Abstract
Conus medullaris/cauda equina injuries typically result in loss of bladder, bowel, and sexual functions, partly as a consequence of autonomic and motor neuron death. To mimic these injuries, we previously developed a rodent lumbosacral ventral root avulsion (VRA) injury model, where both autonomic and motor neurons progressively die over several weeks. Here, we investigate whether minocycline, an antibiotic with putative neuroprotective effects, may rescue degenerating autonomic and motor neurons after VRA injury. Adult female rats underwent lumbosacral VRA injuries followed by a 2-week treatment with either minocycline or vehicle injected intraperitoneally. The sacral segment of the spinal cord was studied immunohistochemically using choline acetyltransferase (ChAT) and activated caspase-3 at 4 weeks post-operatively. Minocycline increased the survival of motoneurons but not preganglionic parasympathetic neurons (PPNs). Further investigations demonstrated that a larger proportion of motoneurons expressed activated caspase-3 compared to PPNs after VRA injury and indicated an association with minocycline’s differential neuroprotective effect. Our findings suggest that minocycline may protect degenerating motoneurons and expand the therapeutic window of opportunity for surgical repair of proximal root lesions affecting spinal motoneurons.
Keywords: spinal cord injury, conus medullaris, apoptosis, preganglionic parasympathetic neuron, ventral root avulsion, neuroprotection
INTRODUCTION
Conus medullaris/cauda equina injuries represent approximately 20% of all traumatic spinal cord injuries and result in paraparesis, sensory disturbance, pain, as well as autonomic dysfunction (Maynard et al. 1997; Hoang and Havton 2006). Injuries to the conus medullaris/cauda equina commonly occur from motor vehicle accidents and include lesions to the lumbosacral roots, which may be avulsed or separated from the spinal cord (Chin and Chew 1997). In adult rodent models, ventral root avulsion (VRA) injuries result in progressive retrograde death of motoneurons (Koliatsos et al. 1994; Novikov et al. 1995) and autonomic neurons (Hoang et al. 2003). As a repair strategy, surgical re-implantation of the avulsed ventral roots into the spinal cord leads to functional reinnervation of skeletal muscles (Carlstedt et al. 1986; Cullheim et al. 1989) and the lower urinary tract (Hoang et al. 2006). Root re-implantation procedures have been successfully performed in humans with traumatic brachial plexus injuries (Carlstedt et al. 2000), but functional improvement is compromised with prolonged lengths of time between the injury and root re-implantation (Carlstedt et al. 2000). This dismal outcome may be explained, in part, by the progressive VRA-induced motoneuron death. Therefore, early neuroprotection is much needed to lengthen the therapeutic window and thereby also augment functional outcome for this otherwise promising repair strategy.
Minocycline, a tetracycline antibiotic, recently emerged as a potentially attractive neuroprotective agent in animal models of stroke (Yrjanheikki et al. 1998), spinal cord injury (Stirling et al. 2004; Teng et al. 2004), as well as neurodegenerative diseases including amyotrophic lateral sclerosis (ALS), Huntington’s disease, and Parkinson’s disease (Blum et al. 2004). Minocycline may also attenuate the development of hyperesthesia and allodynia after an L5 spinal nerve transection injury in the rat (Raghavendra et al., 2003). The latter finding is of special interest to the present studies, as both transection and avulsion injuries of ventral roots may result in persistent neuropathic pain (Li et al., 2002; Bigbee et al., 2007). However, in some studies, minocycline treatment has failed to demonstrate beneficial effects (Smith et al. 2003; Fernandez-Gomez et al. 2005). Although its mechanisms of action are not fully understood, some studies suggest that minocycline may prevent cell death by suppressing the apoptotic pathway (Stirling et al. 2005). Minocycline may also influence non-apoptotic responses to nerve injury. For instance, minocycline has been reported to reduce the activation of microglia after a peripheral nerve injury, in part by inhibiting the expression of p38 mitogen-activated protein kinase (MAPK) (Piao et al., 2006). Minocycline may reduce the production of pro-nerve growth factor in microglia and thereby reduce death of oligodendrocytes after a traumatic spinal cord injury (Yune et al., 2007). Here, we examined whether minocycline may protect preganglionic parasympathetic neurons (PPNs) and motoneurons from retrograde cell death following a lumbosacral VRA injury.
METHODS
All animal procedures were carried out according to the standards established by the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996). The experimental protocols were approved by the Chancellor’s Animal Research Committee at UCLA. All animals were housed in a room with a 12/12 hour light/dark cycle, and they had access to food and water ad libitum. Fourteen adult female Sprague-Dawley rats (175–225g; Charles River Laboratories, Raleigh, NC) were divided into 2 groups: minocycline (n=7) and vehicle (n=7) treatment groups.
All animals underwent a unilateral L5-S2 VRA injury, as previously described in detail (Hoang et al. 2003; Hoang and Havton 2006). Briefly, under gas anesthesia with 2–2.5% Isoflurane (Abbott Laboratories, North Chicago, IL), a unilateral lumbar laminectomy was performed. As PPNs are located in the L6 and mostly S1 segments in the adult rat (Hoang et al. 2003), the lesion included the neighboring roots as well. Under a surgical microscope, the left four (L5, L6, S1 and S2) ventral roots were avulsed from the surface of the spinal cord. A thin sheet of gel foam was first placed over the exposed site followed by the placement of a titanium mesh cage to stabilize the vertebral column and to protect the spinal cord from compression by the overlying muscles and soft tissues (Nieto et al. 2005). The paraspinous muscles and skin were subsequently sutured in layers, and all animals were allowed to recover. All animals maintained bladder and bowel continence during the entire study.
Minocycline (Sigma, St. Louis, MO; 50mg/kg) or vehicle (saline) injections were administered intraperitoneally (i.p.) daily for 7 days post-operatively. The first dose was given on the day of surgery in connection with the root injury procedure. The minocycline dose was decreased in half (25mg/kg i.p. daily) for days 8–14 post-operatively, providing a total of 2 weeks of treatment. This treatment duration was chosen in attempts to maximize minocycline’s neuroprotective effects during the time period with the largest degree of neuronal degeneration and death after a lumbosacral VRA injury (Hoang et al., 2003). The solution containing minocycline was titrated to achieve a near normal pH before its i.p. administration in attempts to reduce the risk of treatment associated side effects, especially chemical irritation at the abdominal injection site. The above protocol for minocycline administration was tolerated well by the rats in the experimental series.
At 4 weeks post-op, under deep anesthesia, the rats were intravascularly perfused with 100 ml of phosphate buffer followed by 500 ml of 4% paraformaldehyde (4°C). A close inspection of the spinal cords was performed under a dissection light microscope to confirm the accuracy and completeness of the multilevel avulsion of ventral roots. The lumbosacral spinal cord segments were removed and postfixed overnight in the perfusion fixative (4°C), cryoprotected in 30% sucrose in phosphate buffered saline (PBS), frozen, and serially sectioned (40 µm thick) in the transverse plane.
Every alternating 2nd spinal cord section of the S1 segment was used for immunohistochemical detection of choline acetyltransferase (ChAT) to identify PPNs and motoneurons or double fluorescent labeling of ChAT and activated caspase-3 to identify neurons undergoing apoptosis. For light stable immunohistochemistry, sections were placed in 0.3% H202 for 10 minutes, rinsed in PBS, and incubated in 5% normal horse serum for 1 hour. The sections were then incubated overnight with anti-goat ChAT (1:200; Chemicon, Temecula, CA) in 0.3% Triton X-100/ PBS at room temperature. This primary antibody for ChAT detection was originally raised against human antigens but cross-reacts with rat antigens. The sections were next rinsed, incubated in solution containing a biotinylated secondary antibody (1:200; Vector Laboratories, Burlingame, CA) for 1 hour, rinsed, incubated with avidin-biotin complex (1:100; Vectastain ABC Elite kit, Vector Laboratories), and visualized using diaminobenzidine (DAB; Sigma). For double immunofluorescence, sections were incubated in ChAT and anti-rabbit activated caspase-3 (1:100 Cell Signaling, Danvers, MA) overnight at room temperature, rinsed, and incubated in anti-rabbit Alexa 594 red and anti-goat Alexa 488 green conjugated secondary antibodies (1:500; Molecular Probes, Eugene, OR) for 1 hour at room temperature. Images were captured with a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI) attached to a Nikon E600 light microscope equipped with fluorescent excitation filter sets.
We used the physical disector method for obtaining stereological counts of ChAT immunolabeled PPNs and motoneurons (Coggeshall and Lekan, 1996; West, 1999) and in accordance with our previous studies on neuronal loss after VRA injuries (Hoang et al., 2003). For this purpose, 10 sections from the S1 spinal cord segment in each animal were analyzed. A blinded observer counted all neuronal profiles except for neurons with cell bodies that were bisected at the rostral surface of each 40 µm section. The cell count was expressed as a ratio of the number of neurons on the avulsed side divided by the number of neurons on the contralateral, non-lesioned side. To obtain the percent of surviving PPNs and motoneurons that expressed activated caspase-3, a minimum of 6 sections per animal were analyzed in the light microscope by two observers. The two independent counts were in agreement and averaged.
Statistical analyses were performed using the non-parametric Mann-Whitney (two-tailed) test (SigmaStat 3.1, Systat Software, Inc., Point Richmond, CA). We regarded p<0.05 as reflecting a statistically significant difference between samples. Quantitative data were expressed as mean ± standard error.
RESULTS
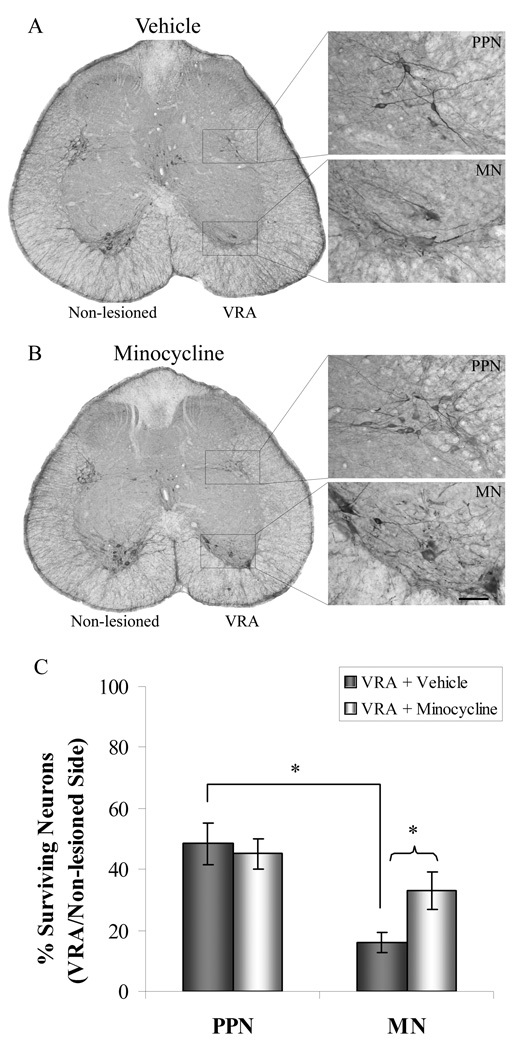
We first performed a quantitative analysis on the effect of a lumbosacral VRA injury on the axotomized PPNs and motoneurons in the S1 spinal cord segment. The PPNs normally innervate autonomic pelvic ganglia (de Groat and Yoshimura, 2006), whereas the S1 motoneurons innervate e.g. the musculus levator ani of the pelvic floor and tail muscles (Schrøder, 1980; Grossman et al., 1982). We here demonstrate that the degree of cell survival for PPNs (48 ± 7%) was significantly greater than that of motoneurons (16 ± 3%; p<0.05; Fig. 1, Table 1) at 4 weeks post-VRA. One possible contributing explanation for this difference in cell death between the studied neuronal types is that motoneuron somata of the S1 segment are located approximately 300–500 µm from the avulsion injury site at ventral root exit zone, whereas PPN somata are located approximately 1,200–1,400 µm from the lesion site. This anatomical aspect may be relevant, as previous studies have demonstrated that neuronal responses to injury may be dependent, in part, on the distance between the axotomy site and the cell body (Lieberman 1971; Herdegen et al., 1997).
Figure 1.
Minocycline protects motoneurons but not PPNs from cell death at 4 weeks after VRA injury. S1 spinal cord sections from vehicle (a) or minocycline (b) groups were immunohistochemically processed for ChAT to quantify the percent of surviving PPNs and motoneurons. Minocycline administration resulted in a significant increase in motoneuron survival, but not PPN survival (* p<0.05; c). Note that significantly more PPNs survived than motoneurons after injury in the vehicle group. Scale bar = 60 µm and applies to all high magnification images.
Table 1.
Minocycline protects motor but not autonomic neurons after VRA injury. PPNs and motoneurons were identified by ChAT immunoreactivity. Non-lesioned and avulsed sides of the lumbosacral spinal cord were compared in 10 sections per animal.
| Total # of PPNs | Total # of MNs | |||
|---|---|---|---|---|
| Vehicle | Non-lesioned | Avulsed | Non-lesioned | Avulsed |
| Rat #1 | 75 | 36 | 44 | 3 |
| Rat #2 | 54 | 22 | 51 | 10 |
| Rat #3 | 96 | 44 | 51 | 9 |
| Rat #4 | 67 | 44 | 57 | 14 |
| Rat #5 | 133 | 27 | 70 | 11 |
| Rat #6 | 83 | 58 | 60 | 7 |
| Minocycline | ||||
| Rat #1 | 94 | 29 | 48 | 7 |
| Rat #2 | 79 | 19 | 49 | 14 |
| Rat #3 | 90 | 52 | 47 | 17 |
| Rat #4 | 105 | 48 | 61 | 7 |
| Rat #5 | 102 | 54 | 50 | 24 |
| Rat #6 | 108 | 65 | 68 | 39 |
| Rat #7 | 102 | 46 | 58 | 20 |
We also assessed the potential effects of minocycline treatment on the survival of axotomized PPNs and motoneurons after the VRA injury. Interestingly, when compared to the retrograde response observed in our above injured control group, rats in the minocycline treatment group (n=7) showed a significantly increased survival for motoneurons (33 ± 6%; p<0.05), but not for PPNs (45 ± 5% Fig. 1c).
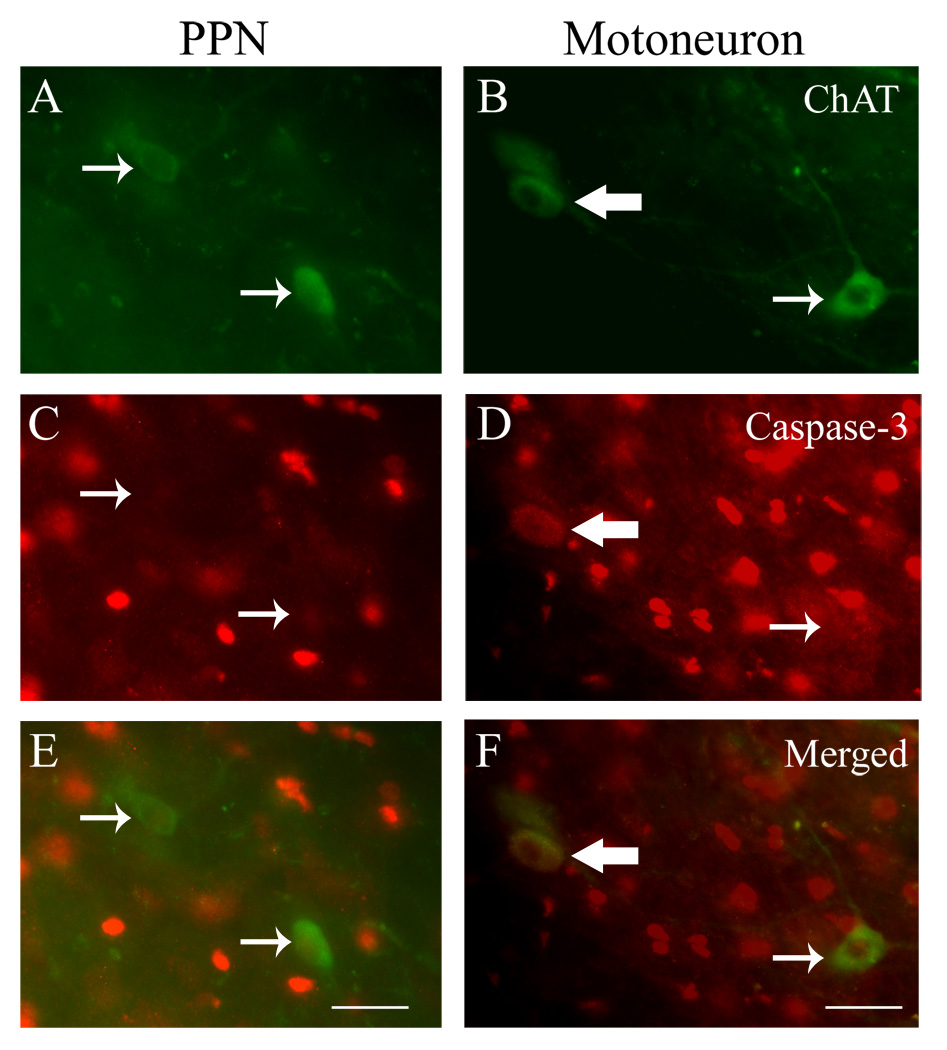
We next examined the expression of activated caspase-3, a marker for the final common pathway of apoptosis, to determine whether specific mechanisms of cell death may be associated with the differential neuroprotective effect provided by minocycline on the axotomized motoneurons and PPNs (Fig. 2). Here, the neuropil of both the lesioned and treatment groups demonstrate immunoreactivity for activated caspase-3 in a subpopulation of glial nuclei. In addition, subpopulations of both motoneurons and PPNs expressed immunoreactivity for the activated caspase-3 in the injured vehicle group and the minocycline treatment group. However, activated caspase-3 was only detected in PPNs and motoneurons on the side ipsilateral to the root injury, with absence of any neuronal localization for activated caspase-3 on the non-lesioned contralateral side of the spinal cord. Quantitative studies showed that there was a significantly larger percent of activated caspase-3 labeled motoneurons (37 ± 4%) than PPNs (10 ± 2%) on the lesioned side in the vehicle group (n=7; p<0.05) (Table 2). It is here interesting to note that the more pronounced loss of motoneurons than PPNs in our sample is also associated with a higher percentage of motoneurons expressing the activated caspase-3. In the minocycline group (n=7), this differential expression of activated caspase-3 was also maintained between motoneurons (28 ± 4%) and PPNs (9 ± 3%) in the minocycline group (n=7) (Table 2). Thus, although the minocycline treatment increased the overall number of surviving motoneurons, the portion of surviving motoneurons showing expression of the activated caspase-3 remained elevated in the treatment group.
Figure 2.
Differential expression of activated caspase-3 between PPNs (a, c, e) and motoneurons (b, d, f) at 4 weeks after VRA injury. Significantly more ChAT-immunolabeled motoneurons co-localized with activated caspase-3 (large arrows) than PPNs (p<0.05). Small arrows indicate no co-localization. Scale bar = 30 µm for a, c, e; 20 µm for b, d, f.
Table 2.
A higher proportion of motoneurons than PPNs expresses activated caspase-3 after VRA injury, and activated caspase-3 expression is not affected by minocyline treatment. Total counts and ratios were determined for all activated caspase-3 and ChAT immunoreactive PPNs and motoneurons. Each value represents an average of counts from 6–14 sections per animal made by two independent observers.
| Counts and ratios for PPNs | Counts and ratios for MNs | ||||||
|---|---|---|---|---|---|---|---|
| Vehicle | Caspase | ChAT | Ratio (%) | Caspase | ChAT | Ratio (%) | |
| Rat#1 | 6.5 | 43.0 | 15.1 | 2.0 | 9.0 | 22.2 | |
| Rat#2 | 8.5 | 53.5 | 15.9 | 12.5 | 32.5 | 38.5 | |
| Rat#3 | 6.5 | 64.0 | 10.1 | 13.5 | 30.0 | 45.0 | |
| Rat#4 | 3.0 | 27.0 | 11.1 | 25.5 | 64.5 | 39.5 | |
| Rat#5 | 4.0 | 47.0 | 8.5 | 4.0 | 16.5 | 24.2 | |
| Rat#6 | 2.0 | 48.5 | 4.1 | 7.0 | 20.5 | 34.1 | |
| Rat#7 | 3.0 | 63.5 | 4.7 | 6.5 | 12.0 | 54.1 | |
| Minocycline | |||||||
| Rat#1 | 5.0 | 24.5 | 20.4 | 4.5 | 12.5 | 36.0 | |
| Rat#2 | 6.5 | 33.0 | 19.7 | 10.0 | 23.0 | 43.5 | |
| Rat#3 | 5.5 | 103.0 | 5.3 | 5.0 | 26.5 | 18.9 | |
| Rat#4 | 2.5 | 54.0 | 4.6 | 3.5 | 12.5 | 28.0 | |
| Rat#5 | 4.0 | 106.0 | 3.8 | 3.5 | 37.5 | 9.3 | |
| Rat#6 | 3.5 | 96.0 | 3.6 | 19.5 | 78.0 | 25.0 | |
| Rat#7 | 1.0 | 20.0 | 5.0 | 6.5 | 19.5 | 33.3 | |
DISCUSSION
Our results demonstrate that minocycline doubled the number of surviving motoneurons, but had no effect on PPNs despite the fact that the two groups of neurons suffered the same trauma. Our further investigations demonstrated a differential expression of activated caspase-3 after VRA injury, which suggests a fundamental difference in death mechanisms between motoneurons and PPNs, perhaps explaining in part why minocycline protected motoneurons but not PPNs.
Minocycline-mediated neuroprotection may be associated with e.g. inhibition of p38 MAPK activation in microglia or with prevention of apoptosis by the inhibition of caspase activation (Stirling et al. 2005). Activated caspase-3 in the spinal cord milieu is reduced by minocycline during the first 24–72 hours after a mid-thoracic spinal cord injury and is associated with functional improvement and spinal cord tissue sparing up to 4 weeks after injury (Lee et al. 2003; Festoff et al. 2006). In our study, we examined the expression of activated caspase-3 following a 2-week treatment to determine whether minocycline may have a sustained influence on neuronal fate at 4 weeks post-VRA. Although minocycline increased motoneuron survival, the percent of motoneurons expressing activated caspase-3 did not differ between the experimental groups, raising the possibility that minocycline may only delay motoneuron death. The neuroprotection of motoneurons in this study shares some resemblance with the effect of minocycline in delaying the onset and halting the progression of motoneuron death in murine ALS models (Zhu et al. 2002), which may be mediated by apoptosis as well (Przedborski 2004). However, degenerative motoneuron conditions in the human are associated with a differential vulnerability between subsets of motoneurons. Although the bladder and anal sphincter functions typically remain intact in ALS, reports have demonstrated that motoneurons of Onuf’s nucleus may also be vulnerable to a degenerative process but to a lesser degree than other somatic motoneurons (Kihira et al., 1997). A differential expression of glutamate receptors between motoneurons of Onuf’s nucleus and other somatic motoneurons has been suggested to offer a clue to the selective vulnerability patterns in ALS (Anneser et al., 2004). Therefore, it is noteworthy to clarify that the motoneurons of the S1 segment in the present study likely innervate the pelvic floor and tail muscles (Schrøder, 1980; Grossman et al., 1982) but do not include the external urethral and anal sphincter-innervating motoneurons of the Onuf’s nucleus homologue in the rat L6 spinal cord segment.
The mechanisms underlying the VRA injury-induced motoneuron death remain controversial, as studies have shown support for both apoptosis and necrosis. Evidence of apoptosis after nerve avulsion injuries include activation of caspase-3, fragmentation of nuclear DNA, nuclear accumulation of p53, and translocation of Bax from the cytoplasm into mitochondria (Martin and Liu 2002). In addition, gene-deficient Bax−/− and p53−/− mice demonstrated absence of motoneuron death (Martin and Liu 2002). Despite the abundant evidence for apoptosis, treatment attempts with caspase inhibitors failed to rescue motoneurons after a cervical VRA injury (Chan et al. 2001). In addition, axotomized motoneurons demonstrated ultrastructural features typical of necrotic cell death, including perturbation, disruption and depletion of cytoplasmic organelles (Li et al. 1998). Furthermore, the lytic membrane attack complex, which leads to necrotic cell death, was increased on the surface and around injured motoneurons, and Clusterin expression was upregulated as a defense mechanism against the complement attack in motoneurons after VRA injury (Ohlsson and Havton, 2006). While the above studies altogether suggest that both apoptosis and necrosis contribute to VRA-induced motoneuron death, the mechanisms as to the concurrent autonomic neuron death are less well understood. Our finding suggests that PPNs do not undergo apoptosis to the same extent as motoneurons after VRA injury, thus different strategies may be needed to protect autonomic neurons.
When using ChAT as a marker for motoneuron or PPN detection in quantitative studies, it is important to take into consideration potential axotomy-induced effects on protein expression. Specifically, the expression of ChAT may be decreased in motoneurons following injury to their axons (Lams et al., 1988; Kou et al., 1995; Bussmann and Sofroniew, 1999). However, the axotomy-induced decrease in the expression of ChAT in motoneurons and PPNs following an injury to their axons is temporary, as ChAT immunoreactivity gradually returns to detectable levels over time with all axotomized motor and autonomic neurons being detectable by ChAT immunohistochemistry by four weeks after the injury (Rende et al., 1995; Hoang et al., 2003). In the present study, we investigated the neuronal counts at four weeks after the VRA injury. Therefore, the present neuronal counts should reflect an accurate assessment of surviving ChAT-immunoreactive neurons.
Here, we demonstrated immunoreactivity for caspase-3 in a subpopulation of glial nuclei. This finding is consistent with previous reports on the spinal cord gray matter of rats in control series and after ventral root lesions (Martin and Liu, 2002; Hoang et al., 2003). It was recently identified that a subpopulation of astrocytic nuclei normally expresses activated caspase-3 throughout the central nervous system, and that this expression pattern is related to a yet to be defined non-apoptotic function (Noyan-Ashraf et al., 2005).
Minocycline is currently being investigated clinically as a potential treatment for a variety of neurological conditions (Blum et al., 2004; Huntington Study Group, 2004). We postulate that minocycline may also be beneficial if combined with root re-implantation surgeries to protect motoneurons against retrograde death, expand the window for surgery, and thus promote improved functional recovery after proximal nerve avulsion injuries. For instance, minocycline may act to partially enhance the function of the lower urinary tract when combined with implantation of lesioned lumbosacral roots into the conus medullaris, as we recently demonstrated a positive correlation between voiding efficiency and motoneuron survival in the adult rat after VRA injury and repair (Hoang et al., 2006).
Acknowledgments
This work was supported by NIH ( NS042719), the Roman Reed Spinal Cord Injury Research Funds of California, and the Adelson Program for Neural Repair and Rehabilitation of the Adelson Medical Research Foundation. We would like to thank Dr. Allison Bigbee for helpful discussions.
REFERENCES
- Anneser JMH, Ince PG, Shaw PJ, Borasio GD. Differential expression of mGluR5 in human lumbosacral motoneurons. NeuroReport. 2004;15:271–273. doi: 10.1097/00001756-200402090-00012. [DOI] [PubMed] [Google Scholar]
- Bigbee AJ, Hoang TX, Havton LA. At-level neuropathic pain is induced by lumbosacral ventral root avulsion injury and ameliorated by root reimplantation into the spinal cord. Exp Neurol. 2007;204:273–282. doi: 10.1016/j.expneurol.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum D, Chtarto A, Tenenbaum L, Brotchi J, Levivier M. Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis. 2004;17:359–366. doi: 10.1016/j.nbd.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Bussmann KA, Sofroniew MV. Re-expression of p75NTR by adult motor neurons after axotomy is triggered by retrograde transport of a positive signal from axons regrowing through damaged or denervated peripheral nerve tissue. Neuroscience. 1999;91:273–281. doi: 10.1016/s0306-4522(98)00562-4. [DOI] [PubMed] [Google Scholar]
- Carlstedt T, Anand P, Hallin R, Misra PV, Noren G, Seferlis T. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J Neurosurg. 2000;93:237–247. doi: 10.3171/spi.2000.93.2.0237. [DOI] [PubMed] [Google Scholar]
- Carlstedt T, Lindå H, Cullheim S, Risling M. Reinnervation of hindlimb muscles after ventral root avulsion and implantation in the lumbar spinal cord in the adult rat. Acta Physiol Scand. 1986;128:645–646. doi: 10.1111/j.1748-1716.1986.tb08024.x. [DOI] [PubMed] [Google Scholar]
- Chan YM, Wu W, Yip HK, So KF, Oppenheim RW. Caspase inhibitors promote the survival of avulsed spinal motoneurons in neonatal rats. Neuroreport. 2001;12:541–545. doi: 10.1097/00001756-200103050-00022. [DOI] [PubMed] [Google Scholar]
- Chin CH, Chew KC. Lumbosacral nerve root avulsion. Injury. 1997;28:674–678. doi: 10.1016/s0020-1383(97)00080-6. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Cullheim S, Carlstedt T, Linda H, Risling M, Ulfhake B. Motoneurons reinnervate skeletal muscle after ventral root implantation into the spinal cord of the cat. Neuroscience. 1989;29:725–733. doi: 10.1016/0306-4522(89)90144-9. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gomez FJ, Gomez-Lazaro M, Pastor D, Calvo S, Aguirre N, Galindo MF, Jordan J. Minocycline fails to protect cerebellar granular cell cultures against malonate-induced cell death. Neurobiol Dis. 2005;20:384–391. doi: 10.1016/j.nbd.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Festoff BW, Ameenuddin S, Arnold PM, Wong A, Santacruz KS, Citron BA. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006;97:1314–1326. doi: 10.1111/j.1471-4159.2006.03799.x. [DOI] [PubMed] [Google Scholar]
- Grossman ML, Basbaum AI, Fields HL. Afferent and efferent connections of the rat tail flick reflex (A model used to analyze pain control mechanisms) J Comp Neurol. 1982;206:9–16. doi: 10.1002/cne.902060103. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Skene P, Bähr M. The c-Jun transcription factor - bipotential mediator of neuronal death, survival and regeneration. TINS. 1997;20:227–231. doi: 10.1016/s0166-2236(96)01000-4. [DOI] [PubMed] [Google Scholar]
- Hoang TX, Havton LA. Novel repair strategies to restore bladder function following cauda equina/conus medullaris injuries. Prog Brain Res. 2006;152:195–204. doi: 10.1016/S0079-6123(05)52012-0. [DOI] [PubMed] [Google Scholar]
- Hoang TX, Nieto JH, Tillakaratne NJK, Havton LA. Autonomic and motor neuron death is progressive and parallel in a lumbosacral ventral root avulsion model of cauda equina injury. J Comp Neurol. 2003;467:477–486. doi: 10.1002/cne.10928. [DOI] [PubMed] [Google Scholar]
- Hoang TX, Pikov V, Havton LA. Functional reinnervation of the rat lower urinary tract after cauda equina injury and repair. J Neurosci. 2006;26(34):8672–8679. doi: 10.1523/JNEUROSCI.1259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington Study Group. Minocycline safety and tolerability in Huntington disease. Neurology. 2004;63:547–549. doi: 10.1212/01.wnl.0000133403.30559.ff. [DOI] [PubMed] [Google Scholar]
- Kihira T, Yoshida S, Yoshimasu F, Wakayama I, Yase Y. Invovement of Onuf's nucleus in amyotrophic lateral sclerosis. J Neurol Sci. 1997;147:81–88. doi: 10.1016/s0022-510x(96)05313-0. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Price WL, Pardo CA, Price DL. Ventral root avulsion: an experimental model of death of adult motor neurons. J Comp Neurol. 1994;342:35–44. doi: 10.1002/cne.903420105. [DOI] [PubMed] [Google Scholar]
- Kou SY, Chiu AY, Patterson PH. Differential regulation of motor neuron survival and choline acetyltransferase expression following axotomy. J Neurobiol. 1995;27:561–572. doi: 10.1002/neu.480270410. [DOI] [PubMed] [Google Scholar]
- Lams BE, Isacson O, Sofroniew MV. Loss of transmitter-associated enzyme staining following axotomy does not indicate death of brainstem cholinergic neurons. Brain Res. 1988;475:401–406. doi: 10.1016/0006-8993(88)90635-x. [DOI] [PubMed] [Google Scholar]
- Lee SM, Yune TY, Kim SJ, Park do W, Lee YK, Kim YC, Oh YJ, Markelonis GJ, Oh TH. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 2003;20:1017–1027. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- Li L, Houenou LJ, Wu W, Lei M, Prevette DM, Oppenheim RW. Characterization of spinal motoneuron degeneration following different types of peripheral nerve injury in neonatal and adult mice. J Comp Neurol. 1998;396:158–168. [PubMed] [Google Scholar]
- Li L, Xian CJ, Zhong JH, Zhou XF. Effect of lumbar 5 ventral root transection on pain behaviors: A novel rat model for neuropathic pain without axotomy to primary sensory neurons. Exp Neurol. 2002;175:23–34. doi: 10.1006/exnr.2002.7897. [DOI] [PubMed] [Google Scholar]
- Lieberman AR. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Liu Z. Injury-induced spinal motor neuron apoptosis is preceded by DNA single-strand breaks and is p53- and Bax-dependent. J Neurobiol. 2002;50:181–197. doi: 10.1002/neu.10026. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Jr, Bracken MB, Creasey G, Ditunno JF, Jr, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International Standards for Neurological and Functional Classification of Spinal Cord Injury American Spinal Injury Association. Spinal Cord. 1997;35:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- Nieto JH, Hoang TX, Warner EA, Franchini BT, Westerlund U, Havton LA. Titanium mesh implantation--a method to stabilize the spine and protect the spinal cord following a multilevel laminectomy in the adult rat. J Neurosci Methods. 2005;147:1–7. doi: 10.1016/j.jneumeth.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Novikov L, Novikova L, Kellerth J-O. Brain-derived neurotrophic factor promotes survival and blocks nitric oxide synthase expression in adult rat spinal motoneurons after ventral root avulsion. Neurosci Lett. 1995;200:45–48. doi: 10.1016/0304-3940(95)12078-i. [DOI] [PubMed] [Google Scholar]
- Noyan-Ashraf MH, Brandizzi F, Juurlink BHJ. Constitutive nuclear localization of activated caspase 3 in subpopulations of the astroglial family of cells. Glia. 2005;49:588–593. doi: 10.1002/glia.20140. [DOI] [PubMed] [Google Scholar]
- Ohlsson M, Havton LA. Complement activation after lumbosacral ventral root avulsion injury. Neurosci Lett. 2006;394:179–183. doi: 10.1016/j.neulet.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Piao ZG, Cho IH, Park CK, Hong JP, Choi SY, Lee SJ, Lee S, Park K, Kim JS, Oh SB. Activation of glia and microglial p38 MAPK in medullary dorsal horn contributes to tactile hypersensitivity following trigeminal sensory nerve injury. Pain. 2006;121:219–231. doi: 10.1016/j.pain.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Przedborski S. Programmed cell death in amyotrophic lateral sclerosis: a mechanism of pathogenic and therapeutic importance. Neurologist. 2004;10:1–7. doi: 10.1097/01.nrl.0000106920.84668.37. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, Deleo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Rende M, Giambanco I, Buratta M, Tonali P. Axotomy induces a different modulation of both low-affinity nerve growth factor receptor and choline acetyltransferase between adult rat spinal and brainstem motoneurons. J Comp Neurol. 1995;363:249–263. doi: 10.1002/cne.903630207. [DOI] [PubMed] [Google Scholar]
- Smith DL, Woodman B, Mahal A, Sathasivam K, Ghazi-Noori S, Lowden PA, Bates GP, Hockly E. Minocycline and doxycycline are not beneficial in a model of Huntington's disease. Ann Neurol. 2003;54:186–196. doi: 10.1002/ana.10614. [DOI] [PubMed] [Google Scholar]
- Schrøder HD. Organization of the motoneurons innervating the pelvic muscles of the male rat. J Comp Neurol. 1980;192:567–587. doi: 10.1002/cne.901920313. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–322. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME, Friedlander RM. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A. 2004;101:3071–3076. doi: 10.1073/pnas.0306239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. TINS. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Yune TY, Lee JY, Jung GY, Kim SJ, Jiang MH, Kim YC, Oh YJ, Markelonis GJ, Oh TH. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27:7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu du C, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]