Abstract
Schistosomiasis is an important parasitic disease for which there is no available vaccine. We have focused on a functionally important antigen of Schistosoma mansoni, Sm-p80, as a vaccine candidate because of its consistent immunogenicity, protective potential and antifecundity effect observed in murine models; and for its pivotal role in the immune evasion process. In the present study we report that a Sm-p80-based DNA vaccine formulation confers 38% reduction in worm burden in a nonhuman primate model, the baboon (Papio anubis). Animals immunized with Sm-p80-pcDNA3 exhibited a decrease in egg production by 32%. Sm-p80 DNA elicited specific immune responses that include IgG; its subtypes IgG1 and IgG2; and IgM in vaccinated animals. Peripheral blood mononuclear cells (PMBCs) from immunized animals when stimulated in vitro with Sm-p80 produced appreciably more Th1 response enhancing cytokines (IL-2, IFN-γ) than Th2 response enhancing cytokines (IL-4, IL-10). PBMCs produced appreciably more spot forming units for INF-γ than for IL-4 in enzyme-linked immunosorbent spot (ELISPOT) assays. Overall it appears that even though a mixed (Th1/Th2) type of humoral antibody response was generated following immunization with Sm-p80; the dominant protective immune response is Th1 type. These data reinforce the potential of Sm-p80 as an excellent vaccine candidate for schistosomiasis.
Keywords: DNA vaccination, Schistosomiasis, Schistosoma mansoni, Sm-p80, Calpain, nonhuman primate, baboons
1. Introduction
Presently there is no vaccine available to control the human parasitic disease, schistosomiasis. This disease afflicts an estimated 207 million people in 74 countries [1] and an additional 779 million people are at risk of acquiring this infection [2]. Treatment with praziquental remains the mainstay of current medical treatment following infection. Therefore advent of a schistosomiasis vaccine would be a significant addition to current methods of the control of this disease.
A major problem that has hindered schistosomiasis vaccine research and development concerns the identification and selection of potential protective antigens encoded by the parasite [3–5]. We have identified a novel schistosome protein that was originally determined to be involved in the surface membrane biogenesis [6;7]. This represents a mechanism of immune evasion utilized by hemo-helminths to evade the protective host immune response [6;8–10]. This novel protein, calpain, has two subunits, the larger of which, Sm-p80, was shown in our previous studies [9;11–15] and of other investigators [16–19] to have a great potential as relevant vaccine antigen for the reduction of the morbidity associated with both Schistosoma mansoni [4] and S. japonicum [17] because of its pronounced antifecundity and anti-worm effects in mice. In the present study we have examined the prophylactic and antifecundity efficacy of a Sm-p80 based DNA vaccine formulation against S. mansoni in a nonhuman primate model. We have utilized baboons to evaluate safety, immunogenicity, protective and antifecundity immune responses in an animal model because this model is more predictive of the human situation [4;20]. This proof of concept study, along with other ongoing work, was designed to serve as the basis for phase I/II human clinical trials.
2. Materials and methods
2.1. Parasites and animals
Biomphalaria glabrata snails, infected with Schistosoma mansoni (Puerto Rican strain) were obtained from the National Institute of Allergy and Infectious Disease Schistosomiasis Resource Center (Biomedical Research Institute, Rockville, MD). Sexually immature baboons (Papio anubis), 1.5 to 2.4 years old, weighing from 5 to 7.7 kilograms, were obtained from the University of Oklahoma Health Sciences Center baboon breeding program and housed in single cages in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited facilities. Baboons were first screened for parasites and for antibodies that were cross-reactive to Sm-p80 before immunization, and were found negative for both. This study was approved by the Institutional Animal Care and Use Committee.
2.2. Naked DNA-vaccine constructs and confirmation of protein expression in COS-7 and CHO K1 cells
Full length cDNA of the large subunit of S. mansoni calpain (Sm-p80) was subcloned into pcDNA3 (Invitrogen Corporation, San Diego, CA) and designated as Sm-p80-pcDNA3, as described earlier [14;15;21;22]. High level expression of Sm-p80-pcDNA3 was ascertained via transient tranfection in CHO K1 [14;15;21;22] and COS-7 cells. The expressed product in COS-7 and CHO K1 cells was analyzed via polyacrylamide gel electrophoresis and western blotting, as described previously [14;21]. For DNA immunization, plasmid DNA was isolated via conventional alkaline lysis method. The plasmid DNA was further purified on Sepharose CL4B columns. The purified DNA was then ethanol precipitated and resuspended in sterile, endotoxin-free saline.
2.3. Baboon immunizations, parasite challenge, worm burden determination and egg counts
Six baboons (3 males and 3 females) were initially immunized with 500 μg Sm-p80-pcDNA3 (prepared in PBS) [22]. Baboons were boosted with 500 μg Sm-p80-pcDNA3 at weeks 4, 8, and 12. For the control group, three baboons (2 males and 1 female) were vaccinated with 500 μg of the control plasmid DNA, pcDNA3 (prepared in PBS) [22]. Baboons in the control group were boosted with 500 μg pcDNA3 at weeks 4, 8, and 12. In both groups, DNA was injected intramuscularly in the quadriceps. At week 16, baboons from both of the groups were challenged with a total of 1000 cercariae each of S. mansoni essentially as provided [23] except that an axillary rather than a groin pouch was used as the cercarial exposure site for each animal. Eight weeks after the challenge, the baboons were immobilized and lightly anesthetized with a mixture of ketamine (Ketaminol – 10 mg/kg body wt) and xylazine (0.5 mg/kg) and then were deeply anesthetized by intravenous injection of heparinized sodium pentobarbital solution. The animals were then euthanized by exsanguination from the heart ventricle. The adult parasites were recovered by perfusion from the mesenteric vasculature and hepatic portal system[24]. Protection (P) was calculated by comparing worm burdens from immunized (I) and control (C) baboons by a standard formula: %P = [(C-I)/C x 100]. After sacrifice, liver and intestine samples were collected, and following digestion in KOH [25], the number of eggs present in each tissue was determined and percent reduction in egg production was calculated.
2.4. Blood collection and peripheral blood mononuclear cell (PBMC) isolation
Blood samples from baboons were collected just prior to the primary immunization, at every booster (i.e., 4, 8 and 12 weeks) and 4 weeks after the final immunization, i.e., before challenge (16 weeks). Sera collected from these bleeds were used in ELISA assays. PBMCs were isolated from baboon blood using HISTOPAQUE®-1077 (Sigma-Aldrich, St. Louis, MO).
2.5. ELISA
Serum samples from each individual animal were used to determine antibody levels/titers for IgG, IgG subtypes (IgG1, IgG2, IgG3, IgG4), IgM, IgA and IgE antibodies as described elsewhere [22;26]. Briefly, 96 well microtiter plates were coated with recombinant Sm-p80 (1.2 μg/well). Various dilutions of individual baboon serum was used and as secondary antibodies, horseradish peroxidase labeled anti-monkey (IgG and IgM) and anti-human IgG1, IgG2, IgG3, IgG4 (Alpha Diagnostic International, San Antonio, TX, USA), IgA and IgE (Sigma-Aldrich, St. Louis, USA) were used at a dilution of 1:1000. All of the samples were assayed in triplicate. Results are expressed as endpoint titers calculated from a curve of optical density verses serum dilution to a cutoff of 2 standard deviations above background control values. Results are expressed as the mean ± S.E.
2.6. PBMC proliferation assays
PBMCs from the two groups of baboons were isolated by density gradient centrifugation using HISTOPAQUE®-1077 (Sigma-Aldrich, St. Louis, MO). For the in vitro proliferation assays, recombinant protein and incubation period was first optimized. A standard assay was then developed which was as follows: in a 96-well flat-bottom plate, PBMCs (5×105cells/200 μl/well) were stimulated with either 0.5 μg ConA or 1.2 μg recombinant Sm-p80 and incubated at 37°C with 5% CO2. After 48h incubation, 100μl supernatant was removed for estimation of cytokine production and to the remainder, 20μl of MTT [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added and incubated for another 4h at 37°C. The contents of the microtiter plates were centrifuged at 1000 g for 10 min and the supernatant was aspirated gently and discarded. In order to dissolve the formazan salt crystals, 100μl of DMSO was loaded into each well and incubated for 30 min at 37°C. The microtiter plates were then read at 550 nm. Stimulation index was calculated as the ratio of OD550nm of stimulated cells to OD550nm of non-stimulated cells.
2.7. Estimation of cytokine production by proliferating PBMCs
To quantitate the cytokine production (IL-2, IL-4, IL-10 and IFN-γ) by the proliferating PBMCs, the medium collected above was used in a Baboon Th1/Th2 ELISA Panel Kit (U-cyTech, The Netherlands), following the manufacturer’s instructions.
2.8. ELISPOT
ELISPOT assay was used to estimate IFN-γ and IL-4 secreting cells following in vitro stimulation with recombinant Sm-p80. Briefly, PBMCs from the two groups of baboons were seeded (3×105cells/100μl/well) on the 96 well pre-coated (anti-IFN-γ or anti-IL-4, U-cyTech, The Netherlands). The cells were stimulated with either 0.5 μg ConA or 1.2 μg recombinant Sm-p80 and incubated at 37°C with 5% CO2 for 48 h, allowing for production and capture of released cytokines. One hundred μl of biotinylated detection antibody was loaded to each well and incubated overnight at 4 °C. The next day GABA(ϕ-labelled anti-biotin antibodies) was added and after 1h incubation at 37°C, freshly prepared Activator I/II solution was added and incubated for 20–30 min at room temperature to develop the spots. The reaction was terminated by washing with distilled water. All assays were run in triplicate. The spot-forming units (SFU) representing single cells were counted using a ELISPOT Bioreader 5000 (ImmunoBioSystem, The Colony, TX, USA). Antigen-specific SFU per well was calculated by subtracting its individual background value (buffer control well without antigen).
2.9. Statistical analyses
Significance between two groups was calculated via one-way ANOVA and in group significance by paired t-test, using the SPSS computer program. Bonferroni adjustments were included for multiple comparisons, to reduce the risk of reaching false conclusions based on chance. P values obtained by these methods were considered significant if they were <0.05.
3. Results
3.1. Expression of Sm-p80-pcDNA3 in COS-7 and CHO K1 cells
The construct used in vaccination experiments (Sm-p80-pcDNA3) was examined for the protein expression in COS-7 and CHO K1 cells. Expression of Sm-p80 was detected via western blotting using an anti-Sm-p80 antibody. A discrete 80 kDa bands was detected in lysates of COS-7 (Fig 1; Lane A) and CHO K1 cells (Fig 1; Lane B) transiently transfected with Sm-p80-pcDNA3 (Fig 1, lane B). No such band was detected in COS-7 or CHO K1 cells transfected with pcDNA3 alone (data not shown).
Figure 1.
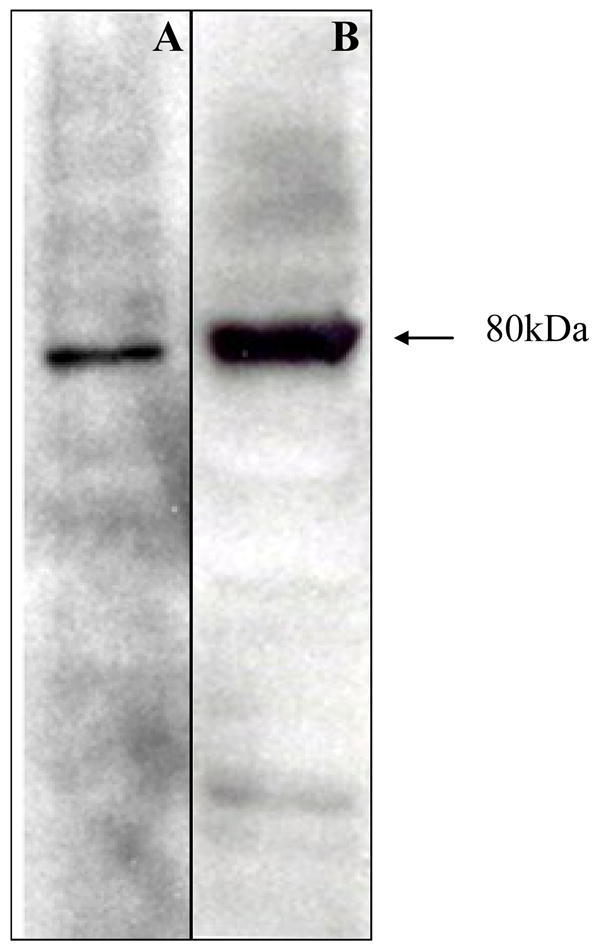
Eukaryotic expression of Sm-p80 in COS-7 and in CHO K1 cells. Western blot of Sm-p80 obtained following transient transfection of the DNA construct, Sm-p80-pcDNA3, in COS-7 cells (lane A) and CHO K1 cells (lane B). This Sm-p80-pcDNA3 construct was used in all of the immunization experiments.
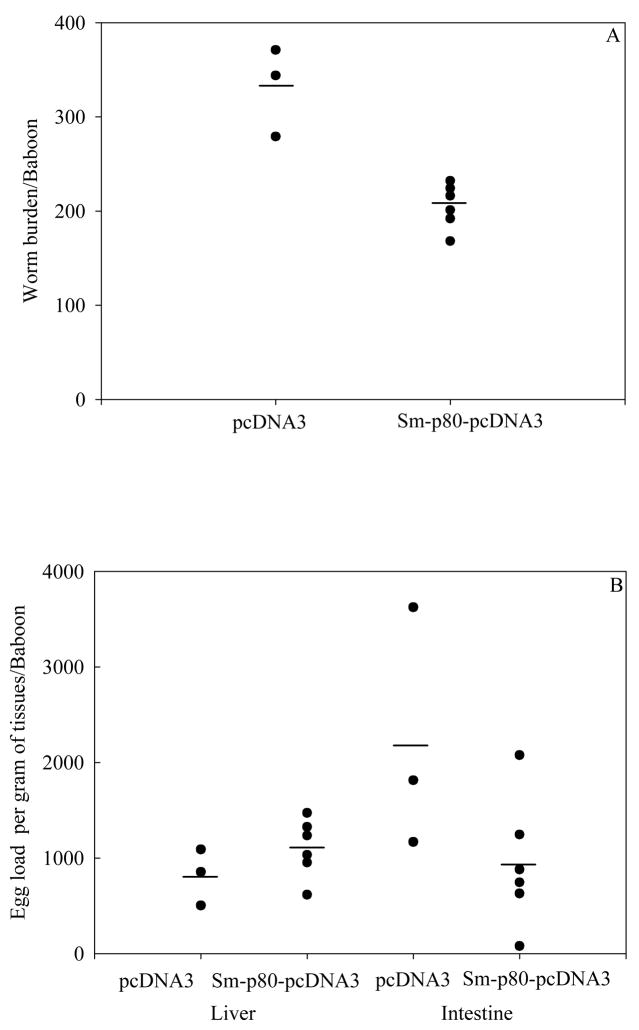
3.2. Reduction in worm burden and in egg production achieved in baboons following vaccination with Sm-p80 in naked DNA immunization protocol
In this study, baboons did not show any difficulty in (i) eating, drinking, urinating, or defecating; (ii) they were alert and responsive; (iii) showed no signs of swelling, hyperemia, indurations at or around the injection site; (iv) no neurological/motor problems were observed. Furthermore, no anti-DNA antibodies were detected in immunized animals.
Using a vaccination strategy that involved priming with plasmid DNA via intramuscular injections and boosting intramuscularly three times at monthly intervals, the protective potential of Sm-p80 was determined. Baboons immunized with Sm-p80-pcDNA3 (three boosts) showed 38% reduction in worm burden when compared with baboons that received only control pcDNA3 (Fig 1; Table 1). Additionally, in the control group of baboons, the breakup of worm types was found to be as follows: males (25.95%), females (11.48%), paired worms (52.91%) and immature worms (9.65%). Whereas in the experimental group, the worm count was as follows: males (30.25%), females (13.94%), paired worms (50.12%) and immature worms (5.66%).
Table 1.
Anti-worm and anti-fecundity effect in baboons following immunization with Sm-p80-pcDNA3
| Immunization Group | n | Worm Burden (Mean ± SE) | Number of eggs/g Liver (Mean ± S.E) | Number of eggs/g Intestine (Mean ± S.E) | % Reduction in worm burden (P<0.05) | % Reduction in egg production (Liver + intestine) (P < 0.05) |
|---|---|---|---|---|---|---|
| pcDNA3 | 3 | 331.33 ± 47.28 | 814.89 ± 170.47 | 2201.12 ± 735.07 | 38%* | - |
| Sm-p80-pcDNA3 | 6 | 205.5 ± 22.74 | 1104.60 ± 129.37 | 941.46 ± 247.95 | - | 32%* |
Antifecundity effect of this vaccine regimen was also found to be pronounced (Fig. 2). Animals immunized with Sm-p80-pcDNA3 exhibited a decrease in egg production (liver + intestine) by 32% (Table 1). These differences in reduction of worm burden and egg counts between Sm-p80-pcDNA3 and control pcDNA3 groups were found to be statistically significant (p<0.05).
Figure 2.
Worm burden distribution (A) and egg load per gram of liver and intestine of individual baboon (B) for groups of animals immunized with control plasmids, pcDNA3 (n =3) and with Sm-p80-pcDNA3 (n =6). Both reduction in worm burden and in egg counts were statistically lower in vaccinated animals (*P< 0.05).
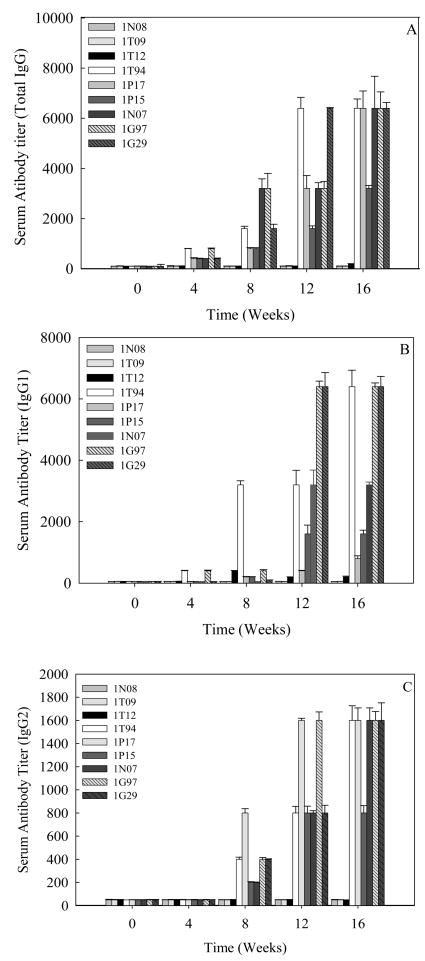
3.3 Protective antibody response to Sm-p80 in immunized baboons
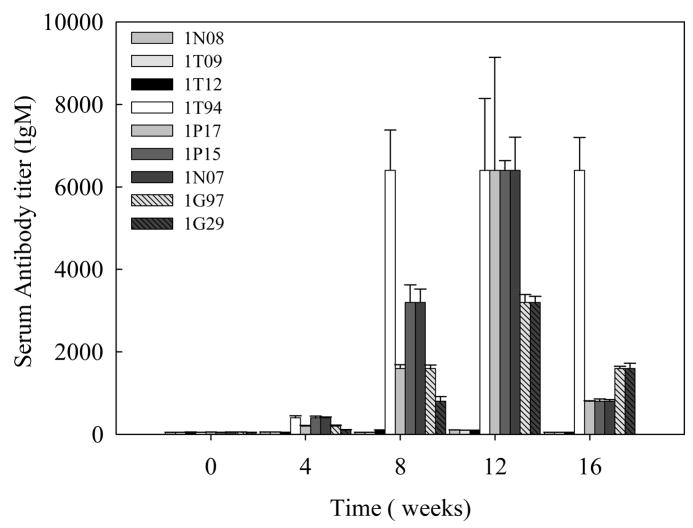
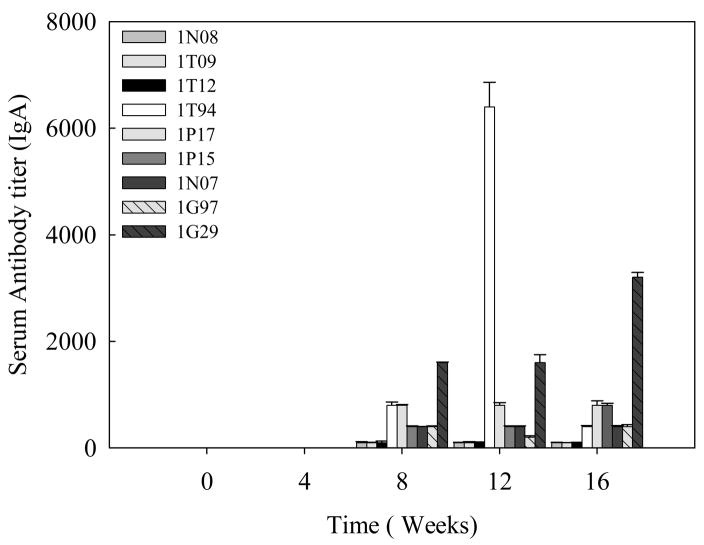
Specific antibody titers were obtained for the total IgG (Fig 3A) and its subtypes; IgG1 (Fig 3B) and IgG2 (Fig 3C) in the sera samples obtained from baboons immunized with Sm-p80-pcDNA3. IgG3 and IgG4 reactivities were not detected in any of the vaccinated animals. Also, no detectable levels of Sm-p80-specific antibodies (total IgG or IgG subtypes) were detected in the group of animals immunized with control plasmid DNA. Antibody titers for the total IgG in the Sm-p80-pcDNA3 group started to rise at week 4 and reached the highest level at week 12 for 2 animals and at week 16 for 4 animals (end point titer = 6400) (Fig 3A). In the Sm-p80-pcDNA3 group the highest level of antibodies were observed for IgG subtype, IgG1; its titer in the vaccinated group showed an increase at week 8 and levels reached a peak at week 12 and maintained at this high level (end point titer = 6400) in 50% of vaccinated animals until the cercarial challenge (Fig 3B). For IgG2 (Fig 3C) antibody levels gradually started to rise at week 8 and reached a peak at week 16 (end point titer = 1600). Similarly, in the vaccinated group, IgM titers had risen by week 8, peaked at week 12 and had decreased by week 16 (Fig 4). Weak to no antibodies titer were observed for specific IgA responses in almost all of the vaccinated animals except one (Fig 5). No IgE, IgG3 or IgG4 were detectable in vaccinated animals (data not shown).
Figure 3.
Antibody titers of anti-Sm-p80 total IgG and IgG subtypes in immunized baboons. ELISA was performed with sera from each baboon (every four weeks) in their respective groups (pcDNA3 and Sm-p80-pcDNA3). Total IgG (A), IgG1 (B), IgG2(C) in individual control (1N08, 1T09 and 1T12) and vaccinated (1T94, 1P17, 1P15, 1N07, 1G97 and 1G29) baboon sera collected every 4 weeks. The values represent the mean of three replicates ± standard error.
Figure 4.
Total IgM titers in individual control (1N08, 1T09 and 1T12) and vaccinated (1T94, 1P17, 1P15, 1N07, 1G97 and 1G29) baboon sera collected every 4 weeks. The values represent the mean of three replicates ± standard error.
Figure 5.
Total IgA titers in individual control (1N08, 1T09 and 1T12) and vaccinated (1T94, 1P17, 1P15, 1N07, 1G97 and 1G29) baboon sera collected every 4 weeks. The values represent the mean of three replicates ± standard error.
3.4. Sm-p80 induced protective T cell response in vaccinated baboons
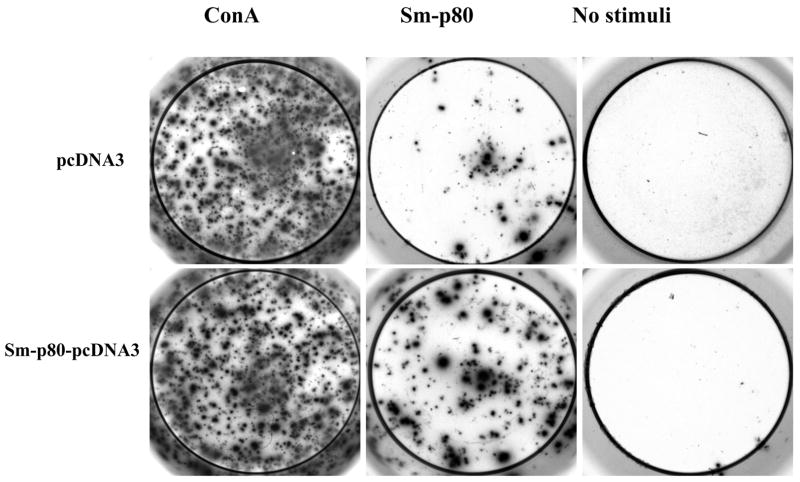
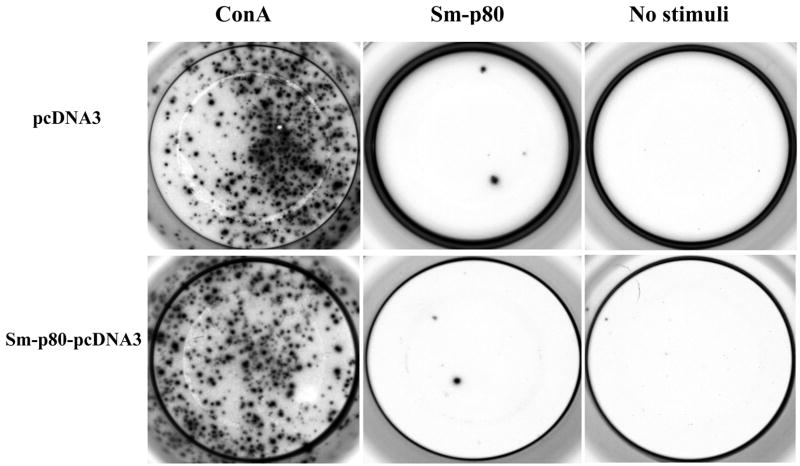
The PBMCs from the vaccinated Sm-p80-pcDNA3 group proliferated to significantly higher levels than compared to their respective control when stimulated in vitro with Sm-p80 recombinant protein (Table 2). However, Sm-p80 driven proliferation of PBMCs was markedly lower when compared to the stimulation induced by ConA (Table 2). Specifically, the PBMCs from the Sm-p80-pcDNA3 vaccinated group exhibited approximately 2-fold higher proliferation compared to their controls (P<0.004) (Table 2). As shown in Table 3, the high degree of proliferation was also correlated by the INF-γ andIL-2 production. IL-2 was produced at over 15-fold higher levels and INF-γ was produced at over 220-fold higher levels compared to their respective pcDNA immunized controls. IL-4 and IL-10 production by PBMCs was negligible in both groups (Table 3). These results were confirmed by ELISPOT analysis of the proliferating PBMCs in response to in vitro stimulation by Sm-p80. In these studies, approximately 65-fold to 200-fold more spot forming unit (SFU) were detected (Table 4) for INF-γ Fig 6, compared to IL-4 (Fig 7) in baboons vaccinated with Sm-p80-pcDNA3.
Table 2. In vitro.
proliferation of baboon PBMCs from control and vaccinated animals
| Baboon | Vaccine group | Stimulation index(SI) |
|
|---|---|---|---|
| ConA | * Sm-p80 | ||
| 1T12 | pcDNA3 | 2.41±0.67 | 0.98±0.02 |
| 1T09 | pcDNA3 | 8.69±6.24 | 1.00±0.17 |
| 1N08 | pcDNA3 | 7.65±0.01 | 0.72±0.34 |
| 1T94 | Sm-p80-pcDNA3 | 2.27±0.57 | 2.50±0.95 |
| 1N07 | Sm-p80-pcDNA3 | 6.13±3.91 | 1.63±0.52 |
| 1G29 | Sm-p80-pcDNA3 | 2.55±0.14 | 2.23±0.11 |
| 1P15 | Sm-p80-pcDNA3 | 3.20±1.88 | 2.09±1.95 |
| 1P17 | Sm-p80-pcDNA3 | 24.53±9.38 | 3.34±2.21 |
| 1G97 | Sm-p80-pcDNA3 | 3.05±0.07 | 1.44±0.05 |
P<0.01)
Table 3.
Levels of cytokines production by PBMCs after in vitro stimulation with recombinant Sm-p80 as measured by ELISA
| Cytokines | Groups |
|
|---|---|---|
| pcDNA3 (mean ± S.D.) | Sm-p80-pcDNA3 (mean ± S.D.) | |
| IL-4 (pg/mL) | 11.05±0.5 | 10.45±0.1 |
| IL-10 (pg/mL) | 2.67±0.6 | 3.66±0.3 |
| IL-2 (pg/mL) | 37.10±6.6 | 543.12±21.0* |
| IFN-γ (pg/mL) | 14.14±1.7 | 3088.64 ±64.0* |
P<0.04)
Table 4.
IFN-γ and IL-4 spot forming unit (SFU) following in vitro stimulation with recombinant Sm-p80
| Baboon | Age in years | sex | Vaccine group | IL-4 | * IFN-γ |
|---|---|---|---|---|---|
| 1T12 | 2.26 | male | pcDNA3 | 1.67±1.16 | 7.67±4.0 |
| 1T09 | 2.00 | male | pcDNA3 | 3.33±1.16 | 99.33±3.0 |
| 1N08 | 1.97 | female | pcDNA3 | 1.67±0.58 | 86.00±6.0 |
| 1T94 | 1.48 | male | Sm-p80-pcDNA3 | 4.00±1.00 | 192.00±11 |
| 1G29 | 2.37 | male | Sm-p80-pcDNA3 | 0.67±0.58 | 161.00±36 |
| 1P17 | 1.89 | male | Sm-p80-pcDNA3 | 2.67±0.58 | 256.33±14 |
| 1G97 | 2.19 | female | Sm-p80-pcDNA3 | 3.33±0.58 | 217.33±33 |
| 1N07 | 1.97 | female | Sm-p80-pcDNA3 | 0.00±0.00 | 65.67±2.0 |
| 1P15 | 1.90 | female | Sm-p80-pcDNA3 | 1.67 ±0.58 | 236.00±8.1 |
The values in the table represent mean ± S.D.;
P<0.03)
Figure 6.
Secretion of IFN-γ products by PBMC cells. Detection of IFN-γ secreting cells by ELISPOT assays in wells seeded with 3×105 cells.
Figure 7.
Secretion of IL-4 products by PBMC cells. Detection of IL-4 secreting cells by ELISPOT assays in wells seeded with 3×105 cells.
4. Discussion
It is widely accepted that human populations in endemic areas develop some degree of immune protection against schistosome infection [27–29]. Additionally, in mice, immunization with radiation-attenuated cercariae can result in up to 80% protection [30]. Furthermore many anti-helminth recombinant vaccines have successfully been used in veterinary practice [31;32]. Taken together these studies provide further credence to the rationale for the continual development and refinement of vaccine formulations against a complex metazoan parasite like S. mansoni.
Immunization regimens that induce immune recognition of functionally important host-interactive proteins in their native state have great potential in enabling the host to “reject” schistosome worms prior to the onset of pathology. In this regard, the antigen understudy, large subunit of calpain (Sm-p80) fits such a target criterion because this protein plays an important role in the surface membrane renewal of schistosomes, a process that is widely considered to be a mechanism employed by blood-dwelling helminths to evade host immunity [6;8–10]. Sm-p80 has been shown to be exposed at the host parasite interface of larval and adult parasites and is naturally immunogenic [6;12;33]. While the natural immunogenicity of the molecule does not provide protection under conditions of natural infection, we have demonstrated that it is possible to present Sm-p80 to a naive host immune system in such a way as to induce immunity in experimental animals [11–14;21]. Thereby we have achieved the goal of developing a reliable, dependable and consistent immunization protocol using Sm-p80 that gives at least 50–60% protection in mice [11–14;21].
As a prelude to Phase I/II human clinical trials, this present proof of concept study was performed to test the prophylactic potential of a Sm-p80-based DNA in a nonhuman primate model. We have utilized the baboon model to evaluate safety, immunogenicity, protective and antifecundity immune responses in an animal model that is more clinically relevant because these animals exhibit human-like acute schistosomiasis syndrome following the cercarial challenge [34;35].
In the present pre-clinical vaccination trial, the Sm-p80-based DNA vaccine was well tolerated; baboons from both control and experimental groups did not exhibit any behavioral or clinical abnormalities. Baboons immunized with Sm-p80-pcDNA3 showed a 38 % reduction in worm burden and a 32 % reduction in egg production. Baboon as a “protection” model has previously been utilized successfully by many investigators to test the efficacy of different schistosome vaccines [34–41]. For example, immunization of baboons with Sm28GST resulted in 38% reduction in the number of parasites and a 33% decrease in female fecundity [36]. Similarly, inoculation of baboons with IrV5 elicited a protection by 28% and noticeable reduction in the sizes of granulomas [40]. In baboons, radiation-attenuated cercariae vaccine is effective but multiple exposures are required to elicit levels of protection from 50% [41] to 80% [40;42]. However, compared to the studies that utilized defined vaccines, a Sm-p80-based DNA vaccine appears to be comparable or superior in its anti-worm and anti-fecundity effect.
Sm-p80 DNA elicited strong immune responses that included IgG1 and IgG2 antibody isotypes and IgM in vaccinated baboons. In this study, total IgG and its subtypes (IgG1 and IgG2), responses increased with each vaccine booster. IgG3 and IgG4 responses were not detectable. Similarly in vaccination studies using Plague antigen LcrV in baboons, IgG3 and IgG4 were found to be under the detection limits [43]. However a cautionary note is warranted here, the non-detection of IgG3 and IgG4 (as well as IgE) could actually be due to the poor cross-reactivity of human reagents being utilized for the detection of baboon antibody subtypes since no secondary antibodies from nonhuman primate origin are available for these subtypes. Conversely, distinct IgM responses to immunization were recorded even though they appeared to be short-lived. Similar trend for IgM levels was also observed with radiation-attenuated cercariae vaccine in baboons [44]. Weak responses were observed for total IgA; however, one animal which appeared to be an outlier showed a robust IgA response. No IgE was detectable in vaccinated animals. As recorded in this study, early emergence with short lives of IgM responses and very small/undetectable levels of IgE were also observed in the “self-curing” S. mansoni-rhesus macaque model [45]. Taken together, our data on Sm-p80 specific humoral responses indicate that even though a mixed Th1/Th2 type of priming was achieved following vaccination with Sm-p80-pcDNA3, Th1 appears to be the dominant of the two.
PBMCs proliferating in response to in vitro stimulation with Sm-p80 produced appreciably more Th1 response enhancing cytokines (IL-2, IFN-γ) than Th2 response enhancing cytokines (IL-4, IL-10). These observations were reaffirmed by ELISPOT analyses in which up to 200-fold more SFUs were detected for INF-γ than for IL-4 in Sm-p80-induced PBMCs. It is evident that Sm-p80 in a DNA formulation is able to elicit a dominant Th1 type Sm-p80-specific response in baboons, as has been observed in the murine model [11;14;15].
Overall our previous data on antifecundity and anti-worm effects of Sm-p80 in the murine model [4;11–15;22] combined with this report in the baboon model and results of other investigators [16;17] clearly indicate that this antigen has a great potential as an important vaccine candidate for the reduction of the morbidity associated with both S. mansoni and S. japonicum infections. The results of this study in the nonhuman primate model have provided the proof of concept and we now believe that Sm-p80 is ready to be tested for safety and efficacy testing in humans.
Acknowledgments
This work is supported by a grant from the Thrasher Research Fund (Award No. 02824-5) to Afzal A. Siddiqui and by the National Center for Research Resources grant (P40-RR-012317) to Gary L. White. We thank David W. Carey, for his excellent technical assistance with the baboon studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006 Sep 23;368(9541):1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006 Jul;6(7):411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 3.Bergquist R, Al-Sherbiny M, Barakat R, Olds R. Blueprint for schistosomiasis vaccine development. Acta Trop. 2002 May;82(2):183–92. doi: 10.1016/s0001-706x(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui AA, Ahmad G, Damian RT, Kennedy RC. Experimental vaccines in animal models for schistosomiasis. Parasitol Res. 2008 Apr;102(5):825–33. doi: 10.1007/s00436-008-0887-6. [DOI] [PubMed] [Google Scholar]
- 5.McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008 Jan;21(1):225–42. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqui AA, Zhou Y, Podesta RB, et al. Characterization of Ca(2+)-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim Biophys Acta. 1993 Mar 24;1181(1):37–44. doi: 10.1016/0925-4439(93)90087-h. [DOI] [PubMed] [Google Scholar]
- 7.Karcz SR, Podesta RB, Siddiqui AA, Dekaban GA, Strejan GH, Clarke MW. Molecular cloning and sequence analysis of a calcium-activated neutral protease (calpain) from Schistosoma mansoni. Mol Biochem Parasitol. 1991 Dec;49(2):333–6. doi: 10.1016/0166-6851(91)90078-k. [DOI] [PubMed] [Google Scholar]
- 8.Silva EE, Clarke MW, Podesta RB. Characterization of a C3 receptor on the envelope of Schistosoma mansoni. J Immunol. 1993 Dec 15;151(12):7057–66. [PubMed] [Google Scholar]
- 9.Young BW, Podesta RB. Complement and 5-HT increase phosphatidylcholine incorporation into the outer bilayers of Schistosoma mansoni. J Parasitol. 1986 Oct;72(5):802–3. [PubMed] [Google Scholar]
- 10.Van Hellemond JJ, Retra K, Brouwers JF, et al. Functions of the tegument of schistosomes: clues from the proteome and lipidome. Int J Parasitol. 2006 May 31;36(6):691–9. doi: 10.1016/j.ijpara.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad G, Torben W, Zhang W, Wyatt M, Siddiqui AA. Sm-p80 based DNA vaccine formulation induces potent protective immunity against Schistosoma mansoni. Parasite Immunology. 2009 March;31(3):156–161. doi: 10.1111/j.1365-3024.2008.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hota-Mitchell S, Siddiqui AA, Dekaban GA, Smith J, Tognon C, Podesta RB. Protection against Schistosoma mansoni infection with a recombinant baculovirus-expressed subunit of calpain. Vaccine. 1997 Oct;15(15):1631–40. doi: 10.1016/s0264-410x(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 13.Hota-Mitchell S, Clarke MW, Podesta RB, Dekaban GA. Recombinant vaccinia viruses and gene gun vectors expressing the large subunit of Schistosoma mansoni calpain used in a murine immunization-challenge model. Vaccine. 1999 Mar 17;17(11–12):1338–54. doi: 10.1016/s0264-410x(98)00391-0. [DOI] [PubMed] [Google Scholar]
- 14.Siddiqui AA, Phillips T, Charest H, et al. Enhancement of Sm-p80 (large subunit of calpain) induced protective immunity against Schistosoma mansoni through co-delivery of interleukin-2 and interleukin-12 in a DNA vaccine formulation. Vaccine. 2003 Jun 20;21(21–22):2882–9. doi: 10.1016/s0264-410x(03)00159-2. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui AA, Pinkston JR, Quinlin ML, et al. Characterization of protective immunity induced against Schistosoma mansoni via DNA priming with the large subunit of calpain (Sm-p80) in the presence of genetic adjuvants. Parasite. 2005 Mar;12(1):3–8. doi: 10.1051/parasite/2005121003. [DOI] [PubMed] [Google Scholar]
- 16.Jankovic D, Aslund L, Oswald IP, et al. Calpain is the target antigen of a Th1 clone that transfers protective immunity against Schistosoma mansoni. J Immunol. 1996 Jul 15;157(2):806–14. [PubMed] [Google Scholar]
- 17.Ohta N, Kumagai T, Maruyama H, Yoshida A, He Y, Zhang R. Research on calpain of Schistosoma japonicum as a vaccine candidate. Parasitol Int. 2004 Jun;53(2):175–81. doi: 10.1016/j.parint.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Ridi RE, Tallima H. Schistosoma mansoni ex vivo lung-stage larvae excretory-secretory antigens as vaccine candidates against schistosomiasis. Vaccine. 2008 Dec 3; doi: 10.1016/j.vaccine.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Yoshida A, Kumagai T, et al. Vaccination with calpain induces a Th1-biased protective immune response against Schistosoma japonicum. Infect Immun. 2001 Jan;69(1):386–91. doi: 10.1128/IAI.69.1.386-391.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy RC, Shearer MH, Hildebrand W. Nonhuman primate models to evaluate vaccine safety and immunogenicity. Vaccine. 1997 Jun;15(8):903–8. doi: 10.1016/s0264-410x(96)00277-0. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui AA, Phillips T, Charest H, et al. Induction of protective immunity against Schistosoma mansoni via DNA priming and boosting with the large subunit of calpain (Sm-p80): adjuvant effects of granulocyte-macrophage colony-stimulating factor and interleukin-4. Infect Immun. 2003 Jul;71(7):3844–51. doi: 10.1128/IAI.71.7.3844-3851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddiqui AA, Pinkston JR, Quinlin ML, et al. Characterization of the immune response to DNA vaccination strategies for schistosomiasis candidate antigen, Sm-p80 in the baboon. Vaccine. 2005 Feb 10;23(12):1451–6. doi: 10.1016/j.vaccine.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Smithers SR, Terry RJ. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965 Nov;55(4):695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- 24.Damian RT, Greene ND, Fitzgerald K. Schistosomiasis mansoni in baboons. The effect of surgical transfer of adult Schistosoma mansoni upon subsequent challenge infection. Am J Trop Med Hyg. 1972 Nov;21(6):951–8. [PubMed] [Google Scholar]
- 25.Cheever AW. Conditions affecting the accuracy of potassium hydroxide digestion techniques for counting Schistosoma mansoni eggs in tissues. Bull World Health Organ. 1968;39(2):328–31. [PMC free article] [PubMed] [Google Scholar]
- 26.Shearer MH, Dark RD, Chodosh J, Kennedy RC. Comparison and characterization of immunoglobulin G subclasses among primate species. Clin Diagn Lab Immunol. 1999 Nov;6(6):953–8. doi: 10.1128/cdli.6.6.953-958.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vereecken K, Naus CW, Polman K, et al. Associations between specific antibody responses and resistance to reinfection in a Senegalese population recently exposed to Schistosoma mansoni. Trop Med Int Health. 2007 Mar;12(3):431–44. doi: 10.1111/j.1365-3156.2006.01805.x. [DOI] [PubMed] [Google Scholar]
- 28.Acosta LP, Waine G, Aligui GD, Tiu WU, Olveda RM, McManus DP. Immune correlate study on human Schistosoma japonicum in a well-defined population in Leyte, Philippines: II. Cellular immune responses to S. japonicum recombinant and native antigens. Acta Trop. 2002 Nov;84(2):137–49. doi: 10.1016/s0001-706x(02)00177-8. [DOI] [PubMed] [Google Scholar]
- 29.Olds GR. New insights into the observed age-specific resistance to reinfection with Schistosoma japonicum. Clin Infect Dis. 2006 Jun 15;42(12):1699–701. doi: 10.1086/504331. [DOI] [PubMed] [Google Scholar]
- 30.Hewitson JP, Hamblin PA, Mountford AP. Immunity induced by the radiation- attenuated schistosome vaccine. Parasite Immunol. 2005 Jul;27(7–8):271–80. doi: 10.1111/j.1365-3024.2005.00764.x. [DOI] [PubMed] [Google Scholar]
- 31.Lightowlers MW. Cestode vaccines: origins, current status and future prospects. Parasitology. 2006;133( Suppl):S27–S42. doi: 10.1017/S003118200600179X. [DOI] [PubMed] [Google Scholar]
- 32.Vercruysse J, Schetters TP, Knox DP, Willadsen P, Claerebout E. Control of parasitic disease using vaccines: an answer to drug resistance? Rev Sci Tech. 2007 Apr;26(1):105–15. [PubMed] [Google Scholar]
- 33.Kumagai T, Maruyama H, Hato M, et al. Schistosoma japonicum: localization of calpain in the penetration glands and secretions of cercariae. Exp Parasitol. 2005 Jan;109(1):53–7. doi: 10.1016/j.exppara.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Damian RT, de la Rosa MA, Murfin DJ, Rawlings CA, Weina PJ, Xue YP. Further development of the baboon as a model for acute schistosomiasis. Mem Inst Oswaldo Cruz. 1992;87(Suppl 4):261–9. 261–9. doi: 10.1590/s0074-02761992000800041. [DOI] [PubMed] [Google Scholar]
- 35.Nyindo M, Farah IO. The baboon as a non-human primate model of human schistosome infection. Parasitol Today. 1999 Dec;15(12):478–82. doi: 10.1016/s0169-4758(99)01569-0. [DOI] [PubMed] [Google Scholar]
- 36.Boulanger D, Reid GD, Sturrock RF, et al. Immunization of mice and baboons with the recombinant Sm28GST affects both worm viability and fecundity after experimental infection with Schistosoma mansoni. Parasite Immunol. 1991 Sep;13(5):473–90. doi: 10.1111/j.1365-3024.1991.tb00545.x. [DOI] [PubMed] [Google Scholar]
- 37.Kanamura HY, Hancock K, Rodrigues V, Damian RT. Schistosoma mansoni heat shock protein 70 elicits an early humoral immune response in S. mansoni infected baboons. Mem Inst Oswaldo Cruz. 2002 Jul;97(5):711–6. doi: 10.1590/s0074-02762002000500022. [DOI] [PubMed] [Google Scholar]
- 38.Kariuki TM, Farah IO, Yole DS, et al. Parameters of the attenuated schistosome vaccine evaluated in the olive baboon. Infect Immun. 2004 Sep;72(9):5526–9. doi: 10.1128/IAI.72.9.5526-5529.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reid GD, Sturrock RF, Harrison RA, Tarara RP. Schistosoma haematobium in the baboon (Papio anubis): assessment of protection levels against either a single mass challenge or repeated trickle challenges after vaccination with irradiated schistosomula. J Helminthol. 1995 Jun;69(2):139–47. doi: 10.1017/s0022149x00014024. [DOI] [PubMed] [Google Scholar]
- 40.Soisson LA, Reid GD, Farah IO, Nyindo M, Strand M. Protective immunity in baboons vaccinated with a recombinant antigen or radiation-attenuated cercariae of Schistosoma mansoni is antibody-dependent. J Immunol. 1993 Nov 1;151(9):4782–9. [PubMed] [Google Scholar]
- 41.Yole DS, Pemberton R, Reid GD, Wilson RA. Protective immunity to Schistosoma mansoni induced in the olive baboon Papio anubis by the irradiated cercaria vaccine. Parasitology. 1996 Jan;112(Pt 1):37–46. doi: 10.1017/s0031182000065057. [DOI] [PubMed] [Google Scholar]
- 42.Kariuki TM, Farah IO. Resistance to re-infection after exposure to normal and attenuated schistosome parasites in the baboon model. Parasite Immunol. 2005 Jul;27(7–8):281–8. doi: 10.1111/j.1365-3024.2005.00783.x. [DOI] [PubMed] [Google Scholar]
- 43.Stacy S, Pasquali A, Sexton VL, Cantwell AM, Kraig E, Dube PH. An age-old paradigm challenged: old baboons generate vigorous humoral immune responses to LcrV, a plague antigen. J Immunol. 2008 Jul 1;181(1):109–15. doi: 10.4049/jimmunol.181.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coulson PS, Kariuki TM. Schistosome vaccine testing: lessons from the baboon model. Mem Inst Oswaldo Cruz. 2006 Sep;101( Suppl 1):369–72. doi: 10.1590/s0074-02762006000900061. [DOI] [PubMed] [Google Scholar]
- 45.Wilson RA, Langermans JA, Van Dam GJ, et al. Elimination of Schistosoma mansoni Adult Worms by Rhesus Macaques: Basis for a Therapeutic Vaccine? PLoS Negl Trop Dis. 2008;2(9):e290. doi: 10.1371/journal.pntd.0000290. [DOI] [PMC free article] [PubMed] [Google Scholar]