Abstract
Resveratrol has been shown to protect against oxidative stress through modulating antioxidant capacity. In this study, we investigated resveratrol-mediated induction of glutathione (GSH) and glutamate cysteine ligase (GCL), and the combined effect of resveratrol and 4-hydroxynonenal (HNE) on GSH synthesis in cultured HBE1 human bronchial epithelial cells. Resveratrol increased GSH and the mRNA contents of both the catalytic (GCLC) and modulatory subunit (GCLM) of GCL. Combined HNE and resveratrol treatment increased GSH content and GCL mRNAs to a greater extent than either compound did alone. Compared to individual agent, combining exposure to HNE and resveratrol also showed more protection against cell death caused by oxidative stress. These effects of combined exposure were additive rather than synergistic. In addition, Nrf2 silencing significantly decreased the combined effect of HNE and resveratrol on GCL induction. Our data suggest that resveratrol increases GSH and GCL gene expression and that there is an additive effect on GSH synthesis between resveratrol and HNE. The results also reveal that Nrf2-EpRE signaling was involved in the combined effects.
Keywords: Resveratrol, Glutathione, Glutamate cysteine ligase, EpRE, Combination effect, Nrf2, Signal transduction, 4-Hydroxynonenal, Antioxidant, Detoxifying enzyme
Resveratrol, a polyphenolic compound (Fig. 1) found in grapes, wine, berries, and herbal medicines such as Polygonum cuspidatum (Japanese knotweed), has been shown to have various beneficial effects including protection against cardiovascular disease and cancer [1–4]. The mechanism underlying these effects is under extensive investigation and multiple biological activities exhibited by resveratrol in regulating cell cycle [5], inducing apoptosis [6], stimulating endothelial nitric oxide synthase [7], and inhibiting platelet aggregation [8] have been demonstrated. Recently the antioxidant property of resveratrol has gained intense attention and studies have demonstrated that resveratrol could increase antioxidant capacity and efficiently protect against oxidative damage [9–18].
Fig. 1.
Structure of resveratrol. Resveratrol (3,5,4′-trihydroxystilbene) is a polyphenolic phytoalexin existing as two geometric isomers: cis- and trans-form. Trans-isomer of resveratrol as shown in the figure is used in current study.
Glutathione (GSH)1 is one of the major antioxidants synthesized in cells and plays key roles in maintaining redox homeostasis, scavenging peroxides, and detoxifying xenobiotics [19,20]. Recent studies have shown that resveratrol could increase GSH levels [10,11,14,21–25], suggesting that modulating the GSH homeostasis may play a significant role in the beneficial effects exhibited by resveratrol. Nonetheless, how resveratrol increases GSH level remains elusive. Furthermore, the concentrations used in most of these studies have been much higher than would be generally achievable in vivo.
Glutamate cysteine ligase (GCL) catalyzes the formation of γ-glutamylcysteine and is the rate-limiting enzyme for de novo GSH synthesis. The gene expression of both the catalytic (GCLC) and modulatory (GCLM) subunit of GCL could be induced in response to various agents including polyphenolic compounds and oxidative stressors. Such an inducible feature makes GCL a crucial component of the cellular adaptation machinery for resistance against oxidative stress [26,27]. Studies have found that the induction of GCL is mainly through the activation of cis-elements, such as the electrophile response element (EpRE, also called antioxidant response element or ARE), NF-κB binding site, and AP-1 binding site [27–29], in the 5′-untranslated region (5′-UTR) of GCLC or GCLM gene. Particularly, EpRE signaling is mainly regulated through the activation of Nrf2 or NF-E2 related factor 2. Nrf2 remains mostly in the cytosol under resting condition through association with Kelch-like ECH-associated protein 1 (Keap1, also called iNrf2) and upon stimulation such as exposure to oxidants or electrophiles, it dissociates from Keap1 and translocates to the nucleus, where it forms heterodimers with other proteins and binds to EpRE to promote target gene transcription [30–32].
Although it has been well documented that GSH content is up regulated by resveratrol and other agents, little is known about the combined effect of them. Therefore, in this study we examined the combined effect between resveratrol and 4-hydroxy-2-nonenal (HNE), an electrophilic inducer of GSH synthesis, on the increase of GSH content and GCL induction, and explored the signaling pathways involved in GCL induction by resveratrol. Our data demonstrate that there is an additive effect between resveratrol and HNE in increasing GSH and inducing GCL. It was also found that Nrf2/EpRE signaling was involved in the GCL induction by resveratrol and in the additive effect of resveratrol and HNE on inducing the GCL mRNA expression.
Methods and materials
Chemicals and reagents
Unless otherwise noted, all chemicals were from Sigma (St. Louis, MO). Antibodies and small interfering RNAs were from Santa Cruz (Santa Cruz, CA). TRIzol Reagent was from Life Technologies (Grand Island, NY). DNA-free reagent was from Ambion (Austin, TX). TaqMan Reverse Transcription Reagent and SYBR Green PCR Master Mix were from Applied Biosystems (Foster City, CA). FuGENE 6 transfection reagent was from Roche (Indianapolis, IN). All chemicals used were at least analytical grade.
Cell culture and treatment
A human bronchial epithelial cell line (HBE1 cell) was cultured in collagen-coated dishes in 5% CO2 at 37 °C as described by Harper et al. [33]. The concentration of oxygen was 20%, which is close to the concentration to which bronchial epithelial cells would be exposed in vivo. Cells were treated when 90% confluent. Resveratrol was freshly made with ethanol and the final concentration of ethanol in medium is 0.05%.
Measurement of GSH content
Total GSH (GSH+GSSG) was measured using a spectrophotometric assay [34]. Briefly, cells lysate in 0.1% Triton X-100/0.1 M sodium phosphate buffer was deproteinized with 5-sulfosalicylic acid. After centrifuge, the supernatant was added to a solution containing 10 µM diethylenetriaminepentaacetic acid (DETAPAC), 1.5 U/ml glutathione reductase, 0.2 mM NADPH, and 0.6 mM DTNB. The reaction rate was monitored at 412 nm and then converted to GSH concentration using a standard curve.
Measurement of mRNA content
The content of GCLC and GCLM mRNAs was determined with real-time RT-PCR using the protocol described before [35]. Briefly cells were treated at 90% confluence and RNA was extracted with Trizol reagent. After reverse transcription, real-time PCR was performed on a Cepheid SmartCycler Real-time PCR machine (Cepheid, Sunnyvale, CA). The result was normalized with GAPDH.
Nrf2 silencing
Cells at 70–80% confluence were transfected with 50 nM Nrf2 siRNA or control siRNA (non-targeting 20–25 nt siRNA designed as negative control) for 24 h before being treated with/without resveratrol for another 12 h.
Cytotoxicity assay (MTT assay)
The MTT assay was performed following protocol provided by the manufacturer. Cells at 70–80% confluence were pretreated with various concentrations of resveratrol for 24 and then exposed to DMNQ for 2 h. MTT was added into the medium and incubated with cells for 2 h. The MTT absorbance was read at wavelength of 570 nm. The survival rate of cells was reported as the percentage of living cells compared with vehicle control.
Statistical analysis
A comparative ΔΔCT method was used for the relative mRNA quantification as described before [36]. All data were expressed as means ± standard error. Sigma Stat software was used for statistical analysis and statistical significance was accepted when P < 0.05. The one-way ANOVA and Tukey test were used for comparison of mRNA level.
Results
Resveratrol increases GSH content in HBE1 cells
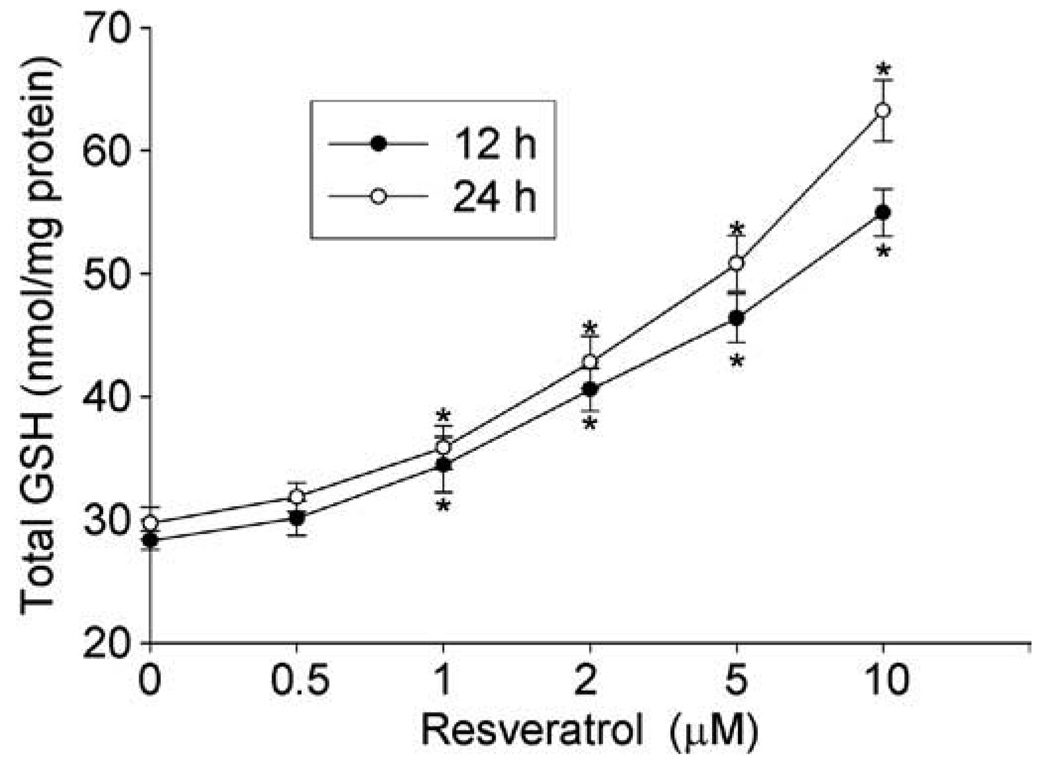
To confirm the GSH increasing effect of resveratrol, HBE1 cells were treated with different concentrations of resveratrol and the total GSH in cells and medium was measured. As shown in Fig. 2, the total GSH content in the cells was increased by resveratrol in a time- and concentration- dependent manner. At concentration of as low as 2 µM, resveratrol significantly increased the GSH content by 1.4-fold (28.33 ± 0.95 vs. 40.59 ± 1.95 nmol/mg protein) after 12 h and the effect was even more remarkable after 24 h of exposure (1.6-fold). The GSH level in the cell medium was also increased by resveratrol with a similar pattern as that of cellular GSH (data not shown). Although 20 µM of resveratrol produced the maximal effect on GSH content, it inhibited the cell growth (by 40%, data not shown). Therefore in the following experiments, less than 5 µM of resveratrol, which showed no adverse effect on cell growth, was used.
Fig. 2.
Resveratrol increased GSH content. HBE1 cells were treated with 0, 0.5, 1, 2, 5, and 10 µM resveratrol for different time as indicated and the total cellular GSH was determined. N = 3, *P < 0.05 compared with control.
Resveratrol increases GCL transcription
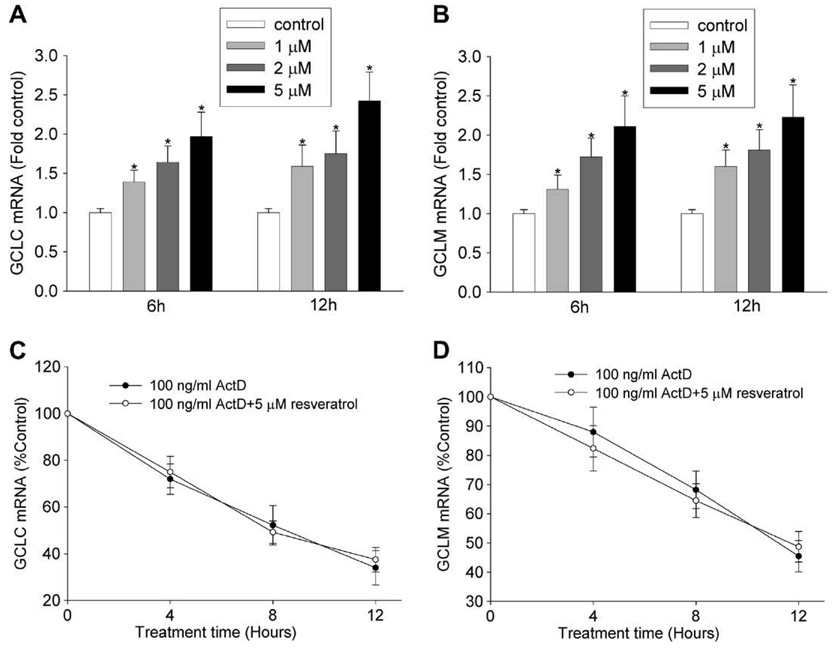
The change of GSH content is largely controlled through the expression level of GCL, the rate-limiting enzyme for de novo GSH synthesis. To test the hypothesis that resveratrol increased the expression of GCL gene, we determined its effects on the mRNA levels of GCLC and GCLM. Fig. 3A and B shows that the mRNA contents of both GCLC and GCLM were increased by resveratrol in a concentration- and time-dependent manner. With pretreatment of 100 ng/ml actinomycin D (an inhibitor of transcription) for 1 h, the induction of GCLC or GCLM by resveratrol was blocked. Furthermore, the rate of decrease of both GCLC and GCLM mRNAs was the same in the presence or absence of resveratrol (Fig. 3C and D), suggesting that resveratrol does not act by increasing mRNA stability. These data together support the conclusion that resveratrol increases GCL mRNA expression at the transcription level.
Fig. 3.
Resveratrol increased GCL mRNAs at transcription level. (A and B) Resveratrol increased the mRNA contents of GCLC (A) and GCLM (B). HBE1 cells were treated with different concentrations of resveratrol for indicated time and the mRNA levels of GCLC and GCLM were determined using RT-real-time PCR assay. N = 3, * P < 0.05 compared with vehicle control. (C and D) The effect of resveratrol on the decay rate of the mRNAs of GCLC (C) and GCLM (D). HBE1 cells were pretreated with 100 ng/ml actinomycin D (ActD) for 1 h and then treated with vehicle control (ethanol) or 5 µM resveratrol for indicated time. The mRNA levels of GCLC and GCLM were determined. The value is shown as percentage of control or the experiment starting point (0 h), N = 3.
Combined effect of resveratrol and HNE on GSH synthesis
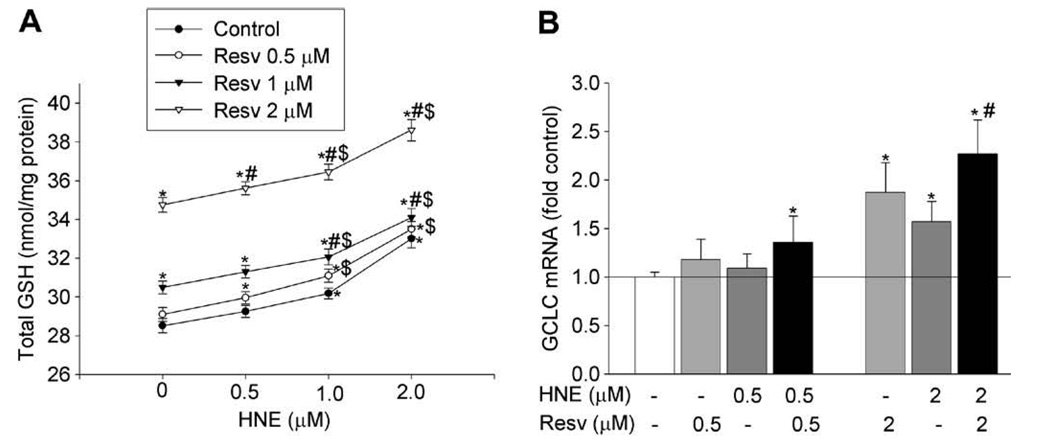
Many agents have been shown to increase GSH synthesis, but little is known about the effect of using them in combination. Here we examined the combined effect of resveratrol and HNE, a lipid peroxidation product, on GSH content and GCLC mRNA expression. As expected, exposure to HNE or resveratrol for 24 h concentration- dependently increased GSH content (Fig. 4A). When cells were sequentially exposed to HNE for 1 h and then resveratrol for 24 h, the increase in GSH content was larger compared to that caused by individual agent at the same concentration. At concentrations of 0.5 and 2 µM, HNE increased GSH by 2.6% (NS) and 15.7% (P < 0.05), and resveratrol increased GSH by 2% (NS) and 21.8% (P < 0.05), respectively, while sequential exposure to both agents increased GSH by 5% (P < 0.05) (0.5 µM HNE and 0.5 µM resveratrol) and 35.4% (P < 0.05) (2 µM HNE and 2 µM resveratrol).
Fig. 4.
Combined effects of HNE and resveratrol on increasing GSH synthesis. (A) Combined effect on the GSH content. Cells were sequentially exposed to HNE for 1 h and then to resveratrol for 24 h, the cellular GSH level was measured. (B) Combined effect on GCLC mRNA expression. Cells were pretreated with HNE for 1 h and then treated with/without resveratrol for 12 h. RNA was extracted and the GCLC mRNA was determined. N = 3, *P < 0.05 compared with vehicle control; #P < 0.05 compared with the same concentration of HNE; $P < 0.05 compared with the same concentration of resveratrol.
Consistently, in comparison to the effects of individual agents, sequential exposure to HNE and resveratrol also had an enhancing effect on GCLC mRNA (Fig. 4B). HNE (0.5 µM) and 0.5 µM resveratrol alone increased GCLC mRNA by 18% and 6%, respectively, while the sequential exposure to HNE (1 h) and then resveratrol for 24 h increased GCLC mRNA by 36%. At the concentration of 2 µM, HNE and resveratrol alone increased GCLC mRNA by 87% and 57%, respectively, and the sequential exposure of both increased GCLC mRNA by 127%. The combined effect of HNE and resveratrol on GCLM mRNA follows the same pattern as that of GCLC (data not shown). This data further support the above finding that there is a combined effect of HNE and resveratrol on increasing GSH synthesis.
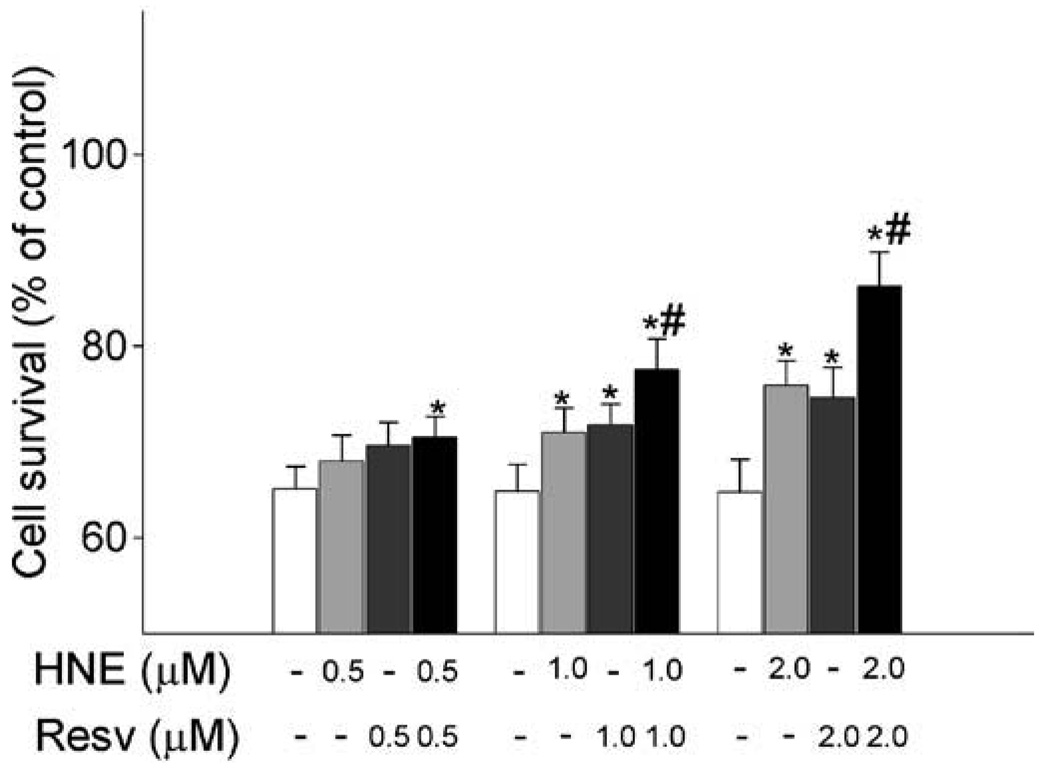
Combined effect of HNE and resveratrol on diminishing cell death caused by DMNQ
After observing that HNE and resveratrol had an additive effect on increasing GSH synthesis, we examined whether these two agents also had a combined effect on increasing cellular resistance against oxidative toxicity. To achieve this, we determined the protection of HNE and resveratrol against cell death caused by 2,3-dimethoxy-1,4-naphthoquinone (DMNQ), a compound that causes toxicity via generating reactive oxygen species. With the MTT assay, it was shown that exposure to 20 µM DMNQ for 2 h significantly caused cell death (Fig. 5). Pretreatment with either resveratrol or HNE alone for 24 h reduced cell death due to DMNQ exposure. Compared to the effects of individual agent, cell survival was further increased when cells were sequentially exposed to HNE for 1 h and then resveratrol for 24 h, demonstrating that combined exposure to both agents enhanced the protection of cells from DMNQ toxicity compared to that produced by the individual agents.
Fig. 5.
Combined exposure of HNE and resverarol exhibits enhancing protective effect against cell death. Cells were sequentially treated with HNE for 1 h and resveratrol for 24 h, then cells were exposed to DMNQ for 2 h and finally the MTT assay was performed to determine cell survival. N = 3, *P < 0.05 compared with vehicle control; #P < 0.05 compared with the same concentration of HNE or resveratrol.
Nrf2/EpRE is involved in resveratrol-mediated induction of GCL genes
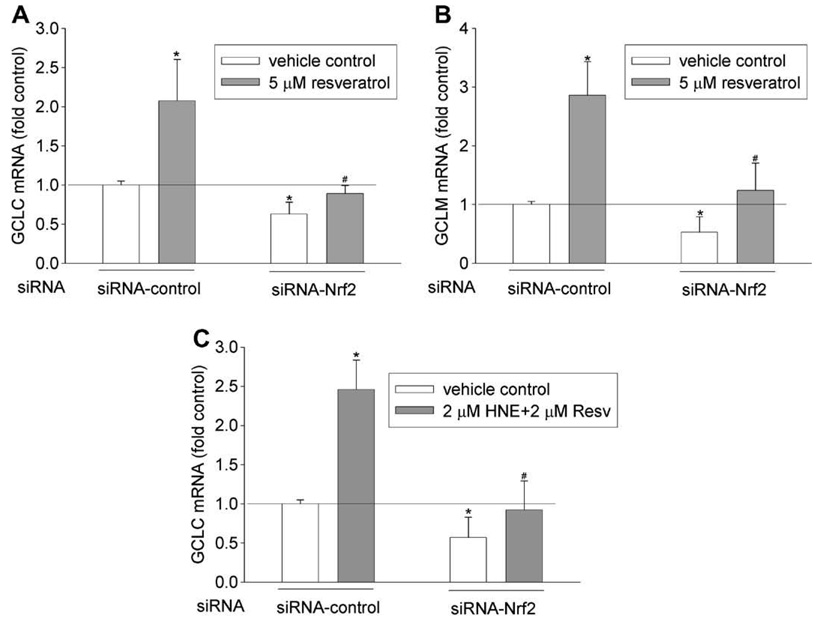
Several cis-elements, including EpRE, TRE, and NF-κB [37] have been reportedly involved in regulating GCL gene expression. To examine the involvement of EpRE-Nrf2 signaling in GCL mRNA induction by resveratrol, Nrf2 siRNA was transfected into HBE1 cells at a concentration (50 nM) that was previously shown to down regulate Nrf2 protein and its nuclear translocation [38]. Nrf2 siRNA treatment attenuated the induction of both GCLC and GCLM mRNAs by resveratrol (Fig. 6 A and B). In addition as shown in Fig. 6 C, Nrf2 siRNA significantly attenuated the induction of GCLC mRNA caused by combined exposure to HNE (2 µM) and resveratrol (2 µM). These data indicate that Nrf2-EpRE signaling is likely involved in resveratrol-mediated GCL mRNA induction and in its combined effect with HNE treatment on GCLC mRNA expression.
Fig. 6.
Effect of Nrf2 silencing on GCL induction. Nrf2 silencing reduced resveratrol-mediated mRNA induction of GCLC (A) and GCLM (B), and the combined effect of HNE and resveratrol on GCLC mRNA induction (C). HBE1 cells were transfected with 50 nM of siRNA-Nrf2 or siRNA-control. Twenty-four hours after the transfection, cells were treated with 5 µM of resveratrol or sequentially with 2 µM of HNE and 2 µM of resveratrol as described above for 12 h. N = 3, *P < 0.05 compared with vehicle control of siRNA- control; #P < 0.05 compared with resveratrol treatment of siRNA-control.
Discussion
Since the identification of resveratrol in red wine in 1992, there has been an intense interest in exploring its biological activities and potential beneficial health effects. In the current study we observed that resveratrol increased GSH content (Fig. 2) and GCL mRNA (Fig. 3) in human HBE1 bronchial epithelial cells at a concentration equivalent to that could be attained in plasma through reasonable dietary consumption [39]. These findings appear to be in agreement with previous reports that resveratrol could increase GSH synthesis; though most of those were performed in animal models and/or using much higher concentrations (>10 µM) [39–41]. GSH plays critical roles in the maintenance of redox homeostasis and the protection against oxidative damage, which is implicated in such diseases as cardiovascular disease, inflammation, and cancer [42]. Therefore, the increase of GSH synthesis by resveratrol at concentrations achievable in vivo further confirms its antioxidant property and suggests that the beneficial health effects of resveratrol is ascribed partly to the GSH increase.
The de novo GSH synthesis can be up regulated by a variety of chemicals including oxidants, electrophiles, and polyphenols. In most studies, higher concentrations of inducers than usually attainable in vivo are used and this raises the concern about the physiological significance of those findings. In the current study we demonstrated that there was an additive effect between HNE and resveratrol on increasing GSH level and GCL mRNA expression (Fig. 4). For example, at 2 µM, HNE and resveratrol alone increased GSH content by 15.7% and 21.8%, respectively, and combined exposure of both agents increased GSH by 35.4%. For GCLC mRNA, the individual effect of 2 µM HNE or 2 µM resveratrol was an increase of 57% and 87%, respectively, while the combined effect was a 127% increase. HNE is an inducer of GSH synthesis and is reportedly present in plasma at concentrations of less than 1 µM (0.3–0.7 µM) under physiological conditions [43,44]. Our results reveal that resveratrol or other dietary GCL inducers could act additively with HNE to increase GSH synthesis in vivo. Furthermore, these data suggest that the effective concentration of individual agent to induce GSH synthesis could be lowered to attainable level in vivo if used in combination with other inducers.
Increasing antioxidant/detoxification capacity such as GSH synthesis is a critical adaptive mechanism in resistance to oxidative stress. Consistent with the reports that resveratrol increased resistance to oxidative damage [9–18], we showed that resveratrol protected cell death caused by DMNQ, a redox cycling compound that exhibits toxicity through generating reactive oxygen species but cannot form adducts with GSH or proteins as do other quinones [45]. More significantly, the combined exposure to HNE and resveratrol produced enhanced protection against cell death (Fig. 5). It should be noted that the induction of protective machinery is a coordinate process and in addition to GSH and GCL, other detoxifying enzymes and/or antioxidants may also contribute to the protective effect of resveratrol and HNE. Nonetheless, the current finding further confirms the above conclusion that HNE and resveratrol have a combined effect on increasing antioxidant capacity.
Nrf2 is the primary transcription factor binds to EpRE and transactivates the expression of GCL genes upon the exposure to many stimuli. We showed that Nrf2-EpRE signaling was involved in the induction of GCL by resveratrol as silencing Nrf2 decreased the resveratrol-mediated increase in both GCLC and GCLM mRNAs significantly (Fig. 6). This result is consistent with reports that resveratrol increased nuclear Nrf2 and the expression of Nrf2/EpRE-regulating genes such as NQO-1 [46] and HO-1 [47]. It was however, noticed that while Nrf2 silencing decreased induction of GCL mRNAs by resveratrol significantly, it did not eliminate it. This was likely due to the residual Nrf2 protein (about 20% of total Nrf2 protein) [38]. We previously reported that Nrf2-EpRE signaling was also involved in HNE-mediated induction of both GCLC and GCLM genes [38]. With the current finding that Nrf2 siRNA decreased the combined effect of HNE and resveratrol on increasing GCLC mRNA (Fig. 6C), it is reasonable to infer that the additive effect of HNE and resveratrol on increasing GSH synthesis occurred at the level of Nrf2 activation as Nrf2 activation caused by the individual agents was additive. This, however, does not exclude the possible involvement of other transcription factors in the additive effect. For example, c-Jun, a main Nrf2 partner, was activated by both resveratrol and HNE [48–50] and its activation was associated with HNE-mediated GCL induction [50]. Therefore the partners of Nrf2 could also be targets of HNE and resveratrol for their additive effect. Most known Nrf2 activators have electrophilic or H2O2 generating chemistry that is thought to act through modification of the critical thiols of Keap1, which until being modified mediates the ubiquination and degradation of Nrf2 [30–32]. There are reports that resveratrol may exhibit prooxidant properties and increase the reactive oxygen species in cells [51,52]. Whether resveratrol activates Nrf2 by this mechanism, however, remains to be determined.
In summary, this study demonstrated that resveratrol was a potential inducer of GSH synthesis and that Nrf2-EpRE signaling is involved in the induction of both GCLC and GCLM genes by resveratrol. The most significant finding here however, was the combined additive effect of HNE and resveratrol in increasing GSH synthesis. At concentrations (0.5 µM) achievable by diet for resveratrol [39] and endogenously for HNE [43,44], at which a single addition of either agent alone failed to increase GSH synthesis, HNE and resveratrol together achieved a significant effect. These findings suggest that various GCL inducers may act together in vivo to regulate GSH synthesis and that the mixing of different inducers could be a promising strategy to increase the antioxidant and detoxification capacity. Indeed, this may be similar to the far more effective protection of a balanced consumption of fruits and vegetables compared with pure antioxidants.
Acknowledgments
This work was supported by Grant ES05511 from National Institutes of Health and Grant 14RT-0059 from the California Tobacco Related Diseases Research Program.
Footnotes
Abbreviations used: GSH, glutathione; GCL, glutamate cysteine ligase; GCLC, catalytic subunit ouf GCL; GCLM, modulatory subunit of GCL; EpRE, electrophile response element; 5′-UTR, 5′-untranslated region; Keap1, Kelch-like ECH-associated protein 1; HNE, 4-hydroxy-2-nonenal; HBE1 cell, human bronchial epithelial line; DMNQ, 2,3-dimethoxy-1,4-naphthoquinone.
References
- 1.Baur JA, Sinclair DA. Nat. Rev. Drug. Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 2.Das DK, Maulik N. Mol. Interv. 2006;6:36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Curr. Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 4.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Toxicol. Appl. Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh TC, Wu JM. Exp. Cell Res. 1999;249:109–115. doi: 10.1006/excr.1999.4471. [DOI] [PubMed] [Google Scholar]
- 6.Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S. Blood. 1998;92:996–1002. [PubMed] [Google Scholar]
- 7.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 8.Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. Clin. Chim. Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 9.Gupta YK, Briyal S, Chaudhary G. Pharmacol. Biochem. Behav. 2002;71:245–249. doi: 10.1016/s0091-3057(01)00663-3. [DOI] [PubMed] [Google Scholar]
- 10.Sharma M, Gupta YK. Life Sci. 2002;71:2489–2498. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- 11.Uguralp S, Mizrak B, Bay Karabulut A. Eur. J. Pediatr. Surg. 2005;15:114–119. doi: 10.1055/s-2004-830359. [DOI] [PubMed] [Google Scholar]
- 12.Olas B, Nowak P, Wachowicz B. Cell. Mol. Biol. Lett. 2004;9:577–587. [PubMed] [Google Scholar]
- 13.Olas B, Wachowicz B, Majsterek I, Blasiak J. Anti-Cancer Drugs. 2005;16:659–665. doi: 10.1097/00001813-200507000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Brito PM, Mariano A, Almeida LM, Dinis TC. Chem. Biol. Interact. 2006;164:157–166. doi: 10.1016/j.cbi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Cetin R, Devrim E, Kilicoglu B, Avci A, Candir O, Durak I. J. Appl. Toxicol. 2006;26:42–46. doi: 10.1002/jat.1103. [DOI] [PubMed] [Google Scholar]
- 16.Eybl V, Kotyzova D, Koutensky J. Toxicology. 2006;225:150–156. doi: 10.1016/j.tox.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Sener G, Topaloglu N, Ozer Sehirli A, Ercan F, Gedik N. Pulm. Pharmacol. Ther. 2007;20:642–649. doi: 10.1016/j.pupt.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, El May M, Gharbi N, Kamoun A, El-Fazaa S. Life Sci. 2007;80:1033–1039. doi: 10.1016/j.lfs.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 19.Meister A. J. Nutr. Sci. Vitaminol. Spec. No. 1992:1–6. doi: 10.3177/jnsv.38.special_1. [DOI] [PubMed] [Google Scholar]
- 20.Hayes JD, Flanagan JU, Jowsey IR. Ann. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 21.Savaskan E, Olivieri G, Meier F, Seifritz E, Wirz-Justice A, Muller-Spahn F. Gerontology. 2003;49:380–383. doi: 10.1159/000073766. [DOI] [PubMed] [Google Scholar]
- 22.Yen GC, Duh PD, Lin CW. Free Radical Res. 2003;37:509–514. doi: 10.1080/1071576031000083099. [DOI] [PubMed] [Google Scholar]
- 23.Ara C, Karabulut AB, Kirimlioglu H, Coban S, Ugras M, Kirimliglu V, Yilmaz S. Renal Failure. 2005;27:435–440. [PubMed] [Google Scholar]
- 24.Li Y, Cao Z, Zhu H. Pharmacol. Res. 2006;53:6–15. doi: 10.1016/j.phrs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Vieira de Almeida LM, Pineiro CC, Leite MC, Brolese G, Tramontina F, Feoli AM, Gottfried C, Goncalves CA. Cell. Mol. Neurobiol. 2007;27:661–668. doi: 10.1007/s10571-007-9152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickinson DA, Levonen AL, Moellering DR, Arnold EK, Zhang H, Darley-Usmar VM, Forman HJ. Free Radic. Biol. Med. 2004;37:1152–1159. doi: 10.1016/j.freeradbiomed.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Rahman I. Biochem. Pharmacol. 2000;60:1041–1049. doi: 10.1016/s0006-2952(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 28.Giovinazzo G, D’Amico L, Paradiso A, Bollini R, Sparvoli F, DeGara L. Plant Biotechnol. J. 2005;3:57–69. doi: 10.1111/j.1467-7652.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- 29.Andrade AC, Del Sorbo G, Van Nistelrooy JG, Waard MA. Microbiology (Reading, England) 2000;146(Pt 8):1987–1997. doi: 10.1099/00221287-146-8-1987. [DOI] [PubMed] [Google Scholar]
- 30.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zipper LM, Mulcahy RT. Biochem. Biophys. Res. Commun. 2000;278:484–492. doi: 10.1006/bbrc.2000.3830. [DOI] [PubMed] [Google Scholar]
- 32.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harper R, Wu K, Chang MM, Yoneda K, Pan R, Reddy SP, Wu R. Am. J. Respir. Cell. Mol. Biol. 2001;25:178–185. doi: 10.1165/ajrcmb.25.2.4471. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe N, Dickinson DA, Liu RM, Forman HJ. Methods Enzymol. 2004;378:319–340. doi: 10.1016/S0076-6879(04)78024-6. [DOI] [PubMed] [Google Scholar]
- 35.Dickinson DA, Iles KE, Wigley AF, Forman HJ. Methods Enzymol. 2004;378:302–318. doi: 10.1016/S0076-6879(04)78023-4. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Liu H, Iles KE, Liu RM, Postlethwait EM, Laperche Y, Forman HJ. Am. J. Respir. Cell. Mol. Biol. 2005;38:463–468. doi: 10.1165/rcmb.2005-0280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wild AC, Mulcahy RT. Free Radic. Res. 2000;32:281–301. doi: 10.1080/10715760000300291. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Court N, Forman HJ. Redox Rep. 2007;12:101–106. doi: 10.1179/135100007X162266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. Drug Metab. Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 40.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Am. J. Physiol. Lung Cell Mol. Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 41.Sekhar KR, Spitz DR, Harris S, Nguyen TT, Meredith MJ, Holt JT, Gius D, Marnett LJ, Summar ML, Freeman ML. Free Radic. Biol. Med. 2002;32:650–662. doi: 10.1016/s0891-5849(02)00755-4. [DOI] [PubMed] [Google Scholar]
- 42.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 43.Selley ML, Bartlett MR, McGuiness JA, Hapel AJ, Ardlie NG. J. Chromatogr. 1989;488:329–340. doi: 10.1016/s0378-4347(00)82957-6. [DOI] [PubMed] [Google Scholar]
- 44.Strohmaier H, Hinghofer-Szalkay H, Schaur RJ. J. Lipid Mediat. Cell Signal. 1995;11:51–61. doi: 10.1016/0929-7855(94)00027-a. [DOI] [PubMed] [Google Scholar]
- 45.Gant TW, Rao DN, Mason RP, Cohen GM. Chem. Biol. Interact. 1988;65:157–173. doi: 10.1016/0009-2797(88)90052-x. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh TC, Lu X, Wang Z, Wu JM. Med. Chem. (Shariqah, United Arab Emirates) 2006;2:275–285. doi: 10.2174/157340606776930709. [DOI] [PubMed] [Google Scholar]
- 47.Chen CY, Jang JH, Li MH, Surh YJ. Biochem. Biophys. Res. Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 48.Kundu JK, Surh YJ. Mutat. Res. 2004;555:65–80. doi: 10.1016/j.mrfmmm.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 49.Jeong WS, Kim IW, Hu R, Kong AN. Pharm. Res. 2004;21:649–660. doi: 10.1023/b:pham.0000022412.69380.d7. [DOI] [PubMed] [Google Scholar]
- 50.Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. Free Radic. Biol. Med. 2002;33:974–979. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- 51.Ahmad KA, Clement MV, Pervaiz S. Ann. NY Acad. Sci. 2003;1010:365–373. doi: 10.1196/annals.1299.067. [DOI] [PubMed] [Google Scholar]
- 52.Azmi AS, Bhat SH, Hanif S, Hadi SM. FEBS Lett. 2006;580:533–538. doi: 10.1016/j.febslet.2005.12.059. [DOI] [PubMed] [Google Scholar]