Over past 10-15 years, a vast collection of studies have provided evidence indicating that reactive oxygen species (ROS), particularly superoxide (O2) •- and hydrogen peroxide (H2O2), contribute to the pathogenesis of cardiovascular diseases, such as heart failure and hypertension. Griendling and colleagues first demonstrated that NADPH oxidase present in the vasculature is a primary source of the elevated ROS levels1. Since these initial studies, NADPH oxidase-derived ROS in the kidney2, heart3, and brain4 have been linked to the development and progression of numerous cardiovascular-related diseases. More recently, however, mitochondria have also been identified as important sources of ROS in controlling cardiovascular function. Considering mitochondria are the primary source of ROS in most cells during normal respiration due to the leaking of electrons from the electron transport chain (ETC), perhaps it should not be all that surprising that mitochondrial-produced ROS are involved in pathophysiological conditions of the cardiovascular system.
To date, most of the evidence linking mitochondrial dysfunction and mitochondrial-produced ROS to the pathogenesis of cardiovascular diseases comes from studies on the peripheral renin-angiotensin system5. For example, using a model of cardiac ischemic reperfusion injury, Kimura et al. reported that angiotensin II (AngII)-induced preconditioning is mediated by mitochondrial-produced ROS6. The authors further demonstrate that AngII-induced NADPH oxidase-derived ROS lie upstream of mitochondrial-produced ROS, thus, implicating a ROS-induced ROS mechanism. Similarly, it was recently demonstrated that in aortic endothelial cells AngII-induced NADPH oxidase activation leads to an increase in mitochondrial ROS production as well as mitochondrial dysfunction, as determined by a decrease in mitochondrial membrane potential and mitochondrial respiration7. Together, these studies and others (detailed elsewhere5) clearly illustrate a role for mitochondrial-produced ROS and mitochondrial dysfunction in peripheral tissues in the pathogenesis of cardiovascular diseases, primarily those associated with increased AngII signaling. However, in the central nervous system (CNS) the contribution of defective mitochondria and mitochondrial-produced ROS in cardiovascular diseases has been mostly overlooked.
In this issue of Hypertension, Chan and colleagues8, studying neurogenic hypertension, provide new evidence that indeed mitochondrial dysfunction and the subsequent production of mitochondrial-localized ROS in the CNS, particularly the rostral ventrolateral medulla (RVLM), play a critical role in cardiovascular function. More specifically, they report a decrease in ETC Complex I and Complex III activity accompanied by an increase in mitochondrial-produced ROS, particularly O2 •- and H2O2, in the RLVM of spontaneously hypertensive rats (SHR). Direct RVLM administration of coenzyme Q10 (CoQ10), a mitochondrial electron transporter and antioxidant, restored electron transport capacity, decreased mitochondrial-localized ROS production, and significantly reduced mean systemic arterial pressure (MSAP) and sympathetic neurogenic vasomotor tone in SHR. Using mitochondrial ETC inhibitors rotenone and antimycin A, Chan et al. provide further evidence that mitochondrial dysfunction in the RVLM results in mitochondrial-produced ROS, which in turn induce changes in cardiovascular function. In normotensive WKY rats or prehypertensive SHR, RVLM administration of rotenone or antimycin A significantly elevated mitochondrial H2O2 production as well as MSAP and power density of vasomotor activity. Similar to its action in hypertensive SHR, CoQ10 markedly attenuated the augmented cardiovascular responses induced by ETC inhibition in normotensive animals. These data indicate that diminished mitochondrial ETC activity and the subsequent production of ROS in the RVLM contribute to the SHR hypertensive phenotype.
To address the hypothesis that a cytoplasm-to-mitochondria ROS-induced ROS mechanism in the RLVM is involved in neurogenic hypertension, Chan and colleagues utilized an intracerebroventricular (ICV) AngII infusion model8. ICV AngII infusion results in an increase in sympathetic tone and blood pressure, at least in part, via NADPH oxidase activation in the RVLM9. Compared to ICV infusion of artificial CSF, AngII infusion decreased ETC Complex I-III activity while increasing mitochondrial-produced H2O2 in the RVLM. Inhibition of NADPH oxidase via p22phox antisense significantly attenuated the AngII-induced increase in mitochondrial H2O2 levels, thus implicating a ROS-induced ROS mechanism initiated by NADPH oxidase-derived ROS. Similar to the SHR experiments, RVLM administration of CoQ10 inhibited the AngII-induced increase in mitochondrial-produced ROS, MSAP, and sympathetic vasomotor tone. Together, these studies strongly suggest that increased sympathetic tone and the pathogenesis of neurogenic hypertension are mediated by mitochondrial ETC dysfunction and an ensuing increase in mitochondrial-produced ROS in the RVLM.
In an attempt to further support the ROS-induced ROS hypothesis, Chan et al. used adenoviral-mediated gene transfer to overexpress three different antioxidants, copper/zinc superoxide dismutase (CuZnSOD or SOD1), manganese superoxide dismutase (MnSOD or SOD2), and catalase in the RVLM of SHR. Previously, this group demonstrated that SOD1, SOD2, or catalase overexpression in the RVLM significantly reduces the elevated arterial pressure of SHR10. In the current study, they report that all three antioxidants restored Complex I and III activity, and reduced the elevated levels of mitochondrial-localized O2 •- and H2O2. Considering SOD1, a O2 •- scavenging enzyme, and catalase, a H2O2 scavenger, are primarily localized in the cytoplasm and peroxisomes, respectively, the authors conclude that ROS generated in the cytosolic compartment induce ETC damage and an increase in mitochondrial-produced ROS. However, alternative interpretations should also be considered. For example, SOD1 is also present in mitochondria11, and thus it is possible that overexpression of mitochondrial-localized SOD1 protects ETC complexes and scavenges mitochondrial-produced ROS. Regarding the catalase overexpression experiments, it remains unclear how the H2O2-scavenging, peroxisome-targeted enzyme reduces both O2 •- and H2O2 in RVLM mitochondria of SHR. Future studies designed to measure cytoplasmic- and mitochondrial-localized ROS simultaneously will help substantiate the ROS-induced ROS mechanism postulated by Chan and colleagues.
Perhaps the most exciting aspect of the study by Chan et al. is that it provides evidence that damaged ETC complexes are a source for mitochondrial-produced ROS in CNS-dependent cardiovascular responses. Until this report, a potential source of mitochondrial-produced ROS in AngII-stimulated neurons, as suggested in previous studies, remained speculative. For example, Zimmerman et al. first proposed a role for mitochondrial-produced ROS in brain angiotensinergic signaling by reporting that overexpressing SOD2, the mitochondrial-targeted isoform of SOD, in the brain significantly attenuates the cardiovascular responses induced by ICV administration of AngII12. However, this earlier study failed to identify a potential source of AngII-induced mitochondrial-produced ROS in central neurons. More recently, Nozoe et al. also showed that SOD2 overexpression in the brain, notably the RVLM, attenuates the acute pressor response of AngII microinjected into the RVLM13. Similar to Chan et al.8, Nozoe and coauthors suggest that AngII signaling in neurons involves a ROS-induced ROS mechanism which starts with NADPH oxidase-derived ROS and ends with mitochondrial-produced ROS. However, in contrast, Nozoe et al. report that AngII does not alter the activity of ETC complexes13. This discrepancy is likely due to the fact that Nozoe and colleagues measured the acute effect of AngII (1 hr stimulation) on ETC complex activity in cultured PC-12 cells, while Chan et al. measured complex activity in vivo in RVLM tissue after 5 days of ICV AngII infusion. As discussed earlier, the fact that rotenone or antimycin A, two ETC inhibitors, microinjected into the RVLM increased mitochondrial-localized ROS, MSAP, and sympathetic tone strengthens the conclusion by Chan and colleagues that in neurons, damaged ETC complexes are a source of mitochondrial-produced ROS. Nevertheless, further experiments, perhaps utilizing genetic strategies to inhibit ETC activity in central neurons are required to corroborate this conclusion.
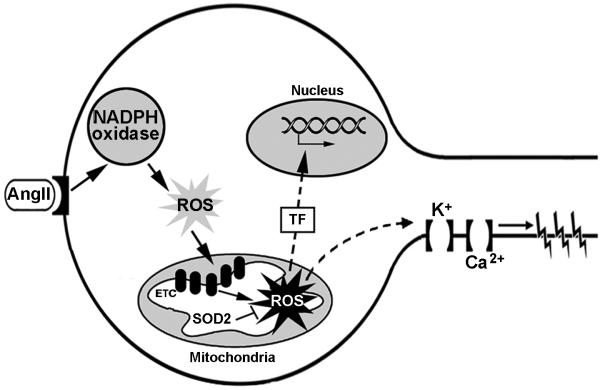
In summary, Chan and coauthors report a role for mitochondrial dysfunction and mitochondrial-produced ROS in the CNS in the pathogenesis of neurogenic hypertension. The data indicate that impaired ETC complexes are a source of mitochondrial-localized ROS and that NADPH oxidase-derived ROS may mediate the impairment of the ETC (Figure). Additional studies are required to examine the downstream mechanism(s) by which mitochondrial-produced ROS increase sympathetic tone and drive the development of hypertension. Such studies should utilize mitochondrial-targeted antioxidants, including SOD2, and focus on the redox sensitivity of neuronal ion channels as well as redox control of transcription factors (Figure). The results of these future experiments may strengthen the conclusions by Chan et al. and may help distinguish damaged ETC complexes and mitochondrial-produced ROS as novel therapeutic targets in neurogenic hypertension.
Figure. Proposed AngII signaling pathway in RVLM neurons involving mitochondrial dysfunction and mitochondrial-produced ROS.
Chan et al.10 provide evidence indicating that AngII stimulation of neurons in the RVLM increases NADPH oxidase-derived ROS, which in turn damage mitochondrial ETC complexes leading to an increase in mitochondrial-produced ROS (solid-line arrows). Additional experiments utilizing mitochondrial-targeted antioxidants, such as SOD2, are needed to determine the downstream signaling events mediated by mitochondrial-produced ROS. Possible targets of mitochondrial-produced ROS include redox sensitive transcription factors (TF) and/or ion channels (broken-line arrows).
Acknowledgments
Sources of Funding M.C.Z's research is supported by a NIH Centers of Biomedical Research Excellence (CoBRE) grant awarded to the Redox Biology Center at the University of Nebraska - Lincoln. I.H.Z's research is supported by NIH grant PO-1 HL62222.
Footnotes
Disclosures None
References
- (1).Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- (2).Wilcox CS. Reactive oxygen species: roles in blood pressure and kidney function. Curr Hyperten Rep. 2002;4:160–166. doi: 10.1007/s11906-002-0041-2. [DOI] [PubMed] [Google Scholar]
- (3).Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- (4).Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol. 2004;84:125–149. doi: 10.1016/j.pbiomolbio.2003.11.009. [DOI] [PubMed] [Google Scholar]
- (5).de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol. 2007;27:545–553. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]
- (6).Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension. 2005;45:860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- (7).Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- (8).Chan SHH, Wu KLH, Chang AYW, Tai MH, Chan JYH. Oxidative impairment of mitochondrial electron transport chain complexes in RVLM contributes to neurogenic hypertension. Hypertension. 2008 doi: 10.1161/HYPERTENSIONAHA.108.116905. [DOI] [PubMed] [Google Scholar]
- (9).Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–H2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- (10).Chan SH, Tai MH, Li CY, Chan JY. Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radic Biol Med. 2006;40:2028–2039. doi: 10.1016/j.freeradbiomed.2006.01.032. [DOI] [PubMed] [Google Scholar]
- (11).Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem. 2001;276:38388–38393. doi: 10.1074/jbc.M105395200. [DOI] [PubMed] [Google Scholar]
- (12).Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- (13).Nozoe M, Hirooka Y, Koga Y, Araki S, Konno S, Kishi T, Ide T, Sunagawa K. Mitochondria-derived reactive oxygen species mediate sympathoexcitation induced by angiotensin II in the rostral ventrolateral medulla. J Hypertens. 2008;26:2176–2184. doi: 10.1097/HJH.0b013e32830dd5d3. [DOI] [PubMed] [Google Scholar]