Abstract
This in vitro study characterized the temporal cytokine expression profile from human monocytes exposed to phagocytosable Ti particles (0.78 ± 0.12 μm) and to Ti discs of comparable surface roughness. Human THP-1 monocytes were cultured in six well tissue culture polystyrene (TCPS) plates. Each well was either bare, contained Ti particles (the particles were clearly engulfed by the monocytes), or contained a Ti disc. Half of the wells were treated with 1 μg/mL lipopolysacchride (LPS), while the other half were left unstimulated. Unstimulated and LPS-stimulated cells in bare wells were the negative and positive controls, respectively. Supernatant was sampled from each well at 1, 6, 24, 48 and 72 h and assayed for the expression of nine different cytokines using a Luminex system. Three cytokines (IL-1β, GM-CSF and IL-13) gave little to no response under all conditions, while six cytokines (TNF-α, IL-6, MIP-1α, MCP-1, VEGF, and IL-1ra) were clearly detectable. Expression levels generally increased with culture time, particle concentration, and LPS stimulation. Most significantly, it was found that cells treated by Ti discs produced in many instances a higher cytokine expression than did particles.
Keywords: Ti, cytokines, monocytes, endotoxin, inflammation and wound healing
Introduction
Wear-induced debris has been well documented to induce inflammation and necrosis in the tissue surrounding implants [1, 2], often leading to loss of implant functionality [3]. A chronic inflammatory condition that compromises the integrity of the implant sites is postulated to arise from cytokines and chemokines released from the persistently activated macrophages that attempt to engulf particulates [4]. A related phenomenon also appears to occur in the tissue that surrounds textured implants with surface features on the order of phagocytosable particles [5], where macrophages attempting to engulf the surface features are hypothesized to prevent the surrounding tissue bed from progress to fibrous encapsulation.
Several studies have cited the relationship between the presence of wear debris, particle phagocytosis, and cytokine expression [3, 6–8], but only a few studies have characterized cytokine expression from monocytes/macrophages on textured surfaces [9, 10]. These studies examined the effect of Ti topography on the activation and cytokine mRNA change of mouse RAW 264.7 monocytes and mouse J774A.1 macrophages using real-time PCR. These studies concluded that Ti topographies induced significant osteogenic growth factor (BMP-2) mRNA expression but had modest effects on the expression of cytokine mRNA.
To our knowledge, no reports have been published on the temporal cytokine release from monocytes/macrophages exposed to materials, nor has anything been published on the differences in cytokine expression between bulk and particulate forms of the same material. By understanding the cellular phenotype and cytokine expression from monocytes on bulk materials and particulates, the effect of materials morphology on inflammation separate from the effect of material chemistry could be observed.
The current study characterized the temporal cytokine expression profile from human THP-1 monocytes exposed to phagocytosable Ti particles (0.78 ± 0.12 μm) and to Ti discs of comparable surface roughness (0.28 ± 0.14 μm). Three cytokines (IL-1β, GM-CSF and IL-13) gave little to no response under all experimental conditions, while six cytokines (TNF-α, IL-6, MIP-1α, MCP-1, VEGF, and IL-1ra) were clearly detectable in culture supernatant. Expression levels generally increased with culture time, Ti particle concentration, exposure to Ti discs, and lipopolysacchride (LPS) stimulation. Most significantly, it was found that cells treated by Ti discs produced in many instances a higher cytokine expression than did particles. We postulate that interrogating monocytes became activated when they attempted to engulf the features in phagocytable size at the disc surface, resulting in a substantial cytokine expression profile with and without LPS stimulation. This study also indicates a synergy between materials stimulation (Ti particles or discs) and LPS stimulation.
Materials and Methods
Ti particles and discs
Ti discs (Advent Research Materials, Oxford, England) with a diameter of 14.00 ± 0.5 mm and a thickness of 1 mm were shipped in dried condition. Ti particles of 2.6 ± 1.8 μm diameter (Corpuscubar Inc, New Jersey, USA) were shipped in water. Received discs and particles were washed in alternating sonicating baths of acetone and ethanol. Discs were placed in a sealed container of ethanol until use and particles were stored in sterile DI water. Discs and particles were washed three times with sterilized PBS immediately before use.
Imaging of Ti particles and discs
Images were taken by a Nanoscope IIIa MultiMode Scanning Probe Microscope (Veeco Instruments Inc., Santa Barbara, CA) using Tapping mode with a J scanner. RTESP probes (Veeco) were used for imaging in air. All images were collected at a scan rate of 0.3 – 1.0 Hz and scan sizes of 20 μm. In each experiment, 3–5 AFM images were captured and analyzed to determine the surface area of disk and particles. A dilute suspension of particles in deionized water was spread on the loading stub then placed in the desiccator until completely dehydrated.
Limulus Amebocyte Lysate (LAL) test
An Limulus Ambebocyte Lysate Kinetic Chromogenic assay, Endochrome-K, purchased from Charles River Laboratories (Wilmington, MA), was run on both the Ti particles and Ti discs, cleaned and uncleaned, according to manufacturer’s instructions. Cleaning involved methanol, acetone, and DIH2O steps as described above in this section. Reagents were rehydrated and handled according to the manufacturer’s certificate of analysis. Each sample was assayed in duplicate. Only labware labeled as non-pyrogenic was used. A standard curve ranging from 5.0 EU/mL to 0.005 EU/mL was prepared. 100 μL of standards, controls, and samples were pipetted into a 96 well plate. For every sample and negative water control, a 10 μL “spike” of the 5.0 EU/mL CSE standard was pipetted into separate wells to achieve “spike recovery” samples, having a final concentration of spike of 0.5 EU/mL. 100 μL of Lysate, a reagent extracted from circulating amebocytes of the horseshoe crab that interacts with endotoxin to form a gelatinous clot, was added to every well and the plate analyzed using a SpectraMax M2 plate reader at 37°C ± 1°C for 1 hour. The concentration level of endotoxin in the sample was determined by back calculating against the standard curve using the onset time. The onset time is the time it takes after the addition of reagent for the optimal density to reach a predetermined optimal density value or the threshold optical density.
Cell Culture
THP-1 human monocytes purchased from Duke Cell Culture Facility (Durham, NC) were suspended in RPMI 1640 media supplemented with 10% FBS, 50 μM 2-Mercaptoethanol, 1 mM sodium pyruvate, 10 mM Hepes, 4.5 g/L glucose, 100 U/ml penicillin G and 100 μg/ml streptomycin. 105 cells/mL were cultured on the 24-well tissue culture polystyrene plates (TCPS) in a fully humidified atmosphere consisting of 95% air, 5% CO2 at 37 °C.
LPS titration
Cells at concentration of 105 cells/mL received dosages of LPS (Escherichia coli; Sigma) at five concentrations: 0 (negative control), 0.01, 0.1, 1, and 10 μg/mL at the time of seeding. Phase contrast images were taken at the time point of interruption post-treatment to analyze potential cell morphological changes due to the different treatment types.
Ti Particles and discs exposure
Each well in the 24-well tissue culture polystyrene plates represented a different treatment condition. Untreated and lipopolysaccharide (LPS)-stimulated (1 μg/mL) monocytes were cultured on three surfaces: 1) Ti particles at either 1 mg/mL (high concentration, will be referred as H-Particle) or 0.1 mg/mL (low concentration, will be referred as L-Particle), 2) Ti discs and 3) tissue culture polystyrene (TCPS) as a control (4 replicates, respectively). Dosages of 1 μg/ml LPS were used because higher dosages of LPS (>1 μg/mL) led to cell death or disability, and low LPS dosage (< 0.01 μg/mL) showed little stimulation of cells. At 1, 6, 24, 48, and 72 hours post-treatment, 200 μL of supernatant was sampled from each well, centrifuged to remove cellular debris, split into 2 of 100 μL aliquots, and frozen at −80°C until assayed. Phase contrast images of the cell culture were taken at the same time points.
Quantification of Cytokine Concentration
Frozen aliquots of supernatant were analyzed by the LINCOplex detection kits (Linco Research, Inc., MO) in a Bio-Plex Suspension Array System (Bio-Rad, Hercules, CA). The Lincoplex assay was shown to produce results comparable with ELISA assay [data is not shown here]. The following cytokines were measured: Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Interleukin-8 (IL-8), Interleukin-10 (IL-10), Interleukin-1 Receptor Antagonist (IL-1ra), Tumor Necrosis Factor-α (TNF-α), Monocyte Chemotactic Protein-1 (MCP-1), Macrophage Inflammatory Protein-1α (MIP-1α), and Vascular Endothelial Growth Factor (VEGF).
Statistical Analyses
Two-way ANOVA was used to evaluate the differences in the mean values among the treatment groups. All Pairwise multiple comparison procedures were performed by a Tukey’s post hoc test. P-values less than 0.05 were considered as representing a significant difference of the characterization step.
Results
LPS Titration
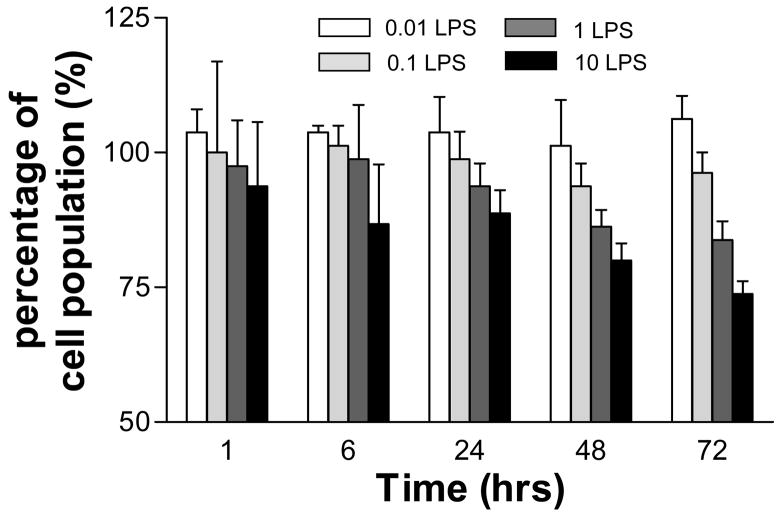
Figure 1 is a bar graph of the percentage of the original cell seeding density (105 cells/mL=100%) as a function of culture time and LPS dosage. Cells receiving no LPS proliferated to over 300% at 72 h post-treatment (data not shown). However, all four concentrations of LPS prevented cell proliferation, with increased LPS doses producing lower cell populations over the length of the experiment. The final population at 72 h was essentially unchanged for the lowest two dosage of LPS (106.3 ± 0.5% for 0.01 μg/mL and 96.3 ± 0.4% for 0.1 μg/mL), while the two higher dosages showed substantial cell losses at 72 h (83.8 ± 0.4% for 1 μg/mL and 73.8 ± 0.2% for 10 μg/mL). Trypan Blue exclusion was used to identify live cells.
Figure 1.
Bar graph of the percentage of the original cell seeding density as a function of culture time and LPS dosage. Cell population (105 cells/mL) at the time of seeding was considered as 100%. Different amounts of LPS (0–10 μg/ml) were added directly into the culture media at a time of cell seeding. Trypan Blue exclusion was used to identify live cells.
LAL Test
The endotoxin concentrations for the as received and cleaned titanium particles and discs are found in Table 1. All the results are valid because the Percent Recovery of the “spiked” samples is between 50 and 200% and the correlation coefficient of the standard curve is > 0.98. There is very little difference in the EU/ml of the cleaned and uncleaned particles or discs. All show very low levels of endotoxin (LPS).
Table 1.
The LAL Chromogenic Assay results for as received and cleaned titanium particles and discs.
| Sample | Replicates | Onset Time (s) | Adjusted (EU/ml) | Mean (EU/ml) | C.V. | % Recovery |
|---|---|---|---|---|---|---|
| PBS | 1 | 2051 | 0.014 | 0.013 | 9.9 | 106 |
| 2 | 2113 | 0.012 | ||||
| Discs cleaned | 1 | 3122 | 0.189 | 0.196 | 4.6 | 86 |
| 2 | 3079 | 0.202 | ||||
| Discs as-received | 1 | 3466 | 0.115 | 0.116 | 1.7 | 119 |
| 2 | 3449 | 0.118 | ||||
| Particles cleaned | 1 | 3313 | 0.014 | 0.014 | 1.5 | 133 |
| 2 | 3327 | 0.014 | ||||
| Particles as received | 1 | 1566 | 0.051 | 0.062 | 24.7 | 120 |
| 2 | 1454 | 0.073 |
Samples were cleaned/washed in acetone, ethanol and D.I water as described in materials and methods.
Cell morphological observation
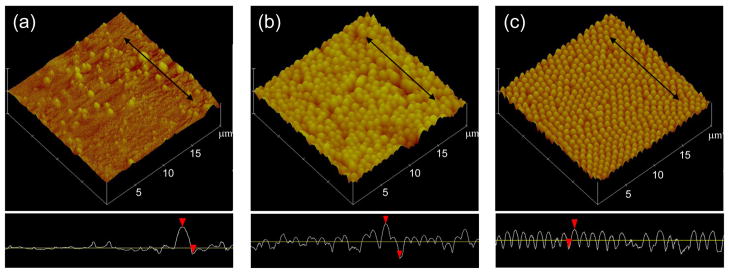
Figure 2 contains AFM images of the Ti disc surface (a), particles at 1 mg/mL (b) and particles at 0.1 mg/mL (c). The disc shows surface features with a long axis 0.28 ± 0.14 μm that are comparable to the size of particles with a long axis of 0.78 ± 0.12 μm. The surface features and particles are both within the size range of dimensions that macrophages are known to engulf (≤ 10 μm) [11].
Figure 2.
Atomic force microscope of Ti disc (a), particles at 1 mg/mL (b) and particles at 0.1 mg/mL (c) used in the study. The disc shows surface features with a long axis of 0.28 ± 0.14 μm that are comparable to the size of particles with a long axis of 0.78 ± 0.12 μm. Black arrows in the images correspond to the line scans in the lower boxes.
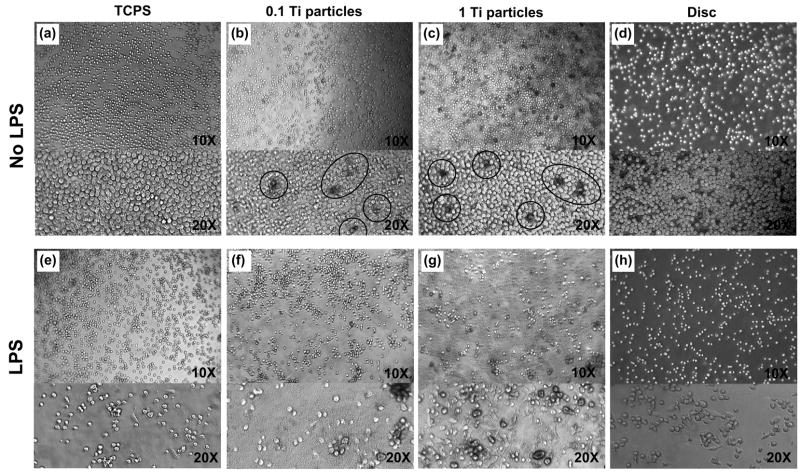
Figure 3 shows phase contrast images of unstimulated and LPS-stimulated THP-1 cells cultured for 72 hrs on bare TCPS (a and e), Ti particles at 0.1 mg/mL (b and f), Ti particles at 1 mg/mL (c and g), and Ti discs (d and h). THP-1 cell morphology at 2 hrs post-treatment was found to be independent of the specific substrate material (bare, particle or disc), while THP-1 cells at 72 hrs post-treatment revealed treatment-dependent morphologies.
Figure 3.
Phage contrast cell images of THP-1 cells cultured for 72 hrs on TCPS (a and e), Ti particles at 0.1 mg/mL (b and f) and at 1 mg/mL (c and g), and Ti discs (d and h). The top panel(a, b, c and d) shows unstimulated cells and the bottom panel (e, f, g and h) shows LPS-stimulated cells (1 μg/mL LPS). Circles in the (b) and (c) demonstrate the blackened THP-1 cells resulted from Ti partilce engulfment. Note that cell density of images may not be representative because the cell distribution of suspended THP-1 cells in the rounded culture wells are not homogeneous along the substrates mainly caused by centrifuge force.
Unstimulated THP-1 cells were typically spherical on TCPS and Ti discs at 72 h (Figure 3a and d). On Ti particles there was increased incidence of cells that were enlarged, spherical and blackened. This change in color and morphology appears to result from Ti particle engulfment. Figure 3b and c show that the number of blackened cells was somewhat higher in the wells with a particle concentration of 1 mg/mL than in wells with a concentration of 0.1 mg/mL. Figure 3f and g demonstrate that the amount of particles engulfed by monocytes appears to be roughly constant whether the cells were treated or untreated with LPS.
LPS stimulation induced a number of the THP-1 cells to adopt an elongated morphology that appeared to be attached to the surface of the culture wells as seen in the Figure 3e [12]. Flattened cells were also observed with LPS-stimulated monocytes in the presence of Ti particles and on Ti discs (Figure 3e–h).
Figure 3f and g shows cells subject to both LPS stimulation and Ti particles. Those cells that have phagocytosed Ti particles, while remaining spherical, show an enlarged radius as well as a dark cytoplasmic rim; whereas the flattened cells appear to lack internalized particles. A few of LPS stimulated cells on Ti discs were also similarly enlarged and spherical (Figure 3h).
Cytokine Expression of Unstimulated THP-1 Monocytes
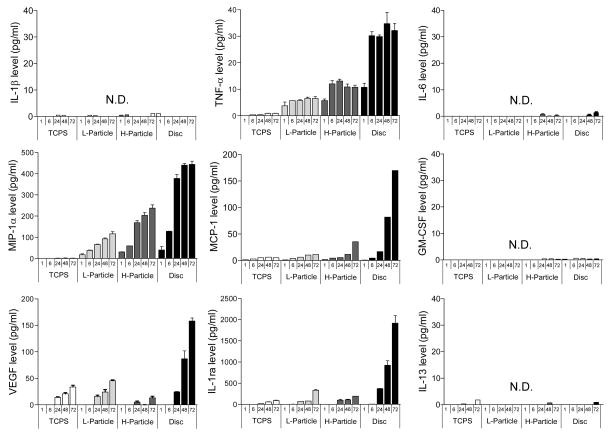
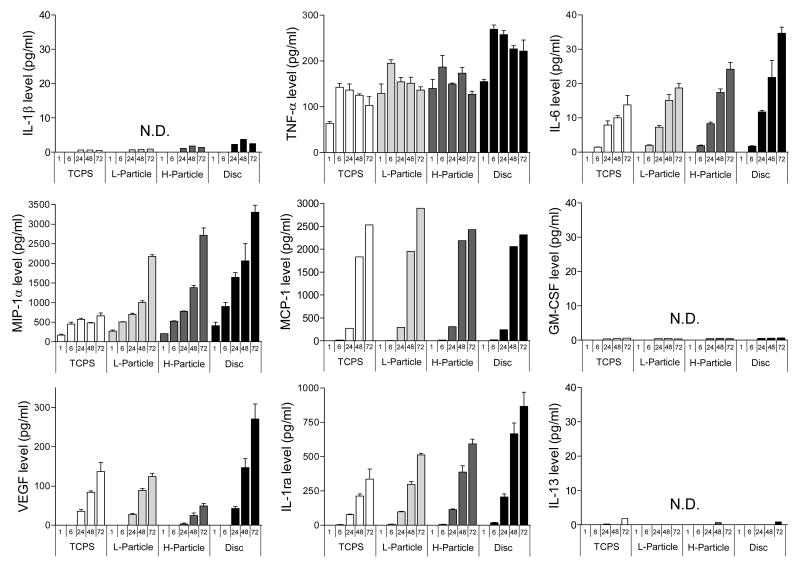
Figure 4 contains bar graphs of nine cytokines and chemokines secreted by unstimulated THP-1 cells cultured on TCPS, Ti particles at concentration of 0.1 mg/ml (L-Particle) and 1 mg/ml (H-particle), and Ti discs at 1, 6, 24, 48, 72 hrs post seeding. Treatment of unstimulated THP-1 cells with Ti particles and discs induced the secretion of the pro-inflammatory cytokine TNF-α, the chemokines MIP-1α and MCP-1, and the pro-wound healing cytokine VEGF, and the anti-inflammatory cytokine IL-1ra. This increased expression of cytokines on Ti particles and discs compared to TCPS shows that unstimulated THP-1 cells are interrogating Ti in both bulk and particulate form. Only trace amounts of IL-1β, IL-6, GM-CSF and IL-13 were detected under all conditions from unstimulated cells.
Figure 4.
9 cytokine expression by unstimulated THP-1 cells cultured on TCPS, Ti particles at concentration of 0.1 mg/ml and 1 mg/ml and Ti discs after 1, 6, 24, 48, 72 hrs. Data are presented as means ± SEM and performed in triplicate. N.D. is non-detectable.
TNF-α assayed from unstimulated THP-1 monocytes was detected at every time point under all conditions (except 1 hr post seeding on TCPS). MIP-1α, MCP-1, VEGF and IL-1ra all gradually increased expression as a function of time up to 72 hrs. Levels of VEGF and IL-1ra treated with either 0.1 mg/ml or 1 mg/ml Ti particles were comparable with control TCPS. The most surprising result was the level of cytokine expression observed with Ti discs, which exceed the corresponding level of expression of TNF-α, VEGF, IL-1ra, MIP-1α and MCP-1 on TCPS or in the presence of Ti particles.
Cytokine Expression of LPS-Stimulated THP-1 Monocytes
Figure 5 shows expression of the same nine cytokines as in Figure 4, except for LPS-stimulated cells. LPS-stimulated THP-1 monocytes on TCPS induced the secretion of pro-inflammatory TNF-α and IL-6, chemokines MIP-1α and MCP-1, pro-wound healing VEGF, anti-inflammatory IL-1ra, and even a slight increase in pro-inflammatory IL-1β. Expression of TNF-α, MIP-1α and MCP-1 from stimulated THP-1 cells on particles and discs was increased about 10 times compared to that from unstimulated THP-1 cells. However, expression of VEGF and IL-1ra from LPS-stimulated and unstimulated THP-1 cells were roughly comparable on all surfaces.
Figure 5.
9 cytokine expression by LPS (1 μg/ml) stimulated THP-1 cells cultured on TCPS, Ti particles at concentration of 0.1 mg/ml and 1 mg/ml and Ti discs after 1, 6, 24, 48, 72 hrs. Data are presented as means ± SEM and performed in triplicate. N.D. is non-detectable.
TNF-α and MIP-1α were sampled for every condition at all time points from LPS-stimulated monocytes, and the general trend was for expression levels to increase with time, with the exception of TNF-α that peaked at 6 hrs culturing time. LPS-stimulation also diminished the distinction between the expression levels observed with particles and Ti discs.
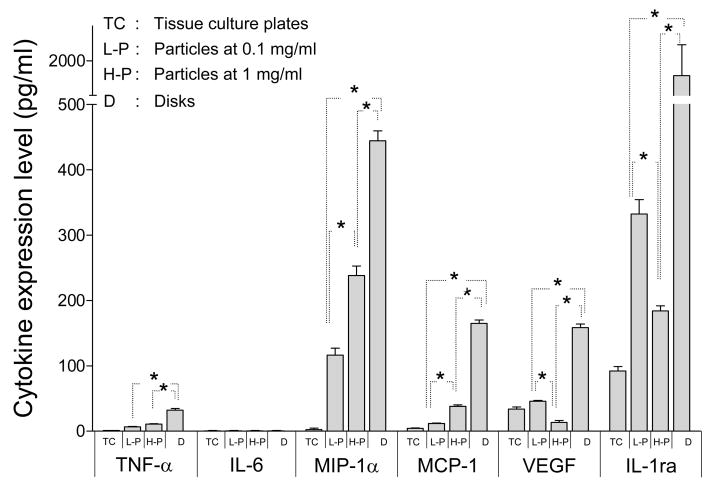
Effect of Ti particles and discs on unstimulated THP-1 cells
Figure 6 compares the expression levels of the six readily detected cytokines and chemokines (TNF-α, IL-6, MIP-1α, MCP-1, VEGF and IL-1ra), but for only unstimulated THP-1 cells at 72 h. In all cases, except for IL-6 that was detected only with LPS stimulation (see Figures 4 and 5), the expression levels for Ti discs were significantly higher (p<0.05) than the corresponding expression levels for Ti particles and bare TCPS. Also note that the expression of levels MIP-1α and MCP-1 on 1 mg/mL particles was significantly higher than that on 0.1 mg/mL particles; whereas the opposite was observed for VEGF and IL-1ra (p<0.05). TNF-α showed the same particle-dependent trend as MIP-1α and MCP-1, except the difference was not significant.
Figure 6.
Cytokine expression at 72 hrs by unstimulated THP-1 cells cultured on TCPS, Ti particles at concentration of 0.1 mg/ml and 1 mg/ml and Ti discs. Data are presented as means ± SEM and performed in triplicate. Two-way ANOVA were performed to test significance p<0.05
Possible synergism between materials and LPS induced cytokine expression
Table 2 is a listing of the 72 hrs data in Figures 4 and 5 (in pg/mL) for the six readily detected cytokines and chemokines (TNF-α, IL-6, MIP-1α, MCP-1, VEGF and IL-1ra) that points to a possible synergism between materials stimulation (Ti particle or disc) and LPS stimulation of monocytes. “LPS measured,” column (a), lists the expression levels measured from LPS stimulated cells on TCPS (from Figure 5). “Materials measured,” column (b), lists the expression levels measured with Ti particles or discs from unstimulated cells (from Figure 4). “LPS+Materials measured,” column (c) lists the expression levels measured with Ti particles or discs from LPS stimulated cells (from Figure 5). “% synergism,” column (d), is the percentage difference between columns (c) and (a)+(b).
Table 2.
A list of 72 hrs data of six readily detected cytokines that poinsts to a possible synergism between materials stimulation and LPS stimulation. (a) LPS-measured cytokine expression was averaged from LPS-stimulated THP-1 cells on TCPS as shown in Figure 5, (b) materials-measured cytokine expression was averaged from unstimulated-THP-1 cells on either Ti particles at concentration of 0.1 mg/mL and 1 mg/mL or Ti discs, (c) LPS+materials-measured cytokine expression was averaged from LPS-stimulated THP-1 cells on either Ti particles at concentration of 0.1 mg/mL and 1 mg/mL or Ti discs, and (d) is the percentage difference between columns (c) and (d), i.e. (d)=((c)−(a+b))/(a+b) indicating that materials synergistically enhance the cytokine expression when combined with LPS stimulation.
| Groups Cytokines |
(a) LPS-measured (pg/mL) | (b) Materials-measured (pg/mL) | LPS+Material | ||
|---|---|---|---|---|---|
| (c) Measured (pg/mL) | (d) % of synergism | ||||
| TNF-α | 103.5 | L-P | 6.5 | 136.5 | 24.1 |
| H-P | 10.8 | 127.3 | 11.4 | ||
| Disc | 32.2 | 221.6 | 63.2 | ||
| IL-6 | 13.8 | L-P | N.D. | 18.7 | 35.5 |
| H-P | N.D. | 24.2 | 75.4 | ||
| Disc | N.D. | 34.7 | 151.4 | ||
| MIP-1α | 640.0 | L-P | 116.4 | 2181.5 | 188.4 |
| H-P | 238.2 | 2721.0 | 209.8 | ||
| Disc | 437.0 | 3307.0 | 207.1 | ||
| MCP-1 | 2392.0 | L-P | 12.0 | 2728.4 | 13.5 |
| H-P | 39.0 | 2335.9 | −3.9 | ||
| Disc | 162.2 | 2417.1 | −5.4 | ||
| VEGF | 136.6 | L-P | 45.5 | 124.2 | −31.8 |
| H-P | 13.2 | 49.7 | −66.8 | ||
| Disc | 158.2 | 270.9 | −8.1 | ||
| IL-1ra | 336.6 | L-P | 343.3 | 512.4 | −24.6 |
| H-P | 179.9 | 593.1 | 14.8 | ||
| Disc | 2002.8 | 865.9 | −63.0 | ||
Note: not detected (N.D) was assigned a value of zero
A positive synergy was observed for pro-inflammatory TNF-α, IL-6 and MIP-1α. Pro-wound healing VEGF exhibited a negative synergy overall, while IL-1ra was negative for the low particle concentration and discs. The percentage differences for MCP-1 were small, suggesting no synergy. There were no consistent trends for particles v. discs or low v. high particle concentrations.
Discussion
This study used the cultured human THP-1 monocyte cell line to explore the cellular morphology and the temporal cytokine and chemokine expression profiles on particulate and disc forms of Ti. The cytokines and chemokines selected for this study were stereotypically pro-inflammatory (IL-1β, TNF-α, MIP-1α, MCP-1, IL-6, IL-13, GM-CSF), anti-inflammatory (IL-1ra) or pro-wound healing (VEGF) [13].
In general, unstimulated THP-1 cells on TCPS and Ti discs displayed a similar rounded and undifferentiated morphology (Figure 3a and d); however, a number of THP-1 cells on particles showed an enlarged, spherical and blackened shape as a result of particle engulfment (Figure 3b and c). It was also interesting that increasing the density of particles also appeared to increase the incidence of cells that adopted a blackened morphology. LPS stimulation of THP-1 cells induced activated/differentiated cells, characterized by thin and elongated bodies that appeared to be attached to the surface of the culture wells (Figure 3e–h). Such morphological changes are indicative of monocyte activation via Toll-Like-Receptor by LPS [14], lending a visual analog to the cytokine expression data seen in Figures 5.
Noted by the presence of both flattened and elongated cells, and spherical and blackened cells, it is clear that cells treated with both LPS and Ti particles were influenced by two different processes (Figure 3f and g). The amount of particles internalized by monocytes appears to be roughly constant whether the cells were treated or untreated with LPS (compare Figure 3c and g), indicating the LPS does not accelerate phagocytosis; however, the larger number of flattened and elongated cells observed with 1 mg/mL Ti particles indicates (compare Figure 3f and g) that the presence of particles may increase LPS-induced activation of THP-1 cells. This latter observation suggests a synergistic effect that possibly mimics the engulfment of a pathogenic particle.
Increased cytokine expression of unstimulated THP-1 cells on Ti discs and particles compared to TCPS clearly showed that unstimulated THP-1 cells were interrogating Ti in both bulk and particulate form. TNF-α expression was unique because it peaked at early time points (6 hrs post seeding) and more or less maintained this level for the length of the experiment. This observation is consistent with TNF-α’s “alarm cytokine” label arising from its early expression compared to other pro-inflammatory cytokines that show a later onset of detectable expression levels (e.g. IL-6). Pro-wound healing VEGF and anti-inflammatory IL-1ra also gradually increased expression as a function of time up to 72 hrs, which is consistent with the previous observation that wound healing and anti-inflammatory cytokines are expressed at a delayed time [15]. Chemokines MIP-1α and MCP-1 also displayed a gradual increase in expression as a function of time up to 72 hrs.
The effect of Ti particles and Ti discs on unstimulated cells is hard to discern from Figure 4, but is more apparent in Figure 6 that looks at 72 h expression levels only. Here, the discs appeared to have a more stimulatory effect on cytokine expression than did particles, and the dependence on particle concentration was positively correlated in some cases (TNF-α, MIP-1α, MCP-1) and negatively correlated in others (VEGF and IL-1ra).
LPS stimulation, with or without either Ti particles or discs, caused about a 10-fold increase in TNF-α, MIP-1α and MCP-1 expression, and caused the appearance of IL-6 expression that was absent with unstimulated cells. These increased cytokine expression levels can be attributed to the ability of LPS to increase monocyte activation, which leads to increased pro-inflammatory cytokine production [16]. However, LPS stimulation did not appear to enhance the expression of VEGF and IL-1ra. LPS-stimulation also appeared to diminish the distinction between the expression levels observed with particles and Ti discs.
The surprising observation that cytokine expression from Ti discs generally exceeded the expression level on Ti particles may have been influenced by 1) the protrusions observed at the disc surface (Figure 2) and 2) the different endotoxin level between particles (0.014 EU/mL) and discs (0.196 EU/mL). Both the Ti particles and the surface features of the Ti discs were roughly the same size (< 10 μm), and within the range of sizes that macrophages will attempt to phagocytose (< 10 μm); however, the particles can be engulfed while the surface features cannot. Consequently, activated macrophages that interrogate but cannot engulf surface protrusions may give rise to a process of frustrated phagocytosis [17] and a concomitant increase in cytokine production [10]. This is also supported by the appearance of clearly activated cells on the Ti disc surface (Figure 3h).
The endotoxin level on discs were about 14 times higher than that on particles even though endotoxin levels of both particles and discs were much lower than the level considered to be safe for implantable device as per the FDA [18]. This difference in endotoxin level might have caused the cytokine expression from unstimulated monocytes on discs to exceed the expression level on particles, although this difference between particles and discs was less pronounced in LPS stimulated monocytes. Note that the difference in intrinsic endotoxin level between particles and discs are trivial in the LPS stimulated monocytes.
The surfaces of Ti discs and substrates decorated with Ti particles were characterized by scanning AFM of 100 × 100 μm2 areas. The surface area of the Ti discs and Ti particles calculated from line integration of scanned data yield 11685.3 ± 493.1 μm2 and 10456.0 ± 126.7 μm2. The feature density on the Ti disc determined by image thresholding was approximately 8 times lower than was the particle density for low and high Ti particle concentration.
Finally, this study suggested the possibility of a synergistic relationship between endotoxin (LPS) stimulation and material stimulation that arises from the presence of either Ti particles or Ti discs. Table 2 is an attempt to extract this information from the 72 hrs cytokine expression data in Figures 4 and 5. A positive synergy in column (d) meant the experimentally measured effect in the presence of materials (Ti particles or discs) and LPS stimulation was larger than the sum of the two effects measured separately. A negative synergy meant the opposite. Expression of pro-inflammatory cytokines TNF-α and IL-6 and the chemokine MIP-1α were synergistically increased when the THP-1 cells were cultured with LPS stimulation with particles and discs, while expression levels of pro-wound healing VEGF and anti-inflammatory IL-1ra were adversely affected by the combination of materials and LPS stimulation. No synergy was observed for MCP-1. These observations are consistent with a cytokine expression profile stimulated by macrophage that engulfs a pathogenic particle (LPS + material), i.e. enhanced expression of cytokines and chemokines that stimulate the inflammatory response, and suppressed expression of cytokines and growth factors that are anti-inflammatory or that promote post-inflammation events.
Conclusions
This in vitro study explored the cellular morphology and characterized the temporal cytokine expression profile from human THP-1 monocytes exposed to phagocytosable Ti particles (2.6 ± 1.8 μm) and to Ti discs of comparable surface roughness. Followed by microscopic images, cytokine expression data show treatment of unstimulated THP-1 cells with Ti particles and discs induced the secretion of the cytokines indicating that unstimulated THP-1 cells are interrogating Ti in both bulk and particulate form. Expression levels generally increased with culture time, particle concentration, and LPS stimulation. Internalization of particles appears to be roughly constant regardless of LPS stimulation. The larger number of flattened and elongated cells however observed with Ti particles indicating that the presence of particles may increase LPS-induced activation of THP-1 cells. This observation relates to the proposed synergistic effect of between LPS and materials induced cytokine expression. The most surprising finding was the high cytokine expression of the monocytes on the discs.
Acknowledgments
This research was supported by NIH (DK054932), the Whitaker Foundation. The authors thank to Rob Schutte, Biomedical Engineering, Duke, for helpful discussions and Jongmyeong Kim, Biomedical Engineering, Duke, for surface area calculation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Human monocyte response to particulate biomaterials generated in-vivo and in-vitro. J Orthop Res. 1995;13(5):792–801. doi: 10.1002/jor.1100130520. [DOI] [PubMed] [Google Scholar]
- 2.Wang ML, Nesti LJ, Tuli R, Lazatin J, Danielson KG, Sharkey PF, Tuan RS. Titanium particles suppress expression of osteoblastic phenotype in human mesenchymal stem cells. J Orthop Res. 2002;20(6):1175–1184. doi: 10.1016/S0736-0266(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell W, Matthews JB, Stone MH, Fisher J, Ingham E. Comparison of the response of human peripheral blood mononuclear cells to challenge with particles of three bone cements in vitro. Biomaterials. 2003;24(5):737–748. doi: 10.1016/s0142-9612(02)00405-2. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JM. Biological responses to materials. Ann Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 5.Karoussis IK, Muller S, Salvi GE, Heitz-Mayfield LJA, Bragger U, Lang NP. Association between periodontal and peri-implant conditions: a 10-year prospective study. Clin Oral Implants Res. 2004;15(1):1–7. doi: 10.1111/j.1600-0501.2004.00982.x. [DOI] [PubMed] [Google Scholar]
- 6.Garrigues GE, Cho DR, Rubash HE, Goldring SR, Herndon JH, Shanbhag AS. Gene expression clustering using self-organizing maps: analysis of the macrophage response to particulate biomaterials. Biomaterials. 2005;26(16):2933–2945. doi: 10.1016/j.biomaterials.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigo AM, Martinez ME, Saldana L, Valles G, Martinez P, Gonzalez-Carrasco JL, Cordero J, Munuera L. Effects of polyethylene and a-alumina particles on IL-6 expression and secretion in primary cultures of human osteoblastic cells. Biomaterials. 2002;23(3):901–908. doi: 10.1016/s0142-9612(01)00200-9. [DOI] [PubMed] [Google Scholar]
- 8.Valles G, Gonzalez-Melendi P, Gonzalez-Carrasco JL, Saldana L, Sanchez-Sabate E, Munuera L, Vilaboa N. Differential inflammatory macrophage response to rutile and titanium particles. Biomaterials. 2006;27(30):5199–211. doi: 10.1016/j.biomaterials.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 9.Takebe J, Champagne CM, Offenbacher S, Ishibashi K, Cooper LF. Titanium surface topography alters cell shape and modulates bone morphogenetic protein 2 expression in the J774A. 1 macrophage cell line. J Biomed Mate Res A. 2003;64A(2):207–216. doi: 10.1002/jbm.a.10275. [DOI] [PubMed] [Google Scholar]
- 10.Smetana K. Cell biology of hydrogels. Biomaterials. 1993;14(14):1046–1050. doi: 10.1016/0142-9612(93)90203-e. [DOI] [PubMed] [Google Scholar]
- 11.Hirayama TT, Fujikawa YY, Itonaga II, Torisu TT. Effect of particle size on macrophage-osteoclast differentiation in vitro. J orthop sci. 2001;6(1):53–8. doi: 10.1007/s007760170025. [DOI] [PubMed] [Google Scholar]
- 12.Benahmed MM, Heymann DD, Pilet PP, Bienvenu JJ, Daculsi GG. LPS increases biomaterial degradation by human monocytes in vitro. J biomed mate res. 1997;34(1):115–9. doi: 10.1002/(sici)1097-4636(199701)34:1<115::aid-jbm15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Thomson AW, Lotze MT. The cytokine handbook. 4. Academicpress: San Diego; 2003. [Google Scholar]
- 14.Rabehi L, Irinopoulou T, Cholley B, Haeffner-Cavaillon N, Carreno MP. Gram-positive and gram-negative bacteria do not trigger monocytic cytokine production through similar intracellular pathways. Infection and Immunity. 2001;69(7):4590–4599. doi: 10.1128/IAI.69.7.4590-4599.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DH, Smith JT, Chilkoti A, Reichert WM. Covalently immobilized rhIL-1ra-ELP fusion protein attenuates inflammatory profile of LPS-stimulated human monocytes. Submitted to Biomaterials. doi: 10.1016/j.biomaterials.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 17.Henson PPM. Mechanisms of exocytosis in phagocytic inflammatory cells. Parke-Davis Award Lecture. Am j pathol. 1980;101(3):494–511. [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Health and Human Services. Public Health Service, Food and Drug Administration. Guideline on validation of the limulus ameboyte lysate test as an end-product endo-toxin test for human and animal parenteral drugs, biological products, and medical devices. 1987.