Summary
Since the discovery of the first histone lysine demethylase in 2004, two protein families with numerous members have been identified that demethylate various histone lysine residues. Initial studies of the histone lysine demethylases focused on their in vitro enzymatic activity but, more recently, model organisms have been used to examine the roles of these enzymes in vivo. Here, we review recent insights into the roles of the histone lysine demethylases in multiple aspects of development across various species, including in germline maintenance and meiosis, in early embryonic development and differentiation, and in hormone receptor-mediated transcriptional regulation.
Introduction
Due to physical and functional constraints, the genetic material must be packaged within the cell in a way that both efficiently minimizes the space it occupies and allows it to be accessed in a regulated manner. The basic unit of this packaging is the nucleosome, which consists of 146 bp of DNA wrapped around a histone octamer. Each histone octamer contains two subunits of four types of histones: H3, H4, H2A and H2B. The DNA between histones is associated with the linker histone H1.
The four histone subunits can be post-translationally modified in a variety of ways, including by phosphorylation, ubiquitylation, sumoylation, acetylation and methylation. Many of these modifications are correlated with particular chromatin environments and biological outcomes (for reviews, see Kouzarides, 2007; Li et al., 2007; Margueron et al., 2005). Certain modifications, such as acetylation, are generally associated with euchromatin. Among the histone modifications, histone lysine methylation is of particular interest given the diverse set of methylation-associated biological processes that exist, including transcriptional activation and repression, heterochromatin-mediated transcriptional silencing, the DNA damage response and X chromosome inactivation (Margueron et al., 2005; Martin and Zhang, 2005).
Each of the methylated lysines exists in four distinct states: un-, mono-, di- or tri-methylated, and each of these states can have independent functions (see Table 1). The most-studied sites of histone lysine methylation are histone H3 lysines 4, 9, 27, 36 and 79, and histone H4 lysine 20 (Martin and Zhang, 2005). Only those four lysines closest to the N-terminus of histone H3 have known demethylases. H3K79- and H4K20-specific demethylases have not yet been identified and there is some controversy over their existence (Frederiks et al., 2008).
Table 1.
Genomic localization of histone demethylase substrates
| Lysine | Methylation | Genomic localization | Gene localization |
|---|---|---|---|
| K4 | me1 | Insulators | Active enhancers, active promoters, intragenic |
| me2 | Tissue-specific genes; insulators | Active enhancers, active promoters, intragenic | |
| me3 | Active chromatin; tissue-specific genes; insulators | Active enhancers, active promoters | |
| K9 | me1 | Pericentric heterochromatin (dm); nuclear interior; insulators | Active enhancers, active promoters |
| me2 | Pericentric heterochromatin (dm); nuclear/nucleolar periphery | Repressed promoters, silenced promoters | |
| me3 | Pericentric heterochromatin (dm); repressed chromatin; silenced genes; centromeres; telomeres | Silenced promoters, intragenic (active genes) | |
| K27 | me1 | Pericentric heterochromatin (dm) | Active promoters, intragenic |
| me2 | Pericentric heterochromatin (dm) | Silenced promoters | |
| me3 | Pericentric heterochromatin (dm); repressed chromatin; tissue-specific silenced genes | Silenced promoters | |
| K36 | me1 | NR | Slight association with active promoters |
| me2 | NR | Intragenic (3′ end) | |
| me3 | Active genes | Intragenic (3′ end) |
Data are from studies performed in Drosophila melanogaster (dm) or in mammalian systems (Barski et al., 2007; Heintzman et al., 2007; Mikkelsen et al., 2007; Regha et al., 2007; Vakoc et al., 2006). NR, data not reported in mammalian systems
Although the histone methylation modification was discovered in the 1960s (Allfrey et al., 1964), histone methyltransferases (KMTs) and histone lysine demethylases (KDMs) have only recently been identified. The existence of enzymes that methylate or demethylate histone lysines is perhaps unsurprising. However, prior to the discovery of the KDMs, the process of histone methylation was widely considered to be irreversible (reviewed by Bannister et al., 2002). In 2004, the amine oxidase-domain-containing mammalian protein LSD1/KDM1 [for lysine-specific demethylase 1, later renamed lysine (K) demethylase 1 to standardize nomenclature; also known as AOF2] (see Table 2) was identified as the first histone lysine demethylase. LSD1/KDM1 demethylates both di- and mono-methylated K4 (H3K4me2/1) (Shi et al., 2004). In 2006, the first jumonji-domain-containing demethylase, JHDM1A/KDM2A (FBXL11), was identified as an H3K36me2/1 demethylase (Tsukada et al., 2006). The jumonji (or JmjC)-domain-containing proteins belong to the dioxygenase superfamily and use a demethylation mechanism distinct from that of LSD1/KDM1 (Anand and Marmorstein, 2007) (see Box 1). These enzymes were predicted to be capable of tri-methyl demethylation (Schneider and Shilatifard, 2006; Trewick et al., 2005), and a tri-methyl demethylase family, the JMJD2/KDM4 family (Fodor et al., 2006; Klose et al., 2006; Whetstine et al., 2006), was indeed reported soon after the first jumonji-domain-containing demethylase was discovered. Over the next few years, a series of studies identified additional jumonji-domain-containing families that have K4me, K9me, K27me and K36me as their substrates (Table 2).
Table 2.
Histone demethylase nomenclature and substrate specificity
| Standardized family name | Family member (mammalian) | Former names | Homologs referenced in this review | Substrate specificity | References |
|---|---|---|---|---|---|
| KDM1 | KDM1 | LSD1; AOF2; BHC110 | Sp: SpLsd1; Swm1 | H3K4me2/1 | (Rudolph et al., 2007; Shi et al., 2004) |
| Dm: SU(VAR)3-3 | H3K9me2/1 | ||||
| p53 | |||||
| KDM2 | KDM2A | JHDM1A; FBXL11 | H3K36me2/1 | (Tsukada et al., 2006) | |
| KDM2B | JHDM1B; FBXL10 | ||||
| KDM3 | KDM3A | JHDM2A; JMJD1A; TSGA | H3K9me2/1 | (Yamane et al., 2006) | |
| KDM3B | JHDM2B; JMJD1B | ||||
| KDM4 | KDM4A | JMJD2A; JHDM3A | Ce: JMJD-2 | H3K9me3/2 | (Whetstine et al., 2006) |
| KDM4B | JMJD2B | H3K36me3/2 | |||
| KDM4C | JMJD2C; GASC1 | ||||
| KDM4D | JMJD2D | ||||
| KDM5 | KDM5A | RBP2; JARID1A | Ce: RBR-2 | H3K4me3/2 | (Christensen et al., 2007; Iwase et al., 2007; Klose et al., 2007; Lee et al., 2007a; Liang et al., 2007) |
| KDM5B | PLU-1; JARID1B | Dm: LID | |||
| KDM5C | SMCX; JARID1C | Sc: Yjr119Cp; Jhd2p | |||
| KDM5D | SMCY; JARID1D | ||||
| KDM6 | KDM6A | UTX | Ce: F18E9.5 | H3K37me3/2 | (Agger et al., 2007; Lan et al., 2007a) |
| KDM6B | JMJD3 |
The substrate specificity column lists the nomenclature and known substrate specificities of each demethylase family and enzyme referenced in this review. In the text, the demethylases are referred to by both the standardized name and one former name (in bold). Many of these demethylases have been described by multiple groups; here we reference the first report to show in vitro demethylase activity for each enzyme. Ce, Caenorhabditis elegans; Dm, Drosophila melanogaster; Sc, Saccharomyces cerevisiae; Sp, Schizosaccharomyces pombe
Box 1. Classes of histone demethylase enzymes
Two classes of enzymes have been reported with histone lysine demethylase activity. The first class of enzyme is solely represented by LSD1/KDM1, whereas the remaining known demethylases fall into the jumonji (JmjC)-domain-containing class. The proposed enzymatic mechanisms of these two distinct classes offer insights into their substrate specificity and necessary co-factors. Demethylases have been identified for histone H3 lysine 4 (H3K4), H3K9, H3K27 and H3K36; in these examples, we use H3K4 as a substrate.
Amine oxidase-domain-containing mechanism:
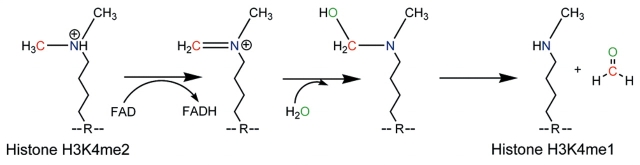
LSD1/KDM1 contains a flavin adenine dinucleotide (FAD)-dependent amine oxidase domain that is responsible for its demethylase activity. The enzyme uses FAD as a co-factor to catalyze an amine oxidation of the protonated nitrogen (blue), creating an iminium (N+) ion. This iminium ion spontaneously hydrolyzes to release a formaldehyde molecule, resulting in a mono-methylated lysine (H3K4me1). This mono-methylated lysine can also undergo the same reaction to become unmethylated. An important difference between this reaction mechanism and that of the JmjC-domain-containing proteins is that LSD1/KDM1 requires a protonated nitrogen as a hydrogen donor. The nitrogen of a tri-methylated lysine is not protonated and therefore cannot be demethylated by LSD1/KDM1. R, amino acid backbone.
JmjC-domain-containing mechanism:
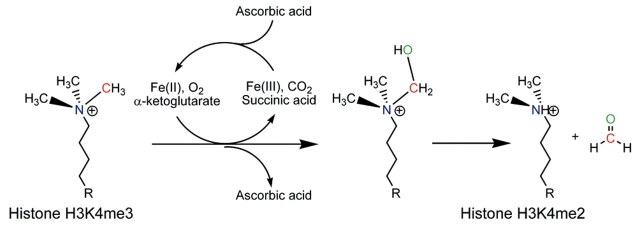
The JmjC-domain-containing enzymes were predicted to be capable of histone demethylation based on their homology to the AlkB dioxygenases, which use oxidation to remove damage-induced alkylation from DNA. The multiple co-factors required for JmjC-mediated demethylation [Fe(II), O2 and α-ketoglutarate] are believed to act coordinately to hydroxylate the methyl group. The reaction then proceeds analogously to a LSD1/KDM1 demethylation, with the unstable carbinolamine group being spontaneously released as formaldehyde. The JmjC domain is capable of demethylating mono-, di- and tri-methylated substrates.
This review focuses on recent studies of the developmental roles of histone lysine demethylases in various model organisms (particularly on the H3K4, K9, K27 and K36me demethylases), beginning at meiosis and gametogenesis, before extending into early embryogenesis and then later to development and differentiation. The histone demethylases have also been shown to play a role in cancer and neurological disorders, a subject that has been covered by a number of recent reviews (Agger et al., 2008; Cloos et al., 2008; Secombe and Eisenman, 2007; Shi, 2007) and so is not reviewed in detail here.
Histone methylation and demethylation in the germline
In the germline – the immortal lineage of cells that gives rise to the haploid gametes in sexually reproducing organisms – numerous DNA-directed events must be coordinated and tightly controlled, both spatially and temporally, as nuclei progress through meiosis. Of the histone modifications examined during meiosis in the mouse and in Caenorhabditis elegans, the methylation of two residues in particular, H3K4me and H3K9me, shows especially interesting dynamics during the early stages of meiotic prophase that correspond to chromosomal pairing, synapsis and the initiation/resolution of DNA double-strand breaks (DSBs). Recent studies are now implicating both KMTs and KDMs in this dynamic regulation.
During early stages of both oogenesis and spermatogenesis, the replicated sister chromatids must find, pair and synapse with their homologs, as well as undergo programmed DSBs that lead to crossover recombination. The identification of paired versus unpaired chromosomes is therefore an essential meiotic process, with meiotic silencing of unpaired chromosomes (MSUC) being an important consequence (reviewed by Turner, 2007). The single X chromosome that is present in mammalian and C. elegans males appears to trigger a version of MSUC that is specific to the sex chromosomes, known as meiotic sex chromosome inactivation (MSCI) (Turner, 2007). H3K9me2 coats unpaired chromosomes in C. elegans pachytene nuclei, including the lone X chromosome in males (Bean et al., 2004; Kelly et al., 2002; Reuben and Lin, 2002; Schaner and Kelly, 2006), suggesting that this methylation state is associated with chromosome mis-alignment and asynapsis. Interestingly, a mutation in the C. elegans him-17 gene (which encodes a chromatin-associated protein that contains six repeats of a putative DNA-binding motif that is also found in proteins implicated in chromatin regulation via their genetic interactions with LIN-35/Rb) leads to mis-patterning of H3K9me2 and to gross defects in DSB initiation and DSB repair (DSBR) progression, as well as to impaired sex chromosome segregation (Reddy and Villeneuve, 2004). These mutant phenotypes highlight the links between the detection of proper chromosome pairing, DSBR and histone methylation in meiosis.
The levels of H3K4me also show distinct and sometimes dramatic changes during early meiosis in both C. elegans and mammals. H3K4me is present on the autosomes and is excluded from the silenced X chromosomes in both sexes in C. elegans (Schaner and Kelly, 2006). Moreover, H3K4me shows a striking global upregulation at the start of male meiosis in mice, followed by a downregulation at the zygotene/pachytene stage (Godmann et al., 2007). This pattern of upregulation and downregulation corresponds to the stages at which chromosomes synapse and DSBR starts to progress.
Given these dynamic methylation patterns, it is not surprising that several enzymes that act on H3K4me and H3K9me have been identified to have meiotic roles. Two classes of KDMs have been identified that recognize H3K4me as a substrate: the amine oxidase-domain-containing LSD1/KDM1 (Shi et al., 2004) and the jumonji-domain-containing JARID1/KDM5 family (Christensen et al., 2007; Iwase et al., 2007; Lee et al., 2007a; Lee et al., 2007c; Secombe et al., 2007; Seward et al., 2007; Tahiliani et al., 2007; Xiang et al., 2007b; Yamane et al., 2007). In metazoans, LSD1/KDM1 acts on H3K4me2/1 (Di Stefano et al., 2007; Rudolph et al., 2007; Shi et al., 2004) and plays important roles in the germline (see below). The KDM5 family acts on H3K4me3/2 (Christensen et al., 2007; Iwase et al., 2007; Lee et al., 2007a; Lee et al., 2007c; Secombe et al., 2007; Seward et al., 2007; Tahiliani et al., 2007; Xiang et al., 2007b; Yamane et al., 2007) and has a less well-defined role in the germline. However, KDM5 mutants in budding yeast (Seward et al., 2007) and in corn fungus (Quadbeck-Seeger et al., 2000) have sporulation defects, hinting at meiotic roles for this demethylase family in these organisms as well. Intriguingly, a recent study in mice has reported that a spermatogenesis-specific association exists between SMCY/KDM5D (JARID1D) and MSH5, a meiosis-specific protein required for the progression of synapsis and for crossover completion (Akimoto et al., 2008). This potential connection between a regulator of H3K4me2/3 and these essential meiotic processes warrants further study.
Homologs of LSD1/KDM1 in C. elegans (Shin-i and Kohara, 2005) and in Drosophila melanogaster (Di Stefano et al., 2007; Rudolph et al., 2007) are also expressed in the germline, and the Drosophila KDM1 mutants have a germline-specific phenotype (Di Stefano et al., 2007; Rudolph et al., 2007). Rudolph et al. identified Drosophila LSD1/KDM1 as SU(VAR)3-3, a suppressor of heterochromatic silencing (Rudolph et al., 2007). Purified SU(VAR)3-3 protein is a H3K4me2/1 demethylase in vitro, and Su(var)3-3 null larvae have increased levels of H3K4me2. Surprisingly, H3K9me is reduced in these animals in heterochromatic regions where SU(VAR)3-3 is not detected, indicating that a finely tuned balance exists between these modifications, in which the absence of a H3K4me2 KDM indirectly influences H3K9me levels (Rudolph et al., 2007). They also reported a severe sterility defect in both male and female Su(var)3-3 mutant flies, with a complete absence of oocytes in the females and severe defects in spermatogenesis in the males (Rudolph et al., 2007). Di Stefano et al. also examined SU(VAR)3-3 (referred to in that study as dLSD1) mutants and reported a similar phenotype of female fertility defects together with H3K4me misregulation. However, unlike Rudolph et al. (Rudolph et al., 2007), they also observed a male-specific embryonic lethality (Di Stefano et al., 2007), perhaps owing to the use of different mutant alleles. Both groups support a model in which the mutation of LSD1/KDM1 leads to the disruption of heterochromatic/euchromatic boundary regions and to a disruption of germline development and meiotic processes. A mechanistic connection between these two phenotypes is still lacking at present, but one possibility is that these phenotypes reflect the transcriptional misregulation of LSD1/KDM1 target genes that are located in these boundary regions.
A set of recent studies in Arabidopsis thaliana supports a transcriptional role for LSD1 in regulating target genes important for reproductive function. These studies show that, FLOWERING LOCUS D (FLD), one of the Arabidopsis LSD1 homologs, promotes the transition from the vegetative to the reproductive phase (flowering) by repressing transcription of the floral repressors FLOWERING LOCUS C (FLC) and FWA (Jiang et al., 2007; Liu et al., 2007). Liu et al. also showed a functional interaction between FLD and the RNA recognition motif (RRM)-domain-containing protein FCA in repressing FLC (Liu et al., 2007). Interestingly, purification of the Schizosaccharomyces pombe LSD1 also identified an RRM-domain-containing protein (SPBPJ758.01) (Nicolas et al., 2006). Perhaps RNA interactions might affect LSD1 function in these protein complexes, but future studies are needed to understand this potential connection between LSD1 and RNA-mediated regulation, chromatin boundary and meiotic function in Arabidopsis and other organisms.
Figure 1.
Figure 2.
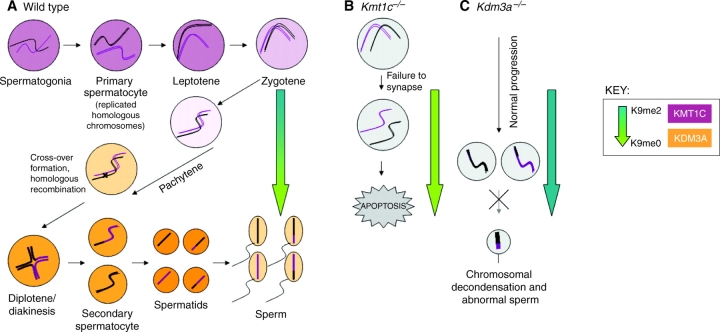
As with the enzymes that regulate H3K4me, several H3K9me KMTs and KDMs have been identified that have meiotic functions. For example, in S. pombe, LSD1/KDM1 functions as an H3K9me2/1 demethylase (Lan et al., 2007b; Opel et al., 2007), and loss of one of the two S. pombe LSD1/KDM1 homologs results in a sporulation defect (Lan et al., 2007b). Both homologs are also important for maintaining heterochromatic boundaries (Gordon et al., 2007; Lan et al., 2007b), which indicates that LSD1 proteins have a conserved role in meiosis and chromatin boundary maintenance across widely divergent organisms. In mice, the H3K9me2/1 methyltransferase G9a/KMT1C (EHMT2) is temporally expressed during the early stages of spermatogenesis, being present only in the pre-meiotic spermatogonia and the early leptotene stage of meiosis (Tachibana et al., 2007). A germline-specific G9a/Kmt1c genetic inactivation produces completely sterile males and partially sterile females, with male germ cell nuclei showing defects in synapsis and DSBR progression, leading to pachytene arrest and apoptosis (Tachibana et al., 2007). The authors also reported a decrease in H3K9me levels as nuclei enter the pachytene stage, coincident with the expression of the testis-specific H3K9me2/1 demethylase JHDM2A/KDM3A (JMJD1A) (Okada et al., 2007; Tachibana et al., 2007), indicating that these enzymes antagonistically regulate this modification during spermatogenesis (Fig. 1).
Fig. 1.
Histone H3 lysine 9 (H3K9) methylation balance in spermatogenesis. (A) H3K9me methyltransferases and demethylases are temporally regulated during spermatogenesis. In wild-type mice, mitotically cycling spermatogonia enter meiosis as primary spermatocytes, which then proceed through stages of early prophase I with concurrently high levels of the H3K9me histone lysine methyltransferase (KMT) G9a/KMT1C (purple shading in nuclei). During leptotene and zygotene, the replicated homologous chromosomes pair and DNA double-strand break (DSB) formation is initiated. During the early-to-late pachytene progression, G9a/KMT1C expression is lost and the H3K9me2 histone lysine demethylase (KDM) JHDM2A/KDM3A is expressed (orange shading in nuclei). The relative levels of H3K9me2 are represented by the blue-to-green arrow: in the earlier stages of meiosis there is increased H3K9me2 (blue), which is dramatically reduced during pachytene while H3K9me0 levels increase (green), reflecting the change from G9a/KMT1C to JHDM2A/KDM3A expression. (B) KMT1C is required for early stages of spermatogenesis. In Kmt1c–/– mutant mice, normal H3K9me2 levels are never achieved (green arrow), homologs are unable to pair/synapse, DSB repair is dysregulated, and cells undergo apoptosis (Tachibana et al., 2007). (C) KDM3A is required for late stages of spermatogenesis. In Kdm3a–/– mice, H3K9me2 levels stay high throughout spermatogenesis (blue arrow), leading to later defects in chromatin condensation as manifested in the few sperm that complete maturation (Okada et al., 2007). Both Kmt1c–/– and Kdm3a–/– mutants are male-sterile.
JHDM2A/KDM3A mediates the demethylation of H3K9me2, and its expression appears to begin in late pachytene and peaks in intensity in the round spermatids (Okada et al., 2007), correlating well with a reduction of the H3K9me2 modification at this stage of meiosis. Male mice that lack JHDM2A/KDM3A are viable but sterile, with smaller testes and severe defects in spermatogenesis, but only in post-meiotic cells (Okada et al., 2007). A defect in chromatin condensation exists in the few detectable mature sperm present in these mutants, and given the normal appearance of pre-meiotic and meiotic cells, the authors concluded that JHDM2A/KDM3A functions primarily after the completion of meiosis (Okada et al., 2007) (Fig. 1). Multiple genes have been shown to affect chromatin condensation in maturing sperm, so the authors examined the expression of several of these genes in Jhdm2a/Kdm3a mutant testes. They observed a downregulation of two essential sperm chromatin-packaging genes: transition nuclear protein 1 (Tnp1) and protamine 1 (Prm1) (Okada et al., 2007). JHDM2A/KDM3A binds to the promoters of both Tnp1 and Prm1 in round spermatids, and Jhdm2a/Kdm3a mutant spermatids show increased H3K9me at these promoters. These data suggest that the normal function of JHDM2A/KDM3A is to activate chromatin condensation genes upon sperm maturation by removing the repressive H3K92/1me modification from their promoters.
Another family of H3K9 KDMs with a meiotic function, the JMJD2/KDM4 family, is noteworthy for its dual substrate specificity for both H3K9me3/2 and H3K36me3/2, which has been shown in vitro (Fodor et al., 2006; Klose et al., 2006; Whetstine et al., 2006). The C. elegans KDM4 homolog, JMJD-2, appears to share this substrate specificity, as its depletion by RNAi leads to a general increase in germline H3K9me3 staining and to an increase in H3K36me3 on one end of the X chromosome during meiotic prophase I (Whetstine et al., 2006) – an intriguing pattern of unknown significance. Although pairing and synapsis appear to be normal, the JMJD-2-depleted animals have a germline phenotype of increased apoptosis and perturbed DSBR, indicating that a potential connection exists between this enzyme, its target modifications and meiotic DSBR (Whetstine et al., 2006). It remains unclear whether the misregulation of one or both tri-methyl modifications is responsible for the meiotic phenotype observed in JMJD-2-depleted C. elegans, opening a novel avenue of research for potential roles of H3K9me3 and/or H3K36me3 in DSBR.
In summary, studies in multiple model systems have shown that H3K4me and H3K9me, in particular, are present in chromatin in defined patterns during meiosis, and that the perturbation of the enzymes that regulate these modifications leads to defects in many essential meiotic steps. Collectively, these studies suggest that the dynamic regulation of histone methylation by both KMTs and KDMs might be one way to monitor meiotic progression. The mechanisms that guide the interplay between histone demethylation and meiotic processes are unknown, and future studies are necessary to explore these interactions. Does the regulation of other histone methylations, such as H3K27me, play important roles in meiosis as well? Although future work might identify unsuspected roles for this modification and the enzymes that regulate it in germline function, as we discuss below, H3K27me KDMs appear to primarily function later in differentiation and development.
Demethylase roles in differentiation
Pluripotency: a role for demethylases?
Studies of several demethylases suggest that they help to modulate the progression of pluripotent progenitor cell types into differentiated cell lineages during development. LSD1/KDM1 has a particularly interesting expression pattern in early mammalian development. Its maternally stored transcript is no longer detectable by the maternal-to-embryo transition (MET), when the embryo becomes transcriptionally competent. LSD1/KDM1 expression then recovers to oocyte levels by the blastula stage (McGraw et al., 2007). In flies, loss of LSD1/KDM1 causes embryonic lethality (Di Stefano et al., 2007; Rudolph et al., 2007), as it does in mice at an early embryonic stage (Wang et al., 2007). The application of the LSD1/KDM1 chemical inhibitor bisguanidine 1c, which is a polyamine analog, to in vitro fertilized mouse embryos not only increases global H3K4me2 (as visualized by immunofluorescence), but induces irreversible arrest at the two-cell stage (Shao et al., 2008), which occurs after the MET in mouse (McGraw et al., 2007), providing further evidence that LSD1/KDM1 has an embryonic role. The authors show that inappropriate upregulation of the pluripotency-maintaining factor OCT4 (POU5F1) occurs in embryos after this chemical inhibition, which indicates a potential mechanism for their early arrest (Shao et al., 2008). However, whether the observed effect brought about by bisguanidine 1c treatment is entirely due to the inhibition of LSD1/KDM1 function requires further investigation.
Several other demethylases are preferentially expressed in undifferentiated cell types, hinting at their having roles in maintaining pluripotency. Of the JMJD2/KDM4 family members, for example, JMJD2C/KDM4C is preferentially expressed in undifferentiated human and mouse ES cells, as compared with blastocysts and both hepatocyte and neural progenitor cells (Katoh and Katoh, 2007). Both JMJD2C/KDM4C and another KDM3 family member, JHDM2A/KDM3A, have been recently implicated in the maintenance of pluripotency in mouse ES cells (Loh et al., 2007). The authors identified predicted OCT4 binding sites within the introns of both the JMJD2C/KDM4C and JHDM2A/KDM3A genes and showed that OCT4-activated transcription occurred via these sites, as assessed by a reporter assay (Loh et al., 2007), findings which together indicate that OCT4 positively regulates the expression of both these demethylases. The RNAi depletion of JMJD2C/KDM4C and JHDM2A/KDM3A in mouse ES cells affected global levels of H3K9me3 and H3K9me2, respectively, and led to a dramatic change in ES cell morphology that is reminiscent of fibroblast differentiation (Loh et al., 2007). Colony formation assays showed that both demethylases were crucial for the maintenance of ES cell pluripotency (Loh et al., 2007). Moreover, the depletion of both JMJD2C/KDM4C and JHDM2A/KDM3A correlated with decreased levels of the pluripotency factors OCT4, SOX2 and NANOG. The depletion of JMJD2C/KDM4C alone led to the increased expression of certain genes, including the endodermal lineage markers Gata4 and Gata6, whereas the depletion of JHDM2A/KDM3A alone led to the induction of genes, including the mesodermal lineage marker brachyury (Loh et al., 2007). JHDM2A/KDM3A was also specifically implicated as a positive regulator of the self-renewal regulatory gene T-cell lymphoma breakpoint 1 (Tcl1), and was shown to be required for the binding of endogenous OCT4 to the Tcl1 promoter (Loh et al., 2007). Although the recruitment mechanism remains to be determined, JMJD2C/KDM4C has been found in association with the Nanog promoter, indicating that Nanog might be directly regulated by this demethylase (Loh et al., 2007). These data suggest a model in which JMJD2C/KDM4C and JHDM2A/KDM3A are downstream targets of the pluripotency factor OCT4 that act in a positive-feedback manner to promote pluripotency by activating target genes, including Nanog and Tcl1, respectively.
In support of this model, the overexpression of JHDM2A/KDM3A in adult neural stem cells (NSCs) has been shown to induce Oct4 expression (Ma et al., 2008). This is consistent with the idea that these H3K9 demethylases might promote pluripotency through a positive-feedback loop that involves both NANOG and OCT4 (Fig. 2A).
Fig. 2.
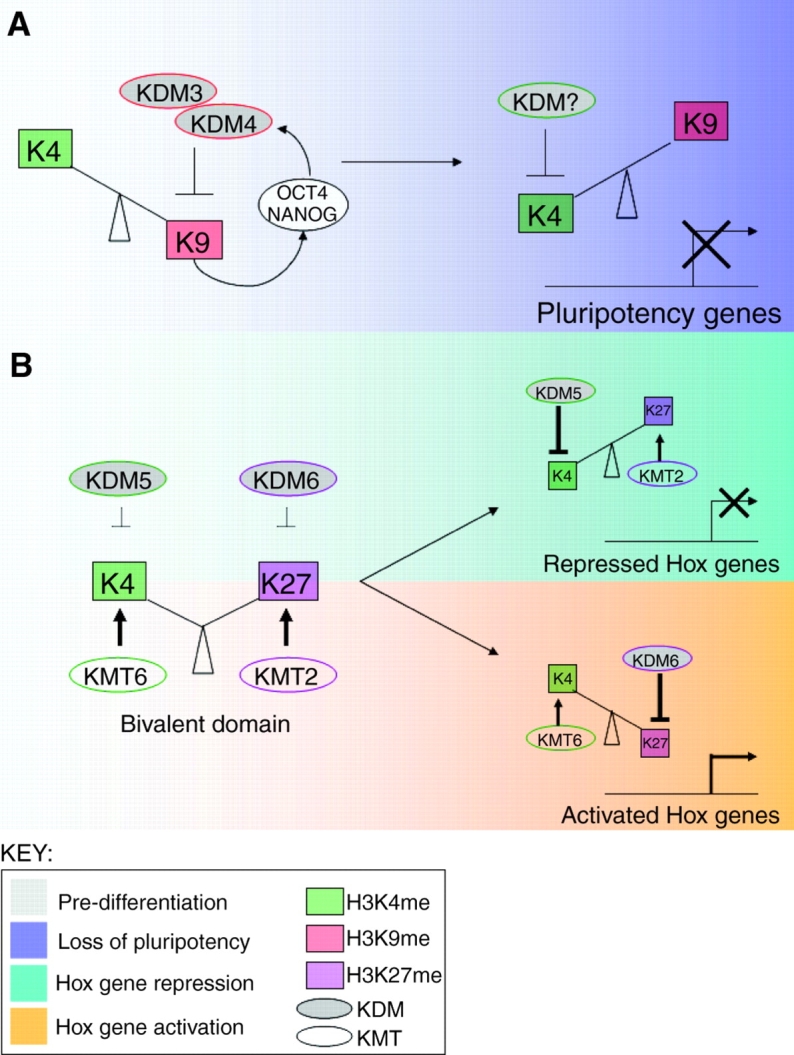
Opposing histone demethylases regulate developmental genes. (A) Model for demethylase regulation of pluripotency genes. Early in mouse development, the pluripotency-maintaining factors OCT4 and NANOG, and the H3K9me histone lysine demethylases KDM3 and KDM4, participate in a positive regulatory feedback loop. Upon differentiation, these demethylases are downregulated, allowing for increased H3K9 methylation and the repression of Oct4/Nanog. This model predicts that the activity of an H3K4me KDM is induced upon differentiation so as to remove the H3K4me modification from the pluripotency genes. LSD1/KDM1 is one candidate for this role, although this model needs to be experimentally confirmed. (B) Model for demethylase regulation of Hox genes. Early in mouse development, Hox genes are marked by a unique `bivalent domain', which features both H3K27me3 and H3K4me3. KDMs for both modifications are present but, for unknown reasons, do not demethylate these marks. Upon differentiation, these bivalent domains resolve into either H3K27me3 (repressed) or H3K4me3 (activated) via the actions of KDMs and KMTs.
In summary, KDMs acting on both H3K4me and H3K9me have been implicated in the cellular decision to either self-renew or to differentiate. The H3K4me2 demethylase LSD1/KDM1 is both maternally stored and embryonically expressed at the blastula stage (McGraw et al., 2007), but the timing of irreversible arrest in mouse embryos that have been exposed to a LSD1/KDM1 inhibitor (Shao et al., 2008) suggests that it is the embryonic expression of this demethylase that is essential for early differentiation events. The observed upregulation of the pluripotency factor OCT4 after mouse embryos are treated with a LSD1/KDM1 inhibitor suggests a possible mechanism (yet to be experimentally addressed) in which LSD1/KDM1 acts to remove the activating H3K4me modification from the Oct4 promoter under normal developmental conditions, repressing the gene and allowing cells to differentiate. In contrast to LSD1/KDM1, the H3K9me demethylases JMJD2C/KDM4C and JHDM2A/KDM3A are expressed in undifferentiated ES cells and appear to act in the maintenance of pluripotency, potentially through the transcriptional activation of pluripotency-maintaining factors, such as OCT4, NANOG and TCL1 (Katoh and Katoh, 2007; Loh et al., 2007). Taken together, these studies hint at a potentially general mechanism for the regulation of developmental events by KDMs, by which antagonistically acting demethylases transcriptionally regulate target genes that are important for pluripotency or differentiation. Further examples of this mode of action are discussed below.
Hox gene regulation: a model for opposing demethylase roles in differentiation
The Hox genes are a developmentally regulated set of evolutionarily conserved genes that are responsible for body plan patterning (reviewed by Akam, 1995). They are transcriptionally silenced in ES cells, but become activated in various lineage-specific patterns upon differentiation (reviewed by Soshnikova and Duboule, 2008). Studies in Drosophila first showed that the regulation of many developmentally important genes, including the Hox genes, is accomplished by the opposing Trithorax (Trx) and Polycomb group (PcG) proteins, which form complexes that are responsible for the transcriptional activation and repression of their targets, respectively (reviewed by Schuettengruber et al., 2007). Both Trx and PcG complexes contain many chromatin-directed activities, such as the H3K4me3-specific histone methyltransferase Mixed-lineage leukemia (MLL/KMT2) family, which can be found in Trx complexes, and the methyltransferase Enhancer of zeste [E(Z)/KMT6], which is found in PcG complexes and is responsible for H3K27 methylation. Surprisingly, given that Trx proteins are gene activators with associated H3K4me3 methylation activity, one Trx group protein, LID, is a Drosophila KDM5 family homolog and an H3K4me3-specific demethylase, which would be expected to repress transcription (Eissenberg et al., 2007; Lee et al., 2007c; Secombe et al., 2007). LID appears to interact with the cell growth regulator dMYC (also known as DM), which binds the jumonji domain of LID to inhibit its demethylase activity (Secombe et al., 2007), but whether and how this connection pertains to Hox gene regulation remains to be determined.
Does the gain or loss of various lysine methylations via these protein complexes play a role in differentiation? Genome-wide studies of the chromatin status of cells as they lose pluripotency and begin to differentiate has proven to be a fruitful means of uncovering key regulatory histone modifications important for differentiation decisions. For example, the unexpected identification of the bivalent domains in the pluripotent genomes of mouse and human ES cells, which contain both `active' H3K4me3 and `repressive' H3K27me3 modifications (Bernstein et al., 2006), is indicative of a novel means of chromatin-based gene regulation that is believed to be especially relevant for development and differentiation. The resolution of these bivalent domains into either H3K4me3 or H3K27me3 upon differentiation is believed to stably mark these regions for activation or repression, respectively (Bernstein et al., 2006; Mikkelsen et al., 2007) (Fig. 2B). In mouse ES cells, these bivalent domains are enriched on the developmentally essential Hox gene loci (Bernstein et al., 2006), rendering these genes an especially useful model for understanding the roles of these unique chromatin domains.
Although H3K4me3 and H3K27me3 co-exist at Hox gene loci in undifferentiated ES cells, upon differentiation only H3K4me3 is found at activated Hox genes, whereas only H3K27me3 is present at silenced Hox genes (Mikkelsen et al., 2007). Is there any connection between the methyltransferases responsible for maintaining these modifications and the demethylases that might act to selectively remove H3K4me3 or H3K27me3? Evidence for coordinated KDMs and KMTs that act to maintain H3K4me3 or H3K27me3 has been found in mammalian systems, as discussed below.
For example, the H3K27me3 demethylase ubiquitously transcribed tetratricopeptide repeat, X chromosome (UTX/KDM6A) (Agger et al., 2007; Lan et al., 2007a; Xiang et al., 2007a) has been identified in the H3K4me3 methyltransferase (MLL/KMT2)-containing complex (Cho et al., 2007; Lee et al., 2007b). Both UTX/KDM6A and the closely related demethylase, JMJD3/KDM6B, bind Hox gene promoters upon differentiating (activating) conditions (Agger et al., 2007; De Santa et al., 2007; Lan et al., 2007a; Lee et al., 2007b) (Fig. 2B). Zebrafish embryos depleted of the UTX/KDM6A homolog (Lan et al., 2007a) and Xenopus embryos that lack the H3K4 methylation patterning protein WDR5 (Wysocka et al., 2005), show surprisingly similar phenotypes of defective somitic patterning (defective posterior development in the zebrafish and axial defects leading to shortened tadpoles in Xenopus) that are indicative of Hox gene misregulation (Lan et al., 2007a; Wysocka et al., 2005). This suggests that H3K27me3 removal is functionally coordinated with H3K4me3 addition, a model that is supported by the physical association of an H3K27me3 demethylase with an H3K4me3 methyltransferase, as well as by the similarities in phenotypes between animals defective in either H3K27me3 demethylation or H3K4me3 patterning.
There is evidence supporting H3K4me3 KDM and H3K27me3 KMT coordination. The H3K4me3 demethylase retinoblastoma binding protein 2 (RBP2/KDM5A; also known as JARID1A) physically interacts with the H3K27me3 methyltransferase-containing Polycomb group repressive complex 2 (PRC2) (Pasini et al., 2008). RBP2/KDM5A and PcG proteins also show an extensive overlap of target genes, and although depletion of RBP2/KDM5A does not prevent PRC2 from binding to its target genes, it appears to inhibit their repression (Pasini et al., 2008). However, the depletion of PRC2 proteins reduces RBP2/KDM5A promoter binding (Pasini et al., 2008), suggesting that the PRC2 complex recruits the demethylase. During ES cell differentiation, both PRC2 and RBP2/KDM5A are lost from the promoters of genes that are induced upon differentiation (Pasini et al., 2008) (Fig. 2B), suggesting that the loss of both the H3K27me3 KMT and the H3K4me3 KDM is required for transcriptional activation. Consistent with this, an earlier study showed that a specific loss of RBP2/KDM5A occurs from Hox gene loci upon ES cell differentiation (Christensen et al., 2007).
In summary, it appears that in mammals, two large biochemical complexes, analogous to the Trx and PcG complexes that exist in flies, might act to coordinately regulate H3K4me3 and H3K27me3 so that only one modification is dominant on the target gene in differentiated cells. These complexes therefore contain opposing enzymatic activities that control and coordinate the balance of histone methylation at H3K4 and K3K27 (Fig. 2B). One complex, analogous to the Trx complex, contains both the H3K4me3 methyltransferase MLL/KMT2 and the H3K27 demethylase UTX/KDM6A. The other, analogous to the PcG complex PRC2, contains the opposite enzymatic capabilities, with the H3K27 methyltransferase subcomplex PRC2/KMT6 and the H3K4me3 demethylase RBP2/KDM5A. Both demethylases are expressed in undifferentiated mouse ES cells (Christensen et al., 2007; Lan et al., 2007a) and RBP2/KDM5A has been reported to bind Hox gene loci before ES cells undergo differentiation (Christensen et al., 2007). Interestingly, the H3K27 demethylase UTX, although expressed in ES cells, appears to be excluded from the Hox gene loci (Lan et al., 2007a), suggesting a possible mechanism for the protection of the bivalent domain in ES cells. How the bivalent domain is protected in stem cells and how it is resolved during differentiation are questions of significant interest and importance, and are likely to involve the relevant histone methylases and demethylases. An answer to these questions might lie in the control of the recruitment of these enzymes and/or their activation upon differentiation, which are poorly understood at the present time.
Demethylases in neural differentiation and disease
In addition to the transition of ES cells from pluripotency to differentiation, both H3K4me3 and H3K27me3 demethylases have been reported to play key roles in neuronal development. A role for H3K27me3 demethylation in mammalian neuronal development has been identified through studies of the co-repressor, silencing mediator for retinoid and thyroid hormone receptor (SMRT; also known as NCOR2), which is crucial for mouse forebrain development and for the maintenance of pluripotency (Jepsen et al., 2007). These authors identified JMJD3/KDM6B as a transcriptional target of SMRT when mouse embryonic NSCs were induced to differentiate with retinoic acid (RA). They also showed that the overexpression of JMJD3/KDM6B in a neuronal stem cell culture induced the expression of neuronal subtype genes, such as distal-less homeobox 5 (Dlx5) (Jepsen et al., 2007), supporting a role for JMJD3/KDM6B in promoting neuronal differentiation. JMJD3/KDM6B is also induced upon macrophage terminal differentiation (De Santa et al., 2007) and is both necessary and sufficient for epidermal differentiation in human keratinocytes (Sen et al., 2008), hinting at a general mechanism in which an H3K27me3 demethylase is activated to remove a pluripotency-maintaining modification upon differentiation (Fig. 2B).
Zebrafish embryos depleted of the H3K4me3 demethylase homolog, selected mouse cDNA on the X (SMCX/KDM5C; also known as JARID1C), show decreased neuronal survival during embryonic development (Iwase et al., 2007), and rat neuronal cultures depleted of SMCX/KDM5C develop defects in dendritic growth (Iwase et al., 2007), indicating a potentially specific role for this demethylase in neuronal development. Consistent with this, mouse studies have also identified the enriched expression of SMCX/KDM5C in the hippocampus, hypothalamus, cerebellum and in differentiated neurons (Xu et al., 2008). SMCX/KDM5C also appears to play a role in the genetic disorder X-linked mental retardation (XLMR), as mutations that compromise its demethylase activity are associated with this disorder (Iwase et al., 2007; Tahiliani et al., 2007) (reviewed by Agger et al., 2008; Cloos et al., 2008; Shi, 2007).
Given the shared involvement of H3K4me3 and H3K27me3 in Hox gene regulation and neural differentiation, it is possible that neuronal lineage-specific genes, such as Dlx5, show other regulatory traits in common with Hox genes, including the bivalent domain chromatin architecture. Interestingly, a recent study also identified a non-coding RNA, HOTAIR, in the regulation of Hox gene transcription (Rinn et al., 2007). Whether and how the action of histone-modifying enzymes is coordinated with that of non-coding RNAs in the regulation of the epigenetic states of Hox loci is an exciting area of future investigation.
Demethylases in organogenesis
Epigenetic regulation clearly plays a role in the earliest stages of development, as discussed above, but there is also a need for the stable patterning of gene regulation as tissues and organs are specified. The regulation of histone methylation at subsets of lineage-specific genes is one way that transcription networks can be guided during development. Methylation appears to be a relatively stable histone modification, and the active addition or removal of this mark might very well play important roles in stably maintaining cellular identity during development and maintenance of organ identity. Not surprisingly, in addition to more general roles early in development, some demethylases also appear to have tissue-specific roles in later development.
Studies of pituitary organogenesis support a role for LSD1/KDM1 as a regulator of differentiation later in development (Wang et al., 2007). Given the early embryonic lethality of mouse LSD1/KDM1 gene knockouts, the authors created a tissue-specific pituitary knockout (because of the high LSD1/KDM1 levels of expression in the adult pituitary) to explore the role of LSD1/KDM1 in organogenesis (Wang et al., 2007). Pituitary gland development was grossly normal in these mutants, but they lacked certain terminal differentiation- and cell-type-specific markers, suggesting a role for LSD1/KDM1 in late differentiation events (Wang et al., 2007). During anterior pituitary development, the growth hormone (GH)-secreting somatotrope cells and the prolactin-secreting lactotrope cells arise from a common lineage (reviewed by Rhodes et al., 1994). The Gh gene is a somatotrope lineage marker that is repressed in lactotropes. In the activated somatotrope condition, LSD1/KDM1 and the H3K4me3 KMT MLL3/KMT2C were found to be co-recruited to the Gh promoter. Upon lactotrope specification (and Gh repression), LSD1/KDM1 co-localizes with the ZEB1-CtBP-CoREST (RCOR2) repressive complex (Wang et al., 2007). These data suggest that LSD1/KDM1 is constitutively present on certain promoters, where it acts as an integral component of both differentially recruited co-activator and co-repressor complexes. Further studies are required to address whether and/or how LSD1/KDM1 demethylase activity is modulated in the context of the various protein complexes it associates with.
LSD1/KDM1 also plays a role in hematopoietic differentiation through interactions with the growth factor-independent (Gfi) proteins (Saleque et al., 2007), which are known to promote the expression of lineage-specific genes. LSD1/KDM1 interacts with GFI1B and is present on 80% of GFI1B binding sites (Saleque et al., 2007). The RNAi depletion of LSD1/KDM1 impaired the differentiation of several different mouse hematopoietic cell types, including erythroid and megakaryocyte cells (Saleque et al., 2007). Surprisingly, LSD1/KDM1 depletion actually induced spontaneous granulocyte differentiation (Saleque et al., 2007), suggesting that it has lineage-specific activities. However, LSD1/KDM1 appears to act predominantly in a pro-differentiation manner in these assays.
The KDM6 family (which includes UTX, UTY and JMJD3 in mammals), as discussed above, has a role in Hox gene regulation. At least one member of this enzyme family has a role in C. elegans gonadal development as well (Agger et al., 2007). During C. elegans larval development, the distal tip cells of the two developing gonad arms lead the migration/elongation of the gonad arms, first distally, away from a mid-ventral location, then proximally back to a mid-body location. Each arm thus forms a U-shape, at which point the gonad is fully formed (Schedl, 1997). Defects in this process can signal pathfinding and/or proliferation defects. Two independent mutations of one of the C. elegans JMJD3/KDM6B homologs, F18E9.5, lead to defects in gonad migration and oocyte accumulation (Agger et al., 2007). It is presently unclear which pathways underlie these phenotypes, but F18E9.5 is an active H3K27me3 demethylase (Agger et al., 2007), indicating a potential connection between H3K27 methylation regulation and this phenotype.
The C. elegans vulva is a valuable model in which to study organogenesis (reviewed by Sternberg, 2005; Sternberg and Horvitz, 1991). As such, studies of the severe vulval defect in KDM5 C. elegans mutants (Christensen et al., 2007) might uncover general mechanisms for these H3K4me3 demethylases in development. C. elegans has only a single KDM5 homolog, rbr-2, which is responsible for global levels of H3K4me3 in later larval stages and adulthood, and the purified protein possesses in vitro H3K4me3 demethylase activity (Christensen et al., 2007). rbr-2 mutants show a highly penetrant (80%) phenotype of either undeveloped or multiple vulvas, a phenotype reminiscent of the synthetic multivulva (synMuv) genes (Fay and Han, 2000; Sternberg, 2005). Given that many of the synMuv genes are homologous to those involved in the mammalian tumor suppressor and cell cycle regulator pRB/E2F complex (which RBP2/KDM5A was originally identified to associate with), the authors genetically addressed a role for rbr-2 in this pathway using various synMuv mutants (Christensen et al., 2007). rbr-2(RNAi) or rbr-2 double mutants showed no enhancement of phenotype with either class of synMuv mutant (Christensen et al., 2007). However, in the genetic background of the sensitized synMuv mutant lin-15(n765ts), rbr-2 depletion dramatically enhanced the multivulva phenotype at the permissive temperature (Christensen et al., 2007), showing a potential role for rbr-2 in this pathway, although its precise position needs to be determined.
Histone demethylases: key regulators of nuclear hormone signaling?
Hormone signaling plays an important role in animal development and differentiation (reviewed by van der Burg et al., 1999). Importantly, several histone demethylases have been shown to participate in hormone receptor (HR)-mediated transcription, particularly androgen receptor (AR) and estrogen receptor (ER) signaling, hinting that they have important roles in the various tissues that express these proteins. For example, before its demethylase activity was reported, the H3K4me3 demethylase RBP2/KDM5A was shown to associate with several nuclear HRs, including ER and retinoic acid receptor (RAR), and to enhance their transcriptional activities (Chan and Hong, 2001). Exactly how an H3K4me3 demethylase helps transcriptional activation remains to be determined. Many lines of evidence also support a role for LSD1/KDM1 in HR-mediated transcription (as discussed below).
Although LSD1/KDM1 was initially identified as an H3K4me2/1-specific demethylase (Shi et al., 2004), later studies also identified a role for LSD1/KDM1 in demethylating H3K9me2/1 when it is associated with the AR (Metzger et al., 2005) and other nuclear hormone receptors (Garcia-Bassets et al., 2007), although in vitro biochemical evidence of mammalian LSD1/KDM1 having an H3K9me2/1 activity is still lacking. Additional demethylases were soon identified as being associated with LSD1/KDM1 and/or the AR complex and as participating in AR-mediated transcriptional regulation. For example, both LSD1/KDM1 and the H3K9me2-specific demethylase JHDM2A/KDM3A were found to co-regulate the AR-responsive genes encoding prostate-specific antigen (PSA; also known as KLK3) and NK3 transcription factor, locus 1 (NKX3.1) (Yamane et al., 2006). The closely related putative demethylase JMJD1C was originally identified as a thyroid hormone receptor-interacting protein (Lee et al., 1995), and later shown to act as an AR co-activator (Wolf et al., 2007). The H3K9/K36me3/2 demethylase JMJD2C/KDM4C was also found to cooperatively participate in LSD1/KDM1 activation of AR-responsive genes (Wissmann et al., 2007), highlighting the importance of collaboration between me3- and me2-specific KDMs in regulating histone methylation and the expression of genes that are important for tissue-specific function, such as PSA in prostate tissue.
LSD1/KDM1 also participates in ER-mediated gene activation, as shown by its presence at ER target genes and by its requirement for ER ligand stimulation, where it participates in H3K9me2 demethylation (Garcia-Bassets et al., 2007). A recent study of LSD1/KDM1 in ER-mediated activation has uncovered fascinating and unexpected evidence that links histone demethylation by LSD1/KDM1 to a localized form of DNA damage that triggers chromatin conformation changes that promote transcription (Perillo et al., 2008) (Fig. 3), a mechanism that might prove to be relevant for the activities of other demethylases at nuclear hormone receptors.
Fig. 3.
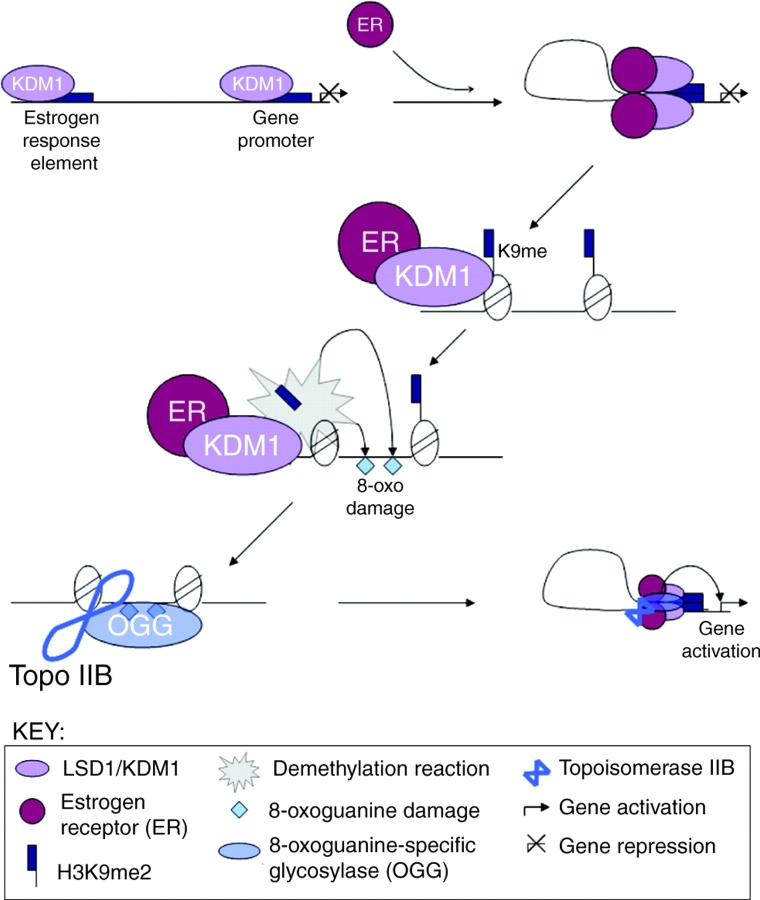
Model for LSD1/KDM1 regulation of estrogen receptor-induced gene expression. LSD1/KDM1 is constitutively associated with upstream estrogen-response elements (EREs) and promoters of estrogen receptor (ER) target genes. Upon addition of ligand (in the form of the hormone estrogen), the activated ER binds to both the enhancer and promoter, and triggers LSD1/KDM1-dependent DNA looping. LSD1/KDM1 then demethylates H3K9me2/1, and hydrogen peroxide generated by the demethylase reaction triggers localized 8-oxoguanine DNA damage. This damage recruits 8-oxoguanine-specific glycosylase (OGG) and topoisomerase IIβ (Topo IIβ), which in turn triggers chromatin conformational changes that promote gene transcription (Perillo et al., 2008).
Perillo et al. examined the three-dimensional aspects of ER stimulation of known target genes and found that ER stimulation induced an upstream estrogen-responsive DNA element (ERE) to come into close proximity with a promoter 1.5 kb away, strongly suggesting the involvement of a DNA looping mechanism (Perillo et al., 2008). The authors found that LSD1/KDM1 is continuously present at these loci and is responsible for H3K4me2 demethylation, but only demethylates H3K9me2 upon ER stimulation (Perillo et al., 2008). Unexpectedly, they discovered that LSD1/KDM1 activity was required for the DNA looping process (Perillo et al., 2008). The authors investigated whether the chemical by-product of the demethylase reaction, hydrogen peroxide, might act as a signal by creating an 8-oxoguanine modification of the DNA (Perillo et al., 2008). Further experiments determined that estrogen treatment induces 8-oxoguanine foci in cells in an ER- and LSD1/KDM1-dependent manner, and that an 8-oxoguanine-specific glycosylase (OGG1) and topoisomerase IIβ are both recruited under these conditions (Perillo et al., 2008). LSD1/KDM1 therefore appears to play a role in ER-induced intragenic movement, bringing elements within the same gene into close proximity. A recent study has reported a role for LSD1/KDM1 in modulating ER-induced chromosomal movement into the interchromatin granules, where active transcription is facilitated by the existing splicing machinery, transcriptional elongation factors and chromatin remodeling activities (Hu et al., 2008), hinting at a potential role for demethylases in modulating both small-scale and large-scale movements of chromatin in response to HR signaling.
A possible difference between differentiation-induced gene regulation and HR signaling is that the demethylases are constitutively present on HR target genes, but demethylase-influenced transcriptional activity seems to be subject to rapid and transient regulation, possibly by protein-protein interactions and/or post-translational modifications (Fig. 3). For example, LSD1/KDM1 is constitutively present at the ER target genes examined but only demethylates H3K9me2 in the presence of ER signaling (Perillo et al., 2008). By contrast, during differentiation, KDM functions appear to be regulated predominantly by recruitment mechanisms, such as through the recruitment of UTX/JMJD6A to the Hox genes during ES cell differentiation (Lan et al., 2007a). It is tempting to speculate that this difference contributes to the relative permanence of methylation patterns after differentiation, as opposed to the transient nature of HR-induced gene regulation.
Conclusion
From the earliest developmental stages, demethylases have been implicated in the decision to maintain pluripotency or to commit to differentiation (see examples in Fig. 2), and these studies have hinted at wider questions that surround histone demethylation regulation. A comparison of the roles of KDMs in regulating pluripotency genes versus lineage-specific genes shows some intriguing differences. Before differentiation, the H3K9 KDMs act to maintain pluripotency genes in an active state, and upon differentiation their activity is reduced and the pluripotency genes become silenced, presumably through contributions of H3K9 KMTs and H3K4me KDMs (Fig. 2A). This model suggests that a KDM-influenced switch occurs between H3K4me and H3K9me. The H3K4me3/H3K27me3 bivalent domain that is present in ES cells on the lineage-specific Hox genes appears to undergo a different mode of KDM regulation. Specifically, both H3K4me3 and H3K27me3 KDMs are expressed in ES cells and are either absent or present at the target gene promoters at a low frequency in order to protect the `poised' genes from inappropriate activation (Agger et al., 2007; Lan et al., 2007a; Lee et al., 2007b; Pasini et al., 2008) (see model in Fig. 2B). Upon differentiation, an unknown mechanism acts to resolve the bivalent domain to either H3K4me3 or H3K27me3 through coordinate regulation of the appropriate KMTs and KDMs. These studies have primarily been performed in cell culture, but the C. elegans organogenesis studies suggest that KDM gene regulation is important for multiple aspects of development, although experiments connecting these proposed molecular mechanisms to the phenotypic observations remain to be done.
Histone demethylases have only been identified and studied for four years, but much has already been uncovered about their important roles in development and disease. However, many questions remain. Specifically, understanding how these demethylases are recruited to target genomic locations and how their activities are regulated is of great importance. Some regulation occurs at the level of demethylase gene expression, but in those cell types in which the demethylase is expressed, there are presumably multiple layers of regulation required to control where the enzymatic activity is directed. Recruitment might involve sequence-specific DNA factors and non-coding RNAs. The local chromatin environment might also contribute to effective recruitment by regulating the stability of protein occupancy (reviewed by Lan et al., 2008). Regulation of demethylase activity may involve post-translational modifications, non-coding RNAs and/or associated partners. These types of regulation might play a role in modulating the H3K4me2/1 to H3K9me2/1 switch in demethylase activity for LSD1/KDM1, which occurs upon its association with the AR. Biochemical purification of the known demethylases from various cell types will help to initiate studies that will ultimately provide mechanistic insights into their recruitment and regulation.
Another question concerns whether histone demethylases exist that target H4K20me and H3K79me. A recent study in yeast suggesting the non-processivity of the only known H3K79me KMT, Dot1, along with the apparent functional redundancy of the three H3K79me states, argues against the existence of H3K79me KDMs (Frederiks et al., 2008). However, like the other methyl modifications with known KDMs, H4K20me does show differing distributions of mono-, di- and tri-methyl states (Karachentsev et al., 2007) and is methylated by SET-domain-containing KMTs. Therefore, at present there is no compelling argument against the existence of H4K20me KDMs. Several jumonji-domain-containing proteins have no known substrates but are believed to be enzymatically active (Shi and Whetstine, 2007; Takeuchi et al., 2006); perhaps one of these or a new chemical class of demethylases will prove to be H4K20me-specific.
Finally, much has been achieved in vitro and in cell culture towards understanding the enzymatic activities of these demethylases, but the truly illuminating studies will be those that seek to understand their roles and mechanism of action in the context of the whole organism. Work has already been done in many model systems that has substantially increased our understanding of the biological roles of these enzymes, and depletion or mutant studies for the as yet undiscovered histone demethylases will no doubt prove equally fruitful.
References
- Agger, K., Cloos, P. A., Christensen, J., Pasini, D., Rose, S., Rappsilber, J., Issaeva, I., Canaani, E., Salcini, A. E. and Helin, K. (2007). UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 449, 731-734. [DOI] [PubMed] [Google Scholar]
- Agger, K., Christensen, J., Cloos, P. A. and Helin, K. (2008). The emerging functions of histone demethylases. Curr. Opin. Genet. Dev. 18, 159-168. [DOI] [PubMed] [Google Scholar]
- Akam, M. (1995). Hox genes and the evolution of diverse body plans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 349, 313-319. [DOI] [PubMed] [Google Scholar]
- Akimoto, C., Kitagawa, H., Matsumoto, T. and Kato, S. (2008). Spermatogenesis-specific association of SMCY and MSH5. Genes Cells 13, 623-633. [DOI] [PubMed] [Google Scholar]
- Allfrey, V. G., Faulkner, R. and Mirsky, A. E. (1964). Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc. Natl. Acad. Sci. USA 51, 786-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand, R. and Marmorstein, R. (2007). Structure and mechanism of lysine-specific demethylase enzymes. J. Biol. Chem. 282, 35425-35429. [DOI] [PubMed] [Google Scholar]
- Bannister, A. J., Schneider, R. and Kouzarides, T. (2002). Histone methylation: dynamic or static? Cell 109, 801-806. [DOI] [PubMed] [Google Scholar]
- Barski, A., Cuddapah, S., Cui, K., Roh, T. Y., Schones, D. E., Wang, Z., Wei, G., Chepelev, I. and Zhao, K. (2007). High-resolution profiling of histone methylations in the human genome. Cell 129, 823-837. [DOI] [PubMed] [Google Scholar]
- Bean, C. J., Schaner, C. E. and Kelly, W. G. (2004). Meiotic pairing and imprinted X chromatin assembly in Caenorhabditis elegans. Nat. Genet. 36, 100-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, B. E., Mikkelsen, T. S., Xie, X., Kamal, M., Huebert, D. J., Cuff, J., Fry, B., Meissner, A., Wernig, M., Plath, K. et al. (2006). A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125, 315-326. [DOI] [PubMed] [Google Scholar]
- Chan, S. W. and Hong, W. (2001). Retinoblastoma-binding protein 2 (Rbp2) potentiates nuclear hormone receptor-mediated transcription. J. Biol. Chem. 276, 28402-28412. [DOI] [PubMed] [Google Scholar]
- Cho, Y. W., Hong, T., Hong, S., Guo, H., Yu, H., Kim, D., Guszczynski, T., Dressler, G. R., Copeland, T. D., Kalkum, M. et al. (2007). PTIP associates with MLL3- and MLL4-containing histone H3 lysine 4 methyltransferase complex. J. Biol. Chem. 282, 20395-20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, J., Agger, K., Cloos, P. A., Pasini, D., Rose, S., Sennels, L., Rappsilber, J., Hansen, K. H., Salcini, A. E. and Helin, K. (2007). RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell 128, 1063-1076. [DOI] [PubMed] [Google Scholar]
- Cloos, P. A., Christensen, J., Agger, K. and Helin, K. (2008). Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev. 22, 1115-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa, F., Totaro, M. G., Prosperini, E., Notarbartolo, S., Testa, G. and Natoli, G. (2007). The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 130, 1083-1094. [DOI] [PubMed] [Google Scholar]
- Di Stefano, L., Ji, J. Y., Moon, N. S., Herr, A. and Dyson, N. (2007). Mutation of Drosophila Lsd1 disrupts H3-K4 methylation, resulting in tissue-specific defects during development. Curr. Biol. 17, 808-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg, J. C., Lee, M. G., Schneider, J., Ilvarsonn, A., Shiekhattar, R. and Shilatifard, A. (2007). The trithorax-group gene in Drosophila little imaginal discs encodes a trimethylated histone H3 Lys4 demethylase. Nat. Struct. Mol. Biol. 14, 344-346. [DOI] [PubMed] [Google Scholar]
- Fay, D. S. and Han, M. (2000). The synthetic multivulval genes of C. elegans: functional redundancy, Ras-antagonism, and cell fate determination. Genesis 26, 279-284. [DOI] [PubMed] [Google Scholar]
- Fodor, B. D., Kubicek, S., Yonezawa, M., O'Sullivan, R. J., Sengupta, R., Perez-Burgos, L., Opravil, S., Mechtler, K., Schotta, G. and Jenuwein, T. (2006). Jmjd2b antagonizes H3K9 trimethylation at pericentric heterochromatin in mammalian cells. Genes Dev. 20, 1557-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiks, F., Tzouros, M., Oudgenoeg, G., van Welsem, T., Fornerod, M., Krijgsveld, J. and van Leeuwen, F. (2008). Nonprocessive methylation by Dot1 leads to functional redundancy of histone H3K79 methylation states. Nat. Struct. Mol. Biol. 15, 550-557. [DOI] [PubMed] [Google Scholar]
- Garcia-Bassets, I., Kwon, Y. S., Telese, F., Prefontaine, G. G., Hutt, K. R., Cheng, C. S., Ju, B. G., Ohgi, K. A., Wang, J., Escoubet-Lozach, L. et al. (2007). Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell 128, 505-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godmann, M., Auger, V., Ferraroni-Aguiar, V., Di Sauro, A., Sette, C., Behr, R. and Kimmins, S. (2007). Dynamic regulation of histone H3 methylation at lysine 4 in mammalian spermatogenesis. Biol. Reprod. 77, 754-764. [DOI] [PubMed] [Google Scholar]
- Gordon, M., Holt, D. G., Panigrahi, A., Wilhelm, B. T., Erdjument-Bromage, H., Tempst, P., Bahler, J. and Cairns, B. R. (2007). Genome-wide dynamics of SAPHIRE, an essential complex for gene activation and chromatin boundaries. Mol. Cell. Biol. 27, 4058-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman, N. D., Stuart, R. K., Hon, G., Fu, Y., Ching, C. W., Hawkins, R. D., Barrera, L. O., Van Calcar, S., Qu, C., Ching, K. A. et al. (2007). Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 39, 311-318. [DOI] [PubMed] [Google Scholar]
- Hu, Q., Kwon, Y. S., Nunez, E., Cardamone, M. D., Hutt, K. R., Ohgi, K. A., Garcia-Bassets, I., Rose, D. W., Glass, C. K., Rosenfeld, M. G. et al. (2008). Enhancing nuclear receptor-induced transcription requires nuclear motor and LSD1-dependent gene networking in interchromatin granules. Proc. Natl. Acad. Sci. USA 105, 19199-19204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase, S., Lan, F., Bayliss, P., de la Torre-Ubieta, L., Huarte, M., Qi, H. H., Whetstine, J. R., Bonni, A., Roberts, T. M. and Shi, Y. (2007). The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell 128, 1077-1088. [DOI] [PubMed] [Google Scholar]
- Jepsen, K., Solum, D., Zhou, T., McEvilly, R. J., Kim, H. J., Glass, C. K., Hermanson, O. and Rosenfeld, M. G. (2007). SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature 450, 415-419. [DOI] [PubMed] [Google Scholar]
- Jiang, D., Yang, W., He, Y. and Amasino, R. M. (2007). Arabidopsis relatives of the human lysine-specific Demethylase1 repress the expression of FWA and FLOWERING LOCUS C and thus promote the floral transition. Plant Cell 19, 2975-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karachentsev, D., Druzhinina, M. and Steward, R. (2007). Free and chromatin-associated mono-, di-, and trimethylation of histone H4-lysine 20 during development and cell cycle progression. Dev. Biol. 304, 46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, Y. and Katoh, M. (2007). Comparative integromics on JMJD2A, JMJD2B and JMJD2C: preferential expression of JMJD2C in undifferentiated ES cells. Int. J. Mol. Med. 20, 269-273. [PubMed] [Google Scholar]
- Kelly, W. G., Schaner, C. E., Dernburg, A. F., Lee, M. H., Kim, S. K., Villeneuve, A. M. and Reinke, V. (2002). X-chromosome silencing in the germline of C. elegans. Development 129, 479-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose, R. J., Yamane, K., Bae, Y., Zhang, D., Erdjument-Bromage, H., Tempst, P., Wong, J. and Zhang, Y. (2006). The transcriptional repressor JHDM3A demethylates trimethyl histone H3 lysine 9 and lysine 36. Nature 442, 312-316. [DOI] [PubMed] [Google Scholar]
- Klose, R. J., Yan, Q., Tothova, Z., Yamane, K., Erdjument-Bromage, H., Tempst, P., Gilliland, D. G., Zhang, Y. and Kaelin, W. G., Jr (2007). The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell 128, 889-900. [DOI] [PubMed] [Google Scholar]
- Kouzarides, T. (2007). Chromatin modifications and their function. Cell 128, 693-705. [DOI] [PubMed] [Google Scholar]
- Lan, F., Bayliss, P. E., Rinn, J. L., Whetstine, J. R., Wang, J. K., Chen, S., Iwase, S., Alpatov, R., Issaeva, I., Canaani, E. et al. (2007a). A histone H3 lysine 27 demethylase regulates animal posterior development. Nature 449, 689-694. [DOI] [PubMed] [Google Scholar]
- Lan, F., Zaratiegui, M., Villen, J., Vaughn, M. W., Verdel, A., Huarte, M., Shi, Y., Gygi, S. P., Moazed, D. and Martienssen, R. A. (2007b). S. pombe LSD1 homologs regulate heterochromatin propagation and euchromatic gene transcription. Mol. Cell 26, 89-101. [DOI] [PubMed] [Google Scholar]
- Lan, F., Nottke, A. C. and Shi, Y. (2008). Mechanisms involved in the regulation of histone lysine demethylases. Curr. Opin. Cell Biol. 20, 316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. W., Choi, H. S., Gyuris, J., Brent, R. and Moore, D. D. (1995). Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Mol. Endocrinol. 9, 243-254. [DOI] [PubMed] [Google Scholar]
- Lee, M. G., Norman, J., Shilatifard, A. and Shiekhattar, R. (2007a). Physical and functional association of a trimethyl H3K4 demethylase and Ring6a/MBLR, a polycomb-like protein. Cell 128, 877-887. [DOI] [PubMed] [Google Scholar]
- Lee, M. G., Villa, R., Trojer, P., Norman, J., Yan, K. P., Reinberg, D., Di Croce, L. and Shiekhattar, R. (2007b). Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquitination. Science 318, 447-450. [DOI] [PubMed] [Google Scholar]
- Lee, N., Zhang, J., Klose, R. J., Erdjument-Bromage, H., Tempst, P., Jones, R. S. and Zhang, Y. (2007c). The trithorax-group protein Lid is a histone H3 trimethyl-Lys4 demethylase. Nat. Struct. Mol. Biol. 14, 341-343. [DOI] [PubMed] [Google Scholar]
- Li, B., Carey, M. and Workman, J. L. (2007). The role of chromatin during transcription. Cell 128, 707-719. [DOI] [PubMed] [Google Scholar]
- Liang, G., Klose, R. J., Gardner, K. E. and Zhang, Y. (2007). Yeast Jhd2p is a histone H3 Lys4 trimethyl demethylase. Nat. Struct. Mol. Biol. 14, 243-245. [DOI] [PubMed] [Google Scholar]
- Liu, F., Quesada, V., Crevillen, P., Baurle, I., Swiezewski, S. and Dean, C. (2007). The Arabidopsis RNA-binding protein FCA requires a lysine-specific demethylase 1 homolog to downregulate FLC. Mol. Cell 28, 398-407. [DOI] [PubMed] [Google Scholar]
- Loh, Y. H., Zhang, W., Chen, X., George, J. and Ng, H. H. (2007). Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 21, 2545-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, D. K., Chiang, C. H., Ponnusamy, K., Ming, G. L. and Song, H. (2008). G9a and Jhdm2a regulate embryonic stem cell fusion-induced reprogramming of adult neural stem cells. Stem Cells 26, 2131-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron, R., Trojer, P. and Reinberg, D. (2005). The key to development: interpreting the histone code? Curr. Opin. Genet. Dev. 15, 163-176. [DOI] [PubMed] [Google Scholar]
- Martin, C. and Zhang, Y. (2005). The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 6, 838-849. [DOI] [PubMed] [Google Scholar]
- McGraw, S., Vigneault, C. and Sirard, M. A. (2007). Temporal expression of factors involved in chromatin remodeling and in gene regulation during early bovine in vitro embryo development. Reproduction 133, 597-608. [DOI] [PubMed] [Google Scholar]
- Metzger, E., Wissmann, M., Yin, N., Muller, J. M., Schneider, R., Peters, A. H., Gunther, T., Buettner, R. and Schule, R. (2005). LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437, 436-439. [DOI] [PubMed] [Google Scholar]
- Mikkelsen, T. S., Ku, M., Jaffe, D. B., Issac, B., Lieberman, E., Giannoukos, G., Alvarez, P., Brockman, W., Kim, T. K., Koche, R. P. et al. (2007). Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas, E., Lee, M. G., Hakimi, M. A., Cam, H. P., Grewal, S. I. and Shiekhattar, R. (2006). Fission yeast homologs of human histone H3 lysine 4 demethylase regulate a common set of genes with diverse functions. J. Biol. Chem. 281, 35983-35988. [DOI] [PubMed] [Google Scholar]
- Okada, Y., Scott, G., Ray, M. K., Mishina, Y. and Zhang, Y. (2007). Histone demethylase JHDM2A is critical for Tnp1 and Prm1 transcription and spermatogenesis. Nature 450, 119-123. [DOI] [PubMed] [Google Scholar]
- Opel, M., Lando, D., Bonilla, C., Trewick, S. C., Boukaba, A., Walfridsson, J., Cauwood, J., Werler, P. J., Carr, A. M., Kouzarides, T. et al. (2007). Genome-wide studies of histone demethylation catalysed by the fission yeast homologues of mammalian LSD1. PLoS ONE 2, e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini, D., Hansen, K. H., Christensen, J., Agger, K., Cloos, P. A. and Helin, K. (2008). Coordinated regulation of transcriptional repression by the RBP2 H3K4 demethylase and Polycomb-Repressive Complex 2. Genes Dev. 22, 1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perillo, B., Ombra, M. N., Bertoni, A., Cuozzo, C., Sacchetti, S., Sasso, A., Chiariotti, L., Malorni, A., Abbondanza, C. and Avvedimento, E. V. (2008). DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science 319, 202-206. [DOI] [PubMed] [Google Scholar]
- Quadbeck-Seeger, C., Wanner, G., Huber, S., Kahmann, R. and Kamper, J. (2000). A protein with similarity to the human retinoblastoma binding protein 2 acts specifically as a repressor for genes regulated by the b mating type locus in Ustilago maydis. Mol. Microbiol. 38, 154-166. [DOI] [PubMed] [Google Scholar]
- Reddy, K. C. and Villeneuve, A. M. (2004). C. elegans HIM-17 links chromatin modification and competence for initiation of meiotic recombination. Cell 118, 439-452. [DOI] [PubMed] [Google Scholar]
- Regha, K., Sloane, M. A., Huang, R., Pauler, F. M., Warczok, K. E., Melikant, B., Radolf, M., Martens, J. H., Schotta, G., Jenuwein, T. et al. (2007). Active and repressive chromatin are interspersed without spreading in an imprinted gene cluster in the mammalian genome. Mol. Cell 27, 353-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben, M. and Lin, R. (2002). Germline X chromosomes exhibit contrasting patterns of histone H3 methylation in Caenorhabditis elegans. Dev. Biol. 245, 71-82. [DOI] [PubMed] [Google Scholar]
- Rhodes, S. J., DiMattia, G. E. and Rosenfeld, M. G. (1994). Transcriptional mechanisms in anterior pituitary cell differentiation. Curr. Opin. Genet. Dev. 4, 709-717. [DOI] [PubMed] [Google Scholar]
- Rinn, J. L., Kertesz, M., Wang, J. K., Squazzo, S. L., Xu, X., Brugmann, S. A., Goodnough, L. H., Helms, J. A., Farnham, P. J., Segal, E. et al. (2007). Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129, 1311-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph, T., Yonezawa, M., Lein, S., Heidrich, K., Kubicek, S., Schafer, C., Phalke, S., Walther, M., Schmidt, A., Jenuwein, T. et al. (2007). Heterochromatin formation in drosophila is initiated through active removal of H3K4 methylation by the LSD1 homolog SU(VAR)3-3. Mol. Cell 26, 103-115. [DOI] [PubMed] [Google Scholar]
- Saleque, S., Kim, J., Rooke, H. M. and Orkin, S. H. (2007). Epigenetic regulation of hematopoietic differentiation by Gfi-1 and Gfi-1b is mediated by the cofactors CoREST and LSD1. Mol. Cell 27, 562-572. [DOI] [PubMed] [Google Scholar]
- Schaner, C. E. and Kelly, W. G. (2006). Germline chromatin. WormBook 1-14, www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Schedl, T. (1997). Developmental genetics of the germline. In C. elegans II (ed. D. L. Riddle, T. Blumenthal, B. J. Meyer and J. R. Priess), pp. 241-269. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [PubMed]
- Schneider, J. and Shilatifard, A. (2006). Histone demethylation by hydroxylation: chemistry in action. ACS Chem. Biol. 1, 75-81. [DOI] [PubMed] [Google Scholar]
- Schuettengruber, B., Chourrout, D., Vervoort, M., Leblanc, B. and Cavalli, G. (2007). Genome regulation by polycomb and trithorax proteins. Cell 128, 735-745. [DOI] [PubMed] [Google Scholar]
- Secombe, J. and Eisenman, R. N. (2007). The function and regulation of the JARID1 family of histone H3 lysine 4 demethylases: the Myc connection. Cell Cycle 6, 1324-1328. [DOI] [PubMed] [Google Scholar]
- Secombe, J., Li, L., Carlos, L. and Eisenman, R. N. (2007). The Trithorax group protein Lid is a trimethyl histone H3K4 demethylase required for dMyc-induced cell growth. Genes Dev. 21, 537-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, G. L., Webster, D. E., Barragan, D. I., Chang, H. Y. and Khavari, P. A. (2008). Control of differentiation in a self-renewing mammalian tissue by the histone demethylase JMJD3. Genes Dev. 22, 1865-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seward, D. J., Cubberley, G., Kim, S., Schonewald, M., Zhang, L., Tripet, B. and Bentley, D. L. (2007). Demethylation of trimethylated histone H3 Lys4 in vivo by JARID1 JmjC proteins. Nat. Struct. Mol. Biol. 14, 240-242. [DOI] [PubMed] [Google Scholar]
- Shao, G. B., Ding, H. M. and Gong, A. H. (2008). Role of histone methylation in zygotic genome activation in the preimplantation mouse embryo. In Vitro Cell Dev. Biol. Anim. 44, 115-120. [DOI] [PubMed] [Google Scholar]
- Shi, Y. (2007). Histone lysine demethylases: emerging roles in development, physiology and disease. Nat. Rev. Genet. 8, 829-833. [DOI] [PubMed] [Google Scholar]
- Shi, Y. and Whetstine, J. R. (2007). Dynamic regulation of histone lysine methylation by demethylases. Mol. Cell 25, 1-14. [DOI] [PubMed] [Google Scholar]
- Shi, Y., Lan, F., Matson, C., Mulligan, P., Whetstine, J. R., Cole, P. A. and Casero, R. A. (2004). Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941-953. [DOI] [PubMed] [Google Scholar]
- Shin-i, T. and Kohara, Y. (2005). NEXTDB: The Nematode Expression Pattern DataBase, http://nematode.lab.nig.ac.jp.
- Soshnikova, N. and Duboule, D. (2008). Epigenetic regulation of Hox gene activation: the waltz of methyls. BioEssays 30, 199-202. [DOI] [PubMed] [Google Scholar]
- Sternberg, P. W. (2005). Vulval development. WormBook 1-28, www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Sternberg, P. W. and Horvitz, H. R. (1991). Signal transduction during C. elegans vulval induction. Trends Genet. 7, 366-371. [DOI] [PubMed] [Google Scholar]
- Tachibana, M., Nozaki, M., Takeda, N. and Shinkai, Y. (2007). Functional dynamics of H3K9 methylation during meiotic prophase progression. EMBO J. 26, 3346-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani, M., Mei, P., Fang, R., Leonor, T., Rutenberg, M., Shimizu, F., Li, J., Rao, A. and Shi, Y. (2007). The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature 447, 601-605. [DOI] [PubMed] [Google Scholar]
- Takeuchi, T., Watanabe, Y., Takano-Shimizu, T. and Kondo, S. (2006). Roles of jumonji and jumonji family genes in chromatin regulation and development. Dev. Dyn. 235, 2449-2459. [DOI] [PubMed] [Google Scholar]
- Trewick, S. C., McLaughlin, P. J. and Allshire, R. C. (2005). Methylation: lost in hydroxylation? EMBO Rep. 6, 315-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada, Y., Fang, J., Erdjument-Bromage, H., Warren, M. E., Borchers, C. H., Tempst, P. and Zhang, Y. (2006). Histone demethylation by a family of JmjC domain-containing proteins. Nature 439, 811-816. [DOI] [PubMed] [Google Scholar]
- Turner, J. M. (2007). Meiotic sex chromosome inactivation. Development 134, 1823-1831. [DOI] [PubMed] [Google Scholar]
- Vakoc, C. R., Sachdeva, M. M., Wang, H. and Blobel, G. A. (2006). Profile of histone lysine methylation across transcribed mammalian chromatin. Mol. Cell. Biol. 26, 9185-9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Burg, B., Sonneveld, E., Lemmen, J. G. and van der Saag, P. T. (1999). Morphogenetic action of retinoids and estrogens. Int. J. Dev. Biol. 43, 735-743. [PubMed] [Google Scholar]
- Wang, J., Scully, K., Zhu, X., Cai, L., Zhang, J., Prefontaine, G. G., Krones, A., Ohgi, K. A., Zhu, P., Garcia-Bassets, I. et al. (2007). Opposing LSD1 complexes function in developmental gene activation and repression programmes. Nature 446, 882-887. [DOI] [PubMed] [Google Scholar]
- Whetstine, J. R., Nottke, A., Lan, F., Huarte, M., Smolikov, S., Chen, Z., Spooner, E., Li, E., Zhang, G., Colaiacovo, M. et al. (2006). Reversal of histone lysine trimethylation by the JMJD2 family of histone demethylases. Cell 125, 467-481. [DOI] [PubMed] [Google Scholar]
- Wissmann, M., Yin, N., Muller, J. M., Greschik, H., Fodor, B. D., Jenuwein, T., Vogler, C., Schneider, R., Gunther, T., Buettner, R. et al. (2007). Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat. Cell Biol. 9, 347-353. [DOI] [PubMed] [Google Scholar]
- Wolf, S. S., Patchev, V. K. and Obendorf, M. (2007). A novel variant of the putative demethylase gene, s-JMJD1C, is a coactivator of the AR. Arch. Biochem. Biophys. 460, 56-66. [DOI] [PubMed] [Google Scholar]
- Wysocka, J., Swigut, T., Milne, T. A., Dou, Y., Zhang, X., Burlingame, A. L., Roeder, R. G., Brivanlou, A. H. and Allis, C. D. (2005). WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell 121, 859-872. [DOI] [PubMed] [Google Scholar]
- Xiang, Y., Zhu, Z., Han, G., Lin, H., Xu, L. and Chen, C. D. (2007a). JMJD3 is a histone H3K27 demethylase. Cell Res. 17, 850-857. [DOI] [PubMed] [Google Scholar]
- Xiang, Y., Zhu, Z., Han, G., Ye, X., Xu, B., Peng, Z., Ma, Y., Yu, Y., Lin, H., Chen, A. P. et al. (2007b). JARID1B is a histone H3 lysine 4 demethylase up-regulated in prostate cancer. Proc. Natl. Acad. Sci. USA 104, 19226-19231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., Deng, X. and Disteche, C. M. (2008). Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS ONE 3, e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane, K., Toumazou, C., Tsukada, Y., Erdjument-Bromage, H., Tempst, P., Wong, J. and Zhang, Y. (2006). JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell 125, 483-495. [DOI] [PubMed] [Google Scholar]
- Yamane, K., Tateishi, K., Klose, R. J., Fang, J., Fabrizio, L. A., Erdjument-Bromage, H., Taylor-Papadimitriou, J., Tempst, P. and Zhang, Y. (2007). PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol. Cell 25, 801-812. [DOI] [PubMed] [Google Scholar]