Abstract
The Kerator is a computer controlled bioreactor for the automated culture and harvest of keratinocytes that can reduce labor and materials involved in the fabrication of engineered skin substitutes (ESS). Previous studies have shown that Kerator is comparable to tissue culture flasks by keratinocyte confluence during culture, clonogenic potential of harvested keratinocytes and microanatomy, cell viability and surface hydration of ESS fabricated with the harvested keratinocytes. In this study, the Kerator and tissue culture flasks were further compared by keratinocyte proliferation in vitro and wound healing after transplantation of ESS to athymic mice. The number of BrdU positive keratinocytes in ESS fabricated with keratinocytes harvested from Kerator after 2 weeks of in vitro maturation was 34 ± 3 per high power field (hpf) (mean ± SEM), which was not significantly different from that fabricated with keratinocytes harvested from flasks (34 ± 1.5 per hpf). Percentage original wound area 6 weeks after surgery of ESS fabricated with keratinocytes from the Kerator was 36 ± 3.3%, which was not significantly different from that of ESS fabricated with keratinocytes from flasks (30 ± 4.3%). In both cases, 78% (7 of 9) mice transplanted were positive for engraftment of human keratinocytes by direct immunofluoresence for HLA-ABC antigens. These results further confirm that the ESS fabricated with keratinocytes harvested from Kerator and flasks are equivalent in vitro and in vivo. Therefore, use of Kerator for large scale production of ESS can lead to increased availability at reduced cost while maintaining ESS quality for grafting.
Keywords: automation, bioreactor, keratinocytes, engineered skin, wound healing
Introduction
Skin is the largest organ in the body and provides a barrier against infections and fluid loss. Loss of this protective barrier contributes to the mortality and morbidity due to burns involving a significant amount of total body surface area (TBSA). Restoration of skin barrier is of definitive importance in the treatment of extensive burn injuries, and this has been accomplished traditionally by autograft or allograft. The limited availability of donor skin for autograft in large burns, and the risks of rejection and transmissible diseases associated with allograft, have led to the development of tissue engineered skin equivalents as alternatives in burn wound treatment. Various approaches have been taken towards the engineering of these therapeutic materials, including cultured epithelial autografts(1;2) (Epicel™); acellular dermal substitutes(3–6) (IntegraDRT™, AlloDerm™), cell populated dressings(7) (TransCyte™); cellular dermal substitutes(8) (Dermagraft™); and bilayered skin substitutes(9), (10) (Apligraf™, Orcel™).
Engineered Skin Substitutes (ESS) are composed of an epidermal substitute of autologous keratinocytes, attached to a dermal analog of bovine collagen-glycosaminoglycan sponge populated with autologous fibroblasts. In vitro prior to grafting, ESS demonstrate morphogenesis similar to native human skin and form a functional epidermal barrier(11;12). After transplantation, ESS have stable engraftment, undergo histogenesis and heal wounds with minimal scarring(13). ESS reduce the requirements for skin autograft in the treatment of massive burns and therefore demonstrate clinical efficacy as adjunctive therapy(14;15). Fabrication of ESS involves: 1) isolation of keratinocytes and fibroblasts from a skin biopsy; 2) primary culture of isolated cells; 3) cryopreservation of cells at the first passage; 4) recovery from cryopreservation and intermediate expansion of cells; 5) sequential inoculation of expanded fibroblasts and keratinocytes on collagen-GAG sponge; and, 6) maturation of ESS at the air-liquid interface prior to grafting(11). These processes are very intensive in terms of the labor and materials used which limits the amount of ESS that can be fabricated at a given time and also impacts the cost of the final product. As a means of increasing product availability and reducing costs we have evaluated the Kerator(16–18), a computer-controlled bioreactor, as an instrument for automation of keratinocyte expansion for fabrication of ESS(19).
The Kerator bioreactor was originally designed for the production of an autologous wound dressing composed of subconfluent keratinocytes attached to a transparent, gas permeable fluorinated ethylene propylene (FEP) film(17;18). To fabricate ESS with keratinocytes cultured in the Kerator, a protocol for keratinocyte harvest was developed to maintain the clonogenic potential of harvested keratinocytes in the subsequent passage. Subconfluent keratinocytes in the Kerator were harvested by incubating in 0.02% EDTA for 8 – 10 minutes, followed by exposure to trypsin for 2 minutes. Keratinocytes harvested from the Kerator in this manner had colony forming efficiencies and growth rates similar to those harvested from flasks, both with and without pre-treatment with 0.02% EDTA. ESS fabricated with keratinocytes cultured in, and harvested from, the Kerator were comparable in vitro to control ESS fabricated with keratinocytes harvested from standard tissue culture flasks by histological organization, cellular viability and surface hydration(19). It is hypothesized based on these in vitro studies, that ESS fabricated with keratinocytes harvested form the Kerator will exhibit wound healing comparable to those fabricated with keratinocytes harvested from flasks, in vivo after transplantation to athymic mice. Demonstration of such comparability between Kerator and tissue culture flasks is an essential step towards the integration of the bioreactor in the process of ESS fabrication for clinical use, where the benefits of automation on cost and availability of the therapeutic product can be fully realized. In this study, engraftment of ESS prepared with normal human keratinocytes from the Kerator or flasks was compared.
Materials and Methods
Kerator Bioreactor
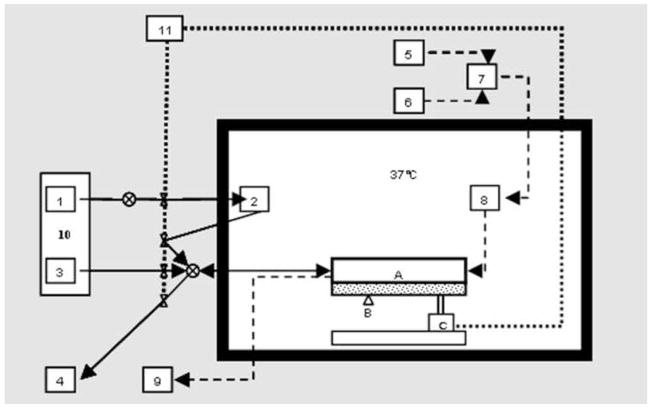
The Kerator has been described in detail in earlier publications(16–18). Briefly, it comprises of a modular polycarbonate growth chamber with fluorinated ethylene propylene (FEP) growth surface, connected to reservoirs of fresh medium, cell suspension and waste by means of sterile silicone tubing. A bidirectional, multichannel peristaltic pump conveys medium and cells to and from the growth chamber. Fluid flow through the various tubes is regulated by means of pneumatic pinch valves (Figure 1). The growth chamber rests on a platform that can be tilted to one side by a pneumatic actuator (Figure 2). The peristaltic pump, pinch valves and pneumatic actuator are controlled by a custom Virtual Instrument (VI) designed in LabVIEW 6.1, which provides automated fluid handling during medium changes and keratinocyte harvest. A mass flow controller mixes air and 100% CO2 to provide 5% CO2 that is filtered and humidified before entering the growth chamber. The growth chamber is located in an insulated box in which the temperature is maintained at 37°C by means of a hot plate and thermostat. The K erator is also equipped with a camera system that allows real time visualization and monitoring of keratinocytes in culture.
Figure 1.
Schematic representation of the Kerator bioreactor, showing A) Growth chamber, B) Camera, C) Tilting actuator, 1) Cold medium reservoir, 2) Warm medium reservoir, 3) Cell seeding and collection reservoir, 4) Waste, 5) CO2 supply, 6) Air supply, 7) Mass flow controller, 8) Humidifier bottle, 9) CO2 detector, 10) Peltier cooler, 11) Compressed air supply.
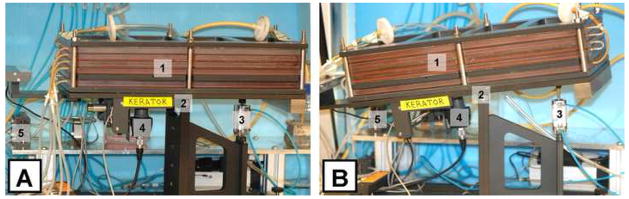
Figure 2.
Frontal view of the Kerator growth chamber in the horizontal (A) and tilted (B) positions. 1) Five-layered growth chamber, 2) Rocking platform, 3) Pneumatic actuator, 4) Camera, and 5) Pneumatic pinch valves. Tilting the resting platform by activating the pneumatic actuator (3) allows medium and cells to be drained from the growth chamber.
Keratinocyte culture and harvest from Kerator
Normal human keratinocytes were inoculated in the Kerator at a density of 4×103/cm2 of growth area. A custom VI was programmed to perform fully automated medium changes in the Kerator once every 48 hours. The growth of keratinocytes was monitored by the online image acquisition and analysis system. When the keratinocytes were considered to be 75 – 90% confluent by image analysis (usually on the seventh day of culture), the cells were harvested by treatment with 0.02% ethylenediamine tetra-acetic acid (EDTA) for 8 –10 minutes followed by brief (1–2 minutes) exposure to trypsin. The resultant cell suspension was collected in a reservoir of 10% fetal bovine serum, following which the growth chamber was rinsed twice with HBS to collect the remaining cells. The cell suspension was centrifuged and the pellet was resuspended in modified MCDB 153. The cells were counted under a microscope using a hemacytometer and used for inoculation of ESS.
Tissue culture flasks were inoculated with keratinocytes at the same density and medium was changed manually every 2 days. When keratinocytes reached 75 – 90% confluence, they were harvested by a 15-second rinse with HBS followed by exposure to trypsin for 5 minutes. The cell suspension was collected in 10% FBS and processed for ESS inoculation as described above.
ESS fabrication
ESS was fabricated as described in earlier publications(12;15), with syngeneic normal human fibroblasts and keratinocytes, and collagen – glycosaminoglycan sponge. Fibroblasts were inoculated on the sponge at a density of 0.5×106/cm2. The day following fibroblast inoculation, keratinocytes harvested from the Kerator or flasks were inoculated on the sponge at a density of 1×106/cm2. The ESS was incubated the air-liquid interface for 3 weeks following keratinocyte inoculation. UCMC 160 medium(20) was used for the first three days of ESS culture and UCMC 161 medium was used for the remaining incubation period, with daily medium changes.
BrdU Immunofluorescence
The number of actively proliferating keratinocytes in the basal layers of ESS fabricated with keratinocytes harvested from Kerator or flasks was quantified by bromo-deoxyuridine (BrdU) immunostaining of CSS in vitro. After 2 weeks of maturation, test and control ESS (n=3) were incubated in 65μM BrdU for 22 h at 37°C and 5% CO 2. Following incubation, ESS were fixed in formalin, embedded in paraffin and sectioned. The sections were processed for BrdU immunostaining by baking at 60°C for 2 h followed by deparaffinizatio n with xylene and rehydration with graded alcohols. The sections were then co-labeled with 1:10 anti-BrdU fluorescein isocyanate and 1:50 primary anti-pancytokeratin by a procedure described previously. After labeling, the slides were washed with PBS and Milli-Q water and coverslipped with Vectashield hard mount media containing 4′, 6′-diamidino-2-phenylindole (DAPI). The slides were examined under the microscope and 10 unique fields per ESS were analyzed for the number of BrdU positive cells (total 30 fields per condition). Data are expressed as mean ± SEM of BrdU positive cells per field.
ESS transplantation to athymic mice
All animal care and handling in this study was performed according to protocols approved by the Institutional Animal Care and Use Committee of the University of Cincinnati. ESS fabricated with keratinocytes harvested from Kerator or flasks were allowed to mature for two weeks in vitro prior to transplantation on athymic mice (nu/nu, Harlan; n = 9 animals per condition). Briefly, a full thickness skin wound measuring 2cm × 2cm was prepared on the dorsolateral aspect of each mouse, sparing the panniculus carnosus. ESS along with an overlying piece of nonadherent dressing was placed orthotopically on the wound and was secured to the wound margin with sutures. The grafted ESS was dressed with a piece of sterile gauze coated with antibiotic ointment (containing Bactroban, Nystatin and Neomycin in equal parts) and the gauze was held in place by tying the sutures over it. The grafted site was covered with an occlusive dressing (OpSite) and the mice were wrapped in Coban. Mice were left undisturbed until two weeks after the surgery when the dressings and sutures were removed. Thereafter, the mice were maintained without dressings until six weeks post-operative, at which time they were euthanized.
Wound areas on athymic mice
Mice were photographed at biweekly intervals from 2 to 6 weeks after surgery. The wound perimeters were traced at the time of surgery and at weekly intervals from 2 to 6 weeks post-operatively (n = 9 per condition at each time point). Wound area at each time point was determined from these tracings using computer planimetry. Percent original wound area was defined as the wound area at serial time points divided by the wound area at the time of surgery × 100%. Data for each time point were expressed as percent original wound area (mean ± SEM).
ESS engraftment on athymic mice
Six weeks after surgery, the mice were euthanized and two biopsies of the graft along with adjoining mouse skin were collected. One biopsy was processed by paraffin embedding and the other for cryomicrotomy. The paraffin embedded specimen was stained by H&E and viewed under the microscope to analyze the histological organization of healed tissue. The frozen sections were stained immunohistochemically for HLA-ABC antigens by a procedure previously described(11;12), to confirm the engraftment of human keratinocytes on the mice. ESS engraftment was expressed as the percentage of animals staining positive for HLA – ABC (n = 9 each for Kerator or flasks).
Statistical Analysis
Data for wound area and ESS engraftment were analyzed for by one-way repeated measures analysis of variance (RM-ANOVA) followed by Student-Neuman-Keul’s test for pair wise comparisons. Data for BrdU incorporation were compared by Student’s t-test. Statistical significance was accepted at the 95% confidence level (p<0.05).
Results
BrdU Immunostaining
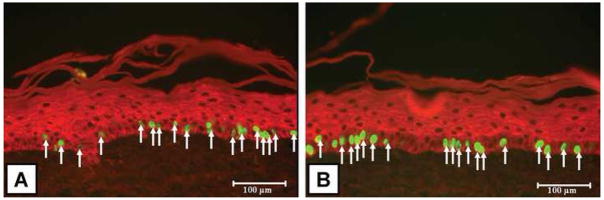
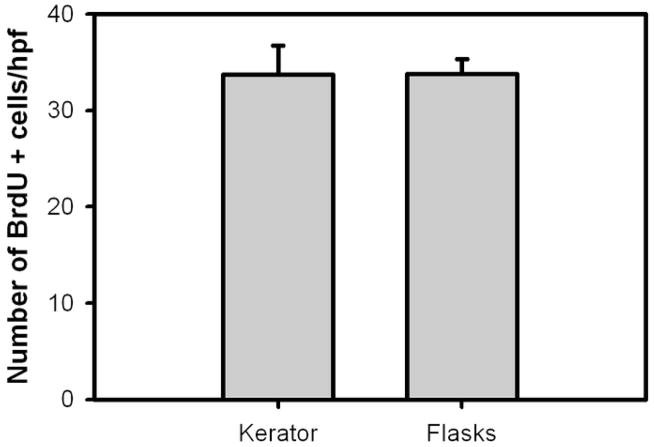
Representative images of BrdU-positive keratinocytes in ESS are shown in Figure 3. In both conditions, the BrdU positive keratinocytes were located in the basal layers of the epidermal portion of the ESS. Results of the number of keratinocytes in ESS which were positive for BrdU uptake day 14 of in vitro maturation is summarized in Figure 4. There were no significant differences between the Kerator (34 ± 3 per hpf) and flasks (34 ± 1.5 per hpf).
Figure 3.
Representative images of ESS sections (viewed at 20X magnification) stained for BrdU positive keratinocytes. (A) Kerator; (B) Flasks. BrdU positive nuclei stain fluorescent green and are located predominantly in the basal layers of the epidermis. Keratinocytes in the epidermis are stained red by anti-pancytokeratin (see methods). Scale bar = 100μm.
Figure 4.
Number of BrdU positive keratinocytes per high power field in ESS fabricated with keratinocytes harvested from Kerator and flasks, after 2 weeks of maturation in vitro. There were no significant differences between the conditions.
Wound closure on athymic mice
Figure 5 shows representative animals grafted with ESS fabricated with keratinocytes harvested from the Kerator and flasks, at 2 and 6 weeks after surgery. In both conditions, the ESS attached to the wound and to the surrounding margins of native mouse skin. The surfaces of the grafted ESS were dry and well keratinized. ESS from both conditions displayed stable engraftment over the six week study period.
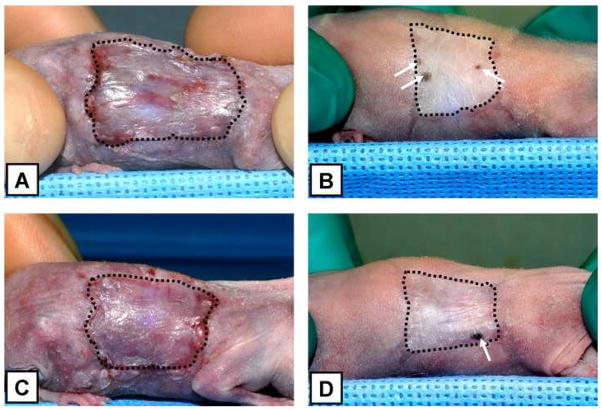
Figure 5.
Representative images, at 2 and 6 weeks after surgery, of athymic mice grafted with ESS fabricated with keratinocytes harvested from Kerator (A and B) and flasks (C and D). The perimeter of grafted area has been delineated with dashed lines. Note the extent of wound contraction in both conditions. Also remarkable is the presence of pigmented spots within the grafted area at 6 weeks in both conditions (arrows), suggesting the presence of human derived cells in those regions.
Wound areas on athymic mice
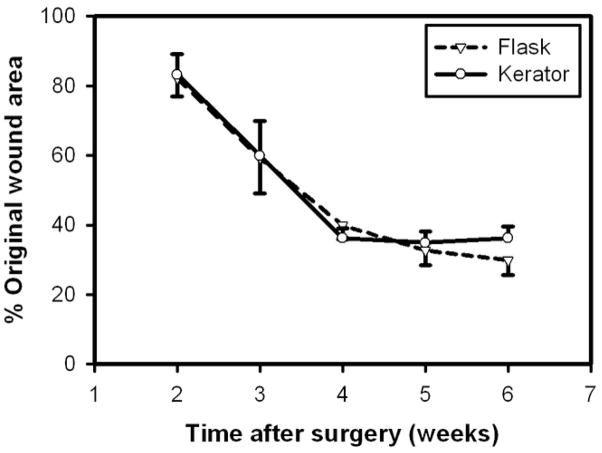
Results of wound area measurements are illustrated in Figure 6. The percent original wound area in mice grafted with ESS fabricated with keratinocytes harvested from the Kerator was 83 ± 6, 60 ± 10, 36 ± 3, 35 ± 3.2 and 36 ± 3.3 at 2, 3, 4, 5 and 6 weeks after surgery, respectively. At parallel time points, the percent wound area in mice grafted with ESS fabricated with keratinocytes harvested from flasks was 82 ± 5.3, 59 ± 10.2, 40 ± 4.1, 33 ± 4.3 and 30 ± 4.3. In both conditions, the wounds areas decreased significantly between 2 and 4 weeks (p < 0.05) but there was no change in wound area between 4 and 6 weeks post-op. There were no significant differences in wound areas between the two conditions at parallel time points.
Figure 6.
Percentage original wound area, of ESS fabricated with keratinocytes harvested from Kerator and flasks transplanted on athymic mice. There were no significant differences between the two conditions at parallel time points.
ESS engraftment on athymic mice
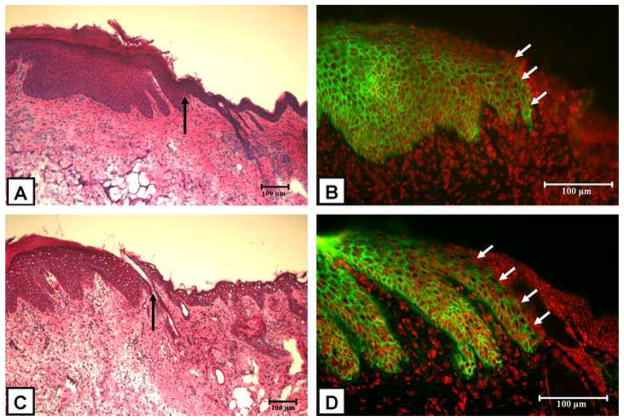
Representative H&E stained paraffin sections of healed ESS from test and control conditions are shown in Figure 7A and 7C, respectively. Confirmation of engraftment of human keratinocytes was performed by direct immunofluoresence for HLA-ABC antigens. Positive staining for HLA-ABC antigens in the epidermis appears net-like, corresponding to the distribution of those antigens on the surface of keratinocytes. The staining is absent in the adjacent edge of normal mouse skin (Figure 7B and 7D, arrow). Results of scoring for HLA-ABC immunostaining showed that in both conditions, 78% of animals (7 of 9) had keratinocytes staining positive for HLA-ABC.
Figure 7.
Representative H&E stained sections and HLA-ABC immunofluorescence of grafted ESS fabricated with keratinocytes harvested from Kerator (A and B, respectively) and flasks (C and D, respectively). In both conditions, H&E sections (A and C) show normal mouse epidermis (to the right of arrow) which is thin and lacks rete ridges. The thicker epithelium with prominent rete ridges (to the left of arrow) resembles human skin and is derived from the grafted ESS. Direct immunofluoresence for HLA-ABC antigens (B and D) shows net-like distribution of HLA-ABC (green) on human derived keratinocytes in the epidermis. Adjacent mouse epidermis (arrow) stains negatively for HLA-ABC. Nuclei have been stained red by propidium iodide. Scale bar = 100μm.
Discussion
The Kerator bioreactor provides automation of keratinocyte culture(16–18) and harvest, thereby reducing the labor and materials involved in the cell expansion phase of ESS fabrication. Data presented in this study demonstrate comparability of ESS with keratinocytes from Kerator and flasks by BrdU incorporation in vitro, and wound healing after transplantation to athymic mice.
Previous studies have shown that ESS fabricated with keratinocytes harvested from Kerator and flasks are comparable in vitro by microanatomy, cell viability and surface hydration(19). In this study, ESS from the two conditions were compared in vitro by BrdU incorporation in the epidermis as a measure of keratinocyte proliferation. No significant differences were found, validating the findings from earlier studies (Figure 2). Also, it is known that the formation of a functional, barrier-forming epithelium both in vitro and in vivo depends on a source of proliferative keratinocytes that give rise to the mature corneocytes of stratum corneum. Because the BrdU studies on ESS were done at the time of their transplantation, the results of these studies served as an indicator of their ability to heal wounds effectively.
The athymic mouse has been the preferred model to study the wound healing capability of human skin substitutes(21–33), because of its immunologic tolerance of xenograft and the limited scarring that occurs during healing. ESS grafts fabricated with keratinocytes harvested from the Kerator had well keratinized and desquamating surfaces after two weeks following surgery (Figure 3). ESS have been shown to form a functional epidermal barrier approaching that of native human skin after transplantation to athymic mice(34;35). Although surface hydration was not measured in vivo in the present study, the dry appearance of these grafts indicated well-formed epithelium. Evaluation of wound areas demonstrated that ESS fabricated with keratinocytes harvested from the Kerator heal wounds in a manner comparable to those fabricated with keratinocytes harvested from standard tissue culture flasks (Figure 4).
Engineered skin fabricated with keratinocytes from flasks were shown to persist for as long as 1 year on athymic mice(36). Tumorigenicity tests done by subcutaneous implantation of such cells in athymic mice ruled out neoplastic transformation after 26-week observation(37). ESS fabricated with keratinocytes harvested form the Kerator showed stable integration with host skin for the six-week period of the present study. However long-term animal studies with these cells and ESS fabricated with them will be necessary to test the stability of ESS. Although FEP has been used as culture surface for growing cell types such as nerve(38) and corneal epithelial cells(39), use of the polymer for clinical will require demonstration of safety and efficacy by such studies.
Pigmented skin was seen within the graft region in 3 out of 9 animals in both conditions, beginning from the fourth week post-op (Fig. 5B and 5D). This gave an early indication of the persistence of human-derived cells because the skin of host animal is devoid of any pigment. This observation also suggests that the FEP culture surface may support the attachment and proliferation of melanocytes, which may be applicable for large scale culture of these cells in the Kerator.
Demonstration of comparability between the Kerator and flasks, both by in vitro and in vivo studies, suggests that the bioreactor can be used for automation of keratinocyte culture for large scale fabrication of engineered skin. Apart from automation, the hands-free operation of cell culture facilitated by the Kerator limits the chances of contamination(40). By integrating online monitoring sensors in the Kerator it can be possible to regulate medium changes according to preset thresholds of biochemical parameters. Such dynamic bioprocess optimization has been shown to enhance cell yield of hybridoma cells(41). Adaptation of this technique to keratinocyte culture can prospectively lead to earlier achievement of an adequate cell yield for ESS fabrication, thereby reducing the lead time between biopsy sourcing and clinical application. Metabolic regulation of keratinocyte culture in the Kerator could also lead to improved cell physiology, thereby resulting in improved ESS quality that may potentially translate into better wound healing in vivo. Although analysis of cost involved in operating the bioreactor has not been performed, automation has historically resulted in greater product availability at lower cost, higher consistency and greater availability. As more tissue engineered products become available commercially, bioreactors such as the Kerator will be valuable tools in making them more available by the manufacturer, and more affordable to the patient
Acknowledgments
The authors extend their gratitude to Andy Supp for assistance with animal surgery; Jodi Miller for providing cell-culture media and assistance with animal surgery; and to Chris Lloyd for fabrication of collagen sponges.
This project was supported by a research grant from the National Institutes of Health (GM050509).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gallico GG, III, O’Connor NE, Compton CC, Kehinde O, Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984 August 16;311(7):448–51. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 2.Green H, Kehinde O, Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc Natl Acad Sci U S A. 1979 November;76(11):5665–8. doi: 10.1073/pnas.76.11.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke JF, Yannas IV, Quinby WC, Jr, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981 October;194(4):413–28. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callcut RA, Schurr MJ, Sloan M, Faucher LD. Clinical experience with Alloderm: a one-staged composite dermal/epidermal replacement utilizing processed cadaver dermis and thin autografts. Burns. 2006 August;32(5):583–8. doi: 10.1016/j.burns.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Heimbach DM, Warden GD, Luterman A, Jordan MH, Ozobia N, Ryan CM, Voigt DW, Hickerson WL, Saffle JR, DeClement FA, Sheridan RL, Dimick AR. Multicenter postapproval clinical trial of Integra dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003 January;24(1):42–8. doi: 10.1097/00004630-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995 June;21(4):243–8. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 7.Noordenbos J, Dore C, Hansbrough JF. Safety and efficacy of TransCyte for the treatment of partial-thickness burns. J Burn Care Rehabil. 1999 July;20(4):275–81. doi: 10.1097/00004630-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Purdue GF, Hunt JL, Still JM, Jr, Law EJ, Herndon DN, Goldfarb IW, Schiller WR, Hansbrough JF, Hickerson WL, Himel HN, Kealey GP, Twomey J, Missavage AE, Solem LD, Davis M, Totoritis M, Gentzkow GD. A multicenter clinical trial of a biosynthetic skin replacement, Dermagraft-TC, compared with cryopreserved human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil. 1997 January;18(1 Pt 1):52–7. doi: 10.1097/00004630-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Curran MP, Plosker GL. Bilayered bioengineered skin substitute (Apligraf): a review of its use in the treatment of venous leg ulcers and diabetic foot ulcers. BioDrugs. 2002;16(6):439–55. doi: 10.2165/00063030-200216060-00005. [DOI] [PubMed] [Google Scholar]
- 10.Still J, Glat P, Silverstein P, Griswold J, Mozingo D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns. 2003 December;29(8):837–41. doi: 10.1016/s0305-4179(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 11.Boyce ST, Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am J Surg. 2002 April;183(4):445–56. doi: 10.1016/s0002-9610(02)00813-9. [DOI] [PubMed] [Google Scholar]
- 12.Boyce ST, Supp AP, Swope VB, Warden GD. Vitamin C regulates keratinocyte viability, epidermal barrier, and basement membrane in vitro, and reduces wound contraction after grafting of cultured skin substitutes. J Invest Dermatol. 2002 April;118(4):565–72. doi: 10.1046/j.1523-1747.2002.01717.x. [DOI] [PubMed] [Google Scholar]
- 13.Boyce ST, Foreman TJ, English KB, Stayner N, Cooper ML, Sakabu S, Hansbrough JF. Skin wound closure in athymic mice with cultured human cells, biopolymers, and growth factors. Surgery. 1991 November;110(5):866–76. [PubMed] [Google Scholar]
- 14.Boyce ST, Kagan RJ, Yakuboff KP, Meyer NA, Rieman MT, Greenhalgh DG, Warden GD. Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Ann Surg. 2002 February;235(2):269–79. doi: 10.1097/00000658-200202000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyce ST, Kagan RJ, Greenhalgh DG, Warner P, Yakuboff KP, Palmieri T, Warden GD. Cultured skin substitutes reduce requirements for harvesting of skin autograft for closure of excised, full-thickness burns. J Trauma. 2006 April;60(4):821–9. doi: 10.1097/01.ta.0000196802.91829.cc. [DOI] [PubMed] [Google Scholar]
- 16.Kino-Oka M, Prenosil JE. Development of an on-line monitoring system of human keratinocyte growth by image analysis and its application to bioreactor culture. Biotechnol Bioeng. 2000 January 20;67(2):234–9. [PubMed] [Google Scholar]
- 17.Prenosil JE, Villeneuve PE. Automated production of cultured epidermal autografts and sub-confluent epidermal autografts in a computer controlled bioreactor. Biotechnol Bioeng. 1998 September 20;59(6):679–83. [PubMed] [Google Scholar]
- 18.Prenosil JE, Kino-Oka M. Computer controlled bioreactor for large-scale production of cultured skin grafts. Ann N Y Acad Sci. 1999 June 18;875:386–97. doi: 10.1111/j.1749-6632.1999.tb08521.x. [DOI] [PubMed] [Google Scholar]
- 19.Kalyanaraman B, Boyce S. Assessment of an automated bioreactor to propagate and harvest keratinocytes for fabrication of engineered skin substitutes. Tissue Eng. 2007 May;13(5):983–93. doi: 10.1089/ten.2006.0338. [DOI] [PubMed] [Google Scholar]
- 20.Swope VB, Supp AP, Schwemberger S, Babcock G, Boyce S. Increased expression of integrins and decreased apoptosis correlate with increased melanocyte retention in cultured skin substitutes. Pigment Cell Res. 2006 October;19(5):424–33. doi: 10.1111/j.1600-0749.2006.00325.x. [DOI] [PubMed] [Google Scholar]
- 21.Caissie R, Gingras M, Champigny MF, Berthod F. In vivo enhancement of sensory perception recovery in a tissue-engineered skin enriched with laminin. Biomaterials. 2006 May;27(15):2988–93. doi: 10.1016/j.biomaterials.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Cooper ML, Spielvogel RL, Hansbrough JF, Boyce ST, Frank DH. Reconstitution of the histologic characteristics of a giant congenital nevomelanocytic nevus employing the athymic mouse and a cultured skin substitute. J Invest Dermatol. 1991 October;97(4):649–58. doi: 10.1111/1523-1747.ep12483707. [DOI] [PubMed] [Google Scholar]
- 23.Cooper ML, Andree C, Hansbrough JF, Zapata-Sirvent RL, Spielvogel RL. Direct comparison of a cultured composite skin substitute containing human keratinocytes and fibroblasts to an epidermal sheet graft containing human keratinocytes on athymic mice. J Invest Dermatol. 1993 December;101(6):811–9. doi: 10.1111/1523-1747.ep12371700. [DOI] [PubMed] [Google Scholar]
- 24.Erdag G, Sheridan RL. Fibroblasts improve performance of cultured composite skin substitutes on athymic mice. Burns. 2004 June;30(4):322–8. doi: 10.1016/j.burns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Geer DJ, Swartz DD, Andreadis ST. In vivo model of wound healing based on transplanted tissue-engineered skin. Tissue Eng. 2004 July;10(7–8):1006–17. doi: 10.1089/ten.2004.10.1006. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg S, Margulis A, Garlick JA. In vivo transplantation of engineered human skin. Methods Mol Biol. 2005;289:425–30. doi: 10.1385/1-59259-830-7:425. [DOI] [PubMed] [Google Scholar]
- 27.Hansbrough JF, Morgan JL, Greenleaf GE, Bartel R. Composite grafts of human keratinocytes grown on a polyglactin mesh-cultured fibroblast dermal substitute function as a bilayer skin replacement in full-thickness wounds on athymic mice. J Burn Care Rehabil. 1993 September;14(5):485–94. doi: 10.1097/00004630-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Kremer M, Lang E, Berger A. Organotypical engineering of differentiated composite-skin equivalents of human keratinocytes in a collagen-GAG matrix (INTEGRA Artificial Skin) in a perfusion culture system. Langenbecks Arch Surg. 2001 August;386(5):357–63. doi: 10.1007/s004230100227. [DOI] [PubMed] [Google Scholar]
- 29.Lam PK, Chan ES, Liew CT, Lau CH, Yen SC, King WW. The efficacy of collagen dermis membrane and fibrin on cultured epidermal graft using an athymic mouse model. Ann Plast Surg. 1999 November;43(5):523–8. doi: 10.1097/00000637-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Lopez Valle CA, Germain L, Rouabhia M, Xu W, Guignard R, Goulet F, Auger FA. Grafting on nude mice of living skin equivalents produced using human collagens. Transplantation. 1996 August 15;62(3):317–23. doi: 10.1097/00007890-199608150-00003. [DOI] [PubMed] [Google Scholar]
- 31.Mis B, Rolland E, Ronfard V. Combined use of a collagen-based dermal substitute and a fibrin-based cultured epithelium: a step toward a total skin replacement for acute wounds. Burns. 2004 November;30(7):713–9. doi: 10.1016/j.burns.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Supp DM, Wilson-Landy K, Boyce ST. Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice. FASEB J. 2002 June;16(8):797–804. doi: 10.1096/fj.01-0868com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swope VB, Supp AP, Boyce ST. Regulation of cutaneous pigmentation by titration of human melanocytes in cultured skin substitutes grafted to athymic mice. Wound Repair Regen. 2002 November;10(6):378–86. doi: 10.1046/j.1524-475x.2002.10607.x. [DOI] [PubMed] [Google Scholar]
- 34.Barai ND, Supp AP, Kasting GB, Visscher MO, Boyce ST. Improvement of epidermal barrier properties in cultured skin substitutes after grafting onto athymic mice. Skin Pharmacol Physiol. 2006 October 11;20(1):21–8. doi: 10.1159/000096168. [DOI] [PubMed] [Google Scholar]
- 35.Boyce ST, Supp AP, Harriger MD, Pickens WL, Wickett RR, Hoath SB. Surface electrical capacitance as a noninvasive index of epidermal barrier in cultured skin substitutes in athymic mice. J Invest Dermatol. 1996 July;107(1):82–7. doi: 10.1111/1523-1747.ep12298286. [DOI] [PubMed] [Google Scholar]
- 36.Guerret S, Govignon E, Hartmann DJ, Ronfard V. Long-term remodeling of a bilayered living human skin equivalent (Apligraf) grafted onto nude mice: immunolocalization of human cells and characterization of extracellular matrix. Wound Repair Regen. 2003 January;11(1):35–45. doi: 10.1046/j.1524-475x.2003.11107.x. [DOI] [PubMed] [Google Scholar]
- 37.Boyce ST, Foreman TJ, Furmanski P, Hansbrough JF. Absence of tumorigenicity in athymic mice by normal human epidermal keratinocytes after culture in serum-free medium. Cancer Lett. 1992 February 29;62(2):141–7. doi: 10.1016/0304-3835(92)90184-w. [DOI] [PubMed] [Google Scholar]
- 38.Kurlander RJ, Tawab A, Fan Y, Carter CS, Read EJ. A functional comparison of mature human dendritic cells prepared in fluorinated ethylene-propylene bags or polystyrene flasks. Transfusion. 2006 September;46(9):1494–504. doi: 10.1111/j.1537-2995.2006.00940.x. [DOI] [PubMed] [Google Scholar]
- 39.Thissen H, Johnson G, Hartley PG, Kingshott P, Griesser HJ. Two-dimensional patterning of thin coatings for the control of tissue outgrowth. Biomaterials. 2006 January;27(1):35–43. doi: 10.1016/j.biomaterials.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 40.Kino-Oka M, Ogawa N, Umegaki R, Taya M. Bioreactor design for successive culture of anchorage-dependent cells operated in an automated manner 32. Tissue Eng. 2005 March;11(3–4):535–45. doi: 10.1089/ten.2005.11.535. [DOI] [PubMed] [Google Scholar]
- 41.Dhir S, Morrow KJ, Jr, Rhinehart RR, Wiesner T. Dynamic optimization of hybridoma growth in a fed-batch bioreactor. Biotechnol Bioeng. 2000 January;67(2):197–205. [PubMed] [Google Scholar]