occipital junction) | Right (50/50) | 29/30 | 98 | 0 |
| Patient8 | 40 | F | 59 | L Cortical (deep frontal) | Right (43/50) | 27/30 | 98 | 0 |
| Patient9 | 57 | M | 87 | R Subcortical (globus pallidus, corona radiata, putamen) | Right (46/50) | 30/30 | 99 | 0 |
| Mean±SE | 55.3±3.76 | 55.7±5.57 | 28.3±0.53 | 94±1.4 |
F=female; M=male; R=right; L=left; MAS= Modified Ashworth Scale; EDS= Edinburgh Handedness Scale; FMS= Fugl-Meyer Scale, percent scores for the paretic upper extremity are given; MMSE= Mini-Mental State Examination
Experimental design
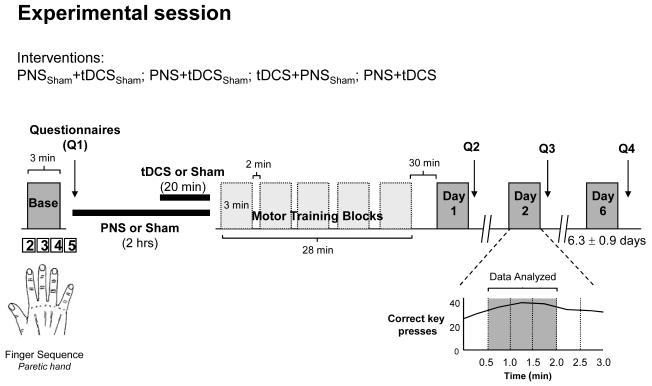
All subjects participated in 5 sessions including one short familiarization and four experimental sessions. The first session was always the familiarization day, in which all patients practiced for 3 minutes a 4-finger key press sequence on a keyboard and got acquainted with the laboratory equipment. Subsequently, they participated in four experimental sessions separated by 6.3±0.9 days (mean±SD). Different forms of stimulation and sham were applied in different sessions. The sessions’ order was randomized across subjects using a computer-generated randomization list.
Stimulation types
Peripheral nerve stimulation of the median and ulnar nerve of the paretic hand (PNS). PNS was applied simultaneously over the median and ulnar nerve at the wrist using 2 electrode bars with the cathode in a proximal position following a setup described in prior studies17-19. In short, trains of electrical stimulation were delivered at 1 Hz for a period of 2 hours (Grass stimulator S 8800, Grass Instrument Division, Astro-Med Inc., West Warwick, RI, USA). Each train consisted of five single pulses of 1ms duration delivered at 10 Hz (inter-pulse interval 100msec, inter-burst interval 500msecs). The stimulus intensity was adjusted to elicit small compound muscle action potentials (CMAPs) of 50-100 μV from the abductor pollicis brevis (APB) and first dorsal interosseus (FDI) in the absence of visible muscle twitches. During the stimulation period, patients remained relaxed and were allowed to read or listen to quiet music. Electromyography activity was monitored throughout the 2-hour stimulation period to ensure relaxation. All subjects perceived mild paraesthesias under the PNS stimulation electrodes associated to the stimulation.
Transcranial direct current stimulation (tDCS) was applied with the anode positioned over the ipsilesional M1 and the cathode over the contralateral supraorbital region for 20 min (1mA), as done in previous studies5, 7, 24. Anodal tDCS (Iomed Phoresor® PM850, Salt Lake City, UT) was delivered through a 3“×3” sponge electrode (Amrex® part2-A103, Carson, CA) placed on the patient’s scalp corresponding to the optimal spot for activation of the paretic APB muscle as determined with magnetic stimulation. In six patients, we confirmed that the anode position overlaid the ipsilesional M1 using a frameless neuronavigation system (Brainsight®, Rogue Research Inc., Montreal, Canada).
PNSSham consisted of PNS delivered to the deep peroneal and posterior tibial nerves of the paretic leg for 120 minutes with the same parameters as previously described for PNS of the median and ulnar nerves stimulation.
tDCSSham consisted of anodal tDCS over the ipsilesional M1 applied for only 1min after which the current was slowly tapered down to 0 for the remainder 19 minutes. This procedure, implemented out of the field of view of the patients, has been shown to blind effectively cutaneous sensations elicited by a longer anodal tDCS stimulation period in both stroke patients and healthy volunteers5, 25. Application of anodal tDCS or tDCSSham (20 min) started 100 min after the onset of PNS or PNSSham (20 minutes prior to completion of the peripheral nerve stimulation). In this manner, both forms of stimulation or sham (PNS and tDCS) were completed at the same time.
The order of the four sessions was randomized across patients, and both patients and investigators carrying out testing of behavioral measurements were blind to the particular types of intervention combination: PNS+tDCS, PNS+tDCSSham, tDCS+PNSSham and PNSSham+tDCSSham.
Motor training was carried out immediately after the end of each stimulation type because: (1) both forms of stimulation (tDCS and PNS) induce changes in motor cortical excitability that outlasts the period of stimulation18 26, (2) simultaneous performance of practice with stimulation could have influenced practice quality, particularly during PNS, and (3) by practicing after stimulation we eliminated potentially distractive effects of each stimulation type as a factor in the interpretation of the results, a strategy used in previous PNS studies15-17, 27.
Motor practice
Participants practiced four different finger sequences that are comparable in difficulty and have minimal carry over effects between them28 (Fig. 1). The practiced sequences were different in each session and were chosen in a counterbalanced order. Subjects were instructed to press each key on a special keyboard containing only 5 keys using the 2nd, 3rd, 4th or 5th digit of the paretic hand. The following four finger sequences were used in random order across subjects for the four testing sessions: 2-5-3-4-2, 4-3-5-2-4, 3-2-4-5-3, 5-2-4-3-5. Subjects were instructed to repeat the five-elements sequence “as quickly and as accurately as possible” for a period of 3 min, which constituted one block. A computer was used to display the sequences to the patient and to record the time and accuracy of each key press (Superlab; Cedrus, San Pedro, CA). In each session, participants read the sequence corresponding to that day 5 times and memorized it. Subsequently, they practiced 5 blocks of 3 minutes each, separated by 2 minutes rest periods for a total of 28 minutes (Fig 1).
Fig. 1.
Experimental design. Patients participated in 4 sessions (order randomized across subjects): PNSSham+tDCSSham, PNS+tDCSSham, tDCS+PNSSham and PNS+tDCS (see text for details). Each session started with 3mins baseline measurement (Base) of performance of a finger sequence task followed by each form of stimulation (2hrs of PNS or Sham, combined with 20min of tDCS or Sham). Each form of stimulation preceded 5 identical blocks of 3mins motor sequence practice performed with 2mins break between blocks. Training was followed by 30mins break after which post training measurements were obtained on Day 1, Day 2 and Day 6 (6.3±0.5 days). Questionnaires (Q) where patients reported the level of attention, fatigue, hand tiredness and perceived difficulty to each sequence were obtained using separate visual analogue scales at four different time points in each session. Inset shows the number of correct key presses for one subject during each 30secs epoch. The mean number of correct key presses per 30secs during the 2nd, 3rd and 4th 30secs epochs (dark grey area) was used to calculate the primary outcome measure (see text).
Testing of motor performance
Motor performance was tested at baseline and in three different opportunities (days 1, 2 and 6) after each form of stimulation + motor training (see Fig 1). In each of these tests, patients performed 1 block of 3 minutes, similar to those implemented in the practice period. For analysis purposes, each 3minute block was divided in six 30sec epochs. We defined the primary outcome measure as the mean number of correct key presses per 30sec relative to baseline. We excluded the initial 30sec epoch during which patients often warmed up after each resting interval and the last 60sec epoch because some patients showed slowing and reported fatigue at that stage. Therefore, the mean number of correct key presses at baseline and post training (day 1, 2 and 6) were calculated on the bases of the 2nd, 3rd and 4th 30secs epochs (Fig 1).
Experimental Sessions
Each experimental session started with baseline determination of motor performance followed by the type of stimulation corresponding to that day, and motor practice. Post-training performance assessments were then done 30 minutes after the end of training (1hr after the completion of the stimulation period; Day 1), at 24hrs (Day 2) and 6.3±0.5 days later (Day 6; Fig. 1).
In each session, participants completed questionnaires about the duration and quality of the previous night sleep (range 0-10; 1=very poor, 10=very good). In addition, we recorded four times in each session the subject’s perceived level of attention (range 0-10; 1=no attention, 10=highest level of attention), fatigue and hand tiredness (range 0-10; 0=highest level of fatigue or tiredness, 10=no fatigue or tiredness), and sense of difficulty in carrying out the training task (range 0-10; 0=very simple, 10=very difficult; see Q1-4 in Fig. 1)17.
Data analysis
Normal distribution of all data was assessed by Kolmogorov-Smirnov tests. The primary outcome measure, the mean number of correct key presses per 30sec at Day 1, 2 and 6 relative to baseline was analyzed using a polynomial repeated measure ANOVA (ANOVARM) with independent factor INTERVENTIONS (PNS+tDCS, PNS+tDCSSham, tDCS+PNSSham and PNSSham+ tDCSSham) and dependent factor TIME (Day 1, Day 2 and Day 6). Additionally, a similar ANOVARM was implemented to evaluate intervention-dependent changes in the total number of key presses using the same TIME and INTERVENTIONS factors. To determine changes in mean number of correct key presses per 30sec during the 5 practice blocks we performed ANOVARM with factors PRACTICE (Training Blocks 1, 2, 3, 4 and 5) and INTERVENTIONS.
To compare the effects of INTERVENTIONS and TIME on attention, fatigue, hand tiredness, perceived difficulty, quality of sleep and the amount of sleep we used separate ANOVARM with INTERVENTIONS as the within-subject factor and TIME (Baseline, Day 1, Day 2 and Day 6) as the repeated measure. Conditioned on significant p-values (p < 0.05), post hoc analyses were conducted and corrected for multiple comparisons with LSD tests. All data are expressed as mean ± SEM.
Results
All participants completed the study and did not experience complications
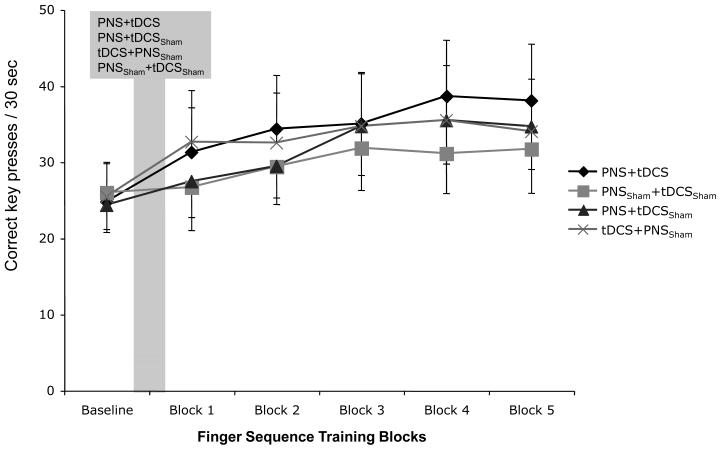
At baseline, the mean number of correct key presses per 30secs was comparable in the 4 sessions (ANOVARM F[3, 24]=1.32, p= 0.9). During the motor practice period, ANOVARM revealed a significant effect of PRACTICE, but not INTERVENTIONS or INTERVENTIONS by PRACTICE interaction on the number of correct key presses (ANOVARM F[4, 32]=8.1, p< 0.001; F[3, 24]=1.1, p= 0.4; and F[12, 96]=0.5, p= 0.8 respectively), indicating that all interventions resulted in comparable performance improvement (Fig. 2).
Fig. 2.
The graph shows the mean number of correct key presses per 30sec at baseline and during finger sequence practice after each stimulation condition (shaded area, mean/SEM). Note the comparable baseline values and the overlapping training-dependent improvements across interventions.
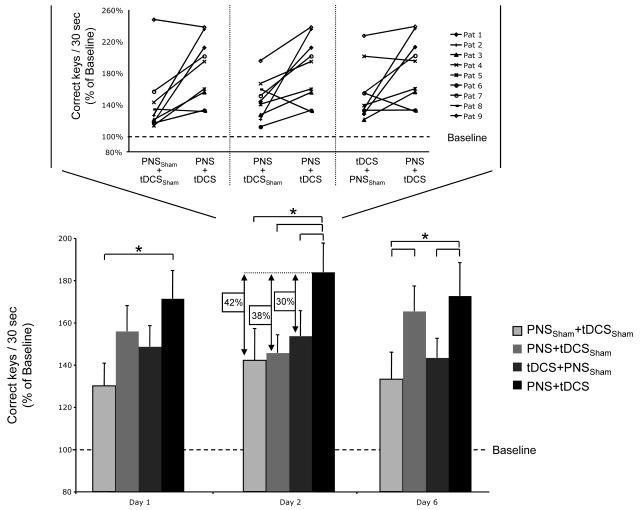
After practice was completed, ANOVARM showed a significant effect of INTERVENTIONS and more importantly INTERVENTIONS by TIME interaction, but not TIME on the percent change of the mean number of correct key presses per 30sec at Day 1, 2 and 6 relative to baseline (ANOVARM INTERVENTIONS F[3, 24]=3.9, p< 0.05; TIME F[2, 16]=0.43, p=0.6; INTERVENTIONS by TIME interaction F[6, 48]=2.6, p< 0.05, Fig. 3). Post hoc testing revealed that, relative to baseline, PNS+tDCS facilitated practice effects to a larger extent than PNSSham+tDCSSham at Day 1 (p<0.05). At Day 2, PNS+tDCS facilitated practice effects more than PNSSham+tDCSSham (p<0.01), PNS+tDCSSham (p<0.01), and tDCS+PNSSham (p<0.05). This Day 2 difference was evidenced by larger PNS+tDCS effects in 7 out of 9 subjects relative to PNSSham+tDCSSham, in 8 out of 9 relative to PNS+tDCSSham and in 6 out of 9 relative to tDCS+PNSSham (Fig 3 inset). At Day 6, PNS+tDCS facilitated practice effects more than PNSSham+tDCSSham (p<0.01) and than tDCS+PNSSham (p<0.05; Fig. 3, Table “online materials”).
Fig. 3.
The bar graph shows the effects of the different interventions on the % change in correct key presses per 30secs relative to baseline (dotted line, 100%). Note that on Day 1, PNS+tDCS was significantly better than PNSSham+tDCSSham. This effect was more pronounced at Day 2 when PNS+tDCS elicited significantly more gains than PNSSham+tDCSSham, PNS+tDCSSham or tDCS+PNSSham (black arrows) and partially present by Day 6. The inset graph shows individual patients’ change in correct key presses relative to baseline between PNSSham+tDCSSham, PNS+tDCSSham, tDCS+PNSSham and PNS+tDCS at Day 2. Values represent mean±SEM; *p < 0.05, **p < 0.01.
Online Materials Only. Percent differences across interventions and sessions on relative improvement of correct key presses.
| Day 1 | Day 2 | Day 3 | |
|---|---|---|---|
| PNS+tDCS vs. PNSSham+tDCSSham | 41.3 | 41.8 | 39.5 |
| PNS+tDCS vs. PNS+tDCSSham | 15.4 | 38.2 | 7.3 |
| PNS+tDCS vs. tDCS+PNSSham | 22.7 | 30.2 | 29.4 |
Values represent the percentage difference of correct key presses between interventions for the different days. Note that combined PNS+tDCS resulted in clear gains relative to all other interventions at all time point measurements.
On the other hand, ANOVARM showed a significant effect of TIME, but not INTERVENTIONS, or TIME by INTERVENTIONS interactions on the total number of key presses (ANOVARM TIME F[3, 24]=13.92, p<0.001; INTERVENTIONS F[3, 8]=0.57, p=0.63; and TIME by INTERVENTIONS interactions F[9, 72]=1.09, p=0.14), indicating that the interventions did not influence the total number of key presses as they did on the percent of correct key presses relative to baseline.
ANOVARM for attention and fatigue did not show effects of INTERVENTIONS (F[3,21]= 1.09, p=ns, and F[3,21]= 1.42, p=ns; respectively), TIME (F[3,21]= 2.45, p=ns, and F[3,21]= 1.75, p=ns; respectively), or INTERVENTIONS by TIME interaction (F[9,63]= 1.61, p=ns, and F[9,63]= 0.93, p=ns; respectively). In contrast, ANOVARM for hand-tiredness showed significant effects of TIME (F[3,21]= 4.11, p < 0.05), but not INTERVENTIONS (F[3,21]= 0.96, p=ns) or INTERVENTIONS by TIME interaction (F[9,63]= 0.92, p=ns), reflecting a comparable increment in hand tiredness over time across conditions (Table 2). Similarly, ANOVARM revealed a significant effect of TIME (F[3,21]= 10.35, p< 0.01), but not INTERVENTIONS (F[3,21]= 0.81, p=ns) or INTERVENTIONS by TIME interaction (F[9,63]= 0.62, p=ns) on the patients’ sense of sequence difficulty, reflecting a comparable decrease of sequence performance difficulty over time but not across interventions (Table 2). Finally, duration and quality of sleep were comparable across INTERVENTIONS (F[3,21]= 0.22, p=ns, and F[3,21]= 1.62, p=ns; respectively) and TIME (F[2,14]=0.75, p=ns, and F[2,14]= 2.79, p=ns; respectively) with no INTERVENTIONS by TIME interaction (F[6,42]= 1.94, p=ns, and F[6,42]= 1.13, p=ns; respectively, Table 3).
Table 2. Questionnaire Data.
| Q1 | Q2 | Q3 | Q4 | Statistics ANOVARM | ||||
|---|---|---|---|---|---|---|---|---|
| Intervention effect | Time effect | Intervention X Time | ||||||
| Attention (0 - 10) | PNSSham+tDCSSham | 9.1±0.6 | 8.5±0.9 | 9.4±0.4 | 9.0±0.6 | ns | ns | ns |
| PNS+tDCSSham | 8.6±0.5 | 9.0±0.6 | 9.3±0.4 | 9.1±0.5 | ||||
| tDCS+PNSSham | 8.9±0.6 | 8.5±0.8 | 9.3±0.5 | 8.5±0.8 | ||||
| PNS+tDCS | 8.5±0.8 | 8.4±1.1 | 9.0±0.6 | 9.3±0.5 | ||||
| Fatigue (0-10) | PNSSham+tDCSSham | 9.0±0.7 | 8.3±0.9 | 9.0±0.6 | 8.8±0.8 | ns | ns | ns |
| PNS+tDCSSham | 8.6±0.5 | 9.0±0.6 | 9.1±0.5 | 8.9±0.6 | ||||
| tDCS+PNSSham | 8.6±0.7 | 8.4±0.7 | 8.9±0.7 | 8.4±0.7 | ||||
| PNS+tDCS | 8.4±0.8 | 8.4±1.1 | 8.8±0.7 | 8.9±0.6 | ||||
| Hand Tiredness (0-10) | PNSSham+tDCSSham | 8.3±1.1 | 7.6±1.2 | 9.0±0.9 | 8.9±0.9 | ns | <0.05 | ns |
| PNS+tDCSSham | 8.5±0.9 | 8.4±1.0 | 8.6±1.0 | 8.8±0.7 | ||||
| tDCS+PNSSham | 8.6±0.8 | 7.4±0.9 | 8.1±0.9 | 8.6±1.0 | ||||
| PNS+tDCS | 8.2±1.1 | 8.0±1.2 | 8.4±1.1 | 8.6±0.8 | ||||
| Subjective Sequence Difficulty (0-10) | PNSSham+tDCSSham | 4.4±1.1 | 4.4±1.1 | 3.0±0.9 | 3.0±1.0 | ns | <0.01 | ns |
| PNS+tDCSSham | 4.5±0.8 | 3.5±0.9 | 3.1±1.1 | 2.6±0.7 | ||||
| tDCS+PNSSham | 4.6±0.9 | 4.5±1.0 | 3.0±1.2 | 3.1±1.1 | ||||
| PNS+tDCS | 4.0±1.0 | 3.3±1.1 | 2.8±1.2 | 3.1±1.0 | ||||
Values represent mean±SEM of responses to attention, fatigue, hand tiredness and subjective sequence difficulty visual analogue scales (0=worst possible answer, 10=best possible response). Statistics were calculated using separate ANOVARM for each scale.
Table 3. Sleep Data.
| Day1 | Day2 | Day6 | Statistics ANOVARM | ||||
|---|---|---|---|---|---|---|---|
| Intervention effect | Time effect | Intervention X Time | |||||
| Sleep Duration (hours) | PNSSham+tDCSSham | 7.3±0.5 | 7.6±0.6 | 7.4±0.5 | ns | ns | ns |
| PNS+tDCSSham | 6.4±0.6 | 7.7±0.3 | 7.5±0.5 | ||||
| tDCS+PNSSham | 7.2±0.6 | 6.9±0.8 | 7.5±0.6 | ||||
| PNS+tDCS | 7.6±0.5 | 7.4±0.5 | 6.9±0.5 | ||||
| Sleep Quality (0-10) | PNSSham+tDCSSham | 8.9±0.4 | 9.1±0.3 | 9.1±0.5 | ns | ns | ns |
| PNS+tDCSSham | 7.8±0.8 | 9.4±0.2 | 8.9±0.4 | ||||
| tDCS+PNSSham | 9.0±0.5 | 8.9±0.5 | 8.3±0.7 | ||||
| PNS+tDCS | 8.6±0.6 | 8.9±0.5 | 8.3±0.6 | ||||
Values represent mean±SEM of responses to sleep duration and sleep quality visual analogue scales (0= worst possible answer, 10= best possible response). Statistics were calculated using separate ANOVARM for each scale.
Discussion
The main finding of this study was that the combination of PNS of the paretic hand with anodal tDCS of the ipsilesional M1 enhanced the beneficial effects of training on motor sequence performance beyond levels reached by solely motor practice or by practice combined with either intervention alone, an effect that outlasted the stimulation and training periods by at least 6 days.
Customarily used neurorehabilitative treatments often result in incomplete recovery of motor function after stroke29, 30. Recent work has led to improved understanding of some mechanisms underlying the beneficial effects of rehabilitative interventions and recovery of function after stroke, including restitution of blood flow to different cortical areas31, cortical plastic reorganization after training interventions30, 32, recovery of diaschisis33, and a better understanding of the mechanisms underlying motor learning34.
It would be important to develop effective adjuvant strategies that could enhance training effects beyond those reached by these interventions. In recent years, tDCS has shown promise as a non-invasive technique capable of modulating cortical excitability and motor behavior in stroke patients35, 36. It has been shown that anodal tDCS can enhance motor cortical excitability for a period of time that outlasts the stimulation window10, 11. In stroke patients, application of tDCS, either anodal to the ipsilesional5 or cathodal to the contralesional4, 7 primary motor cortex, facilitates transiently and to a similar magnitude performance of tasks resembling activities of daily living. The proportion of these changes is also comparable to those induced in pinch force when anodal tDCS is applied over ipsilesional M124. Another recently explored intervention to modulate the effects of training is PNS, which applied to a body part leads to a somatotopically specific increase in corticomotor excitability18 and results in enhanced BOLD signal in the contralateral M1 and dorsal premotor cortex21, 22. In animal models, it leads to changes in receptive fields in the primary somatosensory cortex37. In stroke patients, PNS alone has been shown to elicit transient improvements in swallowing13, pinch force12, use-dependent plasticity16, performance of hand tasks14 and ADL-like tasks15, 17, 38. Interestingly, these studies applying a single session of either tDCS or PNS alone showed only transient discrete behavioral changes in the order of 10 to 20% 4, 5, 7, 15, 17, 27. In this study, we hypothesized that the synchronous application of both forms of stimulation could potentially facilitate motor behavior further.
We studied performance of finger motor sequences that engage activity in a distributed network including M139,40, 41. Patients included were severely paralyzed at the time of the stroke (muscle strength of 2 or less in the neurological exam at stroke onset), but recovered to the extent that they could perform the task required in this experiment (Table 1). At baseline, performance levels across the four sessions were comparable, a finding consistent with previous reports28, 41. Interestingly, all patients learned the task over the training period to a comparable extent, regardless of the preceding stimulation type. However, one hour after PNS+tDCS (Day 1 measure), performance improvements relative to baseline were more prominent than after sham or after either stimulation alone (i.e. at day 1: 41.3% better than sham, 15.4% better than PNS alone and 22.7% better than tDCS alone; table “online materials”), an effect that was more pronounced on Day 2 and that remained present, albeit to a lesser extent, on Day 6. This intervention-dependent improvement was evident in the mean number of correct key presses per 30sec relative to baseline whereas the total number of key presses improved to similar extent with all interventions, suggesting that PNS+tDCS mediated its effect through improvement in accuracy rather than speed. The lack of a significantly different effect of PNS+tDCS relative to the other interventions on speed might be explained by a ceiling effect on motor performance in patients that were otherwise well recovered, or alternatively, due to saturation of the mechanisms of action of the combined intervention. Interestingly, the magnitude of performance improvements measured in this investigation with a single session of PNS+tDCS (approximately 42% better than Sham in Day 2) appear to be superior to those reported before using either tDCS or PNS alone in chronic stroke patients (10 to 20 % range)4, 5, 7, 15, 17, 27.
Our results cannot be explained by fatigue, attention or sleep differences across groups (see table 2 and 3). Not surprisingly, hand tiredness, as reported subjectively using a form of a visual analogue scale, showed worsening over time during the training day, but this was the case for all interventional groups. These results are consistent with those of previous investigations that evaluated corticomotor excitability effects of application of paired associative stimulation protocols (PAS), a form of combined peripheral and central nervous system stimulation, in patients with stroke42, 43. However, this is the first report showing that a combined application of both forms of stimulation may bear behavioral benefits relative to the use of each intervention alone44.
It is possible that the additive effect of PNS+tDCS was mediated through modulation of different pathways where tDCS affected sodium and calcium voltage dependent channels and NMDA receptor activity 10, 11, and PNS modulated GABAergic interneurons activity 18. However, the exact mechanisms underlying this effect remain to be determined. Caveats to keep in mind for future studies include whether application of this combined intervention could facilitate training effects in patients with more profound impairment than those reported here and whether multiple sessions can have longer lasting effects. Additionally, it could not be fully ruled out that PNSSHAM intervention could have influenced the hand cortical representation or induced differential regional effects on attention.
In summary, the present study presents evidence that combining peripheral nerve stimulation to a paretic hand with anodal tDCS to the ipsilesional M1 in association with motor training induces superior improvements in performance of a motor task relative to the use of each stimulation type alone in combination with sham and training. Superiority of behavioral gains with the proposed combined intervention was 4 fold larger than after sham, and 1 to 2 times more robust than using either stimulation alone. Importantly, these effects were maintained 1 and 6 days after the completion of the training. These findings suggest that combining peripheral nerve stimulation with anodal brain polarization prior to physical practice could represent a better adjuvant than application of each intervention alone in neurorehabilitation.
Acknowledgements
The authors thank D. Einstein, J. Samuels and E.R. Buch for data acquisition, P. Gandiga for technical assistance, S. Ravindran and M. Brooks for helping with patient recruitment. This research was supported by the intramural research program of NINDS, NIH, USA. P. Celnik was supported by the American Heart Association (0665347U), NCMRR, NICHD, NIH (R01HD053793) and the Rehabilitation Medicine Scientist Training Program (RMSTP; 5K12HD001097).
Footnotes
Conflicts on Interest Disclosures
None of the authors have any conflicts of interest to disclose.
References
- 1.Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol. 2004;3:528–536. doi: 10.1016/S1474-4422(04)00851-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taub E, Uswatte G, Elbert T. New treatments in neurorehabilitation founded on basic research. Nat Rev Neurosci. 2002;3:228–236. doi: 10.1038/nrn754. [DOI] [PubMed] [Google Scholar]
- 3.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, Rigonatti SP, Marcolin MA, Freedman SD, Nitsche MA, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- 5.Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 6.Hummel FC, Cohen LG. Non-invasive brain stimulation: A new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 7.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain dc stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- 8.Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, Cayer-Meade C, Kobayashi M, Theoret H, Fregni F, Tormos JM, Kurland J, Doron KW, Pascual-Leone A. Improved naming after tms treatments in a chronic, global aphasia patient--case report. Neurocase. 2005;11:182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, Vergari M, Zago S, Priori A. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 2008;79:451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- 10.Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial dc-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- 11.Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conforto AB, Kaelin-Lang A, Cohen L. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Annals of Neurology. 2002;51:122–125. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- 13.Fraser C, Power M, Hamdy S, Rothwell J, Hobday D, Hollander I, Tyrell P, Hobson A, Williams S, Thompson D. Driving plasticity in human adult motor cortex is associated with improved motor function after brain injury. Neuron. 2002;34:831–840. doi: 10.1016/s0896-6273(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 14.Wu CW, Seo HJ, Cohen LG. Influence of electric somatosensory stimulation on paretic-hand function in chronic stroke. Arch Phys Med Rehabil. 2006;87:351–357. doi: 10.1016/j.apmr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Conforto AB, Cohen LG, Santos RL, Scaff M, Marie SK. Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. J Neurol. 2007;254:333–339. doi: 10.1007/s00415-006-0364-z. [DOI] [PubMed] [Google Scholar]
- 16.Sawaki L, Wu CW, Kaelin-Lang A, Cohen LG. Effects of somatosensory stimulation on use-dependent plasticity in chronic stroke. Stroke. 2006;37:246–247. doi: 10.1161/01.STR.0000195130.16843.ac. [DOI] [PubMed] [Google Scholar]
- 17.Celnik P, Hummel F, Harris-Love M, Wolk R, Cohen LG. Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch Phys Med Rehabil. 2007;88:1369–1376. doi: 10.1016/j.apmr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol (Lond) 2002;540:623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res. 2000;131:135–143. doi: 10.1007/s002219900269. [DOI] [PubMed] [Google Scholar]
- 20.McDonnell MN, Ridding MC. Afferent stimulation facilitates performance on a novel motor task. Exp Brain Res. 2006;170:109–115. doi: 10.1007/s00221-005-0192-x. [DOI] [PubMed] [Google Scholar]
- 21.Golaszewski SM, Siedentopf CM, Koppelstaetter F, Rhomberg P, Guendisch GM, Schlager A, Gallasch E, Eisner W, Felber SR, Mottaghy FM. Modulatory effects on human sensorimotor cortex by whole-hand afferent electrical stimulation. Neurology. 2004;62:2262–2269. doi: 10.1212/wnl.62.12.2262. [DOI] [PubMed] [Google Scholar]
- 22.Wu CW, van Gelderen P, Hanakawa T, Yaseen Z, Cohen LG. Enduring representational plasticity after somatosensory stimulation. Neuroimage. 2005;27:872–884. doi: 10.1016/j.neuroimage.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 23.Ridding MC, Pearce SL, Flavel SC. Modulation of intracortical excitability in human hand motor areas. The effect of cutaneous stimulation and its topographical arrangement. Exp Brain Res. 2005;163:335–343. doi: 10.1007/s00221-004-2176-7. [DOI] [PubMed] [Google Scholar]
- 24.Hummel FC, Voller B, Celnik P, Floel A, Giraux P, Gerloff C, Cohen LG. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci. 2006;7:73. doi: 10.1186/1471-2202-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandiga PC, Hummel FC, Cohen LG. Transcranial dc stimulation (tdcs): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial dc motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 27.McDonnell MN, Hillier SL, Miles TS, Thompson PD, Ridding MC. Influence of combined afferent stimulation and task-specific training following stroke: A pilot randomized controlled trial. Neurorehabil Neural Repair. 2007;21:435–443. doi: 10.1177/1545968307300437. [DOI] [PubMed] [Google Scholar]
- 28.Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10:275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The excite randomized clinical trial. Jama. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 30.Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, Schulz JB, Goldberg AP, Hanley DF. Repetitive bilateral arm training and motor cortex activation in chronic stroke: A randomized controlled trial. Jama. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillis AE. Pharmacological, surgical, and neurovascular interventions to augment acute aphasia recovery. Am J Phys Med Rehabil. 2007;86:426–434. doi: 10.1097/PHM.0b013e31805ba094. [DOI] [PubMed] [Google Scholar]
- 32.Wittenberg GF, Chen R, Ishii K, Bushara KO, Eckloff S, Croarkin E, Taub E, Gerber LH, Hallett M, Cohen LG. Constraint-induced therapy in stroke: Magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 33.Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125:1544–1557. doi: 10.1093/brain/awf148. [DOI] [PubMed] [Google Scholar]
- 34.Krakauer JW. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 35.Fregni F, Pascual-Leone A. Technology insight: Noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rtms and tdcs. Nat Clin Pract Neurol. 2007;3:383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- 36.Webster BR, Celnik PA, Cohen LG. Noninvasive brain stimulation in stroke rehabilitation. NeuroRx. 2006;3:474–481. doi: 10.1016/j.nurx.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Recanzone GH, Allard TT, Jenkins WM, Merzenich MM. Receptive-field changes induced by peripheral nerve stimulation in si of adult cats. J Neurophysiol. 1990;63:1213–1225. doi: 10.1152/jn.1990.63.5.1213. [DOI] [PubMed] [Google Scholar]
- 38.Johansson K, Lindgren I, Widner H, Wiklund I, Johansson BB. Can sensory stimulation improve the functional outcome in stroke patients? Neurology. 1993;43:2189–2192. doi: 10.1212/wnl.43.11.2189. [DOI] [PubMed] [Google Scholar]
- 39.Perez MA, Tanaka S, Wise SP, Sadato N, Tanabe HC, Willingham DT, Cohen LG. Neural substrates of intermanual transfer of a newly acquired motor skill. Curr Biol. 2007;17:1896–1902. doi: 10.1016/j.cub.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 40.Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: Fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 42.Jayaram G, Stinear JW. Contralesional paired associative stimulation increases paretic lower limb motor excitability post-stroke. Exp Brain Res. 2008;185:563–570. doi: 10.1007/s00221-007-1183-x. [DOI] [PubMed] [Google Scholar]
- 43.Castel-Lacanal E, Gerdelat-Mas A, Marque P, Loubinoux I, Simonetta-Moreau M. Induction of cortical plastic changes in wrist muscles by paired associative stimulation in healthy subjects and post-stroke patients. Exp Brain Res. 2007;180:113–122. doi: 10.1007/s00221-006-0844-5. [DOI] [PubMed] [Google Scholar]
- 44.Uy J, Ridding MC, Hillier S, Thompson PD, Miles TS. Does induction of plastic change in motor cortex improve leg function after stroke? Neurology. 2003;61:982–984. doi: 10.1212/01.wnl.0000078809.33581.1f. [DOI] [PubMed] [Google Scholar]