Abstract
Photochemically induced cerebral infarction has been considered a clinically relevant model for ischemic stroke. We evaluated various transgenic mice to study the role of platelet adhesion molecules in this model. Infarction to the sensorimotoric cortex was induced by erythrosin B and laser light. Infarct volumes were calculated from triphenyltetrazolium chloride stained brain slices. Thrombus formation and vessel leakage were observed in vivo by multiphoton microscopy. Mice mutant in VWF, GPIbα, β3 integrin, and P-selectin did not show any significant differences in infarct volume compared to wild type (WT). This is in contrast to the intraluminal middle cerebral artery occlusion model in which αIIbβ3 integrin, GPIbα and P-selectin are known to modulate infarct size. Multiphoton microscopy showed that small, non-occlusive embolizing platelet thrombi formed in the photochemically injured brains. Massive vessel leakage was observed within 25 minutes of laser injury. Interestingly, we observed a significant increase in infarct size with aging, accordant with heightened fragility of the blood brain barrier (BBB) in older mice. This model of photochemically induced stroke is closer to a BBB injury model than a thrombotic stroke model in which platelets and their adhesion molecules are crucial. This model will be useful to study mechanisms regulating BBB permeability.
Keywords: photochemically induced stroke, platelet adhesion receptors, aging, blood brain barrier permeability
1. Introduction
Ischemic stroke is a feared complication of atherosclerotic cardiovascular disease occurring in approximately 600,000 individuals per year in the USA (American Stroke Association, 2007). In spite of the serious consequences of stroke and the urgent clinical need to identify and test novel therapeutic agents, there is still a lack of technically accessible and reproducible animal models to determine aspects of pathogenesis or treatment. The most common experimental animal model is based on middle cerebral artery occlusion (MCAO) by an intraluminal filament technique. However, MCAO is technically complex and requires highly specialized skills to ensure reproducibility. Another experimental approach that is technically simpler involves the induction of photochemically induced thrombotic cortical damage. This less invasive animal model produces highly reproducible brain lesions in rats (Yao et al., 1996) and mice (Eichenbaum et al., 2002).
According to different descriptions of this model, stroke induction would in part depend on microvascular thrombotic occlusion in a platelet-dependent manner (Van Reempts and Borgers, 1994) and an increased vascular permeability (Hoff et al., 2005). The formation of a thrombus involves multiple adhesion molecules (such as von Willebrand factor (VWF), collagen, fibrinogen and fibronectin) and their respective receptors (GPIb, GPVI, β1 and β3 integrins) on the platelet surface. However, the contribution of the platelet adhesion molecules to the lesion size has not been documented in the photochemical injury model. Therefore, in order to understand the role of platelet adhesion molecules and their surface receptors in photochemical stroke, we performed a series of experiments using VWF−/−, β3 integrin−/−, transgenic mice lacking the GPIbα extracellular domain (IL4Rα/GPIbα-tg) and P-selectin−/− mice.
2. Results
Infarct area increases with age
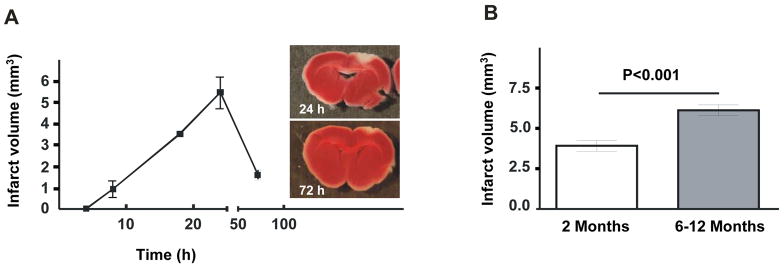
To establish the time of maximal damage in photochemically induced stroke, we did a timeline experiment using WT mice. Infarcted region visualized by the metabolic TTC stain became apparent 8 hours after induction of the injury (0.9 ± 0.6 mm3) and gradually grew in size up to 24 hours (5.5 ± 1.3 mm3). At 72 hours, the infarct volume diminished to one third of 24 hours volume (1.6 ± 0.4 mm3) (Figure 1A). We chose 24 hours as the optimal time point for analyses in subsequent experiments. Next we evaluated the effect of age on the outcome of stroke in the WT mice. We found that 24 hours after stroke, 6–12 months old mice had significantly larger infarct volume compared to 2 months old mice (6.7 ± 0.3 mm3 vs. 3.9 ± 0.3 mm3, P<0.02 respectively) (Figure 1B). Thus it appears that older mice are more susceptible to photochemically induced stroke. In the following experiments, only mice of similar age were compared.
Figure 1. Infarct volume as a function of time and animal age.
(A) Infarct volume at different time points after photochemically induced stroke. (n=4 per time point). Representative photographs of TTC stained sections with maximal infarct volumes from brain at 24 hours and 72 hours post injury (panels). (B) Infarct volumes increase significantly in older mice compared to young mice subjected to photochemical stroke (n=11–13).
Role of adhesion molecules and their receptors on the outcome of photochemically induced stroke
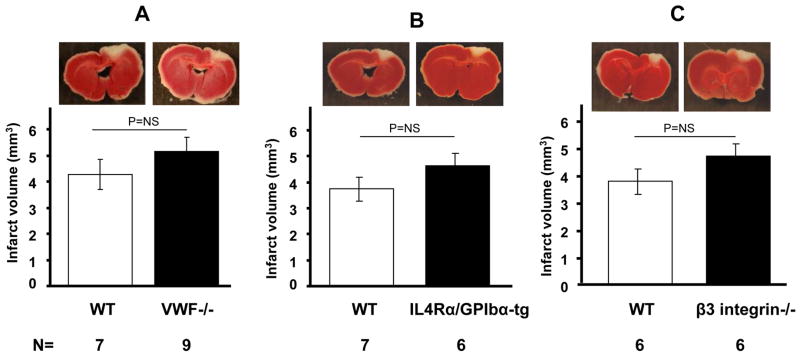
After vascular injury, VWF contributes to thrombus formation by mediating the initial adhesion of platelets to the extracellular matrix and to one another. Two important platelet receptors for VWF are GPIbα in the GPIb-IX-V complex and integrin αIIbβ3. VWF−/− mice demonstrate serious defects in hemostasis and thrombosis (Denis et al., 1998). Despite this, we observed no differences in infarct volume in VWF −/− mice compared to WT (VWF−/− = 5.2 ± 1.7 mm3 vs. WT = 4.3 ± 1.5 mm3, n=7–9, P=0.3) (Figure 2A). Similarly in the IL4Rα/GPIbα-tg mice, which have a defect in platelet adhesion and thrombus formation (Bergmeier et al., 2006), the infarct volumes were comparable to WT (5.4 ± 1.6 mm3 vs. 4.3 ± 1.9 mm3, P=0.26) (Figure 2B). Then we studied the role of β3 integrin, a receptor for adhesive proteins such as fibrinogen, VWF and fibronectin. Inhibition or deficiency of αIIbβ3 integrin and of GPIb were shown to provide protection from intraluminal MCAO induced stroke (Choudhri et al., 1998; Kleinschnitz et al., 2007; Massberg et al., 2005). β3−/− mice lack functional αIIbβ3, the major platelet integrin that mediates platelet aggregation (Hodivala-Dilke et al., 1999). Again, we did not observe any difference in infarct volume of β3−/− mice compared to WT (3.7 ± 1.0 mm3 vs. 4.6 ± 1.2 mm3, P=0.6, Figure 2C). It appears therefore that, in contrast to the intraluminal MCAO model, platelet adhesion molecules do not significantly affect infarct volume produced by the photochemical injury model.
Figure 2. Outcome of photochemically induced stroke in mutant mouse strains defective in thrombosis and hemostasis.
No differences in infarct volume from age-matched wild types (WT) were observed by TTC staining in VWF−/− (A), IL4Rα/GPIbα-tg (B), and β3−/− mice (C). Representative photographs of sections with the largest infarct area are shown.
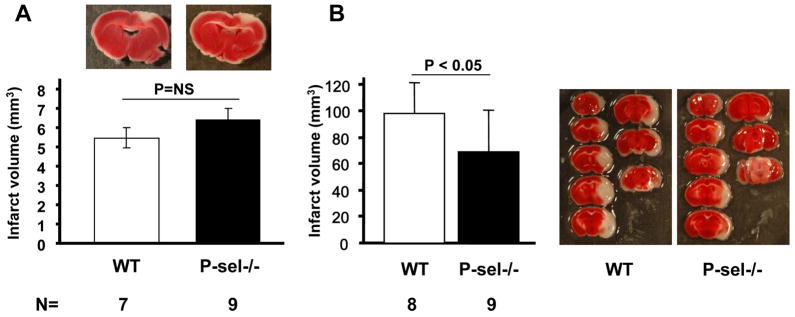
P-selectin (P-sel), a receptor important in platelet rolling (Frenette et al., 1995) and in inflammatory responses, is present in α-granules of platelets and in Weibel-Palade bodies of the endothelial cells (Wagner, 1993). It is released upon platelet stimulation. Previously, it has been shown that P-selectin deficiency provides protection in the intraluminal MCAO model (Connolly et al., 1997). However, in the photochemical stroke model we did not observe any significant difference in infarct volumes in the P-sel−/− mice compared to WT (6.4 ± 1.7 mm3 vs. 5.5 ± 1.3 mm3, P=0.15, Figure 3A). We decided to evaluate the P-sel−/− mice from our own colony in the intraluminal MCAO model. We were able to confirm that deficiency of P-selectin protects mice from stroke in the intraluminal MCAO model (Figure 3B). The infarct volume in P-sel−/− mice was 69.8 ± 31.8 mm3 compared to 98.9 ± 24.5 mm3 in WT mice (Figure 3B). Taken together these data suggest that, in contrast to the intraluminal ischemic stroke produced by MCAO, platelet P-selectin or endothelial P-selectin supporting inflammation do not appear to modulate lesion size produced by photochemical injury.
Figure 3. Comparison of the role of P-selectin in the photochemically induced and intraluminal MCAO stroke models.
(A) Photochemically induced stroke in P-selectin−/− mice and their age matched WT controls. The panels show representative pictures of the sections with maximal infarct for each group. (B) Intraluminal MCAO model on P-selectin−/− mice and their age matched WT controls. Representative photographs of TTC staining of a series of brains sections show protective effects of P-selectin deficiency.
Enhanced vascular leakage in photochemically induced stroke
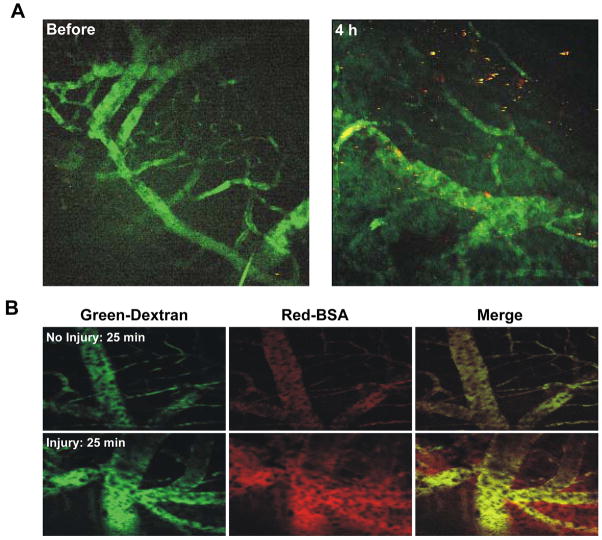
To further clarify the causes of stroke in this particular model, we used multiphoton microscopy, a sensitive technique for viewing labeled platelet accumulation and BBB permeability through a brain window in live mice. We followed the accumulation of labeled platelets in blood vessels exposed to photochemically induced stroke by visualizing the vasculature using a 2×106 Da dextran that is too large to leak from the vessels into the brain tissue. Up to 4 hours after laser treatment, only discrete small platelet clumps formed which embolized frequently but were not sufficient to block any of the visualized microcortical vessels (Figure 4A). Hence, platelet-mediated thrombosis is apparently not the main cause of infarct in this model.
Figure 4. In vivo visualization of photochemical damage by multiphoton microscopy.
Labeled platelets and large dextrans (2×106 Da, for vessel visualization) were infused IV before laser illumination. Vascular leakage was visualized by infusing red BSA. (A) Representative images of microcortical vessels showing vessels (green) and labeled platelets (red). No platelet deposition was visible before laser treatment (left panel). Four hours after laser treatment (right panel) small platelet thrombi could be seen but vessels were still patent. (B) Vascular leakage at a non injured (top panels) site and an injured (bottom panels) site 25 minutes after photochemically induced stroke. Left panels: large dextrans delineating the vessels; middle panels: BSA; right panels: merged images. No vascular leakage of red BSA outside the vessels was observed at a non injured site (top middle panel), whereas BSA leakage was prominent at the site of laser injury (bottom middle panel).
Considering that WT size infarcts were produced in all mice with serious thrombotic defects, we postulated that the primary source of damage would be related to induction of BBB leakage rather than thrombotic ischemia. To examine BBB permeability, WT mice were injected with Alexa labelled BSA (66 kDa), a molecule that leaks across damaged BBB (Kiernan, 1996). Within 25 minutes after illumination, massive leakage became apparent within the site of photochemical injury while the control site in the other hemisphere remained intact (Figure 4B). Our observations indicate that injured vessels become leaky early after photochemically induced cortical infarction and we propose that this is likely the cause of the brain lesion formed in these animals.
3. Discussion
The photochemically induced method of generating infarct is a simple model of ischemic stroke. It has been used preferentially in rats and the presence of activated platelets and fibrin deposition in irradiated areas, as seen by histology, led to an assumption that the model reflects a thrombotic type of stroke (Watson et al., 1985). However, recent studies in rats suggested that not only thrombosis but also BBB leakage occur as part of the pathophysiology of the photochemical model (Hoff et al., 2005). Yet, there is still no genetic or functional evidence that platelets and their adhesion receptors are involved in the pathogenesis of the cortical damage in this stroke model. In human ischemic stroke resulting from arterial occlusion, platelet thrombi play an important role and thrombolysis is the only approved treatment for this condition (Molina, 2007). Since reliable ischemic stroke models with platelet-related microvascular thrombosis features are imperative for research on pathogenesis and novel antithrombotic therapies, we tested the role of a number of key determinants of platelet adhesion in the photochemically induced stroke model.
Comparing the different genetically engineered mouse strains with specific defects in hemostasis and thrombosis to WT mice (Figure 2A–C) we did not find any significant differences in the volume of infarction. The absence of prominent platelet receptors such as GPIbα and β3 integrin, which are necessary for platelet adhesion and thrombus growth (Denis and Wagner, 2007), did not affect the infarct volume. In addition, using intravital microscopy 4 hours after illumination, we observed only scattered aggregates of labelled platelets in the WT mice, without apparent microvascular occlusions in the area of infarct. Although our results do not rule out a minor contribution by platelet thrombi to the infarct, they seem to indicate that platelet accumulation in the photochemically damaged area of the brain is of little pathological importance to the injury process. The involvement of platelet adhesion receptor αIIbβ3 is documented in the intraluminal MCAO model in which antibody blockade to integrin αIIbβ3 or αIIb deficiency provided protection (Choudhri et al., 1998; Massberg et al., 2005). Although a recent study using a different inhibitor of αIIbβ3 did not confirm these results, the authors found major protection with Fab fragments to GPIbα (Kleinschnitz et al., 2007). In contrast, we could not detect any protection in mice mutant for GPIbα, β3 integrin and VWF in the photochemical injury stroke model. We also could not demonstrate a role for P-selectin, a receptor known to be important in ischemia/reperfusion injury (Ishikawa et al., 2004) in the photochemically induced stroke model. However, using the same P-sel−/− mouse colony we could confirm the role of P-selectin in the intraluminal MCAO model as reported by Connolly and colleagues (Connolly et al., 1997). Therefore our results also suggest that the photochemically induced stroke model used in this study is not suitable for studying the inflammatory responses that may affect the outcome of stroke.
We confirmed in the mouse the occurrence of prominent BBB disruption early after injury as documented by a markedly increased leakage of infused labelled BSA in the treated hemisphere compared to the uninjured hemisphere. Thus we think it is likely that the extensive infarct observed may be a result of BBB damage resulting in parenchyma injury rather than microvascular thrombosis. The reasons for the absence of prominent microvascular thrombosis in this model are not clear. Laser induced photothrombosis is an established model to induce thrombosis in different vascular beds although these thrombi are mostly very short-lived (Denis and Wagner, 2007; Furie and Furie, 2005). A functional explanation for the lack of stable injurious microvascular thrombosis might be that the basement membrane was not exposed to conditions where singlet oxygen formation predominantly affects the endothelial cells (Haseldonckx et al., 2000).
A lack of platelet interaction with exposed VWF or collagen may be an explanation for the observed lack of major platelet involvement. The intraluminal MCAO model may also be more prothrombotic as here the stroke is induced by physical occlusion producing blood stasis promoting thrombus formation followed by reperfusion injury. In contrast, in the carotid artery, photochemical occlusion shows platelet dependency, as thrombus formation is inhibited with the specific platelet inhibitor clopidogrel (Umemura et al., 1995). It is possible that at higher erythrosin B dye concentrations one might see more thrombi. However, in our hands, higher doses produced excessive infarct size with high animal mortality (not shown).
We conclude that the present photochemical model causes reproducible cortical lesions that are characterized by BBB leakage. Interestingly, the infarct produced was larger in older animals, suggesting that the impact of BBB injury increases with age. We have previously observed that even in the absence of injury, BBB weakens with increasing mouse age (Hafezi-Moghadam et al., 2007). The important message from our studies is that the photochemical injury model used in this study cannot be considered a model for ischemic stroke in mice, which involves elements of hemostasis, particularly platelets, and of inflammation (Ishikawa et al., 2003; Mitsios et al., 2006). However, the marked vessel permeability makes the current model interesting for studies of molecules and cells regulating BBB permeability as the laser injury may enhance the phenotype of a particular mutation (Kleinschnitz et al., 2007; Umemura et al., 1995). The photochemical injury model may also be a relevant model mimicking the secondary pathological conditions of clinical stroke.
4. Experimental Procedure
Animals
P-selectin−/− (P-sel−/−) (Mayadas et al., 1993) and von Willebrand factor−/− (VWF−/−) (Denis et al., 1998) mice were on C57Bl/6 background. IL4Rα/GPIbα-tg (Kanaji et al., 2002) mice were on mixed background and were a gift from J. Ware and Z. Ruggeri (The Scripps Institute, La Jolla, CA). β3 integrin−/− (β3−/−) (Hodivala-Dilke et al., 1999) mice on BALB/c background were a gift from R.O Hynes (Massachusetts Institute of Technology, Cambridge, MA). Wild-type mice of the same genetic background were used as controls. All animals were housed at the CBR Institute for Biomedical Research (CBRI) and had free access to food and water. Experimental procedures were approved by the CBRI Animal Care and Use Committee.
Photochemical infarction model
Photochemical infarction was induced using a modified protocol previously described for rats (Hoff et al., 2005). Mice were weighed and sedated with an IP injection of ketamine (150 μg/g body weight)/xylazine (15 μg/g body weight), body temperature was maintained at 37°C ± 1.0°C throughout all surgical procedures using a heating pad. The head of the mouse was immobilized in a home-made stereotactic frame. Each mouse was injected IV via the retro-orbital venous plexus with erythrosin B (5 μg/g body weight) dissolved in 0.9% NaCl and subjected to 0.2 μm filtration. The skull was illuminated in a 90 degree angle for 2.5 minutes. Twenty-four hours later the brains were removed and cut into 1-mm-thick coronal sections and stained with 2% triphenyltetrazolium chloride (TTC). From the digitalized image of each brain section, the infarct size was measured using a computerized image analysis program (Zeiss Axiovision), and expressed as absolute infarct volume in mm3.
Transient middle cerebral artery occlusion (MCAO) model
Mice were anesthetized with isoflurane in a mixture of 30% oxygen. Body temperature was maintained at 37°C ± 1.0°C throughout all surgical procedures using a heating pad. The right common carotid, external carotid artery, and internal carotid artery were exposed by midline neck incision. A 7.0 silicon pre-coated monofilament nylon suture was introduced into the internal carotid artery to occlude the right middle cerebral artery to produce transient focal cerebral ischemia. The filament was withdrawn after 90 minutes to allow reperfusion. A Laser Doppler flowmeter (Perimed system 5000, Sweden) was used to confirm induction of ischemia and reperfusion. Twenty-four hours after ischemia, the brains were removed and cut into 1-mm-thick coronal sections and stained with 2% TTC. The infarct size in each section was determined with a computerized image analysis program (NIH image analysis software).
Brain window and multiphoton microscopy
Animals were anesthetized with ketamine (150 μg/g body weight)/ xylazine (15 μg/g body weight) and the head of the mouse was immobilized in a home-made stereotactic frame. The scalp was incised and periosteum removed to expose the skull. The imaging region was thinned using a high-speed air drill (FST). The Bio-Rad Radiance 2000 MP system with both confocal and multiphoton capability and a Spectra Physics MaiTai laser with a continuous tuning range of 720 nm to 920 nm excitation were used for multiphoton observations and vessels were labeled by IV injection of high molecular weight (2×106 Da) fluorescein isothiocyanate labeled dextran (Sigma). Photochemical infarction model was performed as described. Platelets were isolated as described (Chauhan et al., 2006), labeled with calcein red-orange, AM (Molecular Probes) and injected IV before photochemically induced injury. Bovine serum albumin (BSA) Alexa fluor 594 conjugate (Molecular Probes) was used to visualize vascular leakage.
Statistics
Values are expressed as Mean ± SD. Comparisons of groups were performed using paired or unpaired Student’s t-test as appropriate. P<0.05 was considered statistically significant.
Acknowledgments
We thank Dr. Robert van Oostenbrugge, Department of Neurology, AZM, Maastricht, for helpful discussions and Lesley Cowan for help with preparation of manuscript. This work was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health grant HL41002 (to D.D.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Stroke Association. Heart disease and stroke statistics - 2007 update. American Stroke Association; Dallas, TX: 2007. [Google Scholar]
- Bergmeier W, et al. The role of platelet adhesion receptor GPIbalpha far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc Natl Acad Sci U S A. 2006;103:16900–5. doi: 10.1073/pnas.0608207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan AK, et al. Systemic antithrombotic effects of ADAMTS13. J Exp Med. 2006;203:767–76. doi: 10.1084/jem.20051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhri TF, et al. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J Clin Invest. 1998;102:1301–10. doi: 10.1172/JCI3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly ES, Jr, et al. Exacerbation of cerebral injury in mice that express the P-selectin gene: identification of P-selectin blockade as a new target for the treatment of stroke. Circ Res. 1997;81:304–10. doi: 10.1161/01.res.81.3.304. [DOI] [PubMed] [Google Scholar]
- Denis C, et al. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci U S A. 1998;95:9524–9. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis CV, Wagner DD. Platelet adhesion receptors and their ligands in mouse models of thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:728–39. doi: 10.1161/01.ATV.0000259359.52265.62. [DOI] [PubMed] [Google Scholar]
- Eichenbaum JW, et al. A murine photochemical stroke model with histologic correlates of apoptotic and nonapoptotic mechanisms. J Pharmacol Toxicol Methods. 2002;47:67–71. doi: 10.1016/s1056-8719(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Frenette PS, et al. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc Natl Acad Sci U S A. 1995;92:7450–4. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115:3355–62. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, et al. ApoE deficiency leads to a progressive age-dependent blood-brain barrier leakage. Am J Physiol Cell Physiol. 2007;292:C1256–62. doi: 10.1152/ajpcell.00563.2005. [DOI] [PubMed] [Google Scholar]
- Haseldonckx M, et al. Vasogenic oedema and brain infarction in an experimental penumbra model. Acta Neurochir Suppl. 2000;76:105–9. doi: 10.1007/978-3-7091-6346-7_22. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–38. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff EI, et al. In vivo visualization of vascular leakage in photochemically induced cortical infarction. J Neurosci Methods. 2005;141:135–41. doi: 10.1016/j.jneumeth.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, et al. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J Cereb Blood Flow Metab. 2004;24:907–15. doi: 10.1097/01.WCB.0000132690.96836.7F. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, et al. Molecular determinants of the prothrombogenic and inflammatory phenotype assumed by the postischemic cerebral microcirculation. Stroke. 2003;34:1777–82. doi: 10.1161/01.STR.0000074921.17767.F2. [DOI] [PubMed] [Google Scholar]
- Kanaji T, et al. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100:2102–7. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- Kiernan JA. Vascular permeability in the peripheral autonomic and somatic nervous systems: controversial aspects and comparisons with the blood-brain barrier. Microsc Res Tech. 1996;35:122–36. doi: 10.1002/(SICI)1097-0029(19961001)35:2<122::AID-JEMT3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, et al. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–30. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- Massberg S, et al. Platelet adhesion via glycoprotein IIb integrin is critical for atheroprogression and focal cerebral ischemia: an in vivo study in mice lacking glycoprotein IIb. Circulation. 2005;112:1180–8. doi: 10.1161/CIRCULATIONAHA.105.539221. [DOI] [PubMed] [Google Scholar]
- Mayadas TN, et al. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–54. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- Mitsios N, et al. Pathophysiology of acute ischaemic stroke: an analysis of common signalling mechanisms and identification of new molecular targets. Pathobiology. 2006;73:159–75. doi: 10.1159/000096017. [DOI] [PubMed] [Google Scholar]
- Molina CA. Monitoring and imaging the clot during systemic thrombolysis in stroke patients. Expert Rev Cardiovasc Ther. 2007;5:91–8. doi: 10.1586/14779072.5.1.91. [DOI] [PubMed] [Google Scholar]
- Umemura K, et al. Anti-platelet effects of clopidogrel in rat middle cerebral artery thrombosis model. Thromb Res. 1995;80:209–16. doi: 10.1016/0049-3848(95)00169-r. [DOI] [PubMed] [Google Scholar]
- Van Reempts J, Borgers M. Histopathological characterization of photochemical damage in nervous tissue. Histol Histopathol. 1994;9:185–95. [PubMed] [Google Scholar]
- Wagner DD. The Weibel-Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb Haemost. 1993;70:105–10. [PubMed] [Google Scholar]
- Watson BD, et al. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- Yao H, et al. Simplified model of krypton laser-induced thrombotic distal middle cerebral artery occlusion in spontaneously hypertensive rats. Stroke. 1996;27:333–6. doi: 10.1161/01.str.27.2.333. [DOI] [PubMed] [Google Scholar]