Abstract
Objective
The objective of this study was to characterize the clinical features of patients with Niemann-Pick disease Type B and to identify efficacy endpoints for future clinical trials of enzyme replacement therapy.
Patients and Methods
Fifty-nine patients who had Niemann-Pick disease Type B, were at least 6 years of age, and manifested at least 2 disease symptoms participated in this multicenter, multinational, cross-sectional survey study. Medical histories; physical examinations; and assessments of cardiorespiratory function, clinical laboratory data, and liver and spleen volumes; radiographic evaluation of the lungs and bone age; and quality-of-life were obtained during a 2 to 3 day period.
Results
Fifty-three percent of the patients were male, 92% white, and the median age was 17.6 years. The R508del mutation accounted for 25% of all disease alleles. Most patients initially presented with splenomegaly (78%) or hepatomegaly (73%). Frequent symptoms included bleeding (49%), pulmonary infections and shortness of breath (42% each), and joint/limb pain (39%). Growth was markedly delayed during adolescence. Patients commonly had low levels of platelets and high-density lipoprotein, elevated levels of LDL, VLDL, triglycerides, leukocyte sphingomyelin, and serum chitotriosidase, and abnormal liver function tests. Nearly all patients had documented splenomegaly and hepatomegaly and interstitial lung disease. Patients commonly showed restrictive lung disease physiology with impaired pulmonary gas exchange and decreased maximal exercise tolerance. Quality of life was only mildly decreased by standardized questionnaires. The degree of splenomegaly correlated with most aspects of disease, including hepatomegaly, growth, lipid profile, hematologic parameters, and pulmonary function.
Conclusions
This study documents the multisystem involvement and clinical variability of Niemann-Pick B disease. Several efficacy endpoints were identified for future clinical treatment studies. Because of its correlation with disease severity, spleen volume may be a useful surrogate endpoint in treatment trials, whereas biomarkers such as chitotriosidase also may play a role in monitoring treatment responses.
Keywords: Niemann Pick Disease, acid sphingomyelinase deficiency, lysosomal storage disorder, cross-sectional study, natural history
Introduction
Acid sphingomyelinase deficiency (ASM) (sphingomyelin phosphodiesterase; EC 3.1.4.12) is an inborn error of metabolism that leads to the accumulation of sphingomyelin in cells and tissues and causes the clinical condition known as Niemann-Pick disease (NPD).1,2 ASM deficiency is rare, with an estimated incidence of 0.4 in 100,000 to 0.6 in 100,000 newborns.3-5 Historically, two distinct subtypes have been delineated on the basis of their phenotypes (Types A ad B). Although NPD Type C shares the same eponym as NPD caused by ASM deficiency, it is a genetically distinct disorder resulting from defective intracellular trafficking of cholesterol with secondary accumulation of glycosphingolipids. Type A disease, which has an Ashkenazi Jewish predilection, is a severe neurodegenerative disease of infancy characterized by progressive psychomotor retardation, failure to thrive, hepatosplenomegaly, cherry red macula, and death by three to four years of age. In contrast, type B disease, which is panethnic, is characterized by hepatosplenomegaly, thrombocytopenia, interstitial lung disease, and dyslipidemia with most patients having little or no neurologic involvement. Liver dysfunction, retinal stigmata, and growth retardation also may be present but are more variable features. More recent studies suggested that there is a disease spectrum related to the amount of residual in vivo ASM activity, including intermediate neurologic forms of the disease.
The diagnosis of NPD type B is usually made in childhood after organomegaly is noted and survival well into adulthood is known to occur commonly, although age at death and the causes of death have not been extensively studied. Currently there is no specific treatment available for ASM deficiency; however, both enzyme replacement therapy6 and gene therapy7 have shown promising results in an SMPD1 knockout mouse model of NPD, and the first clinical trial of enzyme-replacement therapy for NPD type B has recently begun. The proper evaluation of these potential therapies requires an understanding of both the natural history of the disease and the spectrum of disease manifestations in ASM deficiency in order to select clinically meaningful endpoints.
In this article, we report the detailed clinical findings in the largest series of patients with NPD type B reported to date. We also determined whether the degree of splenomegaly, which is one of the cardinal features of the disease, correlates with other signs of disease severity.
Methods
Study Design and Patients
Fifty-nine patients with NPD type B were enrolled in a cross-sectional survey study to provide prospective natural history data that would assist in the design of future clinical treatment trials. Patients were enrolled between May 5, 2001 and June 17, 2002 at five sites: the United States (n = 26), Brazil (n = 13), Italy (n = 8), France (n = 7), and Germany (n = 5). Baseline data were collected during a 2−3 day period. The study was approved by the Institutional Review Board, Ethics Committee, or Human Subjects Safety Committee at each site and voluntary, written informed consent was obtained from each patient and/or guardian. When appropriate, children also gave assent prior to any study procedures. All study procedures were conducted in accordance with Good Clinical Practice Guidelines.
Eligible patients had to be at least 6 years of age, have a diagnosis of NPD type B based on marked deficiency of ASM activity in peripheral leukocytes or in cultured skin fibroblasts/lymphocytes and the presence of at least two characteristic clinical features, and have a negative pregnancy test if a female of childbearing age. Patients were excluded if they had a diagnosis of NPD type A or non-ASM deficient NPD, such as NPD type C; had received an investigational drug within 30 days of enrollment; had a serious co-morbidity or other circumstance that would interfere with study compliance; had received a bone marrow transplant; or were pregnant or lactating.
Clinical Assessments
Medical histories and physical examinations were obtained on all patients. Height and weight Z scores were determined using normative growth data from the Centers for Disease Control (www.cdc.gov/nccdphp/dnpa/growthcharts/sas.htm). Neurologic examination included assessment of motor and sensory function, cranial nerves, and cognitive status. Ophthalmologic examination, including fundoscopy and retinal photographs, was performed at each site and the findings were reviewed centrally by a single examiner (Dr. Brodie). Routine clinical laboratory studies included blood chemistries, liver function tests, hematology, insulin-like growth factor 1 (IGF-1) and binding protein , thyrotropin, fasting lipid profile, and urinalysis. Specialized testing included SMPD1 and chitotriosidase (chitinase, CHIT1) genotyping, chitotriosidase activity, and sphingomyelin levels in plasma and peripheral blood mononuclear cells.
Cardiopulmonary status was assessed by electrocardiography (ECG), two-dimensional echocardiography, and pulmonary function testing (forced vital capacity, FVC; forced expiratory volume in 1 second, FEV1; and diffusing capacity of the lung, DLCO) by standard clinical techniques. Sub-maximal exercise tolerance was assessed by the 6-minute walk test (6MWT),8 and the values of two tests performed on separate days were averaged. Maximal exercise tolerance was assessed by cycle ergometry with continuous measurements of workload, oxygen (O2) uptake, carbon dioxide output, and tidal volume. FVC, FEV1, DLCO, maximum O2 uptake, and maximum carbon dioxide output were expressed as a percentage of predicted values.
Imaging Studies
Imaging studies included computed tomography scan or magnetic resonance imaging of the abdomen for liver and spleen volumes, chest radiograph and high-resolution computed tomography (HRCT), and, for 28 patients who were younger than 18 years, bone age. Liver and spleen volumes were calculated by integrating cross-sectional images and were expressed as multiples of normal (MN), assuming normal spleen and liver volumes of 0.2% and 2.5% of body weight (with 1 kg equivalent to 1 L). All image data were collected centrally by a medical imaging core laboratory, Bioimaging Technologies, Inc. (Newtown, PA) and reviewed by a board-certified radiologist.
Quality of Life Assessment
Patient quality of life was assessed using the Child Health Questionnaire (CHQ)9 for patients who were younger than 18 years and the Short Form-36 (SF-36)10 for adults.
Specialized Laboratory Tests
For the previously identified common mutations in the SMPD1 gene, dot-blot hybridization by using allele-specific oligonucleotides was used to screen for R496L, L302P, and fsP330 in patients from US and Brazilian sites, and a polymerase chain reaction/restriction enzyme digest test was used to screen for R608del in patients from European sites. for patients with unidentified mutations, the entire coding region and adjacent intronic splice sites of the SMPD1 gene were sequenced.11,12 The CHIT1 gene was genotyped for the common 24-base pair duplication mutation as previously described.13 Chitotriosidase activity was measured in serum using the 4-methylumbelliferyl chito-oligosaccharide assay,14 and sphingomyelin levels were quantitated in plasma and peripheral blood mononuclear cells by liquid chromatography/tandem mass spectrometry.15
Statistical Analysis
Statistical analyses were performed using SAS version 8.0 software (SAS Institute Inc, Cary, NC), and the data were summarized using descriptive statistics. For determination of which features of NPD type B correlate with clinical severity and may be causally related, linear correlation analyses were performed using Pearson's correlation coefficient (r). P < .05 was considered to be statistically significant, and r ≥ 0.4 was considered to be a high correlation. Twenty-four patients (41%) did not satisfactorily complete the cycle ergometry test (9 patients did not perform the test, 4 patients developed medical problems during the test, and 11 patients had sub-maximal effort).
Results
Patient Characteristics and Medical History
Fifty-nine patients (31 males, 28 females) who ranged in age from 7 to 65 years (mean 22.6 ± 13.8, median 17.6) were enrolled in the study and evaluated. One patient discontinued the study prematurely. Thirty patients (51%) were in the pediatric age group (< 18 years), and 54 patients (92%) were white. The mean age at first symptom onset was 5.0 ± 7.0 years (median 2.5, range 1−30.0), and the mean age at diagnosis was 9.8 ± 10.9 years (median 5.4, range 1.0−45.1). Twenty-two patients (37%) had a family history of ASM deficiency: 21 patients with affected siblings and one patient with an affected cousin.
The most frequently reported initial and historical signs and symptoms are listed in Table 1. Most patients presented with splenomegaly (78%) or hepatomegaly (73%). Bleeding (49%), shortness of breath (42%), pulmonary infections (42%), and joint/limb pain (39%) were the most frequent historical complaints. The majority of patients had an intact spleen (92%), one had a partial splenectomy, and four had a total splenectomy. Bleeding episodes most often involved recurrent epistaxis (29%), requiring repeated cauterizations in two patients. Other significant bleeding events occurring in one patient each were subdural hematoma, hematemesis, hemoptysis, hemothorax, excessive bleeding following tonsillectomy and adenoidectomy resulting in a blood transfusion, menorrhagia, and uterine bleeding that required a hysterectomy. Portal hypertension and esophageal varices were described in two patients, one of whom had liver failure. One patient had coronary artery disease requiring triple coronary artery bypass grafting performed at age 29 and repeated at age 39. Other cardiac-related morbidities reported for single patients included aortic and mitral valve replacement because of calcification, aortic valve insufficiency, mitral valve prolapse, excess pericardial fluid, pericarditis, bradycardia, and episodic tachycardia. Two patients required supplemental oxygen, and all were ambulatory.
Table 1.
Presenting and Historical Signs and Symptoms.
| At Presentation | N | % |
|---|---|---|
| Splenomegaly | 46 | 78 |
| Hepatomegaly | 43 | 73 |
| Respiratory disease | 12 | 20 |
| Excessive bleeding/bruising | 6 | 10 |
| Thrombocytopenia | 5 | 8 |
| By History | ||
| Bleeding | 29 | 49 |
| Shortness of breath | 25 | 42 |
| Pulmonary infections | 25 | 42 |
| Joint/limb pain | 23 | 39 |
| Bruising | 16 | 27 |
| Headaches | 14 | 24 |
| Abdominal pain | 12 | 20 |
| Diarrhea | 12 | 20 |
| Fractures | 11 | 19 |
SMPD1 Analysis
Two mutant alleles in the SMPD1 gene were identified in all patients, and overall, 48 different mutations were indentified. The most common individual mutation was R608del (deletion of arginine at amino acid position 608), which occurred in 42% of patients and accounted for 25% of all alleles. The frequencies of all other individual mutations were < 10%. Missense mutations were the most common type of genetic lesion, comprising 59% of alleles. All patients had genotypes consistent with the expression of residual ASM activity, except for one patient who was homozygous for a null mutation (W32X) and a second patient who was homozygous for a missense mutation affecting the initiation methionine. Although these two patients might have been predicted to have Type A disease based on the absence of the full-length, functional ASM gene product, their less severe Type B phenotype is presumably due to translation from a second initiation methionine present at codon 94 that produces a truncated enzyme with partial activity.16,17
Clinical Laboratory Evaluation
The mean complete blood cell counts, fasting lipid values, liver function tests, and hormone assay results are summarized in Table 2. Thrombocytopenia (53%) was more common than anemia (26%) or leukopenia (21%), and all hematologic abnormalities were generally mild to moderate in severity. Most patients had atherogenic lipid profiles with the most characteristic lipid abnormality being a low high-density lipoprotein (HDL) level (74% of patients) when compared with age- and gender-matched control subjects (mean 26 mg/dL). In addition, 41% of patients had a high total cholesterol level, 62% had a high triglyceride level, 46% had a high low-density lipoprotein level, and 62% had a high very-low-density lipoprotein level compared with age- and gender-matched control subjects. The mean cholesterol/HDL level was 10.3, which is 2.3 times the upper limit of normal. The mean cholesterol/HDL ratio was nearly twice as high in patients who had undergone splenectomy compared to those with an intact spleen (17.3 vs 9.8). Alanine aminotransferase and aspartate aminotransferase were elevated in approximately half of the patients, whereas total bilirubin was elevated in a third. Additional laboratory studies revealed that 18 patients (35%) had low IGF-1 levels for their age and gender, and 7 patients (13%) had elevated IGF-1 levels. Two patients (3%) had low thyrotropin levels and 6 patients (10%) had elevated thyrotropin levels.
Table 2.
Laboratory Studies.
| Laboratory Study | N | Mean (SD) | Range | % Abnormal | |
|---|---|---|---|---|---|
| Low | High | ||||
| Hemoglobin, g/L | 58 | 13.3 (1.5) | 9.3 − 16.5 | 26 | 3 |
| Hematocrit, % | 58 | 39.1 (4.5) | 27.8 − 48.3 | 34 | 2 |
| White blood cells, × 109/L | 58 | 6.4 (2.7) | 2.1 − 16.2 | 21 | 7 |
| Neutrophils, % | 58 | 55 (11) | 36 − 82 | 7 | 9 |
| Platelets, × 109/L | 58 | 158 (82) | 59 − 459 | 53 | 3 |
| Cholesterol/HDL ratio* | 58 | 10.3 (5.6) | 2.6 − 34.5 | 0 | 91 |
| HDL, mg/dL | 58 | 26 (10) | 11 − 67 | 74 | 0 |
| Total cholesterol | 58 | 230 (72) | 120 − 517 | 0 | 41 |
| Triglycerides, mg/dL | 58 | 202 (99) | 43 − 495 | 0 | 62 |
| LDL, mg/dL | 57 | 162 (56) | 71 − 283 | 0 | 46 |
| VLDL, mg/dL | 34 | 38 (21) | 4 − 99 | 15 | 62 |
| ALT, U/L | 58 | 69 (60) | 9 − 250 | 0 | 51 |
| AST, U/L | 57 | 63 (50) | 15 − 223 | 0 | 51 |
| Alkaline phosphatase, U/L | 57 | 228 (166) | 51 − 833 | 0 | 42 |
| Total bilirubin, mg/dL | 58 | 3.2 (7.3) | .2 − 40.9 | 0 | 33 |
| Chitotriosidase, nmol/hr/mL | 56 | 549 (832) | 20 − 5792 | 0 | 95 |
| Plasma sphingomyelin, nmol/mL | 41 | 221 (33) | 148 − 278 | 46 | 0 |
| Peripheral blood mononuclear cell | 41 | 77 (68) | 15 − 322 | 22 | 63 |
| sphingomyelin, nmol/mg protein | |||||
| IGF-1, ng/mL | 52 | 198 (164) | 2 − 742 | 35 | 13 |
| IGF-1 BP, ng/mL | 46 | 2100 (1820) | 3 − 6801 | 9 | 35 |
| Thyrotropin, mU/L | 58 | 2.6 (1.3) | .0−5.9 | 3 | 10 |
Cholesterol/HDL ratio > 4.5 is considered abnormally high.
Nearly all patients (95%) had elevated chitotriosidase levels (mean: 549 nmol/hr/mL [reference range: 6 − 98.4]). Patients with two normal CHIT1 genes (n = 34) had higher chitotriosidase levels than those who were heterozygous (n = 22) or homozygous for the null gene duplication. Plasma sphingomyelin levels were within the reference range in all patients (mean: 221 nmol/mL [reference range 22 − 413 nmol/mL]), whereas the mean sphingomyelin level in peripheral blood mononuclear cells was only mildly elevated (mean: 77 nmol/mg protein [reference range 25 − 46]) in 63% of patients.
Growth
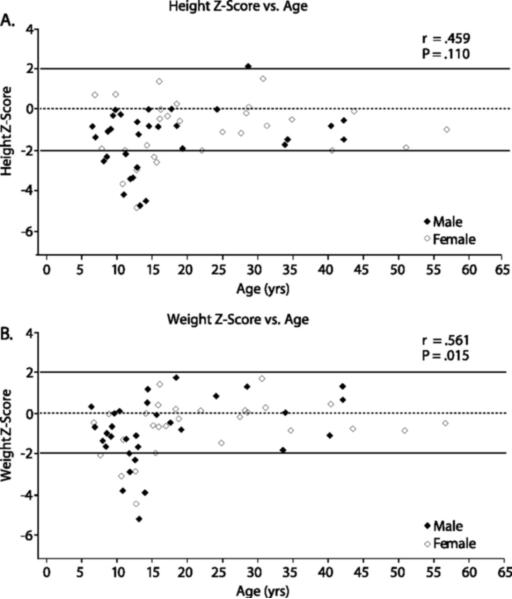
Most patients had heights and weights that were below average when normalized for age and gender. (Figure 1) Height Z-scores ranged from −4.88 to 2.14 (mean −1.3 ± 1.51), and weight Z-scores ranged from −5.22 to 1.80 (mean −.77 ± 1.49). Twenty-nine percent of patients (n = 17) had height Z-scores < −2 and 15% of patients (n = 9) had weight Z-scores < −2. Growth retardation was particularly prominent in adolescents (mean height Z score −2.71), whereas growth was in the low normal range in both children (mean height Z score −1.40, aged < 13 years) and adults (mean height Z score −0.58, aged > 18 years). Overall there was a high correlation between bone age and chronological age (r = 0.512, P < .001), with the majority of adolescent patients having delayed bone ages indicative of delayed puberty.
Figure 1.
Growth Z-scores for height (upper) and weight (lower) vs age in male and female patients.
Cardiopulmonary Assessments
ECG revealed that 16 of 58 patients (28%) had abnormalities, including sinus bradycardia (n = 6), left ventricular hypertrophy (n = 4), conduction abnormalities (n = 6) including one with a short PR interval, and non-specific interventricular conduction block (n=5). Two patients, ages 48 and 58, had evidence of a prior myocardial infarction on ECG. Two-dimensional echocardiograms revealed that 50% of patients (n = 29) exhibited abnormalities. The most common finding was mild mitral valve regurgitation, and there were a few cases of mild ventricular dysfunction. Additional findings included two patients with moderate to severe aortic regurgitation and one patient with pulmonary hypertension.
Most patients had evidence of pulmonary dysfunction (Table 3). In general, patients displayed a low FVC with an FEV1/FVC ratio within the reference range and a low DLCO, which are consistent with restrictive lung disease and impaired gas exchange secondary to interstitial lung disease. Compared to patients with no shortness of breath (n = 34), patients with a history of shortness of breath (n = 25) had lower % predicted FVC (mean 76.4% vs 86.3%, P = .027) and lower percentage of predicted DLCO (51.3% vs 70.6%, P = .009). Nearly all patients (90%; 53 of 59) showed some degree of interstitial lung disease (ground-glass appearance, reticulonodular densities, pleural thickening, and low lung volumes) by both chest radiograph and HRCT.
Table 3.
Cardiorespiratory Function Testing in NPD Type B.
| Test | N | Mean (SD) | Range | % Abnormal* |
|---|---|---|---|---|
| % Predicted FVC | 55 | 82 (16) | 48 − 118 | 47 |
| % Predicted FEV1 | 55 | 80 (18) | 27 − 117 | 49 |
| FEV1:FVC ratio | 55 | .85 (.12) | .32 − 1.00 | 22 |
| % Predicted DLCO | 45 | 62 (25) | 12 − 121 | 76 |
| 6MWT, meters | 56 | 485 (96) | 256 − 721 | 5 |
| % Predicted Maximum | 35 | 83 (26) | 27 − 138 | 46 |
| Workload | ||||
| % Predicted Maximum | 32 | 85 (25) | 40 − 148 | 38 |
| O2 Uptake |
Abnormal values were defined as follows: FVC, FEV1, DLCO, and maximum workload and O2 uptake < 80% of the predicted normal values; FEV1:FVC ratio < .80; and 6MWT < 310 meters. For the FEV1:FVC ratio, the lower limit of normal varies slightly by patient demographics and .80 represents an average value. For the 6MWT, 310 meters is considered to be the lower limit of normal for adult women8, and it also approximates the 320 meter minimum distance for normal community ambulation.
The mean 6MWT distance (485 ± 96 meters) was within the reference range, and only three patients had mean 6MWT distances below the 310-m lower limit of normal for healthy adult women.9 Two patients became hypoxemic (O2 saturation < 89%) by the end of the test after walking 256 and 630m, and both recovered within 2 minutes. Of the 59 patients, 35 (59%) satisfactorily completed the cycle ergometry test. Among these patients, the mean percentage of predicted maximum workload was 83 ± 26% and the percentage of predicted maximum O2 uptake was 85 ± 25%, both of which are in the low normal range. Compared with patients without shortness of breath, patients with a history of shortness of breath had a much lower mean percentage of predicted maximum workload (66% vs 92%), but more similar mean percentage of predicted maximum O2 uptake (80% vs 88%)
Neurological Assessment and Ophthalmologic Examinations
Nearly all patients presented with normal mental status, cranial nerve function, sensation, muscle strength, and coordination. Deep tendon reflexes were normal to hypoactive. Fifteen of 59 patients (25%) had cherry red spots, which were always bilateral. Among these 15 patients, 5 (33%) had cognitive impairment (ie, low IQ) with or without other neurological abnormalities. Among the 44 patients without cherry red spots, none had cognitive impairment but five (11%) had peripheral neuropathy.
Liver and Spleen Volumes
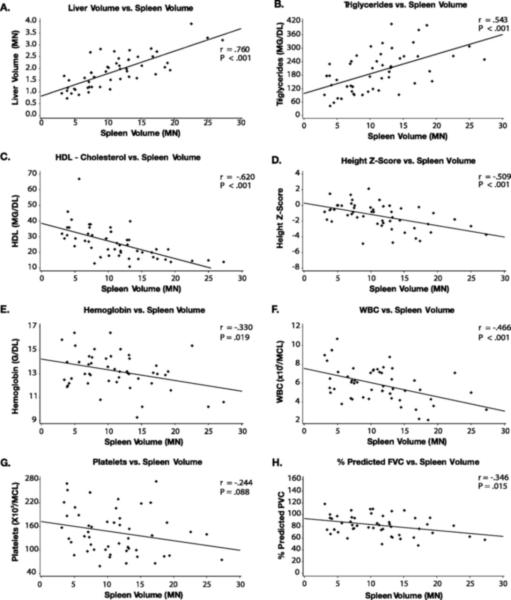
Among the patients who had not undergone splenectomy and available data (n = 51), the mean splenic volume was 11.1 ± 5.7 MN (range 3.1 − 27.3 MN), and 84% of patients had spleen volumes > 5 MN. The mean liver volume was 1.9 MN ± .7 (range: 0 .7 − 3.9 MN), and 71% of patients had liver volumes > 1.5MN. Spleen volume showed no significant trend with age (P = .820) and liver volume showed only a weak correlation (r = −.282, P = .035). Spleen volume was strongly correlated with liver volume (r = .760, P < .001) and triglyceride level (r = .545, P < .001), and negatively correlated with HDL (r = −0.620, P < .001), height Z-score (r = −0.509, P = .0001), hemoglobin (r = −0.330, P = .019), and white blood cell count (r = −0.466, P < .001;Figure 2) Surprisingly, spleen volume did not correlate with platelet count (r = −0.244, P = .088). However, larger spleen volumes were observed in patients with a history of bleeding and/or bruising compared to those without (13.4 ± 5.7 MN vs. 9.1 ± 7.6 MN; P =.006) although their platelet counts were not significantly different (151 ± 96 ×109/L vs 166 ± 64 ×109/L, P = .209).
Figure 2.
Correlations between normalized spleen volume and liver volume (A), triglycerides (B), HDL (C), height Z-score (D), hemoglobin (E), white blood cell count (F), and platelets (G) and predicted FVC (H)
Splenic volume was weakly correlated with various pulmonary measures, including a positive correlation with HRCT interstitial lung disease score (r = .308, P = .033) and was negatively correlated with percentage of predicted DLCO (r = −0.306, P = .052) and percentage of predicted FVC (r = −0.346, P = .015). Splenic volume did not correlate with exercise tolerance as measured by the 6MWT distance (r = −0.260, P = .075) and percentage of predicted peak workload by cycle ergometry (r = −0.198, P = .277).
Liver volume showed strong positive correlations with serum alanine aminotransferase (r = .597, P < .001) and aspartate aminotransferase levels (r = 0.644, P < .001). (Figure 5)
Quality of Life Assessment
For the pediatric patients, the results of the CHQ-PF50 revealed that 4 of the 10 subscale scores (physical functioning, mental health, general health perceptions, and parental impact - emotional) were at least 1 standard deviation below the general population norms, which would suggest diminished quality of life in these areas by parental report. The remaining subscale scores were within the normal range. For the adult patients, 7 of the 8 SF-36 sub-scales (physical functioning, role-physical, bodily pain, vitality, social functioning, role-emotional, and mental health) and both the physical and mental health status summary scores were within 1 standard deviation of the United States general population norms, which would indicate no significant impact of the disease in these areas, or in the overall physical and mental health status of the patients. Only the general health subscale score was > 1SD below United States general population norms, which would indicate that patients do not consider themselves to be as healthy as others, believe they get sick easier than others, and expect their health to worsen over time.
Discussion
Advances in the treatment of inborn errors of metabolism include recent clinical trials of enzyme replacement, substrate deprivation, pharmacologic chaperone therapy, and transplantation of stem cells.18-20 The rational evaluation of any of these approaches requires that the spectrum of disease manifestations and natural history of the disorder be clearly delineated; however, efforts to collect this information systematically for the lysosomal storage diseases have been limited, and most information about the clinical features of these disorders is available only from single case reports or small series of patients. In this study, detailed clinical data were prospectively and systematically collected on the largest series of patients with NPD type B reported to date.
As expected, patients had evidence for involvement of multiple organ systems, including abnormal growth, interstitial lung disease, and hepatosplenomegaly. In addition, there was remarkable variability in the severity of symptoms and clinical findings across patients. In this cohort, the growth Z scores for adolescents were significantly below normal, whereas the Z scores for children and adults were mostly in the low normal range. Notably, the low Z scores that were seen for adolescents were associated with a delayed bone age, indicating delayed puberty, and low IGF-1 levels, which may be indicative of low growth hormone levels as previously observed in a series of mostly prepubertal children with NPD type B 21. Children with Gaucher disease also experience growth retardation and delayed puberty, which have been attributed to a higher resting metabolic rate related to their organomegaly. For these children, linear growth often improves dramatically after splenectomy or enzyme replacement therapy.22 Radiographic evidence for interstitial lung disease was present in nearly all patients studied, as previously reported;23 however, in a separate report, pulmonary function and interstitial lung disease scores by HRCT showed only a weak correlation, indicating that the radiographic appearance of the lung is not a good predictor of lung function.24 Nevertheless, although the clinical presentation of the pulmonary disease was widely variable, both pulmonary function testing, which indicated restrictive lung disease physiology with impaired gas exchange, and cycle ergometry, which was subnormal in half of the patients, suggest that pulmonary disease is a common disease manifestation.
Hepatosplenomegaly also is a consistent feature of the disease, with spleen volume relatively more enlarged than liver volume (mean 11.1 MN vs 1.9 MN). Notably, patients who reported a history of recurrent bleeding events tended to have larger spleen volumes but not lower platelet counts than those without a bleeding diathesis. In addition, there was no overall correlation between spleen volume and platelet count as might be expected based on the pathophysiology of platelet sequestration by the spleen. Possible explanations for this finding include fluctuating platelet counts over time, decreased production by bone marrow in addition to platelet consumption; and/or platelet dysfunction with prolonged coagulation times.
The results reported here also confirm the recently reported finding of abnormal lipid profiles in carriers and in a series of pediatric patients with NPD Type B, 56% of whom had evidence of early atherosclerosis by coronary artery calcium scoring.25 Lee et al 26 reported a pedigree in which low HDL levels segregated with an SMPD1 mutation, lending additional support that ASM plays an important role in cholesterol homeostasis, even in heterozyous carriers. In this series, the lipid profiles were highly atherogenic with a mean total cholesterol/HDL ratio of 10.3, which is associated with a two to three fold increased risk of coronary artery disease based on the Framingham Heart Study.27 Notably, two patients (ages 48 and 58) showed ECG evidence of a prior myocardial infarction, and one patient had undergone two triple coronary artery bypass procedures before age 40. On the basis of the data collected here, it is likely that coronary artery disease is a more common manifestation than has previously been appreciated. Also of importance is the finding that patients who had undergone splenectomy had lipid profiles that were more atherogenic than those with spleens, which may reflect acceleration of the disease secondary to the redistribution of sphingomyelin to other organs following the removal of a major depot organ.
As expected for NPD type B, only 17% of the patients exhibited neurologic features; however, ASMD has a phenotype that is a continuum from the classic type A disease to a recently described intermediate group of patients with later-onset, chronic neurologic disease28-30 to classic type B disease. Cherry red spots, an ophthalmologic finding caused by accumulation of sphingomyelin in the ganglion cells of the macula, originally were thought to reflect neurologic involvement as is typically seen in NPD type A. More recently, cherry red spots have been reported in NPD type B patients.31 In this study, cherry red spots were observed in 25% of patients with NPD type B, confirming that they are not a specific indicator of clinically apparent neurologic disease. Nevertheless, they were present in all five patients with cognitive impairment, suggesting that although in most patients with NPD type B and central nervous system involvement it is confined to the retina, a subset of patients with cherry red spots have clinical symptoms related to the central nervous system.
The SF-36 and CHQ revealed minimal impairment except in the general health domain. A reasonable explanation for this finding is that these generic quality of life questionnaires are insensitive measures when applied to this particular patient population. The identification of a high prevalence of symptoms, coupled with the lack of sensitivity of generic questionnaires, has prompted the development of a disease-specific questionnaire, the ASM-Health Assessment Questionnaire (ASM-HAQ), which will be administered directly to patients to systematically capture this information.
Other major findings of the study include the identification of two potential surrogate markers of disease severity. First, the degree of splenomegaly correlated with multiple disease manifestations in other organ systems; therefore, spleen volume, which can be non-invasively measured at little risk, may be a useful surrogate marker of overall disease severity and treatment response. Similarly, chitotriosidase, which is a marker of activated macrophages, was elevated in nearly all patients. Chitotriosidase may prove to be a useful marker of disease activity as in Gaucher disease, where the level has been proposed for monitoring patient responses to enzyme replacement therapy.32 Approximately 5% of patients are homozygous for a common 24-base duplication mutation and have no demonstrable chitotriosidase activity, which has stimulated the search for other plasma biomarkers, (e.g. PARC/CCL18, a chemokine that is elevated in both Gaucher disease and ASM deficiency).33
As has been reported for other rare diseases, the patients in this study had a substantial interval (mean 4.9 years) between their initial presentation and definitive diagnosis, which may explain why more than one-third had an affected family member, almost always a sibling. This delay in diagnosis has important implications because specific therapies are developed for the lysosomal storage diseases which may be most effective when initiated early in the disease before irreversible apoptosis and/or fibrosis occurs.34, 35 In particular, measurement of lysosomal enzymes in patients who present with hepatosplenomegaly or other evidence of a storage disease would be an important early diagnostic test to carry out. In addition, new methods for the newborn screening of selected lysosomal storage diseases will facilitate early intervention.
Conclusions
The results of this study provide important new information about the spectrum of disease manifestations in NPD type B. The patient cohort was demographically diverse, representing patients from 5 countries on 3 continents (North America, South America and Europe); however, ascertainment bias was likely, particularly with regard to the adult patients, who have survived the longest and may represent a less severely affected cohort. For example, symptomatic pulmonary disease beginning in early childhood has been described in Saudi Arabian patients, while neurological symptoms appear to be more prevalent in Eastern European adolescents and adults. 28, 36 Indeed, the R608del was the most common mutation found in our study patients, accounting for 25% of disease alleles compared to 12% in a worldwide study.32 Because this allele is associated with milder disease manifestations, although widely variable, 38, 39 it is possible that the aggregate clinical data reported here may underestimate disease severity. In addition, the data reported here is from a single time point. To study more fully the natural history of disease, serial evaluations in this large patient cohort will be conducted over several years, which will augment previously obtained longitudinal data showing a worsening of pulmonary function, decreasing platelet and white blood cell counts, and a more atherogenic lipid profile over time.40 Nevertheless the clinical information collected for this large series of patients provides important information about the spectrum of disease manifestations, has identified major morbidities associated with the disease including some previously unrecognized manifestations, and should permit the selection of clinically meaningful endpoints and markers of disease severity for future therapeutic trials.
Acknowledgments
This study was supported by Genzyme Corporation (Cambridge, MA). Dr McGovern is the recipient of Mid Career Patient-Oriented Research Career Development Award K24 RR021991−01 from the National Institutes of Health. The US patient studies were also supported by grant 5 MO1 RR00071 for the Mount Sinai General Clinical Research Center from the National Center for Research Resources, National Institutes of Health.
We acknowledge Drs Susan Richards (chitotriosidase activity and sphingomyelin analysis) and Robert Pomponio (CHIT1 genotyping) at Genzyme and Drs Calogero M. Simonaro and Edward H. Schuchman (SMPD1 genotyping of US and Brazilian patients) and Dr Christophe Marcais (SMPD1 genotyping of European patients) for expert technical assistance. We also acknowledge study site personnel and all of the patients and families whoparticipated in this study.
Footnotes
- MM McGovern: Has performed clinical trials for Genzyme.
- MP Wasserstein: Has performed clinical trials for Genzyme.
- R Giugliani: Has performed clinical trials for Genzyme.
- B Bembi: Has performed clinical trials for Genzyme.
- M Vanier: Has performed clinical trials for Genzyme.
- E Mengel: Has performed clinical trials for Genzyme.
- S Brodie Has performed clinical trials for Genzyme.
- D Mendelson: Has performed clinical trials for Genzyme.
- Gwen Skloot: Has performed clinical trials for Genzyme.
- N Kuriyama: Is an employee of and holds stock in Genzyme.
- RJ Desnick Has received research funding from Genzyme, has received travel expenses for scientific presentations from Genzyme, and has licensed patents for recombinant human acid sphingomyelinase enzyme replacement therapy for Niemann-Pick disease type B and for Fabrazyme for Fabry disease to Genzyme.
- GF Cox: Is an employee of and holds stock in Genzyme.
References
- 1.Schuchman EH, Desnick RJ. Niemann Pick Disease Types A and B: Acid sphingomyelinase deficiencies. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic & Molecular Bases of Inherited Disease. 8th ed. McGraw Hill; New York: 2001. pp. 3589–3610. [Google Scholar]
- 2.Spence M, Callahan J. Sphingomyelin-cholesterol lipidoses: The Niemann Pick group of diseases. McGraw-Hill; New York: 1989. [Google Scholar]
- 3.Meikle P, Hopwood JJ, Clague AR, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 4.Poorthuis BJHM, Wevers RA, Kleijer WJ, et al. The frequency of lysosomal storage diseases in The Netherlands. Hum Genet. 1999;105:151–156. doi: 10.1007/s004399900075. [DOI] [PubMed] [Google Scholar]
- 5.Pinto R, Caseiro C, Lemos M, et al. Prevalence of lysosomal storage diseases in Portugal. Eur J Hum Genet. 2004;12(2):87–92. doi: 10.1038/sj.ejhg.5201044. [DOI] [PubMed] [Google Scholar]
- 6.Miranda SR, He X, Simonaro CM, et al. Infusion of recombinant human acid sphingomyelinase into Niemann-Pick disease mice leads to visceral, but not neurological, correction of the pathophysiology. FASEB J. 2000;14(13):1988–1995. doi: 10.1096/fj.00-0014com. [DOI] [PubMed] [Google Scholar]
- 7.Passini MA, Bu J, Fidler JA, et al. Combination brain and systemic injections of AAV provide maximal functional and survival benefits in the Niemann-Pick mouse. Proc Natl Acad Sci U S A. 2007;104(22):9505–9510. doi: 10.1073/pnas.0703509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enright PL, Sherrill DL. Reference equations for the six-minute walk test in healthy adults. Am J Respir Crit Care Med. 1998;158(5 pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 9.Landgraf JM, Maunsell E, Speechley KN, et al. Canadian-French, German and UK versions of the Child Health Questionnaire: methodology and preliminary item scaling results. Qual Life Res. 1998;7(5):433–445. doi: 10.1023/a:1008810004694. [DOI] [PubMed] [Google Scholar]
- 10.Ware JE, Jr, Kosinski M, Gandek B, et al. The factor structure of the SF-36 Health Survey in 10 countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51:1159–65. doi: 10.1016/s0895-4356(98)00107-3. [DOI] [PubMed] [Google Scholar]
- 11.Levran O, Desnick RJ, Schuchman EH. Identification of a 3’ acceptor splice site mutation (g2610c) in the acid sphingomyelinase gene of patients with Niemann-Pick disease. Hum Mol Genet. 1993;2:205–206. doi: 10.1093/hmg/2.2.205. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell LG, Merril CR. Affinity generation of single-stranded DNA for dideoxy sequencing following the polymerase chain reaction. Anal Biochem. 1989;178:239–242. doi: 10.1016/0003-2697(89)90631-3. [DOI] [PubMed] [Google Scholar]
- 13.Giraldo P, Cenarro A, Alfonso P, et al. Chitotriosidase genotype and plasma activity in patients with type 1 Gaucher's disease and their relatives (carriers and non carriers). Haematologica. 2001;86:977–984. [PubMed] [Google Scholar]
- 14.Aguilera B, Ghauharali-van der Vlugt K, Helmond MTJ, et al. Transglycosidase activity of chitotriosidase. Improved enzymatic assay for the human macrophage chitinase. J Biol Chem. 2003;278:40911–40916. doi: 10.1074/jbc.M301804200. [DOI] [PubMed] [Google Scholar]
- 15.Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: High-throughput, structure-specific and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 2005;36:207–224. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Pittis MG, Ricci V, Guerci VI, et al. Acid sphingomyelinase: Identification of nine novel mutations among Italian Niemann-Pick Type B patients and characterization of in vivo functional in-frame start codon. Hum Mutat. 2004;24:186–187. doi: 10.1002/humu.9263. [DOI] [PubMed] [Google Scholar]
- 17.Dardis A, Zampieri S, Filocamo M, Burlina A, Bembi B, Pittis MG. Functional in vitro characterization of 14 SMPD1 mutations identified in Italian patients affected by Niemann Pick type B disease. Hum Mutat. 2005;26:164. doi: 10.1002/humu.9353. 17. [DOI] [PubMed] [Google Scholar]
- 18.Desnick RJ. Enzyme replacement and enhancement therapies for lysosomal diseases. J Inherit Metab Dis. 2004;27:385–410. doi: 10.1023/B:BOLI.0000031101.12838.c6. [DOI] [PubMed] [Google Scholar]
- 19.Sauer M, Grewal S, Peters C. Hematopoietic stem cell transplantation for mucopolysaccharidoses and leukodystrophies. Klin Padiatr. 2004;216:163–168. doi: 10.1055/s-2004-822629. [DOI] [PubMed] [Google Scholar]
- 20.Grabowski GA, Hopkin RJ. Enzyme therapy for lysosomal storage disease: principles, practice, and prospects. Annu Rev Genomics Hum Genet. 2003;4:403–436. doi: 10.1146/annurev.genom.4.070802.110415. [DOI] [PubMed] [Google Scholar]
- 21.Wasserstein M, Larkin AE, Glass RB, Schuchman EH, Desnick RJ, McGovern MM. Growth restriction in children with type B Niemann-Pick disease. J Pediatr. 2003;142:424–428. doi: 10.1067/mpd.2003.113. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan P, Mazur A, Manor O, et al. Acceleration of retarded growth in children with Gaucher disease after treatment with alglucerase. J Pediatr. 1996;129:149–153. doi: 10.1016/s0022-3476(96)70203-2. [DOI] [PubMed] [Google Scholar]
- 23.Minai OA, Sullivan EJ, Stoller JK. Pulmonary involvement in Niemann-Pick disease: case report and literature review. Respir Med. 2000;94:1241–1251. doi: 10.1053/rmed.2000.0942. [DOI] [PubMed] [Google Scholar]
- 24.Mendelson D, Wasserstein MP, Desnick RJ, et al. Type B Niemann-Pick disease: findings at chest radiography, thin-section CT, and pulmonary function testing. Radiology. 2006;238:339–345. doi: 10.1148/radiol.2381041696. [DOI] [PubMed] [Google Scholar]
- 25.McGovern MM, Pohl-Worgall T, Deckelbaum RJ, et al. Lipid abnormalities in children with types A and B Niemann Pick disease. J Pediatr. 2004;145:77–81. doi: 10.1016/j.jpeds.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 26.Lee CY, Krimbou L, Vincent J, et al. Compound heterozygosity at the sphingomyelin phosphodiesterase-1 (SMPD1) gene is associated with low HDL cholesterol. Hum Genet. 2003;112:552–562. doi: 10.1007/s00439-002-0893-1. [DOI] [PubMed] [Google Scholar]
- 27.Kannel WB, Castelli WP, Gordon T. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann Intern Med. 1979;90:85–91. doi: 10.7326/0003-4819-90-1-85. [DOI] [PubMed] [Google Scholar]
- 28.Harzer K, Rolfs A, Bauer P, et al. Niemann-Pick disease type A and B are clinically but also enzymatically heterogeneous: pitfall in the laboratory diagnosis of sphingomyelinase deficiency associated with the mutation Q292 K. Neuropediatrics. 2003;34(6):301–306. doi: 10.1055/s-2003-44668. [DOI] [PubMed] [Google Scholar]
- 29.Pavlù-Pereira H, Asfaw B, Poupĕtová H, et al. Acid sphingomyelinase deficiency: phenotype variability with prevalence of intermediate phenotype in a series of twenty-five Czech and Slovak patients—a multi-approach study. J Inherit Metab Dis. 2005;28(2):203–227. doi: 10.1007/s10545-005-5671-5. [DOI] [PubMed] [Google Scholar]
- 30.Wasserstein MP, Aron A, Brodie SE, Simonaro C, Desnick RJ, McGovern MM. Acid sphingomyelinase deficiency: prevalence and characterization of an intermediate phenotype of Niemann-Pick disease. J Pediatr. 2006;149(4):554–559. doi: 10.1016/j.jpeds.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 31.McGovern MM, Wasserstein MP, Aron A, Desnick RJ, Schuchman EH, Brodie SE. Ocular manifestations of Niemann Pick disease type B. Ophthalmology. 2004;111:1424–1427. doi: 10.1016/j.ophtha.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 32.Deegan PB, Moran MT, McFarlane I, et al. Clinical evaluation of chemokine and enzymatic biomarkers of Gaucher disease. Blood Cells Mol Dis. 2005;35:259–267. doi: 10.1016/j.bcmd.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Brinkman J, Wijburg FA, Hollak CE, et al. Plasma chitotriosidase and CCL18: early biochemical surrogate markers in type B Niemann-Pick disease. J Inherit Metab Dis. 2005;28:13–20. doi: 10.1007/s10545-005-4416-9. [DOI] [PubMed] [Google Scholar]
- 34.Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 35.Kishnani PS, Corzo D, Nicolino M, et al. Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile onset Pompe disease. Neurology. 2007;68(2):99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- 36.Mihaylova C, Hantke J, Sinigerska I, Cherninkova S, Raicheva M, Bouwer S, Tincheva R, Khuyomdziev R, Bertranpetit J, Chandler D, Angelicheva D, Kremensky I, Seeman P, Tournev I, Kalaydjieva L. Highly variable neural involvement in sphingomyelinase-deficient Niemann–Pick disease caused by an ancestral Gypsy mutation. Brain. 2007;130(4):1050–1061. doi: 10.1093/brain/awm026. [DOI] [PubMed] [Google Scholar]
- 37.Simonaro CM, Desnick RJ, McGovern MM, Wasserstein MP, Schuchman EH. The demographics and distribution of type B Niemann-Pick disease: novel mutations lead to new genotype/phenotype correlations. Am J Hum Genet. 2002;71:1413–1419. doi: 10.1086/345074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanier MT, Ferlinz K, Rousson R, et al. Deletion of arginine (608) in acid sphingomyelinase is the prevalent mutation among Niemann-Pick disease type B patients from northern Africa. Hum Genet. 1993;92(4):325–330. doi: 10.1007/BF01247328. [DOI] [PubMed] [Google Scholar]
- 39.Volders P, Van Hove J, Lories RJU, et al. Niemann-Pick disease type B: an unusual clinical presentation with multiple vertebral fractures. Am J Med Genet. 2002;109(1):42–51. doi: 10.1002/ajmg.10278. [DOI] [PubMed] [Google Scholar]
- 40.Wasserstein MP, Desnick RJ, Schuchman, et al. The natural history of type B Niemann-Pick disease: results from a 10-year longitudinal study. Pediatrics. 2004;114(6) doi: 10.1542/peds.2004-0887. Available at: www.pediatrics.org/cgi/content/full/114/6/e672. [DOI] [PubMed]