Abstract
The two-fold higher prevalence rate of major depression in females may involve vulnerability to omega-3 fatty acid deficiency secondary to a dysregulation in ovarian hormones. However, the role of ovarian hormones in the regulation of brain omega-3 fatty acid composition has not been directly evaluated. Here we determined erythrocyte and regional brain docosahexaenoic acid (DHA, 22:6n-3) composition in intact male and female rats, and in chronically ovariectomized (OVX) rats with or without cyclic estradiol treatment (2 μg/4 d). All groups were maintained on diets with or without the DHA precursor alpha-linolenic acid (ALA, 18:3n-3). We report that both male (−21%) and OVX (−19%) rats on ALA+ diet exhibited significantly lower erythrocyte DHA composition relative to female controls. Females on ALA+ diet exhibited lower DHA composition in the prefrontal cortex (PFC) relative males (−5%). OVX rats on ALA+ diet exhibited significantly lower DHA composition in the hippocampus (−6%), but not in the PFC, hypothalamus, or midbrain. Lower erythrocyte and hippocampus DHA composition in OVX rats was not prevented by estrogen replacement. All groups maintained on ALA− diet exhibited significantly lower erythrocyte and regional brain DHA composition relative to groups on ALA+ diet, and these reductions were greater in males but not in OVX rats. These preclinical data corroborate clinical evidence for gender differences in peripheral DHA composition (female>male), demonstrate gender differences in PFC DHA composition (male>female), and support a link between ovarian hormones and erythrocyte and region-specific brain DHA composition.
Keywords: Omega-3 fatty acids, docosahexaenoic acid, alpha-linolenic acid, ovariectomy, estrogen, prefrontal cortex, hippocampus, hypothalamus, midbrain, gender, rat
1. Introduction
Evidence from cross-national and cross-sectional epidemiological surveys and controlled prospective intervention trials suggest that omega-3 fatty acid deficiency may represent a reversible risk factor for major depression (reviewed in Parker et al., 2006). Importantly, the prevalence rate of major depression is approximately 2-fold greater in women (Kessler, 2003), and some cross-sectional epidemiological surveys have found that low dietary omega-3 fatty acid intake is associated with an elevated risk of depression in females but not in males (Hakkarainen et al., 2004; Tanskanen et al., 2001; Timomen et al., 2004). Moreover, erythrocyte or plasma phospholipid omega-3 fatty acid deficits have been observed in women experiencing postpartum depression (De Vriese et al., 2003; Hibbeln, 2002; Otto et al., 2003), and we have recently found that docosahexaenoic acid (DHA, 22:6n-3), the principal omega-3 fatty acid in brain, was significantly reduced in the postmortem prefrontal cortex (PFC) of female, but not male, patients with major depression (McNamara et al., 2007). Although these findings indicate gender differences in vulnerability to peripheral and central tissue DHA deficits in patients with major depression, the mechanisms mediating this effect remains poorly understood.
The incidence of major depression in women increases during periods associated with ovarian hormonal fluctuations including the perimenopausal period (reviewed in Deecher et al., 2008), and suicidal behavior may be more common during phases of the menstrual cycle when estrogen levels are lowest (Saunders & Hawton, 2006). Emerging clinical evidence suggests that ovarian hormones positively regulate the biosynthesis of DHA from the dietary precursor α-linolenic acid (ALA, 18:3n-3). Specifically, isotope tracer studies have found that DHA biosynthesis from ALA is significantly greater in healthy adult women compared to men (Burdge et al., 2002; Burdge & Wooton, 2002; Pawlosky et al., 2003), and healthy adult women exhibit greater circulating plasma DHA relative to men under similar or habitual dietary conditions (Bakewell et al., 2006; Crowe et al., 2008; Giltay et al., 2004b; Nikkari et al., 1995). Furthermore, chronic treatment of healthy young adult women with oral contraceptives or postmenopausal women with hormone replacement therapy significantly increases plasma DHA content (Bakewell et al., 2006; Burdge & Wooton, 2002; Giltay et al., 2004a,b; Otto et al., 2001; Sumino et al., 2003). Preclinical studies have also observed a positive association between ovarian hormones and liver and plasma DHA composition (Childs et al., 2008), and an interaction between reproductive activity, dietary omega-3 fatty acid composition, and peripheral and central DHA composition (Levant et al., 2006a,b, 2007a,b). However, the role of ovarian hormones in the regulation of brain DHA composition has not been directly evaluated.
Evaluation of a link between ovarian hormones and tissue DHA composition in human subjects is potentially confounded by difficult-to-control factors that influence membrane DHA composition, including dietary omega-3 fatty acid intake, smoking, and alcohol consumption (Hibbeln et al., 2003; Leng et al., 1994; Pawlosky et al., 2001). Definitive evaluation of this relationship therefore requires the use of an animal model so that these variables can be controlled or obviated, and central DHA composition determined. To this end, the present study compared erythrocyte and brain DHA composition in intact male and female rats, and in chronically ovariectomized (OVX) rats with or without cyclic estradiol treatment. All groups were maintained on diets with or without the DHA precursor ALA. Our principal hypotheses were that female rats would exhibit greater erythrocyte and brain DHA composition relative to male rats on the same diet, and that OVX rats would exhibit lower erythrocyte and brain DHA composition relative to sham controls.
2. Materials and Methods
2.1. Diets
Rats were maintained on either ALA-fortified diet (ALA+, TD.04285) or ALA-depleted diet (ALA−, TD.04286). Both diets were prepared by Harlan-TEKLAD, Madison, WI, and were matched for all non-fat nutrients (casein (vitamin-free) 200 g/kg, L-cystine 3 g/kg, sucrose 270 g/kg, dextrose monohydrate 99.5 g/kg, corn starch 200 g/kg, maltodextrin 60 g/kg, cellulose 50 g/kg, mineral mixture AIMN-93G-MX 35 g/kg, vitamin mixture AIN-93-VX 10 g/kg, choline bitartrate 2.5 g/kg, TBHQ (antioxidant) 0.02 g/kg). Both ALA+ and ALA− diets contained omega-3 fatty acid-free hydrogenated coconut (45 g/kg) and safflower (19 g/kg) oils, and the ALA+ diet additionally contained ALA-containing flaxseed oil (6 g/kg). Analysis of diet fatty acid composition by gas chromatography confirmed that the ALA− diet did not contain ALA, but was similar to the ALA+ diet in saturated (C8:0, C10:0, C12:0, C14:0, C16:0, C18:0), monounsaturated (18:1n-9, oleic acid), and omega-6 fatty acid (18:2n-6, linoleic acid) composition (Table 1). ALA represented 4.6% of total fatty acid composition in the ALA+ diet, and neither diet contained preformed DHA, or intermediate omega-3 fatty acid precursors.
Table 1.
Diet Fatty Acid Composition
| Fatty Acid1 | ALA+ | ALA− |
|---|---|---|
| Octanoic acid (8:0) | 4.3 ± 0.1 | 4.6 ± 0.0 |
| Decanoic acid (10:0) | 3.7 ± 0.1 | 3.9 ± 0.0 |
| Lauric acid (12:0) | 29.0 ± 0.4 | 30.5 ± 0.1 |
| Myristic acid (14:0) | 11.0 ± 0.1 | 11.6 ± 0.1 |
| Palmitic acid (16:0) | 8.3 ± 0.0 | 8.5 ± 0.0 |
| Stearic acid (18:0) | 9.4 ± 0.1 | 9.8 ± 0.0 |
| Oleic acid (18:1n-9) | 6.7 ± 0.1 | 5.9 ± 0.1 |
| Linoleic acid (18:2n-6) | 22.7 ± 0.3 | 22.4 ± 0.2 |
| Arachidonic acid (20:4n-6) | - | - |
| α-Linolenic acid (18:3n-3) | 4.6 ± 0.1 | - |
| Docosahexaenoic acid (22:6n-3) | - | - |
Values are means ± SD (mg/100 mg fatty acids).
2.2. Gender
Male and female offspring bred in house to nulliparous female Long-Evans hooded rats were used. Rats were randomly assigned to receive either ALA+ or ALA− diet following weaning on postnatal day 21 (P21) until they were sacrificed on P91 (70 d). Rats were housed 2 per cage with food and water available ad libitum, and maintained under standard vivarium conditions on a 12:12 h light:dark cycle. All experimental procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee, and adhere to the guidelines set by the National Institutes of Health.
2.3. Ovariectomy
Sham (n=20) and ovariectomy (OVX) (n=25) surgeries were performed by Harlan Sprague-Dawley (Indianapolis, IN) in young adult (P56) female Long-Evans hooded rats. One half of OVX rats were randomly assigned to receive cyclic estradiol benzoate (Sigma-Aldrich, E8515) in sesame oil (Sigma-Aldrich, S3547) at a dose of 2 μg (s.c.) every four days from P60-P130 (70 d), and one half were administered an equal volume of sesame oil (Asarian & Geary, 2002). Body weights were recorded every four days immediately prior to injections. Rats from each treatment group were randomly assigned to receive either ALA+ or ALA− diet from P60-P130 (70 d).
2.4. Tissue collection
Rats were sacrificed by decapitation during the light portion of the cycle, brains extracted and immediately immersed in ice-cold 0.9% NaCl for 2 min. The brain was then dissected on ice to isolate the PFC, and the olfactory tubercle and residual striatal tissue were removed the PFC. Midbrain, hypothalamus, and hippocampus were also obtained from rats in the OVX experiments. Tissues were individually placed in cryotubes, flash frozen in liquid nitrogen, and stored at −80°C. At the time of sacrifice, vaginal smears were collected onto glass slides, dried, and stained (Dipp-Kwik Stain kit, American Master/Tech Scientific Inc., Lodi, CA) to determine position in the ovarian cycle (metestrus [diestrus D1], diestrus, proestrus, estrus) based on cell morphology (Becker et al., 2005). Additionally, trunk blood was collected into 1.5 ml tubes containing 200 μl of EDTA (100 mM, pH 7.5) and centrifuged for 20 min (5,000× g, 4°C). In the OVX experiments, plasma was isolated for determination of estradiol concentrations with a DSL-4800 Ultra-sensitive estradiol radioimmunoassay kit (Diagnostic Systems Laboratories, Inc., Webster Texas). Erythrocytes were isolated and washed 3x with 0.9% NaCl, and stored at −80°C for subsequent determination of DHA composition.
2.5. Gas chromatography
Total (triglyceride, phospholipid, and cholesteryl ester) fatty acid composition was determined using the saponification and methylation methods originally described by Mecalfe et al (1966). Brain (100–200 mg) and erythrocyte (~300 mg) samples were placed in a 20 ml glass vial into which 4 ml of 0.5N methanolic sodium hydroxide was added, and the sample heated at 80°C for 5 min. Following a 10 min cooling period, 3 ml of boron trifluoride in methanol was added to methylate the sample. After an additional five minutes of heating in the water bath (80°C), the sample vial was allowed to cool, and 2 ml of a saturated solution (6.2 M) of sodium chloride and 10 ml of hexane was added. The samples were then mixed by vortex for one minute. The hexane fraction was then transferred into a 20 ml vial containing 10 mg of sodium sulfate to dry the sample. The hexane solution was then removed for GC analysis. An injection volume of 1 μL of the hexane solution was analyzed. Samples were analyzed with a Shimadzu GC-2014 equipped with an auto-injector (Shimadzu Scientific Instruments Inc., Columbia MD). Analysis of fatty acid methyl esters is based on areas calculated with Shimadzu Class VP 4.3 software. The column was a DB-23 (123–2332): 30m (length), I.D. (mm) 0.32 wide bore, film thickness of 0.25 μM (J&W Scientific, Folsom CA). Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). The GC conditions were: column temperature ramping by holding at 120°C for one minute followed by an increase of 5°C/min from 120–240°C. The temperature of the injector and flame ionization detector was 250°C. A split (8:1) injection mode was used. The carrier gas was helium with a column flow rate of 2.5 ml/min. DHA data is expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). Samples were processed by a technician that was blinded to treatments.
2.6. Statistical analysis
Gender differences were analyzed with a two-way ANOVA, with Diet (ALA+, ALA−) and Gender (Male, Female) as the main factors. Pairwise comparisons (2-tailed) of simple effects were performed using the Bonferroni correction with a group-wise error rate of α=0.05 to evaluate differences across four groups (α=0.05/4 = 0.01). Effect of position in the ovarian cycle was analyzed with a one-way ANOVA. Effects of OVX were analyzed with a two-way ANOVA, with Diet (ALA+, ALA−) and surgical/post-surgical treatment (Sham, OVX, OVX+E) as the main factors. Pairwise comparisons (2-tailed) of simple effects were performed using the Bonferroni correction to evaluate differences across six treatment groups (α=0.05/6 = 0.008). In cases of statistical significance, effect size was calculated using Cohen’s d, with small, medium, and large effect sizes being equivalent to d-values of 0.30, 0.50, and 0.80, respectively. Parametric linear regression analyses were used to determine the relationship between erythrocyte DHA composition and regional brain DHA composition. All statistical analyses were performed with GB-STAT software (Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Gender differences
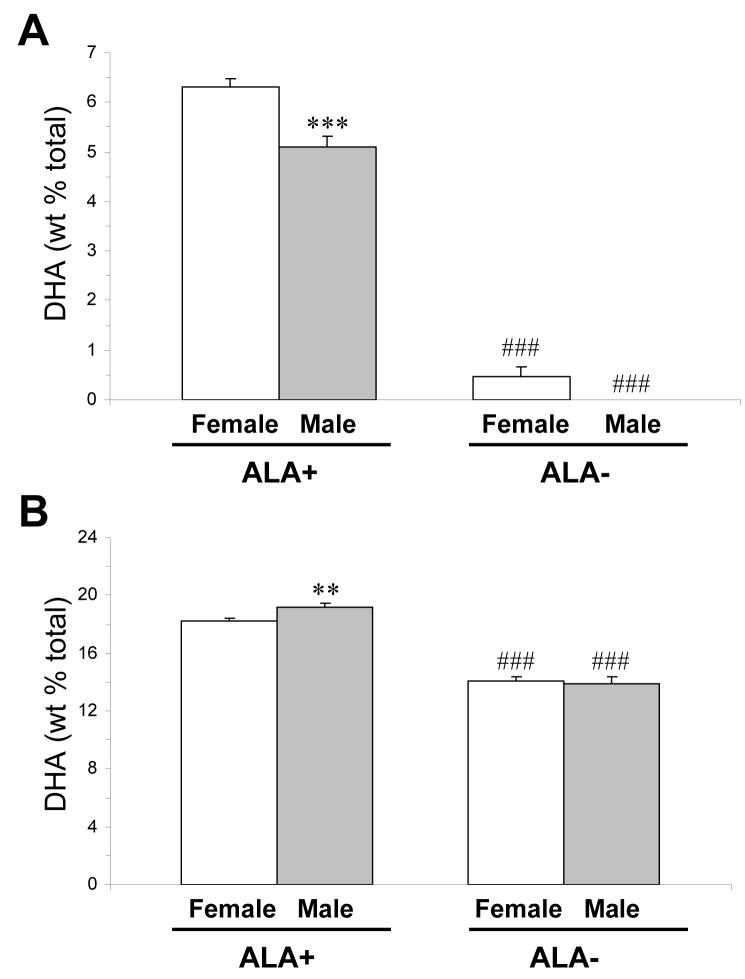
For erythrocyte DHA composition, there was a significant main effect of Gender, F(1,61)=14.8, p=0.0003, and Diet, F(1,61)=525, p≤0.0001, and the Diet × Treatment interaction was not significant, F(1,61)=3.1, p=0.08. Males on ALA+ diet (n=17) exhibited significantly lower erythrocyte DHA composition relative to females on ALA+ diet (n=22)(−21%, p=0.0005, d = 1.2)(Fig. 1A). Males on ALA− diet (n=13) exhibited numerically lower erythrocyte DHA composition relative to females on ALA− diet (n=10)(paired t-test: p=0.04, d = 1.6). Males and females on ALA− diet exhibited significantly lower erythrocyte DHA composition relative to males (−100%, p≤0.0001, d = 7.9) and females (−99%, p≤0.0001, d = 6.2) on ALA+ diet, respectively. For PFC DHA composition, the main effect of Diet, F(1,41)=260, p≤0.0001, and the Diet × Treatment interaction, F(1,41)=3.7, p=0.04, were significant, and the main effect of Gender was not significant, F(1,41)=1.5, p=0.23. Males (n=12) on ALA+ diet exhibited significantly greater PFC DHA composition relative to females (n=14) on ALA+ diet (+5%, p=0.004, d = 1.4)(Fig. 1B). PFC DHA composition did not differ between males (n=12) and females (n=10) on ALA− diet (p=0.72). Both males and females on ALA− diet exhibited significantly lower PFC DHA composition relative to males (−29%, p≤0.0001, d = 4.5) and females (−23%, p≤0.0001, d = 5.4) on ALA+ diet, respectively. Relative to same-gender controls on ALA+ diet, males on ALA− diet exhibited a significantly greater decrease in PFC DHA composition (−29.3%±2.6%) than did females on ALA− diet (−22.7%±1.8%)(p=0.04, d = 1.1).
Fig. 1.
Mean DHA composition in erythrocytes (A) and PFC (B) of male and female rats on ALA+ or ALA− diets (n=10–22/group). Note that male rats on ALA+ diet exhibit lower erythrocyte DHA composition relative to female rats, and (2) male rats on ALA− diet exhibit greater reductions in erythrocyte and PFC DHA composition relative to females. Values are group mean ± S.E.M. ** P≤0.01, ***P≤0.001 vs. females on the same diet, ###P≤0.0001 vs. same gender on ALA+ diet.
3.2. Position in ovarian cycle
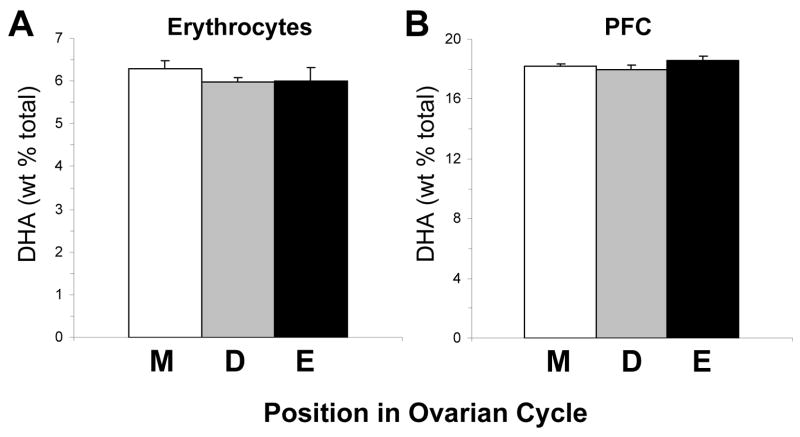
Female rats maintained on ALA+ diet (n=22) were segregated according to their position in the ovarian cycle (metestrus [diestrus D1], diestrus, proestrus, estrus) based on vaginal cell morphology (Becker et al., 2005). The majority of female rats were in metestrus (n=14), and the remainder were in either diestrus (n=4) or estrus (n=4). For erythrocyte DHA composition, the main effect of ovarian phase was not significant, F(2,21)=0.1, p=0.89 (Fig. 2A). Similarly, for PFC DHA composition, the main effect of ovarian phase was not significant, F(2,21)=0.78, p=0.48 (Fig. 2B).
Fig. 2.
Mean DHA composition in erythrocytes (A) and PFC (B) of female rats (n=22) on ALA+ diet segregated according to their position in the ovarian cycle (M, metestrus [D1]; D, diestrus; E, estrus). Note that erythrocyte and PFC DHA composition do not differ significantly between ovarian phases. Values are group mean ± S.E.M.
3.3. Ovariectomy
3.3.1. Plasma estradiol levels and body weight
Depending on the position in the ovarian cycle, plasma estradiol concentrations in sham controls ranged from 19 to 41 pg/mL, values consistent with the physiological range found during the normal rat ovarian cycle (Butcher et al., 1974). Plasma estradiol concentrations in vehicle-treated OVX rats were all below the limit of detection (<5 pg/ml). Regardless of diet, the mean plasma estradiol concentration in estradiol-treated OVX (OVX+E) rats 1 h after the final injection of estradiol benzoate (2 μg) was 13.9±1.2 pg/ml. A prior study found that plasma estradiol levels do not peak until 6 hrs following estradiol benzoate (2 μg) administration in OVX rats (Asarian & Geary, 2002), suggesting the lower plasma estradiol concentration observed in our OVX rats 1 h post-injection is on the rising edge of the curve. Consistent with a prior report (Asarian & Geary, 2002), OVX rats exhibited significantly greater body weight gain relative to sham controls, and cyclic estradiol treatment normalized body weight gain. For baseline body weight (P60), the main effect of Diet, F(1,31)=3.01, p=0.10, and Treatment, F(1,31)=0.325, p=0.73, and the Diet × Treatment interaction, F(1,31)=1.29, p=0.289, were not significant. For post-treatment body weight (P130), the main effect of Treatment was significant, F(1,31)=25.7, p≤0.0001, and the main effect of Diet, F(1,31)=1.90, p=0.177, and the Diet × Treatment interaction, F(1,31)=0.594, p=0.558, were not significant. ALA+ OVX rats and ALA− OVX rats had significantly greater body weights relative to ALA+ shams (p≤0.0001) and ALA-shams (p≤0.0001), respectively. ALA+ OVX+E rats (p≤0.0001) and ALA− OVX+E rats (p≤0.0001) had significantly lower body weights relative to ALA+ untreated OVX and ALA− untreated OVX rats, respectively. Together, these data support the integrity of the OVX surgeries and the physiological efficacy of cyclic estradiol treatment (Asarian & Geary, 2002).
3.3.2. Erythrocytes
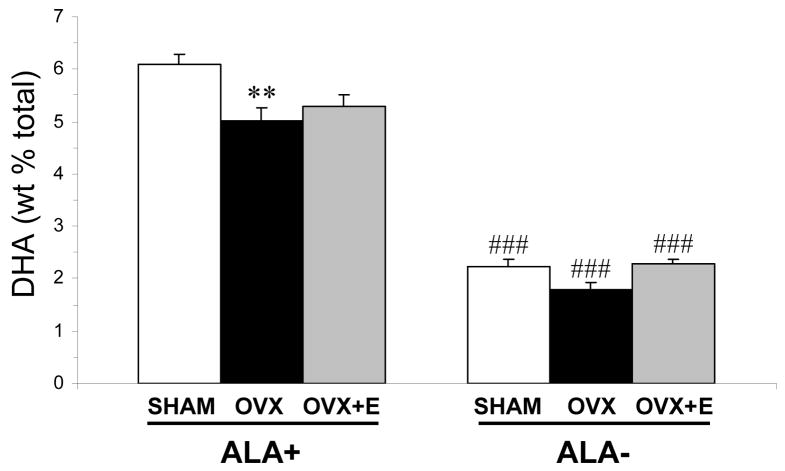
For erythrocyte DHA composition, there was a significant main effect of Diet, F(1,45)=435, p≤0.0001, and Treatment, F(2,45)=6.97, p=0.002, and the Diet × Treatment interaction was not significant, F(2,45)=2.47, p=0.097. OVX rats maintained on ALA+ diet exhibited significantly lower erythrocyte DHA composition relative to shams on ALA+ diet (−19%, p=0.003, d = 1.6)(Fig. 3). OVX+E rats on ALA+ diet did not differ significantly from shams (−13%, p=0.012) or OVX rats (+5%, p=0.43) on ALA+ diet. Shams on ALA− diet exhibited significantly lower erythrocyte DHA composition relative to shams on ALA+ diet (−64%, p≤0.0001, d = 6.3). OVX rats on ALA− diet exhibited a non-significant trend toward lower erythrocyte DHA composition relative to shams on ALA− diet (−20%, p=0.04, d = 1.4)(Fig. 3). OVX+E rats on ALA− diet exhibited a trend toward greater erythrocyte DHA composition relative OVX rats (+22%, p=0.01, d = 1.9), and did not differ from sham controls (+3%, p=0.604) on ALA− diet (Fig. 3).
Fig. 3.
Mean DHA composition in erythrocytes of sham controls, OVX, and OVX+E rats on ALA+ or ALA− diets (n=6–14/group). Note that OVX rats on ALA+ or ALA− diet exhibit a significant reduction in erythrocyte DHA composition relative shams on the same diet, and cyclic estradiol treatment partially normalizes erythrocyte DHA composition in OVX rats on ALA− but not ALA+ diet. Values are group mean ± S.E.M. **P = 0.003 vs. shams on the same diet, ###P≤0.0001 vs. same group on ALA+ diet,
3.3.3. Regional brain
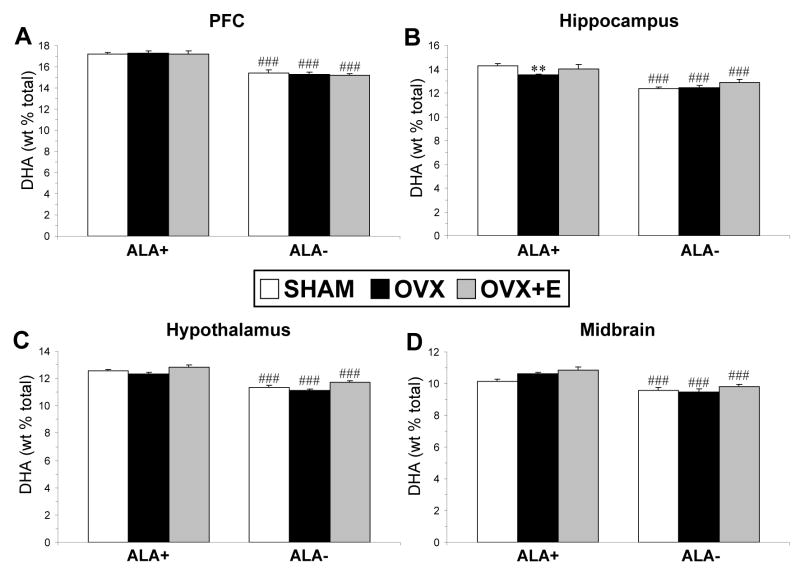
For PFC DHA composition, there was a significant main effect of Diet, F(1,31)=128, p≤0.0001, and the main effect of Treatment, F(2,31)=0.227, p=0.798, and the Diet × Treatment interaction, F(2,31)=0.302, p=0.741, were not significant (Fig. 4A). For hippocampus DHA composition, there was a significant main effect of Diet, F(1,31)=56.9, p≤0.0001, and the main effect of Treatment, F(2,31)=3.65, p=0.053, and the Diet × Treatment interaction, F(2,31)=1.91, p=0.165, were not significant. OVX rats on ALA+ diet (−6%, p=0.006, d = 2.1) but not ALA− diet (p=0.868) exhibited a significantly lower hippocampus DHA composition relative to shams on the same diet (Fig. 4B). For hypothalamus DHA composition, there was a significant main effect of Diet, F(1,31)=104, p≤0.0001, and Treatment, F(2,31)=7.64, p=0.002, and the Diet × Treatment interaction, F(2,31)=0.08, p=0.917, was not significant (Fig. 4C). None of the post-hoc comparisons reached statistical significance after correction for multiple comparisons (p≤0.008). For midbrain DHA composition, there was a significant main effect of Diet, F(1,31)=42.4, p≤0.0001, and Treatment, F(2,31)=3.92, p=0.030, and the Diet × Treatment interaction, F(2,31)=1.52, p=0.233, was not significant (Fig. 4D). OVX (+5%, p=0.026, d = 1.6) and OVX+E (+7%, p=0.023, d = 1.7) rats on ALA+ diet exhibited non-significant trends for greater midbrain DHA composition relative to shams on the same diet.
Fig. 4.
Mean DHA composition in the PFC (A), hippocampus (B), hypothalamus (C), and midbrain (D) of sham controls, OVX, and OVX+E rats on ALA+ or ALA− diets (n=6–7/group). Note that OVX rats on ALA+, but not ALA− diet, exhibit significantly lower hippocampus DHA composition relative to shams on the same diet. Values are group mean ± S.E.M. **P=0.006 vs. shams on ALA+ diet, ###P≤0.0001 vs. same group on ALA+ diet.
3.3.4. Correlations between erythrocyte and regional brain DHA composition
Among all rats on ALA+ diet (n=18), erythrocyte DHA composition was positively correlated with hippocampus DHA composition (r = +0.48, p = 0.043), inversely correlated with midbrain DHA composition (r = −0.56, p = 0.016), and not significantly correlated with PFC (r = −0.13, p = 0.59) or hypothalamus (r = −0.08, p = 0.73) DHA composition. Among all rats on ALA− diet (n=19), erythrocyte DHA composition was not significantly correlated with PFC (r = +0.14, p = 0.55), hippocampus (r = +0.009, p = 0.97), hypothalamus (r = +0.33, p = 0.16), or midbrain (r = +0.27, p = 0.26) DHA composition.
4. Discussion
Based on prior clinical and preclinical evidence, we hypothesized that female rats would exhibit greater erythrocyte and brain DHA composition relative to male rats maintained on the same diet. This hypothesis was supported in part by the finding that female rats on ALA+ diet exhibited significantly greater erythrocyte DHA composition relative to male rats on ALA+ diet (+21%). However, females on ALA+ diet exhibited significantly lower PFC DHA composition relative to males (−5%) on ALA+ diet. Moreover, we also demonstrate that males exhibit greater erythrocyte and PFC DHA loss in response to chronic ALA− diet. Among female rats on ALA+ diet, position in the ovarian cycle did not significantly influence erythrocyte or PFC DHA composition. We also hypothesized that OVX rats would exhibit lower erythrocyte and brain DHA composition relative to sham controls, and that this difference would be attenuated by cyclic estrogen treatment. This hypothesis was supported in part by the finding that OVX rats on ALA+ diet exhibited significantly lower erythrocyte and hippocampus DHA composition relative to shams on ALA+ diet, though no differences were observed in the PFC, hypothalamus, or midbrain. Cyclic estradiol treatment did not significantly attenuate reductions in erythrocyte or hippocampus DHA composition in OVX rats on ALA+ diet. Together, these preclinical data provide evidence for gender differences in rat erythrocyte and brain DHA composition, and that ovarian hormones positively regulate erythrocyte and region-specific DHA composition.
Based on prior evidence that low dietary ALA composition interacts with reproductive activity to reduce regional brain DHA composition (Levant et al., 2006a,b, 2007b), we additionally investigated the combined effects of OVX and chronic dietary ALA depletion. It was predicted that OVX rats on ALA− diet would exhibit greater DHA deficits than OVX rats on ALA+ diet. Contrary to this prediction, the decrease in erythrocyte DHA composition observed in OVX rats maintained on ALA− diet (−20%) was similar to the reduction observed in OVX rats maintained on ALA+ diet (−19%) relative to shams on the same diet. Additionally, we did not observe a significant interaction between OVX and dietary ALA with respect to DHA composition in any brain region including the PFC, a region previously found to exhibit significant DHA loss in response to low dietary ALA composition and reproductive activity (Levant et al., 2007b). These data suggest that reductions in maternal erythrocyte and regional brain DHA composition in response to reproductive activity and low dietary ALA intake cannot be wholly attributed to reductions in maternal ovarian hormones during pregnancy, and may be predominantly due to fetal DHA accrual from maternal stores.
Comparison of total brain DHA composition used in this study and a prior study using isolated brain phospholipids (Levant et al., 2007b) suggest that triglycerides and cholesteryl esters comprise a relatively small percentage of total brain DHA composition. Furthermore, differential DHA composition observed across brain regions in this study is consistent with previous reports (Carrie et al., 2000; Levant et al., 2007b; Xiao et al., 2005), as is the greater reductions in DHA composition in forebrain structures relative to the midbrain (Carrie et al., 2000; Xiao et al., 2005). The finding that DHA composition was significantly reduced in all brain regions of adult sham female rats following chronic (70 d) dietary ALA depletion contrasts with prior studies finding that chronic feeding of low-ALA diets does not alter brain DHA composition in adult virgin female rats (91d, Levant et al., 2006b) or adult male rats (217 d, Bourre et al., 1992). However, other studies have found that a longer duration of feeding a low ALA diet (189 d) can reduce brain DHA composition by 15% in adult virgin females (Levant et al., 2006b), and that chronic (65 d) feeding of an ALA− diet decreased brain DHA in adult male mice (McNamara et al., 2008). Nevertheless, the magnitude of the PFC DHA deficit observed in female rats placed on ALA− diet at P21 (−23%) was greater than that observed when ALA-diet is introduced young adulthood (P60)(−10%), a finding consistent with an age-related reduction in the loss half-life of DHA in response to dietary ALA insufficiency.
Because the ALA+ diet used in this study contained only the omega-3 fatty acid precursor ALA, the significantly lower erythrocyte and hippocampus DHA composition observed in OVX rats maintained on this diet is consistent with ovarian hormones having a positive effect on ALA-DHA biosynthesis. This is also supported by the finding that females on ALA+ diet exhibited greater erythrocyte DHA composition relative to males on ALA+ diet. However, removal of dietary ALA resulted in lower DHA composition in all brain regions, whereas OVX rats on ALA+ diet did not exhibit uniform brain DHA deficits. Although these data would suggest that ovarian hormones do not regulate ALA-DHA biosynthesis, definitive evaluation of this mechanism will require isotope tracer studies. Furthermore, it is not known whether erythrocyte DHA stores are mobilized to compensate for potentially greater reductions in brain DHA composition in OVX rats. In the present study, midbrain DHA composition was inversely correlated with erythrocyte DHA composition, whereas hippocampus DHA composition was positively correlated with erythrocyte DHA composition, in rats on ALA+ diet, suggesting regional differences in DHA accrual from erythrocyte stores. Furthermore, we found that female rats had lower PFC DHA composition relative to male rats despite having greater erythrocyte DHA composition. Although these data suggest that ovarian hormones negatively regulate erythrocyte DHA mobilization and brain accrual, additional studies will be required to evaluate this mechanism.
We previously reported that postmortem PFC DHA composition is significantly lower in female (−32%), but not male (−16%), patients with major depression relative to same-gender controls (McNamara et al., 2007). In the latter study we also found that postmortem PFC DHA composition was numerically lower in female controls than in male controls (−8%), but this difference did not reach statistical significance. In view of clinical evidence for a positive relationship between ovarian hormones and peripheral DHA composition (e.g., Giltay et al., 2004b), we speculated that gender differences in PFC DHA composition in patients with major depression due in part to ovarian hormones. Although we demonstrate in the present study that female rats exhibited lower PFC DHA composition relative males (−5%), chronic depletion of ovarian hormones did not significantly alter PFC DHA composition in OVX rats. Furthermore, the reduction in PFC DHA composition in male rats maintained on ALA− diet (−29%) was significantly greater than the reduction observed in female rats (−23%), suggesting that males are at greater risk for dietary-induced reductions in PFC DHA composition. These data therefore suggest that the greater DHA deficit observed in the postmortem PFC of female patients with major depression is unlikely to be due to chronic deficits in ovarian hormones.
In summary, we provide preclinical evidence for significant gender differences in erythrocyte DHA composition (female>male), as well as evidence that ovarian hormones other than estrogen positively regulate erythrocyte DHA composition. These findings may take on additional significance in view of clinical findings that erythrocyte DHA deficits (Harris, 2007) and the postmenopausal period (Lewis, 2007) are both associated with increased risk for cardiovascular disease. We also provide evidence for significant gender differences in PFC DHA composition (male>female) and in PFC DHA loss following chronic ALA depletion (male>female). However, PFC DHA composition and loss in response to chronic ALA depletion was not altered in OVX rats. Finally, we show that ovarian hormones regulate brain DHA composition in a regional-specific manner, and that the hippocampus is more vulnerable to DHA loss in response to OVX. Although additional studies will be required to elucidate mechanisms, these preclinical data corroborate previous clinical studies implicating ovarian hormones in the positive regulation of peripheral DHA composition, and further indicate that regional brain DHA is also regulated in part by gonadal hormones.
Acknowledgments
This work was supported in part by National Institute of Mental Health grants MH073704 and MH074858 to R.K.M., and DK59630 to P.T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Bakewell L, Burdge GC, Calder PC. Polyunsaturated fatty acid concentrations in young men and women consuming their habitual diets. Br J Nutr. 2006;96:93–99. doi: 10.1079/bjn20061801. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bourre JM, Dumont OS, Piciotti MJ, Pascal GA, Durand GA. Dietary alpha-linolenic acid deficiency in adult rats for 7 months does not alter brain docosahexaenoic acid content, in contrast to liver, heart and testes. Biochim Biophys Acta. 1992;1124:119–122. doi: 10.1016/0005-2760(92)90087-c. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br J Nutr. 2002;88:355–363. doi: 10.1079/BJN2002662. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–420. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Carrié I, Clément M, de Javel D, Francès H, Bourre JM. Specific phospholipid fatty acid composition of brain regions in mice. Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J Lipid Res. 2000;41:465–472. [PubMed] [Google Scholar]
- Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. Gender differences in the n-3 fatty acid content of tissues. Proc Nutr Soc. 2008;67:19–27. doi: 10.1017/S0029665108005983. [DOI] [PubMed] [Google Scholar]
- Crowe FL, Skeaff CM, Green TJ, Gray AR. Serum n-3 long-chain PUFA differ by sex and age in a population-based survey of New Zealand adolescents and adults. Br J Nutr. 2008;99:168–174. doi: 10.1017/S000711450779387X. [DOI] [PubMed] [Google Scholar]
- Deecher D, Andree TH, Sloan D, Schechter LE. From menarche to menopause: exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology. 2008;33:3–17. doi: 10.1016/j.psyneuen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- De Vriese SR, Christophe AB, Maes M. Lowered serum n-3 polyunsaturated fatty acid (PUFA) levels predict the occurrence of postpartum depression: further evidence that lowered n-PUFAs are related to major depression. Life Sci. 2003;73:3181–3187. doi: 10.1016/j.lfs.2003.02.001. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Duschek EJ, Katan MB, Zock PL, Neele SJ, Netelenbos JC. Raloxifene and hormone replacement therapy increase arachidonic acid and docosahexaenoic acid levels in postmenopausal women. J Endocrinol. 2004a;182:399–408. doi: 10.1677/joe.0.1820399. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004b;80:1167–1174. doi: 10.1093/ajcn/80.5.1167. [DOI] [PubMed] [Google Scholar]
- Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lönnqvist J. Is low dietary intake of omega-3 fatty acids associated with depression? Am J Psychiatry. 2004;161:567–569. doi: 10.1176/appi.ajp.161.3.567. [DOI] [PubMed] [Google Scholar]
- Harris WS. Omega-3 fatty acids and cardiovascular disease: a case for omega-3 index as a new risk factor. Pharmacol Res. 2007;55:217–223. doi: 10.1016/j.phrs.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbeln JR. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord. 2002;69:15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Makino KK, Martin CE, Dickerson F, Boronow J, Fenton WS. Smoking, gender, and dietary influences on erythrocyte essential fatty acid composition among patients with schizophrenia or schizoaffective disorder. Biol Psychiatry. 2003;53:431–441. doi: 10.1016/s0006-3223(02)01549-4. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Leng GC, Horrobin DF, Fowkes FG, Smith FB, Lowe GD, Donnan PT, Ells K. Plasma essential fatty acids, cigarette smoking, and dietary antioxidants in peripheral arterial disease. A population-based case-control study. Arterioscler Thromb. 1994;14:471–478. doi: 10.1161/01.atv.14.3.471. [DOI] [PubMed] [Google Scholar]
- Levant B, Radel JD, Carlson SE. Reduced brain DHA content after a single reproductive cycle in female rats fed a diet deficient in n-3 polyunsaturated fatty acids. Biol Psychiatry. 2006a;60:987–990. doi: 10.1016/j.biopsych.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity interact to alter maternal rat brain phospholipid fatty acid composition. J Nutr. 2006b;136:2236–2242. doi: 10.1093/jn/136.8.2236. [DOI] [PubMed] [Google Scholar]
- Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity affect liver and erythrocyte phospholipid fatty acid composition in female rats. J Nutr. 2007a;137:2425–2430. doi: 10.1093/jn/137.11.2425. [DOI] [PubMed] [Google Scholar]
- Levant B, Ozias MK, Carlson SE. Specific brain regions of female rats are differentially depleted of docosahexaenoic acid by reproductive activity and an (n-3) fatty acid-deficient diet. J Nutr. 2007b;137:130–134. doi: 10.1093/jn/137.1.130. [DOI] [PubMed] [Google Scholar]
- Lewis SJ. Risk of cardiovascular disease as a woman ages. J Reprod Med. 2007;52:147–51. [PubMed] [Google Scholar]
- McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Sullivan J, Richtand NM. Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult mice: prevention by chronic lithium treatment. J Psychiatr Res. 2008;42:458–468. doi: 10.1016/j.jpsychires.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Metcalfe LD, Schmitz AA, Pelka JR. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem. 1966;38:514–515. [Google Scholar]
- Nikkari T, Luukkainen P, Pietinen P, Puska P. Fatty acid composition of serum lipid fractions in relation to gender and quality of dietary fat. Ann Med. 1995;27:491–498. doi: 10.3109/07853899709002458. [DOI] [PubMed] [Google Scholar]
- Otto SJ, de Groot RH, Hornstra G. Increased risk of postpartum depressive symptoms is associated with slower normalization after pregnancy of the functional docosahexaenoic acid status. Prostaglandins Leukot Essent Fatty Acids. 2003;69:237–243. doi: 10.1016/s0952-3278(03)00090-5. [DOI] [PubMed] [Google Scholar]
- Otto SJ, van Houwelingen AC, Badart-Smook A, Hornstra G. Changes in the maternal essential fatty acid profile during early pregnancy and the relation of the profile to diet. Am J Clin Nutr. 2001;73:302–307. doi: 10.1093/ajcn/73.2.302. [DOI] [PubMed] [Google Scholar]
- Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- Pawlosky R, Hibbeln J, Lin Y, Salem N. n-3 fatty acid metabolism in women. Br J Nutr. 2003;90:993–994. doi: 10.1079/bjn2003985. [DOI] [PubMed] [Google Scholar]
- Pawlosky RJ, Bacher J, Salem N., Jr Ethanol consumption alters electroretinograms and depletes neural tissues of docosahexaenoic acid in rhesus monkeys: nutritional consequences of a low n-3 fatty acid diet. Alcohol Clin Exp Res. 2001;25:1758–1765. [PubMed] [Google Scholar]
- Saunders KE, Hawton K. Suicidal behaviour and the menstrual cycle. Psychol Med. 2006;36:901–12. doi: 10.1017/S0033291706007392. [DOI] [PubMed] [Google Scholar]
- Sumino H, Ichikawa S, Murakami M, Nakamura T, Kanda T, Sakamaki T, Mizunuma H, Kurabayashi M. Effects of hormone replacement therapy on circulating docosahexaenoic acid and eicosapentaenoic acid levels in postmenopausal women. Endocr J. 2003;50:51–59. doi: 10.1507/endocrj.50.51. [DOI] [PubMed] [Google Scholar]
- Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamäki H. Fish consumption, depression, and suicidality in a general population. Arch Gen Psychiatry. 2001;58:512–513. doi: 10.1001/archpsyc.58.5.512. [DOI] [PubMed] [Google Scholar]
- Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Räsänen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. J Affect Disord. 2004;82:447–452. doi: 10.1016/j.jad.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Huang Y, Chen ZY. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br J Nutr. 2005;94:544–550. doi: 10.1079/bjn20051539. [DOI] [PubMed] [Google Scholar]