Abstract
Proline, a unique proteogenic secondary amino acid, has its own metabolic system with special features. Recent findings defining the regulation of this system led us to propose that proline is a stress substrate in the microenvironment of inflammation and tumorigenesis. The criteria for proline as a stress substrate are: 1) the enzymes utilizing proline respond to stress signaling, 2) there is a large, mobilizable pool of proline and 3) the metabolism of proline serves special stress functions. Studies show that the proline utilizing enzyme, proline oxidase/proline dehydrogenase responds to genotoxic, inflammatory and nutrient stress. Proline as substrate is stored as collagen in extracellular matrix, connective tissue and bone, and it is rapidly released from this reservoir by the sequential action of matrix metalloproteinases, peptidases and prolidase. Special functions include the use of proline by proline oxidase/proline dehydrogenase to generate superoxide radicals which initiate apoptosis by intrinsic and extrinsic pathways. Under conditions of nutrient stress, proline is an energy source. It provides carbons for the tricarboxylic acid cycle and, also participates in the proline cycle. The latter, catalyzed by mitochondrial proline oxidase and cytosolic pyrroline-5-carboxylate reductase, shuttles reducing potential from the pentose phosphate pathway into mitochondria to generate ATP and oxidizing potential to activate the cytosolic pentose phosphate pathway.
Keywords: proline oxidase, proline dehydrogenase, PPARγ, mTOR, apoptosis, bioenergetics
Introduction
The metabolic pathways for proline were first characterized by Elijah Adams and Harold Strecker beginning in the mid-1950s (1–3). Early on, it was recognized that proline was an unusual amino acid, being the only proteogenic secondary amino acid (1, 3). With its alpha nitrogen contained within a pyrrolidine ring, proline is not substrate for the usual amino acid metabolizing enzymes, the decarboxylases, aminotransferases and racemases. Instead, a family of proline-metabolic enzymes evolved with their own tissue and subcellular localization and mechanisms of regulation. Because proline metabolism is distinct from that of primary amino acids, it can play a regulatory role or, alternatively, its metabolism can be reserved for special physiologic or pathophysiologic situations (2). The understanding of these special functions and their mechanisms has made significant advances during the last decade.
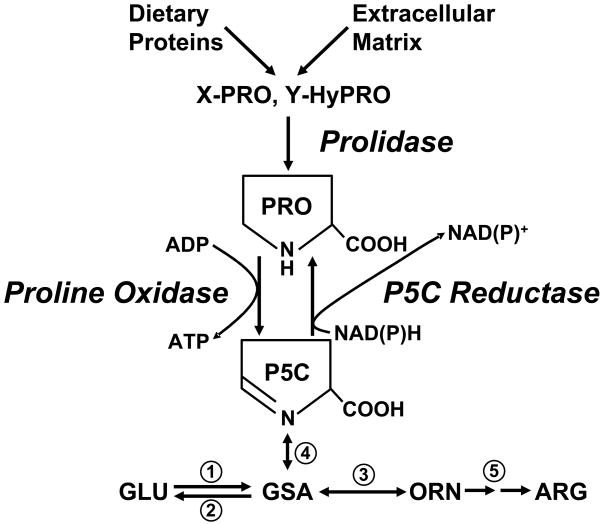
As shown in Figure 1, the oxidized congener of proline, Δ1-pyrroline-5-carboxylate (P5C), which is in tautomeric equilibrium with glutamic-γ-semialdehyde, is in a strategic location in intermediary metabolism. Lying between glutamate and ornithine, P5C serves as an obligate carbon bridge between the two major metabolic cycles, the tricarboxylic acid cycle and the urea cycle (2). Proline can be derived from dietary proteins and from the degradation of endogenous proteins, but the final release of proline requires a specific dipeptidase, prolidase, which can hydrolyze the peptide bond constrained within the pyrrolidine ring. Proline can be biosynthesized from glutamate and ornithine (see Watford M, Metabolism of Glutamine and Proline, this supplement) making it nutritionally nonessential, but as has been previously mentioned (2), the selective preservation of biosynthetic pathways may relate to endpoints more important than simple supply of that amino acid as substrate for protein synthesis (2). Recognizing that P5C is not only the committed precursor of proline, but also its immediate degradative product, we considered that this relationship may be metabolically important. In fact, we showed that these interconversions constitute a catalytic cycle transferring reducing potential into mitochondria, and the cycling of proline-P5C participates in a metabolic interlock with the pentose phosphate pathway (2).
Figure 1.
Proline metabolic pathway. Abbreviations: PRO, proline; P5C, pyrroline-5-carboxylate; X-PRO, imidodipeptide with proline as carboxyl terminus; Y-HyPro, imidodipeptide with hydroxyproline as carboxyl terminus; GLU, glutamate; GSA, glutamic-γ-semialdehyde; ORN, ornithine; ARG, arginine. Numbers designate unlabeled enzymes: 1, P5C synthase; 2, P5C dehydrogenase; 3, ornithine aminotransferase; 4, spontaneous reaction; 5, urea cycle enzymes.
The Proline Cycle
The central enzyme in the proline cycle is proline oxidase (POX) a.k.a. proline dehydrogenase (PRODH). Confusion has arisen in the nomenclature due to the history of the molecular discoveries. Recently, a consensus was reached by a number of laboratories (2nd International Symposium on Proline Metabolism in Health and Disease, September 10–11, 2007, NCI-Frederick, Frederick, Maryland, see Amino Acids, August 2008) that the first enzyme of the proline degradative pathway would be designated proline oxidase or proline dehydrogenase (POX/PRODH). The gene, however, would be referred to as PRODH. The first enzyme of the hydroxyproline degradative pathway, encoded by a distinct gene on a different chromosome and without activity crossover with POX/PRODH, has been the basis for the confusion. It was decided that this hydroxyproline-metabolizing enzyme would be designated as hydroxyproline oxidase (OH-POX) or hydroxyproline dehydrogenase (OH-PRODH) and its gene would be designated PRODH2.
POX/PRODH is tightly bound to mitochondrial inner membranes (Figure 2). The gene will be designated here as PRODH according to the current nomenclature. The catalytic mechanism involves the transfer of electrons from substrate proline to flavine adenine dinucleotide (FAD) with cytochrome c as the subsequent carrier into the electron transport chain. Thus, proline is a direct substrate for the generation of ATP (4, 5). Recently, it was shown that POX/PRODH can reduce oxygen and generate superoxide (6, 7). Although leakage of electrons from an unspecified site in the electron transport chain may be the source of mitochondrial superoxide, recent work by White et al. showed that the FAD of POX has direct access to solvent oxygen, and proline-derived electrons can directly reduce oxygen to produce superoxide autogenously (8). Thus, the trivial name, proline oxidase, is a propos for this enzyme, at least in terms of the monofunctional catalytic mechanism.
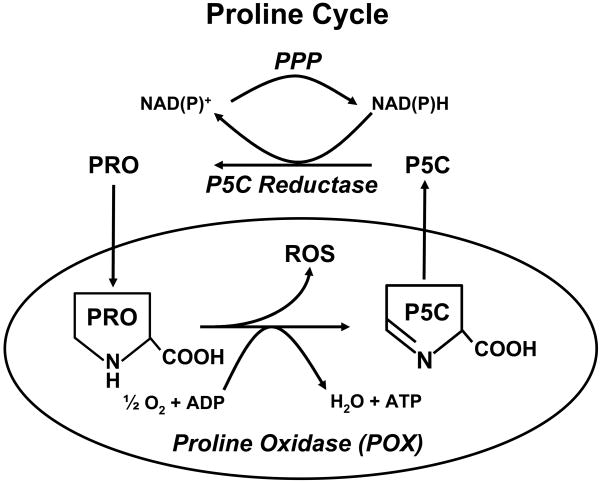
Figure 2.
Proline cycle (Redox shuttle). Abbreviations are as shown in Figure 1. ROS, reactive oxygen species; PPP, Pentose phosphate pathway.
When the proline cycle was proposed as a metabolic interlock to shuttle NADPH reducing equivalents into mitochondria in the form of proline, thereby converting the pentose phosphate pathway into an ATP-generating metabolic pathway (4, 9, 10), critics disparaged the contribution to bioenergetics as quantitatively trivial compared to that of the TCA cycle or even the glycolytic pathway. Recent emphasis on metabolism in cancer (11–12) emphasizes Otto Warburg’s discovery that oxidative phosphorylation and the TCA cycle are dysfunctional in tumors (13). Thus, proline as a substrate may very well have its metabolic niche in certain disease states.
Proline as Nutrient in the Diet or for Interorgan Transfers
As nutrients, proline and hydroxyproline are not especially abundant in the human diet. For a vegetarian diet, the intake of proline and hydroxyproline is even lower. Convincing correlation between dietary intake of proline and hydroxyproline and disease risk is lacking with the exception that populations with a high intake of red meat (presumably the intake of proline and hydroxyproline was high (14), but it was not quantified) may have a slightly increased risk for breast cancer (15). Overall, dietary perturbation of proline and hydroxyproline has no major effects. However, the interaction of dietary proline and hydroxyproline with pharmacologic intervention remains an intriguing possibility. A case in point is the use of inhibitors of matrix metalloproteinases (MMPs) as an antitumor agent. Based on the activation of MMPs by a variety of tumors and the participation of MMPs in invasion and metastases, drugs were designed to inhibit these enzymes. Although the preclinical studies were very successful in retarding tumor growth and metastasis (16), the clinical trials were disappointing (17). The marked differences between animal diets for these studies, i.e. formulated diets (16) containing no animal products (thus little hydroxyproline), versus the ad lib diets during the clinical trials may be a factor. This difference will be discussed in a subsequent section.
Proline/hydroxyproline does not participate significantly in interorgan transfers of substrates. The main paradigm of circulating substrates involves alanine (18) and glutamine (19). Alanine participates in gluconeogenesis and glutamine in the transfer of carbons from muscle to liver and kidney in the postabsorptive state. The latter plays an important role in the physiologic regulation of acid-base balance and the delivery of nitrogen to the kidney to be excreted as ammonium chloride (20).
An “Out of the Box” Consideration of Nutrients
But with newer concepts of metabolism in physiologic and pathophysiologic states, nutritional metabolism must consider more than dietary sources and circulating interorgan transfers. Clinical therapeutic approaches are also relevant. For example, post surgical parenteral nutrition includes not only energy substrates, but also a complement of amino acids, glutamine, vitamins and lipids (21). Parenteral nutrition allows delivery of nutrients to tissue sites without GI absorption and portal transport, the route for dietary nutrients.
Additionally, current technology allows for selective delivery by catheterization or percutaneous delivery of various agents (22). The developing field of nanotechnology makes possible delivery of specific agents in nanoparticles targeted by surface receptors to designated sites (23). Although these methods remain experimental at present and are focused on drug rather than nutrient delivery, they are mentioned here because previously unrecognized metabolic roles for certain nutrients may stimulate innovation to develop novel approaches for local nutritional supplementation.
In disease states, the microenvironment has received considerable attention (24) And may be the critical arena in which nutrients must be considered. When a disease process includes isolation of tissues from their blood supply, whether it is the hypoxemic myocardium, inflammatory nodule, hemorrhagic cerebral tissue or malignant tumor, the nutrition within that microenvironment may be critically important in producing tissue damage, in promoting healing, or in the case of malignant tumors, in expanding invasion and metastasis (25). In this setting, proline as a substrate in the diseased microenvironment has not been appreciated, and this role of proline is the subject of this review. Since the author’s own research has been in the context of cancer, proline will be considered as a substrate primarily in the context of carcinogenesis. However, the contributions of proline as substrate may be generalized to other disease areas.
Criteria for Microenvironmental Stress Substrates
The advances over the last 6–7 years have persuaded us that proline is a microenviron-mental stress substrate. Although the magnitude of the bioenergy generated from proline is much less than that from the TCA cycle or even from glycolysis, the availability of energy from proline may be critical for survival when other substrates, e.g. glucose and glutamine, are unavailable. In a situation where stress is overwhelming, the bioenergetics of proline can be used for programmed cell death. However, for the proline metabolic system to be considered as a special stress responder, the evidence must be persuasive in satisfying the following criteria: 1) the metabolic system for the substrate must respond to stress signals with specific mechanisms for its upregulation. 2) There is a mobilizable reservoir of the substrate. 3) The metabolism of the substrate serves special cellular functions.
Responses of the proline metabolic system to stress
Recent advances in proline metabolism were given impetus by the serendipitous finding by Polyak and co-workers that PRODH, the gene encoding POX/PRODH, is a p53-induced gene (26). Working in Dr. Bert Vogelstein’s lab, they used serial analysis of gene expression (SAGE) to screen the gene targets of p53, a critical cancer suppressor gene. Of 7202 genes monitored, PRODH was one of 14 genes highly induced (> 7-fold) and designated to be p53-induced gene 6 (PIG6). This finding caused considerable excitement in our laboratory at NCI-Frederick and in the laboratory of Dr. David Valle at Johns Hopkins. Our laboratories have collaborated over the years and our mutual interest in proline had been stimulated by the arrival at Hopkins of Chien-An Andy Hu as a postdoctoral fellow. Dr. Hu had worked on plant proline metabolic enzymes at Ohio State University. Dr. Hu and Dr. Jian Yu of the Vogelstein lab made a POX/PRODH expression vector controlled by a tet-off promoter and obtained stable transfectants in DLD-1 colorectal cancer cells. Steve Donald and Xiao-Ya Sun in my laboratory soon showed that overexpression of POX/PRODH produced proline-dependent reactive oxygen species (ROS) and that the proline-dependent ROS were generated with cytotoxic stress in p53 expressing cells but not in p53 nulls (27). Furthermore, the p53-dependent upregulation of POX/PRODH, as documented by enzyme activity and enzyme protein, was reproduced in a number of cell lines. The resultant apoptosis in a proline-dependent fashion was reported at the AACR meeting in 2002 by Andy Hu (28). Thus, clearly, POX/PRODH expression was upregulated by genotoxic stress. Its consequences will be more completely described subsequently (see below).
To elucidate further the mechanisms of regulation of POX/PRODH, Jui Pandhare and Sandra Cooper developed a PRODH promoter luciferase reporter construct, and co-transfected a number of transcription factors including c-jun, c-fos, p65 of NF-kB, etc. They found that although some of these major transcription factors activated the PRODH promoter, their effect was modest (< 2-fold). Surprisingly, peroxisomal proliferator-activated receptor gamma (PPARγ) was a potent activator. Transient transfection with PPARγ, together with Troglitazone treatment, increased reporter expression more than 10-fold (29). We were interested in this finding because the pharmacologic ligands of PPARγ, the thiazolidinediones (TZDs) have been used widely as oral hypoglycemic agents for patients with type 2 diabetes mellitus (30). Additionally, TZDs can retard atherosclerotic processes (31). But most importantly, they have anti-proliferative and apoptotic effects on a variety of cultured cancer cells and animal tumor models, leading to the proposal that TZDs can be used for cancer prevention and/or treatment (32–34). Since the physiologic ligand for PPARγ was found to be prostaglandin J2 (PGJ2) (35), investigators proposed that PPARγ responds to inflammatory stimuli (PGJ2) and may be a mechanism to halt chronic inflammation (36). Thus, PPARγ signaling and the response of POX/PRODH may be considered a response to inflammatory stress. Treatment of a variety of cells with TZDs markedly increased POX/PRODH activity and protein (29) the mechanism of this effect was transcriptional as shown by activation of the promoter and by the binding of PPARγ to the peroxisomal proliferator response element from the PRODH promoter. The latter was shown by electrophoretic mobility shift assays and by chromosomal immunoprecipitation assays. These findings conclusively demonstrated that POX/PRODH is upregulated by PPARγ and its ligands, the TZDs, leading us to conclude that POX/PRODH increases in response to inflammatory stress.
Since POX/PRODH is upregulated directly by both genotoxic and inflammatory stress, we considered whether it would also be responsive to nutrient stress. This stress paradigm plays an important role in cancer since tumor cells detached from the basement membrane are isolated from their blood supply (37). The resultant hypoxic stress and nutrient stress activate several important responses for survival. Neoangiogenesis stimulated by the transcription factor, HIF-1α, is the response to hypoxia; the oxygen-dependent prolylhydroxylation of HIF-1α, and its VHL-directed proteasomal degradation has been characterized (38). For nutrient stress, a constellation of signaling pathways and responses have been described, and the central mediator of these responses has been shown to be the serine-threonine kinase, mammalian target of Rapamycin, mTOR (39).
MTOR integrates metabolic information from several sources to regulate cell behavior (39), including growth factor signaling, amino acid availability and adequate bioenergetics. A serine-threonine kinase, mTOR phosphorylates initiation factors and ribosomal proteins to activate protein translation and cell proliferation, respectively. Using RKO colorectal cancer cells we showed that with decreasing glucose concentrations in the medium, the phosphorylation of mTOR decreased and the mTOR-mediated phosphorylation of S6K was proportionately decreased (40, 41). On the other hand, the activity of POX/PRODH was markedly increased. That this response was mTOR-mediated was shown with Rapamycin, the specific inhibitor of mTOR signaling. At 10 nM, the phosphorylation of mTOR was markedly decreased and the phosphorylation of S6K was essentially abolished. Interestingly, Rapamycin markedly upregulated POX/PRODH, plateauing at a level more than 10-fold higher than controls (40). The details of these studies are being published elsewhere. Thus, a regulatory pathway central to nutrient stress is a robust upregulator of POX/PRODH.
To summarize up to this point, POX/PRODH, the rate-limiting enzyme in proline degradation, was shown to be upregulated under 3 stress situations thereby satisfying criterion #1. It was induced by p53, the primary mechanism for signaling genotoxic stress; by PPARγ and its pharmacologic ligands, a signaling system responding to inflammatory stress; and by modulating mTOR, the major signaling mechanism for nutrient stress, either directly with rapamycin or indirectly by AICAR, the activator of AMP-directed protein kinase (AMPK), a sensing mechanism for cellular bioenergetics.
Reservoir of Mobilizable Proline
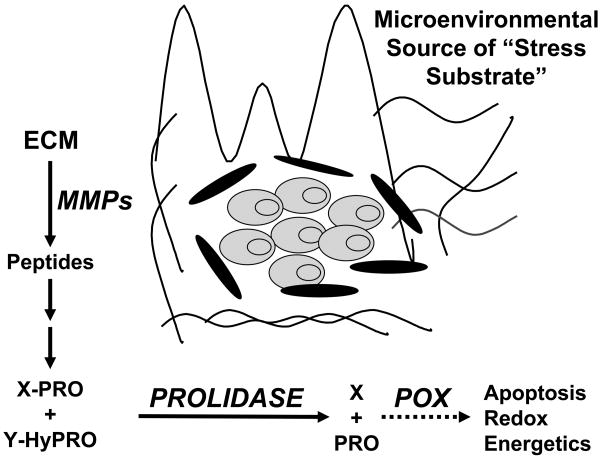
Although POX/PRODH, the enzyme machinery for utilizing proline, is markedly upregulated under stress conditions, the important question remains as to the source of this proline (criterion #2). Since dietary proline is plentiful and proline biosynthesis is ubiquitous, it is unlikely that these sources would be regulated by stress. Although glutamine is the most abundant free amino acid in the body, its delivery requires an intact circulatory system and it is constantly biosynthesized and degraded as part of interorgan transfer (19, 20). Total body glycine (free and protein bound) is also abundant but it is rapidly metabolized into a number of products and the conversions are not amenable to specific regulation (41). The metabolism of proline, on the other hand, is regulated by several stress responses and it is abundant in collagen. Together with hydroxyproline, proline constitutes over 25% of collagen amino acids (42). Collagen is 80% of ECM and 90–95% of the connective tissue and the organic part of bone (42). In fact, collagen makes up 25% of total body protein (43). A crude calculation based on 11.0 kg of total body protein in a 70 kg man (44) results in an estimate of 0.7 kilogram dry weight of proline/hydroxyproline in the body. The degradation of collagen is catalyzed by matrix metalloproteinases (MMPs) (45, 46), a family of metalloenzymes induced under conditions paralleling the stress conditions in which POX is induced, i.e. with genotoxic stress (45), inflammatory stress (46) and nutrient stress (47, 48) (Figure 3). Observing that MMPs are activated during tumor invasion and metastasis (49), investigators proposed that pharmacologic blockade of MMPs would be a useful approach for cancer chemotherapy (17). With encouraging results from preclinical studies (16), clinical trials using broad spectrum inhibitors of MMPs were initiated, but the results were generally disappointing (17). At present, this area is being revisited since the degradation of ECM by MMPs can yield a variety of bioactive factors either as proteolytic products or as defined growth factors bound to ECM (50). These are interesting possibilities and deserve additional studies. However, the metabolic consequences of ECM degradation with the microenvironmental release of proline and hydroxyproline has not been considered. We propose that altered proline/hydroxyproline metabolism is an important endpoint of MMP activation. A decrease in the effects of proline/hydroxyproline metabolism with pharmacologic blockade of MMPs may contribute to the observed anticancer effects, but these may be offset by sources of these stress substrates during clinical trials, e.g. from an ad lib diet.
Figure 3.
Extracellular matrix as mobilizable proline reservoir. Abbreviations: ECM, extracellular matrix; MMPs, matrix metalloproteinases; X-PRO, imidodipeptides with proline as carboxyl terminus; Y-HyPRO, imidodipeptides with hydroxyproline as carboxyl terminus.
If the stress-dependent activation of MMPs results in the availability of proline and hydroxyproline, there should be observable evidence of collagen degradation. Such, indeed, is the case. Fries et al., showed that rats, treated with trinitrobenzenesulphonic acid to induce colitis by inflammatory stress, increased their urinary excretion of hydroxyproline normalized to creatinine more than 2-fold (51). Under these conditions, urinary hydroxyproline is an indication of collagen degradation. In similar studies, Reddy & Dhar showed that Freund’s adjuvent-induced arthritis in rats was accompanied by increased collagen metabolism as indicated by a two-fold increase in urinary hydroxyproline (52). The most relevant study was by Marian and Mazzucco in the skin tumorigenesis model in mice (53). In this model, shaved skin is painted first with a carcinogen, e.g. benzo(a)pyrene or dimethylbenzanthrene, followed by repeated paintings with a tumor promoter, 12-O-tetradecanoylphorbol-13-acetate (TPA). After 5 paintings (2 weeks) with TPA, dermal collagen as measured by hydroxyproline content had decreased by over 20%. Since collagen synthesis was increased as measured by an independent methodology, the decrease in net collagen content must reflect markedly increased collagen degradation (53). Thus, collagen degradation is a notable corollary of skin tumorigenesis. Although this phenomenon has not been emphasized in the carcinogenic process, these findings show that a variety of stress responses is accompanied by collagen degradation.
In our formulation of proline metabolism as a stress substrate, we have shown that the enzyme catalyzing the degradation of proline is upregulated by p53 (26, 27), PPARγ (29) and by rapamycin (40). Furthermore, the literature provided examples by which some of these responses activated the degradation of collagen (54, 55), which would produce an increase in availability of proline as a stress substrate.
Special Functions of Proline Metabolism
The final criterion for proline as stress substrate is whether its metabolism serves special functions for the cell. As we previously mentioned (see above), Donald et al., showed that cytotoxic drugs increased POX/PRODH expression only with functioning p53 and that overexpression of POX/PRODH resulted in proline-dependent generation of ROS (6). Andy Hu and co-workers, using the DLD-1-tet-off- POX cells, showed that overexpression of enzym-atically active POX/PRODH induced proline-dependent apoptosis, which was inhibited by treatment with antioxidants (28). Steve Maxwell and coworkers at Texas A & M University suggested that the mechanism of the apoptotic effect was due to Δ1-pyrroline-5-carboxylic acid (P5C). However, unhydrolyzed P5C-dinitrophenylhydrazone from a commercial source was added directly to the incubation medium as the source of P5C (56). In a subsequent study, Maxwell and colleagues also showed that the effect of POX/PRODH expression was mediated by ROS. They made an important additional discovery: POX/PRODH expression induced the calcineurin-dependent expression of nuclear factor of activated T cells (NFAT) (57), which played a role in apoptosis.
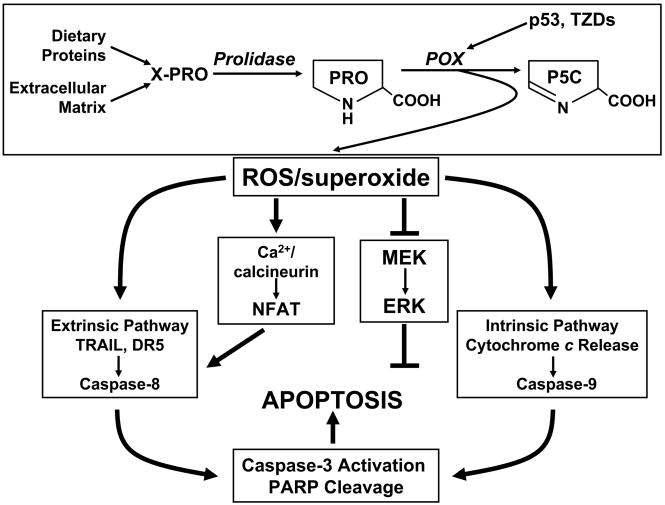
The mechanism of proline-dependent apoptosis was shown by Liu et al (7). Using hydroethidine as a specific fluorescent indicator for superoxide, they showed that superoxide was generated in cells overexpressing POX/PRODH. Additionally, by coexpressing several antioxidant enzymes, i.e. SOD1, SOD2 and catalase, they showed that apoptosis was inhibited by the coexpression of SOD2 (MnSOD) but not by SOD1 (CuZnSOD) or catalase. This was not surprising since MnSOD localizes to mitochondria and the finding supports the interpretation that the apoptotic effect of POX/PRODH is causally related to the proline-dependent generation of mitochondrial superoxide. The following sequence of events occurs. Superoxide alters mitochondrial membrane potential allowing for the release of cytochrome c into the cytosol, followed by the activation of caspase 9 and the caspase cascasde. Downstream events include cell cycle arrest, cleavage of poly (ADP ribose) polymerase and DNA fragmentation, Not only the intrinsic (mitochondrial) apoptotic pathway, but also the extrinsic (death receptor) pathway was activated by POX/PRODH (58). The mechanism for this latter pathway appears to be the upregulation of NFAT (57, 58), which is a potent activator of the tumor necrosis factor-related apoptosis inducing ligand (TRAIL) promoter (58). TRAIL signaling leads to the activation of the caspase 8 limb of the caspase cascade. These effects are shown schematically in Figure 4.
Figure 4.
Proline oxidase produces superoxide which activates both limbs of the apoptotic pathway. Abbreviations: TZDs, thiazolidinediones; TRAIL, tumor necrosis factor related apoptosis-inducing ligand; DR5, death receptor 5, PARP, polyadenoribosyl polymerase; MEK, MAP kinase kinase; ERK, extracellular-signal regulated MAP kinase.
The aforementioned induction of POX/PRODH by PPARγ and its pharmacologic ligands, the TZDs, was of special interest because these agents are potent inhibitors of cancer cell growth (32–34). It has been proposed that this regulator of metabolism may be a promising target for the treatment of cancer (59). A variety of cultured cancer cells will undergo apoptosis when treated by TZDs. Obviously, the induction of POX/PRODH by TZDs suggested that POX/PRODH may be involved. In fact, the generation of ROS with TZD treatment was markedly inhibited by knockdown of POX//PRODH by its siRNA (29). Our findings using colorectal cancer cells were corroborated by studies performed by others using non-small-cell lung cancer cells (60). Thus, it appears that the anticancer effects of PPARγ and TZDs may be critically dependent upon their induction of POX/PRODH. Certainly, the effects on superoxide and apoptosis, and linkage with PPARγ are special functions of proline as stress substrate (Figure 4).
It is tempting to speculate that POX/PRODH is intrinsically able to perform the aformentioned task. Although it can contribute proline-derived electrons to the electron transport chain for use in reduction of oxygen to form superoxide, it would have no advantage over other sources of substrate such as succinate or NADH. The work from Tanner’s lab has shed light on this process. Using the monofunctional POX/PRODH from Thermus thermophilus, White et al., showed that the flavine adenine dinucleotide (FAD) contained in the active site is exposed to solvent oxygen (8). The recombinant enzyme can indeed generate superoxide. If the mammalian enzyme is structurally similar, White’s finding suggests that POX/PRODH may be a controlled metabolic source of superoxide as an oxidizing signal. Although the pool of ROS may be generated by the “leakage of electrons” it is rapidly detoxified by scavenging mechanisms and the complement of antioxidant enzymes (61). The proline-derived superoxide may be part of a special controlled mechanism for apoptotic signaling.
Another special function of proline as stress substrate is related to the induction of POX/PRODH by rapamycin. As described above, treatment of RKO colorectal cancer cells with Rapamycin upregulated POX/PRODH activity more than 10-fold (40, 41). Rapamycin, by inhibiting mTOR, decreases protein synthesis and cell proliferation, switching the cell to a catabolic, survival mode. The maintenance of cellular bioenergetics is a critical mechanism for survival. Importantly, rapamycin treatment maintained ATP levels for at least 24 hours (41) Using dehydroproline (DHP), an inhibitor of POX/PRODH catalytic activity, we showed that the maintenance of ATP levels in the presence or absence of added medium proline was markedly inhibited (62). Thus, the upregulation of POX/PRODH by rapamycin and the contribution of POX/PRODH to the maintenance of ATP suggest that proline also can contribute to the bioenergetics needed for survival.
Although the metabolism of proline sequentially yields P5C, glutamate and α-ketoglutarate, and thus can play an anaplerotic role for the TCA cycle (1, 3), this is not the only source of bioenergetic maintenance activated by proline and POX/PRODH. The proline cycle and its metabolic interlock with the pentose phosphate pathway provide another mechanism (3, 4, 10). The significance of this interlock is to provide an alternative pathway for metabolizing glucose to generate reduced pyridine nucleotide (NADPH), which is shuttled into mitochondria by the cycling of proline (Figure 2). To determine whether such a metabolic interlock is operative, we measured the conversion of 1-14C-glucose to 14CO2 and found that the expression of POX in the DLD-tet-off POX cells increased the pentose phosphate pathway more than 5-fold (62). By contrast, glycolysis as measured by the production of 3H2O from 5-3H-glucose was only modestly increased (25%). Thus, the activation of POX augments bioenergetics not only by supplying carbons to the TCA cycle but also by the recruitment of the pentose phosphate pathway linked by the proline cycle to supplement bioenergetics (Figure 2).
These two special functions of proline as stress substrate (criterion #3), i.e. maintenance of bioenergetics for survival vs. generation of superoxide as an oxidizing signal for programmed cell death, introduce an apparent paradox. Here, the structural biology model again provides an important insight. As previously described, the production of superoxide by the recombinant, purified protein was demonstrated in vitro (8). Furthermore, crystallographic studies identified an adjacent alpha helix shielding the FAD from solvent oxygen so that the enzyme has a switch, which directs electrons from proline either into the electron transport chain for the generation of ATP, or allows exposure to solvent oxygen to generate superoxide (8). Whether this model also applies to the human enzyme has yet to be shown, and the mechanism (s) controlling this switch will be of great interest.
Since the focus of the 7th Amino Acid Assessment Workshop and this special issue is on supplementation of amino acids, a few words here on proline supplementation would be appropriate. Our emphasis is on the mobilization of proline from endogenous stores (ECM) and its utilization. These processes are markedly upregulated by stress signaling. There is no evidence that dietary supplementation of proline modulates this signaling. However, the question of augmentation by supplementation under stress conditions has not been directly addressed experimentally. Under normal conditions, the circulating level of the proline degradative product, P5C, is affected by intake of food, but the relationship to a specific nutrient could not be shown (63). On the other hand, when blockade of MMPs is considered as a therapeutic regimen for cancer, the limitation of the supply of proline from collagen may be a contributing mechanism. Thus, the potential anti-tumor effect of MMP blockade may require the limitation of alternative sources of proline/hydroxyproline, i.e. from the diet.
In summary, we propose that proline is a special microenvironmental stress substrate, and we have provided evidence satisfying the 3 important criteria for such a substrate. 1) The enzyme utilizing proline, proline oxidase/proline dehydrogenase, is responsive to genotoxic stress, to inflammatory stress and to nutrient stress. 2) There is a large reservoir of proline in collagen and MMPs are activated under conditions in which stress signals are activated. Furthermore, evidence from previously published reports show that the degradation of collagen with the release of peptides accompanies these stress conditions. 3) Finally, the degradation of proline serves special functions, programmed cell death, on the one hand, and augmentation of bioenergetics to maintain survival, on the other. Evidence from structural biology provides a potential “switching” mechanism intrinsic to POX/PRODH to mediate alternatively both these processes.
Acknowledgments
This work has been stimulated by many discussions with Dr. David Valle, Johns Hopkins University School of Medicine and Dr. C.-A. Andy Hu, University of New Mexico School of Medicine. I also thank Steven P. Donald and Dr. Lucy Anderson, LCC, NCI-Frederick and Gregory L. Borchert, Basic Research Program, SAIC-Frederick for reading the manuscript and for their helpful suggestions.
This research is supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project also has been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health under Contract No. N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- AICAR
5-aminoimidazole-4-carboxamide-1β-D-ribonucleoside
- AMPK
AMP-activated protein kinase
- HIF-1α
hypoxia-inducible factor-1alpha
- mTOR
mammalian target of rapamycin
- NFAT
nuclear factor of activated T-cells
- P5C
Δ1-pyrroline-5-carboxylic acid
- POX
proline oxidase
- PPARγ
peroxisome proliferator-activated receptor gamma
- PRODH
proline dehydrogenase
- ROS
reactive oxygen species
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- TZDs
thiazolidinediones
- VHL
von-Hippel-Lindau
Footnotes
The International Council on Amino Acid Science paid the expenses for J.M. Phang to attend the 7th Amino Acid Assessment Workshop, including travel expenses and accommodations.
James M. Phang, no conflicts of interest; Jui Pandhare, no conflicts of interest; Yongmin Liu, no conflicts of interest.
References
- 1.Adams E. Metabolism of proline and hydroxyproline. Int Rev Connect Tissue Res. 1970;5:1–91. doi: 10.1016/b978-0-12-363705-5.50007-5. [DOI] [PubMed] [Google Scholar]
- 2.Phang JM. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr Topics Cell Regul. 1985;25:91–132. doi: 10.1016/b978-0-12-152825-6.50008-4. [DOI] [PubMed] [Google Scholar]
- 3.Phang JM, Hu CA, Valle D. Disorders of proline and hydroxyproline metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Metabolic and Molecular Basis of Inherited Disease. McGraw Hill Press; New York: 2001. pp. 1821–38. [Google Scholar]
- 4.Hagedorn CH, Phang JM. Transfer of reducing equivalents into mitochondria by the Interconversions of proline and Δ1-pyrroline-5-carboxylate. Arch Biochem Biophys. 1983;225:95–101. doi: 10.1016/0003-9861(83)90010-3. [DOI] [PubMed] [Google Scholar]
- 5.Adams E, Frank L. Metabolism of proline and the hydroxyprolines. Annu Rev Biochem. 1980;49:1005–61. doi: 10.1146/annurev.bi.49.070180.005041. [DOI] [PubMed] [Google Scholar]
- 6.Baban BA, Vinod MP, Tanner JJ, Becker DF. Probing a hydrogen bond pair and the FAD redox properties in the proline dehydrogenase domain of Escherichia coli Put A. Biochim Biophys Acta. 2004;1701:49–59. doi: 10.1016/j.bbapap.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Borchert GL, Donald SP, Surazynski A, Hu C-A, Weydert CJ, Oberley LW, Phang JM. MnSOD inhibits proline oxidase-induced apoptosis in colorectal cancer cells. Carcinogenesis. 2005;26:1335–42. doi: 10.1093/carcin/bgi083. [DOI] [PubMed] [Google Scholar]
- 8.White TA, Krishnan N, Becker DF, Tanner JJ. Structure and kinetics of monofunctional proline dehydrogenase from Thermus thermophilus. J Biol Chem. 200;282:14316–27. doi: 10.1074/jbc.M700912200. Epub 2007 Mar 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phang JM, Downing SJ, Yeh GC. Linkage of the HMP pathway to ATP generation by the proline cycle. Biochem Biophys Res Commun. 1980;93:462–70. doi: 10.1016/0006-291x(80)91100-6. [DOI] [PubMed] [Google Scholar]
- 10.Hagedorn CH, Phang JM. Catalytic transfer of hydride ions from NADPH to oxygen by the interconversions of proline and delta 1-pyrroline-5-carboxylate. Arch Biochem Biophys. 1986;248:166–74. doi: 10.1016/0003-9861(86)90413-3. [DOI] [PubMed] [Google Scholar]
- 11.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–52. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 12.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 13.Warburg O. Ueber den Stoffwechsel der Tumoren. Constable; London: 1930. [Google Scholar]
- 14.U.S. Deparatment of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 20, Nutrients Data Laboratory Home Page. 2007 http://www.ars.usda.gov/ba/bhnrc/ndl.
- 15.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390–7. [PubMed] [Google Scholar]
- 16.Wang X, Fu X, Brown PD, Crimmin MJ, Hoffman RM. Matrix metalloproteinase inhibitor BB-94 (batimastat) inhibits human colon tumor growth and spread in a patient-like orthotopic model in nude mice. Cancer Res. 1994;54:4726–8. [PubMed] [Google Scholar]
- 17.Wagenaar-Miller RA, Gorden L, Matrisian LM. Matrix metalloproteinases in colorectal cancer: Is it worth talking about? Cancer & Met Rev. 2004;23:119–35. doi: 10.1023/a:1025819214508. [DOI] [PubMed] [Google Scholar]
- 18.Felig P, Pozefsky T, Marliss E, Cahill GF., Jr Alanine: key role in gluconeogenesis. Science. 1970;167:1003–4. doi: 10.1126/science.167.3920.1003. [DOI] [PubMed] [Google Scholar]
- 19.Oehler R, Pusch E, Dungel P, Zellner M, Eliasen MM, Brabec M, Roth E. Glutamine depletion impairs cellular stress response in human leucocytes. Br J Nutr. 2002;87 (Suppl 1):S17–21. doi: 10.1079/bjn2001453. [DOI] [PubMed] [Google Scholar]
- 20.Kamm DE, Fuisz RE, Goodman AD, Cahill GF., Jr Acid-base alterations and renal gluconeogenesis: effect of pH, bicarbonate concentration, and PCO2. J Clin Invest. 1967;46:1172–7. doi: 10.1172/JCI105610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilmore D. Enteral and parenteral arginine supplementation to improve medical outcomes in hospitalized patients. J Nutr. 2004;134(10 Suppl):2863–7S. doi: 10.1093/jn/134.10.2863S. discussion 2895S. [DOI] [PubMed] [Google Scholar]
- 22.Grube E, Buellesfeld L. Rapamycin analogs for stent-based local drug delivery. Everolimus- and tacrolimus-eluting stents. Herz. 2004;29:162–6. doi: 10.1007/s00059-004-2556-6. [DOI] [PubMed] [Google Scholar]
- 23.Al-Jamal WT, Kostarelos K. Liposome-nanoparticle hybrids for multimodal diagnostic and therapeutic applications. Nanomed. 2007;2:85–98. doi: 10.2217/17435889.2.1.85. [DOI] [PubMed] [Google Scholar]
- 24.Laconi E. The evolving concept of tumor microenvironments. Bioessays. 2007;29:738–44. doi: 10.1002/bies.20606. [DOI] [PubMed] [Google Scholar]
- 25.Viola A, Bronte V. Metabolic mechanisms of cancer-induced inhibition of immune responses. Semin Cancer Biol. 2007;17:309–16. doi: 10.1016/j.semcancer.2007.06.005. Epub 2007 Jun 23. [DOI] [PubMed] [Google Scholar]
- 26.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–5. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 27.Donald SP, Sun XY, Hu CA, Yu J, Mei JM, Valle D, Phang JM. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 2001;61:1810–5. [PubMed] [Google Scholar]
- 28.Hu C-AA, Yu J, Lin W-W, Donald SP, Sun X-Y, Almashanu S, Steel G, Phang JM, Vogelstein B, Valle D. Overexpression of proline oxidase, a p53 induced gene (PIG6) induces reactive oxygen species generation and apoptosis in cancer cells. Proc Am Ass Cancer Res. 2001;42:225. [Google Scholar]
- 29.Pandhare J, Cooper SK, Phang JM. Proline oxidase, a proapoptotic gene, is induced by troglitazone: evidence for both peroxisome proliferator-activated receptor gamma-dependent and -independent mechanisms. J Biol Chem. 2006;281:2044–52. doi: 10.1074/jbc.M507867200. Epub 2005 Nov 21. [DOI] [PubMed] [Google Scholar]
- 30.Natali A, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 2006;49:434–41. doi: 10.1007/s00125-006-0141-7. Epub 2006 Feb 14. [DOI] [PubMed] [Google Scholar]
- 31.Van Wijk JP, Rabelink TJ. Impact of thiazolidinedione therapy on atherogenesis. Curr Atheroscler Rep. 2005;7:369–374. doi: 10.1007/s11883-005-0049-6. [DOI] [PubMed] [Google Scholar]
- 32.Li MY, Lee TW, Yim AP, Chen GG. Function of PPARgamma and its ligands in lung cancer. Crit Rev Clin Lab Sci. 2006;43:183–202. doi: 10.1080/10408360600552587. [DOI] [PubMed] [Google Scholar]
- 33.Lu M, Kwan T, Yu C, Chen F, Freedman B, Schafer JM, Lee EJ, Jamesson JL, Jordan VC, Cryns VL. Peroxisome proliferator-activated receptor gamma agonists promote TRAIL-induced apoptosis by reducing survivin levels via cyclin D3 repression and cell cycle arrest. J Biol Chem. 2005;280:6742–51. doi: 10.1074/jbc.M411519200. Epub 2004 Nov 29. [DOI] [PubMed] [Google Scholar]
- 34.McAlpine CA, Barak Y, Matise I, Cormier RT. Intestinal-specific PPARgamma deficiency enhances tumorigenesis in ApcMin/+ mice. Int J Cancer. 2006;119:2339–46. doi: 10.1002/ijc.22115. [DOI] [PubMed] [Google Scholar]
- 35.Monroy MA, Opperman KK, Picciarelli M, Yerrum S, Berg DA, Daly JM. The PPARgamma ligand 15d-PGJ2 modulates macrophage activation after injury in a murine trauma model. Shock. 2007;28:186–91. doi: 10.1097/shk.0b013e3180310982. [DOI] [PubMed] [Google Scholar]
- 36.Strauss DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–8. doi: 10.1016/j.it.2007.09.003. Epub 2007 Nov 5. [DOI] [PubMed] [Google Scholar]
- 37.Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–4. [PubMed] [Google Scholar]
- 38.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007(407):cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 39.Reiling JH, Sabatini DM. Stress and mTOR signaling. Oncogene. 2006;25:6373–83. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- 40.Pandhare J, Cooper SK, Donald SP, Phang JM. Significance of the proline metabolic pathway in the adaptation of cancer cells to nutrient/energy limitation. (Submitted 2007) [Google Scholar]
- 41.Rowsell EV, Al-Naama MM, Rowsell KV. Glycine metabolism in rat kidney cortex slices. Biochem J. 1982;204:313–21. doi: 10.1042/bj2040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dixit SN, Seyer JM, Kang AH. Covalent structure of collagen: Amino-acid sequence of chymotryptic peptides from the carboxyl-terminal region of α2-CB3 of chick-skin collagen. Eur J Biochem. 1977;81:599–607. doi: 10.1111/j.1432-1033.1977.tb11987.x. [DOI] [PubMed] [Google Scholar]
- 43.Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277:4223–31. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Shen W, Kotler DP, Heshka S, Wielopolski L, Aloia JF, Nelson ME, Pierson RN, Jr, Heymsfield SB. Total body protein: a new cellular level mass and distribution prediction model. Am J Clin Nutr. 2003;78:979–84. doi: 10.1093/ajcn/78.5.979. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Wicha M, Leopold WR. Regulation of metastasis-related gene expression by p53: a potential clinical implication. Mol Carcinog. 1999;24:25–28. [PubMed] [Google Scholar]
- 46.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 47.Poulalhon N, Fargo D, Roos N, Tacheau C, Neuzillet C, Michel L, Mauvid A, Verrecchia F. Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin. A direct role as an antifibrotic agent? J Biol Chem. 2006;281:33045–52. doi: 10.1074/jbc.M606366200. Epub 2006 Aug 16. [DOI] [PubMed] [Google Scholar]
- 48.Deryugina EI, Quigley JP. Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin. A direct role as an antifibrotic agent. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. [Google Scholar]
- 49.Stallings-Mann M, Radisky D. Matrix metalloproteinase-induced malignancy in mammary epithelial cells. Cells Tissues Organs. 185:104–10. doi: 10.1159/000101310. [DOI] [PubMed] [Google Scholar]
- 50.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fries W, Giacomin D, Plebani M, Martin A. Effect of experimental colitis on bone metabolism in the rat. Digestion. 1994;55:229–33. doi: 10.1159/000201152. [DOI] [PubMed] [Google Scholar]
- 52.Reddy GK, Dhar SC. Metabolism of collagen in bone of adjuvant induced arthritic rat. Bone. 1989;10:439–45. doi: 10.1016/8756-3282(89)90076-8. [DOI] [PubMed] [Google Scholar]
- 53.Marian B, Mazzucco K. Dermal collagen metabolism during tumor promotion with 12-O-tetradecanoylphorbol-13-acetate in mouse skin. Carcinogenesis. 1985;6:501–4. doi: 10.1093/carcin/6.4.501. [DOI] [PubMed] [Google Scholar]
- 54.Sun Y, Wicha M, Leopold WR. Regulation of metastasis-related gene expression by p53: A potential clinical implication. Mol Carcinogen. 1999;24:25–8. [PubMed] [Google Scholar]
- 55.Poulalhon N, Farge D, Roos N, Tacheau C, Neuzillet C, Michel L, et al. Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin. J Biol Chem. 2006;281:33045–52. doi: 10.1074/jbc.M606366200. [DOI] [PubMed] [Google Scholar]
- 56.Maxwell SA, Davis GE. Differential gene expression in p53-mediated apoptosis-resistant vs. apoptosis-sensitive tumor cell lines. Proc Natl Acad Sci USA. 2000;97:13009–14. doi: 10.1073/pnas.230445997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maxwell SA, Rivera A. Proline oxidase induces apoptosis in tumor cells, and its expression is frequently absent or reduced in renal carcinomas. J Biol Chem. 2003;278:9784–9. doi: 10.1074/jbc.M210012200. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y, Borchert GL, Surazynski A, Hu C-A, Phang JM. Proline oxidase activates both intrinsic and extrinsic pathways for apoptosis: the role of ROS/superoxides, NFAT and MEK/ERK signaling. Oncogene. 2006;25:5640–7. doi: 10.1038/sj.onc.1209564. [DOI] [PubMed] [Google Scholar]
- 59.Han S, Roman J. Peroxisome proliferator-activated receptor gamma: a novel target for cancer therapeutics? Anticancer Drugs. 2007;18:237–44. doi: 10.1097/CAD.0b013e328011e67d. [DOI] [PubMed] [Google Scholar]
- 60.Kim KY, Ahn JH, Cheon HG. Apoptotic action of peroxisome proliferator-activated receptor-gamma activation in human non small-cell lung cancer is mediated via proline oxidase-induced reactive oxygen species formation. Mol Pharmacol. 2007;72:674–85. doi: 10.1124/mol.107.035584. Epub 2007 May 29. [DOI] [PubMed] [Google Scholar]
- 61.Camhi SL, Lee P, Choi AM. The oxidative stress response. New Horiz. 1995;3:170–82. [PubMed] [Google Scholar]
- 62.Phang JM, Donald SP, Pandhare J, Liu Y. Regulation of proline utilization as stress substrate. Amino Acids. 2008 doi: 10.1007/s00726-008-0063-4. (In Press) [DOI] [PubMed] [Google Scholar]
- 63.Fleming GA, Granger A, Rogers QR, Prosser M, Ford DB, phang JM. Fluctuations in plasma pyrroline-5-carboxylate concentrations during feeding and fasting. J Clin Endocrinol Metab. 1989;69:448–52. doi: 10.1210/jcem-69-2-448. [DOI] [PubMed] [Google Scholar]