Abstract
A hallmark of fetal growth restriction (FGR) is restricted placental development and insufficient nutrient supply to the fetus. It has previously been shown that activity levels of telomerase, the enzyme responsible for completing replication of telomeric DNA during cell division, is suppressed in FGR placenta samples as compared to control placenta samples from donors of the same gestational age. Here we examine whether telomere length maintenance is also compromised in FGR placenta samples. Southern analysis of telomere length for placenta and cord blood samples from 32 FGR and 36 control donors, ranging in gestational age from 37 to 40 weeks, revealed significantly shorter telomeres (P≤ 0.001) in FGR placenta samples, but not cord blood samples. Furthermore, analysis of telomerase extracts, RNA and DNA placental samples from donors with and without idiopathic FGR confirmed a direct association between suppression of telomerase activity and reduced telomere length in FGR placenta. In addition, expression levels of markers of telomere-induced senescence, p21, p16 and EF-1α, were significantly elevated in FGR placenta samples (P ≤ 0.01). These observations support a direct affect of reduced telomerase activity levels on the placental pathology associated with FGR.
Keywords: Telomere, Fetal growth restriction, Placenta, Senescence
1. Introduction
Fetal growth restriction (FGR) is a relatively common, pleiotropic complication of pregnancy, affecting ~5-10% of newborns [1,2]. It is associated with substantially increased infant mortality as well as childhood and adulthood morbidity, including increased risk for cardiovascular disease, obesity and diabetes [2]. While the etiology is poorly defined, it is associated with a utero-placental insufficiency, with attenuated placental development and restricted nutrient supply to the fetus. The majority of instances of FGR (~60%) appear to be caused by other known pregnancy complications, including pre-eclampsia, congenital abnormalities and intrauterine viral infections [3,4]. The idiopathic instances of FGR (~40%) are generally characterized by asymmetric growth [4].
Previous studies have shown that FGR is associated with reduced levels of telomerase activity in the placenta [5]. Telomerase is an enzymatic complex that functions to complete the replication of telomeres, genetic elements that cap and protect the ends of chromosomes [6]. In normal human cells, the absence of telomerase leads to gradual reduction in telomere length with each cell division, ultimately compromising telomere function and signaling cell senescence [7-9]. Therefore in the present study, we sought to examine whether FGR is also associated with accelerated telomere loss and aberrant expression of genes associated with telomere-induced senescence in the placenta.
2. Materials and methods
2.1. Study groups and sample collection
All donors for this study were recruited, with informed consent and Institutional Review Board approval, at the Kapiolani Medical Center for Women and Children (KMCWC). All placental samples were obtained thru the Clinical Center for Research Excellence phenotyping core at the John A Burns School of Medicine. For all DNA placental samples used in the initial analysis of telomere length (Fig.1), FGR is defined as any newborn having a birth weight of ≤5th percentile for Filipino newborns at a given gestational age (percentile cut-off was calculated from recent birth weight records from 2004 thru 2006 for KMCWC hospital). The percentile cut-off was calculated from birth weight data for Filipino newborns, since newborns of this ethnic background have the smallest average birth weight, and therefore false positive FGR donors (i.e. small for gestational age donors) should be minimal. The control cohort is defined as any newborn donor having a birth weight of ± 1 standard deviation of the mean birth weight for all newborns at a given gestational age delivered at KMCWC hospital (2004-2006).
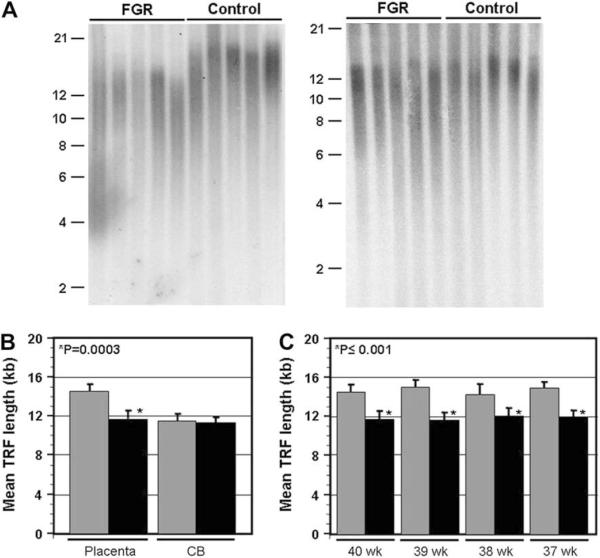
Fig. 1.
Reduced telomere length in placenta samples from FGR newborns. A. Telomere length was assessed by Southern analysis of terminal restriction fragment (TRF) length for placental and cord blood (CB) DNA samples for both FGR and control newborns. Shown are sample blots for placental samples (left panel) and corresponding cord blood samples (right panel) for 5 FGR and control donors at 40 weeks gestational age. Size of molecular weight markers (Kb) is shown on the side. B. Calculation of the average mean TRF length for placenta and cord blood samples for all donors at 40 weeks gestational age (n ≥ 8 for both groups). Control samples are represented by the grey bars, and FGR samples by the black bars. Error bars represent standard deviation from 4 measurements of mean TRF length. The P value (student's t-test) is shown. C. Comparison of mean TRF length for placenta samples for FGR (40 weeks, n = 7; 39 weeks n = 9; 38 weeks n = 8; 37 weeks n = 8) and control (n = 9 for all 4 cohorts) newborns at different gestational ages.
For the fresh placental samples obtained from idiopathic FGR donors and gestational age-matched controls (Fig. 2), the fresh placental biopsies were obtained within 1 h of birth, and were taken near the umbilical cord on the fetal side. All samples were immediately frozen in liquid nitrogen for future work-up (see below). Inclusion criteria for all samples were singleton births delivered at KMCWC for non-incarcerated mothers ranging in age from 18 to 45. The exclusion criteria for all donors were pre-eclampsia, maternal substance abuse, congenital abnormalities, intrauterine viral infections, and chromosomal abnormalities. The idiopathic FGR cohort was additionally defined as having a birth weight of ≤5th percentile for Filipino newborns, and ponderal index ≤10% percentile. It is important to note that there are instances of FGR with birth weights above the 5th percentile that have not been examined in this study, and which may be of interest to assess in future studies. Furthermore, we would like to acknowledge the possibility of the inadvertent inclusion of some small for gestational age donors in this study, despite the tight birth weight percentile cut-off.
Fig. 2.
Reduced telomerase activity and telomere length is associated with elevated expression of markers of telomere-induced senescence in FGR placenta samples. Fresh placental biopsies near the umbilical cord were obtained for 4 idiopathic FGR and 4 control newborns of similar gestational age (38-40 weeks), and telomerase extracts, RNA and DNA were obtained as described (Materials and Methods). A. Telomerase activity was assessed using the TRAP assay. A typical blot for all samples is shown. NC-negative control. The internal control for PCR efficiency in the TRAP assay is indicated at the side (arrow). B. Quantification of TRAP analysis of telomerase activity for samples shown in panel A. Results represent 4 or more measurements of telomerase activity for each sample. The P value (student's t-test) is shown. C. Quantitative analysis of telomere length (mean TRF length) for all placental and CB DNA samples. Results represent 4 or more measurements of mean TRF length for each sample. D. Real time PCR analysis of the expression levels of 3 markers of telomere-induced senescence (p16, p21 and EF-1α) for all placental RNA samples. Expression levels are shown relative to GAPDH. Similar results were also observed using an 18S RNA reference control (not shown). For each gene, results represent 6 or more measurements of relative expression levels per sample. For all quantitative analyses, error bars represent standard deviation.
2.2. Preparation of telomerase extracts, RNA and DNA
Frozen placenta samples were crushed into a fine powder using a cooled stainless steel press, and extracts were divided into 3 parts for extraction of telomerase, RNA, or DNA. Telomerase extracts were prepared using CHAPS lysis buffer according to manufacturers' instructions (Chemicon), and included 20 units of RNasin per sample. RNA was extracted using Trizol according to manufacturers; instructions (Sigma), and DNA was extracted using Phenol: chloroform as previously described [8].
2.3. Telomere length analysis
For all DNA samples, telomere length was measured by Southern analysis of terminal restriction fragment length, as previously described [10].
2.4. Analysis of telomerase activity
Telomerase activity was assessed for all extracts using the TRAP assay [11] according to manufacturers' protocol (Chemicon), with the following exceptions. The TRAP assay was performed on 0.5 μg protein per sample extract. Twenty-five cycles of PCR (94/min, 60/1 min) was performed.
2.5. Real time RT-PCR analysis of gene expression
Reverse transcription (RT) was performed in a 20 μl reaction with an oligo(dT) primer and SuperScript III (Invitrogen) at 50 °C for 60 min using 1.5 mg total RNA that had been pre-treated with RNase-free DNase to remove any contaminating genomic DNA. For all samples, quality of RNA was confirmed beforehand by gel analysis. For all genes analyzed in this study, primers were designed to be 22-24 nucleotides in length, and spanned the most 3′ intron. Each primer set was verified to yield a single amplicon of 100-120 bp in length. Quantitative real time PCR was performed on a MyiQ system using iQ SYBR Green Supermix (BioRad). The reaction mix contained 500 nM of each primer, 200 mM dNTPs, 2 μM MgCl2. All PCR reactions were performed for 40 cycles (95 °C, 15 s; 66 °C, 45 s) followed by continuous melt curve analysis to ensure product accuracy. For each primer set, standard curves (Cp plotted against the log of relative cDNA concentration) generated by serial dilutions of first-strand control cDNA were linear through a range spanning at least one log greater and less than the amount of cDNA used in the test reactions.
3. Results and discussion
To assess telomere length in FGR placental samples, we performed southern analysis of telomere length for 32 FGR donors and 36 gestational age-matched control donors by Southern analysis of terminal restriction fragment (TRF) length [7,10]. As shown in Fig. 1A&B, the TRF lengths for FGR donors at 40 weeks gestational age are noticeably shorter, in general, as compared to gestational age-matched control donors. Quantitative analysis of mean TRF length for all donors at 40 weeks gestational age confirmed that the average telomere length is significantly shorter (P = 0.0003) in the 40-week FGR cohort (Fig. 1B). We have previously shown that telomere length is substantially longer in placenta as compared to cord blood (CB) samples from the same donor [10]. In the present study, we have also assessed TRF length for CB samples from each FGR and control donor, as an internal reference. Importantly, unlike the placenta samples, TRF lengths for FGR CB samples were not reduced in size relative to TRF lengths for control CB samples (P > 0.1; Fig. 1A, B). This observation supports a placental origin of the FGR pathology.
To further assess placental telomere length for FGR donors relative to control donors, we also performed Southern analysis of TRF length for placental DNA samples from donors at 37-39 weeks gestational age. As shown in Fig. 1C, mean TRF length was significantly shorter for FGR samples at all gestational ages assessed (P ≤ 0.001). No difference in TRF length was observed between FGR and control CB samples at these gestational ages (data not shown). These observations suggest that the suppressed telomerase activity levels observed in the FGR placenta affects telomere replication at least as early as 37 weeks gestational age.
It is well established that unabated telomere shortening ultimately causes cell senescence in normal human cells [9]. To assess whether the marked reduction in telomere length observed in the FGR placental samples may have induced elevated frequency of senescent cells in the placenta, we collected fresh placental biopsies from 4 idiopathic FGR donors and 4 gestational age-matched control donors and acquired DNA and RNA samples as well as telomerase extracts from placental biopsies for all donors. Consistent with previous studies and results shown here, we observed significantly reduced levels of telomerase activity and TRF length for the FGR samples relative to control samples (Fig. 2A-C). To assess the proportion of senescent cells in the placenta, we performed real time RT-PCR analysis of the expression levels of 3 established biomarkers of telomere-induced senescence, elongation factor 1 alpha (EF-1α), p21 and p16 [12-16]. We observed significantly higher levels of expression of all of these genes in the FGR samples (P ≤ 0.01) relative to control samples (Fig. 2C), suggesting that the frequency of senescent cells is elevated in the FGR placenta.
The results of the present study provide evidence that suppressed placental telomerase activity may directly affect FGR pathology, at least at 37+ weeks of pregnancy, by causing accelerated loss of telomeric DNA and subsequent elevated levels of telomere-induced senescence. Why are telomerase activity levels diminished in the FGR placenta? Given the pleiotropic characteristic of FGR, the answer to this question is likely to be complex. One possibility is the presence of certain genetic variants of either of the essential components of telomerase, the telomerase RNA component (Terc) or the catalytic component, telomerase reverse transcriptase (Tert). Loss-of-function genetic variants of Terc and Tert have been specifically associated with some human diseases, namely autosomal dominant dyskeratosis congenita (DKC) [17,18] and idiopathic pulmonary fibrosis (IPF) [18,19]. These diseases are rare relative to FGR, and therefore it is unlikely that the genetic variants associated with DKC and IPF are also associated with FGR. It is possible that other unidentified genetic variants of Terc and/or Tert do contribute to the FGR pathology, however the affect of these variants on telomerase function would presumably be tissue specific (ie. in the placenta) since telomere length is not affected in FGR CB samples (Fig. 1). Another possibility is that the onset of telomerase suppression is a downstream, indirect affect of a combination of deleterious genetic factors and/or environmental cues that are ultimately responsible for the inception of FGR. Future studies designed to assess the status of telomerase activity levels and telomere length in FGR at earlier stages of pregnancy (ie. before 37 weeks) should help to more precisely determine whether the suppression of telomerase is an early or late event during FGR pathogenesis. The results presented here suggest that the development of small molecule activators of telomerase may hold promise as a potential therapy for FGR.
Acknowledgements
This work was supported by a Research Centers in Minority Institutions (RCMI) award (RA). We would like to thank Dr. Ivica Zalud and the KMCWC delivery floor nursing staff for help with sample collection, and Jayne Tabata and Omar Sultan for their help and support in the implementation of this project.
References
- [1].Lubchenco LO, Hansman C, Boyd E. Intrauterine growth as estimated from liveborn birthweight data at 24 to 42 weeks of gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- [2].Barker DJ. Intrauterine programming of adult disease. Mol Med Today. 1995;9:418–23. doi: 10.1016/s1357-4310(95)90793-9. [DOI] [PubMed] [Google Scholar]
- [3].Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–6. doi: 10.1016/s0029-7844(01)01780-x. [DOI] [PubMed] [Google Scholar]
- [4].Bianchi CW, Crombleholme TM, D'Alton ME. Fetology: diagnosis and management of the fetal patient. 1st ed. McGraw-Hill; 2000. Chapter 124: intrauterine growth restriction; pp. 929–35. [Google Scholar]
- [5].Izutsu T, Kudo T, Sato T, Nishiya I, Ohyashiki K, Mori M, et al. Telomerase activity in human chorionic villi and placenta determined by TRAP and in situ TRAP assay. Placenta. 1998;19:613–8. doi: 10.1016/s0143-4004(98)90022-4. [DOI] [PubMed] [Google Scholar]
- [6].Cech T. Beginning to understand the end of the chromosome. Cell. 2004;116:273–9. doi: 10.1016/s0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- [7].Harley CB, Futcher AB, Greider CW. Telomeres shorten during aging of human fibroblasts. Nature. 1990;345:458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- [8].Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89:10114–8. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of lifespan by introduction of telomerase into normal human cells. Science. 1998;279:349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- [10].Allsopp R, Shimoda J, Easa D, Ward K. Long telomeres in the mature human placenta. Placenta. 2007;28:324–7. doi: 10.1016/j.placenta.2006.04.003. [DOI] [PubMed] [Google Scholar]
- [11].Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- [12].Lecka-Czernik B, Moerman EJ, Jones RA, Goldstein S. Identification of gene sequences over-expressed in senescent and Werner syndrome human fibroblasts. Exp Gerontol. 1996;31:159–74. doi: 10.1016/0531-5565(95)02014-4. [DOI] [PubMed] [Google Scholar]
- [13].Jiang H, Schiffer E, Song Z, Wang J, Zürbig P, Thedieck K, et al. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc Natl Acad Sci U S A. 2008;105:11299–304. doi: 10.1073/pnas.0801457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–8. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- [15].Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–4. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- [16].Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16Ink4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–23. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- [17].Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–5. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- [18].Garcia CK, Wright WE, Shay JW. Human diseases of telomerase dysfunction: insights into tissue aging. Nucleic Acids Res. 2007;35:7406–16. doi: 10.1093/nar/gkm644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–26. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]