Abstract
Objective
To examine the impact of age, sex, ethnicity, and vascular disease on measures of brain morphology, including relative brain volume, ventricle volume, hippocampus and entorhinal cortex volume, and white matter hyperintensity (WMH) burden in a large community-based cohort of non-demented, ethnically diverse older adults.
Design
Beginning in 2003, high-resolution quantitative magnetic resonance imaging (MRI) was acquired on 769 participants without dementia. The relations of age, sex, self reported vascular disease history, and ethnicity, with brain morphology was examined in a cross-sectional study using multiple linear regression analyses. Sex and ethnicity interactions were also considered.
Setting
The Washington Heights/Hamilton Heights Aging Project (WHICAP), a community-based epidemiological study of older adults from three ethnic groups (i.e., Caucasian, Hispanic, African American) from northern Manhattan.
Main outcome measures
Relative brain volume (absolute brain volume/cranial volume), ventricular volume, hippocampus and entorhinal cortex volumes were derived manually on high-resolution MRI scans. White matter hyperintensities were quantified semi-automatically on FLAIR-weighted MRI.
Results
Increased age was associated with decreased relative brain volume and increased ventricular and WMH volume. Hispanic and African American participants had larger relative brain volumes and more severe WMH burden than Caucasians, but their associations with age were similar across ethnic groups. Compared with men, women had larger relative brain volumes. Vascular disease was associated with smaller relative brain volume and higher WMH burden, particularly among African Americans.
Conclusions
Increased age and vascular disease particularly among African Americans are associated with increased brain atrophy and WMH burden. African American and Hispanic participants have larger relative brain volumes and more WMH than Caucasians. Ethnic group differences in WMH severity appear to be partially attributable to differences in vascular disease. Future work will focus on the determinants and cognitive correlates of these differences.
Aging is accompanied by a diminution in brain volume and increase in cerebrovascular disease1. Magnetic resonance imaging (MRI) provides the capacity to visualize and quantify these changes in vivo. Several population- and clinic-based cross-sectional and longitudinal studies have shown that normal aging is associated with decreases in total brain volume (e.g.,2–8) and with increases in white matter hyperintensities (WMH; e.g.,7,9–11), which appear as areas of increased signal intensity on T2-weighted, including FLAIR, MRI scans and are thought to reflect small vessel vascular disease12–14. Age-associated changes in brain and WMH volume account for some degree of age-associated cognitive decline15–19 and are particularly vulnerable to the effects of vascular risk factors10,20–26.
The population of older adults is rapidly becoming more ethnically diverse27 and the importance of including ethnic minorities in aging studies is well established28. Investigations that have examined the associations between aging and brain morphology have generally relied on small samples with limited ethnic diversity (e.g.,11, 15). Even large community-based samples that incorporated MRI have been generally limited to Caucasians7, with rare exception29,30. Studies that have included ethnic minorities have found substantial differences in brain morphology that corresponded to differences in prevalent cardiovascular risk10,31–34. These studies, however, were limited to comparison of Caucasians to African Americans.
The Washington/Hamilton Heights-Inwood Columbia Aging Project (WHICAP) is an ongoing, community-based study of aging and dementia comprising elderly participants from an urban community. A unique aspect of the cohort is the inclusion of Caribbean Hispanics and African American participants, which facilitates the examination of ethnicity as a modifying factor in cognitive aging. We have previously shown that the prevalence and incidence of both cerebrovascular disease and dementia is higher in African Americans and Hispanics than in Caucasians in this cohort35. Thus, it is important to examine ethnic differences in brain morphology that may affect cognitive aging. In 2003, we began systematically acquiring structural MRI scans on active, non-demented participants in the study cohort. The purpose of the current study was to examine the impact of age, sex, ethnicity, and vascular disease history on common measures of brain morphology. Analyses focused on global atrophy (i.e., total relative brain volume and ventricular volume), hippocampus and entorhinal cortex volume, and the severity of white matter hyperintensities (WMH).
Methods
Subjects
Data were included from individuals (n=2,776) participating in a prospective study of aging and dementia in Medicare-eligible northern Manhattan residents, age 65 years and older (Washington/Hamilton Heights-Inwood Columbia Aging Project: WHICAP II). The WHICAP II cohort represents a combination of continuing members of a cohort originally recruited in 1992 (WHICAP I; n=602) and members of a new cohort recruited between 1999 and 2001 (n= 2,174). The sampling strategies and recruitment outcomes of these two cohorts have been described in detail elsewhere35. The population from which participants were drawn comprises individuals from three broadly defined ethnic categories (i.e., Hispanic, African American, and non-Hispanic White). Participants have been followed at approximately 18-month intervals with similar assessments at each interval. Ethnic group was determined by self-report using the format of the 2000 US Census36. All individuals were first asked to report their race (i.e., American Indian/Alaska Native, Asian, Native Hawaiian or other Pacific Islander, Black or African American, or White), then, in a second question, were asked whether or not they were Hispanic. Recruitment, informed consent and study procedures were approved by the Institutional Review Boards of Columbia Presbyterian Medical Center and Columbia University Health Sciences and the New York State Psychiatric Institute.
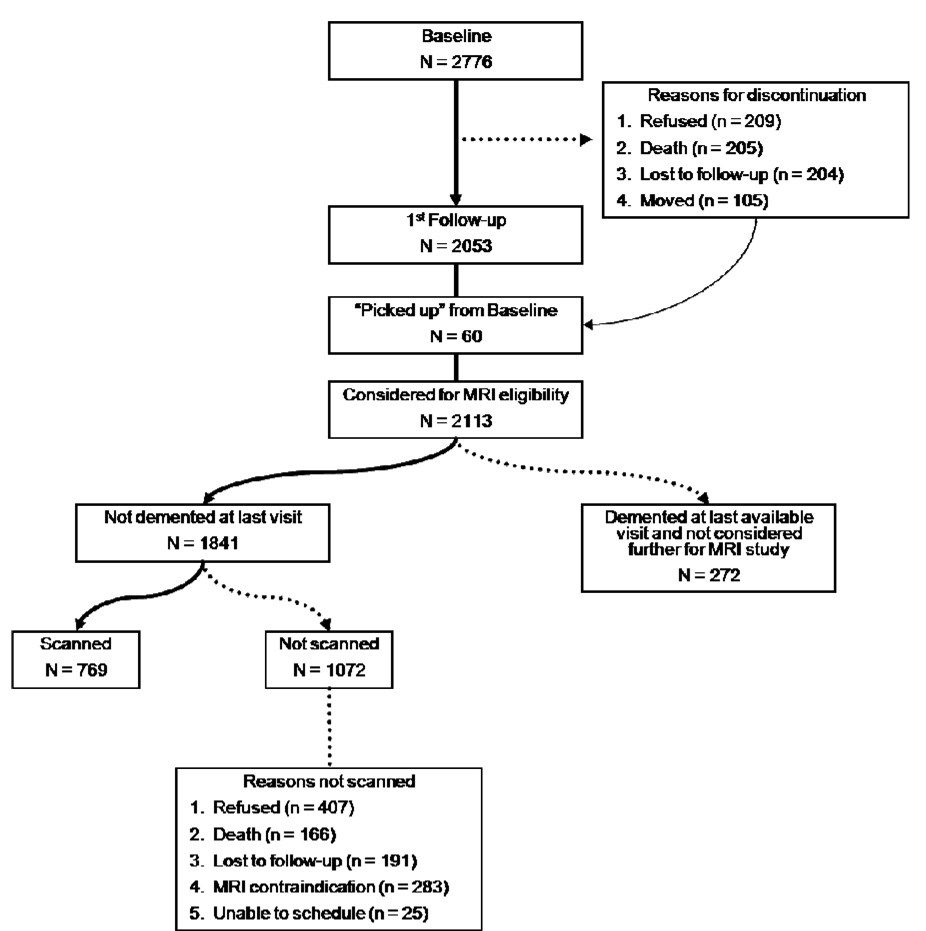
Figure 1 schematically displays the derivation of the WHICAP imaging sample. The imaging project was concurrent with the second follow-up of the WHICAP II cohort. Participants were deemed potentially eligible for MRI if they did not meet criteria for dementia at the most recent visit prior to the second follow-up. At the conclusion of the first follow-up, a total of 2113 participants were considered for MRI eligibility; 2053 of these individuals had been seen at the first follow-up, and for 60 of these participants, their most recent visit was baseline (i.e., they were not seen during the 1st follow-up wave). Dementia was diagnosed in 272 (12.9%) participants of these 2113 participants. Of the remaining 1841 participants, 769 (41.8%) received MRI scans. As detailed in Figure 1, 407 (38.0%) of the remaining 1072 refused participation, 166 (15.4%) died before they were able to be scheduled for imaging, 191 (17.8%) were lost to follow-up, 283 (26.3%) had MRI contraindications, and 25 (2.3%) were unable to be scheduled. Frequencies and specific reasons why these participants did not receive MRI scans are in Figure 1.
Figure 1.
Schematic representation of derived MRI sample.
We compared the demographic characteristics of individuals who received MRI scans (n=769) to those who refused participation in the MRI study, but otherwise met inclusion criteria (n=407; see Table 1). Those who refused participation were a year older and more likely to be women. African Americans were more frequent among those who were scanned. Education level was similar between the two groups. These findings were essentially identical when we compared those who received scans (n=769) to those who were eligible but did not receive scans (n=1072).
Table 1.
Demographic comparison of participants who received MRI scans to those who were eligible, but refused participation in MRI study. Data are presented from the total sample and then stratified by ethnicity (Caucasian, African American, and Hispanic).
| Variable | Refused | Scanned | Statistic | P | |
|---|---|---|---|---|---|
|
Total Sample |
N | 407 | 769 | ||
| Age, Mean (SD) | 81.55 (6.36) | 80.433 (5.63) | t (1174) = 3.098 | 0.002 | |
| Education, Mean (SD | 10.69(4.78) | 10.48 (4.89) | t (1170) = 0.716 | 0.474 | |
| Sex (% women) | 75.4 | 67.1 | χ2 (1) = 8.79 | 0.003 | |
| Ethnicity |
χ2 (3) = 23.93 |
<0.001 |
|||
| % Caucasian | 37.3 | 26.7 | |||
| % African American |
22.6 | 34.3 | |||
| % Hispanic | 39.1 | 37.1 | |||
| %Other | 1.0 | 2.0 | |||
|
Caucasians |
N | 152 | 205 | ||
| Age, Mean (SD) | 81.49 (6.74) | 80.39 (5.85) | t (355) = 1.65 | 0.100 | |
| Education, Mean (SD | 13.97 (2.99) | 13.71 (3.22) | t (351) = 0.770 | 0.442 | |
| Sex (% women) | 68.4 | 59.5 | χ2 (1) = 2.98 | 0.084 | |
|
African Americans |
N | 92 | 264 | ||
| Age, Mean (SD) | 80.85 (6.17) | 80.22 (5.85) | t (354) = 0.871 | 0.384 | |
| Education, Mean (SD) | 11.95 (3.66) | 11.99 (3.72) | t (354) = 0.104 | 0.917 | |
| Sex (% women) | 84.8 | 68.2 | χ2 (1) = 9.42 | 0.002 | |
|
Hispanics |
N | 159 | 285 | ||
| Age, Mean (SD) | 82.07 (6.04) | 80.72 (5.30) | t (442) =2.44 | 0.015 | |
| Education, Mean (SD | 6.76 (3.86) | 6.74 (4.35) | t (442) = 0.041 | 0.967 | |
| Sex (% women) | 77.4 | 73.3 | χ2 (1) = 0.877 | 0.349 |
Subject evaluation
Clinical Evaluation
At each assessment, each participant underwent an in-person interview of general health and functional ability followed by a semi-structured standardized assessment, including medical history, physical and neurological examination, and a neuropsychological battery that included measures of memory, orientation, language, abstract reasoning, and visuospatial ability37. The neuropsychological test battery and its validity in the diagnosis of dementia has been described in detail in a previous publication37. All participants received structured neurologic, medical and functional assessments. The diagnosis of dementia was based on standard research criteria38 and was established using all available information (except the MRI results) gathered from the initial and follow-up assessments and medical records at a consensus conference of physicians, neurologists, neuropsychologists and psychiatrists.
Vascular disease history
History of diabetes, hypertension, heart disease, and clinical stroke was ascertained by self report39. History of heart disease included arrhythmias (e.g., atrial fibrillation), coronary artery disease, and congestive heart failure. Stroke was defined according to the WHO criteria40, based on self report, supplemented by a neurological examination. These 4 dichotomous variables were summed to create a ‘vascular disease history’ variable (range 0–4).
MRI protocol
Acquisition
Scan acquisition was performed on a 1.5T Philips Intera scanner at Columbia University Medical Center and transferred electronically to the university of California at Davis for morphometric analysis in the Imaging of Dementia and Aging Laboratory. For measures of total brain volume, ventricular volume, and WMH volume, fluid attenuated inverse recovery (FLAIR) weighted images (TR=11,000 ms, TE=144.0 ms, 2800 inversion time, FOV 25 cm, 2 nex, 256×192 matrix with 3 mm slice thickness) were acquired in the axial orientation. T1-weighted images acquired in the axial plane and re-sectioned coronally were used to quantify hippocampus and entorhinal cortex volumes (TR=20 ms, TE = 2.1 ms, FOV 240 cm, 256×160 matrix with 1.3 mm slice thickness).
Quantification of WMH volume, total relative brain volume, lateral ventricle volume, hippocampus volume, and entorhinal cortex volume
Five morphological variables were derived for the current analyses: WMH volume, total relative brain volume (ratio of absolute brain volume to intracranial volume), lateral ventricle volume, hippocampus volume, and entorhinal cortex volume. User operated image analysis was performed on a Sun Microsystems Ultra 5 workstation using the Quantum 6.2 package. Subject identifying information was not available to the operator.
Total brain and WMH volumes were derived on FLAIR-weighted images following a two-step process, as previously described41, 42. First, an operator manually traced the dura mater within the cranial vault, including the middle cranial fossa but not the posterior fossa and cerebellum. Intracranial volume was defined as the number of voxels contained within the manual tracings, multiplied by voxel dimensions and slice thickness. These manual tracings also defined the border between brain and non-brain elements and permitted for the removal of the latter.
Nonuniformities in image intensity were removed 43 and two Gaussian probability functions, representing brain matter and cerebrospinal fluid (CSF), were fitted to the skull-stripped image41, 43. Once brain matter was isolated, a single Gaussian distribution was fitted to image data and a segmentation threshold for WMH was set a priori at 3.5 SDs in pixel intensity above the mean of the fitted distribution of brain matter. Erosion of two exterior image pixels was applied to the brain matter image before modeling to remove partial volume effects and ventricular ependyma on WMH determination. White matter hyperintensity volume was calculated as the sum of voxels greater to or equal to 3.5 SD above the mean intensity value of the image and multiplied by voxel dimensions and slice thickness. Similarly, total brain volume was the sum of voxels designated as brain volume from the segmentation process. Relative brain volume was the ratio of total brain volume to intracranial volume. White matter hyperintensity volumes were also adjusted by intracranial volume.
Lateral ventricle volumes
Lateral ventricle volumes were calculated based on previously reported methods44. In brief, after segmentation of the image into CSF and brain matter tissue types, the operator returned to the image and outlined the ventricles according to a standardized protocol. Voxels belonging to the CSF tissue class were then counted within each region of interest (ROI) and summed across the ROIs to form the ventricular measures.
Hippocampus
Boundaries for the hippocampus were manually traced from the coronal 3D-T1 weighted images after reorientation along the axis of the left hippocampus. While the borders were traced on the coronal slices, corresponding sagittal and axial views were simultaneously presented to the operator in separate viewing windows in order to verify hippocampal boundaries. The rostral end of the hippocampus was identified using the sagittal view to distinguish between amygdala and the head of the hippocampus. The axial view was used as a separate check. In anterior sections, the superior boundary of the hippocampus was the amygdala. In sections in which the uncus lies ventral to caudal amygdala, the uncus was included in the hippocampus. In more posterior sections that do not contain amygdala, the hippocampal (choroid) fissure and the superior portion of the inferior horn of the lateral ventricle formed the superior boundary. The fimbria were excluded from the superior boundary of the hippocampus. The inferior boundary of the hippocampus was the white matter of the parahippocampal gyrus. The lateral boundary was the inferior (temporal) horn of the lateral ventricle, taking care in posterior sections to exclude the tail of the caudate nucleus. The posterior boundary of the hippocampus was the first slice in which the fornices were completely distinct from any gray/white matter of the thalamus.
Intra-rater reliability determined for both right and left hippocampus using this method was good with intraclass correlation coefficients of 0.98 for right hippocampus and 0.96 for left hippocampus.
Entorhinal cortex
Measurement of entorhinal cortex area was performed following a protocol developed and described by Killiany and colleagues 45. In brief, the entorhinal cortex area was outlined on three consecutive coronal images centered at the level of the mammillary bodies. The first image (in the rostral to caudal direction) in which the white matter tracts of the fornix were seen was used as the center image for measurement. The outline of the entorhinal cortex region began at the angle formed by the junction of the rhinal sulcus and the surface of the brain (the posterior end of this region may be continuous with the collateral sulcus in some subjects). The rhinal (or collateral) sulcus is the first sulcus encountered on the inferior/medial surface of the brain when following along the surface of the entorhinal cortex from the hippocampus outward towards the lateral surface. The outline then transected the angle formed by the rhinal sulcus and the inferior/medial surface of the brain, cutting across the gray matter to the level of the white matter. The edge of the white matter was then followed to the inferior surface of the hippocampus. The outline continues along the surface of the brain back to the starting point. This procedure was repeated using the same landmarks on the immediately adjacent rostral and caudal slices to calculate the total entorhinal area. Inter-rater reliabilities for this process averaged 0.90.
Statistical analysis
Distribution and differences across groups in demographic data and dichotomous vascular disease history variables (i.e., hypertension, diabetes, heart disease, stroke) were determined with Chi-squared analysis and general linear models. The associations of demographic and vascular disease history with the morphology data were examined with multiple regression analysis, with relative brain volume, lateral ventricular volume, hippocampus and entorhinal cortex volume, and WMH volume as dependent variables. For these analyses, ethnicity was ‘dummy’ coded, with ‘Caucasian’ as the reference group. Interaction terms (ethnicity by vascular disease history, ethnicity by age, sex by vascular disease history, and sex by age) were calculated and included in additional multiple regression models examining relative brain volume and WMH volume. Effects of ethnicity, sex, and their interaction on morphology were also examined with analysis of variance (ANOVA).
Results
Sample
Of the 769 participants with available structural MRI data, 52 met diagnostic criteria for dementia at the clinical evaluation closest to the neuroimaging study. These individuals were excluded from the current analyses. Because we were interested in studying effects of the three most representative ethnic groups in the cohort (i.e., Caucasians, African Americans, and Hispanics), we excluded 15 participants who self-identified as belonging to a different ethnic group, resulting in a study sample of 702. Of these 702 participants, total brain, total ventricular, and WMH volumes were calculated on 686, hippocampus volume was calculated on 663, and entorhinal cortex volume was calculated on 555. Demographic characteristics, including vascular disease variables, for these participants are displayed in Table 2 stratified by ethnicity and sex.
Table 2.
Demographic and risk factor profiles stratified by ethnicity and sex for participants in the current study.
| Caucasian (n=203) | African American (n=243) | Hispanic (n=256) | Total Sample (n=702) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (n)1 | Men (n=82) | Women (n=121) | Total | Men (n=74) | Women (n=169) | Total | Men (n=67) | Women (n=189) | Total | Men (n=223) | Women (n=479) | Total |
| Age (mean ± SD)2 | 79.12 ± 5.03 | 81.01 ± 6.03 | 80.25 ± 5.71 | 78.40 ± 5.07 | 80.28 ± 5.97 | 79.71 ± 5.77 | 79.77 ± 5.17 | 80.45 ± 5.22 | 80.27 ± 5.21 | 79.08 ± 5.09 | 80.53 ± 5.70 | 80.07 ± 5.55 |
| Education (mean ± SD)3 | 14.19 ± 3.23 | 13.42 ± 3.17 | 13.73 ± 3.21 | 12.07 ± 3.69 | 12.42 ± 3.42 | 12.31 ± 3.50 | 6.82 ± 4.37 | 6.88 ± 4.35 | 6.86 ± 4.35 | 11.26 ± 4.83 | 10.48 ± 4.77 | 10.73 ± 4.80 |
| Hypertension (% with history)4 | 56.1 | 52.9 | 54.2 | 59.5 | 74.0 | 69.5 | 64.2 | 76.7 | 73.4 | 59.6 | 69.7 | 66.5 |
| Diabetes (% with history)5 | 22.0 | 9.1 | 14.3 | 20.3 | 29.6 | 26.7 | 25.4 | 22.2 | 23.0 | 22.4 | 21.5 | 21.8 |
| Heart disease (% with history)6 | 31.7 | 22.3 | 26.1 | 24.3 | 20.1 | 21.5 | 23.9 | 15.9 | 18.0 | 26.9 | 19.0 | 21.5 |
| Stroke (% with history)7 | 38.3 | 30.8 | 33.8 | 47.3 | 28.6 | 34.3 | 32.8 | 26.6 | 28.2 | 39.6 | 28.4 | 31.9 |
| Vascular disease history (mean ± SD; range 0–4)8 | 1.82 ± 1.13 | 1.29 ± 0.93 | 1.50 ± 1.05 | 2.12 ± 1.12 | 1.82 ± 1.15 | 1.92 ± 1.15 | 1.97 ± 1.18 | 1.51 ± 1.05 | 1.63 ± 1.12 | 1.96 ± 1.14 | 1.57 ± 1.08 | 1.69 ± 1.12 |
More women among Hispanics (73.8%) compared with African Americans (69.5%) or Caucasians (59.6%)%)(χ2 (2) = 10.86, p = 0.004)
Women older than men overall (F (1, 701) = 10.81, p = 0.001), among Caucasians (F (1,202)5.47, p = 0.020), among African Americans (F (1,242) = 5.53, p = 0.020), but not among Hispanics (F (1, 255) = 0.848, p = 0.358). Age was similar across ethnic groups (F (2, 701) = 1.236, p = 0.291.
Education similar in men and women (F (1, 700) = 0.144, p = 0.704), Caucasians had significantly more years of education than African Americans who had significantly more years of education than Hispanics (F (2, 700) = 185.91, p < 0.001).
Hypertension more frequent among women (χ2 (1) = 6.953, p = 0.008), primarily in African Americans (χ2 (1) = 5.113, p = 0.024) and Hispanics ((χ2 (1) 3.988, p = 0.046), but not Caucasians (χ2 (1) = 0.202, p = 0.653. Hispanics more likely to have hypertension than African Americans and Caucasians (χ2 (2) = 20.585, p < 0.001)
Rates of diabetes mellitus similar in men and women (χ2(1) = 0.075, p = 0.784), although among Caucasians, a greater proportion of men reported history of diabetes (χ2(1) = 6.502, p = 0.010). African Americans were more likely to have diabetes mellitus than Hispanics or Caucasians (χ2(2) = 10.582, p = 0.005).
Men more likely to have history of heart disease than women (χ2(1) = 5.636, p = 0.018). Rates of heart disease similar across ethnic groups (χ2(2) = 4.446, p = 0.108).
Men more likely to report history of stroke than women among African Americans (χ2(1) = 7.994, p = 0.005), but not Caucasians (χ2(1) = 1.195, p = 0.274) or Hispanics (χ2(1) =0.949, p = 0.330. The percent of participants who reported past signs or symptoms of stroke did not differ by ethnic group (χ2(2) = 2.559, p = 0.278).
African Americans had higher degrees of vascular disease than Caucasians (p<0.001) and Hispanics (p = 0.03); women had lower degrees of vascular disease than men (p < 0.001), but there was no ethnicity by sex interaction.
Relative brain volume
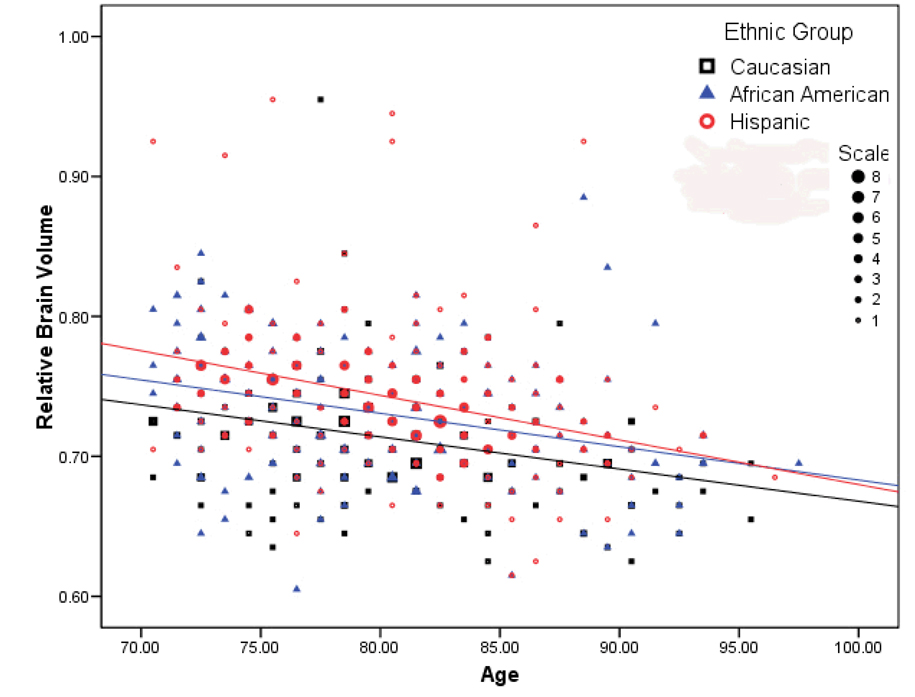
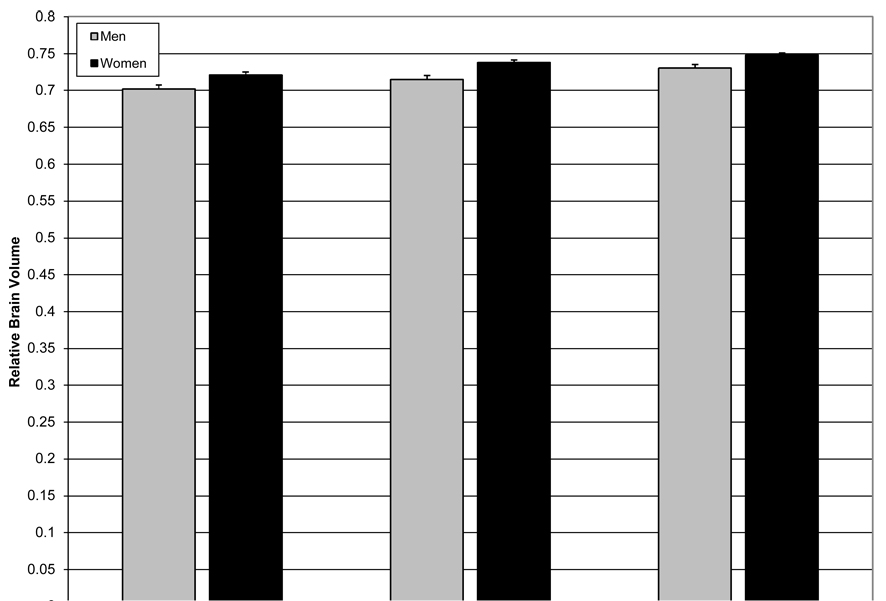
Results of the regression analysis revealed significant effects of age, sex, vascular disease history, and ethnicity on relative brain volume (F(5,685)=38.290, p<0.001). For each additional year in age, there was an associated 0.3% decrease in relative brain volume (β=−0.003, t=10.341, p<0.001; see Figure 2). Relative brain volume among women was 2% larger than men (β=0.020, t=5.927, p<0.001). Hispanic (β=0.028, t=7.198, p<0.001) and African American (β=0.016, t=4.085, p<0.001) participants had 2.8% and 1.6% larger relative brain volumes than Caucasians, respectively. Finally, for each additional vascular disease there was a 0.5% associated reduction in relative brain volume (β=-0.005, t=−2.697, p<0.001). When interaction terms were entered into the model, none was significant, demonstrating that the association of vascular disease history and age with relative brain volume did not differ across ethnicity or sex. Analysis of variance controlling for age and vascular disease history confirmed main effects of sex (F(1,685)=34.906, p<0.001) and ethnicity (F(2,685)=23.528, p<0.001), but no sex by ethnicity interaction (F(2,685)=0.167, p=0.847; see Figure 3).
Figure 2.
The relationship among chronological age, ethnicity, and relative brain volume. While the three ethnicity groups significantly differed in relative brain volume, the relationship between age and relative brain volume was similar across groups. Note that data have been grouped into 20 bins on the y-axis and 30 bins on the x-axis; the number of participants represented by each data point is indicated by its size.
Figure 3.
Relative brain volume across ethnic groups and sex. Women had significantly larger relative brain volumes than men and African American and Hispanics had significantly larger relative brain volumes than Caucasians. However, the sex by ethnicity interaction was not significant. Error bars are standard errors. Data are adjusted for vascular disease history and age.
Lateral ventricles
Findings regarding relative ventricular volumes paralleled the observations with relative brain volume (F(5,685)=21.323, p<0.001). Increasing age (β=.001, t=7.627, p<0.001) was associated with larger ventricle size, but being female (β=−0.005, t=−0.005, p<0.001), Hispanic (β=−0.005, t=−4.861, p<0.001), or African American (β=−0.002, t=−2.00, p=0.046) was associated with smaller relative ventricular volume. Vascular disease history was not significantly associated with ventricular volume (β=0.001, t=1.806, p=0.071).
Hippocampal and entorhinal cortex volume
The regression model for hippocampal volume was not statistically significant (F(5,660)=0.898,p=0.482. On post hoc inspection, none of the independent variables was significantly associated with hippocampal volume. Similarly, the regression analysis for entorhinal cortex volume did not reach statistical significance (F(5,552)=1.774,p=0.116. On post hoc inspection, increased age was associated with smaller entorhinal cortex volume (βstandardized=−0.116,t=−2.707,p=0.007).
White matter hyperintensity volume
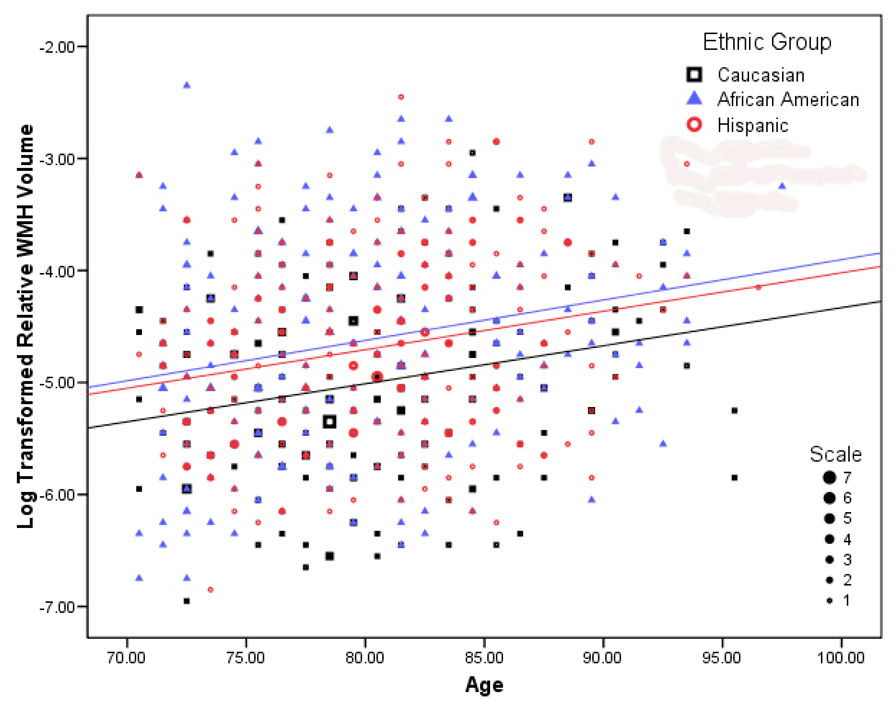
The regression model examining WMH volume was statistically significant (F(5,684)=9.489,p<0.001). With increasing age, there was a significant increase in WMH volume (β=0.035, t=6.097,p<0.001; see Figure 4). Further, increased WMH volume was associated with being Hispanic (β=0.275,t=3.441,p=0.001), being African American (β=0.359, t=4.443, p < 0.001), and increased vascular disease history (β=0.085,t=2.442,p=0.015). There were no sex differences in WMH burden (β=0.079,t=1.146,p=0.252). When interaction terms were entered into the regression model, there was a significant effect for the African American by vascular disease history interaction (β=0.158,t=2.113,p=0.035), indicating that the association between increased vascular disease history and WMH burden was greatest among African Americans (see Figure 5). Analysis of variance controlling for age and vascular disease history, confirmed a main effect of ethnicity (F(2,684)=9.722,p<0.001) and no effect of sex (F(1,684)=1.821,p<0.987) or ethnicity by sex interaction (F(2,684)=0.014,p=0.987).
Figure 4.
The relationship among chronological age, ethnicity, and log transformed relative WMH volume. The three groups significantly differed in severity of WMH, but the relationship between chronological age and relative WMH volume was similar across groups. Note that data have been grouped into 25 bins on the y-axis and 30 bins on the x-axis; the number of participants represented by each data point is indicated by its size.
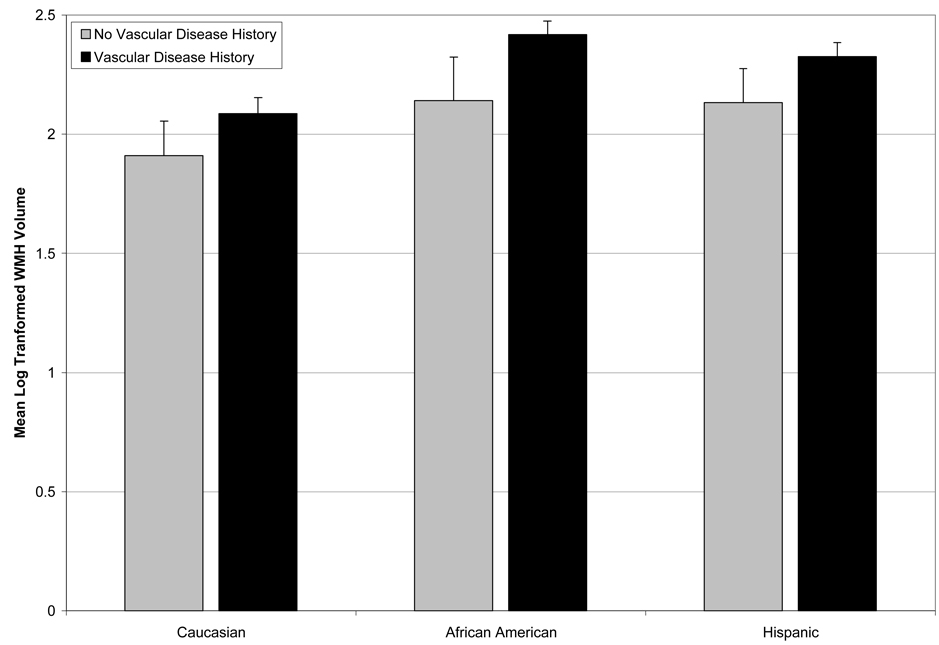
Figure 5.
Interaction between ethnicity and vascular disease history on WMH burden. For illustration purposes, vascular disease history was dichotomized into no vascular disease history versus any vascular disease history. African Americans with vascular disease had a disproportionately greater degree of WMH than their Caucasian and Hispanic counterparts. Error bars represent standard errors. Data presented are adjusted for age and sex.
Discussion
The current study examined the impact of age, sex, ethnicity, and vascular disease history on measures of cerebral atrophy, hippocampus and entorhinal cortex volume, and WMH volume among ethnically diverse elderly adults from an urban community. Consistent with most reports2,3,6–8, increasing age was associated with increased atrophy, indexed by relative brain volume and ventricular volume. In addition, relative brain volumes were larger among women than men. Interestingly, relative brain volumes were higher among African American and Hispanic participants than among Caucasians, but the relationship between age and relative brain volume was similar across ethnic groups. A history of vascular disease was also associated with markers of diminished brain volume, but similarly in men and women and across ethnic groups.
Hippocampus and entorhinal cortex volumes were no associated with demographic data or history of vascular disease in this cognitively healthy group of participants. Overall, age, sex, ethnicity, and vascular disease did not appear to be related to hippocampal and entorhinal cortex volumes, although on post hoc inspection increased age was associated with smaller entorhinal cortex volume. The findings are in contrast with previous studies by Raz and colleagues46,47, which demonstrated volumetric reduction with age in the hippocampus and entorhinal cortex particularly prominent among those with hypertension. Our findings could be explained by the fact that variability in hippocampus and entorhinal cortex volume may be specific to Alzheimer’s disease or other degenerative process48–50 less detectable in normal aging, and our sample excluded individuals with dementia. Indeed, in one morphological study with rhesus monkeys, who do not develop Alzheimer’s pathology, there was little effect of age on hippocampal volume51. Participants in the current study who met formal criteria for dementia were excluded from the analyses. Future work will focus on whether these medial temporal lobe measures have utility in predicting future development of dementia or in distinguishing those with dementia from those without. The functional impact of hippocampus and entorhinal cortex volume in this population will also be examined by determining their relationship with cognitive function in a cross sectional study.
White matter hyperintensity volumes were associated with increased age and were greater among Hispanic and African American participants than among Caucasian participants, but the relationship between age and WMH volume did not vary by ethnic group. These findings are consistent with previous reports demonstrating that age is a primary predictor of WMH severity52 and that WMH burden is greater among African Americans and Hispanics53,54. As expected, vascular disease history was associated with increased WMH severity. The relationship was strongest among African American participants. African Americans in the WHICAP cohort have a higher degree of vascular disease than Caucasians, coinciding with the fact that African Americans in the WHICAP cohort, particularly men, had the highest degree of vascular disease. Our findings of increased vascular disease and WMH among African Americans and Hispanics in this northern Manhattan community suggest that cerebrovascular disease may contribute substantially to cognitive impairment in this subgroup35,55. Future studies will closely examine the relative contribution of WMH burden and MRI evidence of cerebral infarction to cognitive functioning in this cohort.
While many of the associations among morphology, vascular disease, and demographic data in the current study were statistically reliable, effect sizes tended to be relatively small. The magnitude of the relationships suggests that the observed effects are quite subtle, but further evaluation of their functional consequences is clearly warranted. The implications of larger relative brain volumes among those groups with greater vascular disease (i.e., African Americans and Hispanics) and more severe WMH need to be explored further.
A unique feature of the WHICAP cohort is that it includes participants that are underrepresented in most studies that examine brain aging. WHICAP participants are older, come from diverse educational and socioeconomic backgrounds, and vary widely in their health status. A somewhat unexpected finding in the current study was of larger relative brain volumes among ethnic groups and between sexes even after age and vascular disease variables were controlled, although significantly higher relative brain volumes among women have been previously reported7. Few reports have compared explicitly morphological features across ethnic groups. The finding, however, is similar to that of one study that showed relatively smaller lateral ventricle volume among Hispanic patients with probable Alzheimer’s disease compared with non-Hispanic whites56. It is important to emphasize that despite the main effects of ethnicity and sex on relative brain volume, there were no interactions with age. The findings suggest either that these differences are likely to have existed throughout the adult lifespan although differential rates of volume change may have occurred earlier in life.
The use of ethnic comparisons in biomedical research is controversial due to the possibility of the assumption of ethnic biological differences when none exist57. It is important to emphasize that the rationale for ethnic comparisons in this study was based on the previous observations that dementia risk factors, cerebrovascular disease, and dementia are more prevalent amongst African American and Hispanics as compared to Caucasians. We believe that the ethnic differences in brain structure found in our study are due to lifetime exposures that include differences in the prevalence of risk factors and socioeconomic exposures that need to be more fully characterized.
The current study is among the first to compare specific brain morphometry among the elderly in three ethnic groups. Because the study cohort was large, drawn from a community, and quite diverse along a number of dimensions, these findings are more likely to reflect the general population where ethnic diversity is increasing. Furthermore, WHICAP participants are evaluated with comprehensive neuropsychological, medical, and behavioral assessments and followed longitudinally. Future work will examine the association between these MRI measurements and current functioning and we will be able to evaluate their value in predicting future cognitive decline.
Despite these strengths, some weaknesses should be noted. First, although the three main ethnic groups represented in the WHICAP imaging sample are similar in terms of age, they vary systematically and substantially on other factors. For example, Hispanic participants have markedly fewer years of formal education than African American and Caucasian participants, which may be related with life exposures and consequently brain development. Second, as with many cross-sectional neuroimaging studies, we assume that the proportion of brain volume to intracranial volume (i.e., relative brain volume) is a reflection of the amount of brain atrophy. However, despite the strong relationship between relative brain volume and chronological age, it is possible that group effects reflect life-long differences. We will examine this issue through examination of early life nutritional status, clinical correlates, and prediction of future and past longitudinal change.
Acknowledgments
This work was supported by National Institutes of Health grants AG007232 and AG029949.
References
- 1.Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007 Mar;21(2):149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- 2.Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000 Sep;216(3):672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 3.Tang Y, Whitman GT, Lopez I, Baloh RW. Brain volume changes on longitudinal magnetic resonance imaging in normal older people. J Neuroimaging. 2001 Oct;11(4):393–400. doi: 10.1111/j.1552-6569.2001.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 4.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001 Jul;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 5.Resnick SM, Goldszal AF, Davatzikos C, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000 May;10(5):464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 6.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003 Apr 15;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005 Apr;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Brickman AM, Buchsbaum MS. Alzheimer's disease and normal aging: Neurostructures. In: Byrne J, editor. Learning and Memory: A Comprehensive Review. New York: Elsevier; in press. [Google Scholar]
- 9.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996 Aug;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 10.Liao D, Cooper L, Cai J, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke. 1996 Dec;27(12):2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- 11.Jernigan TL, Archibald SL, Fennema-Notestine C, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001 Jul–Aug;22(4):581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 12.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993 Sep;43(9):1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 13.Thomas AJ, O'Brien JT, Davis S, et al. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002 Sep;59(9):785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- 14.Udaka F, Sawada H, Kameyama M. White matter lesions and dementia: MRI-pathological correlation. Ann N Y Acad Sci. 2002 Nov;977:411–415. doi: 10.1111/j.1749-6632.2002.tb04845.x. [DOI] [PubMed] [Google Scholar]
- 15.Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998 Jan;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 16.Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Ann N Y Acad Sci. 2007 Feb;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brickman AM, Habeck C, Ramos MA, Scarmeas N, Stern Y. Multivariate-defined spatial networks of age-associated atrophy. Poster Presentation at the annual meeting of the International Society of Magnetic Resonance in Medicine (Berlin) 2007 April [Google Scholar]
- 18.Brickman AM, Habeck C, Ramos MA, Scarmeas N, Stern Y. A forward application of age associated gray and white matter networks. Human Brain Mapping. doi: 10.1002/hbm.20452. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000 Apr;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 20.Manolio TA, Kronmal RA, Burke GL, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994 Feb;25(2):318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 21.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001 Jan;70(1):9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology. 1997;16(3):149–162. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- 23.DeCarli C, Reed T, Miller BL, Wolf PA, Swan GE, Carmelli D. Impact of apolipoprotein E epsilon4 and vascular disease on brain morphology in men from the NHLBI twin study. Stroke. 1999 Aug;30(8):1548–1553. doi: 10.1161/01.str.30.8.1548. [DOI] [PubMed] [Google Scholar]
- 24.Strassburger TL, Lee HC, Daly EM, et al. Interactive effects of age and hypertension on volumes of brain structures. Stroke. 1997 Jul;28(7):1410–1417. doi: 10.1161/01.str.28.7.1410. [DOI] [PubMed] [Google Scholar]
- 25.Salerno JA, Murphy DG, Horwitz B, et al. Brain atrophy in hypertension. A volumetric magnetic resonance imaging study. Hypertension. 1992 Sep;20(3):340–348. doi: 10.1161/01.hyp.20.3.340. [DOI] [PubMed] [Google Scholar]
- 26.Meyer JS, Rauch GM, Crawford K, et al. Risk factors accelerating cerebral degenerative changes, cognitive decline and dementia. Int J Geriatr Psychiatry. 1999 Dec;14(12):1050–1061. doi: 10.1002/(sici)1099-1166(199912)14:12<1050::aid-gps56>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 27.Bureau UC. U.S. Interim Projections by Age, Sex, Race, and Hispanic Origin. 2004 [Google Scholar]
- 28.Brown DR, Alexander M. Recruiting and retaining people of color in health research studies: Introduction. Journal of Aging and Health. 2004;16:S5–S8. [Google Scholar]
- 29.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003 Jan–Feb;22(1):1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 30.Mosley TH, Jr., Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology. 2005 Jun 28;64(12):2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 31.Longstreth WT, Jr, Arnold AM, Manolio TA, et al. Clinical correlates of ventricular and sulcal size on cranial magnetic resonance imaging of 3,301 elderly people. The Cardiovascular Health Study. Collaborative Research Group. Neuroepidemiology. 2000;19:30–42. doi: 10.1159/000026235. 2000/01// [DOI] [PubMed] [Google Scholar]
- 32.Fox ER, Taylor HA, Jr, Benjamin EJ, et al. Left ventricular mass indexed to height and prevalent MRI cerebrovascular disease in an African American cohort: the Atherosclerotic Risk in Communities study. Stroke. 2005 Mar;36(3):546–550. doi: 10.1161/01.STR.0000154893.68957.55. [DOI] [PubMed] [Google Scholar]
- 33.Kase CS, Wolf PA, Chodosh EH, et al. Prevalence of silent stroke in patients presenting with initial stroke: the Framingham Study. Stroke. 1989 Jul;20(7):850–852. doi: 10.1161/01.str.20.7.850. [DOI] [PubMed] [Google Scholar]
- 34.Sundquist J, Winkleby MA, Pudaric S. Cardiovascular disease risk factors among older black, Mexican-American, and white women and men: an analysis of NHANES III, 1988–1994. Third National Health and Nutrition Examination Survey. JAGS. 2001;49:109–116. doi: 10.1046/j.1532-5415.2001.49030.x. 2001/02// [DOI] [PubMed] [Google Scholar]
- 35.Tang MX, Cross P, Andrews H, et al. Incidence of Alzheimer's disease in African-Americans, Caribbean Hispanics and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. 2001/// [DOI] [PubMed] [Google Scholar]
- 36.Census PaH. Census of Population and Housing Summary Tape File1, Technical Documentation. WAshington, DC: Bureau of the Census; 1991. [Google Scholar]
- 37.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992 May;49(5):453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 38.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- 39.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005 Aug 23;65(4):545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatano S. Plans for prevention of stroke formulated by WHO and practice in Japan. Nippon Rinsho. 1976 Jan 10;34(1):131–136. [PubMed] [Google Scholar]
- 41.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992 Mar–Apr;16(2):274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 42.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995 Nov;45(11):2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 43.DeCarli C, Murphy DG, Teichberg D, Campbell G, Sobering GS. Local histogram correction of MRI spatially dependent image pixel intensity nonuniformity. J Magn Reson Imaging. 1996 May–Jun;6(3):519–528. doi: 10.1002/jmri.1880060316. [DOI] [PubMed] [Google Scholar]
- 44.Murphy DG, DeCarli C, Schapiro MB, Rapoport SI, Horwitz B. Age-related differences in volumes of subcortical nuclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging. Arch Neurol. 1992 Aug;49(8):839–845. doi: 10.1001/archneur.1992.00530320063013. [DOI] [PubMed] [Google Scholar]
- 45.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease [see comments] Annals of Neurology. 2000;47(4):430–439. [PubMed] [Google Scholar]
- 46.Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004 Feb 10;62(3):433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J Neurosci. 2004 Jan 28;24(4):956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barkhof F, Polvikoski TM, van Straaten EC, et al. The significance of medial temporal lobe atrophy: a postmortem MRI study in the very old. Neurology. 2007 Oct 9;69(15):1521–1527. doi: 10.1212/01.wnl.0000277459.83543.99. [DOI] [PubMed] [Google Scholar]
- 49.Jack CR, Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002 Mar 12;58(5):750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitwell JL, Jack CR., Jr Neuroimaging in dementia. Neurol Clin. 2007 Aug;25(3):843–857. doi: 10.1016/j.ncl.2007.03.003. viii. [DOI] [PubMed] [Google Scholar]
- 51.Shamy JL, Buonocore MH, Makaron LM, Amaral DG, Barnes CA, Rapp PR. Hippocampal volume is preserved and fails to predict recognition memory impairment in aged rhesus monkeys (Macaca mulatta) Neurobiol Aging. 2006 Oct;27(10):1405–1415. doi: 10.1016/j.neurobiolaging.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 52.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yue NC, Arnold AM, Longstreth WT, Jr, et al. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology. 1997 Jan;202(1):33–39. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- 54.Wu CC, Mungas D, Petkov CI, et al. Brain structure and cognition in a community sample of elderly Latinos. Neurology. 2002 Aug 13;59(3):383–391. doi: 10.1212/wnl.59.3.383. [DOI] [PubMed] [Google Scholar]
- 55.Gurland BJ, Wilder DE, Lantigua R, et al. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999 Jun;14(6):481–493. [PubMed] [Google Scholar]
- 56.Minagar A, Sevush S, Bertran A. Cerebral ventricles are smaller in Hispanic than non-Hispanic patients with Alzheimer's disease. Neurology. 2000 Aug 8;55(3):446–448. doi: 10.1212/wnl.55.3.446. [DOI] [PubMed] [Google Scholar]
- 57.Oppenheimer GM. Paradigm lost: race, ethnicity, and the search for a new population taxonomy. Am J Public Health. 2001 Jul;91(7):1049–1055. doi: 10.2105/ajph.91.7.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]