Abstract
Pathological cardiac hypertrophy is considered a precursor to clinical heart failure. Understanding the transcriptional regulators that suppress the hypertrophic response may have profound implications for the treatment of heart disease. We report the generation of transgenic mice that overexpress the transcription factor CHF1/Hey2 in the myocardium. In response to the α-adrenergic agonist phenylephrine, they show marked attenuation in the hypertrophic response compared with wild-type controls, even though blood pressure is similar in both groups. Isolated myocytes from transgenic mice demonstrate a similar resistance to phenylephrine-induced hypertrophy in vitro, providing further evidence that the protective effect of CHF1/Hey2 is mediated at the myocyte level. Induction of the hypertrophy marker genes ANF, BNP, and β-MHC in the transgenic cells is concurrently suppressed in vivo and in vitro, demonstrating that the induction of hypertrophy-associated genes is repressed by CHF1/Hey2. Transfection of CHF1/Hey2 into neonatal cardiomyocytes suppresses activation of an ANF reporter plasmid by the transcription factor GATA4, which has previously been shown to activate a hypertrophic transcriptional program. Furthermore, CHF1/Hey2 binds GATA4 directly in coimmunoprecipitation assays and inhibits the binding of GATA4 to its recognition sequence within the ANF promoter. Our findings demonstrate that CHF1/Hey2 functions as an antihypertrophic gene, possibly through inhibition of a GATA4-dependent hypertrophic program.
Keywords: transgenic mouse
Congestive heart failure is a growing public health concern, given that the incidence and prevalence continue to rise worldwide (11). Heart failure is the end result of many pathological processes affecting the heart, including infarction, cardiomyopathy, and pathological hypertrophy. Hypertrophy has been a subject of intense study, not only because it can be a precursor to heart failure but also because it is a physiological process accounting for normal postnatal enlargement and adaptive response to hemodynamic load. A large body of experimental data has been generated describing the molecular signaling and transcriptional pathways that promote hypertrophy; however, the critical steps controlling the progression from physiological to pathological hypertrophy are poorly understood (reviewed in Refs. 29, 34). In addition, the progression from hypertrophy to heart failure, though commonly accepted, is not well-characterized at the molecular level, although apoptosis may be involved (17). The factors that prevent the development of pathological hypertrophy and progression to heart failure are even less understood, although they are of significant scientific interest and are growing in number (reviewed in Ref. 12).
We and others have previously cloned and characterized the hairy-related basic helix-loop-helix (bHLH) transcriptional repressor CHF1 (also called Hey2, Hesr-2, Hrt2, HERP1, and gridlock) (3, 15, 19, 24, 32, 46). CHF1/Hey2 is notable for its expression in the developing heart and vasculature and is thought to play an important role in cardiovascular development (reviewed in Refs. 8, 14). Targeted disruption of CHF1/Hey2 in mice leads to congenital heart abnormalities and cardiomyopathy (5, 10, 20, 35, 36). In our line of knockout mice, the development of cardiomyopathy in adults appeared to be independent of congenital anomalies (35). This finding indicates that CHF1-dependent transcriptional programs are important in maintenance of normal ventricular function. To date, the mechanism of the cardiomyopathy remains unknown but does not appear to be related to altered expression of known cardiac structural genes or transcription factors (35). If CHF1/Hey2 loss of function results in cardiomyopathy, a corollary hypothesis would be that CHF1/Hey2 gain of function may provide protection against the onset of hypertrophy and progression to heart failure, presumably through transcriptional repression of prohypertrophic genes.
The transcription factor GATA4 is known to induce hypertrophy both in vitro and in vivo, through the activation of a variety of hypertrophy-associated genes, and is considered a key regulator of a hypertrophic transcriptional program (25). Expression of atrial natriuretic factor (ANF), brain natriuretic peptide (BNP), and β-myosin heavy chain (β-MHC) is commonly used as a molecular marker of hypertrophy, and their expression is partially dependent on GATA4. GATA4 also is activated by MAP kinase signaling pathways downstream of many hypertrophic stimuli (26). GATA4 also has been shown to play an important role in cardiac development (22, 30) and human septation defects (9). GATA4 is clearly an important nodal regulator of both developmental and disease-related processes within the cardiovascular system, and factors that regulate the activity of GATA4 may have profound effects on GATA4-dependent processes.
In this article we report that overexpression of CHF1/Hey2 in the myocardium prevents phenylephrine-induced cardiac hypertrophy and expression of hypertrophy marker genes in vivo and in vitro and therefore functions as an antihypertrophic gene. Furthermore, we provide evidence that CHF1/Hey2 can directly block activation of a hypertrophy-associated gene, ANF, in reporter assays and that it interacts with GATA4, an important transcriptional activator of ANF and other hypertrophy-associated genes. Our findings demonstrate that CHF1/Hey2 can act as a suppressor of hypertrophy in vivo and in vitro, possibly through attenuation of a GATA4-dependent transcriptional program.
MATERIALS AND METHODS
Generation and characterization of transgenic mice that overexpress CHF1/Hey2 in myocardium
To examine the consequences of persistent overexpression of CHF1 in the developing and adult heart, we generated two independent FvB transgenic mouse lines expressing CHF1/Hey2 under the control of the ventricular regulatory myosin light chain (MLC2v) promoter. The minimal 250-bp MLC2v promoter has been shown to direct ventricular expression from embryonic day 9.0 through adulthood (33). We cloned a CHF1 cDNA consisting of nucleotides 76 to 1164 downstream of the minimal 250-bp MLC2v promoter and upstream of a bovine growth hormone polyadenylation signal before pronuclear injection. Pups were screened by Southern blotting and PCR of the MLC2v transgene unit. We chose this cDNA because it contains the entire protein coding sequence but will give rise to a smaller or “minigene” transcript (1.2 kb) compared with the endogenous transcript (∼2.6 kb), thereby allowing careful quantitation of transgene expression. Transgene copy number and expression level were determined using Southern blotting and Northern blotting, respectively. To assess expression of a variety of cardiac-specific genes, we isolated RNA from wild-type and transgenic hearts for semiquantitative RT-PCR as described previously (35). Primer sequences are listed in Table 1. Echocardiographic assessments of left ventricular dimensions, wall thickness, and fractional shortening were essentially as described previously (35). Generation and manipulation of transgenic animals was approved by the Institutional Animal Care and Use Committee of Harvard Medical School and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Table 1.
Oligonucleotide primers used
| ANF_L | 5′-GTC AAT CCT ACC CCC GAA GCA GCT-3′ |
| ANF_R | 5′-CAG CAT GGG CTC CTT CTC CA-3′ |
| BNP-S | 5′-ATG GAT CTC CTG AAG GTG CT-3′ |
| BNP-AS | 5′-AAG AGG GCA GAT CTA TCG GA-3′ |
| CHF1-S | 5′-GAC AAC TAC CTC TCA GAT TAT GGC-3′ |
| CHF1-AS | 5′-CGG GAG CAT GGG AAA AGC-3′ |
| CHF2-S | 5′-CAT GAA GAG AGC TCA CC-3′ |
| CHF2-AS | 5′-AAT GTG TCC GAG GCC AC-3′ |
| CHF3-S | 5′-ATG GAC CCA TCG ATG TGG-3′ |
| CHF3-AS | 5′-AAA GGC CAG GGC ACT GG-3′ |
| HPRT-S | 5′-CTC GAA GTG TTG GAT ACA GG-3′ |
| HPRT-AS | 5′-TGG CCT ATA GGC TCA TAG TG-3′ |
| αMHC-S | 5′-CTG CTG CAG AGG TTA TTC CTC G-3′ |
| αMHC-AS | 5′-GGA AGA GTG AGC GGC GCA TCA AGG-3′ |
| βMHC-S | 5′-TGC AAA GGC TCC AGG TCT GAG GGC-3′ |
| βMHC-AS | 5′-GCC AAC ACC AAC CTG TCC AAG TTC-3′ |
| MLC2v-S | 5′-CTCAGTCCTTCTCTTCTCCG-3′ |
| MLC2v-AS | 5′-TGTTCCTCACGATGTTTGGG-3′ |
| Nkx2.5/Csx-S | 5′-GCAGAAGGCAGTGGAGCTGGACAAAGCC-3′ |
| Nkx2.5/Csx-AS | 5′-TTGCACTTGTAGCGACGGTTCTGGAACCAG-3′ |
| MEF2c-S | 5′-GCCCTGAGTCTGAGGACAAG-3′ |
| MEF2c-AS | 5′-ATCGTGTTCTTGCTGCCAG-3′ |
| GATA4_L | 5′-CAC TAT GGG CAC AGC AGC TCC-3′ |
| GATA4_R | 5′-TTG CAG CTG GCC TGC GAT GTC-3′ |
| GAPDH_L | 5′-GCT TCA CCA CCT TCT TG-3′ |
| GAPDH_R | 5′-TCA CCA TCT TCC AGG AG-3′ |
| ANF GATA4 BINDING SITE 1 | 5′-CTT TTA AAG TTA TCA GCT CAG CGA AGC-3′ |
| ANF GATA4 BINDING SITE 2 | 5′-GCT TCG CTG GAC TGA TAA CTT TAA AAG-3′ |
| ANF GATA4 BINDING SITE 1 MUTANT | 5′-CTT TTA AAG TTA AGA GCT CAG CGA AGC-3′ |
| ANF GATA4 BINDING SITE 2 MUTANT | 5′-GCT TCG CTG GAC TCT TAA CTT TAA AAG-3′ |
Induction and assessment of phenylephrine-induced hypertrophy in vivo
Briefly, osmotic minipumps (Durect, Cupertino, CA) were loaded with phenylephrine or vehicle according to the manufacturer’s instructions. Pumps were implanted subcutaneously between the scapula of 8- to 12-wk-old male transgenic and wild-type mice with the use of a sterile technique. Pumps infused either phenylephrine at a dose of 30 mg·kg−1·day−1 or vehicle for a 2-wk period. At the end of the study period, hearts were harvested for gravimetry, histology, RNA, protein, and cardiac myocyte isolation. Before death, echocardiographic measurement of ventricular wall thickness was performed as described previously (35).
To assess effects of the transgene and phenylephrine on systemic blood pressure, we measured blood pressure by using the tail-cuff method. Mice were trained by placement in the apparatus daily between the hours of 1:00 and 5:00 PM for 1 wk before any measurements were made. We subsequently measured blood pressure in wild-type and transgenic mice before, during, and after phenylephrine infusion. Blood pressure was measured on a daily basis beginning 1 wk after training and 1 wk before osmotic minipump implantation and then for the 2 wk following implantation.
Isolation and culture of mouse cardiac myocytes and assessment of cellular hypertrophy in vitro
Adult cardiac myocytes were isolated from male wild-type or transgenic mice after 2 wk of phenylephrine or vehicle treatment according to the Alliance for Cell Signaling protocol (38). Twenty-four hours after plating was completed, cells were immunostained with MF-20 monoclonal antibody against sarcomeric myosin (Developmental Studies Hybridoma Bank) and digitally photographed. Cell area was measured by quantitative morphometry of 50 consecutive cells for each condition with NIH Image software.
Neonatal cardiac myocytes were harvested using a modification of the method of Springhorn and Claycomb (41). Hearts were removed and trimmed of atria and vascular tissue, and the remaining ventricular tissue from each heart was cut into several pieces. Tissue was incubated in 4 ml of collagenase type II solution (1 mg/ml) in HBSS and then transferred to a P60 dish and placed at 37°C. Cells were pipetted every 10 min until dispersed (up to 30–40 min) and then filtered with a 70-µm nylon cell strainer (FALCON 35–2350) on a 50-ml tube to remove tissue debris. Collagenase was neutralized in the filtrate by the addition of DMEM-20% FCS. Cells were collected by centrifugation at 700 rpm for 5 min, resuspended in 10 ml of DMEM-20% FCS, and incubated on a P100 dish at 37°C for 1–2 h to remove fibroblasts. The nonadherent cells were collected by centrifugation of the culture medium at 700 rpm for 5 min and resuspended in 10.5 ml of DMEM-20% FCS, and the number of cells was quantified by Coulter counting. Cells were seeded onto fibronectin-coated dishes at the density of 1 × 105 cells/well in a 24-well plate for hypertrophy assays and 3–5 × 106 cells onto P60 dishes for other purposes. Ara-C (20 µM) was included in the culture medium to inhibit proliferation of contaminating fibroblasts.
For hypertrophy assays, cells were allowed to attach for 24 h in DMEM-20% FCS and then changed to serum-free DMEM (catalog no. 11995-065, with penicillin streptomycin, GIBCO) containing 10 µg/ml insulin [1.744 mM stock as 1,000× (10 mg/ml); catalog no. I-1882, Sigma], 10 µg/ml transferrin (catalog no. T-8158, Sigma), 1 mg/ml BSA, and 20 µM Ara-C. After 24 h in serum-free medium, cells were stimulated with phenylephrine (20 µM) and timolol (2 µM) or vehicle for 48 h. Cells were immunostained for sarcomeric actin with MF-20 monoclonal antibody (Developmental Studies Hybridoma Bank), and cell size was quantified using morphometry as described above.
Transfection, coimmunoprecipitation assays, and Western blotting
For transfection assays, neonatal cardiac myocytes were cultured in DMEM-10% FCS and transfected with FuGENE 6 according to the manufacturer’s protocol (Roche). Cells were transfected with plasmids driving expression of CHF1/Hey2 (3) or GATA4 (43) under the control of the human cytomegalovirus promoter, in conjunction with an ANF-luciferase reporter construct (40). An expression vector containing the CMV promoter with no insert (pcDNA3; Promega, Madison, WI) was used to normalize the total DNA for each transfection. Cells were harvested for luciferase assays after 48 h, and firefly luciferase activity driven by the ANF promoter (40) was normalized to Renilla luciferase activity according the manufacturer’s protocol (Promega).
For coimmunoprecipitation, COS-7 cells were transfected with plasmids expressing FLAG-tagged CHF1/Hey2 and myc-tagged GATA4 by using FuGENE 6 as described above. Lysates were made, and immunoprecipitation with 9E10 monoclonal antibody against the myc tag or M2 antibody against the FLAG tag followed by Western blotting was performed essentially as described previously (42), except that SDS was omitted from the lysis buffer.
Electrophoretic mobility shift assays
GATA4 and CHF1/Hey2 proteins were synthesized from plasmid templates by in vitro transcription and translation with a commercially available kit according to the manufacturer’s instructions (Promega). Oligonucleotides containing the binding site for GATA4 within the mouse ANF promoter were synthesized, annealed, and labeled using standard methods. The primer sequences are listed in Table 1. Binding conditions and electrophoresis for CHF1/Hey2 and GATA4 were similar to those previously described (3, 42), except that the binding buffer consisted of 10 mM Tris, pH 7.4, 50 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, and 4% glycerol, and electrophoresis was performed with 1×Tris-borate-EDTA at room temperature.
RESULTS
Generation and characterization of CHF1/Hey2 transgenic mice
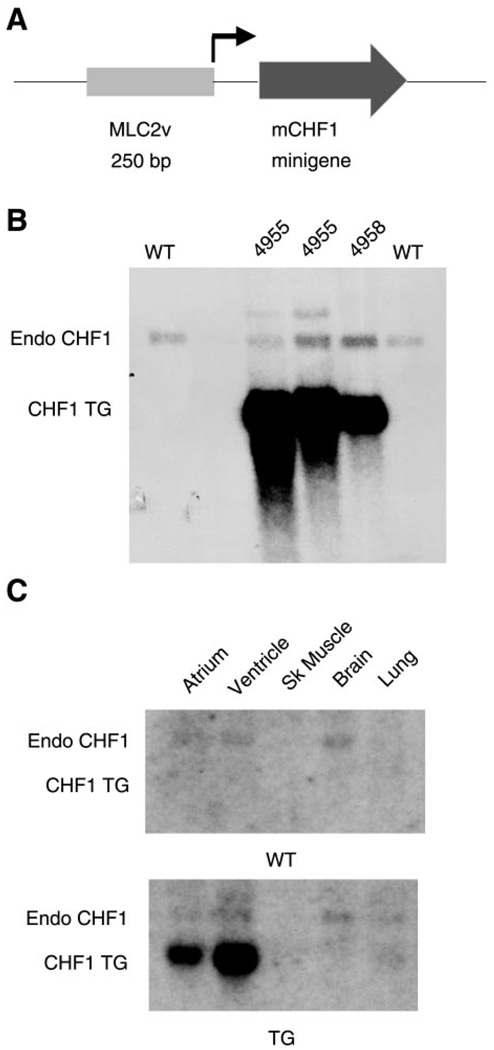
Our group has previously reported that CHF1/Hey2 is expressed at high levels in the developing ventricle from approximately embryonic day 8.5 until birth and is then expressed at lower levels in the postnatal heart (3). Furthermore, our group has reported that targeted disruption of the gene for CHF1/Hey2 leads to cardiomyopathy (35). To examine the consequences of persistent overexpression of CHF1/Hey2 in the developing and adult heart, we generated transgenic mice expressing CHF1/Hey2 under the control of the MLC2v promoter. Two independent FvB transgenic lines that express the cDNA were generated. The transgene copy number for both lines was between 1 and 5 (data not shown). As shown in Fig. 1, the transgene mRNA was expressed at high levels in all transgenic lines and was specific to myocardium, although there was some ectopic atrial expression that was not expected.
Fig. 1.
Generation and characterization of CHF1/Hey2 transgenic (TG) mice. A: construct containing MLC2v promoter and CHF1 cDNA “minigene.” B: Northern blot analysis showing expression of CHF1 in hearts from 2 different transgenic lines. Endo, endogenous transcript; TG, transgene; WT, wild type. C: Northern blot analysis demonstrating expression of CHF1 TG in the heart but not other tissues. Sk, skeletal.
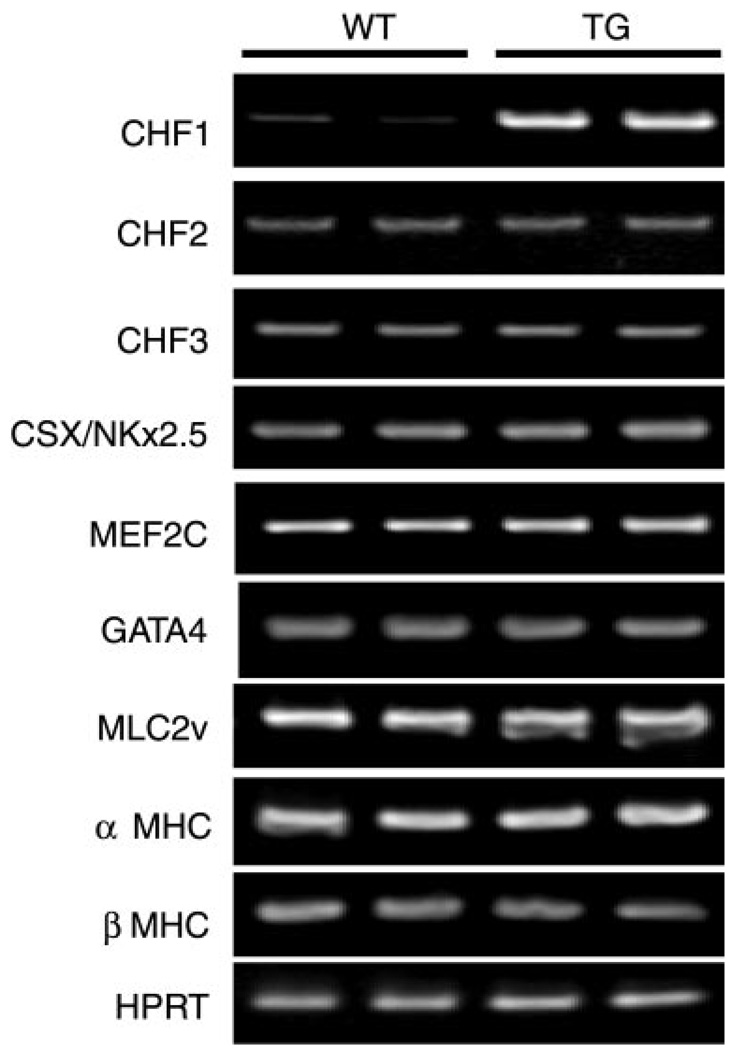
Careful embryonic and anatomic pathological analysis of transgenic mice revealed no alterations in cardiovascular development (data not shown). Furthermore, transgenic mice were indistinguishable from wild-type littermates in terms of survival to weaning or adulthood (up to 52 wk). Serial echocardiograms (up to 40 wk) showed no significant differences in wall thickness, left ventricular dimensions, heart rate, or fractional shortening (data not shown). To assess for baseline alterations in cardiac gene expression, we analyzed RNA from wild-type and transgenic hearts by using RT-PCR. As shown in Fig. 2, transgenic overexpression of CHF1/Hey2 did not alter basal expression of the related transcription factors CHF2/Hey1 and CHF3/HeyL, and it did not alter basal expression of the cardiac-specific genes Nkx2.5, GATA4, MLC2v, myocyte enhancer factor 2c (MEF2c), α-MHC, or β-MHC. Similar results were seen with both transgenic lines. Together, these results suggest that overexpression of CHF1/Hey2 in the myocardium has no obvious effects on normal cardiovascular development or function.
Fig. 2.
Analysis of cardiac gene expression in CHF1 TG mice. RNA was extracted from the ventricles of 10-wk-old TG mice and WT controls, and the indicated transcripts were quantified using RT-PCR. No alterations in expression of a variety of cardiac-specific genes are evident at baseline. MHC, myosin heavy chain.
CHF1/Hey2 transgenic mice are resistant to phenylephrine-induced hypertrophy and do not demonstrate induction of hypertrophy-associated markers
As mentioned, the absence of CHF1/Hey2 leads to cardiomyopathy (35), and overexpression of CHF1/Hey2 does not lead to any obvious developmental, anatomical, or functional abnormalities at baseline and does not have any effect on overall viability and mortality. To test the hypothesis that CHF1/Hey2 may prevent the development of hypertrophy, we challenged the transgenic mice with phenylephrine infusion. Phenylephrine, an α-adrenergic agonist, is a commonly used hypertrophic agent, both in vitro on isolated cardiac myocytes and in vivo through the use of osmotic minipump infusion.
Briefly, osmotic minipumps were implanted into 8-wk-old transgenic and wild-type mice subcutaneously. Pumps infused either phenylephrine at a dose of 30 mg·kg−1·day−1 or vehicle for a 2-wk period. At the end of the study period, hearts were harvested for gravimetry, histology, RNA, protein, and cardiac myocyte isolation. In Fig. 3A, histological sections from wild-type mice stained with hematoxylin and eosin show the expected increase in wall thickening after phenylephrine treatment. Transgenic mice, in contrast, did not show any obvious increase in wall thickness or heart size. No differences were seen in fibrosis or apoptosis as measured using Masson trichrome staining and TdT-mediated dUTP nick end labeling staining (data not shown). To quantify the degree of hypertrophy, we calculated the ventricular weight-to-body weight ratio (VW/BW) for wild-type and transgenic mice before and after phenylephrine treatment. As shown in Fig. 3B, VW/BW increased for wild-type mice after phenylephrine treatment but did not change significantly in the transgenic mice. Echocardiographic assessment of wall thickness before and after treatment also showed no significant increase in transgenic mice treated with phenylephrine compared with wild-type mice. Left ventricular function was preserved before and after treatment for both groups (data not shown). Although this finding is somewhat surprising, it may reflect the limited dose and timing of phenylephrine infusion such that hypertrophy develops, but progression to left ventricular dysfunction has not yet occurred. Prolonged infusion would be expected to cause hypertrophy and decrease left ventricular function in wild-type mice. Similar results were seen in both transgenic lines. ANF, BNP and β-MHC mRNAs are induced in wild-type but not transgenic animals treated with phenylephrine, demonstrating that expression of these marker genes correlates with the development of hypertrophy seen in vivo. CHF1/Hey2 and GATA4 mRNAs were unaffected in both wild-type and transgenic animals (Fig. 3D). These results were quantified using densitometry, as shown in Fig. 3E. Interestingly, the expression of endogenous CHF1/Hey2 was reproducibly decreased with phenylephrine treatment, consistent with its potential function as an antihypertrophic gene.
Fig. 3.
CHF1 TG mice are resistant to phenylephrine (PE)-induced hypertrophy. A: histological sections from 8- to 10-wk-old WT and TG mice treated with PE or vehicle for 2 wk. Veh, vehicle. Gravimetric (B) and echocardiographic assessment (C) was performed of hypertrophy in WT and TG mice treated with PE or vehicle for 2 wk. Asterisks indicate significant differences. D: analysis of atrial natriuretic peptide (ANF), brain natriuretic peptide (BNP), β-MHC, CHF1/Hey2, GATA4, and GAPDH mRNA by RT-PCR in WT and TG hearts after PE infusion. E: quantitation of RT-PCR signal by densitometry. Densitometric data are representative of at least 3 independent experiments. TG mouse hearts showed no significant increase in ventricular weight-to-body weight ratio, left ventricular wall thickness, or hypertrophic marker gene expression in response to phenylephrine.
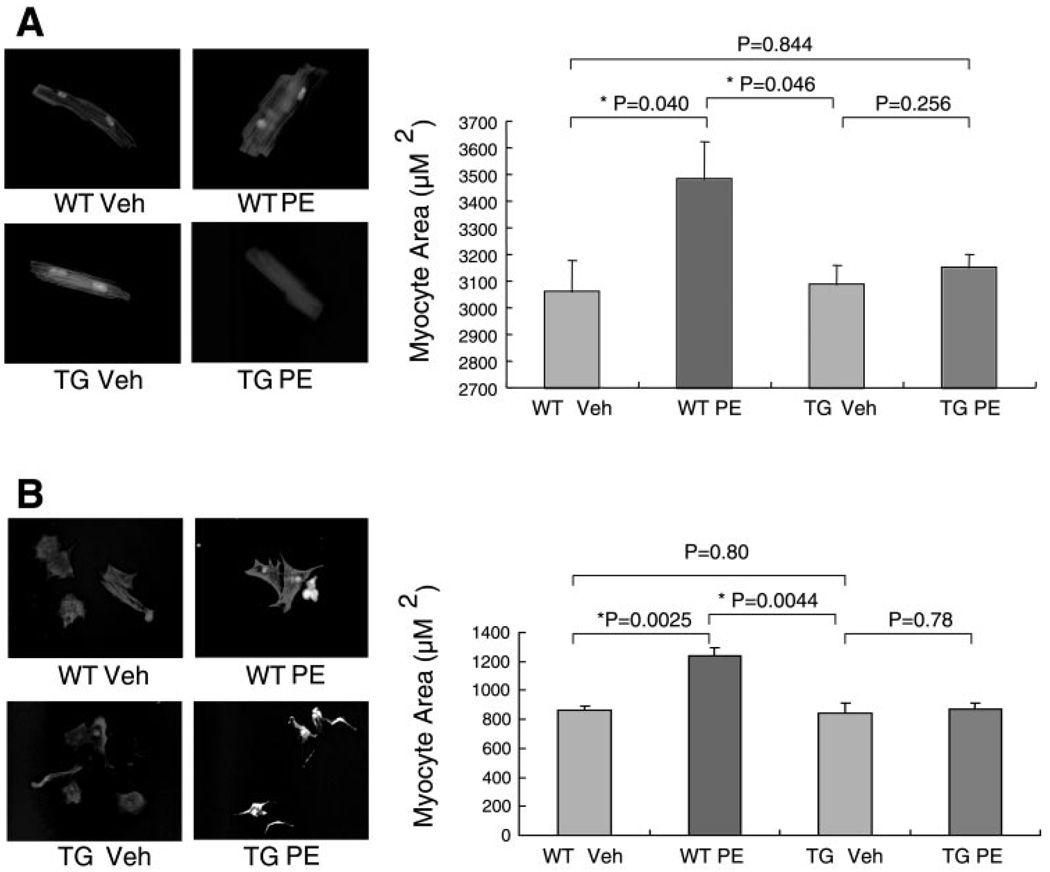
Cardiac myocytes from transgenic mice are resistant to phenylephrine-induced hypertrophy in vivo and in vitro
To determine that the effect of CHF1/Hey2 on cardiac hypertrophy is a primary effect on the myocyte rather than caused by effects on other cells, we initially isolated cardiac myocytes from adult wild-type or transgenic mice after 2 wk of phenylephrine or vehicle treatment. As shown in Fig. 4A, two-dimensional cell area was not significantly different for wild-type cells and transgenic cells treated with vehicle. With phenylephrine treatment, wild-type cells demonstrated increased size compared with vehicle controls, but transgenic cells did not. Three independent preparations were analyzed. We also isolated neonatal cardiac myocytes from wild-type and transgenic animals and treated them with phenylephrine in vitro. Although others (4) have reported that mouse neonatal myocytes do not develop hypertrophy in response to phenylephrine in vitro, in our hands wild-type myocytes did develop hypertrophy in response to phenylephrine. The reasons for this discrepancy are unclear but may be related to the different protease treatments used in the isolation protocols. Transgenic myocytes, in contrast, did not develop hypertrophy in response to phenylephrine in vitro (Fig. 4B). Three independent preparations were analyzed. These findings are consistent with the failure of the transgenic animals to develop increased VW/BW and demonstrate that the effect is mediated at the myocyte level.
Fig. 4.
CHF1 TG cardiac myocytes are resistant to PE-induced hypertrophy. A: WT and TG mice were treated with PE or vehicle for 2 wk, and cardiac myocytes were harvested using the Alliance for Cell Signaling Protocol. Immediately after cell plating, rod-shaped myocytes were immunostained with MF-20 antibody and digitally photographed (left). Two-dimensional myocyte area was quantified using morphometry for 50 consecutive cells with NIH Image software (right). These data are representative of 3 independent experiments. B: neonatal cardiac myocytes were isolated from WT and TG mice. Myocytes were cultured in serum-free medium and treated with PE or vehicle. After 48 h, cells were stained with MF-20 (left). Cell size was quantified using digital image capture and planimetry (right). Statistical significance was determined using Student’s t-test; asterisks indicate significant differences. TG myocytes showed no significant increase in size after PE treatment. These data are representative of 3 independent experiments.
Cardiac myocytes from transgenic mice do not demonstrate induction of hypertrophy markers after phenylephrine treatment in vitro
To assess whether induction of a hypertrophic transcriptional program accompanies the observed changes in myocyte cell size, we isolated RNA from cultured neonatal myocytes treated with phenylephrine. Semiquantitative RT-PCR of ANF, BNP, and β-MHC mRNAs from cultured wild-type and transgenic neonatal myocytes treated with either vehicle or phenylephrine demonstrated that ANF, BNP, and β-MHC expression is induced in wild-type myocytes by phenylephrine, as expected. Transgenic myocytes, in contrast, did not demonstrate induction of these mRNAs (Fig. 5). These findings indicate that the resistance to hypertrophy observed in the transgenic myocytes is accompanied by absence of induction of a hypertrophic transcriptional program. Endogenous CHF1/Hey2 mRNA was reproducibly decreased, whereas transgenic CHF1/Hey2 and GATA4 mRNAs were not affected by phenylephrine treatment in vitro, which mirrors their responses to phenylephrine infusion in vivo (Figs. 3D and 5).
Fig. 5.
Neonatal cardiac myocytes from CHF1/Hey2 myocardial TG mice do not demonstrate induction of hypertrophic marker genes after PE treatment. Neonatal myocytes were harvested and treated with PE or vehicle as described in MATERIALS AND METHODS and the legend to Fig. 4. After 48 h, cells were harvested for RNA. A: RT-PCR for ANF, BNP, β-MHC, CHF1/Hey2, GATA4, and GAPDH mRNA was performed using standard methods. B: quantitation of RT-PCR data by densitometry. Data are representative of 3 independent experiments.
CHF1/Hey2 transgenic mice have blood pressures comparable to those of wild-type mice before and during phenylephrine infusion
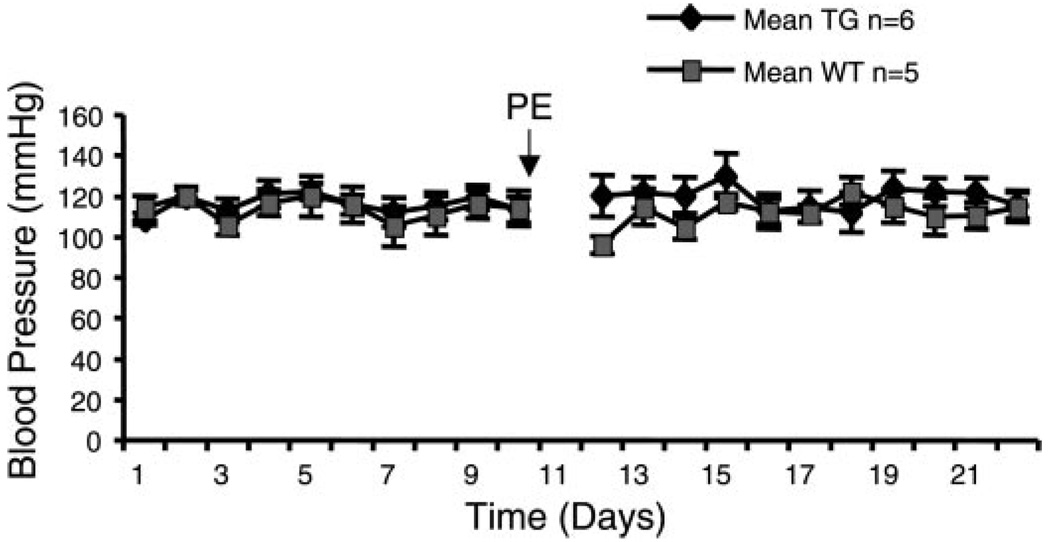
To assess whether the myocardial CHF1/Hey2 transgene may affect systemic blood pressure, we carefully measured blood pressure before, during, and after phenylephrine infusion. As shown in Fig. 6, blood pressure was not significantly different in wild-type and transgenic mice before and during phenylephrine treatment. These findings indicate that transgenic expression of CHF1/Hey2 in the myocardium does not alter baseline blood pressure and does not affect the blood pressure response to phenylephrine at the doses used. Consequently, the hypertrophy seen in wild-type myocytes is likely due to a primary effect on the myocardium.
Fig. 6.
CHF1/Hey2 TG mice have blood pressure similar to that of WT mice before and during PE treatment. Blood pressure was measured on a daily basis 1 wk before and during 2 wk of PE infusion with a computer-controlled tail cuff apparatus after mice were trained for 1 wk before measurement.
CHF1/Hey2 suppresses GATA4-dependent transcription of ANF promoter in primary neonatal cardiac myocytes, interferes with GATA4 binding to DNA, and interacts directly with GATA4
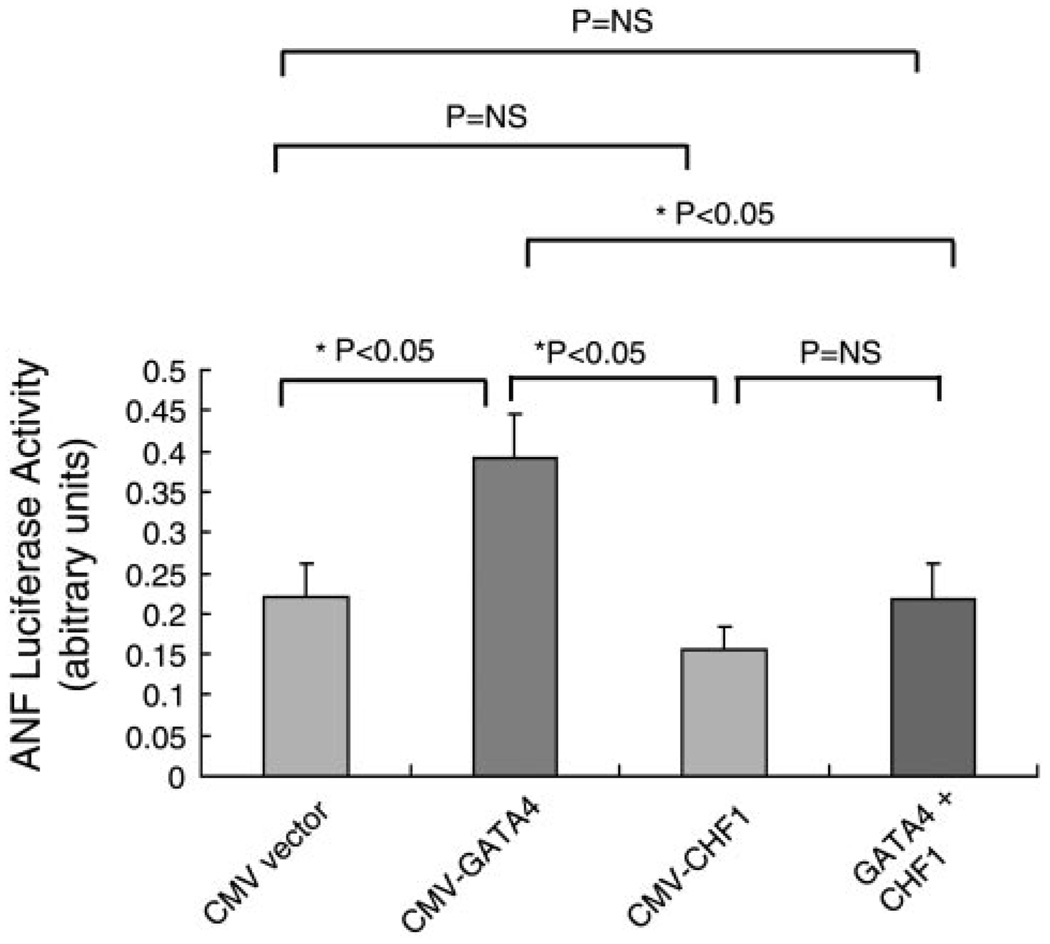
GATA4 previously has been shown to activate hypertrophy-associated genes in vivo and in vitro (25). ANF expression is commonly induced by hypertrophic stimuli, and its promoter is GATA4 dependent and often used as a reporter for induction of hypertrophy (40). GATA4 mutations also have been associated with septal defects (9), which we also have observed in CHF1/Hey2 knockout mice (35). Furthermore, deletion of the GATA4-interacting repressor protein gene, FOG2, gives rise to a spectrum of anomalies reminiscent of those seen in the CHF1/Hey2 null mice, such as tricuspid stenosis and septal defects (43, 44). Given that CHF1/Hey2 prevents the development of hypertrophy in vivo and in vitro and blocks induction of ANF mRNA in cultured cardiac myocytes, we assessed whether CHF1/Hey2 can directly inhibit GATA4-dependent activation of ANF in reporter assays. As shown in Fig. 7, transfection of neonatal cardiac myocytes with the ANF promoter demonstrates that GATA4 can activate the promoter, as expected, and that cotransfection of CHF1/Hey2 can block this activation.
Fig. 7.
CHF1/Hey2 inhibits GATA4-dependent activation of the ANF promoter. Neonatal cardiac myocytes from WT mice were harvested and transfected with an ANF-luciferase reporter plasmid in the presence of CMV expression vector alone, CMV-GATA4, CMV-CHF1/Hey2, or GATA and CHF1 in combination. The results represent the average of 3 independent experiments. Statistical significance was determined using Student’s t-test. Asterisks indicate a significant difference; NS, not significant.
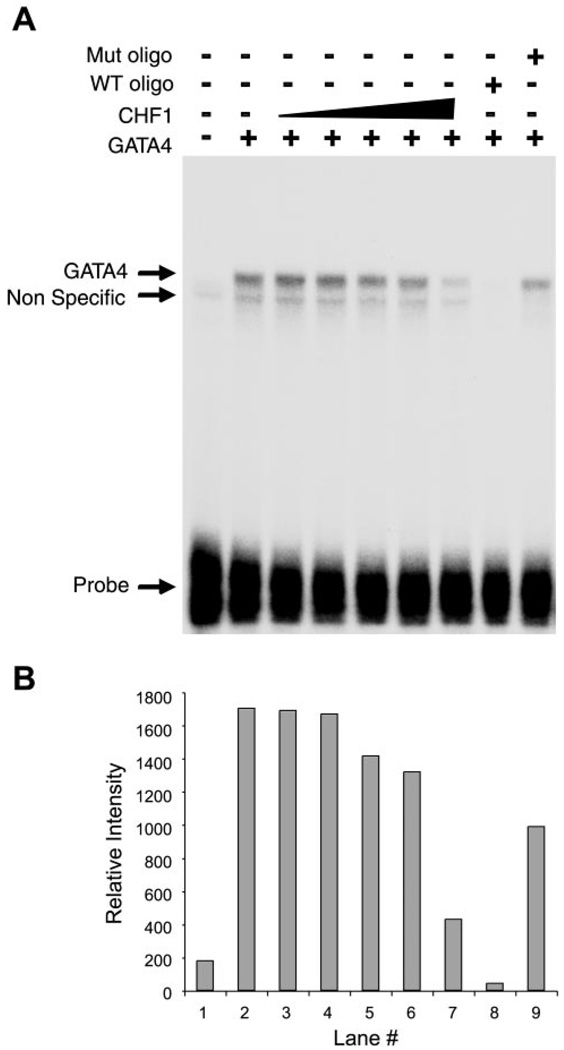
To determine the mechanism by which CHF1/Hey2 blocks GATA4-dependent activation of the ANF promoter, we tested the hypothesis that CHF1/Hey2 may interfere with the binding of GATA4 to its specific binding sequence within the ANF promoter. GATA4 is known to bind specifically to the ANF promoter and activate it in conjunction with the cardiac-specific factor Nkx2.5 (6, 23). To test this hypothesis, we performed electrophoretic mobility shift assays essentially as described previously (3, 42), with minor modifications as described in MATERIALS AND METHODS. As shown in Fig. 8A, GATA4 bound to the ANF promoter oligonucleotide (lane 2). CHF1/Hey2, when added to GATA4, inhibited formation of the GATA4 complex in a dose-dependent fashion (lanes 3–7). The specificity of the GATA4 complex could be further demonstrated by competition with an unlabeled ANF promoter oligonucleotide (lane 8) and by failure of a mutant ANF promoter oligonucleotide to compete for binding (lane 9). Quantification by densitometry confirmed these findings (Fig. 8B). These findings demonstrate that CHF1/Hey2 directly inhibits binding of GATA4 to its recognition sequence within the ANF promoter.
Fig. 8.
CHF1/Hey2 inhibits binding of GATA4 to its recognition site in the ANF promoter. A: GATA4 protein was incubated with radiolabeled oligonucleotides containing the GATA4 binding site from the ANF promoter either alone or in combination with increasing amounts of CHF1/Hey2 protein. The ratio of CHF1 plasmid to GATA4 plasmid in the in vitro transcription/translation reactions was 4-, 8-, 12-, 14-, and 16-fold, respectively, in lanes 3–7. Total plasmid DNA was kept constant with pcDNA3 vector. Unlabeled WT and mutant oligonucleotides also were included as competitors to demonstrate the specificity of the GATA4 binding complex (lanes 8 and 9). The total amount of protein lysate was kept constant in all lanes. B: quantitation of GATA4 binding by densitometry. These data are representative of 4 independent experiments.
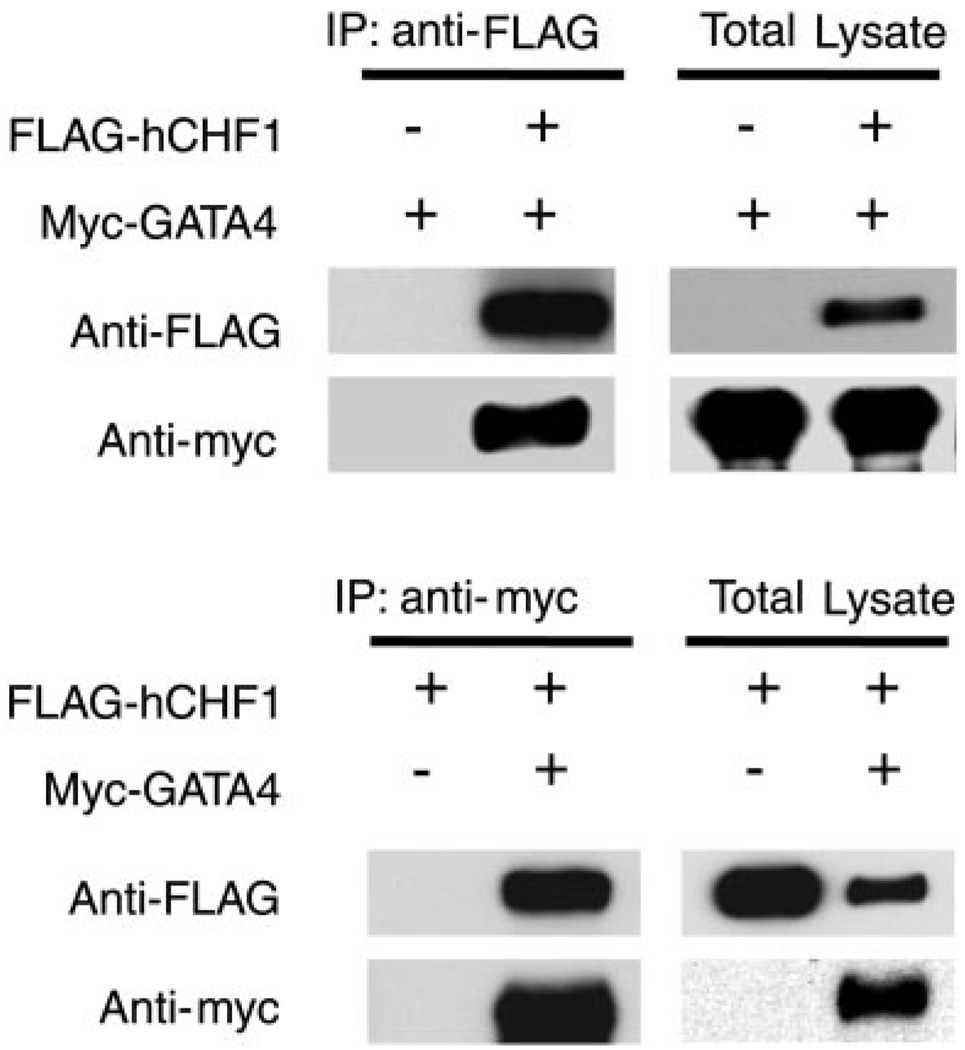
To examine further the potential mechanism by which CHF1/Hey2 regulates GATA4 activity, we tested the hypothesis that CHF1/Hey2 may interact directly with GATA4. CHF1/Hey2 and its relatives have been shown to interact with several important transcriptional regulatory proteins, such as the arylhydrocarbon receptor nuclear translocator ARNT (3), MyoD (42), mSin3, and HES proteins (16). To assess the ability of GATA4 to interact with CHF1/Hey2, we coexpressed myc-tagged GATA4 and FLAG-tagged CHF1/Hey2 in COS-7 cells. As shown in Fig. 9, immunoprecipitation of myc-tagged GATA4 coimmunoprecipitated FLAG-tagged CHF1/Hey2. Similarly, immunoprecipitation of FLAG-tagged CHF1/Hey2 coimmunoprecipitated myc-tagged GATA4. Our findings indicate that CHF1/Hey2 interacts directly with GATA4 and forms an inactive complex that prevents DNA binding and transcriptional activation.
Fig. 9.
CHF1/Hey2 coimmunoprecipitates with GATA4. Expression plasmids for FLAG-CHF1/Hey2, myc-GATA4, and vector were transfected into COS-7 cells as indicated. Cells were lysed, and proteins were immunoprecipitated (IP) with 9E10 anti-myc antibody or M2 anti-FLAG antibody. FLAG-tagged CHF1 and myc-tagged GATA4 were detected using Western blotting. These data are representative of 4 independent experiments.
DISCUSSION
We have found that the bHLH transcriptional repressor CHF1/Hey2 functions as an antihypertrophic protein, both in vitro and in vivo. Furthermore, we have found that CHF1/Hey2 can directly inhibit activation of a hypertrophy-associated gene, ANF, through a direct inhibitory interaction with the transcription factor GATA4 that interferes with DNA binding. Our findings imply that CHF1/Hey2 functions as an antihypertrophic protein by inhibiting the activation of a GATA4-dependent hypertrophy transcriptional program.
The interaction between CHF1/Hey2 and GATA4 was independently described in a recent publication, and a similar effect on ANF promoter activity in vitro was demonstrated (18). Our findings, however, provide additional evidence that this interaction is relevant to the hypertrophic response in vivo. An interaction between the related factor CHF2/Hey1 (also known as HERP2) and the erythroid factor GATA1 was recently reported to inhibit hematopoiesis in vitro (7). A similar report demonstrating an interaction between HES-1 and GATA1 resulting in decreased hematopoiesis in vitro was also published recently (13). These findings suggest that interactions between hairy-related transcription factors and GATA-related transcription factors to regulate important biological processes is likely to be a general phenomenon.
Analysis of our data on ANF reporter gene activity (Fig. 7), GATA4 DNA binding (Fig. 8), and coimmunoprecipitation (Fig. 9) suggests that the effect of CHF1/Hey2 on GATA4 DNA binding is relatively weak compared with the effect on reporter gene transcription and in relation to the relative robustness of the protein-protein interaction. A previous report (18) presents data that the CHF1/Hey2 (also known as HRT2) interaction with GATA4 does not prevent DNA binding, although different binding conditions were used. The relatively weak effect of CHF1/Hey2 in vitro compared with inside cells may be due to an additional effect of CHF1/Hey2 on GATA4 interactions with coactivators such as p300 or corepressors such as FOG2, or to an effect on GATA4 posttranslational modification such as phosphorylation at serine 105 (26).
To date, relatively few antihypertrophic transcriptional regulatory genes have been described, and little is known about the mechanisms by which the majority of these genes function. An antihypertrophic transcriptional program has been postulated to explain the phenotype of mice overexpressing the homeodomain only protein, HOP (2, 39). HOP has been shown to inhibit SRF-dependent transcription in vitro by preventing the binding of SRF to its target CArG boxes, and targeted disruption of the gene for HOP in mice leads primarily to embryonic death due to abnormalities in cardiac development (2, 39). Transgenic mice that overexpress HOP in the myocardium, in contrast, develop spontaneous cardiac hypertrophy through recruitment of class I inhibitory histone deacetylases (HDACs) and inhibition of an SRF-dependent antihypertrophic program (21). The identities of these putative downstream antihypertrophic genes remain generally unknown, although there are multiple reports of individual transcriptional regulatory genes exerting an antihypertrophic effect, such as HDAC9 (45) and the RNA helicase CHAMP (27). The relationship of CHF1/Hey2 to these other proteins associated with suppression of hypertrophy remains under investigation, but it is intriguing to speculate that CHF1/Hey2 may act as an antihypertrophic protein downstream of some of these antihypertrophic molecules.
Although we have demonstrated that CHF1/Hey2 can suppress the activation of GATA4-dependent hypertrophy genes, other signaling and transcriptional mechanisms also may be involved. GATA4 is phosphorylated at serine 105 by ERK1/2 after phenylephrine stimulation, which enhances its DNA binding and transcriptional activity (26). CHF1/Hey2 may additionally interfere with this phosphorylation by its association with GATA4, further inhibiting hypertrophy in vivo. This hypothesis is currently under investigation. The integration of transcriptional regulation and hypertrophic signaling has been partially elucidated for members of the transcription factor families MEF2 and NFATs (nuclear factors of activated T cells), in addition to GATA4 (reviewed in Ref. 1). These families of factors have been implicated in the regulation of fetal cardiac genes associated with hypertrophy (1, 25, 28, 31). NFATs are regulated by interaction with the calcium-dependent phosphatase calcineurin, which dephosphorylates NFATs and allows them to translocate to the nucleus and activate gene transcription. MEF2 factors can be activated by at least two mechanisms, such as direct activation by MAP kinases or dissociation from class II HDACs through calcium-dependent phosphorylation and nuclear export of these HDACs (28, 31, 45). We have measured the expression of MEF2c, NFATc1–4, calcineurin Aβ, and HDAC1–9 in wild-type and transgenic hearts treated with vehicle or phenylephrine by using semiquantitative RT-PCR; however, no significant differences were seen either at baseline or after phenylephrine treatment in both whole hearts and cultured myocytes (data not shown). The relationship of CHF1/Hey2 and these prohypertrophic transcriptional pathways, however, continues to be explored.
We recently reported that vascular smooth muscle cells lacking CHF1/Hey2 demonstrated decreased responsiveness to the growth factors PDGF (platelet-derived growth factor) and HB-EGF (heparin-binding epidermal growth factor-like growth factor) in vitro through attenuation of Rac1 activation and decreased expression of the Rac guanine exchange factor Sos1 (37). These findings, in conjunction with the data presented in this report, suggest that CHF1/Hey2 acts as a regulator of cellular responsiveness to exogenous signals. We have measured activation of the proximal signaling molecules AKT, ERK1/2, p38, and p70S6k in wild-type and transgenic myocytes after phenylephrine stimulation, but we have not seen any differences in activation of these prohypertrophic pathways (data not shown). The exact intracellular targets of CHF1/Hey2 in cardiac myocytes other than GATA4 that may additionally contribute to the blunted response to phenylephrine are under continuing investigation.
Two surprising findings of our study are that the transgenic mice do not have any apparent developmental or adult spontaneous phenotype and that the GATA4-dependent hypertrophy marker genes do not have different levels of baseline expression in the wild-type and transgenic mice. Although an exact mechanistic explanation for these findings requires further experimentation, one possible explanation for the lack of phenotype would be that the function of CHF1/Hey2 during development and disease processes is to function as a homeostatic repressor and regulator of various developmental and disease processes, which may be much more sensitive to loss of function rather than gain of function. The lack of effect on basal gene expression may be ascribed to the combinatorial nature of gene expression, where regulation of the marker genes studied is dependent on various other transcription factors such as SRF, Nkx2.5, MEF2c, and others, and that basal expression is less dependent on GATA4 than the inducible expression provoked by hypertrophic stimuli.
One caveat to our study is that we have not yet demonstrated that CHF1/Hey2 and GATA4 interact in vivo. Our study is limited by lack of a suitable antibody to CHF1/Hey2 for coimmunoprecipitation, chromatin immunoprecipitation, Western blotting, and electrophoretic mobility supershifting. We hope to address this deficiency in a future study.
Another issue unresolved by our study is whether the antihypertrophic effect of CHF1/Hey2 overexpression is general for many kinds of hypertrophic stimuli or is specific to phenylephrine. Our unpublished data suggest that it may be general, because neonatal transgenic myocytes show resistance to angiotensin II and serum in vitro, whereas wild-type cells develop hypertrophy. We also have found that rats undergoing aortic constriction show decreased expression of CHF1/Hey2, which is consistent with our current study. Further studies of these transgenic mice in other in vivo models of hypertrophy such as angiotensin II infusion, isoproterenol infusion, and aortic constriction are clearly warranted.
Inhibition of hypertrophic signaling or activation of “antihypertrophic” genetic programs is of profound scientific and clinical interest. Insights into mechanisms for preventing the development of pathological hypertrophy and progression to heart failure may have tremendous impact on development of therapy for cardiovascular disease. Our findings demonstrate the novel finding that CHF1/Hey2 can function as an antihypertrophic protein and raise the possibility that future therapy directed toward CHF1/Hey2-dependent pathways may eventually lead to improved treatments for heart failure.
ACKNOWLEDGMENTS
We thank Gordon Huggins for the GATA4 and myc-tagged GATA4 expression constructs. We thank Chris Glembotski for the ANF reporter plasmid.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-67141-04 and HL-076232-01A1 (to M. T. Chin) and an American Heart Association Postdoctoral Fellowship Grant (Northeast Affiliate) to F. Xiang.
REFERENCES
- 1.Akazawa H, Komuro I. Roles of cardiac transcription factors in cardiac hypertrophy. Circ Res. 2003;92:1079–1088. doi: 10.1161/01.RES.0000072977.86706.23. [DOI] [PubMed] [Google Scholar]
- 2.Chen F, Kook H, Milewski R, Gitler AD, Lu MM, Li J, Nazarian R, Schnepp R, Jen K, Biben C, Runke G, Mackay JP, Novotny J, Schwartz RJ, Harvey RP, Mullins MC, Epstein JA. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002;110:713–723. doi: 10.1016/s0092-8674(02)00932-7. [DOI] [PubMed] [Google Scholar]
- 3.Chin MT, Maemura K, Fukumoto S, Jain MK, Layne MD, Watanabe M, Hsieh CM, Lee ME. Cardiovascular basic helix loop helix factor 1, a novel transcriptional repressor expressed preferentially in the developing and adult cardiovascular system. J Biol Chem. 2000;275:6381–6387. doi: 10.1074/jbc.275.9.6381. [DOI] [PubMed] [Google Scholar]
- 4.Deng XF, Rokosh DG, Simpson PC. Autonomous and growth factor-induced hypertrophy in cultured neonatal mouse cardiac myocytes. Comparison with rat. Circ Res. 2000;87:781–788. doi: 10.1161/01.res.87.9.781. [DOI] [PubMed] [Google Scholar]
- 5.Donovan J, Kordylewska A, Jan YN, Utset MF. Tetralogy of Fallot and other congenital heart defects in Hey2 mutant mice. Curr Biol. 2002;12:1605–1610. doi: 10.1016/s0960-9822(02)01149-1. [DOI] [PubMed] [Google Scholar]
- 6.Durocher D, Charron F, Warren R, Schwartz RJ, Nemer M. The cardiac transcription factors Nkx2–5 and GATA-4 are mutual cofactors. EMBO J. 1997;16:5687–5696. doi: 10.1093/emboj/16.18.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elagib KE, Xiao M, Hussaini IM, Delehanty LL, Palmer LA, Racke FK, Birrer MJ, Shanmugasundaram G, McDevitt MA, Goldfarb AN. Jun blockade of erythropoiesis: role for repression of GATA-1 by HERP2. Mol Cell Biol. 2004;24:7779–7794. doi: 10.1128/MCB.24.17.7779-7794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer A, Gessler M. Hey genes in cardiovascular development. Trends Cardiovasc Med. 2003;13:221–226. doi: 10.1016/s1050-1738(03)00082-3. [DOI] [PubMed] [Google Scholar]
- 9.Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, Matsuoka R, Cohen JC, Srivastava D. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- 10.Gessler M, Knobeloch KP, Helisch A, Amann K, Schumacher N, Rohde E, Fischer A, Leimeister C. Mouse gridlock: no aortic coarctation or deficiency, but fatal cardiac defects in Hey2 −/− mice. Curr Biol. 2002;12:1601–1604. doi: 10.1016/s0960-9822(02)01150-8. [DOI] [PubMed] [Google Scholar]
- 11.Givertz MM, Colucci WS, Braunwald E. Clinical aspects of heart failure: high-output failure; pulmonary edema. In: Braunwald E, Zipes DP, Libby P, editors. Heart Disease: A Textbook of Cardiovascular Medicine. 6th. Philadelphia, PA: Saunders; 2001. pp. 534–561. [Google Scholar]
- 12.Hardt SE, Sadoshima J. Negative regulators of cardiac hypertrophy. Cardiovasc Res. 2004;63:500–509. doi: 10.1016/j.cardiores.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Ishiko E, Matsumura I, Ezoe S, Gale K, Ishiko J, Satoh Y, Tanaka H, Shibayama H, Mizuki M, Era T, Enver T, Kanakura Y. Notch signals inhibit the development of erythroid/megakaryocytic cells by suppressing GATA-1 activity through the induction of HES1. J Biol Chem. 2005;280:4929–4939. doi: 10.1074/jbc.M406788200. [DOI] [PubMed] [Google Scholar]
- 14.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 15.Iso T, Sartorelli V, Chung G, Shichinohe T, Kedes L, Hamamori Y. HERP, a new primary target of Notch regulated by ligand binding. Mol Cell Biol. 2001;21:6071–6079. doi: 10.1128/MCB.21.17.6071-6079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iso T, Sartorelli V, Poizat C, Iezzi S, Wu HY, Chung G, Kedes L, Hamamori Y. HERP, a novel heterodimer partner of HES/E(spl) in Notch signaling. Mol Cell Biol. 2001;21:6080–6089. doi: 10.1128/MCB.21.17.6080-6089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang PM, Izumo S. Apoptosis in heart: basic mechanisms and implications in cardiovascular diseases. Trends Mol Med. 2003;9:177–182. doi: 10.1016/s1471-4914(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 18.Kathiriya IS, King IN, Murakami M, Nakagawa M, Astle JM, Gardner KA, Gerard RD, Olson EN, Srivastava D, Nakagawa O. Hairy-related transcription factors inhibit GATA-dependent cardiac gene expression through a signal-responsive mechanism. J Biol Chem. 2004;279:54937–54943. doi: 10.1074/jbc.M409879200. [DOI] [PubMed] [Google Scholar]
- 19.Kokubo H, Lun Y, Johnson RL. Identification and expression of a novel family of bHLH cDNAs related to Drosophila hairy and enhancer of split. Biochem Biophys Res Commun. 1999;260:459–465. doi: 10.1006/bbrc.1999.0880. [DOI] [PubMed] [Google Scholar]
- 20.Kokubo H, Miyagawa-Tomita S, Tomimatsu H, Nakashima Y, Nakazawa M, Saga Y, Johnson RL. Targeted disruption of hesr2 results in atrioventricular valve anomalies that lead to heart dysfunction. Circ Res. 2004;95:540–547. doi: 10.1161/01.RES.0000141136.85194.f0. [DOI] [PubMed] [Google Scholar]
- 21.Kook H, Lepore JJ, Gitler AD, Lu MM, Wing-Man Yung W, Mackay J, Zhou R, Ferrari V, Gruber P, Epstein JA. Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J Clin Invest. 2003;112:863–871. doi: 10.1172/JCI19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y, Shioi T, Kasahara H, Jobe SM, Wiese RJ, Markham BE, Izumo S. The cardiac tissue-restricted homeobox protein Csx/Nkx2.5 physically associates with the zinc finger protein GATA4 and cooperatively activates atrial natriuretic factor gene expression. Mol Cell Biol. 1998;18:3120–3129. doi: 10.1128/mcb.18.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leimeister C, Externbrink A, Klamt B, Gessler M. Hey genes: a novel subfamily of hairy- and Enhancer of split-related genes specifically expressed during mouse embryogenesis. Mech Dev. 1999;85:173–177. doi: 10.1016/s0925-4773(99)00080-5. [DOI] [PubMed] [Google Scholar]
- 25.Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J Biol Chem. 2001;276:30245–30253. doi: 10.1074/jbc.M102174200. [DOI] [PubMed] [Google Scholar]
- 26.Liang Q, Wiese RJ, Bueno OF, Dai YS, Markham BE, Molkentin JD. The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol Cell Biol. 2001;21:7460–7469. doi: 10.1128/MCB.21.21.7460-7469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu ZP, Olson EN. Suppression of proliferation and cardiomyocyte hypertrophy by CHAMP, a cardiac-specific RNA helicase. Proc Natl Acad Sci USA. 2002;99:2043–2048. doi: 10.1073/pnas.261708699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J, McKinsey TA, Nicol RL, Olson EN. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc Natl Acad Sci USA. 2000;97:4070–4075. doi: 10.1073/pnas.080064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molkentin JD, Dorn IG., II Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 30.Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 31.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa O, Nakagawa M, Richardson JA, Olson EN, Srivastava D. HRT1, HRT2, and HRT3: a new subclass of bHLH transcription factors marking specific cardiac, somitic, and pharyngeal arch segments. Dev Biol. 1999;216:72–84. doi: 10.1006/dbio.1999.9454. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien TX, Lee KJ, Chien KR. Positional specification of ventricular myosin light chain 2 expression in the primitive murine heart tube. Proc Natl Acad Sci USA. 1993;90:5157–5161. doi: 10.1073/pnas.90.11.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17:1937–1956. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- 35.Sakata Y, Kamei CN, Nakagami H, Bronson R, Liao JK, Chin MT. Ventricular septal defect and cardiomyopathy in mice lacking the transcription factor CHF1/Hey2. Proc Natl Acad Sci USA. 2002;99:16197–16202. doi: 10.1073/pnas.252648999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakata Y, Koibuchi N, Xiang F, Youngblood JM, Kamei CN, Chin MT. The spectrum of cardiovascular anomalies in CHF1/Hey2 deficient mice reveals roles in endocardial cushion, myocardial and vascular maturation. J Mol Cell Cardiol. In press doi: 10.1016/j.yjmcc.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Sakata Y, Xiang F, Chen Z, Kiriyama Y, Kamei CN, Simon DI, Chin MT. Transcription factor CHF1/Hey2 regulates neointimal formation in vivo and vascular smooth muscle proliferation and migration in vitro. Arterioscler Thromb Vasc Biol. 2004;24:2069–2074. doi: 10.1161/01.ATV.0000143936.77094.a4. [DOI] [PubMed] [Google Scholar]
- 38.Sambrano GR, Fraser I, Han H, Ni Y, O’Connell T, Yan Z, Stull JT. Navigating the signalling network in mouse cardiac myocytes. Nature. 2002;420:712–714. doi: 10.1038/nature01306. [DOI] [PubMed] [Google Scholar]
- 39.Shin CH, Liu ZP, Passier R, Zhang CL, Wang DZ, Harris TM, Yamagishi H, Richardson JA, Childs G, Olson EN. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell. 2002;110:725–735. doi: 10.1016/s0092-8674(02)00933-9. [DOI] [PubMed] [Google Scholar]
- 40.Sprenkle AB, Murray SF, Glembotski CC. Involvement of multiple cis elements in basal- and α-adrenergic agonist-inducible atrial natriuretic factor transcription. Roles for serum response elements and an SP-1-like element. Circ Res. 1995;77:1060–1069. doi: 10.1161/01.res.77.6.1060. [DOI] [PubMed] [Google Scholar]
- 41.Springhorn JP, Claycomb WC. Preproenkephalin mRNA expression in developing rat heart and in cultured ventricular cardiac muscle cells. Biochem J. 1989;258:73–78. doi: 10.1042/bj2580073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun JX, Kamei CN, Layne MD, Jain MK, Liao JK, Lee ME, Chin MT. Regulation of myogenic terminal differentiation by the hairy-related transcription factor CHF2. J Biol Chem. 2001;276:18591–18596. doi: 10.1074/jbc.M101163200. [DOI] [PubMed] [Google Scholar]
- 43.Svensson EC, Huggins GS, Lin H, Clendenin C, Jiang F, Tufts R, Dardik FB, Leiden JM. A syndrome of tricuspid atresia in mice with a targeted mutation of the gene encoding Fog-2. Nat Genet. 2000;25:353–356. doi: 10.1038/77146. [DOI] [PubMed] [Google Scholar]
- 44.Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- 45.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhong TP, Rosenberg M, Mohideen MAPK, Weinstein B, Fishman MC. Gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science. 2000;287:1820–1824. doi: 10.1126/science.287.5459.1820. [DOI] [PubMed] [Google Scholar]