Abstract
Erk activation is often used as a downstream pathway indicator of TCR signaling, generally in terms of both Erk1 and Erk2 isoforms measured together. In order to investigate potential distinctions between Erk1 and Erk2 regulation and effects downstream of TCR ligation, we generated a series of stable and independent Erk1 and Erk2 shRNA knockdown lines in the 1B6 T cell hybridoma. We observed no compensatory effect by opposite isoform upregulation, and found similar fractions of total phosphorylated Erk1/2 across this epi-allelic series in response to both anti-CD3 and peptide-MHC stimulation of TCR. Moreover, a previous prediction of an isoform-independent linear relationship between Erk activation and IL-2 production was confirmed. The effect of the shRNA-mediated knockdowns in reducing IL-2 production was observed to be stronger than that arising from pharmacological MEK inhibition at comparable degrees of ERK1/2 phosphorylation levels.
Keywords: Signal transduction, cell activation, T cells, shRNA
INTRODUCTION
T cell receptor (TCR) ligation and cross-linking is known to result in the activation of Erk1/2 (MAPK1/2). Erk activation has been shown to be necessary for Jun and Fos complex to form the AP-1 transcription factor. AP-1 along with other transcription factors, binds to the IL-2 promoter resulting in IL-2 production (2–4).
Erk dynamics have been implicated in significantly helping the regulation of T cell phenotypic behaviors. Sustained versus transient Erk dynamics have been observed in naïve T lymphocytes, corresponding to positive and negative selection, respectively (5). Furthermore, the duration of Erk signaling appears to influence CD4+CD8+ lineage commitment (6). Multiple mechanisms have been postulated to generate these differences in Erk dynamics. Recently, a positive feedback loop in which Erk phosphorylates Lck in a regulatory manner to prevent SHP-1 deactivation of Lck was shown to sustain TCR signaling (7). A mathematical modeling effort has demonstrated how the balance between this novel Erk positive feedback and negative feedback via phosphatase-mediated deactivation of molecules proximal to the TCR allows for sensitivity in ligand discrimination (8). A downstream positive feedback loop between Erk-mediated immediate early gene (9) expression and Erk has also been described (10). The dynamic integration of multiple feedbacks both upstream and downstream may yield a strong dependence of T cell responses to Erk activity. To this point, we have recently experimentally validated a computational model prediction of a linear relationship between Erk activation and IL-2 production in response to peptide-MHC stimulation of TCR signaling (11).
In the midst of increasingly intense focus on MAPK activation mechanisms broadly (10), the potentially independent roles of Erk1 and Erk2 in T cell signaling are open to question. Our recent data-driven statistical model determined how multiple kinase signals distal to the TCR and upstream of IL-2 promoter elements integrate to influence IL-2 production in response to peptide-MHC stimulation. This model indicated an especially strong dependence on Erk1 and Erk2 -- in quantitative combination with other key kinase pathway signals such as Akt -- for successful prediction of the effects of PLP peptides possessing varying TCR avidities for the 1B6 TCR (11). The model elucidated similar correlations of Erk1 and Erk2 to IL-2 production from an experimental data compendium of 1B6 hybridoma data.
These observations concerning Erk activation and function have lead us to the hypothesis that in our 1B6 system, IL-2 production in response to TCR stimulation may be highly sensitive to total Erk1/2 activation levels but with indistinguishable contributions from Erk1 versus Erk2. Studying the independent contributions of Erk1 and Erk2 in T cell signaling poses significant technical challenges. Clearly, it is important to vary the level of each isoform independently. In addition the ability to measure a cell response at multiple cellular concentrations of active isoform is also desirable for a quantitative understanding of the contributions of that isoform. Pharmacological inhibitors have the advantage that inhibitor doses can be varied, resulting in a range of active protein. One disadvantage of pharmacological inhibitors is that typically these cannot distinguish between the two isoforms because they act upon the common Erk upstream kinase, MEK. Traditional knockout experiments focusing on multiple aspects of development have been performed (12, 13, 14, 9). The disadvantage of these experiments is the difficulty of generating multiple levels of active protein.
Our approach takes advantage of the varying levels of efficiency in RNA interference between non-overlapping sequences for a target protein. We generated an epi-allelic series (15) of Erk1 and Erk2 stable shRNA T cell lines that resulted in a quantitative range of respective knockdown degrees in either isoform individually. These seven knockdown cell lines enabled rigorous examination of the relationship between total Erk, phospho-Erk, and IL-2 secretion, yielding a strong quantitative relationship between Erk, phospho-Erk and IL-2 production across the range of Erk1 and Erk2 knockdown levels, with Erk1 and Erk2 effects quantitatively similar as predicted.
METHODS
Generation of stable knockdown cell lines
Replication-deficient lentivirus was produced in 293FT packaging cells (Invitrogen) as described previously (16). The pLKO.1 vector and shRNA sequences were obtained from the RNAi Consortium (17). 48 hrs after infection, viral supernatants were collected, filtered through a 0.45 micrometer filter and used for infection immediately or stored at 4C for several days. 105 1B6 hybridoma cells were spin-infected with viral supernatant in 24 well-plates for 1.5 hrs at 2500 rpm at 30C in the presence of 8 microgram/ml polybrene. 4 hrs after infection, fresh media was added and cells were grown at 37C for 48 hrs. To eliminate uninfected cells, puromycin (2.5 microgram/ml) was added to the cells for additional 5 days. Cells were maintained in puromycin-containing media prior to stimulation. The entire infection process was repeated for the L144 peptide results such that independent infections for different batches of virus under different stimulation conditions are reported. One Erk2 shRNA virus did not yield viable 1B6 cells following puromycin selection during the repeat, therefore 8 lines were produced for the anti-CD3 results and 7 lines produced for the L144 peptide results.
Semi-quantitative western blotting
2×106 cells were resuspended in 200 ml media without FBS. For anti-CD3 stimulated samples, 2 μg/ml anti-CD3 (2C11, BD Biosciences) was added for 10 minutes at 37C. Cells were then lysed and boiled for 5 minutes in Laemmli reducing sample buffer. 10 μl of each sample was load per well. For L144 stimulated samples, 5×106 1B6 cells were added to DAS cell (18) seeded- L144 peptide coated- 6-well plates, centrifuged at 1000 rpm for 1 minute, and incubated for 10 minutes at 37C and lysed as previously described (11). 10 μg of protein was loaded per well. After electrophoresis, samples were transferred to polyvinylidene difluoride (PVDF) membrane and blocked with 3% BSA in TBST. Blots were visualized and quantified using a Kodak Image Station 1000. The net intensity of individual bands was determined. Anti-total and anti-phospho p44/42 (Thr202/Tyr204) antibody was from Cell Signaling Technologies, and anti-rabbit HRP from Santa Cruz Biotechnology.
To determine the level of protein knockdown for each cell line, the intensity of each Erk band was first normalized to its tubulin control. This normalization accounts for differences in protein loading from one sample to another. To account for differences in intensity across multiple membranes, both Erk bands across a single membrane are then expressed as fold change relative to the Luciferase control band. The standard deviations reported are representative of both biological and experimental replicates.
IL-2 ELISA
Supernatant was collected from 100,000 1B6 cells stimulated for 4 hours on DAS cell (18)-seeded and L144 peptide-coated 24-well plates analyzed using quantitative sandwich ELISA for mouse IL-2 (BD Biosciences).
RESULTS AND DISCUSSION
A range of stable Erk1 and Erk2 knockdown cell lines can be used as a tuning tool for quantitatively modulating the TCR activation pathway
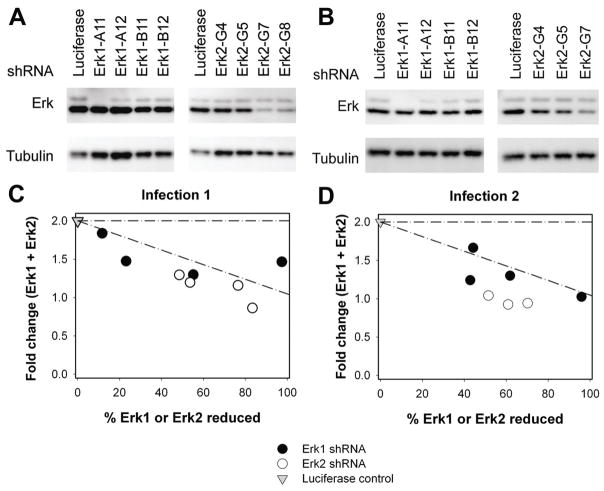
To determine whether total Erk levels could be modulated using established shRNA techniques, four unique sequences in Erk1 and four unique sequences in Erk2 mRNA transcripts were targeted for knockdown in a mouse 1B6 T cell hybridoma, generating an epi-allelic series for both Erk1 and Erk2. Stable cell lines were generated for each shRNA using lenti-viral infections and puromycin selection. As a non-specific control, stable cell lines were generated by infection with shRNAs targeting Luciferase. The level of total protein knockdown for each sequence was determined by western blotting for two separate infections (Figure 1A, B). The fold change in Erk levels relative to Luciferase control reported in Table I are averages of three experimental replicates for each infection. Normalization allows for comparison of multiple experimental replicates by expressing band intensities in knockdown cell lines as fraction of luciferase control.
Figure 1. Generation of a range of stable knockdown cell lines.
(A, B) Total Erk Western blot of the knockdown cell lines under two separate infection A) infection 1 and B) infection 2. Band intensities shown are normalized as described in text. (C, D) Sum of Erk1 and Erk2 is plotted versus the fraction of either Erk1 in an Erk1 knockdown or Erk2 in an Erk2 knockdown expressed as fold change from luciferase control for (C) infection 1 and (D) infection 2. In the case of complete compensation by one isoform for the decreased levels of another, a horizontal line at y = 2 is expected.
TABLE I. Measured levels of total and phospho Erk in knockdown cell lines.
A summary of the values used in the figures +/− standard deviation.
| Anti-CD3 | L144 peptide | |||||||
|---|---|---|---|---|---|---|---|---|
| Cell line | Erk1 | Erk2 | Erk1phos | Erk2phos | Erk1 | Erk2 | Erk1phos | Erk2phos |
| Erk1-A11 | 0.04±0.04 | 0.77±0.13 | 0.07±0.06 | 1.19±0.36 | 0.04±0.05 | 0.98±0.19 | 0.24±0.08 | 0.79±0.23 |
| Erk1-A12 | 0.45±0.18 | 0.85±0.17 | 0.57±0.28 | 1.58±0.46 | 0.56±0.29 | 1.11±0.13 | 0.50±0.13 | 1.05±0.28 |
| Erk1-B11 | 0.77±0.47 | 0.71±0.10 | 0.33±0.22 | 1.15±0.41 | 0.38±0.09 | 0.92±0.20 | 0.82±0.13 | 0.97±0.23 |
| Erk1-B12 | 0.88±0.21 | 0.96±0.40 | 0.78±0.24 | 1.29±0.31 | 0.57±0.27 | 0.67±0.13 | 0.95±0.50 | 0.83±0.38 |
| Erk2-G4 | 0.73±0.10 | 0.46±0.04 | 1.28* | 1.01±0.40 | 0.56±0.23 | 0.48±0.08 | 0.74±0.63 | 0.53±0.18 |
| Erk2-G5 | 0.78±0.26 | 0.51±0.05 | 0.93* | 0.40±0.29 | 0.53±0.13 | 0.39±0.04 | 0.96±0.58 | 0.46±0.28 |
| Erk2-G7 | 1.02±0.36 | 0.15±0.03 | 0.59* | 0.31±0.06 | 0.64±0.16 | 0.30±0.04 | 0.59±0.24 | 0.35±0.20 |
| Erk2-G8 | 0.92±0.31 | 0.24±0.04 | 1.19* | 0.40±0.27 | N/A | N/A | N/A | N/A |
replicate measurements not made
A quantitative range of knockdown levels was generated for both Erk1 and Erk2 individually, as seen in Figure 1. These results demonstrate our ability to generate a spectrum of stable knockdowns using shRNA constructs possessing diverse target sequences. The slight differences in knockdown levels seen in Figure 1A, B are likely due to differences in virus titer during each infection.
No compensation occurs between Erk isoforms due to knockdown
To determine whether one Erk isoform was being up-regulated in the presence of an shRNA to the other Erk isoform, the level of the knockdown isoform was plotted against the total cumulative level of both Erk1 and Erk2 (Figure 1C, D). We evaluated the degree of compensation of one isoform by another by probing for deviation from a linear relationship between total Erk1+Erk2 and the percentage reduced of a given isoform. Complete compensation would be delineated by a horizontal line at fold change from luciferase control (Erk1 + Erk2) = 2. A lack of compensation would be delineated by a perfect line drawn from the luciferase control at the ordinate intercept to fold change from luciferase control (Erk1 + Erk2) = 1 at 100% knockdown for one isoform. Although slight differences can be noted between the anti-CD3 and peptide stimulation conditions, significant compensation between isoforms was not found in the 1B6 cell line.
IL-2 production is dependent on total Erk level
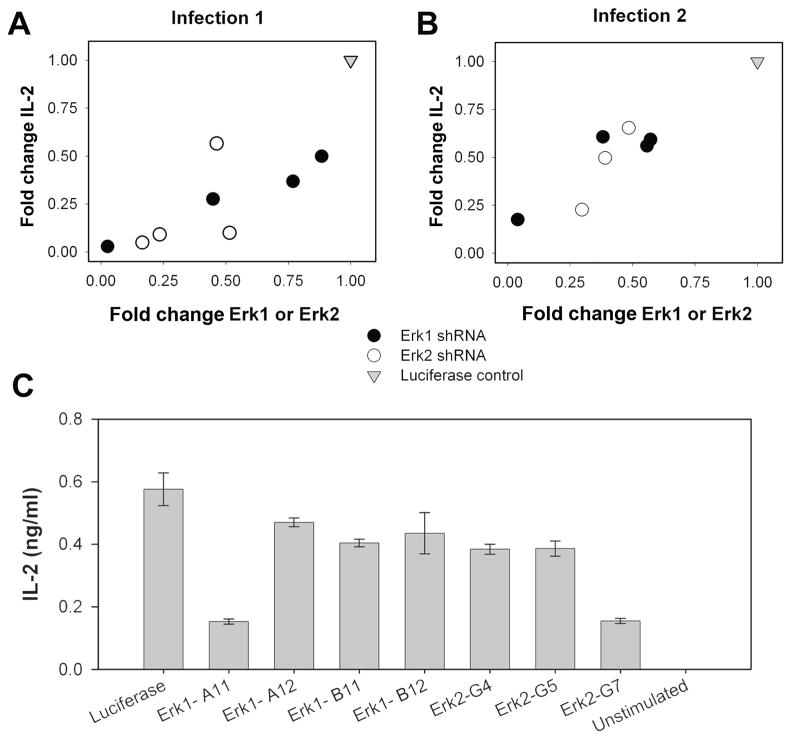
To determine whether there is a direct correlation between total Erk levels and IL-2 production in response to TCR stimulation, IL-2 production in response to an altered peptide ligand of PLP, L144, was measured. L144 peptide presentation to 1B6 T cells was previously described in (11). IL-2 production by 1B6 Erk1/Erk2 knockdown cell lines was measured four hours after peptide stimulation by ELISA (Figure 2C). Both Erk1 and Erk2 levels were correlated for the two infections in a similar fashion with IL-2 production for L144 peptide stimulation (Figure 2A, B). The correlation coefficients for total levels of knockdown Erk with IL-2 production for the two infections are 0.83 and 0.93, respectively (Table 2). This suggests that IL-2 production is related to Erk levels, and demonstrates that IL-2 production can be modulated by varying the level of cellular Erk protein via either isoform.
Figure 2. IL-2 production is dependent on total Erk protein level.
IL-2 levels following stimulation by for 4 hours in (A) infection 1 and (B) infection 2, expressed as fold change relative to Luciferase control. IL-2 levels are plotted versus total Erk1 levels in an Erk1 knockdown or total Erk2 in an Erk2 knockdown. (C) Representative experiment of absolute values of IL-2 produced by 4 μg/ml L144 for 4 hours; units are in ng/ml.
Table II. Correlation Coefficients for select plots.
Phospho-Erk levels are related to total Erk levels
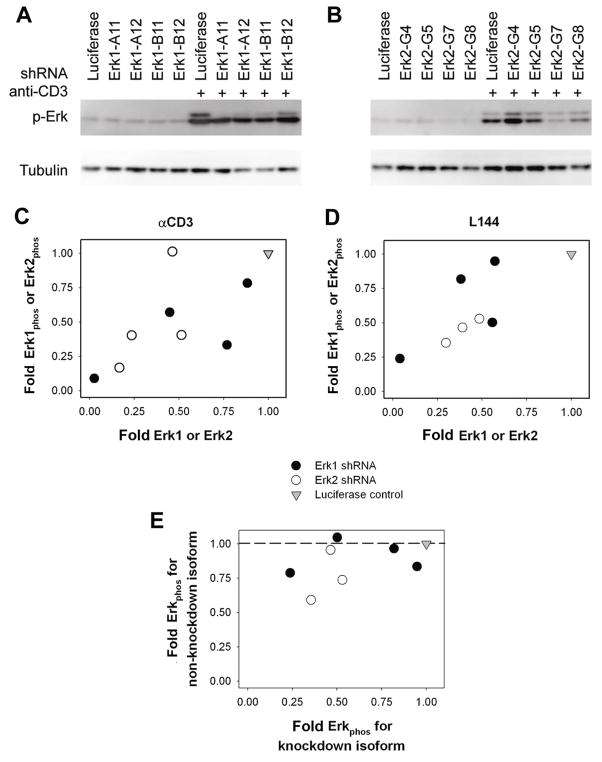
To determine the relationship between an Erk knockdown and its associated phospho-Erk levels upon stimulation, stable knockdown cell lines were stimulated using anti-CD3, or L144 peptide loaded DAS cells and phospho-Erk levels measured via immunoblotting then normalized and depicted as fold change from Luciferase control as described above (Figure 3A, B). By comparing total-Erk levels to phospho-Erk levels in these knockdown cell lines (Figure 3C, D), we obtain a positive correlation between the two measurements (Table 2), indicating that a constant fraction of the Erk1 and Erk2 present in these cells is activated in response to TCR stimulation. The observation that phosphorylated fractions of Erk in knockdown cell lines are constant is consistent with the idea that MEK, the upstream Erk kinase, distinguishes between its substrate ERK isoforms. In addition, the amount of phosphorylation of the knockdown isoform compared to the non-targeted isoform (Figure 3E) shows a lack a compensation at the phosphorylation level, consistent with the lack of compensation in total protein (Figure 1C, D).
Figure 3. Phospho-Erk levels are related to total Erk levels.
(A, B) phospho-Erk levels as measured by Western blot of the knockdown cell lines following 4 hours stimulation by (A) 2 μg/ml anti-CD3 for infection 1 and (B) 4 μg/mL L144 peptide for infection 2. Band intensities shown are normalized and depicted as described in text. Standard deviations reported include both experimental and biological replicates.
(C, D) phospho-Erk1 level is plotted versus total Erk1 level for Erk1 knockdown and phospho-Erk2 level is plotted versus total Erk2 level for Erk2 knockdown for 4 hours stimulation by (C) 2 μg/ml anti-CD3 and (D) 4 μg/mL L144 peptide. Levels are expressed as fold change with respect to luciferase control.
(E) Phosphorylation levels of the knockdown Erk isoform versus the phosphorylation levels of the untargeted isoform. In the case of complete compensation by one isoform for the decreased levels of another, a horizontal line at 1 would be expected.
Individual Erk isoforms influence IL-2 production to similar degrees
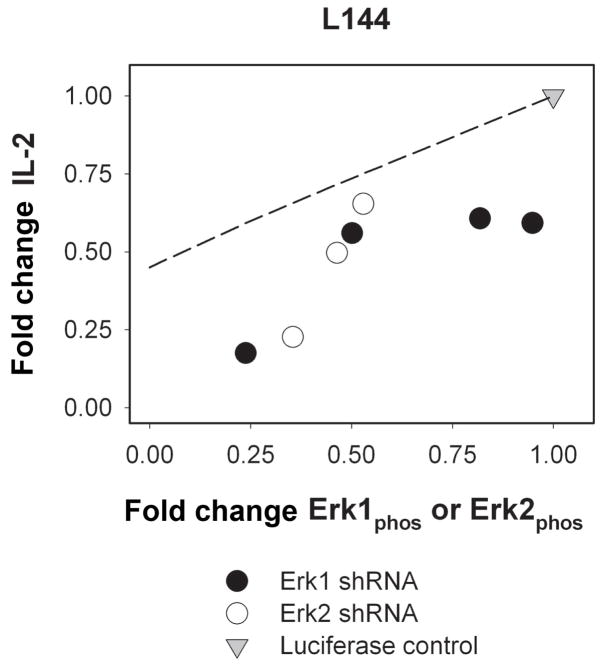
Because phospho-Erk levels might be expected to be more proximally predictive than total Erk protein levels of IL-2 production, we tested the relationship between phospho-Erk levels and IL-2 following TCR stimulation by the L144 peptide (Figure 4, Table 2). Similarly to the case for total Erk levels, IL-2 production appears to strongly depend on both phospho-Erk1 and Erk2 levels, indicating that IL-2 levels can be quantitatively modulated through Erk activation. Moreover, the quantitative dependencies on phospho-Erk1 and phospho-Erk2 appear to be essentially indistinguishable yet both impact IL-2 to a greater extent than would be predicted for redundant isoforms. Therefore, it is possible that each isoform is necessary and contributes uniquely to IL-2 promotion. Interestingly, Erk1 and Erk2 knockout mice exhibit different phenotypes (12), and conditional Erk2 knockouts have more severe T cell defects than Erk1 knockout mice (13), indicating that individual Erk isoforms might indeed be important in various cellular contexts.
Figure 4. IL-2 production is dependent on phospho-Erk level, with Erk1 and Erk2 yielding similar influences.
IL-2 production versus phospho-Erk1 levels in an Erk1 knockdown or phospho-Erk2 in an Erk2 knockdown are shown for 4 μg/ml L144 peptide. IL-2 measurements were made at 4 hours post-stimulation and phosphorylation was quantified 10 minutes post-stimulation. All measurements are expressed as fold change with respect to the luciferase control. The broken line denotes the effect of phospho-Erk diminution by the upstream MEK inhibitor PD98059 as found in our previous work (11).
Finally, the dashed line in Figure 4 denotes the effect of phospho-Erk diminution by means of the small-molecule MEK inhibitor PD98059, as found in our earlier work (11). Clearly, shRNA knockdown of Erk appears to lead to a greater reduction in IL-2 production than did pharmacological inhibition of the upstream kinase MEK, for equivalent degree of phospho-Erk diminution. This raises an intriguing aspect of comparing reduced protein levels to pharmacological inhibition in that Erk protein may facilitate signal transduction by more than its activity alone; the greater reduction in IL-2 for the RNAi conditions may be indicative of Erk being used as part of macromolecular complexes for other signaling reactions. Another difference between the pharmacological condition and the shRNA cell lines is the perturbation in both isoforms versus the effects of one isoform; however, reduction of the levels of both isoforms simultaneously could not be tested due to loss of cell viability for Erk1/Erk2 double knockdown cell lines.
Concluding note
By generating an epi-allelic series, an approach previously shown useful for determining the effect of knocking down protein levels to various extents on phenotypic outcomes (15), we have demonstrated here that an incomplete kinase knockdown can yield information consistent with the level to which the kinase is knocked-down. It remains to be seen whether these results are upheld for different proteins in multiple systems. As ever-increasing numbers of researchers in immunology and other fields of biology rely on incomplete knockdown methods along the lines we describe here, our work here may provide a helpful framework for quantitative analysis and interpretation.
Footnotes
Funding for this work was provided by grants from the National Institutes of Health (NIGMS Cell Decision Processes Center P50-GM68762 and NIAID R01-AI65824) and the Cambridge-MIT Institute.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Priatel JJ, Teh SJ, Dower NA, Stone JC, Teh HS. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity. 2002;17:617–627. doi: 10.1016/s1074-7613(02)00451-x. [DOI] [PubMed] [Google Scholar]
- 2.Whitehurst CE, Geppert TD. MEK1 and the extracellular signal-regulated kinases are required for the stimulation of IL-2 gene transcription in T cells. J Immunol. 1996;156:1020–1029. [PubMed] [Google Scholar]
- 3.Li QJ, Dinner AR, Qi S, Irvine DJ, Huppa JB, Davis MM, Chakraborty AK. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nat Immunol. 2004;5:791–799. doi: 10.1038/ni1095. [DOI] [PubMed] [Google Scholar]
- 4.Dumont FJ, Staruch MJ, Fischer P, DaSilva C, Camacho R. Inhibition of T cell activation by pharmacologic disruption of the MEK1/ERK MAP kinase or calcineurin signaling pathways results in differential modulation of cytokine production. J Immunol. 1998;160:2579–2589. [PubMed] [Google Scholar]
- 5.Werlen G, Hausmann B, Palmer E. A motif in the alphabeta T-cell receptor controls positive selection by modulating ERK activity. Nature. 2000;406:422–426. doi: 10.1038/35019094. [DOI] [PubMed] [Google Scholar]
- 6.Nishida T, Matsuki Y, Ono T, Oguma T, Tsujimoto K, Sato M, Tadakuma T. The novel murine CD4+CD8+ thymocyte cell line exhibits lineage commitment into both CD4+ and CD8+ T cells by altering the intensity and the duration of anti-CD3 stimulation in vitro. J Immunol. 2004;172:6634–6641. doi: 10.4049/jimmunol.172.11.6634. [DOI] [PubMed] [Google Scholar]
- 7.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 8.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 2005;3:e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 11.Kemp ML, Wille L, Lewis CL, Nicholson LB, Lauffenburger DA. Network Signal Combinations Downstream of T Cell Receptor Activation Predict IL-2 Response. 2006 doi: 10.4049/jimmunol.178.8.4984. Submitted. [DOI] [PubMed] [Google Scholar]
- 12.Saba-El-Leil MK, Vella FD, Vernay B, Voisin L, Chen L, Labrecque N, Ang SL, Meloche S. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4:964–968. doi: 10.1038/sj.embor.embor939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pages G, Guerin S, Grall D, Bonino F, Smith A, Anjuere F, Auberger P, Pouyssegur J. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal A, Dillon S, Denning TL, Pulendran B. ERK1−/− Mice Exhibit Th1 Cell Polarization and Increased Susceptibility to Experimental Autoimmune Encephalomyelitis. The Journal of Immunology. 2006;176:5788–5796. doi: 10.4049/jimmunol.176.10.5788. [DOI] [PubMed] [Google Scholar]
- 15.Hemann MT, Fridman JS, Zilfou JT, Hernando E, Paddison PJ, Cordon-Cardo C, Hannon GJ, Lowe SW. An epi-allelic series of p53 hypomorphs created by stable RNAi produces distinct tumor phenotypes in vivo. Nat Genet. 2003;33:396–400. doi: 10.1038/ng1091. [DOI] [PubMed] [Google Scholar]
- 16.Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 17.Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, Carpenter AE, Foo SY, Stewart SA, Stockwell BR, Hacohen N, Hahn WC, Lander ES, Sabatini DM, Root DE. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 2006;124:1283–1298. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Munder M, Bettelli E, Monney L, Slavik JM, Nicholson LB, Kuchroo VK. Reduced self-reactivity of an autoreactive T cell after activation with cross-reactive non-self-ligand. J Exp Med. 2002;196:1151–1162. doi: 10.1084/jem.20020390. [DOI] [PMC free article] [PubMed] [Google Scholar]