Abstract
Objective
Intraventricular extension of intracerebral hemorrhage (IVH) is an independent predictor of poor outcome. IVH volume may be important in outcome prediction and management; however, it is difficult to measure routinely.
Design and Patients
We reviewed the charts and computed tomographies of a cohort of consecutive patients with IVH. The cohort was divided into two groups: index and validation by random sampling. IVH and intracerebral hemorrhage (ICH) volume were measured manually in all patients. IVH was also graded using a simple classification system termed IVH score (IVHS). Clinical outcome was determined by the modified Rankin Scale (mRS) at discharge and in-hospital death. Poor outcome was defined as mRS 4–6.
Main Results
One hundred seventy-five patients were analyzed, 92 in the index group and 83 in the validation group. Exponential regression yielded the following formula for estimating IVH volume (mL): eÎVHS/5 (R2 = .75, p < 0.001). The IVH estimation formula was then verified in the validation group (R2 = .8, p < 0.001). The following correlations with mRS were obtained: IVH volume R = .305; ICH volume R = .468; total volume [TV] R = .571 (p < 0.001 for all three correlations). Partial correlation of TV with mRS controlling for ICH volume yielded R = .3 for TV (p < 0.001). Logistic regression model comparing ICH and TV association with poor outcome yielded the following: ICH odds ratio = 5.2, 95% confidence interval 2.3–11.6, p < 0.001; TV odds ratio = 41.6, 95% confidence interval 9.6–180.6, p < 0.001. Substituting TV for ICH volume in the ICH score resulted in a significant increase in the specificity from 64% to 87% for predicting mortality.
Conclusions
IVHS enables clinicians to rapidly estimate IVH volume. The addition of IVH to ICH volume increases its predictive power for poor outcome and mortality significantly. IVHS and TV may be used in clinical practice and clinical trials of patients with ICH.
Keywords: intracranial hemorrhage, intraventricular hemorrhage, outcome prediction
Spontaneous intracerebral hemorrhage (ICH) accounts for one fifth of all strokes. It is a devastating condition with 30% mortality and a high rate of morbidity among survivors (1-5). Most cases are attributed to hypertension or amyloid angiopathy (2, 5, 6). Intraventricular extension of intracerebral hemorrhage (IVH) that occurs in 45% of cases (7) is a known independent predictor of poor outcome (1, 7-14) and several studies have demonstrated a direct relation between IVH volume and poor outcome or mortality (20, 25, 26). Yet, most studies investigating IVH volume use sophisticated and time-consuming volumetric analyses (15) that are impractical for routine clinical use and clinicians still lack a method for easily obtaining an estimate of the IVH volume. The purpose of this study was to create a useful tool for rapid determination of IVH volume and to further explore the prognostic significance of IVH volume. Specifically, we tried to assess the relationship between IVH volume or total volume (TV the combination of ICH and IVH volume) and clinical outcome.
METHODS
Study Design and Population
A retrospective chart review of all patients admitted to our stroke center between April 2003 and June 2006 with the diagnosis of ICH. Patients were included if they suffered from spontaneous nontraumatic ICH and had evidence of IVH on computed tomography (CT) during admission done within 24 hours of onset. Patients were excluded from the analysis if they had ICH secondary to vascular malformations, tumor, or hemorrhagic conversion of an infarct. All CT scans were done using identical technique (slice thickness 5 mm, gantry tilt −16). We also excluded patients who had CT of inadequate quality for measurement purposes. The study cohort was divided into an index group used for model development and a validation group by random sampling.
Measurements
Two of the authors (HH and AB) were blinded to clinical outcomes and independently reviewed all the admission CT scans to verify ICH location, ICH volume, and IVH presence. Hydrocephalus was coded as present or absent (questionable cases were coded based on the official radiology report). ICH location was determined by the anatomical structure that contained the majority of the hematoma. ICH volume was determined using the ABC/2 method (16). Two of the authors (HH and ND) independently measured IVH volume by hand-drawn regions of interest around each area of intraventricular blood in every slice and in each lateral ventricle, third ventricle, and fourth ventricle separately. The regions of interest area were calculated automatically by the Picture Archiving and Communication Systems software (General Electric Centricity workstation). Each region of interest area was then multiplied by the slice thickness (5 mm) and added together to obtain the IVH volume in milliliter in each ventricle. IVH volume was obtained as the sum of the volumes from the four ventricles. Maximal ventricular volume (the potential volume of blood in each ventricle) was determined for each ventricle.
IVH Scoring
Our primary goal was to develop a simple grading system to estimate IVH volume measurement. This system was partially based on scores previously developed for grading IVH (17-20). Yet, we chose to develop a new score because none of the previous scores were intended to estimate IVH volume.
The a priori assumptions underlying our grading system were as follows: 1) the third and fourth ventricles contribute much less to the ventricular volume than the lateral ventricles and 2) in the presence of hydrocephalus, the ventricular volume increases through expansion. We graded each lateral ventricle with a score of 0 (no blood or small amount of layering), 1 (up to one third filled with blood), 2 (one to two thirds filled with blood), or 3 (mostly or completely filled with blood). The third and fourth ventricles received a score of 0 for no blood or 1 if they were partially or completely filled with blood. Hydrocephalus was coded as present (1) or absent (0).
Two of the authors (HH and AB) were blinded to volume measurements and outcomes while they independently scored the IVH in each ventricle for all patients. This was done before and was unrelated to the volume measurements. All medical charts were reviewed for baseline demographics, clinical presentation, laboratory values, external ventricular drainage insertion, and outcome measures. Clinical outcome was assessed on hospital discharge using the modified Rankin Scale (mRS). Patients with mRS 4–6 on hospital discharge were considered to have a poor outcome. Do-not-resuscitate status was captured at admission. The study was approved by the Institutional Review Board.
Statistical Analysis
The analysis was performed using SPSS version 15 (SPSS, Chicago, IL). Interclass correlation was used to assess inter-rater reliability for ICH volume, IVH volume, and IVH scoring. A sub-sample of IVH cohort was randomly selected using a Bernouli function (using 0.5 as the probability coefficient) as the index group for developing IVH scoring system and the conversion formula to IVH volume. The second half of the cohort was used to assess the validity of the IVH grading system for predicting IVH volume. The measured IVH volume was log transformed to achieve normality. A linear regression of the IVH grade with hydrocephalus to IVH volume was done to obtain the correction factor for hydrocephalus and produce the final formula for IVH score (IVHS). Additional regression was then performed to obtain the conversion formula from IVHS to IVH volume. After calculating the volume using the conversion formula, the calculated volume and measured volume were entered into a regression model to study the correlation between the two in the validation cohort. Cronbach’s alpha assessed internal reliability of the IVHS within each cohort.
Receiver operating characteristics analysis was used to determine the sensitivity and specificity of the different volumes and scores in predicting mortality and poor outcome and obtain volume cutoffs for both poor outcome and mortality. Partial correlation was used to explore the association of IVH, ICH, and TV with outcome (mRS) as well as their relative contribution to the prediction. In addition, we used logistic regression of individual volumes to compare the fit of the volume cutoffs with poor outcome and mortality between volumes using the log-likelihood ration method (21). Cohen’s method was used to compare the strength of correlation between the different outcome scores and mRS (22). Logistic regression was used to determine independent association of variables with poor outcome and mortality. p value <0.05 was considered significant.
RESULTS
We identified 178 patients with IVH. Four patients were excluded because of poor quality CT (severe motion artifacts) and the final cohort consisted of 174 patients who met inclusion criteria. The patients’ characteristics are shown in Table 1. The interclass correlation between the two raters for IVH volume was 0.995 with a 95% confidence interval (CI) of 0.973–0.999 (p < 0.001). The interclass correlation between the two raters for IVH grading was 0.915 with a 95% CI of 0.835–0.955 (p < 0.001). Additional sample of 20 patients was scored by two other people with far less clinical training after a brief explanation of the score: a neurology resident and a medical student. The interclass correlation for their scoring compared with the stroke neurologist was resident 0.941 (p < 0.001, 95% CI 0.846–0.977) and medical student 0.8 (p = 0.001, 95% CI 0.48–0.92).
Table 1.
Baseline characteristics
| Index Group (n = 91) | Validation Group (n = 83) | p | |
|---|---|---|---|
| Age (mean ± SD) | 59 ± 15 | 61 ± 14 | 0.3 (TT) |
| NIHSS (mean ± SD) | 18 ± 10 | 19 ± 9 | 0.7 (TT) |
| Mortality (%) | 29 (31.5) | 26 (31) | 0.97 (CS) |
| Poor outcome (%) | 69 (75) | 63 (76) | 0.7 (CS) |
| Mode of arrival | 0.9 (CS) | ||
| Direct admission | 38 | 35 | |
| Transfer | 53 | 48 | |
| Early limitation of care (%) | 10 (11) | 12 (14.5) | 0.5 (CS) |
| EVD insertion (%) | 30 (33) | 14 (16.9) | 0.015 (CS) |
| IVT (%) | 5 (5.5) | 0 (0) | 0.06 (FE) |
NIHSS, National Institutes of Health Stroke Scale; TT, Student’s t test; CS, chi-square test; EVD, extraventricular drainage; IVT, intraventricular tPA; SD, standard deviation.
Baseline characteristics of the index and validation groups.
Model Development in the Index Group
Ninety-one patients were randomly selected from the IVH cohort of 174 patients for the index cohort. Cronbach’s alpha for the five components of IVH scoring was 0.741. The maximal ventricular volume is shown in Table 2. We observed a volume ratio of 9–10:1 between the lateral ventricles and the third and fourth ventricles, and therefore weighted the lateral ventricle score by a factor of 3 (thus, yielding a ratio of 9:1 between the lateral and midline ventricles). Linear regression of the IVH grade and hydrocephalus to IVH volume yielded the following formula (p < 0.001 for both components):
Table 2.
Maximal ventricular volume
| Ventricle | Median Volume in Milliliter (Interquartile Range) |
|---|---|
| Right lateral | 24.2 (11–37.4) |
| Left lateral | 25.7 (16.8–34.6) |
| Third | 2.4 (0.7–4.2) |
| Fourth | 3.0 (1.5–4.5) |
Maximal ventricular volume of each ventricle. The maximal volume was calculated based on the intraventricular extension of intracerebral hemorrhage volume in each ventricle. Only ventricles that were noted to be completely filled with blood were included.
Therefore, the final formula for calculating the IVHS is
where RV stands for right ventricle score (0–3), LV for left ventricle score (0–3), III for third ventricle score (0, 1), IV for fourth ventricle score (0, 1), and H for the presence of hydrocephalus (0, 1). IVHS ranges from 0 (no IVH) to 23 (all ventricles filled with blood and hydrocephalus present). The formula for converting IVHS to volume is
Two examples of IVH grading are shown in Figure 1. Table 3 provides a rapid-conversion table. A reference card and a conversion calculator may be found as supplementary material to the article for easy conversion.
Figure 1.
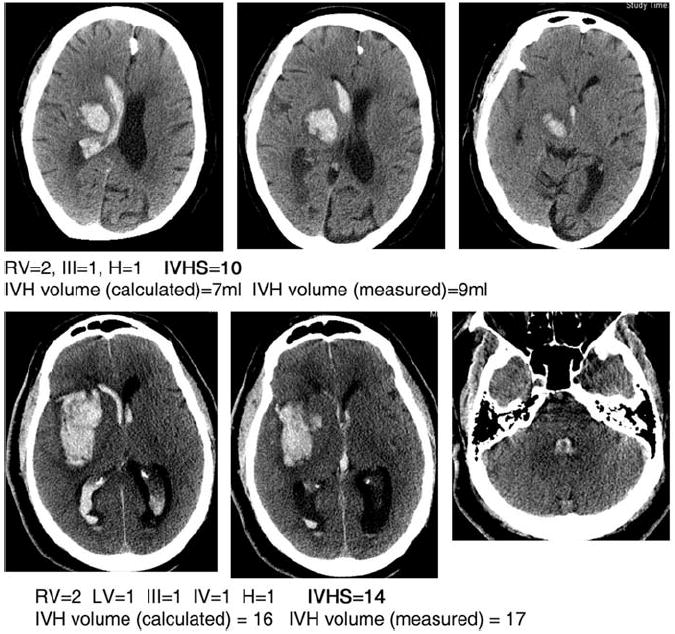
Intraventricular extension of intracerebral hemorrhage (IVH) grading. LV, left ventricle; RV, right ventricle; III, third ventricle score; H, hydrocephalus; IVHS, IVH score.
Table 3.
A quick reference for converting IVHS to IVH volume
| IVH Score | IVH Volume (mL) | IVH Score | IVH Volume (mL) |
|---|---|---|---|
| 1 | 1.2 | 13 | 13.5 |
| 2 | 1.5 | 14 | 16.4 |
| 3 | 1.8 | 15 | 20.1 |
| 4 | 2.2 | 16 | 24.5 |
| 5 | 2.7 | 17 | 30.0 |
| 6 | 3.3 | 18 | 36.6 |
| 7 | 4.1 | 19 | 44.7 |
| 8 | 5.0 | 20 | 54.6 |
| 9 | 6.0 | 21 | 66.7 |
| 10 | 7.4 | 22 | 81.5 |
| 11 | 9.0 | 23 | 99.5 |
| 12 | 11.0 |
IVHS, intraventricular extension of intracerebral hemorrhage (IVH) score.
A rapid conversion table for converting IVHS to IVH volume (in mL).
Validation Group
The validation group consisted of 83 patients. Cronbach’s alpha of the five components of IVHS was 0.714. Regression of calculated IVH volume to measured IVH volume (Fig. 2) yielded a good fit with R2 = .8 (p < 0.001). This indicates that the IVHS conversion formula performs equally well in the validation and index cohort.
Figure 2.
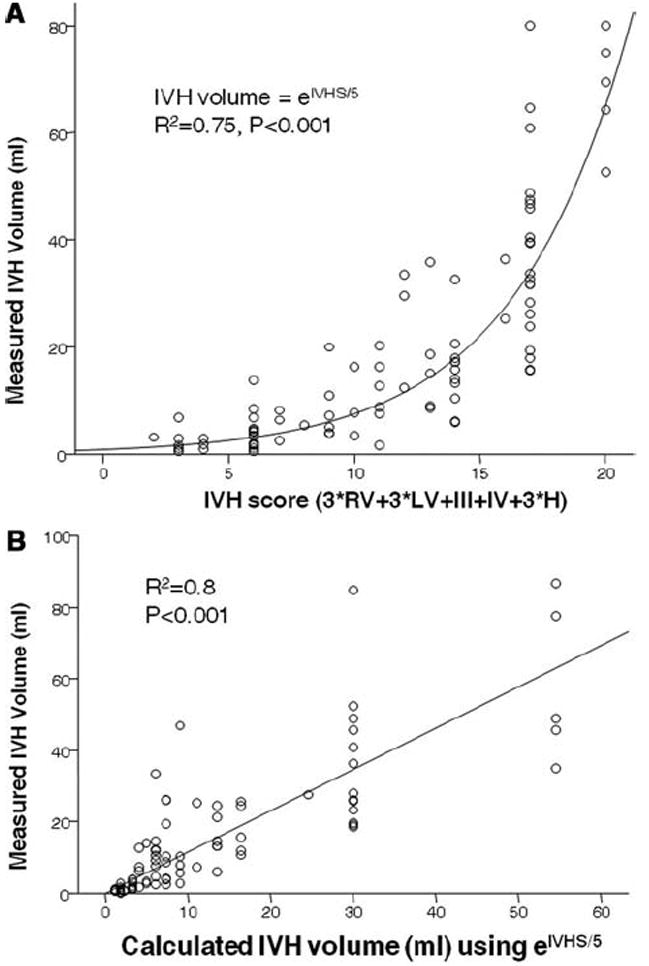
Curve fitting of intraventricular extension of intracerebral hemorrhage (IVH) score (IVHS) to IVH volume in the index cohort (A) and calculated to measured IVH volume in the validation cohort (B). IVHS-IVH Score. LV, left ventricle; RV, right ventricle; III, third ventricle score.
Exploring IVH, ICH, and Total Volume to Clinical Outcome
Using the entire cohort of 174 patients, we explored the correlation of IVH, ICH, and TV (TV, the sum of ICH and IVH volume) with outcome (mRS) using bivariate correlation: IVH volume R = .305; ICH volume R = .468; TV R = .571 (p < 0.001 for all three correlations). Partial correlation of TV with mRS controlling for ICH volume yielded R = .3 for TV (p < 0.001). This represents the additional variance of the outcome explained by adding IVH to ICH volume.
Further exploration of TV using receiver operating characteristics curve showed a cutoff of 40 mL for poor outcome and 60 mL for mortality (Fig. 3). In fact, beyond 50 mL of TV, 100% of patients had a poor outcome. Receiver operating characteristics analysis of ICH volume yielded a cutoff of 30 mL for mortality and 25 mL for poor outcome. We then ran logistic regression assessing the association of the cutoffs for ICH volume and TV (both measured and calculated) with poor outcome and mortality (Table 4). In both categories, TV was a significantly better predictor than ICH volume alone. The measured and calculated values performed similarly.
Figure 3.
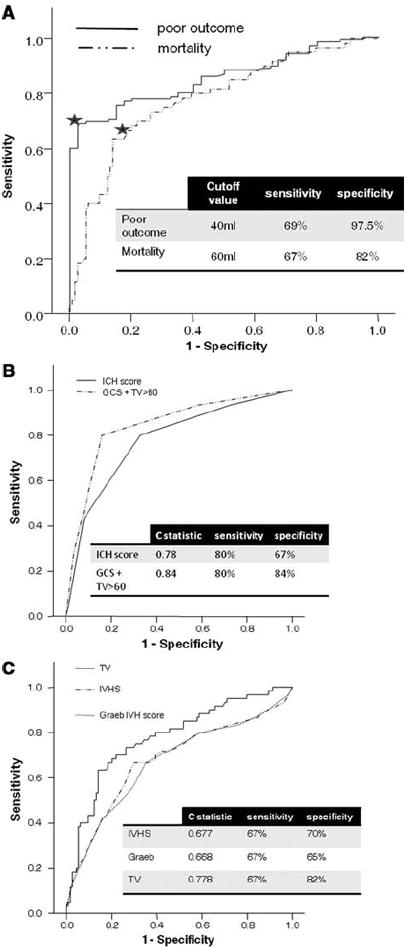
Receiver operating characteristic curves. A, Total volume (TV) as a predictor of poor outcome (modified Rankin Scale 4–6) and mortality. The star marks the cutoff point. B, Comparing Graeb Score, intraventricular extension of intracerebral hemorrhage (IVH) score (IVHS) and TV as predictors of mortality. C, Comparing intracerebral hemorrhage (ICH) Score and composite score (Glasgow Coma Scale [GCS ] + TV >60 mL) as predictors of mortality.
Table 4.
Logistic regression models for the association of ICH and TV (both measured and calculated) cutoffs with poor outcome and mortality
| Poor Outcome Model |
Mortality Model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICH >25 mL | TV >40 mL |
LRT Comparing ICH and TV (M/C) | p (M/C) | ICH >30 mL | TV >60 mL (M/C) |
LRT Comparing ICH and TV (M/C) | p (M/C) | |||
| M | C | M | C | |||||||
| 2-log likelihood | 168.8 | 130.0 | 139.3 | 38.8/29.5 | <0.001 | 204.6 | 184.0 | 187.4 | 20.6/17.2 | <0.001 |
| OR | 5.2 | 41.6 | 31.9 | 4.3 | 8.9 | 8.1 | ||||
| 95% CI for OR | 2.3–11.6 | 9.6–180.6 | 7.4–138 | 2.2–8.5 | 4.3–18.1 | 4–16.5 | ||||
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
M, measured TV (intracerebral hemorrhage [ICH] volume + measured intraventricular extension of ICH [IVH] volume); C, calculated TV (ICH volume + calculated IVH volume using eIVHS/5); LRT, likelihood ratio test; OR, odds ratio; CI, confidence interval.
Logistic regression of individual volume cutoffs to poor outcome (modified Rankin Scale 4–6) and mortality.
Predictors of Mortality and Poor Outcome
The role of TV in predicting poor outcome and mortality was explored using logistic regression. The following known predictors were entered into the model: age, Glasgow Coma Scale (GCS), infratentorial location, hydrocephalus, and TV. The only variables retaining independent association with mortality were GCS (p < 0.001, odds ratio [OR] = 0.73, 95% CI 0.64–0.83) and TV (p = 0.001, OR = 4.4, 95% CI 1.8–10.6). The variables retaining independent association with poor outcome were GCS (p < 0.001, OR = 0.65, 95% CI 0.52–0.8) and TV (p < 0.001, OR = 20.1, 95% CI 4.3–95.7).
Self-fulfilling Prophecy
The role of early care limitation (23) in predicting mortality was explored using logistic regression with death as the dependent variable. The variables previously found as predictors of mortality (TV and GCS) were entered into the model along with admission code status (coded as limitation of care vs. full code) and age. Early limitation of care was independently associated with mortality (p = 0.003, OR = 11, 95% CI 2.3–53.2). A second model with GCS, TV, and age was used to explore the role of withdrawal of care (as opposed to other causes of death) in predicting death. The decision to withdraw care was not an independent predictor of mortality (p = 0.9).
Comparing IVHS with Previous Scores
To further validate our score, we compared the predictive value of IVHS for mortality with two previously published scores: the IVH scoring system by Graeb et al (17) and the ICH score by Hemphill et al (24). The comparison with the Graeb score was performed using both IVHS and TV whereas for the ICH score we used a composite score of GCS (scored the same way as in the ICH score) and TV >60 (scored as 1 point). Figure 3a, b shows the receiver operating characteristic curves for both scores. Although IVHS and the Graeb score performed similarly, once TV was used the specificity of the score increased substantially. We further analyzed the correlation of the same volumes and scores with outcome (mRS) using Spearman’s correlation: IVHS R = .346; Graeb score R = .332 (p = 0.52 comparing the correlation of IVHS and Graeb score with mRS); TV (calculated) R = .571 (p = 0.004 comparing Graeb score and TV); ICH score R = .589; composite score (GCS + TV >60) R = .689 (p < 0.001 comparing ICH score and composite score; p < 0.001 for all correlations). Taken together, these results suggest that measuring IVH volume using IVHS to produce TV achieved the most robust correlation with outcome. Substituting TV for ICH volume in the ICH score produces a score that is more specific for mortality and has a better correlation with outcome.
DISCUSSION
IVH remains a poorly understood phenomenon. Previous work has demonstrated that IVH volume is an important factor contributing to outcome and survival. Two investigators studying IVH using a 12-point grading system found an association between high IVH grade and poor outcome (17, 20). The relationship of IVH volume and outcome has also been studied in a series of 47 patients (25), which identified a “lethal volume” of 20 mL, and there was an observed association between TV and poor outcome. Another study showed that IVH volume predicts mortality independently of the GCS (13). Most recently, the dynamic nature of IVH was studied in a subanalysis of the activated factor VII phase II trial (26) demonstrating the importance of changes in IVH volume. However, the study of IVH volume and its clinical application has been hampered by the difficulty of measuring the volume of blood in routine clinical practice. Unlike ICH where the hematoma is relatively well defined and lends itself to volume approximation methods such as ABC/2 (16), IVH is more diffuse and involves multiple structures. The results from this study demonstrate that using a simple grading system, IVH volume can be closely estimated within minutes after acquisition of the head CT, requiring only visual inspection of the CT and a reference card. Our findings are in agreement with previous studies showing the important impact of IVH volume on clinical outcome (13, 25, 26).
The mechanisms by which IVH volume affects outcome likely include increased intracranial pressure with reduced cerebral perfusion (27), mechanical disruption, ventricular wall distension (28), and possibly an inflammatory response (29, 30). Although IVH volume in itself is associated with poor outcome, we observed the previously reported (25) stronger association with TV. We identified a cutoff of TV >40 mL beyond which the patients were 41 times more likely to have a poor outcome and a “poor-outcome threshold” of 50 mL above which 100% of patients had a poor outcome. A cutoff of TV >60 mL was similarly identified for mortality; however, we did not identify a “lethal volume” (in our cohort patients with TV >60 mL had a 60% rate of mortality) as previously reported (25). This discrepancy may be explained by differences in measurement techniques, improved image quality and resolution in our study, and advances in the intensive care of IVH patients.
The ability to estimate volume and, thus, generate TV sets IVHS as a unique measure of prognostic value separate from previously published scores. From the clinical standpoint, numerous scales have been designed to predict outcome after ICH (10, 12, 24-26, 31, 32). Most scales code IVH as absent or present. We have shown that for patients with IVH, our composite score using only TV and GCS is superior to the prevalent ICH score (24) in predicting mortality. This may reflect the additional impact of IVH volume on outcome. TV may be used in two ways in clinical research rather than using the traditional ICH volume: to stratify patients when assessing treatment effect; and as a signal for treatment success by showing an increase in the proportion of patients achieving favorable outcome with TV >40 mL.
Limitations
This study has several limitations. It is retrospective and as such the outcome correlation may need to be validated prospectively. Our clinical endpoint occurs at discharge from the hospital and not at a set time. Because patients with severe strokes tend to have longer stays, this also introduces bias. In addition, we did not have 90-day outcome and it is possible that some patients discharged with a poor outcome may improve by that time point. Our choice to define poor outcome as mRS 4–6 on discharge (instead of the traditional mRS 3–6 at 90 days) is meant to partially compensate for this deficiency. To score the IVHS, hydrocephalus needs to be identified. This may present a problem with inexperienced clinicians or when hydrocephalus is mild and in places when radiology advice is not readily available. Finally, the efficacy of surgical intervention could not be assessed in this work. Rather, the outcome of our cohort represents patients who received routine ICU and neurosurgical care in a large stroke center. Finally, our study reaffirms the concept of “self-fulfilling prophecy” (23) in IVH patients. Even after controlling for age, TV, and GCS early limitation of care predicted mortality in our cohort.
CONCLUSION
IVHS allows for rapid estimation of actual IVH volume within minutes from the initial CT scan of patients with ICH. IVH volume combined with ICH volume allows for estimating TV, which is a powerful determinant of outcome. This information may be used in the acute setting to inform patients and families and in research settings to stratify patients in treatment vs. control groups.
Acknowledgments
We thank Alice Z. Chuang, PhD for valuable statistical support.
Footnotes
See also p. 1152.
The authors have not disclosed any potential conflicts of interest.
References
- 1.Gates PC, Barnett HJ, Vinters HV, et al. Primary intraventricular hemorrhage in adults. Stroke. 1986;17:872–877. doi: 10.1161/01.str.17.5.872. [DOI] [PubMed] [Google Scholar]
- 2.Lang EW, Ren Ya Z, Preul C, et al. Stroke pattern interpretation: The variability of hypertensive versus amyloid angiopathy hemorrhage. Cerebrovasc Dis. 2001;12:121–130. doi: 10.1159/000047691. [DOI] [PubMed] [Google Scholar]
- 3.Naff NJ. Intraventricular hemorrhage in adults. Curr Treat Options Neurol. 1999;1:173–178. doi: 10.1007/s11940-999-0001-0. [DOI] [PubMed] [Google Scholar]
- 4.Naff NJ, Tuhrim S. Intraventricular hemorrhage in adults: Complications and treatment. New Horiz. 1997;5:359–363. [PubMed] [Google Scholar]
- 5.Woo D, Broderick JP. Spontaneous intracerebral hemorrhage: Epidemiology and clinical presentation. Neurosurg Clin N Am. 2002;13:265–279. v. doi: 10.1016/s1042-3680(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty ML, Woo D, Haverbusch M, et al. Racial variations in location and risk of intracerebral hemorrhage. Stroke. 2005;36:934–937. doi: 10.1161/01.STR.0000160756.72109.95. [DOI] [PubMed] [Google Scholar]
- 7.Hallevi H, Albright K, Aronowski J, et al. Intraventricular hemorrhage: Anatomic relationships and clinical implications. Neurology. 2008;70:848–852. doi: 10.1212/01.wnl.0000304930.47751.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattathiri PS, Gregson B, Prasad KS, et al. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: Results from the STICH trial. Acta Neurochir Suppl. 2006;96:65–68. doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- 9.Diamond P, Gale S, Stewart K. Primary intracerebral haemorrhage—Clinical and radiologic predictors of survival and functional outcome. Disabil Rehabil. 2003;25:689–698. doi: 10.1080/0963828031000090470. [DOI] [PubMed] [Google Scholar]
- 10.Hallevy C, Ifergane G, Kordysh E, et al. Spontaneous supratentorial intracerebral hemorrhage. Criteria for short-term functional outcome prediction. J Neurol. 2002;249:1704–1709. doi: 10.1007/s00415-002-0911-1. [DOI] [PubMed] [Google Scholar]
- 11.Lisk DR, Pasteur W, Rhoades H, et al. Early presentation of hemispheric intracerebral hemorrhage: Prediction of outcome and guidelines for treatment allocation. Neurology. 1994;44:133–139. doi: 10.1212/wnl.44.1.133. [DOI] [PubMed] [Google Scholar]
- 12.Portenoy RK, Lipton RB, Berger AR, et al. Intracerebral haemorrhage: A model for the prediction of outcome. J Neurol Neurosurg Psychiatry. 1987;50:976–979. doi: 10.1136/jnnp.50.8.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuhrim S, Horowitz DR, Sacher M, et al. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999;27:617–621. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 14.Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman RD, Maldjian JA, Brun NC, et al. Radiologic estimation of hematoma volume in intracerebral hemorrhage trial by CT scan. AJNR Am J Neuroradiol. 2006;27:666–670. [PMC free article] [PubMed] [Google Scholar]
- 16.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 17.Graeb DA, Robertson WD, Lapointe JS, et al. Computed tomographic diagnosis of intra-ventricular hemorrhage. Etiology and prognosis. Radiology. 1982;143:91–96. doi: 10.1148/radiology.143.1.6977795. [DOI] [PubMed] [Google Scholar]
- 18.Hanley D. Evaluating accelerated resolution of intraventricular hemorrhage, rt-PA (Cathflo®) treatment of brain hemorrhage. [July 1, 2008]; Available at: http://www.clinicaltrials.gov.
- 19.Hijdra A, Brouwers PJ, Vermeulen M, et al. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. 1990;21:1156–1161. doi: 10.1161/01.str.21.8.1156. [DOI] [PubMed] [Google Scholar]
- 20.Ruscalleda J, Peiro A. Prognostic factors in intraparenchymatous hematoma with ventricular hemorrhage. Neuroradiology. 1986;28:34–37. doi: 10.1007/BF00341763. [DOI] [PubMed] [Google Scholar]
- 21.Tabachnick BG, Fidell LS. Using Multivariate Statistics. Third edition. New York: Harper Collins; 1996. [Google Scholar]
- 22.Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Hillsdale, NJ: Erlbaum Associates; 1983. p. 57. [Google Scholar]
- 23.Zahuranec DB, Brown DL, Lisabeth LD, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68:1651–1657. doi: 10.1212/01.wnl.0000261906.93238.72. [DOI] [PubMed] [Google Scholar]
- 24.Hemphill JC, III, Bonovich DC, Besmertis L, et al. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 25.Young WB, Lee KP, Pessin MS, et al. Prognostic significance of ventricular blood in supratentorial hemorrhage: A volumetric study. Neurology. 1990;40:616–619. doi: 10.1212/wnl.40.4.616. [DOI] [PubMed] [Google Scholar]
- 26.Steiner T, Diringer MN, Schneider D, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: Risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59:767–773. doi: 10.1227/01.NEU.0000232837.34992.32. discussion 773–764. [DOI] [PubMed] [Google Scholar]
- 27.Mayer SA, Thomas CE, Diamond BE. Asymmetry of intracranial hemodynamics as an indicator of mass effect in acute intracerebral hemorrhage. A transcranial Doppler study. Stroke. 1996;27:1788–1792. doi: 10.1161/01.str.27.10.1788. [DOI] [PubMed] [Google Scholar]
- 28.Mayfrank L, Kissler J, Raoofi R, et al. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28:141–148. doi: 10.1161/01.str.28.1.141. [DOI] [PubMed] [Google Scholar]
- 29.Wasserman JK, Zhu X, Schlichter LC. Evolution of the inflammatory response in the brain following intracerebral hemorrhage and effects of delayed minocycline treatment. Brain Res. 2007;1180:140–154. doi: 10.1016/j.brainres.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X, Zhang Y, Strong R, et al. Distinct patterns of intracerebral hemorrhage-induced alterations in NF-kappa B subunit, iNOS, and COX-2 expression. J Neurochem. 2007;101:652–663. doi: 10.1111/j.1471-4159.2006.04414.x. [DOI] [PubMed] [Google Scholar]
- 31.Juvela S. Risk factors for impaired outcome after spontaneous intracerebral hemorrhage. Arch Neurol. 1995;52:1193–1200. doi: 10.1001/archneur.1995.00540360071018. [DOI] [PubMed] [Google Scholar]
- 32.Kwak R, Kadoya S, Suzuki T. Factors affecting the prognosis in thalamic hemorrhage. Stroke. 1983;14:493–500. doi: 10.1161/01.str.14.4.493. [DOI] [PubMed] [Google Scholar]


