Abstract
Apurinic/apyrimidinic endonuclease-1/Redox factor-1, a multifunctional DNA base excision repair and redox regulation enzyme, plays an important role in oxidative signalling, transcription factor regulation, and cell cycle control. Recently, we have demonstrated that following the triggering of CD40 on B cells, APE/Ref-1 translocates from the cytoplasm to the nucleus and regulates the activity of B cell-specific transcription factors. In the present paper we investigate whether APE/Ref-1 plays a role in controlling CD40-mediated B cell proliferation too. We demonstrate a concurrent increase in proliferation and decrease in apoptosis of primary mouse B cells activated by CD40 cross-linking and transfected with functional APE/Ref-1 antisense oligonucleotide. Moreover, we provide evidence that a redox-mediated signalling mechanism is involved in this process and we propose that APE/Ref-1, controlling the intracellular redox state, may also affect the cell cycle by inducing nucleus-cytoplasm redistribution of p21. Together, these findings suggest that APE/Ref-1 could act as a negative regulator in an adaptive response to elevated ROS levels following CD40 cross-linking. Considering the important role of ROS and APE/Ref-1 in CD40-mediated B cell proliferation, our data will contribute to understand the mechanisms of tumor escape and suggest APE/Ref-1 as a novel target for tumor therapeutic approaches.
Keywords: CD40, APE/Ref-1, Proliferation
1. Introduction
The multifunctional protein apurinic/apyrimidinic endonuclease-1/Redox factor-1 (APE/Ref-1) [also referred to as human apurinic/apyrimidinic endonuclease (APE, HAP1, and APEX)] has a DNA repair domain at the C-terminus and a redox regulation domain at the N-terminus. The latter is involved in the regulation of the DNA binding activity of a group of nuclear factors including activator protein-1 (AP-1), NF-κB, p53, hypoxia-inducible factor (HIF)-1, HIF-like factor, Myb, BSAP, EBF and others via redox modification of a cysteine residue in the target protein (Fritz et al., 2003; Grillo et al., 2006; Merluzzi et al., 2004; Tell et al., 1998a,b). The redox activity of APE/Ref-1 is regulated by chemical reduction and oxidation in vitro. In cultured cells, APE/Ref-1 acts as an intermediary between thioredoxin and AP-1 or in numerous others signalling pathways in response to oxidant stress induced by PMA (Hirota et al., 1997), ionizing radiation (Wei et al., 2000) or cellular metabolism and receptor activation (D'Autreaux and Toledano, 2007). However, whether APE/Ref-1 is involved in redox regulation of cell proliferation either in B cells or in other cell types is currently unknown.
Survival, proliferation and apoptosis of lymphocytes are critical events to the maintenance of immune system homeostasis during development and responses to antigenic stimuli. Several cellular processes, including proliferation, can be regulated by ROS (D'Autreaux and Toledano, 2007). High levels of ROS are known to determine oxidative stress and DNA damage, while ROS low levels can stimulate proliferation and regulate protein function (Valko et al., 2007).
Cellular proliferation is a highly coordinated process requiring sequential assembly and activation of phase-specific protein kinase complexes, consisting of cyclin and cyclin-dependent kinase (cdk). Apart from these positive regulators, the cell cycle has negative regulators known as cdk inhibitors (p21 and p27), that bind the kinase complexes and prevent their spontaneous activation. Many cell cycle regulatory proteins, like cyclin D1, p21, and retinoblastoma have been shown to be sensitive to fluctuations in intracellular redox environment (Esposito et al., 1997; Menon et al., 2003).
ROS are produced by receptor-activated B lymphocytes after CD40 ligation (Lee, 2003; Lee and Koretzky, 1998) and their subtoxic levels transiently induce APE/Ref-1 activation and cellular relocalization. Recently, we have demonstrated, both in murine primary B cells and in human B cell line, a novel role of APE/Ref-1 in mediating CD40-downstream signalling events (Merluzzi et al., 2008, 2004).
APE/Ref-1 is critical for mouse development, as Ref-1-/- mouse is lethal at early stage of embryogenesis (Xanthoudakis et al., 1996) and heterozygous mutation in the Ref-1 gene in Xpc mutant mice results in skin cancer predisposition (Friedberg et al., 2000). Many studies have reported elevated APE/Ref-1 levels or altered subcellular localization in various types of cancer, such as cervical, prostate, breast and epithelial ovarian cancers, as well as in pediatric rabdomyosarcomas and melanomas, suggesting a prognostic relevance and a possible role of APE/Ref-1 in regulating cell proliferation in many tumors (Evans et al., 2000; Freitas et al., 2003; Puglisi et al., 2002; Tanner et al., 2004; Yang et al., 2005). Moreover, Gaiddon et al. (1999) have reported that APE/Ref-1 potentiates p53-induced apoptosis in the human lung adenocarcinoma cell line H1299 cells and Yao et al. (1995) have shown a possible relationship between APE/Ref-1 induction and the occurrence of apoptosis induced by hypoxia in human adenocarcinoma HT29 cells, suggesting that the role of APE/Ref-1 in contributing to apoptosis seems to rely on different types of cell lines and experimental stresses used in specific studies.
In the present study, we investigate whether APE/Ref-1 is involved in CD40-induced B cell proliferation. We demonstrate that repression of APE/Ref-1, by an antisense (AS) overexpression, determines an additional increase in CD40-mediated B cell proliferation, suggesting that APE/Ref-1 could act as “tuning molecule” in activated B cells. Moreover, we provide evidence that a redox-mediated signalling mechanism is involved in this proliferative response, as the observed increase in proliferation is abolished by pre-treatment of cells with the antioxidant N-acetyl-L-cysteine (NAC). Finally, we show that APE/Ref-1 may also affect the cell cycle by inducing nucleus-cytoplasm translocation of the cdk inhibitor p21. We propose that in primary B cells, APE/Ref-1 plays a critical role in CD40-induced B cell proliferation by modulating the extent of the proliferative response to ROS generated following B cell activation. Decreasing APE/Ref-1 levels could influence CD40-dependent activity of cell cycle regulatory pathways, which in turn regulates progression from one cell cycle phase to the next (Menon et al., 2007). We identify the nucleus-cytoplasm translocation of inhibitor p21 as a possible downstream critical event following CD40-mediated B cell proliferation.
2. Experimental procedures
2.1. Cell preparation, transfections and culture conditions
Murine B cells were isolated from spleen by a negative depletion method as previously described (Merluzzi et al., 2004) and the purity was more than 95%. APE/Ref-1 AS oligonucleotide and the AS reverse oligonucleotide were synthesized and HPSF purified by MWG-Biotech AG (Firenze, Italy). The oligonucleotides were phosphorotioated at 3′ and 5′ ends (at the position marked by *) to confer nuclease resistance. The sequence of the APE/Ref-1 AS probe was 5′-T*T*CCCCGCTTTGGCATC*G*C*-3′ and the AS reverse was 5′-G*C*GATCCCAAAGCGGGG*A*A*-3′ (nucleotides -3 to 16). Transfection of purified splenic B cells was performed by Lipofectin (Invitrogen) as described (Merluzzi et al., 2004). B cells were incubated with purified anti-mouse CD40 mAb HM40-3 at 1 μg/ml (BD Pharmingen) for the indicated times. When cells were treated with N-acetyl-L-cysteine (NAC) (Sigma), theywere preincubated for 30 min prior to the experiments at a density of 106 cells/ml with 10 mM NAC and experiments were performed in the continuous presence of 10 mM NAC.
2.2. Preparation of cell extracts and Western blot analysis
Preparation of cells extract and Western blot analysis were performed as described (Merluzzi et al., 2004). The experimental conditions used for extract preparation ensured that nuclear and cytoplasmic fractions were complementary as no material was lost during the preparation and no cross-contamination was observed. Antibodies used in Western blot included: anti-p21 WAF-1 (Immunological Sciences) 1:500, anti-APE/Ref-1 (C-20) 1:1000, anti-C23/nucleolin (C-11) 1:1000 (Santa Cruz Biotechnology), anti-actin (C-11) 1:2000, (Sigma).
2.3. MTT
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide MTT (Sigma) was used according to the manufacturer's instructions. The MTT test consists of measuring the mitochondrial succinate dehydrogenase activity of cells. This enzyme, by cutting the tetrazolium cycle, makes the yellow MTT, turn into blue formazan crystals. Dead cells do not cause this change. The results are read on a multiwell scanning spectrophotometer (ELISA reader) and the optical densities obtained are directly proportional to the number of living cells.
2.4. [3H]TdR incorporation assay
Proliferation of cells was assayed by measuring [3H]TdR incorporation during the final 16 h of cell culture. In brief, 5 × 104 splenic B cells were cultured in a final volume of 200 μl and stimulated with purified anti-mouse CD40 mAb in round bottom 96-well plates (Costar Corp., Cambridge, MA). During the last 16 h of cell culture, 0.5 μC of [3H]TdR was added to each well; incorporated 3[H]thymidine radioactivity was measured in a scintillation counter (Packard). Each incubation experiment was done in triplicate.
2.5. Flow cytometry assays
2.5.1. Carboxyfluorescein succinimidyl ester (CFSE) assays
Freshly purified mouse B cells were labeled with 1 μM CFSE (Molecular Probes) at room temperature for 8 min, washed twice with RPMI 1640 containing 10% FCS. After stimulation for 2 and 3 day, the CFSE profiles were evaluated by flow cytometry and the proliferation index was calculated using MODFIT software (Verity Software House, Topsham, ME). The proliferation Wizard module of ModFit LT™ V2.0 indicates the proliferation index as the sum of the cells in all generations divided by the computed number of original parent cells present at the start of the experiments and it is therefore a measure of the increase in cell number in the culture over the course of the experiment.
2.5.2. Propidium iodide staining for DNA content measurement
Cells washed in phosphate-buffered saline (PBS) were fixed in 70% ethanol for 30 min at -20 °C. Then the cells were washed twice in cold PBS and incubated in staining solution (PBS containing 100 μg/ml ribonuclease A, 50 μg/ml propidium iodide) for 30 min at room temperature. After staining, DNA content was analyzed by flow cytometry on FACSCalibur (Becton Dickinson) using CellQuest software (Becton Dickinson). Percentage of cells in each phase of cell cycle was analyzed using MODFIT software.
2.5.3. Flow cytometric detection of apoptosis: FITC-Annexin V/PI double staining
Double staining for FITC-Annexin V binding and for cellular DNA using Propidium Iodide (PI) was performed. After washing twice with PBS, 1 × 106 cells were resuspended in 1 ml of binding buffer (10 mM Hepes pH 7.4, 140 mM NaCl, and 2.5 mM CaCl2). A 0.1 vol. was added of FITC-Annexin V and PI at final concentration of 1 μg/ml cell suspension. The mixture was incubated for 15 min in the dark, at room temperature and then cellular fluorescence was measured by flow cytometry analysis with a FACSCalibur (Becton Dickinson).
2.6. Statistical analysis
The two-tailed Student's t-test was used to determine the statistical significance of the data. A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. Enhanced proliferation of mouse B cells transfected with APE/Ref-1 AS and stimulated with anti-CD40 mAb
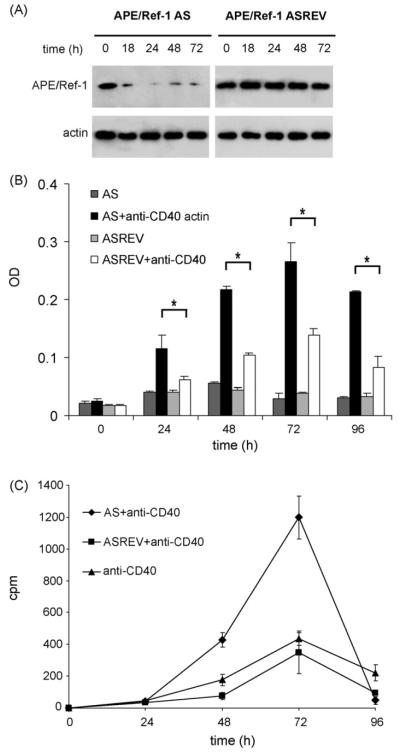
To investigate the role of APE/Ref-1 in the regulation of CD40-mediated B cell proliferation, we have transfected mouse B cells with phosphorothioate oligonucleotide, which is complementary to the APE/Ref-1 mRNA sequence and overlaps the translation initiation site (AS oligonucleotide) (Daily et al., 2001). As control, an AS reverse oligonucleotide was used. The cells were pre-treated with oligonucleotide plus Lipofectin and then stimulated with soluble purified anti-mouse CD40 mAb for different times. Western blot analysis showed the ability of the AS oligonucleotide, but not the AS reverse (ASREV) oligonucleotide (control), to efficiently down-regulate APE/Ref-1 protein production (Fig. 1A). Next, we have examined the effect of such down-regulation on CD40-mediated B cell proliferation. The MTT test provided an indication of mitochondrial function, which is proportional to the viable cell number and generates a signal, which is dependent on the degree of cell activation. As shown in Fig. 1B the number of living cells was similar in B cells transfected with APE/Ref-1 AS or control oligonucleotide. However after CD40 stimulation, B cells activation was significantly enhanced in cells transfected with APE/Ref-1 AS oligonucleotide compared with the control oligonucleotide. To confirm these data on B cell proliferation, we have used the conventional [3H] thymidine uptake assay. As shown in Fig. 1C stimulation of purified mouse B cells with anti-CD40 mAb induced cell proliferation that reached the highest level 72 h after stimulation. B cells transfected with APE/Ref-1 AS oligonucleotide proliferated three times more than B cells transfected with the control oligonucleotide or non-transfected B cells (p < 0.01), demonstrating that the control oligonucleotide used was not affecting activated B cells proliferation. This response appeared to be specific to CD40 stimulus because it was distinct from that activated by LPS. Indeed, mouse B cells transfected with APE/Ref-1 AS or control oligonucleotide presented similar proliferation responses when stimulated with LPS (data not shown).
Fig. 1.
Effect of APE/Ref-1 silencing on the B cells viability and proliferation. (A) Western blot analysis using anti-APE/Ref-1 Ab (upper panel) and actin (lower panel) on equal amount of total extracts from primary mouse B cells stimulated with anti-CD40 mAb and pre-treated with APE/Ref-1 AS or with APE/Ref-1 ASREV oligonucleotides. Representative blots from one of three independent experiments (B) B cell viability determined by the MTT assay, after incubating the B cells transfected with APE/Ref-1 AS or with control (APE/Ref-1 ASREV) oligonucleotides and stimulated with or without anti-CD40 mAb for 24, 48, 72 and 96 h. The data show the mean±S.D. *p < 0.05 from the triplicate samples. (C) B cells transfected with APE/Ref-1 AS or control (APE/Ref-1 ASREV), stimulated with anti-CD40 mAb were pulsed with [3H]thymidine for 16 h before harvesting at indicated time; incorporated 3[H] radioactivity was measured in a scintillation counter. The data show the mean counts per minute (cpm)±S.D. from the triplicate samples.
These data demonstrated that repression of APE/Ref-1 determines an additional increase in CD40-mediated B cell proliferation, suggesting that APE/Ref-1 could act as “moderator” regulating the levels of CD40-induced B cell proliferation.
3.2. CD40-activated B cells transfected with APE/Ref-1 AS proliferate faster than control-transfected cells
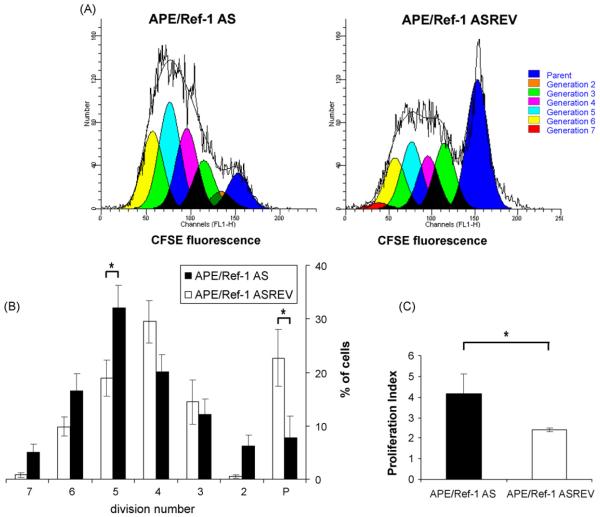
To further examine the growth behavior of CD40-activated B cells in which APE/Ref-1 was silenced, proliferation assays was performed with B cells, transfected with APE/Ref-1 AS or control oligonucleotides and then labeled with the fluorescent dye CFSE. As the cells divide, each successive generation contains a decreasing amount of CFSE. After 72 h of CD40 stimulation, cells were stained with a PE conjugated anti CD45R (B220) antibody, to specifically identify B lymphocytes and analyzed by FACS. The loss of CFSE signal in cells was modeled using the Proliferation Wizard algorithm of the ModFit LT program. Data were used to calculate percentages of events in each division cycle. The proliferation profile of cells transfected with APE/Ref-1 oligonucleotides and stimulated for 72 h with anti-CD40mAb is shown in Fig. 2A. Data from three similar independent experiments were used to make CFSE histograms of blasting B cells, reporting percentages of events in each division cycle. As shown in Fig. 2B, cells transfected with control oligonucleotides progress through the generations more slowly than cells transfected with APE/Ref-1 AS; after 3 days, 22±8% of cells transfected with control oligonucleotides were still in parent generation compared with only 7.8±3% of cells transfected with APE/Ref-1 AS and on the contrary, 54±12% of cells transfected with APE/Ref-1 AS distribute over the fourth generation (total number of cells in the 5th, 6th and 7th generation) compared with 28±11% of cells transfected with control oligonucleotides, suggesting that decreasing intracellular APE/Ref-1 expression results in increasing B cells proliferation rate. These data were confirmed by cellular proliferation index in Fig. 2C (4.2 vs. 2.3, p < 0.01).
Fig. 2.
B cells transfected with APE/Ref-1 AS and activated with anti-CD40 mAb proliferate faster than control cells. Mouse B cells were transfected with APE/Ref-1 AS or control (APE/Ref-1 ASREV) oligonucleotides, labeled with CFSE and stimulated with anti-CD40 mAb for 72 h. Cell divisions were determined using the Proliferation Wizard algorithm ModFit LT program (Version 2.0). (A) CFSE histograms of blasting B cells of one out three independent experiments; (B) the statistic analysis from data from three independent experiments expressed as percentages of cells in each generation and presented as means±S.D. *p < 0.05. Black and open histograms represent the percentage of APE/Ref-1 AS and control (APE/Ref-1 ASREV) cells in each generation, respectively; P indicates the parent generation. (C) The average of proliferation index from three independent experiments.
3.3. Effect of APE/Ref-1 repression in CD40-mediated apoptosis
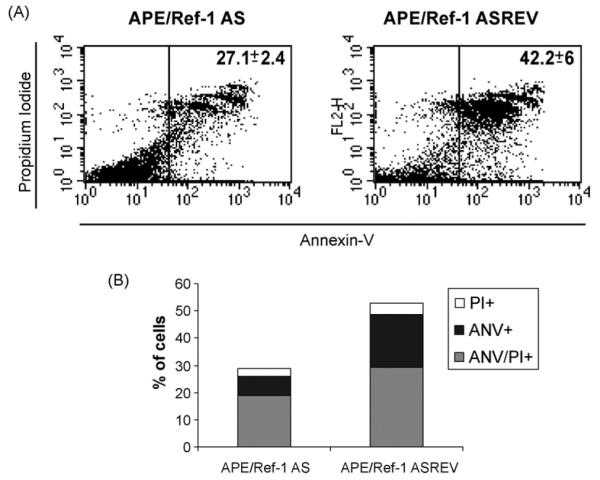
Functional outcomes of CD40 engagement in B cells is complex, varying from stimulation to apoptosis and depending on cell activation and differentiation state as well as on degree of CD40 ligation (Miyashita et al., 1997). Under the same experimental conditions, after 72 h of CD40 stimulation we have observed a percentage of untransfected B cells undergoing apoptosis. To investigate whether APE/Ref-1 is also involved in CD40-mediated apoptosis, we have used AnnexinV/propidium iodide staining analysis. A significantly reduced level of apoptosis in primary B cells transfected with APE/Ref-1 AS oligonucleotide, compared to control transfected cells, was detectable (Fig. 3A, 27.1± course 2.4% vs. 42.2± 6%, respectively). Time analysis revealed that CD40-mediated apoptosis was a slow-rate process, since significant difference in apoptosis between cells transfected with APE/Ref-1 AS and control oligonucleotide were detectable 48 h (data not shown) and further increased 72 h after CD40 activation (Fig. 3A and B). Furthermore, as shown in Fig. 3B, a progressive transition from early apoptotic cells (AnnexinV+/PI−) to late apoptotic cells (Annexin V+/PI+) was evident, indicating that intracellular APE/Ref-1 levels may affect CD40-stimulated B cells to undergo apoptosis.
Fig. 3.
Effect of APE/Ref-1 AS oligonucleotide on apoptosis of B cells activated by CD40 cross-linking. At 72 h after APE/Ref-1 AS transfection, cells were collected and resuspended in binding buffer containing Annexin V and PI, and then processed for flow cytometry analysis. (A) Events were gated on a dot plot showing forward and side scatter in order to exclude debris. Gated events were plotted on a dot plot showing Annexin V staining (FL1-H) and PI staining (FL2-H). Data are representative of three independent experiments. Number represents the percentage of gated cells counted for Annexin V plus Annexin V-PI positivity±S.D. from three independent experiments. (B) The graph bars represent the percentage of cells positive for a particular staining. One of three independent experiments with similar results was shown.
The decreased apoptotic rate measured in B cells transfected with APE/Ref-1 AS oligonucleotide was in agreement with the enhanced survival rate observed in MTT assay (Fig. 1B). In addition, inhibiting APE/Ref-1 by AS might render B cells less sensitive to apoptosis induced by CD40-stimulation.
Taken together, these results demonstrated a concurrent reduced apoptosis and increased mitogenesis in B cells transfected with APE/Ref-1 AS oligonucleotide.
3.4. A redox mechanism is involved in the control of APE/Ref-1-mediated proliferation
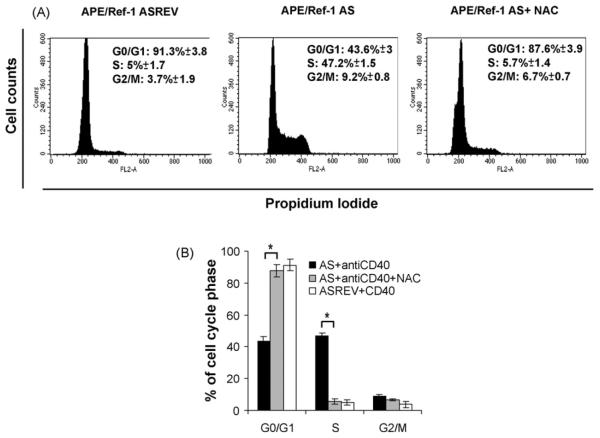
To determine which phase of the cell cycle, in addition to S phase, was affected by APE/Ref-1 silencing upon CD40-activation, we have examined nuclear DNA content by PI staining and FACS analysis. The cell cycle status of B cells transfected with APE/Ref-1 AS or control oligonucleotide was analyzed 24, 48, and 72 h after CD40-activation. As shown in Fig. 4A, 24 h after activation more cells were arrested in G1 phase (91.3±3.8% vs. 43.6 3%, p < 0.01) and fewer in S phase (5±1.7% vs. 47.2± 1.5%, p < 0.01) in control cells compared with APE/Ref-1 AS treated ones. This finding suggests that the repression of APE/Ref-1 results in enhanced cell cycle progression from G0/G1 to S phase in CD40-stimulated B cells.
Fig. 4.
Effect of APE/Ref-1 AS oligonucleotide on cell cycle phases of B cells activated by CD40 cross-linking. B cells transfected with APE/Ref-1 AS or control were intracellularly stained with PI and analyzed by flow cytometry to determine the cell cycle status. (A) Representative DNA histograms of B cells transfected with control (APE/Ref-1 ASREV) or APE/Ref-1 AS oligonucleotide were untreated or pre-treated with NAC and then stimulated for 24 h with anti-CD40 mAb. The percentage of cells in each phase of the cell cycle±S.D. from three independent experiments is indicated. (B) Histograms showing the means±S.D. of percentage of cells at G0/G1, S and G2/M of B cell transfected with control or APE/Ref-1 AS oligonucleotides untreated or pre-treated with NAC for 24 h from three independent experiments.
To investigate whether a redox-sensitive mechanism was involved in APE/Ref-1 AS-mediated proliferation, we have employed the antioxidant NAC, which is a sulfidryl containing thiol antioxidant, widely used as modulator of the intracellular redox state and recently discovered as inhibitor of cell progression from G1 to S phase in normal fibroblast (Menon et al., 2007).
We have observed that NAC induced changes in cell cycle status within 24 h of CD40-mediated activation; under these conditions, a significant increase in the percentage of G0/G1 cells (from 43.6 3 to 87.6±3.9%, p < 0.01), a concomitant decrease in the percentage of S phase cells (from 47.2±1.5% to 5.7 1.4%, p < 0.01) and a partial inhibition of cells in G2/M phase (from 9.2% to 6.7%) in APE//Ref-1 AS treated cells incubated with NAC was observable (Fig. 4A and B).
Since NAC mediated its effect through reduction of ROS, the finding that APE/Ref-1 AS-induced proliferation enhancement was reversed by NAC indicated that a redox-mediated signalling mechanism was involved in APE/Ref-1 activity.
3.5. Nucleus-cytoplasm translocation is under the control of APE/Ref-1 mediated proliferation
It has been recently demonstrated that alteration in the intracellular redox state may affect the cell cycle due to nucleus-cytoplasm translocation of p21 (Hwang et al., 2007), a protein that binds to and inhibits both the DNA synthesis regulator, proliferating cell nuclear antigen (PCNA), (Gulbis et al., 1996; Waga et al., 1994) and cyclin A/E-cdk2 complexes (Sherr and Roberts, 1999; Maddika et al., 2007) thus controlling the cell cycle. Indeed, p21 sequestration within the cytoplasm may serve to block p21's inhibitory activity toward cyclinA-E/cdk2 complexes, allowing increased proliferation rate (Porter, 1999; Hwang et al., 2007). To determine whether p21 molecule was involved in the APE/Ref-1 redox-mediated proliferation described in this paper, we have investigated the expression and translocation of p21 by Western blot.
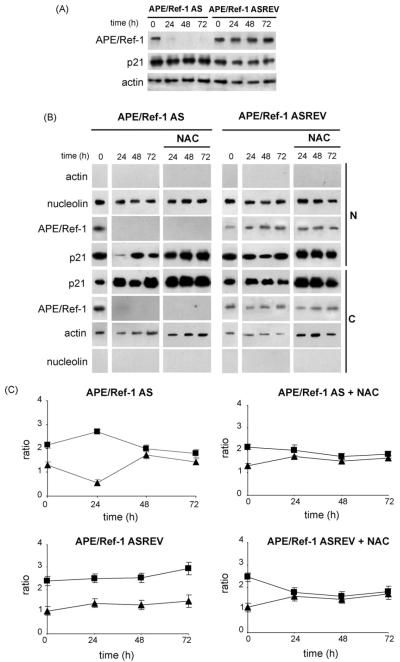
As shown in Fig. 5A, we have observed that the total levels of p21 protein remain constant in both B cells transfected with APE/Ref-1 AS or control oligonucleotides.
Fig. 5.
Analysis of p21 expression in B cells transfected with APE/Ref-1 AS and control oligonucleotides (A) Western blot analysis using anti-APE/Ref-1 Ab (upper panel) anti-p21 (middle panel) and actin (lower panel) on equal amount of total extracts prepared from primary mouse B cells stimulated with anti-CD40 mAb for 24, 48 and 72 h and pre-treated with APE/Ref-1 AS or control (APE/Ref-1 ASREV) oligonucleotides. Representative blots from one of three independent experiments are shown. (B) Nuclear (N) and cytoplasmic (C) proteins were prepared from activated B cells transfected with APE/Ref-1 AS or control (APE/Ref-1 ASREV) and treated with or without NAC, harvested at the indicated times and analyzed by SDS-PAGE and Western blot for p21 protein. Nucleolin and actin were assayed as controls for the purity of the nuclear and cytosolic fractionation. Results are representative of at least three independent experiments. (C) Densitometric scanning of bands is reported as ratio between the p21 protein levels and the actin or nucleolin levels in the same sample using MultiAnalyst program. Bars indicate the means±S.D. of three independent experiments. (■) indicates cytoplasmic values and (▲) nuclear values in cells activated by CD40 cross-linking.
As the cell cycle inhibitory activity of p21 is intimately correlated with its nuclear localization (Porter, 1999; Hwang et al., 2007), we have analyzed also the subcellular distribution of p21 during cell cycle progression. At time 0, APE/Ref-1 was equally distributed between the nucleus and the cytoplasm (Fig. 5B) whereas at 24 h the CD40-induced-B cell activation relocalized APE/Ref-1 in the nucleus. Moreover, CD40-stimulation caused a time dependent distribution of p21 to the cytoplasm in B cells transfected with APE/Ref-1 AS, but not as much as in control transfected cells (Fig. 5B and C). In fact, in APE/Ref-1 AS-treated cells, p21 was primarily localized in the nucleus (Fig. 5B and C, time 0) while became mainly cytoplasmic at 24 h. At 48 and 72 h p21 levels remained equally distributed in both the nucleus and the cytoplasm. These data demonstrated that APE/Ref-1 AS treatment induces nucleus to cytoplasm translocation of p21. The finding that cytoplasmic translocation of p21 was abolished by NAC indicated that a redoxmediated signalling mechanism is involved and it regulates the cell cycle-inhibitory role of p21 (Fig. 5B and C).
4. Discussion
APE/Ref-1 is a ubiquitously expressed multifunctional protein involved in DNA repair and redox regulation of TFs in response to oxidative stress. Levels of APE/Ref-1 were found to be elevated in several malignant tissues and the localization of the protein was found modified between the tumor and normal cellular counterpart (Kelley et al., 2001; Xu et al., 1997), suggesting that APE/Ref-1 might regulate the rate of tumor cell growth.
A growing number of reports indicate that intracellular redox status regulates various aspects of cellular function and that ROS are important chemical mediators involved in a number of cellular processes (Finkel, 2003; Valko et al., 2007). The cumulative generation of ROS through either endogenous or exogenous insults is common for many types of diseases that are linked with altered redox regulation of cellular signalling pathways (Palmieri and Sblendorio, 2007; Valko et al., 2007). ROS is known to cause oxidative stress and DNA damage at high levels; however, at low physiologic levels, ROS can stimulate proliferation and regulate protein function by inactivating many protein tyrosine phosphatases, activating some kinases and TFs and controlling cyclin levels. Cellular levels of ROS are also tightly regulated throughout the cell cycle (Havens et al., 2006; Menon et al., 2003).
Although intracellular ROS are generated by several sources, including mitochondria, they are also produced during signalling processes following ligation of the TNFR superfamily members, such as CD40 cross-linking (Lee, 2003; Lee and Koretzky, 1998). The primary sources of ROS involved in receptor-mediated signalling cascades are plasma membrane oxidases, preferentially NADPH oxidases, with a rapid kinetics of activation and inactivation. This allows a tight up- and down-regulation of intracellular ROS levels within the short time required for the transduction of signals from the plasma membrane to the cell nucleus. The mode of action of ROS may involve direct interaction with specific receptors, and/or redox-activation of members of signalling pathways. Furthermore, ROS act in concert with intracellular Ca2+ in signalling pathways that regulate the balance of cell proliferation vs. cell cycle arrest and cell death (Valko et al., 2007). The delicate intracellular interplay between oxidizing and reducing equivalents allows ROS to function as second messengers in the control of cell proliferation (D'Autreaux and Toledano, 2007).
Redox signals can elicit positive responses such as cellular activation or proliferation, as well as negative responses such as growth arrest or apoptosis. For instance, following the intracellular increase of H2O2, APE/Ref-1 is rapidly translocated into the nucleus of B-lymphocytes where it regulates the DNA binding activity of Pax-5 (Tell et al., 1998b, 2000), which is a TF acting as a master regulator of B lymphocyte growth and differentiation (Wakatsuki et al., 1994). Recently, we have demonstrated a novel role of APE/Ref-1 in CD40-mediated B cell activation, as it translocates from the cytoplasm to the nucleus and regulates B cell-specific TFs following the triggering of CD40 (Merluzzi et al., 2008, 2004).
Since ROS are involved in many tumors and diseases (Valko et al., 2007), can induce nuclear translocation of APE/Ref-1 (Frossi et al., 2002; Tell et al., 2000) and APE/Ref-1 acts as a key signalling molecule in B cell CD40-mediated activation (Merluzzi et al., 2004, 2008), we investigate whether APE/Ref-1 is also involved in controlling of B cell CD40-mediated proliferation.
First, APE/Ref-1 protein production was repressed by AS transfection techniques. Then, the cells were activated by CD40 mAb and their proliferative response was measured in terms of thymidine incorporation and MTT assay. Based on these data and the number of cells divisions using CFSE staining, we observed that B cells, transfected with APE/Ref-1 AS oligonucleotides and activated with anti-CD40 mAb, proliferate more and faster than control cells suggesting that APE/Ref-1 could have a role as “moderator” regulating the level of CD40-induced cell proliferation. Moreover, we have measured apoptosis by flow cytometry after Annexin V and PI staining and cell cycle distribution using DNA staining with PI. The decreased apoptotic rate measured in B cells transfected with APE/Ref-1 AS may explain the observed enhanced survival observed in MTT assay. Inhibiting APE/Ref-1 by AS may render B cells less sensitive to apoptosis induced by 72 h of CD40 stimulation. Measurement of cell cycle progression suggests that the absence of APE/Ref-1 results in enhanced cell cycle progression from G1 to S phase of activated B cells. Moreover, using NAC we demonstrate that a redox-sensitive mechanism is involved in APE/Ref-1 AS CD40-mediated B cell proliferation, because in cells treated with NAC, APE/Ref-1 AS induced proliferation is strongly inhibited.
Since recently it has been demonstrated that ROS induce nucleus-cytoplasm translocation of p21 and its subsequent degradation (Hwang et al., 2007), thus confirming that alteration of the intracellular redox state may affect cell cycle regulation, we investigated by Western blot the expression of p21 protein and in particular its subcellular distribution. We observed that CD40 stimulation causes a time dependent distribution of p21 in the cytoplasm in B cells transfected with APE/Ref-1 AS, compared to B cells transfected with the control oligonucleotide. Sequestration of p21 in the cytoplasm may serve to block its inhibitory activity toward cyclin A-E/cdk2 complexes and to allow increased proliferation, suggesting that altering the intracellular redox state by APE/Ref-1 repression affects the cell cycle. The finding that nucleus to cytoplasmic translocation of p21 is abolished by NAC indicates that a redox-mediated signalling mechanism is involved in APE/Ref-1 AS-induced cytoplasmic localization of p21.
Our findings demonstrate for the first time that APE/Ref-1 could act as a regulator of proliferation/apoptosis in an adaptive response to elevated ROS levels following CD40 cross-linking, thereby protecting cells from excess ROS stresses possibly through both DNA repair and activation of transcription factors such as Pax5.
B-lymphocytes are exposed to a reduction/oxidation environment during activation or inflammatory processes, and the antioxidant systems are efficient in protecting themselves against harmful ROS. These data, highlighting the crucial role of APE/Ref-1 in CD40-mediated B cell proliferation, could contribute to understand how oxidative stress could participate in normal physiology and disease states. ROS alteration in normal regulatory processes of cell growth may lead to cancer development (Mates and Sanchez-Jimenez, 2000; Valko et al., 2007). Because of its involvement in CD40-mediated proliferation and apoptosis, APE/Ref-1 could be considered as a novel target for tumor therapeutic approaches.
This study opens interesting questions regarding the mechanism by which ROS, produced following CD40 cross-linking in primary mouse B cells, could modulate the function of APE/Ref-1. We have recently demonstrated that, following CD40 cross-linking, APE/Ref-1 translocates in the nucleus of B cells (Merluzzi et al., 2004, 2008) where it regulates the activity of TFs. In this study, we demonstrate that APE/Ref-1 itself can modulate the CD40-mediated proliferation and directly or indirectly can affect the nucleus-cytoplasm translocation of inhibitor p21 and regulates cell cycle progression. However the mechanism by which APE/Ref-1 modulates the nucleus-cytoplasm translocation remains to be established.
Supplementary Material
Acknowledgements
We are grateful to Alfonso Colombatti, Orietta D'Orlando and Luca Danelli for helpful suggestions.
This work was supported by grants from Ministero dell'Istruzione Università e Ricerca (PRIN 2005), Agenzia Spaziale Italiana.(OSMA, MOMA), Associazione Italiana Ricerca sul Cancro (AIRC) and L.r. n. 26/2005 del Friuli Venezia Giulia.
Abbreviations
- CD40L
CD40 ligand
- ROS
Reactive Oxygen Species
- NAC
N-acetyl-L-cysteine
- PI
Propidium Iodide
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.molimm.2008.06.003.
References
- Daily D, Vlamis-Gardikas A, Offen D, Mittelman L, Melamed E, Holmgren A, Barzilai A. Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by activating NF-kappa B via Ref-1. J. Biol. Chem. 2001;276:1335–1344. doi: 10.1074/jbc.M008121200. [DOI] [PubMed] [Google Scholar]
- D'Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- Esposito F, Cuccovillo F, Vanoni M, Cimino F, Anderson CW, Appella E, Russo T. Redox-mediated regulation of p21(waf1/cip1) expression involves a post-transcriptional mechanism and activation of the mitogen-activated protein kinase pathway. Eur. J. Biochem. 1997;245:730–737. doi: 10.1111/j.1432-1033.1997.00730.x. [DOI] [PubMed] [Google Scholar]
- Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat. Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Freitas S, Moore DH, Michael H, Kelley MR. Studies of apurinic/apyrimidinic endonuclease/ref-1 expression in epithelial ovarian cancer: correlations with tumor progression and platinum resistance. Clin. Cancer Res. 2003;9:4689–4694. [PubMed] [Google Scholar]
- Friedberg EC, Bond JP, Burns DK, Cheo DL, Greenblatt MS, Meira LB, Nahari D, Reis AM. Defective nucleotide excision repair in xpc mutant mice and its association with cancer predisposition. Mutat. Res. 2000;459:99–108. doi: 10.1016/s0921-8777(99)00068-3. [DOI] [PubMed] [Google Scholar]
- Fritz G, Grosch S, Tomicic M, Kaina B. APE/Ref-1 and the mammalian response to genotoxic stress. Toxicology. 2003;193:67–78. doi: 10.1016/s0300-483x(03)00290-7. [DOI] [PubMed] [Google Scholar]
- Frossi B, Tell G, Spessotto P, Colombatti A, Vitale G, Pucillo C. H(2)O(2) induces translocation of APE/Ref-1 to mitochondria in the Raji B-cell line. J. Cell Physiol. 2002;193:180–186. doi: 10.1002/jcp.10159. [DOI] [PubMed] [Google Scholar]
- Gaiddon C, Moorthy NC, Prives C. Ref-1 regulates the transactivation and pro-apoptotic functions of p53 in vivo. EMBO J. 1999;18:5609–5621. doi: 10.1093/emboj/18.20.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo C, D'Ambrosio C, Scaloni A, Maceroni M, Merluzzi S, Turano C, Altieri F. Cooperative activity of Ref-1/APE and ERp57 in reductive activation of transcription factors. Free Radic. Biol. Med. 2006;41:1113–1123. doi: 10.1016/j.freeradbiomed.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Gulbis JM, Kelman Z, Hurwitz J, O'Donnell M, Kuriyan J. Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell. 1996;87:297–306. doi: 10.1016/s0092-8674(00)81347-1. [DOI] [PubMed] [Google Scholar]
- Havens CG, Ho A, Yoshioka N, Dowdy SF. Regulation of late G1/S phase transition and APC Cdh1 by reactive oxygen species. Mol. Cell Biol. 2006;26:4701–4711. doi: 10.1128/MCB.00303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CY, Kim IY, Kwon KS. Cytoplasmic localization and ubiquitination of p21(Cip1) by reactive oxygen species. Biochem. Biophys. Res. Commun. 2007;358:219–225. doi: 10.1016/j.bbrc.2007.04.120. [DOI] [PubMed] [Google Scholar]
- Kelley MR, Cheng L, Foster R, Tritt R, Jiang J, Broshears J, Koch M. Elevated and altered expression of the multifunctional DNA base excision repair and redox enzyme Ape1/ref-1 in prostate cancer. Clin. Cancer Res. 2001;7:824–830. [PubMed] [Google Scholar]
- Lee JR. Reactive oxygen species play roles on B cell surface receptor CD40-mediated proximal and distal signaling events: effects of an antioxidant, N-acetyl-L-cysteine treatment. Mol. Cell Biochem. 2003;252:1–7. doi: 10.1023/a:1025529704480. [DOI] [PubMed] [Google Scholar]
- Lee JR, Koretzky GA. Production of reactive oxygen intermediates following CD40 ligation correlates with c-Jun N-terminal kinase activation and IL-6 secretion in murine B lymphocytes. Eur. J. Immunol. 1998;28:4188–4197. doi: 10.1002/(SICI)1521-4141(199812)28:12<4188::AID-IMMU4188>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, Eshraghi M, Manda KD, Wiechec E, Los M. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist. Updat. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Mates JM, Sanchez-Jimenez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int. J. Biochem. Cell Biol. 2000;32:157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- Menon SG, Sarsour EH, Kalen AL, Venkataraman S, Hitchler MJ, Domann FE, Oberley LW, Goswami PC. Superoxide signaling mediates N-acetyl-L-cysteine-induced G1 arrest: regulatory role of cyclin D1 and manganese superoxide dismutase. Cancer Res. 2007;67:6392–6399. doi: 10.1158/0008-5472.CAN-07-0225. [DOI] [PubMed] [Google Scholar]
- Menon SG, Sarsour EH, Spitz DR, Higashikubo R, Sturm M, Zhang H, Goswami PC. Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res. 2003;63:2109–2117. [PubMed] [Google Scholar]
- Merluzzi S, D'Orlando O, Leonardi A, Vitale G, Pucillo C. TRAF2 and p38 are involved in B cells CD40-mediated APE/Ref-1 nuclear translocation: a novel pathway in B cell activation. Mol. Immunol. 2008;45:76–86. doi: 10.1016/j.molimm.2007.05.010. Epub 2007 Jun 27. [DOI] [PubMed] [Google Scholar]
- Merluzzi S, Moretti M, Altamura S, Zwollo P, Sigvardsson M, Vitale G, Pucillo C. CD40 stimulation induces Pax5/BSAP and EBF activation through a APE/Ref-1-dependent redox mechanism. J. Biol. Chem. 2004;279:1777–1786. doi: 10.1074/jbc.M305418200. [DOI] [PubMed] [Google Scholar]
- Miyashita T, McIlraith MJ, Grammer AC, Miura Y, Attrep JF, Shimaoka Y, Lipsky PE. Bidirectional regulation of human B cell responses by CD40-CD40 ligand interactions. J. Immunol. 1997;158:4620–4633. [PubMed] [Google Scholar]
- Palmieri B, Sblendorio V. Oxidative stress detection: what for? Part II. Eur. Rev. Med. Pharmacol. Sci. 2007;11:27–54. [PubMed] [Google Scholar]
- Porter AG. Protein translocation in apoptosis. Trends Cell Biol. 1999;9:394–401. doi: 10.1016/s0962-8924(99)01624-4. [DOI] [PubMed] [Google Scholar]
- Puglisi F, Barbone F, Tell G, Aprile G, Pertoldi B, Raiti C, Kelley MR, Damante G, Sobrero A, Beltrami CA, Di Loreto C. Prognostic role of Ape/Ref-1 subcellular expression in stage I-III breast carcinomas. Oncol. Rep. 2002;9:11–17. [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Tanner B, Grimme S, Schiffer I, Heimerdinger C, Schmidt M, Dutkowski P, Neubert S, Oesch F, Franzen A, Kolbl H, Fritz G, Kaina B, Hengstler JG. Nuclear expression of apurinic/apyrimidinic endonuclease increases with progression of ovarian carcinomas. Gynecol. Oncol. 2004;92:568–577. doi: 10.1016/j.ygyno.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Tell G, Pellizzari L, Cimarosti D, Pucillo C, Damante G. Ref-1 controls pax-8 DNA-binding activity. Biochem. Biophys. Res. Commun. 1998a;252:178–183. doi: 10.1006/bbrc.1998.9548. [DOI] [PubMed] [Google Scholar]
- Tell G, Scaloni A, Pellizzari L, Formisano S, Pucillo C, Damante G. Redox potential controls the structure and DNA binding activity of the paired domain. J. Biol. Chem. 1998b;273:25062–25072. doi: 10.1074/jbc.273.39.25062. [DOI] [PubMed] [Google Scholar]
- Tell G, Zecca A, Pellizzari L, Spessotto P, Colombatti A, Kelley MR, Damante G, Pucillo C. An `environment to nucleus' signaling system operates in B lymphocytes: redox status modulates BSAP/Pax-5 activation through Ref-1 nuclear translocation. Nucleic Acids Res. 2000;28:1099–1105. doi: 10.1093/nar/28.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369:574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Wei SJ, Botero A, Hirota K, Bradbury CM, Markovina S, Laszlo A, Spitz DR, Goswami PC, Yodoi J, Gius D. Thioredoxin nuclear translocation and interaction with redox factor-1 activates the activator protein-1 transcription factor in response to ionizing radiation. Cancer Res. 2000;60:6688–6695. [PubMed] [Google Scholar]
- Wakatsuki Y, Neurath MF, Max EE, Strober W. The B cell-specific transcription factor BSAP regulates B cell proliferation. J. Exp. Med. 1994;179:1099–1108. doi: 10.1084/jem.179.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthoudakis S, Smeyne RJ, Wallace JD, Curran T. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8919–8923. doi: 10.1073/pnas.93.17.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Moore DH, Broshears J, Liu L, Wilson TM, Kelley MR. The apurinic/apyrimidinic endonuclease (APE/ref-1) DNA repair enzyme is elevated in premalignant and malignant cervical cancer. Anticancer Res. 1997;17:3713–3719. [PubMed] [Google Scholar]
- Yang S, Irani K, Heffron SE, Jurnak F, Meyskens FL., Jr. Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1) in human melanoma and identification of the therapeutic potential of resveratrol as an APE/Ref-1 inhibitor. Mol Cancer Ther. 2005;4:1923–1935. doi: 10.1158/1535-7163.MCT-05-0229. [DOI] [PubMed] [Google Scholar]
- Yao KS, Clayton M, O'Dwyer PJ. Apoptosis in human adenocarcinoma HT29 cells induced by exposure to hypoxia. J. Natl. Cancer Inst. 1995;87:117–122. doi: 10.1093/jnci/87.2.117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.