Abstract
Blood vessels, either in insufficient numbers or in excess, contribute to the pathogenesis of many diseases. Agents that stimulate angiogenesis can improve blood flow in patients with ischemic diseases, whereas anti-angiogenic agents are used to treat disorders ranging from macular degeneration to cancer. In this review I describe in vitro assays that can be used to assess the activity of agents that affect angiogenesis. Means of quantifying endothelial cell matrix degradation, migration, proliferation, apoptosis and morphogenesis are discussed, as are embryoid body, aortic ring and metatarsal assays of vessel outgrowth. Strengths and limitations of these techniques are also addressed.
Keywords: angiogenesis assays, endothelial cells, organ culture
Introduction
Development of new blood vessels occurs during embryonic development and during normal and pathologic periods of tissue growth. New vessels can form via two distinct processes, namely vasculogenesis and angiogenesis. Vasculogenesis, which involves the differentiation of vascular cells from undifferentiated precursors, forms the initial vascular network during early embryonic development and contributes to the vascularization of some tissues. Postnatal vascular development, too, may involve vasculogenesis, as cells derived from bone marrow have been shown to incorporate into the endothelium of new vessels in adults (reviewed in Ribatti et al., 2001).
Angiogenesis is the formation of new vessels from a pre-existing vascular network. This process is responsible for most vascularization in the embryo and growing tissues, as well as in the ovarian and uterine cycles, tissue repair, and disorders such as cancer, rheumatoid arthritis, and various retinopathies. Angiogenesis involves a variety of coordinated events, including degradation of the extracellular matrix surround the parent vessel, migration and proliferation of the endothelial cells and mural cells to assemble the new vessel, lumen formation, and construction of the mural cell layer of the vessel wall with associated pericytes and/or smooth muscle cells (reviewed in Carmeliet, 2000).
Either insufficient vascularization or excessive vessel formation can contribute to disease pathogenesis. Therapeutic strategies may therefore be designed to enhance or decrease vessel growth. Angiogenic agents have been used to treat ischemic disorders such as peripheral vascular disease and coronary artery disease (reviewed in Khan et al., 2003; Zhou et al., 2007). Anti-angiogenic agents have been used as treatments for cancer, diabetic retinopathy, macular degeneration, psoriasis and other diseases (reviewed in Liekens et al., 2001; Quesada et al., 2006).
Angiogenic and antiangiogenic activities can be assessed using both in vitro and in vivo assays. In vivo assays may better mimic angiogenesis as it occurs in normal and pathologic states, but in vitro assays offer several critical advantages over their in vivo counterparts. In vitro assays allow identification of direct effects on endothelial cell function, whereas in vivo assays involve multiple cell types and potential metabolic processing of the agent, complicating analysis of the agent's mechanism of action. In vitro assays allow analysis of isolated processes that contribute to angiogenesis whereas in vivo assays model angiogenesis as a whole. In vitro assays allow analysis of variables such as matrix components in isolation; tissue effects and other complexities of environment contribute to angiogenesis in vivo. In vitro assays do not require the technical expertise in animal handling required for in vivo assays. In vitro assays are typically less expensive than in vivo assays, and may be adapted for large-scale screening. Many in vitro angiogenesis assays can be more easily quantified than vessel outgrowth in vivo, and for some assays this quantification can be automated. Finally, in vitro assays allow genetic manipulation of the endothelial cells by transfection or adenoviral infection, as well as utilization of cells and tissue from transgenic mice for stem cell and organ culture assays.
In this review I discuss in vitro techniques used to assess various aspects of angiogenesis, suitable for analysis of both angiogenic and anti-angiogenic effects. Due to constraints of space, only selected in vitro angiogenesis assays are described; descriptions of additional assays can be found in the following excellent reviews: (Auerbach et al., 2000; Auerbach et al., 2003; Eccles et al., 2005; Entschladen et al., 2005; Huerta et al., 2007; Renvoizé et al., 1998; Staton et al., 2004; Vailhé et al., 2001).
Assessment of endothelial cell functions relating to angiogenesis
Endothelial cells are the primary constituents of new vessels, and many endothelial cell functions are required for angiogenesis, including matrix degradation, migration, proliferation, and morphogenesis (Figure 1). Numerous techniques are used to assess these functions in endothelial cells (Table 1). The endothelial cell culture assays described below are generally quick to conduct and easy to interpret, involve well-controlled conditions, and allow assessment of both angiogenic and anti-angiogenic effects.
Figure 1.
Endothelial cell functions involved in angiogenesis.
Table 1.
In vitro assays of angiogenesis
| Assay | Angiogenic functions assessed |
|---|---|
| Endothelial cell assays | |
| Zymogen assay | Matrix degradation |
| Scrape wound assay | Migration |
| Transwell/Boyden chamber assay | Migration |
| Under-agarose assay | Migration |
| Cell counting | Proliferation |
| Thymidine incorporation | Proliferation |
| BrdU incorporation | Proliferation |
| MTT assay | Proliferation |
| TUNEL assay | Apoptosis |
| Annexin V assay | Apoptosis |
| On-Matrigel assay | Morphogenesis |
| In-collagen/Matrigel assay | Morphogenesis |
| Collagen sandwich assay | Morphogenesis |
| Matrix invasion assay | All |
| Microbead assay | All |
| Stem cell and organ culture assays | |
| Embryoid body assay | All |
| Aortic ring assay | All |
| Mouse metatarsal assay | All |
Matrix degradation
Vessel sprouting requires degradation of both the laminin-rich basement membrane surrounding the endothelial cells and proteolysis of the collagen-rich extracellular matrix of the surrounding connective tissue. Matrix degradation can facilitate angiogenesis by activating angiogenic proteins or releasing matrix- or membrane-bound growth factors. This process can also release anti-angiogenic matrix fragments, including endostatin, angiostatin and tumstatin. Families of proteases responsible for matrix degradation during angiogenesis include matrix metalloproteinases (MMPs), other metalloproteinases, cysteine cathepsins, serine proteases and aminopeptidases (reviewed in Davis and Senger, 2005; Pepper, 2001; Rundhaug, 2005; van Hinsbergh et al., 2006). Of these, the MMPs are most frequently assessed as contributors to angiogenesis.
Some MMPs are secreted extracellularly, frequently in an inactive form, whereas others are tethered to the endothelial cell surface. Membrane-tethered MMPs (MT-MMPs) and secreted MMP zymogens must be activated by intracellular or secreted proteases. Upon activation MMPs digest matrix components such as collagen, fibrin, laminin and fibronectin (reviewed in Rundhaug, 2005; van Hinsbergh et al., 2006). Since MMPs and other proteases are required for both tumor invasion and angiogenesis (reviewed in Eccles, 2004), many inhibitors of these enzymes have been tested as anti-cancer agents (reviewed in Egeblad and Werb, 2002; Liekens et al., 2001; Mannello et al., 2005; Overall and Lopez-Otin, 2002).
MMP activity can be assessed using zymogen assays or matrix invasion assays. In a zymogen assay, an MMP substrate such as collagen, fibrinogen or gelatin is co-polymerized with polyacrylamide in an SDS-PAGE gel. Supernatants or lysates from treated endothelial cells are electrophoresed through the gel, followed by incubation in non-denaturing conditions to permit proteolysis. When zymogen gels are stained with a dye such as Coomassie blue, the hydrolyzed area appears transparent (reviewed in Lombard et al., 2005). The gel zymogen technique is inexpensive and provides information about the identities and relative levels of MMPs. This technique is time-consuming, however, and has not been adapted for large-scale screening.
Zymogen assays can also be conducted by incubating cells, lysates or conditioned medium with biotin-linked or fluorescence-linked matrix components. When endothelial cells are incubated on a matrix component linked to a fluorescent dye, MMP activity is observed as non-fluorescent areas of the substrate. Stimulation or inhibition of MMP activity can be observed using this technique (Gálvez et al., 2001). Large-scale screening of MMP stimulators or inhibitors can be conducted by adding cell lysates or conditioned medium to biotin-conjugated gelatin or to fluorescent dye-conjugated MMP substrates in 96-well plates (Bickett et al., 1993; Menges et al., 1997; Ratnikov et al., 2000). Although these zymogen techniques allow much faster and larger-scale analysis of MMP activity, they do not allow analysis of active vs. inactive MMPs or visualization of their relative levels of expression. Identification of the exact MMPs involved can be difficult with these assays, since multiple MMPs may degrade a single substrate, and non-MMP proteolytic enzymes can contribute to matrix-digesting activity.
Endothelial cells plated on collagen or fibrin gels containing migratory factors invade the gel and form lumen-containing tubes, processes that can be inhibited by addition of endogenous or pharmacologic MMP inhibitors (Bayless and Davis, 2003; Davis and Saunders, 2006); these invasion assays are described in greater detail below. Interestingly, certain MMPs cause vessel regression rather than angiogenesis in this in vitro model (Davis et al., 2001; Saunders et al., 2005), highlighting the importance of identifying MMPs affected by potential therapeutic agents.
Migration
Following matrix degradation, endothelial cells migrate into the surrounding tissue in response to angiogenic chemokines. Growth factors can contribute to endothelial cell motility by causing random cell movement (chemokinesis) or directed migration toward a stimulatory factor (chemotaxis). Cell motility is of particular interest in the design of anti-cancer therapeutics, as cell migration is required for both tumor invasion and tumor angiogenesis (reviewed in Eccles, 2004). Assays that allow measurement of endothelial cell motility in response to added factors include the scrape wound assay, transwell assay and under-agarose assay.
In the scrape wound or scratch wound assay, endothelial cells are grown to confluence and a wound is introduced by clearing an area of the monolayer using a pipet tip, needle or cell scraper. Cell filling of the cleared space initially occurs by migration, though cells in the cleared area will eventually also proliferate (Lampugnani, 1999). Since some growth factors stimulate both migration and proliferation, migration can be specifically addressed by adding anti-proliferative agents to the culture medium. Quantification can involve measuring the distance moved by the endothelial cells, the area covered by the endothelial cells, or the amount of time required to close the wound area. This assay can be adapted for large-scale screening (Yarrow et al., 2004), and multiple time points can be assessed using the same wells. Disadvantages include the difficulty of creating scraped areas of equal size and with even boundaries, variability between wells or experiments due to variations in degree of initial confluence, and difficulty of quantification. This assay is a measure of cell motility, but does not address whether a treatment causes chemokinesis or chemotaxis.
The transwell assay is more easily quantified than the scrape wound assay. The use of porous filters to assess migration was first described by Stephen Boyden, who examined chemotaxis of immune cells (Boyden, 1962). The transwell assay is therefore also known as the Boyden chamber assay, or modified Boyden chamber assay. With this technique, endothelial cells are plated on one side of a porous membrane, and a solution containing the potential migratory factor is placed on the opposite side of the membrane from the cells. For endothelial cells, a pore size of 3 μm is most appropriate, and the membrane is coated with fibronectin or collagen prior to plating the cells to facilitate adherence (reviewed in Eccles et al., 2005). After an incubation period of 3-18 hr, the migrated cells are stained and counted (Figure 2A). Since the concentrations of the angiogenic or angiogenic agent quickly normalize between the upper and lower chambers, movement of the cells may occur in response to chemokinesis rather than directed migration. A checkerboard series of conditions can be tested, should investigators wish to determine if increased motility is caused by chemotaxis or chemokinesis (Zigmond and Hirsch, 1973). Traditionally the migrated cells have been counted manually following removal of the non-migrated cells from the upper surface of the membrane, since automated counting techniques do not easily differentiate between the membrane pores and the migrated cells. Newer fluorescent staining techniques and light-blocking membranes permit automated cell counting, however (reviewed in Eccles et al., 2005). Advantages of the transwell technique include the sensitivity of the assay to low levels of chemotactic factors (Kreutzer et al., 1978) and the high degree of reproducibility relative to other migration assays. Disadvantages include the high cost of the membranes and the fact that migration is usually only assessed for a fraction of the total membrane surface.
Figure 2.
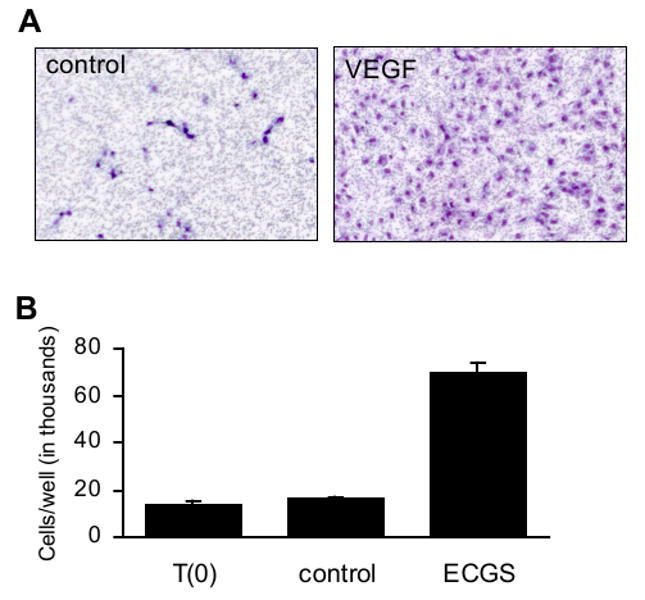
Cell culture assays assessing endothelial cell function. A) Cell migration as measured using a transwell assay. Starved HUVEC were plated onto a fibronectin-coated transwell with pores 3 μm pore diameter. Medium on the opposing side of the membrane lacked (left panel) or contained (right panel) recombinant VEGF at 10 ng/ml. After 18 hr, nonmigrated cells were removed with a cotton swab. Migrated cells were fixed and stained with hematoxylin and eosin, then photographed using a 5× objective. Unpublished images courtesy of Magali Saint-Geniez. B) Cell proliferation as measured by direct cell counting. HUVEC were treated with endothelial cell growth supplement and cultured for three days. The mean numbers of cells present in each well prior to the start of treatment (T(0)) and following three days of treatment (control, VEGF) are shown. Error bars indicate standard deviation. Unpublished data courtesy of Sandie Smith.
A migration assay that allows measurement of directed migration as opposed to chemokinesis is the under-agarose assay. In this assay, agarose gels are prepared in tissue culture plates, then two wells are punched into the gels, leaving 2 mm of agarose between them (Heit and Kubes, 2003; Nelson et al., 1975). Endothelial cells are plated into one well, and the potential migratory factor is added to the other well (Hoying and Williams, 1996). Migration is defined as the distance moved by the endothelial cells in the direction of the stimulus (chemotaxis), relative to the distance moved by endothelial cells on the opposite side of the well (chemokinesis). The under-agarose assay is inexpensive and allows clear differentiation between chemokinesis and directed migration, but it is less sensitive than the transwell assay (Kreutzer et al., 1978), difficult to quantify, and not suited to large-scale analysis. This assay has also been used to examine endothelial cell recruitment of mural cells (Hirschi et al., 1999; Kashiwagi et al., 2005), an important component of vessel maturation.
Proliferation
Endothelial cell proliferation, combined with increased survival, supplies the cells that make up a new vessel. Effects of angiogenic and anti-angiogenic factors on proliferation can be measured by direct cell counts, quantification of DNA synthesis, or assessment of metabolic activity. Proliferation assays can be used to measure angiogenic activity when low-serum or low-growth factor conditions are used, or anti-angiogenic activity when the culture medium contains normal levels of serum and/or growth factors.
The most-simple means of assessing cell number involves directly counting cells following treatment with angiogenic or anti-angiogenic factors (Figure 2B). Viable cells can be stained with trypan blue and counted with a hemocytometer, or trypsinized and counted with a Coulter particle counter. Although direct cell counts are simple and inexpensive, this technique does not indicate whether changes in cell number are caused by alterations in proliferation, apoptosis or both.
Mitotic cell division can be assessed by measuring labeled reagents incorporated during DNA synthesis. In the thymidine incorporation assay, levels of tritiated thymidine incorporated into newly synthesized DNA are measured using a scintillation counter. In BrdU assays, bromodeoxyuridine, a pyrimidine analog, is incorporated during DNA synthesis and assessed using immunohistochemistry; this assay yields results similar to those of thymidine incorporation (Messele et al., 2000). The key advantage of the BrdU assay over thymidine incorporation is that radioactivity is not required. The immunohistochemical techniques required for BrdU detection may be time-consuming, however, and quantification more difficult.
The MTT assay measures activity of mitochondria as a means of assessing numbers of living cells. Active mitochondria convert the yellow substrate 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan, a purple product. This colorimetric change can be quantified using spectrophotometry and correlated with cell number (Denizot and Lang, 1986). The MTT assay is well-suited to large-scale screening, but since some agents affect MTT processing without affecting endothelial cell viability (Ahmad et al., 2006; Trevisi et al., 2006), MTT assay results should be confirmed using an alternate measure of cell proliferation.
Survival
Many angiogenic factors increase endothelial cell number by enhancing both proliferation and survival. Cell death in response to toxic stimuli can occur by necrosis, a passive response, or by apoptosis, which is programmed cell death. During apoptosis, extrinsic or intrinsic signals activate caspases 3, 6 and 7, which in turn induce the DNA fragmentation, DNA budding and chromatin condensation characteristic of programmed cell death (reviewed in Saraste and Pulkki, 2000). Endothelial cell apoptosis can be induced by serum starvation in vitro, whereas normal cell culture conditions are used when assessing apoptotic effects of anti-angiogenic factors. Two commonly used techniques for assessing endothelial cell apoptosis are TUNEL and annexin V assays.
Terminal deoxynucleotidyl transferase-dUTP nick end labeling (TUNEL) measures DNA fragmentation. In TUNEL, terminal deoxynucleotide transferase binds to the 3′-OH region of fragmented DNA, then incorporates a fluorescent dye. Labeled cells can be identified by microscopy or flow cytometric analysis, and the number of apoptotic cells relative to the number of total cells calculated. Since TUNEL labels both necrotic and apoptotic cells (Grasl-Kraupp et al., 1995), however, cells should be visually examined or an alternate apoptosis technique used to confirm that cell death is due to apoptosis.
During early apoptosis, the cell membrane component phosphatidylserine is translocated from the inner surface to the outer surface of the membrane (reviewed in Saraste and Pulkki, 2000). Annexin V is a protein that binds to phosphatidylserine with high affinity (Martin et al., 1995); fluorescence-conjugated annexin V can therefore be used to identify apoptotic cells, either by microscopy or flow cytometry. Like TUNEL, annexin V may label necrotic cells in addition to apoptotic cells. These processes can be differentiated by staining the cells with propidium iodide, which labels necrotic cells but not apoptotic cells (Vermes et al., 1995).
Morphogenesis
Angiogenesis requires the assembly of endothelial cells into vessel tubes. The tube formation stage of angiogenesis can be modeled in vitro by plating endothelial cells with extracellular matrix components. Morphogenesis assays utilize substrates such as Matrigel or type I collagen, and endothelial cells may be plated on, in or between these substrates.
The most widely used assay for endothelial cell morphogenesis involves plating human umbilical vein endothelial cells (HUVEC) on Matrigel, an extracellular matrix isolated from Engelbreth-Holm-Swarm mouse sarcoma cells. HUVEC plated on Matrigel at low densities form a network of branching structures that is typically maintained for 12-24 hr (Kubota et al., 1988). Wells are photographed and morphogenesis quantified by measuring the length or area of capillary-like structures (CLS) per unit area. The CLS mostly form in a two-dimensional plane (Figure 3A), facilitating photography. This assay can be conducted in a short time period, is easy to set up, and can be established and quantified in 96-well plates. It should be noted, however, that Matrigel is a tumor endothelial cell matrix, and is consequently rich in angiogenic growth factors. Indeed, CLS formation is not stimulated above baseline by the potent angiogenic factor VEGF when HUVEC are plated on regular Matrigel (Donovan et al., 2001). Growth factor-reduced preparations of Matrigel are not free of endogenous growth factors, but do permit stimulation of CLS formation above baseline (Donovan et al., 2001). Further limitations include the observation that while at least some of the CLS on Matrigel contain lumens (Kubota et al., 1988), many of the extensions between endothelial cells resemble cords or cell processes more than tubes (Figure 3A). Finally, it should be noted that other cell types, including fibroblasts and cancer cells, also form networks on Matrigel (Donovan et al., 2001). Due to the high baseline levels of morphogenesis with this assay, results are most striking when antiangiogenic agents are used, though stimulatory effects can also be measured.
Figure 3.
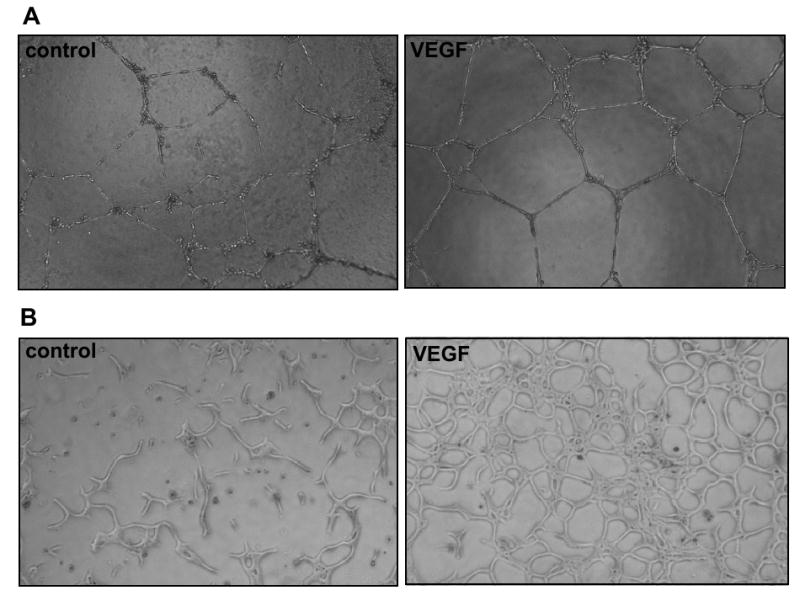
Endothelial cell morphogenesis. A) HUVEC plated on Matrigel. HUVEC were plated onto Matrigel pads and cultured in the absence (left panel) or presence (right panel) of VEGF (10 ng/ml). Capillary-like structures were photographed (10× objective) 20 hr after plating. B) Bovine aortic endothelial cells suspended in diluted Matrigel. The endothelial cells were suspended in a matrix containing 50% Matrigel and 50% culture medium. The following day, the medium was replaced with serum-free medium lacking (left panel) or containing (right panel) VEGF at 10 ng/ml. Capillary-like structures were photographed (10× objective) after three days of treatment. Unpublished images courtesy of Tony Walshe.
CLS have a more uniform diameter when endothelial cells are plated within an extracellular matrix substrate. HUVEC suspended in gels containing rat tail collagen at 3.75-5.0 μg/ml form lumen-containing CLS within 24 hr, structures that are maintained over several days (Davis and Camarillo, 1996; Kamei et al., 2006; Salazar et al., 1999). Similar morphogenesis is observed with bovine aortic and microvascular endothelial cells plated in gels of diluted Matrigel or type I collagen (Stitt et al., 2005; A.G. and Tony Walshe, unpublished observations). Quantification of the structures in thick gels requires sectioning of the gel or photography at multiple focal planes, since the tubes form in three dimension; three-dimensional assessment can also be done using confocal analysis or two-photon imaging. Tube organization in two dimensions can be maximized by plating a 25-100 μl drop of the cell-matrix mixture on tissue culture plastic (Figure 3B; Davis and Camarillo, 1996; A.G. and Tony Walshe, unpublished observations). Suspension of endothelial cells within a gel is technically more complicated than plating of HUVEC on Matrigel, but the CLS in gel suspension assays form in more-dense networks, and non-HUVEC endothelial cells readily form CLS in these assays. Endothelial tube formation within matrix has been used to show both angiogenic and anti-angiogenic effects (Figure 3B; Saunders et al., 2006).
A third means of producing endothelial cell tubes in vivo is the collagen sandwich assay, developed by Montesano and others. In this assay a collagen gel is plated and allowed to solidify. Endothelial cells are plated at subconfluence onto the gel and allowed to adhere before being covered with a second layer of collagen (Montesano et al., 1983). CLS form within several days of culture with HUVEC (Ashikari-Hada et al., 2005), bovine adrenal cortex endothelial cells (Montesano et al., 1983), bovine carotid endothelial cells (Kanayasu et al., 1989) or bovine retinal epithelial cells (Ramsauer and D'Amore, 2007). The surrounding of cells by collagen more accurately reflects the in vivo environment than does plating cells on collagen, and the CLS formed with this assay are maintained for many days. The CLS form primarily in two dimensions, which facilitates photography, but the assay setup is time-consuming and some invasive vessel growth does occur in three dimensions. This assay is particularly well-suited for stimulation of angiogenesis, as exogenous growth factors are needed for formation of extensive CLS networks.
Assessment of sprouting angiogenesis using endothelial cell cultures
The morphogenesis assays described above involve CLS formation from endothelial cells present at subconfluent levels atop or within extracellular matrix components. Angiogenesis in vivo, however, involves sprouting from confluent endothelium in the pre-existing vessel. Several in vitro assays have been developed to model formation of vessel sprouts from confluent endothelial cell monolayers.
Cells grown to confluence on collagen gels or fibrin gels can be induced to invade the matrix and form lumen-containing tubes in response to factors such as PMA, bFGF and VEGF (Davis et al., 2000; Montesano and Orci, 1985; Montesano and Orci, 1987; Montesano et al., 1986; Pepper et al., 1990), a response that can be inhibited by TGF-β (Pepper et al., 1990). Sprouting is assessed by sectioning the gels or by photographing intact cultures. Observation of sprouting with phase microscopy can be facilitated by plating thin gels onto miroscope slides (Davis et al., 2000). The invading sprouts have lumens and more closely resemble capillaries in vivo than do the CLS generated by culturing cells on extracellular matrix. Quantification can be difficult, as vessels grow in three dimensions, but sectioning and microscopy techniques can be used for three-dimensional analysis.
In another technique for modeling sprouting angiogenesis, endothelial cells are grown to confluence on microbeads, which are then embedded into fibrin gels. Endothelial sprouts in bovine pulmonary artery endothelial cell cultures treated with angiogenic factors are observed within 3 days of culture, and factors that induce sprouting can be distinguished from those that simply induce migration (Nehls and Drenckhahn, 1995). Adrenal cortex-derived endothelial cells, too, have been used to assess angiogenic agents in this assay (Koblizek et al., 1998). Vessel outgrowth can clearly be seen in phase micrographic images with this assay and quantified as vessel length or number of vessels per bead. Since the endothelial cells cover the entire surface of the bead, however, vessels may grow out in any direction; lumen-containing tubes must also be distinguished from migrating cells. The microbead assay has also been adapted to demonstrate angiogenic activity with HUVEC (Nakatsu et al., 2003), but HUVEC sprouting was only observed in the presence of a supporting layer of fibroblasts (Nakatsu et al., 2003). This is not surprising, given that HUVEC have a greater requirement for growth factors than do many other cell types, but presence of another cell type could complicate analysis of direct effects on endothelial cells.
Assessment of sprouting angiogenesis using stem cell and organ culture assays
All assays previously described involve use of a single cell type – endothelial cells. Embryoid body and organ culture assays, on the other hand, allow in vitro analysis angiogenesis in an environment composed of multiple cell types. Like the assays of sprouting angiogenesis using endothelial cell cultures, multiple cell processes involved in angiogenesis are modeled (Table 1), but in the embryoid body and organ culture assays additional stages of angiogenesis such as recruitment of pericytes to new vessels can also be observed.
Embryoid body assay
The embryoid body assay is unique among the assays described in this review in that it models vasculogenesis and angiogenesis at distinct stages in the culture period. This assay utilizes mouse embryonic stem cells derived from the blastocyst inner cell mass. During normal cell culture, the embryonic stem cells are maintained in an undifferentiated state using leukemia inhibitory factor (LIF). When the cells are cultured in suspension (Wang et al., 1992), spinning flasks (Wartenberg et al., 1998) or hanging drops (Goumans et al., 1999) in the absence of LIF, cystic embryoid bodies form. The walls of these structures contain vessels that initially form by vasculogenesis (Vittet et al., 1996), though when the embryoid bodies are subsequently placed in collagen, vessel growth continues by angiogenesis and can be modulated by angiogenic or antiangiogenic factors (Feraud et al., 2001). The vasculature can be visualized by whole-mount immunostaining for endothelial cell markers such as PECAM (Figure 4A; Feraud et al., 2003). Alternatively, embryoid bodies can be generated from using stem cells from transgenic mice in which fluorescence is regulated by endothelial-specific promoters (Gimond et al., 2006). The embryoid body technique allows use of cells from transgenic mice to identify factors necessary for angiogenesis (Ng et al., 2004), and vascularized embryoid bodies can also be formed using human embryonic stem cells (Gerecht-Nir et al., 2005). Vessels in the embryoid body form a disorganized plexus of interconnected endothelial cells, though the vessels do have lumens and contain hematopoietic cells such as erythroblasts and macrophages (Bautch et al., 1996). Vascularization is difficult to quantify, and mural cell recruitment to the vessels has not been well-described. Many tissue types are present within the embryoid body, and factors secreted by these non-vascular cells may indirectly influence angiogenesis.
Figure 4.
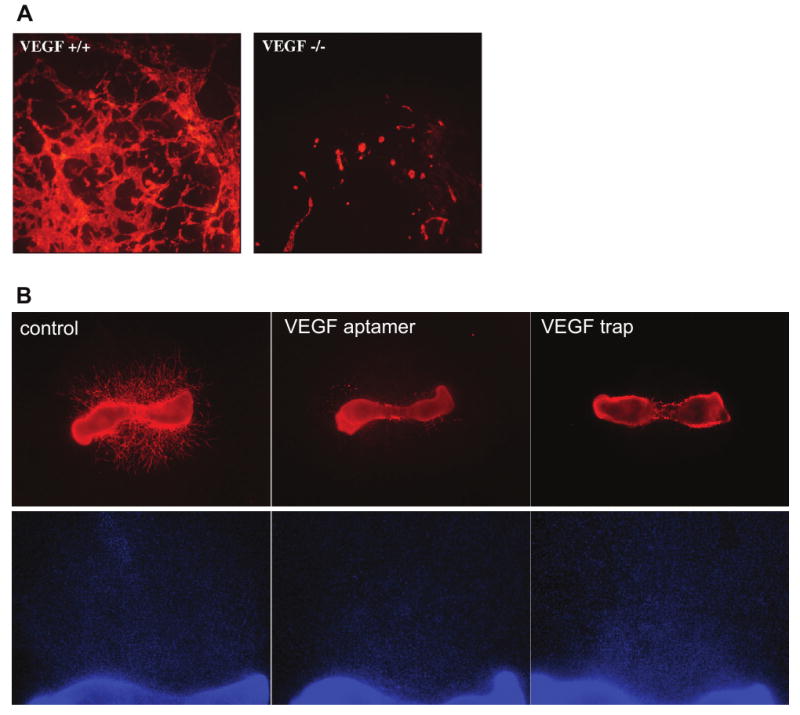
Embryoid body and mouse metatarsal models of angiogenesis. A) Vascular structures in embryoid bodies. Embryoid bodies were differentiated for 10 days from VEGF-deficient (left panel) or wild-type (right panel) mouse embryonic stem cells. The vascular structures were labeled using an antibody to PECAM and photographed at using a 5× objective. Unpublished images courtesy of Robyn Loureiro. B) Vessel outgrowth from mouse metatarsal bones. Metatarsal bones were isolated from wild type embryonic day 18 mouse embryos and cultured with control medium (left panels), an aptamer to VEGF (Ruckman et al., 1998; middle panels) or a soluble, truncated VEGF receptor (VEGF trap; Holash et al., 1992; right panels). Vessel outgrowth in the top panels is visualized by fluorescently labeling the endothelial cell marker PECAM and photographing using a 2× objective. The DAPI staining in the lower panels, photographed using a 4× objective, shows that the inhibitors do not prevent outgrowth of non-endothelial cells from the metatarsal bone. Unpublished images courtesy of Yin-Shan Ng.
Rat and mouse aortic ring assays
The aortic ring assay is an organ culture model in which angiogenic vessels grow from a segment of the aorta. In this assay, the thoracic aorta is excised, the adventitia is removed, and rings approximately 1 mm in length are cut and embedded into thick (Nicosia and Ottinetti, 1990) or thin (Zhu and Nicosia, 2002) collagen gels. The rings are then cultured in serum-free medium for approximately one week. Neovessel outgrowth can be quantified using phase microscopy or immunohistochemical analysis (reviewed in Go and Owen, 2003). Since the neovessels grow above a fibroblast layer on the tissue culture surface, vessels are best imaged with endothelial-specific labeling, e.g. using Griffonia simplicifolia isolectin B4 or antibodies to Tie2 (Zhu and Nicosia, 2002).
The mouse aortic ring assay was developed to take advantage of the transgenic tools available for this species (Masson et al., 2002). Angiogenesis assays can be conducted using endothelial cells with specific characteristics or labels; for example, aortas from mice whose endothelial cells express green-fluorescent protein can be used to facilitate visualization of vessel outgrowth (Zhu et al., 2003). Angiogenic outgrowth occurs over a shorter time period with the mouse aortic rings (Masson et al., 2002), and both angiogenic and anti-angiogenic factors can be assessed (e.g. Kojima et al., 2007; van der Schaft et al., 2002). Each mouse yields fewer aortic rings than does a rat, however, and removal of the adventitial surrounding the aorta is more difficult with these smaller vessels. Furthermore, vessel outgrowth from the mouse aortic rings requires culture in serum-containing medium.
The rat and mouse aortic ring assays have been use to assess both angiogenic and anti-angiogenic agents (e.g. Nicosia et al., 1994; Nicosia and Ottinetti, 1990). The vessels that grow out from aortic rings are anatomically similar to neovessels in vivo, in that they recruit smooth muscles and pericytes to associate with the endothelial cell tube (Nicosia et al., 1992; Nicosia and Villaschi, 1995). Disadvantages of this assay, however, are numerous. Variability in the handling of the rings and the amount of adventitia remaining on the vessel can influence vessel outgrowth within an experiment. Use of rings from different aortas and, particularly with mice, different ages and strains of animals (Zhu et al., 2003) can lead to variability in angiogenic responses. Vessel outgrowth usually occurs in three dimensions, complicating photography and quantification. Finally, angiogenic outgrowth in vivo occurs from microvessels, not from major vessels such as the aorta.
Mouse metatarsal assay
An organ culture assay that does promote vessel outgrowth from smaller vessels is the mouse metatarsal assay, in which angiogenesis is observed from embryonic mouse bones. In this assay, metatarsal bones are isolated from embryonic day 17 mouse embryos and plated onto tissue culture plastic (Deckers et al., 2001). Vessel outgrowth is robust within one week of culture, and can be quantified by measuring vessel area. This assay has been used to assess angiogenic and anti-angiogenic agents (Figure 4B; Deckers et al., 2001; van der Pluijm et al., 2003). The neovessel outgrowth is robust and derived from the bone microvasculature, which is more typical of angiogenesis in vivo than is outgrowth from a major vessel like the aorta. It is, however, very time-consuming to isolate the metatarsal bones and, as with the aortic ring assay, variability in removal of surrounding tissue and handling of the bones can alter angiogenic activity. Also, since the embryonic bones are themselves undergoing angiogenesis, this technique does not model angiogenic outgrowth from quiescent microvessels.
Considerations in choosing an assay
In addition to the advantages and limitations of the individual assays described above, certain properties of the agent in question, general classes of assay, and types of endothelial cell used should be taken into consideration.
Angiogenic vs. anti-angiogenic effects
In vitro assays can be used to assess both angiogenic and anti-angiogenic factors, though the choice of assays and exact assay conditions may vary depending on which type of factor is being tested. Angiogenic factors are typically tested in low-serum or low-growth factor conditions, and using assays in which baseline angiogenesis is low, as with the mouse aortic ring assay. Assessment of anti-angiogenic factors requires higher levels of baseline angiogenesis or angiogenic activity. This can be done by using higher levels of serum in cell culture assays, by including angiogenic factors such as VEGF in the culture media, or by using assays such as the mouse metatarsal assay that provide high baseline levels of angiogenic activity. It should also be noted that in many assays cytotoxic effects will not be easily distinguished from specific anti-angiogenic effects (reviewed in Auerbach et al., 2003). It is therefore important to visually inspect cell cultures to look for signs of necrotic cell death. In embryoid body and organ culture assays, detrimental effects on the cells surrounding or underlying the vasculature may also indicate toxicity of the agent. Specificity of angiogenic and anti-angiogenic effects can be confirmed by repeating assays using non-endothelial cell types
Cell culture vs. organ culture
Cell culture and organ culture assays are often used in conjunction to assess angiogenic and anti-angiogenic effects. Cell culture assays can be carried out under well-controlled conditions and can help to define the mechanism of action for an agent. Cultured endothelial cells are similar in many ways to angiogenic endothelial cells in vivo (Pauly et al., 1992), and can be obtained from several species and tissue types.
It is important, however, to verify cell culture findings with organ culture or in vivo angiogenesis models. Culture can lead to changes in the growth characteristics and cell surface antigens of endothelial cells, among other differences (reviewed in Auerbach et al., 2000). Agents that show promise in in vitro studies may therefore have different effects in vivo. Furthermore, most cell culture assays measure angiogenic cell functions in a two-dimensional plane rather than the three-dimensional environment for vessel outgrowth that is found in vivo. Even morphogenesis assays that take place in three- dimensional matrices do not fully model vessel assembly, as the vessel tube formation occurs in the absence of surrounding mural cells and tissue. Although coculture assays – assays in which endothelial cells are cultured with pericytes or smooth muscle cells to better recapitulate in vivo vessel assembly – are beyond the scope of this review, many of the assays can also be adapted for analysis of heterotypic cell-cell interactions. Addition of multiple cell types does, however, complicate interpretation of direct effects on endothelial cells.
Embryoid body and organ culture assays allow three-dimensional vessel outgrowth, and multiple cell types, including perivascular cells such as smooth muscle cells and pericytes, participate in angiogenesis. These assays have greater intraexperimental variability than do cell culture assays, however, and quantification of vessel outgrowth in three dimensions is challenging. Furthermore, it can be difficult to identify the mechanism of action for an angiogenic or anti-angiogenic agent using these complex models.
Endothelial cell types
Endothelial cells in vivo have different functions and characteristics depending on the vessel size and tissue, and these differences may be reflected in the phenotype of cultured endothelial cells. There are differences, for example, in growth factor receptor expression between arterial and venous endothelial cells (Moyon et al., 2001; Villa et al., 2001; Wang et al., 1998); in expression of MMPs and TIMP1 between HUVEC and human dermal microvascular endothelial cells (Jackson and Nguyen, 1997); and in ecto-5′-nucleotidase activity between human and porcine endothelial cells (Smolenski et al., 2006). Different endothelial cell types may behave differently in cell culture assays. HUVEC, for example, form CLS when plated at low densities on Matrigel (Kubota et al., 1988), but bovine aortic endothelial cells do not (Darland and D'Amore, 2001). The cell type used for cell culture-based assays of angiogenesis should therefore be chosen to resemble the tissue of interest as closely as possible, and in vitro assays must be optimized for each cell type used.
Conclusions
In vitro assays provide a valuable tool for assessing effects of angiogenic and antiangiogenic agents. Cell culture techniques can be used to identify endothelial cell functions affected, and angiogenic vessel growth can be measured in well-controlled organ culture conditions. The limitations of in vitro assays should be understood, however, and results validated by using multiple assay types and conditions, by using combinations of cell culture and organ culture assays, and by comparing in vitro effects to results observed with in vivo assays.
Acknowledgments
I would like to thank Robyn Loureiro, Patricia D'Amore, Diane Darland, Yin-Shan Ng, Magali Saint-Geniez and Tony Walshe for providing critical comments, and members of the laboratory of Patricia A. D'Amore (Harvard University and the Schepens Eye Research Institute, Boston, MA) for providing unpublished data for use in this article. My work on in vitro angiogenesis assays was partially supported by EY05318.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad S, Ahmad A, Schneider KB, White CW. Cholesterol interferes with the MTT assay in human epithelial-like (A549) and endothelial (HLMVE and HCAE) cells. Int J Toxicol. 2006;25:17–23. doi: 10.1080/10915810500488361. [DOI] [PubMed] [Google Scholar]
- Ashikari-Hada S, Habuchi H, Kariya Y, Kimata K. Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins. J Biol Chem. 2005;280:31508–31515. doi: 10.1074/jbc.M414581200. [DOI] [PubMed] [Google Scholar]
- Auerbach R, Akhtar N, Lewis RL, Shinners BL. Angiogenesis assays: problems and pitfalls. Cancer Metastasis Rev. 2000;19:167–172. doi: 10.1023/a:1026574416001. [DOI] [PubMed] [Google Scholar]
- Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- Bautch VL, Stanford WL, Rapoport R, Russell S, Byrum RS, Futch TA. Blood island formation in attached cultures of murine embryonic stem cells. Dev Dyn. 1996;205:1–12. doi: 10.1002/(SICI)1097-0177(199601)205:1<1::AID-AJA1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Bayless KJ, Davis GE. Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. Biochem Biophys Res Commun. 2003;312:903–913. doi: 10.1016/j.bbrc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Bickett DM, Green MD, Berman J, Dezube M, Howe AS, Brown PJ, Roth JT, McGeehan GM. A high throughput fluorogenic substrate for interstitial collagenase (MMP-1) and gelatinase (MMP-9) Anal Biochem. 1993;212:58–64. doi: 10.1006/abio.1993.1291. [DOI] [PubMed] [Google Scholar]
- Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Darland DC, D'Amore PA. TGFβ is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis. 2001;4:11–20. doi: 10.1023/a:1016611824696. [DOI] [PubMed] [Google Scholar]
- Davis GE, Black SM, Bayless KJ. Capillary morphogenesis during human endothelial cell invasion of three-dimensional collagen matrices. In Vitro Cell Dev Biol Anim. 2000;36:513–519. doi: 10.1290/1071-2690(2000)036<0513:CMDHEC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Davis GE, Camarillo CW. An α2 β 1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- Davis GE, Pintar Allen KA, Salazar R, Maxwell SA. Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci. 2001;114:917–930. doi: 10.1242/jcs.114.5.917. [DOI] [PubMed] [Google Scholar]
- Davis GE, Saunders WB. Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Investig Dermatol Symp Proc. 2006;11:44–56. doi: 10.1038/sj.jidsymp.5650008. [DOI] [PubMed] [Google Scholar]
- Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- Deckers M, van der Pluijm G, Dooijewaard S, Kroon M, van Hinsbergh V, Papapoulos S, Löwik C. Effect of angiogenic and antiangiogenic compounds on the outgrowth of capillary structures from fetal mouse bone explants. Lab Invest. 2001;81:5–15. doi: 10.1038/labinvest.3780207. [DOI] [PubMed] [Google Scholar]
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Donovan D, Brown NJ, Bishop ET, Lewis CE. Comparison of three in vitro human ‘angiogenesis’ assays with capillaries formed in vivo. Angiogenesis. 2001;4:113–121. doi: 10.1023/a:1012218401036. [DOI] [PubMed] [Google Scholar]
- Eccles SA. Parallels in invasion and angiogenesis provide pivotal points for therapeutic intervention. Int J Dev Biol. 2004;48:583–598. doi: 10.1387/ijdb.041820se. [DOI] [PubMed] [Google Scholar]
- Eccles SA, Box C, Court W. Cell migration/invasion assays and their application in cancer drug discovery. Biotechnol Annu Rev. 2005;11:391–421. doi: 10.1016/S1387-2656(05)11013-8. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Entschladen F, Drell TL, Lang K, Masur K, Palm D, Bastian P, Niggemann B, Zaenker KS. Analysis methods of human cell migration. Exp Cell Res. 2005;307:418–426. doi: 10.1016/j.yexcr.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Feraud O, Cao Y, Vittet D. Embryonic stem cell-derived embryoid bodies development in collagen gels recapitulates sprouting angiogenesis. Lab Invest. 2001;81:1669–1681. doi: 10.1038/labinvest.3780380. [DOI] [PubMed] [Google Scholar]
- Feraud O, Prandini MH, Vittet D. Vasculogenesis and angiogenesis from in vitro differentiation of mouse embryonic stem cells. Methods Enzymol. 2003;365:214–228. doi: 10.1016/s0076-6879(03)65015-9. [DOI] [PubMed] [Google Scholar]
- Gálvez BG, Matìas-Román S, Albar JP, Sánchez-Madrid F, Arroyo AG. Membrane type 1-matrix metalloproteinase is activated during migration of human endothelial cells and modulates endothelial motility and matrix remodeling. J Biol Chem. 2001;276:37491–37500. doi: 10.1074/jbc.M104094200. [DOI] [PubMed] [Google Scholar]
- Gerecht-Nir S, Dazard JE, Golan-Mashiach M, Osenberg S, Botvinnik A, Amariglio N, Domany E, Rechavi G, Givol D, Itskovitz-Eldor J. Vascular gene expression and phenotypic correlation during differentiation of human embryonic stem cells. Dev Dyn. 2005;232:487–497. doi: 10.1002/dvdy.20247. [DOI] [PubMed] [Google Scholar]
- Gimond C, Marchetti S, Pages G. Differentiation of mouse embryonic stem cells into endothelial cells: genetic selection and potential use in vivo. Methods Mol Biol. 2006;330:303–329. doi: 10.1385/1-59745-036-7:303. [DOI] [PubMed] [Google Scholar]
- Go RS, Owen WG. The rat aortic ring assay for in vitro study of angiogenesis. Methods Mol Med. 2003;85:59–64. doi: 10.1385/1-59259-380-1:59. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Zwijsen A, van Rooijen MA, Huylebroeck D, Roelen BA, Mummery CL. Transforming growth factor-β signalling in extraembryonic mesoderm is required for yolk sac vasculogenesis in mice. Development. 1999;126:3473–3483. doi: 10.1242/dev.126.16.3473. [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- Heit B, Kubes P. Measuring chemotaxis and chemokinesis: the under-agarose cell migration assay. Sci STKE. 2003;2003:PL5. doi: 10.1126/stke.2003.170.pl5. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D'Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Nat Acad Sci USA. 2002;99:11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoying JB, Williams SK. Measurement of endothelial cell migration using an improved linear migration assay. Microcirculation; Presented at the 1995 Microcirculatory Society Meeting; 1996. pp. 167–174. [DOI] [PubMed] [Google Scholar]
- Huerta S, Goulet EJ, Huerta-Yepez S, Livingston EH. Screening and Detection of Apoptosis. J Surg Res. 2007 doi: 10.1016/j.jss.2006.07.034. doi:101016. [DOI] [PubMed] [Google Scholar]
- Jackson CJ, Nguyen M. Human microvascular endothelial cells differ from macrovascular endothelial cells in their expression of matrix metalloproteinases. Int J Biochem Cell Biol. 1997;29:1167–1177. doi: 10.1016/s1357-2725(97)00061-7. [DOI] [PubMed] [Google Scholar]
- Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- Kanayasu T, Nakao-Hayashi J, Asuwa N, Morita I, Ishii T, Ito H, Murota S. Leukotriene C4 stimulates angiogenesis in bovine carotid artery endothelial cells in vitro. Biochem Biophys Res Commun. 1989;159:572–578. doi: 10.1016/0006-291x(89)90032-6. [DOI] [PubMed] [Google Scholar]
- Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, Huang PL, Buerk DG, Munn LL, Jain RK, Fukumura D. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest. 2005;115:1816–1827. doi: 10.1172/JCI24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan TA, Sellke FW, Laham RJ. Gene therapy progress and prospects: therapeutic angiogenesis for limb and myocardial ischemia. Gene Ther. 2003;10:285–291. doi: 10.1038/sj.gt.3301969. [DOI] [PubMed] [Google Scholar]
- Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8:529–532. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- Kojima T, Chang JH, Azar DT. Proangiogenic role of ephrinB1/EphB1 in basic fibroblast growth factor-induced corneal angiogenesis. Am J Pathol. 2007;170:764–773. doi: 10.2353/ajpath.2007.060487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer DL, O'Flaherty JT, Orr W, Showell HJ, Ward PA, Becker EL. Quantitative comparisons of various biological responses of neutrophils to different active and inactive chemotactic factors. Immunopharmacology. 1978;1:39–47. doi: 10.1016/0162-3109(78)90007-3. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG. Cell migration into a wounded area in vitro. Methods Mol Biol. 1999;96:177–182. doi: 10.1385/1-59259-258-9:177. [DOI] [PubMed] [Google Scholar]
- Liekens S, De Clercq E, Neyts J. Angiogenesis: regulators and clinical applications. Biochem Pharmacol. 2001;61:253–270. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- Lombard C, Saulnier J, Wallach J. Assays of matrix metalloproteinases (MMPs) activities: a review. Biochimie. 2005;87:265–272. doi: 10.1016/j.biochi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Mannello F, Tonti G, Papa S. Matrix metalloproteinase inhibitors as anticancer therapeutics. Curr Cancer Drug Targets. 2005;5:285–298. doi: 10.2174/1568009054064615. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson VV, Devy L, Grignet-Debrus C, Bernt S, Bajou K, Blacher S, Roland G, Chang Y, Fong T, Carmeliet P, Foidart JM, Noel A. Mouse aortic ring assay: a new approach of the molecular genetics of angiogenesis. Biol Proced Online. 2002;4:24–31. doi: 10.1251/bpo30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges DA, Ternullo DL, Tan-Wilson AL, Gal S. Continuous assay of proteases using a microtiter plate fluorescence reader. Anal Biochem. 1997;254:144–147. doi: 10.1006/abio.1997.2408. [DOI] [PubMed] [Google Scholar]
- Messele T, Roos MT, Hamann D, Koot M, Fontanet AL, Miedema F, Schellekens PT, Rinke de Wit TF. Nonradioactive techniques for measurement of in vitro T-cell proliferation: alternatives to the [(3)H]thymidine incorporation assay. Clin Diagn Lab Immunol. 2000;7:687–692. doi: 10.1128/cdli.7.4.687-692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985;42:469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Montesano R, Orci L. Phorbol esters induce angiogenesis in vitro from large-vessel endothelial cells. J Cell Physiol. 1987;130:284–291. doi: 10.1002/jcp.1041300215. [DOI] [PubMed] [Google Scholar]
- Montesano R, Orci L, Vassalli P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J Cell Biol. 1983;97:1648–1652. doi: 10.1083/jcb.97.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986;83:7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001;128:3359–3370. doi: 10.1242/dev.128.17.3359. [DOI] [PubMed] [Google Scholar]
- Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez-del-Pulgar S, Carpenter PM, Hughes CC. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res. 2003;66:102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D. A novel, microcarrier-based in vitro assay for rapid and reliable quantification of three-dimensional cell migration and angiogenesis. Microvasc Res. 1995;50:311–322. doi: 10.1006/mvre.1995.1061. [DOI] [PubMed] [Google Scholar]
- Nelson RD, Quie PG, Simmons RL. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975;115:1650–1656. [PubMed] [Google Scholar]
- Ng YS, Ramsauer M, Loureiro RM, D'Amore PA. Identification of genes involved in VEGF-mediated vascular morphogenesis using embryonic stem cell-derived cystic embryoid bodies. Lab Invest. 2004;84:1209–1218. doi: 10.1038/labinvest.3700150. [DOI] [PubMed] [Google Scholar]
- Nicosia RF, Bonanno E, Villaschi S. Large-vessel endothelium switches to a microvascular phenotype during angiogenesis in collagen gel culture of rat aorta. Atherosclerosis. 1992;95:191–199. doi: 10.1016/0021-9150(92)90022-9. [DOI] [PubMed] [Google Scholar]
- Nicosia RF, Nicosia SV, Smith M. Vascular endothelial growth factor, platelet-derived growth factor, and insulin-like growth factor-1 promote rat aortic angiogenesis in vitro. Am J Pathol. 1994;145:1023–1029. [PMC free article] [PubMed] [Google Scholar]
- Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta: a quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–122. [PubMed] [Google Scholar]
- Nicosia RF, Villaschi S. Rat aortic smooth muscle cells become pericytes during angiogenesis in vitro. Lab Invest. 1995;73:658–666. [PubMed] [Google Scholar]
- Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- Pauly RR, Passaniti A, Crow M, Kinsella JL, Papadopoulos N, Monticone R, Lakatta EG, Martin GR. Experimental models that mimic the differentiation and dedifferentiation of vascular cells. Circulation. 1992;86:III-68–III-73. [PubMed] [Google Scholar]
- Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–1117. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Belin D, Montesano R, Orci L, Vassalli JD. Transforming growth factor-β 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990;111:743–755. doi: 10.1083/jcb.111.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada AR, Munoz-Chapuli R, Medina MA. Anti-angiogenic drugs: from bench to clinical trials. Med Res Rev. 2006;26:483–530. doi: 10.1002/med.20059. [DOI] [PubMed] [Google Scholar]
- Ramsauer M, D'Amore PA. Contextual role for angiopoietins and TGF- in blood vessel stabilization. J Cell Sci. 2007 doi: 10.1242/jcs.003533. in press. [DOI] [PubMed] [Google Scholar]
- Ratnikov B, Deryugina E, Leng J, Marchenko G, Dembrow D, Strongin A. Determination of matrix metalloproteinase activity using biotinylated gelatin. Anal Biochem. 2000;286:149–155. doi: 10.1006/abio.2000.4798. [DOI] [PubMed] [Google Scholar]
- Renvoizé C, Biola A, Pallardy M, Bréard J. Apoptosis: identification of dying cells. Cell Biol Toxicol. 1998;14:111–120. doi: 10.1023/a:1007429904664. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Nico B, Roncali L, Dammacco F. Postnatal vasculogenesis. Mech Dev. 2001;100:157–163. doi: 10.1016/s0925-4773(00)00522-0. [DOI] [PubMed] [Google Scholar]
- Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, Claesson-Selsh L, Janjic N. 2′-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998;273:20556–67. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar R, Bell SE, Davis GE. Coordinate induction of the actin cytoskeletal regulatory proteins gelsolin, vasodilator-stimulated phosphoprotein, and profilin during capillary morphogenesis in vitro. Exp Cell Res. 1999;249:22–32. doi: 10.1006/excr.1999.4460. [DOI] [PubMed] [Google Scholar]
- Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45:528–537. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118:2325–2340. doi: 10.1242/jcs.02360. [DOI] [PubMed] [Google Scholar]
- Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolenski RT, Khalpey Z, Osborne FN, Yuen A, Slominska EM, Lipinski M, Lavitrano M, Rose M, Yacoub MH. Species differences of endothelial extracellular nucleotide metabolism and its implications for xenotransplantation. Pharmacol Rep. 2006;58(Suppl):118–125. [PubMed] [Google Scholar]
- Staton CA, Stribbling SM, Tazzyman S, Hughes R, Brown NJ, Lewis CE. Current methods for assaying angiogenesis in vitro and in vivo. Int J Exp Pathol. 2004;85:233–248. doi: 10.1111/j.0959-9673.2004.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt AW, McGoldrick C, Rice-McCaldin A, McCance DR, Glenn JV, Hsu DK, Liu FT, Thorpe SR, Gardiner TA. Impaired retinal angiogenesis in diabetes: role of advanced glycation end products and galectin-3. Diabetes. 2005;54:785–794. doi: 10.2337/diabetes.54.3.785. [DOI] [PubMed] [Google Scholar]
- Trevisi L, Pighin I, Bazzan S, Luciani S. Inhibition of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) endocytosis by ouabain in human endothelial cells. FEBS Lett. 2006;580:2769–2773. doi: 10.1016/j.febslet.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Vailhé B, Vittet D, Feige JJ. In vitro models of vasculogenesis and angiogenesis. Lab Invest. 2001;81:439–452. doi: 10.1038/labinvest.3780252. [DOI] [PubMed] [Google Scholar]
- van der Pluijm G, Deckers M, Sijmons B, de Groot H, Bird J, Wills R, Papapoulos S, Baxter A, Lowik C. In vitro and in vivo endochondral bone formation models allow identification of anti-angiogenic compounds. Am J Pathol. 2003;163:157–163. doi: 10.1016/S0002-9440(10)63639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaft DW, Dings RP, de Lussanet QG, van Eijk LI, Nap AW, Beets-Tan RG, Bouma-Ter Steege JC, Wagstaff J, Mayo KH, Griffioen AW. The designer anti-angiogenic peptide anginex targets tumor endothelial cells and inhibits tumor growth in animal models. FASEB J. 2002;16:1991–1993. doi: 10.1096/fj.02-0509fje. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006;26:716–728. doi: 10.1161/01.ATV.0000209518.58252.17. [DOI] [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001;108:161–164. doi: 10.1016/s0925-4773(01)00469-5. [DOI] [PubMed] [Google Scholar]
- Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, Dejana E. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424–3431. [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang R, Clark R, Bautch VL. Embryonic stem cell-derived cystic embryoid bodies form vascular channels: an in vitro model of blood vessel development. Development. 1992;114:303–316. doi: 10.1242/dev.114.2.303. [DOI] [PubMed] [Google Scholar]
- Wartenberg M, Gunther J, Hescheler J, Sauer H. The embryoid body as a novel in vitro assay system for antiangiogenic agents. Lab Invest. 1998;78:1301–1314. [PubMed] [Google Scholar]
- Yarrow JC, Perlman ZE, Westwood NJ, Mitchison TJ. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004;4:21. doi: 10.1186/1472-6750-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Poon MC, Pu WT, Han ZC. Therapeutic neovascularization for peripheral arterial diseases: advances and perspectives. Histol Histopathol. 2007;22:677–686. doi: 10.14670/HH-22.677. [DOI] [PubMed] [Google Scholar]
- Zhu WH, Iurlaro M, MacIntyre A, Fogel E, Nicosia RF. The mouse aorta model: influence of genetic background and aging on bFGF- and VEGF-induced angiogenic sprouting. Angiogenesis. 2003;6:193–199. doi: 10.1023/B:AGEN.0000021397.18713.9c. [DOI] [PubMed] [Google Scholar]
- Zhu WH, Nicosia RF. The thin prep rat aortic ring assay: a modified method for the characterization of angiogenesis in whole mounts. Angiogenesis. 2002;5:81–86. doi: 10.1023/a:1021509004829. [DOI] [PubMed] [Google Scholar]
- Zigmond SH, Hirsch JG. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973;137:387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



