Abstract
Seasonal changes in the neuroendocrine actions of gonadal steroid hormones are triggered by fluctuations in daylength. The mechanisms responsible for photoperiodic influences upon the feedback and behavioral effects of testosterone in Siberian hamsters are poorly understood. We hypothesized that daylength regulates the expression of androgen receptor (AR) and/or steroid receptor coactivator-1 (SRC-1) in specific forebrain regions. Hamsters were castrated and implanted with either oil-filled capsules or low doses of testosterone; half of the animals remained in 16L/8D and the rest were kept in 10L/14D for the ensuing 70 days. The number of AR-immunoreactive (AR-ir) cells was regulated by testosterone in medial amygdala and caudal arcuate, and by photoperiod in the medial preoptic nucleus and the posterodorsal medial amygdala. A significant interaction between photoperiod and androgen treatment was found in medial preoptic nucleus and posterodorsal medial amygdala. The molecular weight and distribution of SRC-1 were similar to reports in other rodent species, and short days reduced the number of SRC-1-ir cells in posteromedial bed nucleus of the stria terminalis (BNST) and posterodorsal medial amygdala. A significant interaction between androgen treatment and daylength in regulation of SRC-1-ir was found in anterior medial amygdala. The present results indicate that daylength-induced fluctuations in SRC-1 and AR expression may contribute to seasonally changing effects of testosterone.
Keywords: Photoperiod, Androgen, Testosterone, SRC-1, Androgen receptor, Pineal
1. Introduction
Dramatic seasonal fluctuations of reproductive function characterize most mammals. Daylength (photoperiod) provides the critical environmental signal which regulates neuroendocrine function. Transfer of male hamsters to short daylengths precipitates drastic reductions in gonadotropin secretion which trigger testicular regression. Reproductive function is significantly suppressed within approximately 6 weeks, and reaches a nadir after about 12 weeks of short days [13]. Two striking changes occur in neuroendocrine function upon exposure to such inhibitory photoperiods. First, short days exert a “steroid-dependent” effect, in which gonadal steroid hormones gain an increased capacity to suppress serum LH and FSH concentrations [7,17,43,48,51] but become less potent as activators of mounting, intromission, and ejaculation [27,37]. Such effects are monitored by challenging long- and short-day castrates with various doses of exogenous testosterone and assessing serum gonadotropin concentrations and sexual behavior. Second, “steroid-independent” changes occur, whereby LH and FSH levels are suppressed by short-day exposure even in castrated hamsters which are not given steroid replacement [7,43,51]. Furthermore, sexual behavior declines more rapidly after the removal of androgen than in long-day hamsters [27,37].
Previous studies have investigated central mechanisms responsible for the effects of photoperiod upon the negative feedback and behavioral effects of testosterone. To a lesser extent, investigators have sought to explain the steroid-independent effects of daylength. Early work provided inconsistent evidence for influences of photoperiod on the metabolism of androgen, or upon the number and affinity of androgen receptors (AR) extracted from brain and pituitary homogenates, and failed to localize any such effects [6,38,45]. More recently, immunocytochemical techniques have been used to assess effects of daylength on the number of cells expressing these steroid hormone receptors in particular brain regions. For example, short days suppress the induction of progestin receptors by estrogen in the preoptic area and hypothalamus of female Syrian hamsters [23]. Less systematic study has been devoted to the possible influence of daylength on androgen receptors [53]. We recently reported a significant effect of relatively short-term (11 day) exposure to short days on androgen receptor immunostaining in specific regions of the bed nucleus of the stria terminalis and the arcuate nucleus of Siberian hamsters [8]. While such changes in AR immunostaining may represent important events in the initiation of reproductive responses to photoperiod, the effects upon AR immunostaining of longer-term exposure to short days, which would be adequate to trigger gonadal regression, have not been tested. One aim of the present study was to address this issue by assessing AR at the time maximal reproductive suppression is reached.
Influences of photoperiod on steroid hormone action may not be restricted to changes in expression of the cognate receptors. A variety of in vitro studies reveal that nuclear receptor coactivators dramatically enhance the transcriptional activity of steroid receptors (for reviews, see Refs. [12,24,41]). These nuclear receptor coactivators are thought to enhance steroid-dependent gene transcription via a two-step mechanism [20] in which coactivators bridge the steroid receptor complex with the basal transcription machinery and remodel chromatin structure through their intrinsic histone acetyltransferase activity [5,44]. Steroid receptor coactivator-1 (SRC-1, also known as NCoA-1) was the first coactivator of steroid receptors to be discovered and belongs to a larger p160 family of nuclear receptor coactivators [35]. This family of coactivators also includes SRC-2 (NCoA-2/GRIP-1/TIF-2) [14,52] and SRC-3 (RAC3/AIB1/pCIP/ACTR/TRAM1; 119, [1,19,47]). SRC-1 enhances the transcriptional activity of steroid receptors, including AR, ER and PR, in a ligand-dependent manner in vitro [34]. SRC-1 is expressed in a variety of hormone-responsive tissues including brain, uterus, prostate, and breast (for review, see Ref. [31]). In brain, SRC-1 is expressed in many regions in which gonadal steroids act to regulate gonadotropin secretion and behavior (for review, see Refs. [31,49]). In addition, studies suggest that expression of nuclear receptor coactivators in brain is regulated by hormones [4,9,15,28]. Moreover, functional studies reveal that SRC-1 modulates steroid-dependent brain development [3,33], gene transcription in brain, and the expression of reproductive behavior [2,30]. Despite their potentially pivotal influence, the hypothesis that daylength regulates steroid hormone action through an influence on SRC-1 expression has not been tested in a species which breeds seasonally. In hamsters, light-regulated expression of pCREB-binding cofactors p300 and CBP have been examined only in the suprachiasmatic nucleus and in the context of circadian rhythms [11]; the role of steroid response cofactors in photoperiodic responses has not been investigated. Thus, in the present studies, we sought to quantify effects of androgen and daylength on SRC-1 expression in neuroendocrine regions of the Siberian hamster brain.
2. Methods
All procedures were approved by the University of Massachusetts IACUC and conformed to the NIH Guidelines for Animal Care and Use. Thirty-four young adult male Siberian hamsters (Phodopus sungorus, age 5-11 weeks), born and raised in 16L/8D (lights on 04:15), were castrated under sodium pentobarbital anesthesia (60 mg/kg) and immediately implanted with subcutaneous Silastic capsules (0.145 cm i.d., 0.193 cm o.d.) containing either sesame oil or 12.5 mg/ml testosterone in sesame oil (Sigma, St. Louis, MO) as previously described [8]. Acetominophen was added to the drinking water to insure post-operative analgesia. Half of the animals given each type of capsule were transferred to 10L/14D immediately after surgery. After a survival period of 70 days, hamsters were anesthetized with sodium pentobarbital (100 mg/kg). The thoracic cavity was opened, a blood sample was taken, and the animal was heparinized (500 U, Rugby laboratories) and transcardially perfused with 0.1 M phosphate buffer (pH 7.4) followed by 5% acrolein containing 0.25% glutaraldehyde in phosphate buffer. The brains were removed and post-fixed for 4 h before infiltration with 30% sucrose in 0.1 M phosphate buffer. Sections were cut at a thickness of 40 μm on a rotary microtome. A 1-in-6 series was collected into cryoprotectant and stored at -20 °C until the time of immunostaining. The small size of the Siberian hamster and the choice of 40-μm section thickness made it impossible to focus on all subregions of the BNST and the arcuate nucleus of individual animals when analyzing both AR-ir and SRC-1ir in the same brain. Our previous study [8] indicated the importance of carefully examining AR-ir across the rostral-caudal extent of the arcuate nucleus. We elected to examine immunostaining in both anterior and posterior BNST in our study of SRC-1 ir, but could only analyze one intermediate plane of the BNST when analyzing AR-ir.
Serum was harvested and frozen at -20 °C until radioimmunoassays were performed. LH was measured in a single assay whose sensitivity was 1.21 pg/tube. Testosterone was extracted from 50 μl aliquots of serum using 1.2 ml of diethyl ether (Burdick-Jackson), evaporated, and assayed using GDN#250 as antibody (1:50,000) and (1,2,6,7,16,17-[3H])-T as trace (NET553, New England Nuclear).
2.1. Western blot analysis
In order to characterize SRC-1 immunoreactive peptide in Siberian hamster brain, Western blots were performed on tissue extracts obtained from additional long-day hamsters. Immediately following decapitation, hypothalamic brain tissue was excised, placed in chilled microfuge tubes, snap frozen on dry ice and stored at -80 °C. Tissue was homogenized in TEDG (consisting of 10 mM Tris-base, pH=7.4, 1 mM EDTA, 1 mM Dithiothreitol (DTT), 10% glycerol) containing 400 mM NaCl and protease inhibitors (1 μg/ml of aprotinin, leupeptin, and pepstatin) using a Teflon homogenizer. After tissue homogenization, samples were centrifuged at 12,000×g for 30 min at 4°C to sediment cellular debris and nuclei. The supernatant fraction was collected, and the protein concentration was determined by Bradford assay. Eighty micrograms of total protein from each tissue sample was gel electrophoresed on 7.5% polyacrylamide gels containing 1% SDS and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA). The membrane was washed briefly in TBS-T (12 mM Tris, 180 mM NaCl, 0.06% Tween 20, pH=7.4) and then incubated for 1 h in TBS-T containing 5% nonfat dry milk with constant agitation to block nonspecific binding. The membrane was then incubated with 1135/H4, a mouse monoclonal antibody generated against amino acids 477-947 of human SRC-1 (0.05 μg/ml, kindly provided by D. Edwards, University of Colorado HSC and B. O'Malley, M. Tsai and S. Onate, Baylor College of Medicine) overnight in TBS-T containing 0.02% NaN3 at 4 C. After washes in TBS-T, the membrane was incubated in a sheep anti-mouse horseradish peroxidase-linked secondary antibody (Amer-sham, Sweden), diluted at 1:6000 in TBS-T, for 1 h at room temperature with agitation. Following washes in TBS-T, immunoreactive bands were detected with an enhanced chemiluminescence kit (ECLPlus, Amersham), followed by exposing the membrane to Biomax film (Kodak Film, NY). Recombinant human SRC-1 protein, extracted from Sf9 insect cells (Tissue Culture CORE Facility of the University of Colorado Cancer Center) infected with the appropriate recombinant transfer plasmids as described previously [50], was used as a positive control for Western blots.
2.2. Immunohistochemistry
Immunocytochemical detection of AR was performed as described previously [8]. Sections were initially rinsed in 0.05 M Tris-buffered saline (TBS), followed by a pretreatment of 1% sodium borohydride for 10 min to remove residual aldehydes. Tissue was then rinsed in TBS and incubated in a solution of 1% H2O2, 20% normal goat serum and 1% bovine serum albumin in TBS for 20 min to decrease nonspecific staining and reduce endogenous peroxidase activity. Sections were rinsed in 0.05 M TBS, and incubated overnight in rabbit anti-AR (PG-21, generously supplied by Dr. Geoffrey Greene, Ben May Institute, Chicago, IL) at 1:1000. After thorough rinsing, sections were incubated in biotinylated goat anti-rabbit (1:500, Vector Laboratories, Burlingame, CA) followed by avidin-biotin peroxidase (Vector). Immunostaining was visualized by incubation in 5 μM nickel ammonium sulfate, 2 M sodium acetate, 49 mM ammonium chloride, 0.3% benzi-dine in TBS. After rinsing in TBS, sections were mounted onto slides, dried, and coverslipped with Permount.
For detection of SRC-1, sections were incubated for 48 h in the SRC-1 monoclonal antibody, 1135/H4 (1 μg/ml), in TBS containing 0.02% sodium azide (NaN3), 1% normal goat serum, 0.1% gelatin and Triton-X (pH 7.6 at 4 °C). Tissue was rinsed in TBS containing NaN3, gelatin and Triton-X prior to incubation in a biotinylated goat anti-mouse IgG (3 μg/ml, Jackson Laboratory, West Grove, PA) in TBS containing NaN3 and Triton X-100 and 1.5% normal goat serum for 90 min. Tissue was rinsed in TBS containing NaN3, gelatin and Triton X-100 followed by rinsing in TBS. Sections were then incubated for 90 min in TBS containing 1% avidin DH: biotinylated horseradish peroxidase H complex (Vectastain ABC Elite Kit, Vector) followed by rinsing in TBS. Finally, sections were exposed to 0.5% diaminobenzine (DAB) with 3% hydrogen peroxide with TBS for approximately 3 min. The sections were rinsed in TBS and then mounted on microscope slides and coverslipped.
2.3. Quantification of immunostaining
In order to quantify AR- or SRC-1 immunostained cells, relevant brain areas were inspected at 200× magnification on a Zeiss Axioscope. The observer was blind to the experimental treatment of the animals. Images were captured using an MTI CCD72 camera and an Apple Power Macintosh 7100 computer. The NIH Image program was utilized to count the number of cells stained to densities 3 or more standard deviations above background as previously described [7]. Validation experiments employing linear regression verified that application of this criterion resulted in numbers of cells which correlated well with the number estimated by eye (r2=0.92).
Matched sections from long and short-day hamsters were processed simultaneously in order to quantify AR and SRC-1 immunostaining (AR-ir and SRC-1ir) in brain regions which participate in feedback and behavioral effects of testosterone. We quantified SRC-1-ir in the portions corresponding to the anteromedial and posteromedial bed nucleus of the stria terminalis (BNST) as described by Ju and Swanson [16], and we counted AR-ir cells in an intermediate plane of the BNST. Both AR- and SRC-1-immunoreactivity were also quantified in the anterior and posterior portions of the medial amygdaloid nucleus, the medial preoptic nucleus, and in the rostral, middle and caudal portions of the arcuate nuclei. Since no published atlas of the Siberian hamster brain is available, the rat brain atlas of Paxinos and Watson [36] (plates 26-28, 29-32, and 33-36, respectively) and the Syrian hamster atlas of Morin and Wood [32] were used to identify relevant areas; for a schematic depiction of brain regions analyzed, see Fig. 1.In general, immunostained cells of each hamster were counted in a single focal plane of two non-consecutive sections of the 1-in-6 series containing the same anatomical region. A standard shape (square or oval as illustrated in Fig. 1) fitting the approximate dimensions of the region of interest was applied to each section for purposes of quantification; the length of the side or the major axis of the shape varied between nuclei but was approximately 125-175 μm.
Fig. 1.
Schematic illustration of coronal sections of Siberian hamster brain. Crosshatching indicates regions in which AR-ir and SRC-ir were quantified. BNST, bed nucleus of the stria terminalis; MPN, medial preoptic nucleus; MeA, anteromedial amygdala; MePD, posterodorsal amygdala; rARC, rostral arcuate nucleus; mARC, middle arcuate nucleus; cARC, caudal arcuate nucleus. Modified after Morin and Wood [32].
Data were analyzed by ANOVA for the main effects of androgen treatment and photoperiod, and their interaction as described previously [8]. The Student-Newman-Keuls test was used for post-hoc comparisons. Differences were considered significant at p<0.05.
3. Results
3.1. Body weight
Hamsters did not differ in body weight at the time of their assignment to different groups. ANOVA indicated significant main effects of photoperiod on body weight (p=0.001): after 10 weeks, castrated short-day hamsters were lighter than those held in long days (28.7±1.1 vs. 35.1±1.5, mean±S.E.M.; p=0.003). A similar trend did not achieve statistical significance in steroid-implanted castrates (28.8±0.9 g vs. 31.5±1.3 vs. in short vs. long-day hamsters, respectively). Low-dose androgen replacement had no significant effect on final body weight in either photoperiod (28.7±1.1 vs. 28.8±0.9 g in short days, and 35.1±1.5 vs. 31.5±1.3 g in long days in castrated vs. androgen-treated hamsters, respectively; pN0.1). Thus, short-day exposure precipitated a 17% decline in body weight in both T-treated castrates and oil-treated control hamsters (6.2±1.7 and 6.0±0.8 g, respectively). There was no interaction between daylength and T treatment.
3.2. Hormone concentrations
Serum androgen concentrations were generally undetectable in castrated hamsters which received oil capsules. In hamsters implanted with T capsules and perfused 70 days after capsule implantation, serum testosterone values were near the limits of detection, averaging 20.3±5.2 pg/ml. This value is approximately 10% of that found in intact long-day Siberian hamsters in the same assay.
Analysis of variance indicated a significant influence of photoperiod upon serum LH concentrations (p<0.01). Short-day exposure significantly reduced serum LH concentrations in hamsters given either oil or 12.5 mg/ml capsules (p<0.05 and p<0.001, respectively). However, this dose of testosterone did not influence LH values (p=0.55), and there was no significant interaction between photo-period and androgen dose (p=0.56).
3.3. AR immunostaining
No significant effects of either photoperiod or testosterone treatment on the number of AR-ir cells were evident in the BNST of hamsters killed 70 days after the implantation of blank or testosterone capsules (Fig. 2; Table 1). Furthermore, there was no interaction between photoperiod and testosterone treatment. In the MPN, photoperiod exerted a significant main effect (p=0.002) but testosterone treatment did not, and there was an interaction between these factors (p<0.005; Fig. 3). This may be explained by the significant suppression of AR staining by short days in blank-implanted castrates (p<0.02), whereas short days tended to increase AR staining in animals receiving testosterone (p=0.07).
Fig. 2.
Representative photomicrographs illustrating AR-ir cells (top) and SRC-1-ir cells (bottom) in the BNST. SD, sections from hamsters exposed to 10L/14D for 10 weeks prior to sacrifice; LD, hamsters exposed to 16L/8D. Scale bar in top left panel (applies to all micrographs), 40 μm.
Table 1.
Effects of photoperiod and androgen treatment on number of cells immunostained for androgen receptor in Siberian hamsters
| Long days |
Short days |
|||
|---|---|---|---|---|
| Castrate | Castrate+T | Castrate | Castrate+T | |
| BNST | 87.3±21.6 | 90.0±14.4 | 84.0±14.3 | 54.3±11.1 |
| MeA | 47.0±3.4* | 39.3±3.6** | 48.3±8.8* | 20.0±5.3 |
| MePD | 32.0±4.7 | 30.1±3.9** | 46.2±4.7* | 20.0±3.4 |
| MPN | 95.7±43.7**,* | 35.8±5.6 | 44.0±13.8 | 89.1±18.1 |
| rARC | 51.0±7.0 | 46.5±5.6 | 42.8±6.3 | 48.9±6.3 |
| mARC | 81.5±4.2 | 64.0±7.8 | 63.2±9.1 | 70.1±7.9 |
| cARC | 98.3±12.1* | 70.5±4.3 | 98.4±12.2 | 86.1±8.7 |
p≤0.05 vs. corresponding T-treated group.
p≤0.05 vs. corresponding SD group.
Fig. 3.
Effects of testosterone (T capsules) and photoperiod on AR-ir in BNST (top) and MPN (bottom) of Siberian hamsters after 70 days of treatment. Bars represent mean±S.E.M. number of immunoreactive cells. Hamsters were maintained in 16L/8D (long days, open bars) or 10L/14D (short days, shaded bars) after castration. Asterisk indicates significant suppression of AR staining in short-day, oil-treated castrates (p<.02).
In neither the anterior nor the posterior nuclei of the medial amygdala was the effect of photoperiod statistically significant (p=0.08 and 0.47, respectively; Table 1). Testosterone treatment exerted significant effects in both portions of the amygdala (p<0.005), and the interaction between photoperiod and androgen was statistically significant in the posterodorsal (p<0.01), but not the anteromedial amygdala (0.05<p<0.10). This interaction may result from a short-day-induced reduction in the number of AR-ir cells in animals which received T capsules (p<0.02), but not in blank-implanted castrates (p=0.15). Furthermore, low-dose androgen replacement reduced the number of AR-ir cells in the anterior medial amygdala of short-day animals (p=0.001) but had only a marginal effect in long-day hamsters (p=0.05).
Photoperiod was without significant effect upon AR-ir staining in any portion of the arcuate nucleus, and there was no interaction between photoperiod and low-dose androgen replacement in this region (Table 1). Low-dose androgen replacement was without significant effect in either the rostral or middle arcuate, but there was a significant effect of androgen treatment on the number of AR-ir cells in the caudal arcuate (p<0.05). This was apparently due to a decrease in the number of AR-ir cells in the caudal arcuate upon androgen treatment in long-day hamsters (p<0.04); there was no such effect in short-day animals (p=0.4).
3.4. SRC-1 immunostaining
Analysis by Western blot using the SRC-1 monoclonal antibody, 1135/H4, revealed that SRC-1 protein is expressed in the hypothalamus of Siberian hamsters (Fig. 4) at a molecular mass of 160 kDa, which is similar to reports from other species [46]. SRC-1 immunoreactivity was observed in many brain regions, including particularly high levels of expression in the hippocampus, cortex, amygdala, preoptic area and hypothalamus (Fig. 2). This expression pattern of SRC-1 protein is consistent with reports of SRC-1 mRNA and protein expression in rat [15,25,30] and mouse [22,29,33,42] brain. Taken together with our Western blot data, these findings indicate that SRC-1 protein is expressed in brain regions known to regulate reproductive functions in hamsters.
Fig. 4.
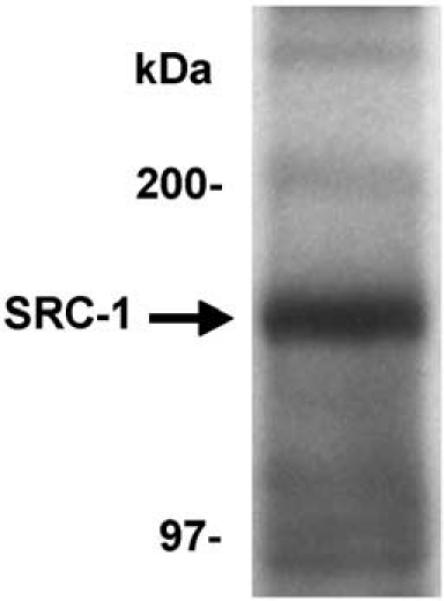
SRC-1 is expressed in Siberian hamster brain. Western blot analysis shows SRC-1 expressed at 160 kDa in the male hamster hypothalamus.
Photoperiod exerted a significant effect on the number of SRC-1-ir cells in the posteromedial BNST (p<0.05; Table 2; Fig. 5). The short-day induced reduction in SRC-1 immunostaining was evident in both castrated and T-treated hamsters, but there were neither significant effects of low-dose androgen replacement nor an interaction between T condition and photoperiod. In contrast, photoperiod and androgen were without significant effect in the anterior BNST, and these factors did not interact to regulate the number of SRC-1-ir cells.
Table 2.
Effects of photoperiod and androgen treatment on number of cells immunostained for steroid receptor coactivator-1 (SRC-1) in Siberian hamsters
| Long days |
Short days |
|||
|---|---|---|---|---|
| Castrate | Castrate+T | Castrate | Castrate+T | |
| BNSTa | 89.5±5.6 | 88.6±3.0 | 87.7±4.0 | 89.4±5.9 |
| BNSTpm | 95.2±3.8* | 95.7±3.4 | 77.2±6.0 | 83.5±7.7 |
| MeA | 81.0±2.9*, ** | 71.0±2.8 | 71.8±3.4 | 75.9±2.7 |
| MePD | 109.3±2.9* | 108.0±2.9 | 98.5±4.2 | 103.1±4.0 |
| MPN | 99.2±4.2 | 95.9±2.6 | 88.5±3.3 | 93.6±4.8 |
| mARC | 81.0±3.8 | 81.5±5.2 | 83.3±4.7 | 83.4±0.7 |
p≤0.05 vs. corresponding SD group.
p≤0.05 vs. corresponding T-treated group.
Fig. 5.
Effects of testosterone and photoperiod on number of SRC-1 immunoreactive cells in Siberian hamster BNST. In the posteromedial BNST, short days reduced SRC-1-ir (p<0.02, asterisk). Legends as in Fig. 3.
In the anteromedial amygdala, overall ANOVA the number of SRC-1-ir cells was not affected by photoperiod or T (Table 2). Nevertheless, ANOVA indicated a significant interaction between photoperiod and androgen treatment (p<0.03). In the posterodorsal medial amygdala, photoperiod exerted a significant effect on SRC-1-ir cells (p<0.05), which arose from an effect of daylength on castrated animals (p=0.05). There was no significant effect of androgen treatment and no interaction between hormonal condition and photoperiod.
In neither the medial preoptic nucleus nor arcuate nucleus was the number of SRC-1-ir cells affected by photoperiod or androgen treatment (p>0.10), although there was a trend towards a reduction in the number of immunostained cells in the MPN of short-day castrates (p=0.07). There was no interaction between photoperiod and low-dose androgen treatment.
4. Discussion
The present results indicate that photoperiodic regulation of body weight, gonadotropin secretion, and male sexual behavior may be accompanied by changes in androgen receptor and SRC-1 expression in specific regions of hamster brain. These changes may contribute to seasonal fluctuations in the response to gonadal steroids and/or steroid-independent effects of daylength.
Despite its dramatic response to photoperiod and popularity as a subject for studies of seasonal reproduction, few studies have addressed androgen receptor expression in the Siberian hamster. Our previous work concentrated on initial responses to short-day exposure at survival intervals that precede gonadal regression [8]. Although photoperiod effects upon numbers of AR-ir cells were restricted to the middle arcuate, effects of androgen were evident in all regions of the neuroendocrine forebrain. At this survival time, photoperiod and androgen were found to interact in the regulation of AR-ir in the BNST, medial amygdala and arcuate. It was not clear in that experiment, however, whether more dramatic changes in AR-ir might take place as the nadir of reproductive function approaches. In the current study, therefore, we focused on AR-ir in animals that had experienced 10 weeks of short-day exposure, which is adequate to precipitate the full gonadal response in intact animals. We found no dramatic differences in the effects of photoperiod on AR-ir at the short and long survival times. After 70 days of low-dose androgen replacement, the main effects of testosterone achieved statistical significance in the medial amygdala and the caudal arcuate nucleus. The main effects of photoperiod upon AR-ir, which were few in our previous study, were non-significant in each of the neuroendocrine regions we examined in hamsters allowed to survive for 10 weeks. As we found at 11 days of short-day exposure, interactions between photoperiod and androgen occur in several regions. At 10 weeks, such interactions were found in the MPN and posteromedial amygdala. Thus, the influence of testosterone and daylength on AR-ir expression in these regions is largely consistent at these two survival times, and interactions between photoperiod and androgen are also generally evident. The relatively minor discrepancies may reflect the transient nature of photoperiodic responses; hamsters which have experienced 10 weeks of inhibitory photoperiods are preparing for gonadal recrudescence and refractoriness to short days and this may explain some of the differences from the patterns of AR-ir in animals beginning to experience the effects of winter photoperiods at 11 days. Furthermore, although both studies utilized low doses of androgen, the testosterone-implanted hamsters in the present studies had particularly low serum testosterone concentrations. This most likely reflects exhaustion of the Silastic capsules after many weeks. Although short days clearly influenced LH secretion and body weight, the levels of testosterone were probably too low to trigger the differential feedback response reported in earlier studies [7,43] and thus may have led us to underestimate the potential for photoperiod effects on AR-ir and interactions between daylength and testosterone. Further experimentation utilizing a wider range of androgen doses and survival times is warranted in order to characterize fully the influence of photoperiod and testosterone on androgen receptor expression.
In recent years, it has become clear that nuclear receptor coactivators are essential to the influences of gonadal hormones on their target cells [31,49]. To our knowledge, the present study is the first to explore the possibility that photoperiod modulates expression of these coactivators. Short photoperiod significantly reduced the number of SRC-1-ir cells in the posteromedial BNST and tended to reduce staining in the MPN. This effect may contribute to the reduction in the ability of androgen to activate male sexual behavior in short-day hamsters [27,37]. Furthermore, lesion studies indicate the BNST in photoperiodic regulation of reproduction in Syrian hamsters [39,40]; photoperiodic and androgen influences on AR-ir and SRC-1-ir in this region may thus represent a mechanism important in seasonal reproduction. The influence of photoperiod and androgen on AR-ir and SRC-1-ir in the posteromedial amygdala may also contribute photoperiodic regulation of testosteroneregulated sexual behavior, and help to explain the photoperiod-dependent remodeling of this brain region described by Cooke et al. [10].
In light of previous reports that gonadal steroid hormones can regulate SRC-1 expression, we were surprised not to find significant effects of T treatment on SRC-ir in the present study. This may be related to the low-dose of androgen administered. There was evidence for regionally restricted interactions between daylength and androgen. The observation that photoperiod can modulate SRC-1 expression in the BNST of castrated hamsters may be particularly relevant to the steroid-independent effect of daylength, particularly in light of the possibility that unliganded steroid receptors may interact with SRC-1 or other cofactors in order to regulate gene expression.
It would be worthwhile to determine whether SRC-1 ir is particularly affected by androgen treatment and/or photoperiod in cells that express gonadal steroid hormone receptors. Such double-label studies might provide evidence of a physiologically significant interaction that is not evident when all SRC-1 ir cells are surveyed in single label experiments such as the present study. It is also possible that quantitatively minor changes in SRC-1 and AR expression which result from fluctuations in photoperiod and/or androgen levels exert a multiplicative influence on feedback or behavioral responses to testosterone. The changes in SRC-1-ir seen in this study must again be interpreted keeping in mind the low doses of testosterone employed and the relatively long survival time. As is the case for AR-ir staining, exploration of changes in SRC-1 expression across a wider range of androgen treatments and survival times is needed. It is essential to better our understanding of the mechanisms by which photoperiodic effects influence neuronal responsiveness to steroid hormones. Our present findings suggest that regulation of nuclear receptor coactivators may be an important event in this fine-tuning of neuronal responsiveness specific brain regions. Thus, the possibility of changes in other nuclear receptor coactivators (e.g., SRC-2, SRC-3...) upon photoperiodic challenges and steroid hormone treatment deserves further exploration. Such studies may pave the way for examination of effects of manipulation of coactivator protein expression (e.g. Refs. [3,30,41]) on gonadal responses to photoperiod.
Acknowledgments
We thank Richard Hurlbut for capable animal care, Sima Patel for histological assistance, and Drs. Sandra I. Sulsky and Carol Bigelow for advice on statistical analyses. This research was supported by NIH RO1 HD59166 and NSF 98-17252 to ELB and By NIH R01 DK61935 and NSF IBN-0080818 to MJT.
References
- [1].Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- [2].Apostolakis EM, Ramamurphy M, Zhou D, Onate S, O'Malley B. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol. Endocrinol. 2002;16:1511–1523. doi: 10.1210/mend.16.7.0877. [DOI] [PubMed] [Google Scholar]
- [3].Auger AP, Tetel MJ, McCarthy MM. Steroid receptor co-activator-1 mediates the development of sex specific brain morphology and behavior. Proc. Natl. Acad. Sci. U. S. A. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Auger AP, Perrot-Sinai TS, Auger CJ, Ekas L, Tetel MJ, McCarthy MM. Expression of the nuclear receptor coactivator, cAMP response element-binding protein, is sexually dimorphic and modulates sexual differentiation of neonatal rat brain. Endocrinology. 2002;143:3009–3016. doi: 10.1210/endo.143.8.8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- [6].Bittman EL, Krey LC. Influences of photoperiod on nuclear androgen receptor occupancy in neuroendocrine tissues of the golden hamster. Neuroendocrinology. 1988;47:61–67. doi: 10.1159/000124892. [DOI] [PubMed] [Google Scholar]
- [7].Bittman EL, Jetton AE, Villalba C, DeVries GJ. Effects of photoperiod and androgen on pituitary function and neuropeptide staining in Siberian hamsters. Am. J. Physiol. 1996;271:R64–R72. doi: 10.1152/ajpregu.1996.271.1.R64. [DOI] [PubMed] [Google Scholar]
- [8].Bittman EL, Ehrlich DA, Ogdahl JL, Jetton AE. Photoperiod and testosterone regulate androgen receptor immunostaining in the Siberian hamster brain. Biol. Reprod. 2003;69:876–884. doi: 10.1095/biolreprod.102.010900. [DOI] [PubMed] [Google Scholar]
- [9].Charlier TD, Balthazart J, Ball GF. Sex differences in the distribution of the steroid coactivator SRC-1 in the song control nuclei of male and female canaries. Brain Res. 2003;959:263–274. doi: 10.1016/s0006-8993(02)03758-7. [DOI] [PubMed] [Google Scholar]
- [10].Cooke BM, Hegstrom CD, Breedlove SM. Photoperiod-dependent response to androgen in the medial amygdala of the Siberian hamster, Phodopus sungorus. J. Biol. Rhythms. 2002;17:147–154. doi: 10.1177/074873002129002438. [DOI] [PubMed] [Google Scholar]
- [11].Fiore P, Gannon RL. Expression of the transcriptional coactivators CBP and p300 in the hamster suprachiasmatic nucleus: possible molecular components of the mammalian circadian clock. Brain Res. Mol. Brain Res. 2003;111:1–7. doi: 10.1016/s0169-328x(02)00663-0. [DOI] [PubMed] [Google Scholar]
- [12].Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- [13].Hoffman K, Illnerova H. Photoperiodic effects in the Djungarian hamster. Neuroendocrinology. 1986;43:317–321. doi: 10.1159/000124562. [DOI] [PubMed] [Google Scholar]
- [14].Hong H, Kohli K, Garabedian MJ, Stallcup MR. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol. Cell. Biol. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iannacone EA, Yan AW, Gauger KJ, Dowling ALS, Zoeller RT. Thyroid hormone exerts site-specific effects on SRC-1 and NCoR expression selectively in the neonatal rat brain. Mol. Cell. Endocrinol. 2002;186:49–59. doi: 10.1016/s0303-7207(01)00672-4. [DOI] [PubMed] [Google Scholar]
- [16].Ju G, Swanson LW. Studies on the cellular architecture of the bed nucleus of the stria terminalis in the rat: I. Cytoarchitecture. J. Comp. Neurol. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- [17].Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE. Neuroendocrine basis of seasonal reproduction. Recent Prog. Horm. Res. 1984;40:185–232. doi: 10.1016/b978-0-12-571140-1.50010-4. [DOI] [PubMed] [Google Scholar]
- [18].Kemppainen JA, Lane MV, Sar M, Wilson EM. Androgen receptor phosphorylation, turnover, nuclear transport, and transcription activation. J. Biol. Chem. 1992;267:968–974. [PubMed] [Google Scholar]
- [19].Li H, Gomes PJ, Chen JD. RAC3, a steroid/nuclear receptorassociated coactivator that is related to SRC-1 and TIF2. Proc. Natl. Acad. Sci. U. S. A. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu Z, Wong J, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator-1 (SRC-1) enhances ligand-dependent and receptordependent cell-free transcription of chromatin. Proc. Natl. Acad. Sci. U. S. A. 1999;96:9485–9490. doi: 10.1073/pnas.96.17.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lu S-F, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139:1594–1601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- [22].Ma H, Hong H, Huang SM, Irvine RA, Webb P, Kushner PJ, Coetzee GA, Stallcup MR. Multiple signal input and output domains of the 160-kilodalton nuclear receptor coactivator proteins. Mol. Cell. Biol. 1999;19:6164–6173. doi: 10.1128/mcb.19.9.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mangels RA, Powers JB, Blaustein JD. Effect of photoperiod on neural estrogen and progestin receptor immunoreactivity in female Syrian hamsters. Brain Res. 1998;796:63–74. doi: 10.1016/s0006-8993(98)00318-7. [DOI] [PubMed] [Google Scholar]
- [24].McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- [25].Meijer OC, Steenbergen PJ, de Kloet ER. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology. 2000;141:2192–2199. doi: 10.1210/endo.141.6.7489. [DOI] [PubMed] [Google Scholar]
- [26].Mercer JG, Moar KM, Logie TJ, Findlay PA, Adam CL, Morgan PJ. Seasonally inappropriate body weight induced by food restriction: effect on hypothalamic gene expression in male Siberian hamsters. Endocrinology. 2001;142:4173–4181. doi: 10.1210/endo.142.10.8454. [DOI] [PubMed] [Google Scholar]
- [27].Miernicki MW, Pospichal MW, Powers JB. Short photoperiods affect male hamster sociosexual behaviors in the presence and absence of testosterone. Physiol. Behav. 1990;47:95–106. doi: 10.1016/0031-9384(90)90046-7. [DOI] [PubMed] [Google Scholar]
- [28].Misiti S, Schomburg I, Yen PM, Chin WW. Expression and hormonal regulation of coactivator and corepressor genes. Endocrinology. 1998;139:2493–2500. doi: 10.1210/endo.139.5.5971. [DOI] [PubMed] [Google Scholar]
- [29].Misiti S, Koibuchi N, Bei M, Farsetti A, Chin MW. Expression of steroid receptor coactivator-1 mRNA in the developing mouse embryo: a possible role in olfactory epithelium development. Endocrinology. 1999;140:1957–1960. doi: 10.1210/endo.140.4.6782. [DOI] [PubMed] [Google Scholar]
- [30].Molenda HA, Griffin L, Auger AP, McCarthy MM, Tetel MJ. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinology. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- [31].Molenda HA, Kilts C, Allen RL, Tetel MJ. Nuclear receptor coactivator function in reproductive physiology and behavior. Biol. Reprod. 2003;69:1449–1457. doi: 10.1095/biolreprod.103.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain. Academic Press; San Diego: 2001. p. 146. [Google Scholar]
- [33].Nishihara E, Yoshida-Kimoya H, Chan C, Liao L, Davis RL, O'Malley BW, Xu J. SRC-1 null mice exhibit moderate motor dysfunction and delayed development of cerebellar Purkinje cells. J. Neurosci. 2003;23:213–222. doi: 10.1523/JNEUROSCI.23-01-00213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oñate SA, Tsai SY, Tsai MJ, O'Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- [35].Oñate SA, Boonyaratanakornkit V, Spencer TE, Tsai SY, Tsai MJ, Edwards DP, O'Malley BW. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J. Biol. Chem. 1998;273:12101–12108. doi: 10.1074/jbc.273.20.12101. [DOI] [PubMed] [Google Scholar]
- [36].Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Orlando: 1986. [Google Scholar]
- [37].Poschipal MW, Karp JD, Powers JB. Influence of daylength on male hamster sexual behavior: masking effects of T. Physiol. Behav. 1991;49:417–422. doi: 10.1016/0031-9384(91)90258-p. [DOI] [PubMed] [Google Scholar]
- [38].Prins GS, Bartke AJ, Steger RW. Influence of photoinhibiton, photostimulation and prolactin on pituitary and hypothalamic nuclear androgen receptors in the male hamster. Neuroendocrinology. 1990;52:511–516. doi: 10.1159/000125636. [DOI] [PubMed] [Google Scholar]
- [39].Ratiere MN, Garyfallou VT, Urbanski HF. Lesions in the bed nucleus of the stria terminalis, but not in the lateral septum, inhibit short-photoperiod induced testicular regression in Syrian hamsters. Brain Res. 1995;705:159–167. doi: 10.1016/0006-8993(95)01152-8. [DOI] [PubMed] [Google Scholar]
- [40].Ratiere MN, Garyfallou VT, Urbanski HF. Lesions in the anterior bed nucleus of the stria terminalis in Syrian hamsters block short photoperiod-induced testicular regression. Biol. Reprod. 1997;57:796–806. doi: 10.1095/biolreprod57.4.796. [DOI] [PubMed] [Google Scholar]
- [41].Robyr D, Wolffe AP, Wahli W. Nuclear hormone receptor coregulators in action: diversity for shared tasks. Mol. Endocrinol. 2000;14:329–347. doi: 10.1210/mend.14.3.0411. [DOI] [PubMed] [Google Scholar]
- [42].Shearman LP, Zylka MJ, Reppert SM, Weaver DR. Expression of basic helix-loop-helix/PAS genes in the mouse suprachiasmatic nucleus. Neuroscience. 1999;89:387–397. doi: 10.1016/s0306-4522(98)00325-x. [DOI] [PubMed] [Google Scholar]
- [43].Simpson SM, Follett BK, Ellis DH. Modulation by photoperiod of gonadotrophin secretion in intact and castrated Djungarian hamsters. J. Reprod. Fertil. 1982;66:243–250. doi: 10.1530/jrf.0.0660243. [DOI] [PubMed] [Google Scholar]
- [44].Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Oñate SA, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–197. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- [45].Stankov B, Lucini V, Snochowski M, Cozzi B, Fumagalli P, Maccarinelli G, Fraschini F. Cytosolic androgen receptors in the neuroendocrine tissues of the golden hamster: influence of phtoperiod and melatonin treatment. Endocrinology. 1989;125:1742–1744. doi: 10.1210/endo-125-3-1742. [DOI] [PubMed] [Google Scholar]
- [46].Takashita A, Yen PM, Misiti S, Cardona GR, Liu Y, Chin WW. Molecular cloning and properties of a full-length putative thyroid hormone receptor coactivator. Endocrinology. 1996;137:3594–3597. doi: 10.1210/endo.137.8.8754792. [DOI] [PubMed] [Google Scholar]
- [47].Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW. TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J. Biol. Chem. 1997;272:27629–27634. doi: 10.1074/jbc.272.44.27629. [DOI] [PubMed] [Google Scholar]
- [48].Tamarkin L, Hutchison JS, Goldman BD. Regulation of serum gonadotropins by photoperiod and testicular hormone in the Syrian hasmster. Endocrinology. 1976;99:1528–1533. doi: 10.1210/endo-99-6-1528. [DOI] [PubMed] [Google Scholar]
- [49].Tetel MJ. Nuclear receptor coactivators in neuroendocrine function. J. Neuroendocrinol. 2000;12:927–932. doi: 10.1046/j.1365-2826.2000.00557.x. [DOI] [PubMed] [Google Scholar]
- [50].Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol. Endocrinol. 1999;13:910–924. doi: 10.1210/mend.13.6.0300. [DOI] [PubMed] [Google Scholar]
- [51].Turek FW, Elliot JA, Alvis JD, Menaker M. The interaction of castration and photoperiod in the regulation of hypophyseal and serum gonadotropin levels in male golden hamsters. Endocrinology. 1975;96:854–860. doi: 10.1210/endo-96-4-854. [DOI] [PubMed] [Google Scholar]
- [52].Voegel JJ, Heine MJS, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- [53].Wood RI, Newman SW. Intracellular partitioning of androgen recptor immunoreactivity in the brain of the male Syrian hamster: effects of castration and steroid replacement. J. Neurobiol. 1993;24:925–938. doi: 10.1002/neu.480240706. [DOI] [PubMed] [Google Scholar]






