Abstract
Mitochondrial electron transport generates the ATP that is essential for the excitability and survival of neurons, and the protein phosphorylation reactions that mediate synaptic signaling and related long-term changes in neuronal structure and function. Mitochondria are highly dynamic organelles that divide, fuse and move purposefully within axons and dendrites. An Major functions of mitochondria in neurons include the regulation of Ca2+ and redox signaling, developmental and synaptic plasticity, and the arbitration of cell survival and death. The importance of mitochondria in neurons is evident in the neurological phenotypes in rare diseases caused by mutations in mitochondrial genes. Mitochondria-mediated oxidative stress, perturbed Ca2+ homeostasis and apoptosis may also contribute to the pathogenesis of prominent neurological diseases including Alzheimer’s, Parkinson’s and Huntington’s diseases, stroke, ALS and psychiatric disorders. Advances in understanding the molecular and cell biology of mitochondria are leading to novel approaches for the prevention and treatment of neurological disorders.
The Structure and Regulatory Systems of Mitochondria
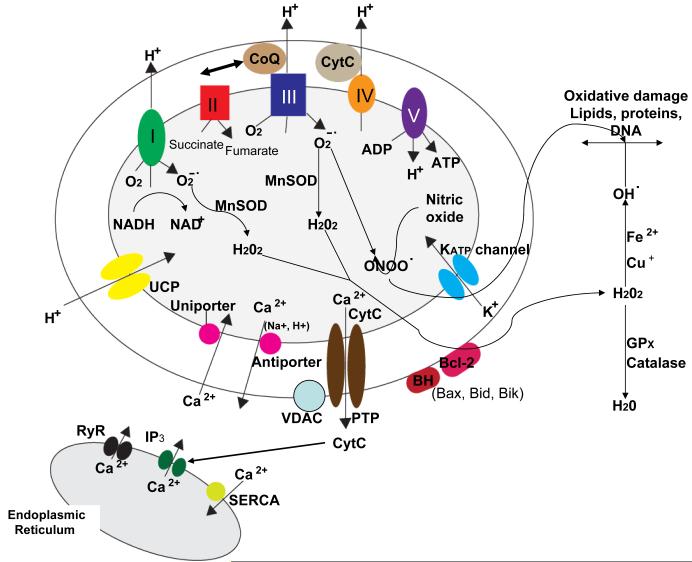
More than 1 billion years ago, a primitive bacteria invaded a single-cell anaerobic organism and, rather than killing the cell, established a symbiotic relationship which has been maintained and refined during the subsequent evolution of complex multicellular organisms (Dyall et al., 2004). Mitochondria, the descendants of the original symbiont, are organelles highly efficient in their ability to utilize O2 and substrates such as glucose and pyruvate to produce cellular energy in the form of ATP (Wallace, 2005). Mitochondria consist of two membranes, an inter-membrane space and an internal ‘matrix’. The molecular machinery for energy production, the electron transport chain (ETC), is organized in an assembly line-like manner within and across the inner mitochondrial membrane (Fig. 1). The ETC consists of five protein complexes. Three of the complexes (I, III and IV) pump protons (H+) outwardly across the inner membrane to establish a H+ gradient necessary for the production of ATP at complex V (ATP synthase). Thirteen of the proteins of the ETC are encoded by genes in the mitochondrial genome. The remaining proteins in mitochondria (more than 1000) are encoded by genes in the cell nucleus and mediate processes such as the regulation of ion homeostasis, stress responses, cell survival and signal transduction. The activity of complex I converts NADH to the energy substrate NAD+ and complex II converts succinate to fumarate. During electron transport, O2 is converted to H2O and, particularly at complexes I and III the free radical superoxide (O2-.) is also generated. Two important cofactors that modulate energy and free radical production are coenzyme Q10 at complex III and cytochrome c at complex IV.
Figure 1.
Proteins involved in mitochondrial bioenergetics, oxygen radical metabolism and Ca2+ regulation. The electron transport chain consists of four protein complexes (I-IV) and the ATP synthase (complex V) located in the mitochondrial inner membrane. The activity of complex I converts NADH to NAD+ and the activity of complex II converts succinate to fumarate. Complexes I, III and IV transport protons (H+) across the membrane and complexes I and III generate superoxide anion radical (O2-.) during the electron transfer process. The enzymatic activity of mitochondrial manganese superoxide dismutase (MnSOD) converts O2-. to hydrogen peroxide (H2O2) which may then diffuse to the cytoplasmic compartments where glutathione peroxidase and catalase convert H2O2 to H2O. However, H2O2 can interact with Fe2+ or Cu+ to generate hydroxyl radical (OH.), a highly reactive free radical that can induce lipid peroxidation and oxidative damage to proteins and DNA. Mitochondrial uncoupling proteins (UCP) function as H+ leak channels which decrease mitochondrial membrane potential results in decreased generation of O2-. and ATP. Several mitochondrial proteins are involved in regulating movement of Ca2+ into and out of the mitochondria including the Ca2+ uniporter which moves Ca2+ into the mitochondrial matrix and the Ca2+ antiporter which extrudes Ca2+ into the cytosol. In addition, movement of K+ through ATP-sensitive potassium channels (KATP) in the inner membrane can result in decreased mitochondrial Ca2+ uptake. An important transmembrane protein complex which includes the voltage-dependent anion channel (VDAC) forms large permeability transition pores (PTP). The PTP open during the process of apoptosis resulting in the release of cytochrome c into the cytoplasm. Several cytoplasmic proteins may also interact with mitochondrial membranes resulting in a change its permeability including Bcl-2 and BH-only proteins such as Bax, Bid and Bik. Finally, there are interactions between mitochondria and the endoplasmic reticulum (ER) such that Ca2+ released through ER IP3 receptors and ryanodine receptors (RyR) is rapidly transferred into mitochondria. On the other hand, cytochrome c released from mitochondria can trigger the release of Ca2+ from the ER.
Much of the O2-. generated during mitochondrial respiration is converted to hydrogen peroxide in a reaction catalyzed by manganese superoxide dismutase (MnSOD). Hydrogen peroxide is, in turn, converted to water by glutathione peroxidase and catalase. However, by reacting with Fe2+ or Cu+ hydrogen peroxide generates the highly reactive hydroxyl radical (OH.) which can induce membrane lipid peroxidation, and can damage proteins and DNA. To further guard against oxidative damage, mitochondria contain several prominent antioxidant molecules including coenzyme Q10 (ubiquinone), creatine and nicotinamide (Beal, 2003). Another reaction of interest from the viewpoints of neuronal signaling and degeneration involves the interaction of nitric oxide with O2-. to produce peroxynitrite (ONOO-), a reactive molecule that can induce nitration of proteins on tyrosine residues thereby impairing the function of those proteins (Goldstein and Merenyi, 2008). Free radicals are also generated by the activity of mitochondrial monoamine oxidases (MAOA and MAOB), enzymes involved in the metabolism of serotonin, norepinphrine and dopamine. Mitochondria-derived O2-. and hydrogen peroxide serve important signaling functions in physiological processes including synaptic plasticity and learning and memory (Kishida and Klann, 2007). The membrane potential maintained by the H+ gradient, and several other properties of mitochondria can be evaluated in living neurons using technologies described in Supplemental Figure 1.
While the ability of mitochondria to remove Ca2+ from the cytoplasm and accumulate it in their matrix is well known, mitochondria also play roles in the regulation of rapid changes of intracellular Ca2+ dynamics, and participate in many Ca2+-mediated signaling processes (for review see Nicholls et al., 2003; Giacomello et al., 2007). The functional properties of Ca2+ transporters and channels in mitochondria are being characterized, although in most cases the identities of the proteins that comprise the Ca2+-handling systems are unknown. The outer mitochondrial membrane is relatively permeable to Ca2+ and the inner membrane contains key Ca2+-regulating proteins including a Ca2+ uniporter that transfers Ca2+ into the mitochondrial matrix and Na+/Ca2+ and H+/Ca2+ antiporters that move Ca2+ out of the mitochondria (Fig. 1). Thus, gradients of Ca2+, H+ and Na+ concentrations greatly influence Ca2+ flux across the inner mitochondrial membrane. Interestingly, mitochondria are often located immediately adjacent to the endoplasmic reticulum (ER) where they rapidly remove Ca2+ released into the cytoplasm from the ER through activated inositol triphosphate (IP3) receptors and ryanodine receptors (Fig. 1). In this article we describe mitochondrial proteins involved in neuronal plasticity and disease processes.
Mitochondrial Dynamics
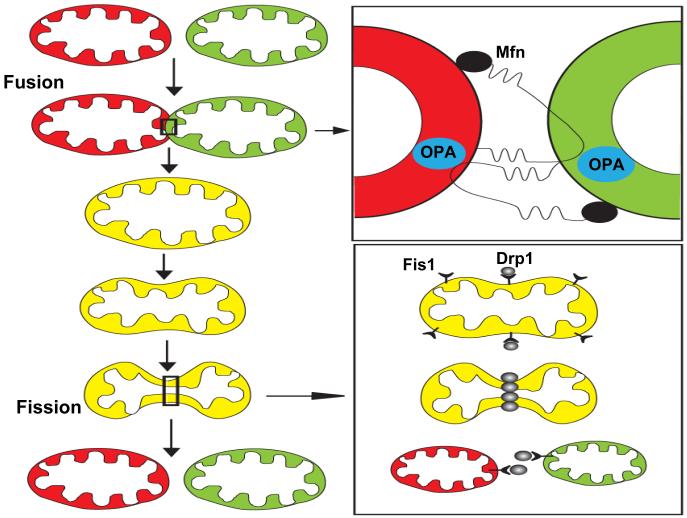
Mitochondria function within an integrated reticulum that is continuously remodeled by growth and fission of individual mitochondria, and the fusion of different mitochondria (Fig. 2). Key molecular mechanisms involved in mitochondrial fission and fusion have recently been elucidated (see Berman et al., 2008 for review). Before dividing, mitochondria replicate their DNA and levels of mitochondrial proteins encoded by nuclear DNA increase in a process called biogenesis described (Supplemental Figure 2). Mitochondrial fission is mediated by two key proteins, dynamin-related protein 1 (Drp1) and Fis1. Drp1 is located in the cytoplasm and in punctate arrays on mitochondrial tubules, and includes a GTPase domain and a GTPase effector domain. Fis1 is located throughout the outer mitochondrial membrane, with the bulk of the protein consisting of six antiparallel helices (with the central four helices consisting of two tandem tetratricopeptide repeats) on the cytosolic side of the membrane. Additional proteins implicated in mitochondrial fission include endophilin B1 and MTP18. Endophilin B1 is a fatty acyl transferase that is required for maintenance of mitochondrial morphology. Knockdown of endophilin B1 causes the formation of vesicles and tubules of outer mitochondrial membrane, and knockdown of both endophilin B1 and Drp1 leads to a mitochondrial phenotype identical to that of the Drp1 single knockdown, suggesting that endophilin B1 functions upstream of Drp1 in the process of mitochondrial fission (Karbowski et al., 2004). Fission of mitochondria involves the recruitment of Drp1 (from remote cytosolic and mitochondrial sites) to discrete foci within the mitochondria, the sites where fission is initiated. MTP18 is a mitochondrial membrane protein that when overexpressed increases mitochondrial fission and when knocked down increases fusion (Tondera et al., 2005). Overexpression of Fis1 does not induce mitochondrial fission in cells lacking MTP18 indicating that MTP18 is required for mitochondrial fission. The nature of the interactions between the different proteins that mediate mitochondrial fission, and how they induce the separation of the mitochondrial membranes remains to be established. However, it has been suggested that Drp1 functions as a “mechanoenzyme” that uses GTP hydrolysis to induce constriction at mitochondrial fission sites (Shaw and Nunnari, 2002).
Figure 2.
Mitochondrial fusion and fission mechanisms. Mitochondria can fuse with each other and exchange their membrane, intermembrane and matrix components (upper left). Fusion of the outer mitochondrial membranes is mediated by homophilic and heterophilic interactions of cytosolic domains of mitofusins (Mfn1 and Mfn2) which are proteins located in the outer mitochondrial membrane (upper right). Fusion of the inner mitochondrial membranes is believed to be mediated by OPA1 which is located in the intermembrane space. Mitochondrial fission (lower left) involves recruitment of dynamin-related protein 1 (Drp1) to discrete foci within the mitochondria and also requires Fis1, a protein located in the outer mitochondrial membrane (lower right).
Mitochondrial fusion is mediated by proteins called mitofusins (Mfn1 and Mfn2). Mfn1 and Mfn2 are integral membrane proteins located in the outer mitochondrial membrane with both the C- and N-terminal regions protruding into the cytosol (Koshiba et al., 2004). The N-terminal region contains a GTPase domain and a hydrophobic heptad repeat region, and the C-terminal region also contains a heptad repeat region which may facilitate homomeric binding and fusion of membranes of interacting mitochondria. Mutations in Mfn2 cause Charcot-Marie-Tooth disease (CMT) type 2A, a peripheral neuropathy characterized by axonal degeneration. Wild type Mfn2 cannot counteract the mitochondrial fusion defect caused by mutant Mfn2 but, interestingly, wild type Mfn1 can overcome the pathological effect of mutant Mfn2 (Detmer and Chan, 2007). Another protein critical for mitochondrial fusion is OPA1 (optic atrophy 1), a member of the dynamin family of GTPases located in the mitochondrial intermembrane space where it is associated with the inner membrane.
Fusion can occur rapidly (within less than one minute), and the underlying events must therefore involve the coordinated fusion of the outer and inner mitochondrial membranes. A simplified model therefore involves outer membrane fusion mediated by homotypic (Mfn1 - Mfn1 or Mfn2 - Mfn2) or heterotypic (Mfn1 - Mfn2) binding of mitofusins in trans, followed by OPA1-mediated fusion of the inner membranes (Fig. 2). Mitochondrial fusion requires the proton gradient generated by a functioning electron transport chain and, therefore, metabolically compromised mitochondria are fusion incompetent. Mitochondrial fission often occurs soon after two mitochondria fuse. Immediately following fusion mitochondrial contents are segregated so as to enrich one of the mitochondria generated by subsequent fission with well-functioning components while targeting the impoverished fission partner for autophagic degradation (Twig et al., 2008); in this way cells are able to retain healthy mitochondria during the lifetime of the organism. Another possible function of mitochondrial fission is to generate variability in mitochondrial phenotypes which are then deployed to different regions of the cell or respond to different environmental demands.
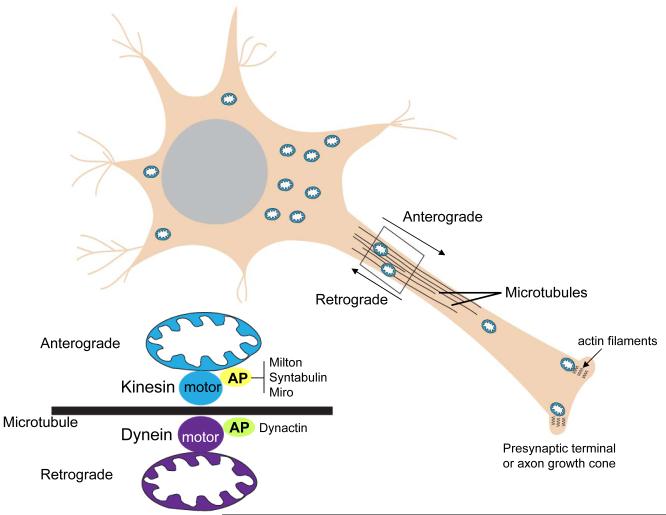
Because of their highly complex morphologies that include long axons and elaborate dendritic arbors, it is essential that mechanisms exist to transport mitochondria throughout the neuron, and to recruit mitochondria to regions with particularly high metabolic demands such as presynaptic terminals (Hollenbeck and Saxton, 2005). Mitochondrial transport occurs by microtubule motor-driven translocation along microtubule tracks that are arranged in polarized parallel arrays. Mitochondria can be actively transported in both the anterograde and retrograde directions by ATP-dependent “motor” proteins (Fig. 3). Anterograde transport is mediated by kinesins, while dynein motor proteins mediate retrograde transport. Mitochondria do not bind directly to the motor proteins but, instead, bind to adaptor proteins that link the mitochondrial membrane to the motor protein. Milton, syntabulin and Miro are adaptor proteins for kinesins, and dynactin is an adaptor protein for dynein (Rice and Gelfand, 2006; Frederick and Shaw, 2007). While progress has been made in identifying the proteins involved in mitochondrial transport within neurons, the signaling mechanisms that control mitochondrial transport in response to local changes in neuronal activity and energy metabolism are largely unknown. For example, what is the mechanism that couples mitochondrial membrane potential to transport such that mitochondria with a greater membrane potential undergo anterograde transport whereas mitochondria with a low membrane potential undergo retrograde transport (Miller and Sheetz, 2004)?
Figure 3.
Mitochondrial trafficking mechanisms within the axon. Microtubules serve as tracks along which mitochondria move either towards the presynaptic terminal (anterograde transport) or towards the cell body (retrograde transport). ATP-dependent motor proteins that move mitochondria along microtubules include kinesins (anterograde) and dynein (retrograde). Mitochondria associate with the motor proteins through specific adaptor proteins. Adaptor proteins (AP) for kinesins include Milton, syntabulin and a Rho GTPase called Miro. Dynactin is an adaptor protein for dynein. Within the axonal growth cone and presynaptic terminal, mitochondria may be anchored and moved along actin filaments by a myosin-mediated mechanism.
It is likely that neurotransmitters and neurotrophic factors control mitochondrial dynamics because of their influences on neuronal energy metabolism, Ca2+ homeostasis and dendritic and axonal motility. Indeed, a recent study showed that cerebellar Purkinje cells in mice deficient in the glutamate receptor Grid2 exhibit extremely long mitochondria and abnormal spines and synapses, and severe ataxia providing evidence that glutamate receptor-mediated signaling affects mitochondrial fission and/or fusion (Liu and Shio, 2008). Further studies in which the effects of neurotransmitters and neurotrophic factors on the molecular machineries that regulate mitochondrial fission, fusion and movement within neurons will expand our appreciation of the integration of mitochondrial motility with neuronal functional and structural dynamics.
Developmental Roles for Mitochondria
During development of the nervous system, neural stem cells proliferate and then differentiate into neurons in the process of neurogenesis. The newborn neurons then grow an axon and dendrites and eventually form synapses; during this process many newly generated neurons undergo programmed cell death (apoptosis). What are the roles of mitochondria in these highly complex and dynamic developmental events? Changes in mitochondrial energy metabolism occur in brain cells during embryonic and early postnatal development with a shift from the use of fatty acids as fuels during early development to the use of glucose later on (Erecinska et al., 2004), suggesting roles for mitochondria in supporting the different bioenergetic requirements of highly proliferative neural stem cells and postmitotic neurons. During neuronal differentiation the number of mitochondria per cell increases, but the velocity at which individual mitochondria move decreases as neurite outgrowth slows and synaptogenesis occurs (Chang and Reynolds, 2006). Neuronal differentiation is accompanied by an increase in the amount of mitochondria per cell; treatment with chloramphenicol (an inhibitor of mitochondrial protein synthesis) prevents differentiation of the cells whereas oligomycin (an inhibitor of the mitochondrial ATP synthase) does not, suggesting that increased mitochondrial mass (but not ATP production) is required for neuronal differentiation (Vayssiere et al., 1992). In addition, signals that influence mitochondrial biogenesis and function, including nitric oxide (Barsoum et al., 2006) and BDNF (Markham et al., 2004), may regulate the proliferation and differentiation of neural progenitor cells in the developing and adult brain (Cheng et al., 2003).
Soon after differentiating from stem cells, neurons extend several neurites, one of which begins to grow rapidly and acquires the molecular, structural and functional characteristics of the axon, while the other neurites become dendrites. Shortly before axogenesis occurs mitochondria congregate at the base of the neurite that is destined to become the axon (Mattson and Partin, 1999), and during axogenesis there is increased entry of mitochondria into the nascent axon and the mitochondrial density in the remaining short processes (dendrites) decreases (Ruthel and Hollenbeck, 2003). When electron transport is impaired (by selectively damaging mitochondrial DNA) and the cells are provided an alternative energy source to maintain ATP levels, axogenesis is abolished but growth of dendrites is relatively unaffected (Mattson and Partin, 1999). Mitochondria may play a key role in establishing neuronal polarity by reducing the Ca2+ concentration at the base of presumptive axon, thereby promoting polymerization of microtubules and the rapid growth and differentiation of the axon.
After axons and dendrites differentiate, their growth and synaptic connectivity may be influenced by mitochondrial motility and functions. During the formation of axonal branches mitochondria respond to changes in growth by modifying their entry into branches; however, the latter process does not require an active growth cone, suggesting the involvement of a mechanism different than that by which mitochondria accumulate in the growth cone (Ruthel and Hollenbeck, 2003). Focal application of NGF to growing axons results in the accumulation of mitochondria near the site of NGF stimulation by a mechanism involving docking interactions with the actin cytoskeleton, suggesting a role for mitochondria in facilitating growth cone responses to neurotrophic factors (Chada et al., 2004).
Many newly generated neurons undergo programmed cell death (PCD), a process regulated by neurotrophic factors and controlled by mitochondria (Kirkland and Franklin, 2003). Changes in the levels of expression of Bcl-2 family members that regulate mitochondria-mediated cell death occur during the processes of neurogenesis and neuronal differentiation. For example, in the developing mouse brain levels of anti-apoptotic Bcl-xL are highest at the peak of neurogenesis, whereas the peak of pro-apoptotic Bax expression coincides with the astrocyte production (Chang et al., 2007). Interestingly, overexpression of Bcl-xL and Bax in neural progenitors induces neuronal and astrocytic differentiation, respectively, and these roles of Bcl-xL and Bax are apparently independent of their effects on cell survival and death (Chang et al., 2007).
To date, the evidence that mitochondria play important roles in sculpting cytoarchitecture during development of nervous systems is based largely on correlational data - the location or properties of mitochondria change in association with developmental processes. Several approaches could be employed to establish the function of mitochondria in development including: knocking down mitochondrial function globally under conditions where cellular energy levels are maintained (low dose ethidium bromide together with pyruvate and uridine supplementation); knocking down the expression or function of proteins involved in specific mitochondrial processes (Ca2+ regulation, ROS metabolism, fission, fussion, PTP function, etc.); targeted inhibition of mitochondrial motility and functions selectively in subcellular regions (only in dendrites, axons or synapses). Time lapse analysis of mitochondrial movements within subcellular compartments in response to physiological stimuli (electrical activity, activation of neurotrophic factor receptors, cell adhesion cues, etc.) will elucidate potential roles of mitochondria in plasticity. Selective ablation and artificial movement of mitochondria (using 2-photon laser technology, for example) may establish the requirements of mitochondria for specific physiological events (neurite outgrowth dendritic remodeling, etc.).
Mitochondria in Synaptic Plasticity
Mitochondria presumably produce much of the ATP required to maintain synaptic ion homeostasis and phosphorylation reactions and, indeed presynaptic terminals typically contain multiple mitochondria (Figs. 3 and 4). Dendritic spines of excitatory glutamatergic synapses, the most abundant type of synapse in the mammalian CNS, experience large amounts of Ca2+ influx through NMDA receptors and voltage-dependent Ca2+ channels, as well as release of Ca2+ from the ER (Toresson and Grant, 2005). Dendrites also contain mitochondria, although they are typically absent from spines (Fig. 4c). One exception is olfactory bulb dendritic spines which subserve both pre- and post-synaptic functions, and which contain mitochondria that can move back-and-forth between spines and the parent dendrite (Cameron et al., 1991). The behaviors and functional properties of mitochondria differ in axons and dendrites. For example, twice as many mitochondria are motile in the axons compared to the dendrites of cultured hippocampal neurons, and there is a greater proportion of highly charged, more metabolically active mitochondria in dendrites than in axons (Overly et al., 1996).
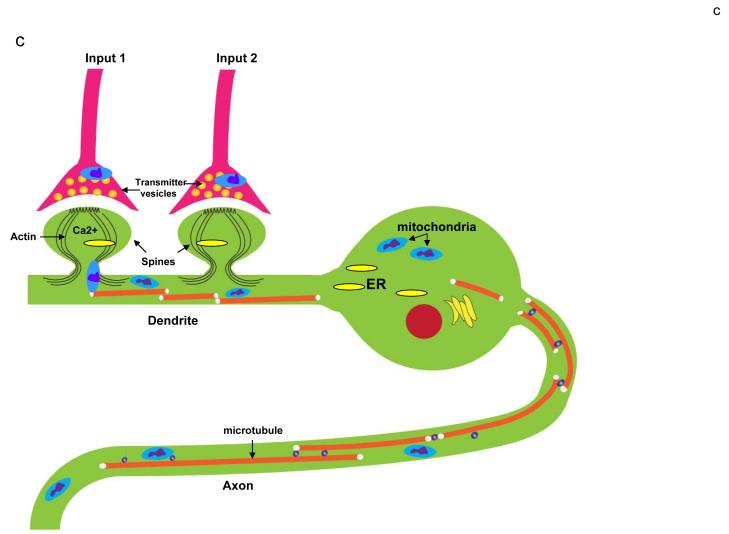
Figure 4.
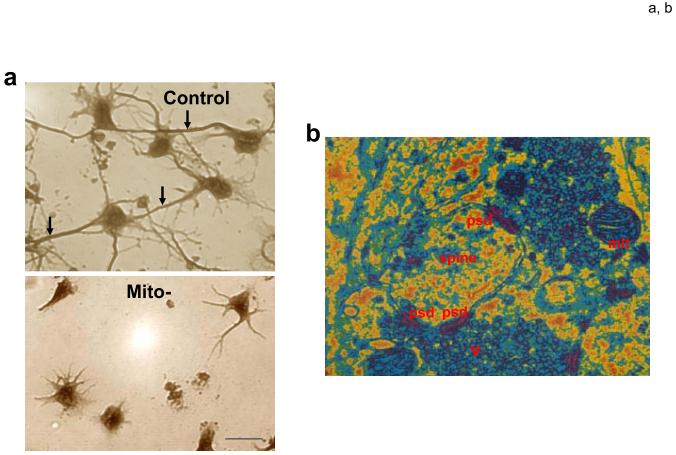
Roles of mitochondria in developmental and synaptic plasticity. a. Mitochondria play a pivotal role in axogenesis. At the time of plating in cell culture, embryonic rat hippocampal neurons were not (control) or were (Mito-) treated with ethidium bromide which damages mitochondrial DNA thereby rendering the ETC dysfunctional. Images show neurons approximately 4 days later; the neurons in the control culture elaborated long axons (arrows) and shorter dendrites, whereas the Mito- neurons formed only shorter process and no axon. b. Electron microscopic image of synapses in the adult rodent brain showing a dendritic spine with three postsynaptic densities (psd), two presynaptic terminals with numerous synaptic vesicles (v) and a mitochondrion (mit) in one presynaptic terminal. c. Involvement of mitochondrial motility in synaptic plasticity. This example shows a neuron receiving synaptic inputs onto two dendritic spines. Activation of Input 1 results in Ca2+ influx into the dendritic spine which induces the local engagement of cytoskeleton-mitochondria interactions resulting in the translocation of a mitochondrion to the base of that spine. In contrast, mitochondria are not recruited to an adjacent inactive synapse (Input 2). Mitochondrial transport along axons and dendrites may also be influenced by action potentials. By moving to regions of active synapses mitochondria may contribute to plasticity by increasing the local supply of ATP and by buffering and releasing Ca2+. ER, endoplasmic reticulum.
Synapses that are very active become potentiated resulting in long-term increases in the size and functional “strength” of those synapses, a form of synaptic plasticity implicated in learning and memory (Harms et al., 2008). Recent findings in which the motility and function of mitochondria have been visualized in experimental models suggest that mitochondria play active roles in synaptic plasticity. During synaptogenesis the movement of mitochondria into dendritic protrusions correlates with the morphological plasticity of developing spines; impairment of the dynamin-like GTPases Drp1 and OPA1 reduces dendritic mitochondria content and causes a loss of synapses and dendritic spines, whereas increasing dendritic mitochondrial content enhances the number and plasticity of spines and synapses (Li et al., 2004). Genetic manipulations of syntaphilin have revealed a role for this protein as a negative regulator of mitochondrial motility in axons; syntaphilin mutant neurons exhibit enhanced short-term facilitation during prolonged stimulation, probably by affecting mitochondria-mediated Ca2+ signaling at presynaptic terminals (Kang et al., 2008). Moreover, in Drosophila Milton interacts with kinesin and is required for transport of mitochondria to presynaptic terminals; photoreceptors mutant for Milton show aberrant synaptic transmission despite normal phototransduction (Stowers et al., 2002). It remains to be established if and how recruitment of mitochondria to active synapses contributes to long-term changes in synaptic strength.
In addition to movements of mitochondria within axons and dendrites, changes in mitochondrial functions (Ca2+ regulation, energy metabolism and oxyradical production) also play roles in synaptic plasticity. Post-tetanic potentiation, a form of plasticity that arises from a persistent presynaptic Ca2+ elevation following tetanic stimulation, is blocked by inhibitors of mitochondrial Ca2+ uptake and release (Tang and Zucker, 1997). Blocking the PTP with cyclosporin A results in an increase of basal synaptic transmission and impairs synaptic plasticity (Levy et al., 2003). Other findings suggest that presynaptic mitochondria play a role in the maintenance of synaptic transmission by sequestering Ca2+ and thereby accelerating recovery from synaptic transmission during periods of moderate to high synaptic activity (Billups and Forsythe, 2002). Neurons in Drosophila Drp1 mutants exhibit synapses devoid of mitochondria and elevations in resting Ca2+ levels at neuromuscular junctions (Verstreken et al., 2005). Basal synaptic transmission is largely normal in Drp1 mutant flies, but during intense stimulation mutants fail to maintain normal neurotransmission. Although exo- and endocytosis are normal in the mutants, the ability to mobilize reserve pool vesicles is impaired as the result of reduced ATP availability. Moreover, age-related cognitive impairment, and presumably the synaptic plasticity subserving learning and memory, is associated with structural abnormalities in mitochondria and oxidation of RNA and DNA (Liu et al., 2002a).
Emerging findings suggest roles for mitochondria as mediators of at least some of the effects of glutamate and BDNF on synaptic plasticity. An increasing number of signaling functions for mitochondria are being discovered (Supplemental Figure 3). For example, glutamate receptor-mediated patterned synaptic activity, such as occurs during stimulus train-induced bursting, results in slow and prolonged changes in mitochondrial potential that exhibit both temporal and spatial correlations with the intensity of the electrical activity (Bindokas et al., 1998). The patterned changes in mitochondrial membrane potential involve glutamate receptor-mediated Ca2+ influx. Synaptic activation of glutamate receptors may also affect mitochondrial bioenergetics independently of Ca2+ influx as suggested by studies of stimulus-evoked changes in NAD(P)H fluorescence at CA1 synapses in hippocampal slices (Shuttleworth et al., 2003). Several neurotrophic factors have been shown to modify synaptic plasticity including BDNF, which plays a pivotal role in hippocampus-dependent learning and memory (Lu et al., 2008). BDNF promotes synaptic plasticity, in part, by enhancing mitochondrial energy production because it increases glucose utilization in cultured cortical neurons in response to enhanced energy demand (Burkhalter et al., 2003) and increases mitochondrial respiratory coupling at complex I (Markham et al., 2004). Consistent with this possibility, BDNF expression and signaling is increased in response to environmental factors such as exercise and cognitive stimulation that increase cellular energy demand (Mattson et al., 2004).
Many questions remain unanswered concerning the roles of mitochondria in synaptic plasticity. Are mitochondria in presynaptic terminals phenotypically different than those in dendrites? If and how does synaptic activity affect mitochondrial function and motility? This could be determined by combining manipulations of specific signaling components (glutamate receptors, Ca2+-dependent and other kinases, cyclic nucleotides, nitric oxide, etc.) with high resolution imaging-based measurements of mitochondrial motility and functional status (membrane potential, ROS and Ca2+ levels, etc.). Behavioral and electrophysiological evaluation of synaptic plasticity in mice with a conditional knockdown of proteins with specific roles in mitochondrial motility (Drp1, Fis1, Mfn and OPA) and function (components of the mitochondrial ETC, PTP, Ca2+ handling systems, for example) should establish the roles of these proteins in synaptic plasticity.
Pivotal Roles for Mitochondria in Neuronal Cell Death
Two general types of cell death are widely recognized - necrosis and PCD (Supplemental Figure 4). Necrosis is sometimes referred to as accidental cell death, implying that cells do not execute preexisting programs; instead they are caught “off guard” in these situations. In PCD the cell “decides” to die and so activates a molecular suicide cascade that involves the production and mobilization of various proteins that can be considered as excecutioners.
Necrosis
Necrosis is not typical for developmental or tissue turnover-related cell death. Instead, neuronal necrosis occurs under conditions of severe energy deprivation and oxidative and ionic stress. A neuron undergoing necrosis dies rapidly as the result of cell swelling, activation of proteases and the rupture of cell membranes (Supplemental Figure 4b). During necrosis intracellular contents, which contain neurotoxic substances such as glutamate and lysosomal enzymes, are spewed into the extracellular space (Nieminen, 2003). A typical trigger for necrosis is ATP depletion caused by severe ischemia, glutamate toxicity or mitochondrial toxins. Moreover, apoptotic stimuli can cause necrosis when cytosolic ATP is depleted because apoptosis requires ATP for its execution. During necrosis mitochondria accumulate Ca2+, their inner membrane becomes structurally disorganized and damaged, and the mitochondria swell and may rupture. In some instances, antagonists of ionotropic glutamate receptors or activate ATP-sensitive K+ channels can preserve mitochondrial function and prevent necrosis (Rogawski, 1993; Liu et al., 2002b). It may also be possible to protect neurons against necrosis by blocking pivotal enzymatic events, such as activation of c-jun N-terminal kinase (JNK) (Arthur et al., 2007) and calpains (Widdowson et al., 1997). The rapidity of the necrosis process severely limits the ability of the cell to survive, therefore emphasizing the importance of preventing exposure to the necrotic insult - eating well and exercising to reduce the chance of a stroke, for example.
Programmed Cell Death
Apoptosis is the prototypical form of PCD in neurons during development and adult cell turnover, and it may also occur in a range of neurodegenerative conditions (see section on neurological disorders below). Morphologically it is characterized by cell shrinkage, membrane blebbing and karyorrhexis. On a biochemical level two apoptotic cascades exist, an intrinsic pathway in which mitochondria play a pivotal role and an extrinsic pathway that bypasses mitochondria (see Stefanis, 2005 for review). The intrinsic pathway (Supplemental Figure 4c), which predominates in neurons, is initiated with a cell death signal such as trophic factor withdrawal, moderate overactivation of glutamate receptors, oxidative stress and DNA damage. The apoptotic trigger often activates kinases such as JNK and transcription factors such as p53 that induce the expression and mitochondrial translocation of the pro-apoptotic Bcl-2 family members Bax and Bak which form pores in the outer mitochondrial membrane. The increased permeability of the outer mitochondrial membrane also involves the formation of a large multiprotein permeability transition pore (PTP) through which cytochrome c passes from the mitochondrial inter-membrane space to the cytosol. In the cytosol cytochrome c binds to apoptosis protease activating factor 1 (Apaf-1) and ATP (or dATP) to form the apoptosome which then recruits and activates the initiator caspase 9 (Riedl and Salvesen, 2007). Caspase 9 then cleaves and activates caspase 3 which, in turn, cleaves numerous protein substrates that execute the cell death process.
There are several additional points of regulation of the apoptotic process. For example, Bax and Bak actions can be inhibited by Bcl-2, Bc1-xL or other anti-apoptotic members of the Bcl-2 family, and caspases can be inhibited by inhibitor of apoptosis proteins (IAPs). IAPs on the other hand can be inhibited by the mitochondrial protein secondary mitochondria-derived activator of caspases (Smac/DIABLO) and high temperature requirement protein A2 (HtrA2/Omi). Bax and Bak action can be enhanced/initiated by proapoptotic members of the same family, most importantly by caspase 3-cleaved (truncated) Bid (tBid). In the nucleus caspase 3 activates caspase activated DNase (CAD) (Enari et al., 1998) which cleaves nuclear DNA between histones, creating DNA fragments of ∼160 bp or multiples thereof. Thus, cytochrome c release, caspase 3 activation and nucleosomal DNA cleavage are three biochemical hallmarks of apoptosis.
While caspase inhibition can prevent cell death, it does not prevent cytochrome c release into the cytosol, and may thereby compromise mitochondrial ATP generation while simultaneously increasing superoxide production. Consequently, caspase inhibiton can render neurons vulnerable to necrosis. Similarly, AIF and HtrA2 are essential components of mitochondrial structure and function, and their release into the cytosol impairs mitochondrial function (Cheung et al., 2006, Martins et al., 2004). Some evidence suggests that mitochondria can release cytochrome c, Smac, HtrA2 and AIF with some degree of selectivity, but the mechanism is unclear (Huang et al., 2001, Wang et al., 2004). Nevertheless, PCD is reduced in mice deficient in Apaf-1(Yoshida et al., 1998), caspase 3 (Kuida et al., 1996) or caspase 9 (Hakem et al., 1998), and in mice in which cytochrome c is mutated so that it cannot activate caspase 9 but still functions as an electron carrier (Hao et al., 2005). The phenotype of each of the latter mice is characterized by a lack of cell death in CNS precursor populations resulting in gross misdevelopment of the brain and either embryonic or early postnatal death. Remarkably, caspase 3-deficient mice do not have any overt phenotype when bred on a C57BL/6 genetic background, in contrast to the cortical dysgenesis observed on a mixed genetic background (Leonard et al., 2002); the reason for this effect of genetic backgrounds is unknown.
In most cells Bax and Bak can compensate for each other; however, in cultured cerebellar granule neurons and sympathetic neurons Bax deficiency alone very effectively inhibits trophic factor withdrawal-induced apoptosis (Miller et al, 1997). Bax deficiency does not prevent neuronal atrophy or downregulation of cell metabolism, but rather prevents the execution of apoptosis. Combined deficiency of Bax and Bak is very effective in preventing outer mitochondrial membrane permeability and apoptosis, resulting in CNS dysgenesis (Lindsten et al., 2000). In contrast, Bid-deficient mice survive into adulthood without obvious developmental abnormalities (Yin et al., 1999), yet their neurons exhibit increased resistance to ischemic injury (Plesnila et al., 2001). The proteins that form the PTP downstream of Bax, Bak and Bid interactions with the mitochondrial membrane, and the mechanism of pore formation remain elusive. The longstanding concept that the adenine nucleoside transporter (ANT) and voltage dependent anion channel (VDAC) constitute the mitochondrial PTP has been recently challenged by the phenotypes of mice deficient in these proteins (see Juhaszova et al., 2008 for review).
While apoptotic PCD involves the entire cell, apoptotic biochemical cascades that involve mitochondria can be triggered locally within dendrites and axons, where they may play roles in neuritic and synaptic structural and functional plasticity. Activation of glutamate receptors in the dendrites of embryonic hippocampal neurons results in a local increase of mitochondrial membrane permeability and activation of caspase 3 in the dendrites (Mattson et al., 1998). The latter study also showed that exposure of isolated synapses to triggers of apoptosis results in mitochondrial membrane depolarization, ROS production and Ca2+ accumulation and activation of caspase 3. Prostate apoptosis response 4, a mediator of neuronal apoptosis that acts upstream of mitochondrial alterations, can also be activated locally in synapses (Duan et al., 1999). Other studies have shown that inhibitors of caspases 1 and 3 can modify long-term potentiation of synaptic transmission (LTP) at hippocampal synapses (Gulyaeva et al., 2003; Lu et al., 2006), suggesting roles for apoptotic cascades in synaptic plasticity. Cleavage of the GluR1 and GluR4 subunits of AMPA glutamate receptors by caspase 3 may mediate some of the effects of the intrinsic apoptotic pathway on synaptic plasticity, and may also provide a mechanism that (by reducing Na+ and Ca2+ influx) prevents excitotoxic necrosis (Glazner et al., 2000; Lu et al., 2002). A role for mitochondrial apoptotic cascades in synaptic remodeling in vivo is suggested by the demonstration that caspase 3 activity increases transiently in dendritic spines in auditory forebrain of the zebra finch in response to exposure to tape-recorded birdsong (Huesmann and Clayton, 2006). Selective blockade of caspase 3 impairs consolidation of a persistent physiological trace of the song stimulus, thus establishing a role for caspase 3 in learning and memory.
In addition to their role in the most common caspase-mediated form of PCD, mitochondria also mediate a different type of neuronal death that is caspase independent (CIPCD) in which the outer mitochondrial membrane becomes permeable, but instead of cytochrome c, apoptosis inducing factor (AIF) is released (Supplemental Figure 4d). CIPCD in neurons has been most clearly demonstrated in cell death paradigms in which activation of poly (ADP-ribose) polymerase 1 (PARP-1) and the generation of poly ADP-ribose (PAR) polymers occurs (Yu et al., 2002, 2006). PARP-1 is an NAD+-dependent enzyme that is activated in neurons by overactivation of glutamate receptors and in response to DNA damage and is required for AIF release from mitochondria. PAR polymers associate with mitochondria and induce the release of AIF which then translocates to the nucleus and triggers nuclear DNA fragmentation.
Despite the evidence that mitochondria are pivotal arbitrators of neuronal cell survival and death decisions, critical issues remain unresolved. The identities of the proteins that mediate key death-regulatory systems within mitochondria are unknown including components of the PTP and ATP-sensitive K+ channels. In addition, the physiological roles of subthreshold levels of activation of “death events” (PTP opening, cytochrome c release, Ca2+ uptake and release, etc.) remain to be established. The evidence that the latter events can occur locally and transiently in synaptic terminals, dendrites and axons suggests important roles in synaptic plasticity and neurite outgrowth. We believe that future studies will reveal a range of non-apoptotic functions for different caspases and other “apoptotic” proteins (Bcl-2 and p53 family members and PARP-1, for example) in processes ranging from neurogenesis to synaptic plasticity.
Neurological Disorders
Several inherited diseases are caused by mutations in mitochondrial DNA, and the cell types most affected these disorders are those with high energy demands including muscle cells and neurons (Wallace, 2005). However, the most common neurological disorders (Alzheimer’s, Parkinson’s and Huntington’s diseases, stroke and psychiatric disorders) are not caused by mitochondrial mutations, but instead typically involve interactions of nuclear genetic and environmental variables. In this section we describe the current state of understanding of the roles of mitochondria in the dysfunction and degeneration of neurons in major neurological disorders.
Ischemic stroke
During a stroke the drastic reduction in blood supply to neurons normally perfused by the affected cerebral blood vessel results in cellular hypoxia and glucose deprivation leading to greatly diminished ETC activity and ATP depletion (Moustafa and Baron, 2008). Histologically, the brain tissue affected by a stroke consists of a core region where ischemia is severe and the neurons die rapidly by necrosis, and a surrounding penumbra where the neurons may die over a period of hours to several days by a PCD mechanism(s). The involvement of mitochondria in ischemic neuronal death in the penumbra is supported by considerable data from experimental models (particularly middle cerebral artery occlusion - reperfusion studies in rodents) reviewed in detail elsewhere (Zheng et al., 2003) including: Ca2+ influx through NMDA receptors and accumulation of Ca2+ in mitochondria (Zhang and Lipton, 1999); mitochondrial O2-. production and consequent oxidative damage to proteins, lipids and DNA (Keller et al., 1998); p53-mediated Bax expression and facilitation of PTP formation (Culmsee and Mattson, 2005; Endo et al., 2006); and PTP opening, cytochrome c release and activation of caspases 9 and 3 (Friberg et al., 1998; Korde et al., 2007). In addition to Bax, Bid has been shown to be a pivotal promoter of mitochondrial PTP formation and cytochrome c release in neurons undergoing ischemic cell death (Plesnila et al., 2001). Several putative components of the PTP have been implicated in ischemic neuronal death including cyclophilin D (Schinzel et al., 2005) and porin (Perez Velazquez et al., 2000). Recently a novel protein called pancortin-2 was shown to promote mitochondria-mediated apoptosis during cerebral ischemia by interacting with the actin-associated protein WAVE1 and sequestering Bcl-xL thereby preventing Bcl-xL from stabilizing mitochondrial membranes (Cheng et al., 2007). Interestingly, mitochondrial fission occurs in neurons during ischemic conditions and may contribute to their death because prevention of fission by knockdown of Drp1 or overexpression of Mfn1 protects neurons against hypoxic death (Barsoum et al., 2006).
Two additional classes of mitochondrial proteins that play important roles in protecting neurons against excitotoxic and ischemic injury are K+ channels and uncoupling proteins (UCPs). Treatment with the mitochondrial K+ channel opener diazoxide reduces mitochondrial Ca2+ uptake and oxidative stress, prevents PTP formation and protects neurons against excitotoxic and ischemic injury (Liu et al., 2002b). Mitochondrial UCPs reduce mitochondrial oxyradical production by dissipating the hydrogen ion gradient across the inner mitochondrial membrane. Neurons in transgenic mice overexpressing UCP-2 exhibit increased resistance to death in models of focal ischemic stroke and traumatic brain injury (Mattiasson et al., 2003). Studies of cultured neurons in which UCP-4 levels were reduced using RNA interference methods demonstrated roles for UCP-4 in modifying cellular energy metabolism (decreasing ETC activity and increasing glucose uptake and glycolysis) and reducing oxyradical production in ways that increase the resistance of the neurons to excitotoxic and oxidative insults (Liu et al., 2006). UCP-4 activity reduces mitochondrial Ca2+ accumulation and store-operated Ca2+ entry, a process in which depletion of ER Ca2+ stores triggers Ca2+ influx through plasma membrane Ca2+ channels (Chan et al., 2006).
Finally, studies of ischemic death have revealed roles for mitochondria in a process called preconditioning hormesis in which the expression of genes that encode cytoprotective proteins including chaperones such as heat-shock protein 70 (HSP-70) and glucose-regulated protein 78 (GRP-78), anti-apoptotic proteins such as Bcl-2 and antioxidant enzymes is increased (Arumugam et al., 2006). Ischemic preconditioning hormesis has been shown to block ischemia-induced translocation of BAD to mitochondria, Bcl-xL cleavage and PTP formation in mitochondria (Miyawaki et al., 2008). Bcl-2 may protect neurons against excitotoxicity and ischemic injury by enhancing the ability of mitochondria to sequester large quantities of Ca2+ while their respiratory function is maintained (Murphy et al., 1996). Several therapeutic strategies for stroke may act, in part, by inducing an adaptive mitochondrial stress response including dietary energy restriction (Yu and Mattson, 1999) and mitochondrial uncouplers (Korde et al., 2005). Thus, in addition to reducing risk factors such as hypertension, a lifestyle that includes regular exposure to dietary and behavioral factors that induce an adaptive cellular stress response in neurons would be expected to improve the outcome in individuals who do suffer a stroke (Mattson and Cheng, 2006).
Alzheimer’s disease
Alzheimer’s disease (AD) affects nearly 5 million Americans, and will soon become the second leading cause of death as more people live into their seventh and eighth decades. AD involves progressive synaptic dysfunction and then death of neurons in brain regions critical for learning and memory processes (hippocampus and entorhinal and frontal cortices, and associated structures), and is characterized histopathologically by the accumulation of extracellular plaques comprised of amyloid β-peptide (Aβ) and intracellular neurofibrillary tangles which are aggregates of the microtubule-associated protein tau (Goedert and Spillantini, 2006). Proteolytic processing of the amyloid precursor protein (APP) by β- and γ-secretases results in increased production of Aβ, particularly the longer 42 amino acid form (Fig. 5). The critical role of Aβ production, self-aggregation and neurotoxicity in AD has been established from genetic studies that identified mutations in APP and presenilin-1 (the enzymatic component of the γ-secretase protein complex) as the causes of many cases of early-onset dominantly inherited AD, and by investigations of animal and cell culture models of AD (see Mattson, 2004; Hardy, 2006 for review). The mechanisms of neuronal degeneration downstream of Aβ involve membrane-associated oxidative stress, perturbed Ca2+ homeostasis, impaired energy metabolism and possibly apoptosis, suggesting roles for mitochondrial alterations in the disease process.
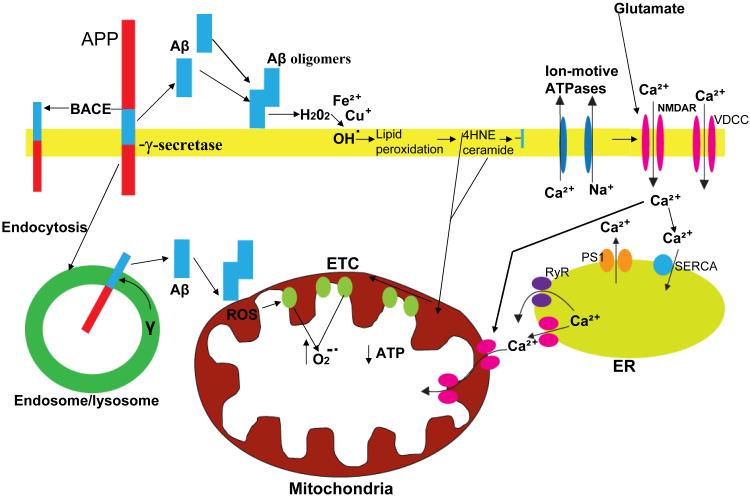
Figure 5.
Involvement of amyloidogenic APP processing, oxidative stress and perturbed cellular Ca2+ homeostasis in mitochondrial dysfunction in Alzheimer’s disease. Amyloidogenic processing of the β-amyloid precursor protein (APP) involves sequential cleavages by β-secretase (BACE) which cleaves APP at the cell surface and γ-secretase which cleaves within the membrane-spanning domain of APP resulting in the liberation of the amyloid β-peptide (Aβ). Aβ monomers interact to form oligomers and during this process Aβ may interact with Fe2+ or Cu+ to generate H2O2 and OH. resulting in membrane lipid peroxidation and the generation of 4-hydroxynonenal (HNE) and ceramide. By impairing the function of plasma membrane ion-motive ATPases (Na+ and Ca2+ pumps) and glucose and glutamate transporters (not shown) HNE promotes excessive Ca2+ influx through N-methyl-D-aspartate (NMDA) receptors and voltage-dependent Ca2+ channels (VDCC). Ab may also form Ca2+-conducting pores in the plasma membrane. Excessive Ca2+ influx and release from endoplasmic reticulum (ER) stores may then result in excessive Ca2+ uptake into mitochondria and impairment of their function. Alternatively, HNE and ceramide may diffuse to mitochondria and directly damage mitochondrial membranes. Aβ may also be generated intracellularly in an endosomal/lysosomal compartment; intracellular Aβ may interact with and damage mitochondrial membranes. In these ways, Aβ may impair mitochondrial ATP production and Ca2+ regulation, with adverse consequences for neuronal plasticity and survival.
Positron emission tomography measurements of radiolabeled 2-deoxyglucose uptake into brain cells of living subjects have demonstrated reduced energy metabolism in affected brain regions of AD patients, and recent prospective imaging studies suggest that the cellular energy deficit precedes cognitive symptoms (Mosconi et al., 2008). Cytochrome c oxidase activity is decreased in the brains of AD patients compared to age-matched control subjects (Maurer et al., 2000). Neurons that exhibit increased oxidative stress in AD also exhibit damaged mitochondria and increased autophagy (Hirai et al., 2001; Moreira et al., 2007). Studies of cybrid cells prepared by fusion of platelets from AD patients and control subjects with an immortal cell line have provided evidence for a widespread defect in mitochondrial function characterized by reduced cytochrome c oxidase activity and increased free radical production (Swerdlow et al., 1997). The cause of the mitochondrial alterations in cells of AD patients is unknown, but may involve aging and disease-related increases in Aβ levels, oxidative stress and reduced cellular energy availability.
Aβ may promote neuronal mitochondrial dysfunction in neurons in AD because exposure of cultured neurons to Aβ results in increased mitochondrial O2-. production, decreased ATP production and increased mitochondrial Ca2+ uptake that can trigger opening of the PTP and apoptosis (Hashimoto et al., 2003). Aβ impairs mitochondrial function by inducing membrane lipid peroxidation and the production of 4-hydroxynonenal (Bruce-Keller et al., 1998), a toxic aldehyde that can impair the function of synaptic mitochondria (Keller et al., 1997). In addition, Aβ can increase the hydrolysis of membrane sphingomyelin by sphingomyelinases, resulting in the production of ceramides (Cutler et al., 2004), thus triggering mitochondria-mediated neuron death by a mechanism involving dephosphorylation of Akt, BAD and GSK-3β (Stoica et al., 2003). Ceramide alters mitochondrial Ca2+ homeostasis and triggers apoptosis by inducing cyclin-dependent kinase 5-mediated phosphorylation of tau resulting in the clustering of mitochondria with the ER thereby increasing the mitochondrial uptake of Ca2+ released from the ER (Darios et al., 2005). Aβ may also act directly on mitochondria by impairing electron transport (Manczak et al., 2006; Veereshwarayya et al., 2006) and interacting with the mitochondrial protein Aβ-binding alcohol dehydrogenase (ABAD) which impairs the binding of NAD to ABAD (Lustbader et al., 2004). It has also been suggested that APP is present in mitochondria where it can be processed by Aβ-generating presenilin-1/γ-secretase (Hansson et al., 2004) and by the mitochondrial protease HtrA2 which generates a 161 amino acid non-amyloidogenic C-terminal APP fragment (Park et al., 2006). A remaining unanswered question is how Aβ interacts with membrane lipids and proteins to perturb mitochondrial function, but peptide oligomerization and metal (Fe2+ and Cu+) generation of hydrogen peroxide are implicated (Mattson, 2004).
Mechanisms of mitochondrial dysfunction in AD that are independent of Aβ have also been suggested. For example, presenilin mutations may promote mitochondrial dysfunction by perturbing ER Ca2+ handling which, in turn, promotes synaptic mitochondrial Ca2+ overload and can trigger apoptosis (Bezprozvanny and Mattson, 2008). Wild type presenilin-1 may function as a Ca2+ leak channel in the ER, and AD-causing mutations impair this Ca2+-regulating function of presenilin-1. It has also been shown that presenilin-1 mutations impair kinesin-based axonal transport (Pigino et al., 2003), suggesting that presenilin-1 may influence the movement of mitochondria to axon terminals. Increasing evidence also suggests a role for mitochondrial alterations upstream of Aβ and tau pathologies in AD. Cytochrome c oxidase deficiency in neurons results in decreased Aβ accumulation and lower levels of oxidative stress in a mouse model of AD (Fukui et al., 2007). Mitochondrial superoxide accumulation may contribute to the AD process because APP mutant mice with reduced levels of manganese SOD exhibit worsening of behavioral deficits and dendritic pathology (Esposito et al., 2006). Altogether, the available data identify several possible targets for therapeutic interventions that stabilize mitochondria in AD including β- and γ-secretase inhibitors, antioxidants, mitochondrial membrane-stabilizing agents, and agents that target Ca2+-regulating proteins. Exercise, cognitive stimulation and dietary energy restriction may also protect mitochondria against AD-associated dysfunction, in part, by increasing neurotrophic factor signaling (Arumugam et al., 2006).
In order to better understand the roles of mitochondrial alterations in AD, longitudinal studies of molecular, structural and functional aspects of mitochondria in neurons in animal models of AD are needed. Parallel studies in which the effects of specific manipulations of mitochondrial functions (ATP and ROS production, Ca2+ handling, etc.) on Aβ and tau pathologies and synaptic plasticity are evaluated would help clarify if and how mitochondria modify AD pathogenic processes.
Parkinson’s disease
Evidence supports a major role for mitochondrial alterations in Parkinson’s disease (PD), the most common movement disorder (see Schapira, 2008 for review). Degeneration of dopaminergic neurons in the substantia nigra underlies motor dysfunction in PD, and the disease also involves degeneration of neurons in regions of the brain controlling autonomic functions, cognition and mood (Braak et al., 2003). Neurons that degenerate in PD often contain intracellular inclusions of the protein α-synuclein which form the so-called Lewy bodies. The causes of sporadic cases of PD are unknown, but decreased levels of mitochondrial complex I activity are associated with the disease process (Swerdlow et al., 1996). Complex I in PD has oxidatively damaged and misassembled protein subunits (Keeney et al., 2006). In addition, the environmental toxins rotenone and MPTP cause degeneration of dopaminergic neurons and PD-like symptoms in animal models by a mechanism involving selective inhibition of complex I (Gerlach et al., 1991; Panev et al., 2005) suggesting that complex I impairment may be sufficient to cause the disease. Mitochondrial DNA deletions have also been implicated in the degeneration of dopaminergic neurons (Biskup and Moore, 2006), although whether mitochondrial DNA damage is pivotal for PD remains unknown.
Some cases of PD are inherited and have an early age of disease onset (see Bogaerts et al., 2008 for review). Mutations in α-synuclein (PARK1), LRRK (PARK8; leucine-rich repeat kinase 2) and UCHL1 (PARK5; ubiquitin C-terminal hydrolase L1) cause PD that is inherited in an autosomal dominant manner, while mutations in Parkin (PARK2), DJ-1 (PARK7) and PINK1 (PARK6; PTEN induced putative kinase 1) cause PD when inherited from both parents (recessive mutations). Mutations in the gene encoding the mitochondrial serine protease HTRA2 (PARK13; high temperature requirement A2) have also been linked to PD in several families. α-Synuclein is located in presynaptic terminals where it may play a role in dopaminergic signaling (Abeliovich et al., 2000). α-Synuclein is normally degraded by the ubiquitin - proteasome pathway, and studies of cultured cells (Saha et al., 2000) and a family with inherited PD caused by a triplication of the α-synuclein gene (Singleton et al., 2003) suggest that even modest intracellular accumulation of α-synuclein can result in the dysfunction and degeneration of dopaminergic neurons. (Fig. 6).
Figure 6.
The degeneration of dopaminergic neurons in Parkinson’s disease involves impaired ETC function, proteasomal overload and excitotoxicity. Mitochondrial complex I activity is reduced in vulnerable neurons in PD, likely as the result of a combination of normal aging, exposures to environmental toxins and genetic factors. The resulting ATP depletion and increased levels of ROS render neurons vulnerable to excitotoxic Ca2+ overload. Mutations in genes that cause inherited PD (α-synuclein, Parkin, DJ-1, PINK1, UCHL1 and LRRK2) may adversely affect mitochondrial function either indirectly or directly. Mutations of α-synuclein (or increased amounts of wild-type α-synuclein caused by increased expression or decreased proteasomal degradation) results in the formation of α-synuclein oligomers which may exacerbate ROS-mediated damage to mitochondrial membranes and proteins. UCHL2 mutations may contribute to proteasomal overload in PD. Parkin is a ubiquitin E3 ligase that plays important roles in removing damaged proteins from neurons; this E3 ligase activity is reduced in PD resulting in excessive accumulation of damaged/neurotoxic proteins. Parkin may affect one or more proteins of the PTP, thereby preventing cytochrome c release and apoptosis. DJ-1 is a mitochondrial protein that reduces ROS and blocks PTP formation. PINK1 is important for the maintenance of membrane potential and suppression of oxidative stress. Thus, mutations in DJ-1 and PINK1 promote damage to mitochondria. E1, ubiquitin E1 ligase; E2, ubiquitin E2 ligase; LRRK2, leucine-rich repeat kinase 2; UCHL1, ubiquitin C-terminal hydrolase L1.
Overexpression of wild-type α-synuclein in cultured cells results in the formation of α-synuclein-immunopositive inclusions, increased levels of oxidative stress and mitochondrial alterations that are ameliorated by treatment with antioxidants (Hsu et al., 2000). Inducible expression of mutant α-synuclein in PC12 cells impairs proteasome activity and increases the vulnerability of the cells to mitochondria-mediated (PTP- and caspase-3-dependent) apoptosis (Tanaka et al., 2001). Mitochondrial dysfunction and damage have been documented in brain cells of α-synuclein mutant mice and Parkin-deficient mice (Stichel et al., 2007). α-Synuclein A53T mutant mice develop mitochondrial DNA damage and neuronal degeneration, and evidence for apoptosis of neocortical, brainstem and motor neurons (Martin et al., 2006). A proteomic analysis of brain tissue samples from transgenic mice overexpressing A30P α-synuclein revealed increased oxidative modification and impaired enzymatic activity of the metabolic proteins carbonic anhydrase 2, alpha-enolase and lactate dehydrogenase 2 (Poon et al., 2005). Mutant α-synuclein may adversely affect mitochondria by actions at the outer mitochondrial membrane or by accumulating inside the mitochondria (Devi et al., 2008).
Parkin is an ubiquitin E3 ligase that associates with mitochondrial membranes and can protect mitochondria against apoptotic PTP opening and cytochrome c release (Darios et al., 2003). Drosophila deficient in Parkin exhibit locomotor deficits due to apoptotic death of muscle cells and reduced lifespan; mitochondrial abnormalities occur early in the process of muscle degeneration (Greene et al., 2003). A proteomic analysis of Parkin-deficient mice revealed decreased levels of several subunits of complexes I and IV, and striatal cells from the mice exhibited reduced mitochondrial respiratory capacity and decreased antioxidant capacity (Palacino et al., 2004). Parkin may directly interact with DJ-1, and PD DJ-1 mutants reduce this interaction resulting in failure of Parkin to ubiquitinate and enhance the degradation of mutant DJ-1 (Moore et al., 2005).
Cell fractionation and immuno-ultrastructural analysis showed that DJ-1 is present in the mitochondrial inter-membrane space and in the matrix, and that PD-linked DJ-1 mutants are similarly localized in mitochondria (Zhang et al., 2005). DJ-1 functions to reduce mitochondrial oxidative stress and recent findings suggest that DJ-1 exhibits peroxiredoxin-like peroxidase activity (Andres-Mateos et al., 2007). Oxidation of DJ-1 to form cysteine-sulfinic acid at cysteine 106 results in mitochondrial localization of DJ-1 and protection against cell death (Canet-Aviles et al., 2004). DJ-1 is also located in mitochondria in Drosophila neurons, and Drosophila DJ-1 mutants exhibit locomotor dysfunction which is exacerbated by oxidative stress (Park et al., 2005). Overexpressing α-synuclein or reducing levels of Parkin or DJ-1 render C. elegans vulnerable to mitochondrial complex I inhibitors, but not direct oxidative insults (Ved et al., 2005), consistent with mitochondrial impairment being a common mechanism by which different familial PD mutations promote neuronal degeneration.
PINK1 is a serine/threonine kinase located in mitochondria where it may associate with outer and inner membranes. PINK1 appears to play an important role in mitochondrial maintenance because depletion of PINK1 in cultured cells results in abnormal mitochondrial morphology and membrane depolarization, and similar mitochondrial alterations are present in primary cells from patients with PINK1 mutations (Exner et al., 2007). Inactivation of PINK1 in Drosophila results in degeneration of dopaminergic neurons and muscle cells that is preceded by mitochondrial enlargement and disintegration (Yang et al., 2006). In Drosphila PINK1 is required for normal mitochondrial function, and Parkin can rescue mitochondrial function in PINK1 mutants (Clark et al., 2006; Park et al., 2006b). PINK1 may regulate mitochondrial biogenesis because it has been shown to interact with proteins that control the processes of mitochondrial fission and fusion; overexpression of PINK1 increases mitochondrial fission, whereas depletion of PINK1 increases fusion (Yang et al., 2008). Loss-of-function mutations of Drp1, a mitochondrial fission-promoting protein, are largely lethal in a PINK1 or parkin mutant background, whereas cell degeneration and mitochondrial morphological alterations caused by PINK1 and Parkin mutations are suppressed by Drp1 (Poole et al., 2008). PINK1 may protect mitochondria against oxidative stress by phosphorylating the mitochondrial chaperone protein TRAP1 (Pridgeon et al., 2007). Thus, mutations in several proteins that cause familial PD serve important roles in the physiology and plasticity of mitochondria, strongly suggesting a pivotal role for mitochondrial abnormalities in PD.
The details of the molecular events by which α-synuclein accumulation and mutations in Parkin, DJ-1 and PINK1 impair mitochondrial function and trigger dopaminergic neuron death remain to be established. Candidate mechanisms include interactions with mitochondrial ETC proteins, Ca2+-regulating systems, Bcl-2 family proteins and PTP proteins. Despite the lack of a full understanding of molecular pathogenesis, the availability of mitochondrial toxin- and genetic mutation-based cell culture and animal models of PD provide abundant opportunities for preclinical screening of drugs, and dietary and behavioral interventions. Indeed, a range of promising agents (monoamine oxidase inhibitors, anti-apoptotic agents and creatine) are in transition from bench to bedside (Schapira, 2008).
Huntington’s disease
Huntington’s disease (HD), the most common inherited neurodegenerative disorder, is caused by expansions of CAG repeats in the huntingtin gene resulting in polyglutamate repeats in the huntingtin protein (Walker, 2007). Medium spiny neurons in the striatum, and cerebral cortical and brainstem neurons are among the cell populations most severely affected in HD resulting in characteristic motor, cognitive/behavioral and autonomic deficits. Several adverse effects of mutant huntingtin on mitochondria have been reported including: impaired mitochondrial trafficking (Chang et al., 2006); reduced ATP levels in synaptic terminals (Orr et al., 2008); mitochondrial depolarization at lower calcium loads than do mitochondria from controls (Panov et al., 2002); increased sensitivity to Ca2+ overload and NMDA receptor-mediated neuronal apoptosis (Fernandes et al., 2007); enhanced sensitivity to Ca2+-induced decreases in state 3 respiration and membrane potential (Milakovic et al., 2006); a decreased threshold for PTP opening and cytochrome c release (Choo et al., 2004). Impaired mitochondrial electron transport may also contribute to the pathogenesis of HD because exposure of rodents to the succinate dehydrogenase inhibitor 3-nitropropionic acid results in selective damage to striatal medium spiny neurons and motor dysfunction similar to that of humans with HD (Brouillet et al., 2005).
Other findings suggest that mutant huntingtin can adversely affect mitochondria by modifying gene transcription. For example, mutant huntingtin represses the transcription of PGC-1α by interacting with the promoter and interfering with the CREB-dependent PGC-1 α gene expression (Cui et al., 2006). Inhibition of PGC-1α expression limits the ability of the vulnerable neurons to adequately respond to energy demands in HD. Direct toxic effects of mutant huntingtin on mitochondria and energy-dependent neuronal processes such as axonal transport may worsen when protective function of PGC-1α is inhibited. Indeed, crossbreeding of PGC1α deficient mice with HD knockin mice leads to increased neurodegeneration of striatal neurons and motor abnormalities in the HD mice (Cui et al., 2006). In a related study, Weydt et al., (2006) reported reduced expression of PGC1α target genes in caudate nucleus of HD patient postmortem brain tissues using microarray gene expression methods. Overexpression of PGC1-α protects striatal neurons against mutant huntingtin. The transcriptional activity of the pro-apoptotic protein p53 is increased by binding to mutant huntingtin, and levels of p53 are increased in the brains of HD patients and huntingtin mutant mice (Bae et al., 2005). Selective depletion or inhibition of p53 preserves mitochondrial membrane potential and protects cultured cells against mutant huntingtin-induced death, and abrogates behavioral deficits in hungtingtin mutant mice. Drugs that selectively inhibit p53 (Zhu et al., 2002) therefore have a potential for preventing the death of neurons in HD.
Because individuals with HD can be identified by genetic screening it is possible to initiate treatments before they become symptomatic, a fact that provides the opportunity to preserve vulnerable neurons and thereby delay disease onset. In this regard, several drugs in use for other disorders have proved effective in animal models of HD and will hopefully soon be tested in patients including the anti-excitotoxic agent tiagabine (Masuda et al., 2008), the antidepressant paroxetine (Duan et al., 2004) and the histone deacetylase inhibitor sodium butyrate (Ferrante et al., 2003). Advances in molecular biology have led to the development of small interfering RNAs (siRNAs) that selectively suppress expression of mutant huntingtin and retard the disease process in huntingtin mutant mice (DiFiglia et al., 2007). Perhaps someday such approaches can be used in HD patients.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is characterized clinically by progressive neuromuscular dysfunction resulting from degeneration of lower motor neurons (see Bromberg, 2002 for review). While most cases of ALS are sporadic, the disease can be inherited as the result of mutations in Cu/Zn-SOD (SOD1/ALS1), Alsin (ALS2), TDP-43 and other yet-to-be identified genes (Gros-Louis et al., 2006; Sreedharan et al., 2008). Lines of transgenic mice expressing an ALS SOD1 mutation provide a valuable model of the human disease because they reliably develop progressive degeneration of lower motor neurons and die from the disease (see Kato, 2008 for review). Several aspects of the phenotype of lower motor neurons suggest possible reasons why they are selectively vulnerable in ALS (Boillee et al., 2006 for review). Motor neurons are the largest type of neuron with very long (in some cases a meter or more in length) large diameter axons resulting in a both a high energy requirement and the necessity for efficient axonal transport. As with other neurodegenerative disorders, considerable evidence suggests that motor neurons in ALS suffer from increased levels of oxidative stress (Beal et al., 1997; Pedersen et al., 1998; Cutler et al., 2002; Cai et al., 2005), perturbed cellular Ca2+ homeostasis involving an excitotoxic component (Rothstein, 1995; Kruman et al., 1999), cytoskeletal abnormalities (Binet and Meininger, 1988; Manetto et al., 1988), mitochondrial dysfunction (von Lewinski and Keller, 2005) and the triggering of apoptotic biochemical cascades (Sathasivam et al., 2001).
Mitochondria are transported in axons and are present in relatively high amounts in axon terminals of motor neurons where they provide the ATP necessary to support the maintenance and restoration of ion gradients during neuromuscular activity. Ultrastructural analysis of neuromuscular junctions in tissue biopsy samples from ALS patients revealed abnormalities in mitochondrial morphology (Atsumi, 1981). Mitochondrial dysfunction and degeneration have been observed in motor neurons in SOD1 mutant mice. At the onset of motor symptoms there is a massive degeneration of mitochondria in motor neurons which occurs long before the motor neurons die (Kong and Xu, 1998). Other findings suggest that mutant SOD1 impairs fast axonal transport resulting in reduced delivery of mitochondria to axon terminals (De Vos et al., 2007). Mutant Cu/Zn-SOD misfolds and self-aggregates, and it has been reported that misfolded Cu/Zn-SOD binds to mitochondrial membranes (Vande Velde et al., 2008). The interaction of mutant Cu/Zn-SOD with mitochondria may cause a shift in the redox potential resulting in an increased production of oxyradicals (Ferri et al., 2006). Impaired function of Bcl-2 may also contribute to mitochondrial dysfunction in ALS because mutant SOD1 proteins form aggregates that sequester Bcl-2 in spinal cord cells (Pasinelli et al., 2004). A role for perturbed copper metabolism in ALS is suggested be a study showing that overexpression of the copper chaperone for SOD1 in SOD1 mutant ALS mice resulted in severe mitochondrial pathology and a dramatic acceleration of motor neuron degeneration and death of the mice (Son et al., 2007). Mutant SOD1 may not only have untoward effects on mitochondria in motor neurons, but may also cause dysfunction of mitochondria in astrocytes resulting in increased oxyradical production which may adversely affect neurons (Cassina et al., 2008).
Several treatments or genetic manipulations that prevent or counteract mitochondrial impairment have been reported effective in suppressing the disease process in SOD1 mice. Two inhibitors of mitochondrial PTP formation, cyclosporine A and nortriptyline delayed disease onset and increased survival of ALS mice (Keep et al., 2001; Wang et al., 2007). Dietary supplementation with creatine, which enhances cellular energy availability and reduces oxidative stress, protected motor neurons and extended survival of SOD1 mutant mice (Klivenyi et al., 1999). Deletion of the pro-apoptotic protein Bax protected motor neurons from mutant SOD1-induced death, delayed disease onset and extended survival, but failed to prevent neuromuscular denervation and mitochondrial vacuolization (Gould et al., 2006). Such preclinical findings suggest a therapeutic potential for interventions that preserve or enhance mitochondrial energy metabolism, reduce mitochondrial ROS production and/or stabilize mitochondrial membranes.
Psychiatric disorders
Evidence that patients with psychiatric disorders (depression, bipolar disorder and schizophrenia) exhibit mitochondrial abnormalities at the structural, molecular and functional levels has been reviewed recently (Shao et al., 2008). There have been at least 19 case reports of patients with mitochondrial disease presenting with symptoms of a psychiatric disorder which in most cases occurred prior to diagnosis of mitochondrial disease (Fattal et al., 2006). Of these cases, five had major depressive disorder, eight had psychosis, one had bipolar disorder, 3 had an anxiety disorder and one had a major personality change. The latter findings suggest the possibility that a mitochondrial deficit is sufficient to trigger one or more psychiatric disorders. Mitochondrial deficits in idiopathic psychiatric disorders are suggested from positron emission tomography (PET) analysis of brain energy metabolism. Patients with depression exhibit reduced glucose utilization in the prefrontal cortex, anterior cingulate gyrus and caudate nucleus (Videback, 2000). The energy metabolism deficits in patients with depression may be widespread as suggested from data demonstrating reduced mitochondrial ATP production rate and increased mitochondrial DNA deletions in patients compared to control subjects (Gardner et al., 2003). Patients with bipolar disorder also exhibit impaired brain energy metabolism and reductions in the levels of mitochondrial proteins involved in energy metabolism, and may also have increased mtDNA mutations (Kato, 2007). Schizophrenia patients exhibit reduced Complex IV activity in the frontal cortex and caudate nucleus which are associated with increased emotional and cognitive impairment (Prince et al., 2000). Alterations in the expression of genes encoding mitochondrial proteins in brain tissue samples from patients with bipolar disorder and schizophrenia have been documented (for example see Iwamoto et al., 2005).
Thus far the data suggesting roles for mitochondrial alterations in psychiatric disorders are correlations only and it therefore remains to be determined whether the alterations contribute to the disease process or are epiphenomena. The development of animal models of psychiatric disorders in which the effects of genetic and pharmacological manipulations of mitochondrial functions on the behavioral phenotype are examined would shed light on the role of mitochondria in the disease process. There have been several reports of improvement in the symptoms of psychiatric patients during treatment with coenzyme Q10 (Onishi et al., 1997; Shinkai et al., 2000) suggesting a potential clinical benefit of mitochondria-directed treatments.
Future Directions
The information reviewed above reveals enabling and regulatory roles for mitochondria in the function and plasticity of neurons, and implicate mitochondrial dysfunction in the pathogenesis of a range of neurological disorders. However, there remain large gaps in our understanding of major aspects of mitochondrial contributions to neuronal morphogenesis, function and responses to physiological and pathological challenges. Questions that must be addressed in order to fully understand mitochondrial neurobiology include: 1) What are the molecular mechanisms by which mitochondria respond to changes in the activation state of major classes of neuronal signals including neurotrophic factors, neurotransmitters, neuropeptides and cytokines? 2) How do specific second messengers (Ca2+, cyclic nucleotides, etc.), kinases and transcription factors affect mitochondrial energy metabolism, and Ca2+ and redox signaling? 3) What are the mechanisms of and roles for mitochondrial dynamics in the development and plasticity of neuronal circuitry? 4) Does mitochondrial dysfunction play only a contributory role to neurodegenerative disorders downstream of the major pathogenic abnormality, or are mitochondrial alterations early seminal events? 5) Can neuronal dysfunction and degeneration be prevented by interventions that sustain mitochondrial function? 6) Can synaptic plasticity and neurological functions (learning and memory, motor function, emotional health) be enhanced by interventions that target mitochondria? 7) If and how do changes in mitochondria mediate the effects of environmental factors that either improve (exercise, dietary energy restriction and cognitive stimulation) or worsen (diabetes, overeating and a sedentary lifestyle) brain health? The molecular and functional complexity of mitochondria, and their ability to fuse, divide and move within neurons, portends of new revelations regarding their roles in neuroplasticity and disease.
Acknowledgements
The authors thank KC Alexander for preparing the illustrations for the Figures of this manuscript. This work was supported by the National Institute on Aging Intramural Research Program of the NIH.
References
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Andres-Mateos E, Perier C, Zhang L, Blanchard-Fillion B, Greco TM, Thomas B, Ko HS, Sasaki M, Ischiropoulos H, Przedborski S, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur PG, Matich GP, Pang WW, Yu DY, Bogoyevitch MA. Necrotic death of neurons following an excitotoxic insult is prevented by a peptide inhibitor of c-jun N-terminal kinase. J. Neurochem. 2007;102:65–76. doi: 10.1111/j.1471-4159.2007.04618.x. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Gleichmann M, Tang SC, Mattson MP. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Res. Rev. 2006;5:165–178. doi: 10.1016/j.arr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Atsumi T. The ultrastructure of intramuscular nerves in amyotrophic lateral sclerosis. Acta Neuropathol. 1981;55:193–198. doi: 10.1007/BF00691318. [DOI] [PubMed] [Google Scholar]
- Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward SD, Moran TH, Montell C, Ross CA, et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron. 2005;47:29–41. doi: 10.1016/j.neuron.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RH., Jr. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- Beal MF. Bioenergetic approaches for neuroprotection in Parkinson’s disease. Ann. Neurol. 2003;53(Suppl 3):S39–47. doi: 10.1002/ana.10479. [DOI] [PubMed] [Google Scholar]
- Berman SB, Pineda FJ, Hardwick JM. Mitochondrial fission and fusion dynamics: the long and short of it. Cell Death Differ. 2008;15:1147–1152. doi: 10.1038/cdd.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J. Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindokas VP, Lee CC, Colmers WF, Miller RJ. Changes in mitochondrial function resulting from synaptic activity in the rat hippocampal slice. J. Neurosci. 1998;18:4570–4587. doi: 10.1523/JNEUROSCI.18-12-04570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binet S, Meininger V. Modifications of microtubule proteins in ALS nerve precede detectable histologic and ultrastructural changes. Neurology. 1988;38:1596–1600. doi: 10.1212/wnl.38.10.1596. [DOI] [PubMed] [Google Scholar]
- Biskup S, Moore DJ. Detrimental deletions: mitochondria, aging and Parkinson’s disease. Bioessays. 2006;28:963–967. doi: 10.1002/bies.20471. [DOI] [PubMed] [Google Scholar]
- Bogaerts V, Theuns J, van Broeckhoven C. Genetic findings in Parkinson’s disease and translation into treatment: a leading role for mitochondria? Genes Brain Behav. 2008;7:129–151. doi: 10.1111/j.1601-183X.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillée S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Bromberg MB. Diagnostic criteria and outcome measurement of amyotrophic lateral sclerosis. Adv. Neurol. 2002;88:53–62. [PubMed] [Google Scholar]
- Brouillet E, Jacquard C, Bizat N, Blum D. 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. J. Neurochem. 2005;95:1521–1540. doi: 10.1111/j.1471-4159.2005.03515.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Li YJ, Lovell MA, Kraemer PJ, Gary DS, Brown RR, Markesbery WR, Mattson MP. 4-Hydroxynonenal, a product of lipid peroxidation, damages cholinergic neurons and impairs visuospatial memory in rats. J. Neuropathol. Exp. Neurol. 1998;57:257–267. doi: 10.1097/00005072-199803000-00007. [DOI] [PubMed] [Google Scholar]
- Burkhalter J, Fiumelli H, Allaman I, Chatton JY, Martin JL. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J. Neurosci. 2003;23:8212–8220. doi: 10.1523/JNEUROSCI.23-23-08212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Lin X, Xie C, Laird FM, Lai C, Wen H, Chiang HC, Shim H, Farah MH, Hoke A, et al. Loss of ALS2 function is insufficient to trigger motor neuron degeneration in knock-out mice but predisposes neurons to oxidative stress. J. Neurosci. 2005;25:7567–7574. doi: 10.1523/JNEUROSCI.1645-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Kaliszewski CK, Greer CA. Organization of mitochondria in olfactory bulb granule cell dendritic spines. Synapse. 1991;8:107–118. doi: 10.1002/syn.890080205. [DOI] [PubMed] [Google Scholar]
- Canet-Avilés RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de León A, Robinson KM, Mason RP, Beckman JS, Barbeito L, Radi R. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J. Neurosci. 2008;28:4115–4122. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr. Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- Chan SL, Liu D, Kyriazis GA, Bagsiyao P, Ouyang X, Mattson MP. Mitochondrial uncoupling protein-4 regulates calcium homeostasis and sensitivity to store depletion-induced apoptosis in neural cells. J. Biol. Chem. 2006;281:37391–37403. doi: 10.1074/jbc.M605552200. [DOI] [PubMed] [Google Scholar]
- Chang DT, Reynolds IJ. Differences in mitochondrial movement and morphology in young and mature primary cortical neurons in culture. Neuroscience. 2006;141:727–736. doi: 10.1016/j.neuroscience.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol. Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Chang MY, Sun W, Ochiai W, Nakashima K, Kim SY, Park CH, Kang JS, Shim JW, Jo AY, Kang CS, et al. Bcl-XL/Bax proteins direct the fate of embryonic cortical precursor cells. Mol. Cell Biol. 2007;27:4293–4305. doi: 10.1128/MCB.00031-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev. Biol. 2003;258:319–333. doi: 10.1016/s0012-1606(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Cheng A, Arumugam TV, Liu D, Khatri RG, Mustafa K, Kwak S, Ling HP, Gonzales C, Xin O, Jo DG, et al. Pancortin-2 interacts with WAVE1 and Bcl-xL in a mitochondria-associated protein complex that mediates ischemic neuronal death. J. Neurosci. 2007;27:1519–1528. doi: 10.1523/JNEUROSCI.5154-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung EC, Joza N, Steenaart NA, McClellan KA, Neuspiel M, McNamara S, MacLaurin JG, Rippstein P, Park DS, Shore GC, McBride HM, et al. Dissociating the dual roles of apoptosis-inducing factor in maintaining mitochondrial structure and apoptosis. EMBO J. 2006;25:4061–4073. doi: 10.1038/sj.emboj.7601276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Choo YS, Johnson GV, MacDonald M, Detloff PJ, Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum. Mol. Genet. 2004;13:1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem Biophys Res Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann. Neurol. 2002;52:448–457. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darios F, Corti O, Lücking CB, Hampe C, Muriel MP, Abbas N, Gu WJ, Hirsch EC, Rooney T, Ruberg M, Brice A. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum. Mol. Genet. 2003;12:517–526. doi: 10.1093/hmg/ddg044. [DOI] [PubMed] [Google Scholar]
- Darios F, Muriel MP, Khondiker ME, Brice A, Ruberg M. Neurotoxic calcium transfer from endoplasmic reticulum to mitochondria is regulated by cyclin-dependent kinase 5-dependent phosphorylation of tau. J. Neurosci. 2005;25:4159–4168. doi: 10.1523/JNEUROSCI.0060-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J. Cell Biol. 2007;176:405–414. doi: 10.1083/jcb.200611080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, Brownlees J, Ackerley S, Shaw PJ, McLoughlin DM, et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum. Mol. Genet. 2007;16:2720–2728. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sena-Esteves M, Chase K, Sapp E, Pfister E, Sass M, Yoder J, Reeves P, Pandey RK, Rajeev KG, et al. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc. Natl. Acad. Sci. U.S.A. 2007;104:17204–17209. doi: 10.1073/pnas.0708285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Rangnekar VM, Mattson MP. Prostate apoptosis response-4 production in synaptic compartments following apoptotic and excitotoxic insults: evidence for a pivotal role in mitochondrial dysfunction and neuronal degeneration. J. Neurochem. 1999;72:2312–2322. doi: 10.1046/j.1471-4159.1999.0722312.x. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ladenheim B, Xu X, Cadet JL, Mattson MP. Paroxetine retards disease onset and progression in Huntingtin mutant mice. Ann. Neurol. 2004;55:590–594. doi: 10.1002/ana.20075. [DOI] [PubMed] [Google Scholar]
- Dyall SD, Brown MT, Johnson PJ. Ancient invasions: from endosymbionts to organelles. Science. 2004;304:253–257. doi: 10.1126/science.1094884. [DOI] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Endo H, Kamada H, Nito C, Nishi T, Chan PH. Mitochondrial translocation of p53 mediates release of cytochrome c and hippocampal CA1 neuronal death after transient global cerebral ischemia in rats. J. Neurosci. 2006;26:7974–7983. doi: 10.1523/JNEUROSCI.0897-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Cherian S, Silver IA. Energy metabolism in mammalian brain during development. Prog. Neurobiol. 2004;73:397–445. doi: 10.1016/j.pneurobio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, Puoliväli J, Scearce-Levie K, Masliah E, Mucke L. Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J. Neurosci. 2006;26:5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner N, Treske B, Paquet D, Holmström K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken HH, Gasser T. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J. Neurosci. 2007;27:12413–12418. doi: 10.1523/JNEUROSCI.0719-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattal O, Budur K, Vaughan AJ, Franco K. Review of the literature on major mental disorders in adult patients with mitochondrial diseases. Psychosomatics. 2006;47:1–7. doi: 10.1176/appi.psy.47.1.1. [DOI] [PubMed] [Google Scholar]
- Fernandes HB, Baimbridge KG, Church J, Hayden MR, Raymond LA. Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA-induced apoptosis in YAC128 model of Huntington’s disease. J. Neurosci. 2007;27:13614–13623. doi: 10.1523/JNEUROSCI.3455-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch SM. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J. Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri A, Cozzolino M, Crosio C, Nencini M, Casciati A, Gralla EB, Rotilio G, Valentine JS, Carrì MT. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13860–13865. doi: 10.1073/pnas.0605814103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick RL, Shaw JM. Moving mitochondria: establishing distribution of an essential organelle. Traffic. 2007;8:1668–1675. doi: 10.1111/j.1600-0854.2007.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg H, Ferrand-Drake M, Bengtsson F, Halestrap AP, Wieloch T. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J. Neurosci. 1998;18:5151–5159. doi: 10.1523/JNEUROSCI.18-14-05151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui H, Diaz F, Garcia S, Moraes CT. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14163–14168. doi: 10.1073/pnas.0705738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Johansson A, Wibom R, Nennesmo I, von Döbeln U, Hagenfeldt L, Hällström T. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J. Affect. Disord. 2003;76:55–68. doi: 10.1016/s0165-0327(02)00067-8. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Riederer P, Przuntek H, Youdim MB. MPTP mechanisms of neurotoxicity and their implications for Parkinson’s disease. Eur. J. Pharmacol. 1991;208:273–286. doi: 10.1016/0922-4106(91)90073-q. [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007;14:1267–1274. doi: 10.1038/sj.cdd.4402147. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Chan SL, Lu C, Mattson MP. Caspase-mediated degradation of AMPA receptor subunits: a mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J. Neurosci. 2000;20:3641–3649. doi: 10.1523/JNEUROSCI.20-10-03641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Goldstein S, Merenyi G. The chemistry of peroxynitrite: implications for biological activity. Methods Enzymol. 2008;436:49–61. doi: 10.1016/S0076-6879(08)36004-2. [DOI] [PubMed] [Google Scholar]
- Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, Milligan CE, Oppenheim RW. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J. Neurosci. 2006;26:8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros-Louis F, Gaspar C, Rouleau GA. Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochim. Biophys. Acta. 2006;1762:956–972. doi: 10.1016/j.bbadis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Gulyaeva NV, Kudryashov IE, Kudryashova IV. Caspase activity is essential for long-term potentiation. J. Neurosci. Res. 2003;73:853–864. doi: 10.1002/jnr.10730. [DOI] [PubMed] [Google Scholar]
- Harms KJ, Rioult-Pedotti MS, Carter DR, Dunaevsky A. Transient spine expansion and learning-induced plasticity in layer 1 primary motor cortex. J. Neurosci. 2008;28:5686–5690. doi: 10.1523/JNEUROSCI.0584-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromolecular Med. 2003;4:21–36. doi: 10.1385/NMM:4:1-2:21. [DOI] [PubMed] [Google Scholar]
- Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- Hansson CA, Frykman S, Farmery MR, Tjernberg LO, Nilsberth C, Pursglove SE, Ito A, Winblad B, Cowburn RF, Thyberg J, Ankarcrona M. Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. J. Biol. Chem. 2004;279:51654–51660. doi: 10.1074/jbc.M404500200. [DOI] [PubMed] [Google Scholar]
- Hao Z, Duncan GS, Chang CC, Elia A, Fang M, Wakeham A, Okada H, Calzascia T, Jang Y, You-Ten A, et al. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, et al. Mitochondrial abnormalities in Alzheimer’s disease. J. Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J. Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 2000;157:401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhai D, Huang Y. Dependence of permeability transition pore opening and cytochrome C release from mitochondria on mitochondria energetic status. Mol. Cell. Biochem. 2001;224:1–7. doi: 10.1023/a:1011990300114. [DOI] [PubMed] [Google Scholar]
- Huesmann GR, Clayton DF. Dynamic role of postsynaptic caspase-3 and BIRC4 in zebra finch song-response habituation. Neuron. 2006;52:1061–1072. doi: 10.1016/j.neuron.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol. Genet. 2005;14:241–253. doi: 10.1093/hmg/ddi022. [DOI] [PubMed] [Google Scholar]
- Juhaszova M, Wang S, Zorov DB, Nuss HB, Gleichmann M, Mattson MP, Sollott SJ. The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Ann. N. Y. Acad. Sci. 2008;1123:197–212. doi: 10.1196/annals.1420.023. [DOI] [PubMed] [Google Scholar]
- Kang J-S, Tian J-H, Pan P-Y, Zald P, Li, Cuiling, Deng C, Sheng Z-H. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Jeong SY, Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. J. Cell Biol. 2004;166:1027–1039. doi: 10.1083/jcb.200407046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T. Mitochondrial dysfunction as the molecular basis of bipolar disorder: therapeutic implications. CNS Drugs. 2007;21:1–11. doi: 10.2165/00023210-200721010-00001. [DOI] [PubMed] [Google Scholar]
- Kato S. Amyotrophic lateral sclerosis models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:97–114. doi: 10.1007/s00401-007-0308-4. [DOI] [PubMed] [Google Scholar]
- Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keep M, Elmér E, Fong KS, Csiszar K. Intrathecal cyclosporin prolongs survival of late-stage ALS mice. Brain Res. 2001;894:327–331. doi: 10.1016/s0006-8993(01)02012-1. [DOI] [PubMed] [Google Scholar]
- Keller JN, Mark RJ, Bruce AJ, Blanc E, Rothstein JD, Uchida K, Waeg G, Mattson MP. 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience. 1997;80:685–696. doi: 10.1016/s0306-4522(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, Steiner SM, Bruce-Keller AJ, Hutchins JB, Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J. Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland RA, Franklin JL. Bax, reactive oxygen, and cytochrome c release in neuronal apoptosis. Antioxid. Redox Signal. 2003;5:589–596. doi: 10.1089/152308603770310257. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, Mueller G, Wermer M, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat. Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J. Neurosci. 1998;18:3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korde AS, Pettigrew LC, Craddock SD, Maragos WF. The mitochondrial uncoupler 2,4-dinitrophenol attenuates tissue damage and improves mitochondrial homeostasis following transient focal cerebral ischemia. J. Neurochem. 2005;94:1676–1684. doi: 10.1111/j.1471-4159.2005.03328.x. [DOI] [PubMed] [Google Scholar]
- Korde AS, Pettigrew LC, Craddock SD, Pocernich CB, Waldmeier PC, Maragos WF. Protective effects of NIM811 in transient focal cerebral ischemia suggest involvement of the mitochondrial permeability transition. J. Neurotrauma. 2007;24:895–908. doi: 10.1089/neu.2006.0122. [DOI] [PubMed] [Google Scholar]
- Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- Kruman II, Pedersen WA, Springer JE, Mattson MP. ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Exp. Neurol. 1999;160:28–39. doi: 10.1006/exnr.1999.7190. [DOI] [PubMed] [Google Scholar]
- Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Leonard JR, Klocke BJ, D’Sa C, Flavell RA, Roth KA. Strain-dependent neurodevelopmental abnormalities in caspase-3-deficient mice. J. Neuropathol. Exp. Neurol. 2002;61:673–677. doi: 10.1093/jnen/61.8.673. [DOI] [PubMed] [Google Scholar]
- Levy M, Faas GC, Saggau P, Craigen WJ, Sweatt JD. Mitochondrial regulation of synaptic plasticity in the hippocampus. J. Biol. Chem. 2003;278:17727–17734. doi: 10.1074/jbc.M212878200. [DOI] [PubMed] [Google Scholar]
- Li Z, Okamoto K-I, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-α-lipoic acid. Proc. Natl. Acad. Sci. U.S.A. 2002a;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J. Cereb. Blood Flow Metab. 2002b;22:431–543. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Liu D, Chan SL, de Souza-Pinto NC, Slevin JR, Wersto RP, Zhan M, Mustafa K, de Cabo R, Mattson MP. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med. 2006;8:389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- Lu C, Fu W, Salvesen GS, Mattson MP. Direct cleavage of AMPA receptor subunit GluR1 and suppression of AMPA currents by caspase-3: implications for synaptic plasticity and excitotoxic neuronal death. Neuromolecular Med. 2002;1:69–79. doi: 10.1385/NMM:1:1:69. [DOI] [PubMed] [Google Scholar]
- Lu C, Wang Y, Furukawa K, Fu W, Ouyang X, Mattson MP. Evidence that caspase-1 is a negative regulator of AMPA receptor-mediated long-term potentiation at hippocampal synapses. J. Neurochem. 2006;97:1104–1110. doi: 10.1111/j.1471-4159.2006.03800.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum. Mol. Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Manetto V, Sternberger NH, Perry G, Sternberger LA, Gambetti P. Phosphorylation of neurofilaments is altered in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 1988;47:642–653. doi: 10.1097/00005072-198811000-00007. [DOI] [PubMed] [Google Scholar]
- Markham A, Cameron I, Franklin P, Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur. J. Neurosci. 2004;20:1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, Price DL, Lee MK. Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, Abuin A, Grau E, Geppert M, Livi GP, et al. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol. Cell. Biol. 2004;24:9848–9862. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda N, Peng Q, Li Q, Jiang M, Liang Y, Wang X, Zhao M, Wang W, Ross CA, Duan W. Tiagabine is neuroprotective in the N171-82Q and R6/2 mouse models of Huntington’s disease. Neurobiol. Dis. 2008;30:293–302. doi: 10.1016/j.nbd.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat. Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Keller JN, Begley JG. Evidence for synaptic apoptosis. Exp. Neurol. 1998;153:35–48. doi: 10.1006/exnr.1998.6863. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Partin J. Evidence for mitochondrial control of neuronal polarity. J. Neurosci. Res. 1999;56:8–20. doi: 10.1002/(SICI)1097-4547(19990401)56:1<8::AID-JNR2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res. Rev. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Maurer I, Zierz S, Möller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol. Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Milakovic T, Quintanilla RA, Johnson GV. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. J. Biol. Chem. 2006;281:34785–34795. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J. Cell Sci. 2004;117:2791–804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- Miller TM, Moulder KL, Knudson CM, Creedon DJ, Deshmukh M, Korsmeyer SJ, Johnson EM., Jr. Bax deletion further orders the cell death pathway in cerebellar granule cells and suggests a caspase-independent pathway to cell death. J. Cell Biol. 1997;139:205–217. doi: 10.1083/jcb.139.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Mashiko T, Ofengeim D, Flannery RJ, Noh KM, Fujisawa S, Bonanni L, Bennett MV, Zukin RS, Jonas EA. Ischemic preconditioning blocks BAD translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4892–4897. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Zhang L, Troncoso J, Lee MK, Hattori N, Mizuno Y, Dawson TM, Dawson VL. Association of DJ-1 and parkin mediated by pathogenic DJ-1 mutations and oxidative stress. Hum. Mol. Genet. 2005;14:71–84. doi: 10.1093/hmg/ddi007. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Siedlak SL, Wang X, Santos MS, Oliveira CR, Tabaton M, Nunomura A, Szweda LI, Aliev G, Smith MA, et al. Increased autophagic degradation of mitochondria in Alzheimer disease. Autophagy. 2007;3:614–615. doi: 10.4161/auto.4872. [DOI] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol. Aging. 2008;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa RR, Baron JC. Pathophysiology of ischaemic stroke: insights from imaging, and implications for therapy and drug discovery. Br. J. Pharmacol. 2008;153(Suppl 1):S44–54. doi: 10.1038/sj.bjp.0707530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AN, Bredesen DE, Cortopassi G, Wang E, Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Vesce S, Kirk L, Chalmers S. Interactions between mitochondrial bioenergetics and cytoplasmic calcium in cultured cerebellar granule cells. Cell Calcium. 2003;34:407–424. doi: 10.1016/s0143-4160(03)00144-1. [DOI] [PubMed] [Google Scholar]
- Nieminen AL. Apoptosis and necrosis in health and disease: role of mitochondria. Int. Rev. Cytol. 2003;224:29–55. doi: 10.1016/s0074-7696(05)24002-0. [DOI] [PubMed] [Google Scholar]
- Onishi H, Kawanishi C, Iwasawa T, Osaka H, Hanihara T, Inoue K, Yamada Y, Kosaka K. Depressive disorder due to mitochondrial transfer RNALeu(UUR) mutation. Biol. Psychiatry. 1997;41:1137–1139. doi: 10.1016/S0006-3223(97)00005-X. [DOI] [PubMed] [Google Scholar]
- Orr AL, Li S, Wang CE, Li H, Wang J, Rong J, Xu X, Mastroberardino PG, Greenamyre JT, Li XJ. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J. Neurosci. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overly CC, Rieff HI, Hollenbeck PJ. Organelle motility and metabolism in axons vs dendrites of cultured hippocampal neurons. J. Cell Sci. 1996;109:971–980. doi: 10.1242/jcs.109.5.971. [DOI] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat. Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Park J, Kim SY, Cha GH, Lee SB, Kim S, Chung J. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim SS, Seong YM, Kim KH, Goo HG, Yoon EJ, Min do S, Kang S, Rhim H. Beta-amyloid precursor protein is a direct cleavage target of HtrA2 serine protease. Implications for the physiological function of HtrA2 in the mitochondria. J. Biol. Chem. 2006a;281:34277–34287. doi: 10.1074/jbc.M603443200. [DOI] [PubMed] [Google Scholar]
- Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, Brown RH., Jr. Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43:19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Fu W, Keller JN, Markesbery WR, Appel S, Smith RG, Kasarskis E, Mattson MP. Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann. Neurol. 1998;44:819–824. doi: 10.1002/ana.410440518. [DOI] [PubMed] [Google Scholar]
- Perez Velazquez JL, Frantseva MV, Huzar DV, Carlen PL. Mitochondrial porin required for ischemia-induced mitochondrial dysfunction and neuronal damage. Neuroscience. 2000;97:363–369. doi: 10.1016/s0306-4522(99)00569-2. [DOI] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. Alzheimer’s presenilin 1 mutations impair kinesin-based axonal transport. J. Neurosci. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesnila N, Zinkel S, Le DA, Amin-Hanjani S, Wu Y, Qiu J, Chiarugi A, Thomas SS, Kohane DS, Korsmeyer SJ, Moskowitz MA. BID mediates neuronal cell death after oxygen/ glucose deprivation and focal cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15318–15323. doi: 10.1073/pnas.261323298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon HF, Frasier M, Shreve N, Calabrese V, Wolozin B, Butterfield DA. Mitochondrial associated metabolic proteins are selectively oxidized in A30P alpha-synuclein transgenic mice--a model of familial Parkinson’s disease. Neurobiol. Dis. 2005;18:492–498. doi: 10.1016/j.nbd.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5(7):e172. doi: 10.1371/journal.pbio.0050172. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince JA, Harro J, Blennow K, Gottfries CG, Oreland L. Putamen mitochondrial energy metabolism is highly correlated to emotional an intellectuarl impairment in schizophrenics. Neuropsychopharmacology. 2000;22:284–292. doi: 10.1016/S0893-133X(99)00111-6. [DOI] [PubMed] [Google Scholar]
- Rice SE, Gelfand VI. Paradigm lost: milton connects kinesin heavy chain to miro on mitochondria. J. Cell Biol. 2006;173:459–461. doi: 10.1083/jcb.200604071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. Therapeutic potential of excitatory amino acid antagonists: channel blockers and 2,3-benzodiazepines. Trends Pharmacol Sci. 1993;14:325–331. doi: 10.1016/0165-6147(93)90005-5. [DOI] [PubMed] [Google Scholar]
- Rothstein JD. Excitotoxicity and neurodegeneration in amyotrophic lateral sclerosis. Clin. Neurosci. 1995;3:348–359. [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. Response of mitochondrial traffic to axon determination and differential branch growth. J. Neurosci. 2003;23:8618–8624. doi: 10.1523/JNEUROSCI.23-24-08618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha AR, Ninkina NN, Hanger DP, Anderton BH, Davies AM, Buchman VL. Induction of neuronal death by alpha-synuclein. Eur. J. Neurosci. 2000;12:3073–3077. doi: 10.1046/j.1460-9568.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- Sathasivam S, Ince PG, Shaw PJ. Apoptosis in amyotrophic lateral sclerosis: a review of the evidence. Neuropathol. Appl. Neurobiol. 2001;27:257–274. doi: 10.1046/j.0305-1846.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008;7:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM, Jones EG, Bunney WE, Vawter MP. Mitochondrial involvement in psychiatric disorders. Ann. Med. 2008;40:281–295. doi: 10.1080/07853890801923753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JM, Nunnari J. Mitochondrial dynamics and division in budding yeast. Trends Cell Biol. 2002;12:178–184. doi: 10.1016/s0962-8924(01)02246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai T, Nakashima M, Ohmori O, Terao T, Nakamura J, Hiramatsu N, Hashiguchi H, Tsuji S. Coenzyme Q10 improves psychiatric symptoms in adult-onset mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes: a case report. Aust. N. Z. J. Psychiatry. 2000;34:1034–1035. doi: 10.1080/000486700286. [DOI] [PubMed] [Google Scholar]
- Shuttleworth CW, Brennan AM, Connor JA. NAD(P)H fluorescence imaging of postsynaptic neuronal activation in murine hippocampal slices. J. Neurosci. 2003;23:3196–3208. doi: 10.1523/JNEUROSCI.23-08-03196.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Son M, Puttaparthi K, Kawamata H, Rajendran B, Boyer PJ, Manfredi G, Elliott JL. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6072–6077. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis L. Caspase-dependent and -independent neuronal death: two distinct pathways to neuronal injury. Neuroscientist. 2005;11:50–62. doi: 10.1177/1073858404271087. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Zhu XR, Bader V, Linnartz B, Schmidt S, Lübbert H. Mono- and double-mutant mouse models of Parkinson’s disease display severe mitochondrial damage. Hum. Mol. Genet. 2007;16:2377–2393. doi: 10.1093/hmg/ddm083. [DOI] [PubMed] [Google Scholar]
- Stoica BA, Movsesyan VA, Lea PM, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol. Cell. Neurosci. 2003;22:365–382. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Stowers RS, Megeath LJ, GorskaAndrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on Milton, a novel drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Jr, Davis RE, Parker WD., Jr. Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann. Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Jr., Davis RE, Parker WD., Jr. Cybrids in Alzheimer’s disease: a cellular model of the disease? Neurology. 1997;49:918–925. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Engelender S, Igarashi S, Rao RK, Wanner T, Tanzi RE, Sawa A, Dawson VL, Dawson TM, Ross CA. Inducible expression of mutant alpha-synuclein decreases proteasome activity and increases sensitivity to mitochondria-dependent apoptosis. Hum. Mol. Genet. 2001;10:919–926. doi: 10.1093/hmg/10.9.919. [DOI] [PubMed] [Google Scholar]
- Tang Y-G, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Tondera D, Czauderna F, Paulick K, Schwarzer R, Kaufmann J, Santel A. The mitochondrial protein MTP18 contributes to mitochondrial fission in mammalian cells. J. Cell Sci. 2005;118:3049–3059. doi: 10.1242/jcs.02415. [DOI] [PubMed] [Google Scholar]
- Toresson H, Grant SG. Dynamic distribution of endoplasmic reticulum in hippocampal neuron dendritic spines. Eur. J. Neurosci. 2005;22:1793–1798. doi: 10.1111/j.1460-9568.2005.04342.x. [DOI] [PubMed] [Google Scholar]
- Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: The bioenergetic view. Biochim. Biophys. Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Velde C, Miller TM, Cashman NR, Cleveland DW. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4022–4027. doi: 10.1073/pnas.0712209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vayssière JL, Cordeau-Lossouarn L, Larcher JC, Basseville M, Gros F, Croizat B. Participation of the mitochondrial genome in the differentiation of neuroblastoma cells. In Vitro Cell Dev. Biol. 1992;28A:763–772. doi: 10.1007/BF02631065. [DOI] [PubMed] [Google Scholar]
- Ved R, Saha S, Westlund B, Perier C, Burnam L, Sluder A, Hoener M, Rodrigues CM, Alfonso A, Steer C, et al. Similar patterns of mitochondrial vulnerability and rescue induced by genetic modification of alpha-synuclein, parkin, and DJ-1 in Caenorhabditis elegans. J. Biol. Chem. 2005;280:42655–42668. doi: 10.1074/jbc.M505910200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veereshwarayya V, Kumar P, Rosen KM, Mestril R, Querfurth HW. Differential effects of mitochondrial heat shock protein 60 and related molecular chaperones to prevent intracellular beta-amyloid-induced inhibition of complex IV and limit apoptosis. J. Biol. Chem. 2006;281:29468–29478. doi: 10.1074/jbc.M602533200. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken JTK, Koh T-W, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Videbech P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatrica Scand. 2000;101:11–20. doi: 10.1034/j.1600-0447.2000.101001011.x. [DOI] [PubMed] [Google Scholar]
- von Lewinski F, Keller BU. Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends Neurosci. 2005;28:494–500. doi: 10.1016/j.tins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Walker FO. Huntington’s disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu SW, Koh DW, Lew J, Coombs C, Bowers W, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor substitutes for caspase executioners in NMDA-triggered excitotoxic neuronal death. J. Neurosci. 2004;24:10963–10973. doi: 10.1523/JNEUROSCI.3461-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Guan Y, Wang X, Smith K, Cormier K, Zhu S, Stavrovskaya IG, Huo C, Ferrante RJ, Kristal BS, Friedlander RM. Nortriptyline delays disease onset in models of chronic neurodegeneration. Eur. J. Neurosci. 2007;26:633–641. doi: 10.1111/j.1460-9568.2007.05663.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Groom L, Cheng A, Yin J, Mattson MP, Kao JPY, Lakatta EG, Sheu SS, Dirksen RT, Cheng H. Redox flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, Gilbert ML, Morton GJ, Bammler TK, Strand AD, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metab. 2006;4:349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Widdowson PS, Gyte A, Upton R, Foster J, Coutts CT, Wyatt I. Calpain activation and not oxidative damage mediates L-2-chloropropionic acid-induced cerebellar granule cell necrosis. Toxicol. Appl. Pharmacol. 1997;142:248–255. doi: 10.1006/taap.1996.7940. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J. Neurosci. Res. 1999;57:830–839. [PubMed] [Google Scholar]
- Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lipton P. Cytosolic Ca2+ changes during in vitro ischemia in rat hippocampal slices: major roles for glutamate and Na+-dependent Ca2+ release from mitochondria. J. Neurosci. 1999;19:3307–3315. doi: 10.1523/JNEUROSCI.19-09-03307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawson TM, Dawson VL. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum. Mol. Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Lee JE, Yenari MA. Stroke: molecular mechanisms and potential targets for treatment. Curr. Mol. Med. 2003;3:361–372. doi: 10.2174/1566524033479717. [DOI] [PubMed] [Google Scholar]
- Zhu X, Yu QS, Cutler RG, Culmsee CW, Holloway HW, Lahiri DK, Mattson MP, Greig NH. Novel p53 inactivators with neuroprotective action: syntheses and pharmacological evaluation of 2-imino-2,3,4,5,6,7-hexahydrobenzothiazole and 2-imino-2,3,4,5,6,7-hexahydrobenzoxazole derivatives. J. Med. Chem. 2002;45:5090–5097. doi: 10.1021/jm020044d. [DOI] [PubMed] [Google Scholar]