Abstract
Understanding the homeostatic mechanisms governing lymphocyte pools achieves critical importance as lymphocyte-targeted therapies expand in use and scope. The primacy of B lymphocyte stimulator (BLyS) family ligands and receptors in governing B lymphocyte homeostasis has become increasingly clear in recent years, affording insight into novel opportunities and potential pitfalls for targeted B cell therapeutics. Interclonal competition for BLyS-BR3 interactions determines the size of naïve B cell pools and can regulate the stringency of selection applied as cells complete maturation. Thus one of the predicted consequences of ablative therapies targeting primary pools is relaxed negative selection. This suggests that BLyS levels and B cell reconstitution rates may serve useful prognostic roles and that BLyS itself might be targeted to circumvent relapse. Alternatively, manipulations that allow rare, minimally autoreactive specificities to survive and mature may lead to opportunities in cases where antibody-based vaccine development has heretofore been unsuccessful. BLyS family ligands and receptors also play a role in activated and memory B cell pools, suggesting they might likewise be targeted to promote or delete particular antigen-experienced subpopulations in a similar way.
Keywords: B cell, homeostasis, BLyS, targeted therapy
INTRODUCTION
During the last decade, the central role of homeostatic mechanisms in determining the behavior, composition, and size of lymphocyte pools has become increasingly apparent. These strong influences stem in part from the critical requisite that lymphocyte numbers be tied to organism volume in order to accommodate adequate immune surveillance [17]. Accordingly, lymphocyte production and selection are integrated with mechanisms that ensure functional pools can be maintained at sizes sufficient for protection [86]. These considerations are compounded by the notion that different lymphocyte subsets, such as memory versus naïve pools, are likely to be regulated independently of each other [19, 132]. A global outcome of these strong homeostatic controls is that perturbations in normal lymphocyte production, longevity, or numbers will be countered by compensatory mechanisms attempting to keep pool sizes at minimally acceptable levels.
The need for a sophisticated mechanistic understanding of lymphocyte homeostasis has increased with the growing application of therapeutics that ablate lymphocyte subsets. In particular, the selective elimination or reduction of B cell pools is rapidly emerging as a therapeutic intervention in a variety of diseases. Currently, patients with autoimmune diseases and non--Hodgkin’s lymphoma comprise the majority of cases in which B cell ablative therapies are used [29]. Early success in these conditions prompted additional applications, leading to trials in Sjögren’s syndrome, Graves’ disease, multiple sclerosis, and solid organ transplantation [26, 41, 60, 97, 106, 107]. Currently, these therapies focus on ablating most peripheral B cells [10, 12, 22, 24, 25, 64, 67, 74, 78, 125, 130, 133, 134], but the ability to target specific subsets precisely is forthcoming, and may yield even greater efficacy. The most extensive trials have been with Rituximab®, a monoclonal CD20-specific antibody, and favorable outcomes with this therapy have generated keen interest in targeting other B cell markers. For example, monoclonal antibodies against CD22, CD80, CD52, CD30, and CD40 are now being investigated for use in lymphoma [29]. Similarly, antagonists for FcγRIIB, BLyS®, and B lymphocyte stimulator (BLyS) receptors are also in clinical trials [91, 102, 113, 119, 124]. Some examples of ablative therapies are presented in Table 1. Inasmuch as the expressions of these markers, receptors, and cytokines differ among B cell subsets, the direct and downstream effects of these reagents will likely diverge.
Table 1.
Examples of targeted ablative strategies in use or development
| Therapy | Example | Target | Stage | |
|---|---|---|---|---|
| Non-specifically targeted | all hematopoietic cells | total body irradiation | all hematopoietic cells | clinic |
| Cell-specific targeted | anti-idiotype | customized | clonal population of cells (lymphoma) | clinic |
| chemical inhibitors | Gleevec1 | specific tumor cells bearing BCR/ABL protein | clinic | |
| anti-CD20 | Rituxan2, Zevalin3 | mature B cells | clinic (GENE, IDEC) | |
| anti-CD30 | MDX-06044, SGN-3055 | activated B cells (NHL cells) | phase II (MEDX, SGEN) | |
| anti-CD52 | Campath6 | mature lymphocytes | clinic | |
| anti-CD80 | Galiximab7 | activated B cells (NHL cells) | phase III | |
| anti-CD23 | Lumiliximab8 | late transitional and mature B cells (B-CLL) | phase II | |
| anti-CD40 | SGN-409 | antigen-presenting cells (CLL and NHL) | phase I/II | |
|
| ||||
| Homeostasis regulator targeted | anti-BLyS/TACI-Ig/ | Belimumab10 | cytokine required for B | phase III (HGS/GSK), phase I (AMG, IDEC/GENE, ZGEN) |
| /BR3-Fc/anti-BR3 | AMG-62311, Atacicept12, anti-BR313 | cell survival | ||
| anti-APRIL | cytokine required for B cell survival | |||
Novartis, reviewed in ref. [152];
Genentech, reviewed in ref. [147];
Biogen Idec, reviewed in ref. [148];
Medarex, reviewed in ref. [145];
Seattle Genetics, reviewed in ref. [154];
Ilex, reviewed in ref. [144];
Biogen Idec, reviewed in ref. [149];
Biogen Idec, reviewed in ref. [153];
Seattle Genetics reviewed in ref. 150];
Human Genome Sciences, reviewed in ref. [146];
Amgen, unpublished;
Zymogenetics/Serono, reviewed in ref. [151];
Biogen Idec, unpublished.
B-CLL – B cell chronic lymphocytic leukemia.
These expanding efforts in toto underscore the need for a thorough understanding of the homeostatic and selective processes active within B cell populations. Indeed, the effective conceptualization, development, and application of targeted ablative therapies demands a detailed understanding of three fundamental, interrelated aspects of B cell physiology: the molecular mechanisms that merge selection with homeostatic demands, the basis for independent homeostatic control of different functional subsets, and the cues that mediate transit between such independently regulated subsets. Accordingly, this review examines principles of homeostatic control within naïve and antigen-experienced B cell subsets, with particular emphasis on how the BLyS family of receptors and ligands impacts each of these. In the first section we describe the development and homeostatic control of primary B cell populations, summarizing current thinking, identifying gaps in knowledge, and offering speculation regarding implications for effective clinical manipulation. In the second we present a parallel discussion of antigen-experienced B cell subsets.
PRIMARY B CELL POOL SIZES ARE MAINTAINED BY COMPETITION IN THE PERIPHERY
In adults, B cells differentiate from bone marrow (BM) hematopoietic stem cells after genetic and epigenetic changes yielding B lineage specification and commitment, reviewed elsewhere [15, 85]. Although several nomenclatures are used to divide committed B lineage differentiation intermediates, all are based on Ig gene rearrangement status [46, 100, 111]. Prior to antigen receptor expression, developing B cells in the pro- and pre-B compartments rely on marrow stromal elements for continued commitment, survival, and differentiation [20, 65, 66, 99, 105, 122]. As evidenced by early depletion studies [101] as well as mutations and manipulations that selectively alter mature pool sizes, there do not appear to be any direct feedback mechanisms to either lineage commitment rates or transit through these early differentiative intermediates. Accordingly, homeostatic controls that dictate primary B cell pool sizes act primarily at later stages.
Once a complete antigen receptor is expressed, B cells enter the immature BM compartment. This is a critical step in B cell maturation, since from this point forward cell fate can be influenced by B cell receptor (BCR) specificity. Stringent specificity-based selection occurs here, such that avid BCR ligation leads to either secondary V gene rearrangements or death [40, 42, 93, 94, 129]. It is estimated that only about 10% of the immature BM B cells formed survive these selective processes and exit to the periphery, where they undergo further specificity-based selection in the transitional (TR) compartment [2–4, 32, 33, 73, 112]. Residency in the TR pool is short, as cells either die or complete differentiation to enter the naïve peripheral pools within three days [4, 32, 112]. Only about 30% of cells exiting the BM complete TR differentiation [4, 112]. At normal steady state, most surviving TR cells enter the follicular (FO) compartment, with a small proportion joining the marginal zone (MZ) pool. Cells within the FO pool display a relatively long half-life of 80–120 days [4, 120].
Because the TR stage is the last before entrance to mature naïve B cell populations, throughput at this point is effectively the production rate of primary B cells. This rate, in combination with the lifespan of primary cells themselves, determines the steady-state size of naïve B cell pools. Accordingly, identifying the molecular determinants of these two parameters is crucial to understanding the homeostatic control mechanisms operative within primary pools.
Observations demonstrating that interclonal competition determines the ability to survive within mature peripheral pools provided some of the earliest clues to these mechanisms [31, 37, 84, 121]. Most of these studies employed mixed marrow radiation chimeras and yielded results implying that primary B cells compete for limited, viability-promoting resources. Moreover, all of these studies suggested that BCR specificity impacts clonotypic fitness to capture or utilize these resources. The notion that interclonal competition moderated by BCR specificity underlies the homeostatic control of primary B cells carries two critical implications. First, the probability of survival in TR and FO pools is plastic rather than absolute, depending on the abundance of viability-promoting resources. Moreover, this plasticity will itself be tied to total B cell numbers and their relative competitive advantage. Second, because BCR specificity plays a dominant role in determining intrinsic competitive fitness, the molecular metric for biological space must be integrated with BCR signaling.
THE BLyS FAMILY OF LIGANDS AND RECEPTORS
The BLyS family of receptors and ligands is a relatively recent addition to the large array of tumor necrosis factor (TNF) superfamily ligands and receptors and plays a central role in determining the selection and maintenance of naïve and antigen-experienced B cell subsets. Consequently, intense investigation has been focused on these molecules and their mechanisms of action. A brief summary of this family’s characteristics is provided here inasmuch as numerous excellent reviews detailing their discovery and general characteristics have been published [14, 16, 49, 50, 61, 76–78, 117, 123].
The BLyS family consists of two ligands, BLyS (also known as BAFF) [89, 118] and a proliferation-inducing ligand (APRIL) [45], and three receptors, BLyS receptor 3 (BR3 also known as BAFFr) [115, 128, 142], transmembrane activator and CAML interactor (TACI) [135, 136], and B cell maturation antigen (BCMA) [44]. BLyS can bind to all three receptors, but interacts most strongly with BR3. In contrast, APRIL can interact only with TACI and BCMA. APRIL also binds to sulfated proteoglycans on cell surfaces [53, 59]. While the consequences of this interaction are unclear, recent evidence indicating that TACI requires oligomeric ligands suggests such tethered arrays may be important for fostering TACI signaling [11, 59].
BLyS is produced by many cell types, including dendritic cells, neutrophils, and radioresistant stromal elements [62, 71, 92]. The soluble trimeric form of BLyS generated by furin cleavage at the cell surface is currently the only known biologically active form. Splice variants that might afford membrane-tethered or regulatory forms remain a possibility [38, 39]; however, whether these or other membrane-bound forms might possess biological activity, especially in anatomically discrete sites, warrants further investigation. While the soluble trimeric form of BLyS is able to interact with BR3 efficiently, oligomerization into a 60-mer allows signaling through TACI [11]. Similar to BLyS, APRIL is also cleaved by furin convertase, but this event occurs within the Golgi apparatus, making soluble APRIL the only biologically active form [75]. APRIL is produced by some tumors and by osteoclasts in humans [90] and in mice (Quinn, unpublished). These differences in production and availability may help to explain the different roles these cytokines play in the maintenance, selection, and differentiation of B cells.
THE BLyS-BR3 AXIS REGULATES THE PRIMARY B CELL POOL SIZE AND COMPOSITION
Extensive evidence indicates that BLyS/BR3 interactions are critical to TR throughput and FO B cell lifespan, thus serving as the chief determinant of primary pool size and composition. Initial clues to this relationship were provided by studies showing that exogenous BLyS administration could nearly double the size of FO and MZ pools within days [89] and that BR3 mutants or knockouts displayed profound reductions in TR, FO, and MZ cell numbers [48, 58, 68, 69, 115, 142]. Moreover, a similar phenotype was seen in BLyS-deficient mice [115]. The dominance of BR3 in maintaining naïve B cells was further revealed by both TACI and BCMA knockout mice [21, 137, 141], where relatively normal FO and MZ compartments indicated that neither of these receptors is functionally redundant with BR3. Likewise, exogenous administration of BR3 antagonists [109, 110] as well as neutralizing BLyS antibody (Scholz and Crowley, unpublished) yields a reduction in all primary B cell subsets.
Several observations link the BLyS-BR3 axis to prior observations showing that primary B cell homeostasis involves interclonal competition. First, in mixed BM chimeras, B cells derived from BR3-deficient progenitors cannot compete effectively with their BR3-sufficient counterparts for survival [48]. Second, the lifespan and proportional throughput of TR B cells increase during sustained exogenous BLyS administration [58]. Perhaps most importantly in this context, this increased TR throughput reflects relaxation of the specificity--mediated selection occurring at this checkpoint. Thus, in several transgenic models, increased BLyS levels allowed cells that are normally deleted to survive and enter the FO and MZ compartments [70, 127]. Similarly, mice transgenic for a DNA reactive BCR that were given exogenous BLyS had a dramatic increase in the number of mature autoreactive B cells [56]. Together, these studies demonstrate that TR selection and BLyS sensitivity are linked, such that changes in BLyS levels can alter the stringency of TR selection.
IMPLICATIONS FOR HOMEOSTATIC INTERVENTION AND MANIPULATION
The interplay between TR selection and homeostatic competition raises implications, both cautionary and exciting, for manipulation of the primary pools. In particular, these considerations suggest that ablative strategies should be undertaken with cognizance that BLyS-mediated compensatory homeostatic mechanisms may ensue, especially during autoreconstitution. Indeed, because the threshold for TR negative selection is directly affected by BLyS, cells that would normally be lost at this point might proceed into the mature peripheral pools. Although experimental systems demonstrating this principle have employed either exogenous administration or transgenes to elevate BLyS, precipitous ablation of primary B cells, the major consumers of BLyS, is likely to increase systemic BLyS levels. This possibility has significant repercussions for effective clinical management of ablative treatments and raises intriguing possibilities for new therapeutic strategies.
MAINTAINING SELECTIVE STRINGENCY REQUIRES CAREFUL CALIBRATION
Ironically, despite the clear promise of B cell ablative therapies for autoimmunity, these approaches may actually promote the survival of autoreactive TR cells as peripheral autoreconstitution progresses. Temporary elimination of primary B cells and subsequently elevated BLyS levels is predicted to alter TR selection in such a way as to allow maturation of specificities that would normally undergo elimination. Further, while minimally autoreactive clones are unlikely to be pathogenic in most people, studies from mice demonstrate the role of different genetic backgrounds in creating permissive environments for the development of autoimmune disease. Likewise, elevated serum BLyS levels and autoimmune disease severity are correlated [9, 27, 28, 82]. In non-diseased states, appropriately functioning control mechanisms governing antigen presentation, T cell help, and selection within the germinal center (GC) prevent autoreactive naïve clones from developing into longer--lived variations [30, 57]. However, in autoimmuneprone backgrounds, these control parameters may be aberrant. For example, B cells in mice with an autoimmuneprone background resist depletion with anti--CD20 antibody [1]. Likewise, increased BLyS levels can induce autoimmunity in mice possessing an autoimmune genetic background by three months of age, whereas mice without such predisposition fail to develop disease until advanced age [126].
CORRELATES OF TR SELECTION MAY YIELD PROGNOSTIC INFORMATION
While elevated BLyS levels may be either causative or correlative, either possibility suggests that the development of successful targeted therapies will benefit from following homeostatic parameters in both animal and clinical studies. Accordingly, careful monitoring of BLyS levels and the reconstitution rates of different B cell subsets might yield prognostic information. For example, if TR pools reach steady state and are followed by rapid reconstitution of the FO and MZ pools, then a window of reduced TR selective stringency is likely. On the other hand, slower FO reconstitution rates may indicate maintenance of “normal” TR selection, indicating it is less likely that mature pools are being repopulated with autoreactive cells. Ratios of TR to FO cells might prove the most robust figure, since normal ratios will be subject to B cell production rates and will thus vary according to age, gender, and other factors. Likewise, prospectively tracking serum BLyS levels might detect conditions conducive to relaxed TR selection. Finally, determining the occurrence of markers for negative selection, such as specific V gene usage, CDR3 lengths and sequences, or expression of cell surface markers, may also have predictive value in identifying patients at risk for relaxed selection.
CAN HOMEOSTATIC RESPONSES TO ABLATIVE STRATEGIES BE MODULATED?
Tracking biomarker surrogates of selective stringency might allow intervention when the probability of admitting autoreactive cells into mature pools appears high. Such interventions might exploit recent advances in our knowledge of how homeostasis and selection intersect during TR differentiation. A general model for such homeostatic manipulation is shown in Fig. 1. In contrast to the selective stringency imposed at normal steady state (panel A), ablated autoreconstituting individuals may offer an environment in which autoreactive B cells can mature. Following B cell ablation, BLyS consumption will be minimal and available BLyS levels consequently high (panel B). This may yield a window during which virtually all TR B cells, regardless of specificity, mature. With time, the FO pool will fill and TR throughput should return to normal. During the intervening period however, such cells may have had the opportunity to progress into downstream niches, such as memory or plasma cell (PC) pools, which no longer compete with primary cells and are beyond all checkpoints guarding against autoimmune effector function and pathology. This model can be generalized inasmuch as analogous homeostatic controls likely operate at each level of B cell development and differentiation. For example, a similar representation might be constructed for cells occupying short- or long-lived plasma cell (LLPC) niches, memory niches, or any other independently regulated subset (see below). Thus, in each situation there may be both opportunity and danger in ablative manipulations or other approaches perturbing homeostatic regulation. Based on this model, immediately accessible interventions might involve reducing available BLyS by targeting either BLyS per se or BLyS-producing cells (panel C). This possibility assumes that BLyS production rates are relatively stable, and further work is needed to examine this assumption. Alternatively, blocking BR3 interactions with synthetic antagonists might reduce fruitful BLyS-BR3 interactions, regardless of how BLyS production is controlled.
Fig. 1.
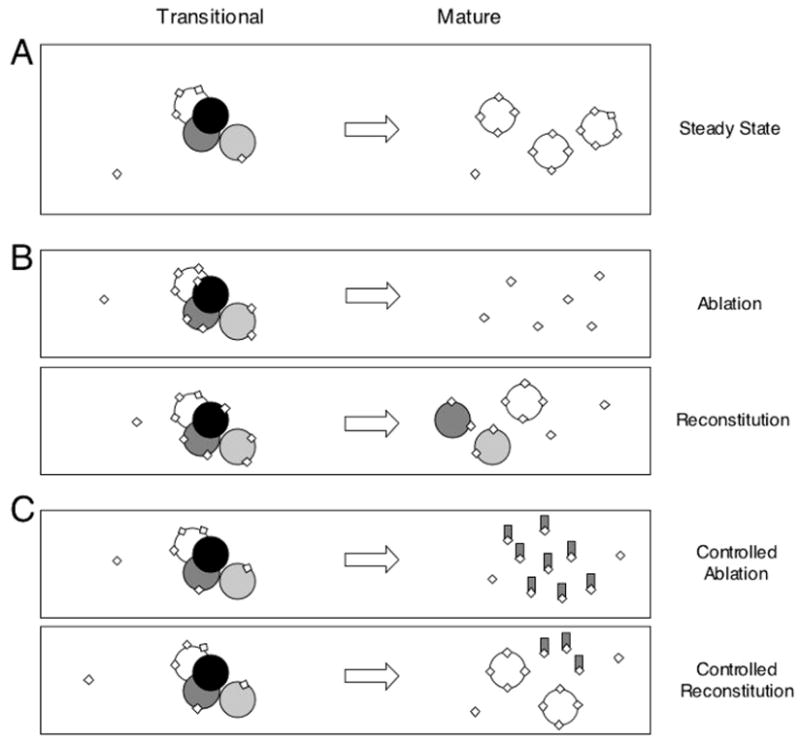
Peripheral BLyS availability regulates the quality and quantity of developing B cells in the steady state as well as during ablation/reconstitution. Developing B cells become responsive to BLyS (diamonds) as they emigrate from the BM to the periphery. Once they have emerged as TR cells, they require signals from BLyS receptors for survival and subsequent progression into the mature pool. Panel A: At normal steady state, clonotypes that effectively compete for BLyS mature and, because autoreactive clonotypes (shaded) are comparatively poor competitors, they are eliminated from the emerging repertoire. Panel B: In ablative regimes, mature peripheral B cells are eliminated, yielding surplus free BLyS. Competition for BLyS is thus reduced among TR cells, relaxing selective stringency so that the emerging primary pool may include self reactive clonotypes. Panel C: In controlled ablative regimes where compensatory homeostatic responses are blunted by blocking BLyS/BR3 interactions, steady state BLyS can be functionally mimicked, resulting in TR throughput and selection similar to that found in unmanipulated animals.
A more distant option for influencing TR selective stringency includes targeting the intracellular mediators of signaling via BLyS receptors. For example, BR3 preferentially utilizes the alternative NF-κB pathway, while TACI relies upon classical NF-κB signaling [63, 108]. Further, these two receptors employ different TNF receptor-associated factors (TRAFs), with TACI using TRAF2, 5, and 6 and BR3 relying exclusively on TRAF3 [51, 96, 139, 140]. Thus, by pharmacologically targeting these mediators in a lineage- or subset-specific manner, BLyS receptor signal strength might be “tuned” to afford appropriate selective stringency.
HOMEOSTATICALLY RELAXED SELECTION AS A MEANS TO MANIPULATE DIVERSITY AND TOLERANCE
Although potentially detrimental in the context of autoimmunity, interventions that deliberately relax TR selection might be a useful tool when an expanded repertoire diversity is sought. Situations where this might prove beneficial include the generation of vaccines where some degree of self reactivity is wanted (e.g. tumor vaccines) as well as settings in which clonotypes with neutralizing activity are normally eliminated at TR stages. While intentionally increasing BLyS levels per se would carry obvious risks even on a temporary basis, the directed augmentation of BLyS responsiveness among desired clonotypes may eventually prove possible.
The potential to modulate TR selection also has exciting implications for transplantation. For example, the ablation of B cell pools coupled with controlled TR selection during autoreconstitution might facilitate graft acceptance inasmuch as emerging B cells would undergo appropriate tolerogenic selection to peripheral autoantigens, including those of the graft, with appropriate stringency. Consistent with this idea, anti-CD20 treatment improved the survival of pancreatic islet transplants in non-human primates and protracted return to normal TR cell proportions were correlated with extended graft acceptance [72].
HOMEOSTATIC CONTROLS IN ANTIGEN-EXPERIENCED SUBSETS
While the mechanistic understanding of primary B cell homeostasis has advanced substantially in recent years, our knowledge of the analogous mechanisms operating in antigen-experienced subsets remains rudimentary. Ample evidence indicates that antigen-experienced pools are free of the controls governing primary B cells. For example, antigen-activated B cell clones expand considerably, indicating their release from interclonal competition with quiescent naïve B cells and suggesting that they utilize different resources for survival. Moreover, several alternative fates can be adopted by daughter cells generated during antigen-driven activation, yielding multiple subsets with disparate homeostatic properties. Indeed, some descendants of activated B cells display relatively short lifespans of days to weeks, whereas others persist indefinitely [79, 81, 104, 116]. Analogous to emerging primary cells, specificity-based selective events play a strong role in determining these fates [34]. Finally, trafficking, homing, and residency attributes vary, raising the possibility of local microenvironmental contributions. For example, early PCs appear primarily in extrafollicular areas of the spleen [13, 35], whereas LLPCs rely on elements of the BM for survival [55, 87, 88].
The prompt formation of plasmablasts, proliferating cells that produce low levels of antibody, is a feature common to most early humoral responses. These cells are found predominantly in extrafollicular spaces and have a short lifespan, with most dying within three days [54]. Plasmablasts can also terminally differentiate into PCs, which produce large amounts of antibody but have lost proliferative capacity. Short-lived PCs can isotype switch and, under certain circumstances, undergo somatic hypermutation despite residence outside GC reactions [138]. While nearly all forms of antigenic stimuli generate short-lived plasma cells (SLPCs), some forms of activation, such as Toll-like receptor (TLR) ligands and T-independent (TI) antigens, yield SLPCs almost exclusively. Little is known about the control mechanisms governing either the initial expansion of plasmablasts or the survival of SLPCs. However, increasing evidence suggests that TACI may play a pivotal role among these cells. For example, stimulation via either TLR4 or TLR9 increases TACI expression substantially, and TACI-deficient mice show reduced TI responses [132, 137]. Additionally, since SLPCs are maintained in anatomically distinct locations, ligand availability may afford a mechanism for local regulation.
Although T-dependent responses also generate early PCs, they are unique in their ability to form GCs in secondary lymphoid organs. These structures are the primary sites of somatic hypermutation and affinity maturation; they are initiated by costimulation provided via cognate T cell help. Again, the ultimate fates of B cells that enter GCs vary widely. It is generally accepted that positive selection of high-affinity and deletion of lower-affinity clones occurs within the confines of a GC reaction, but these mechanisms are poorly understood (reviewed in [52]). Those GC B cells that maintain or improve antigen affinity may become LLPCs or memory cells [83]. LLPCs maintain serum antibody levels, providing protection against known antigens, and have a half-life of greater than six months [36, 57]. Memory cells do not produce antibody but are long lived [116] and are thought to differentiate rapidly into PCs during a recall response [23]. The exact physical and molecular niches required to support memory B cells have been difficult to define. LLPCs and memory cells primarily reside in the BM and in the spleen, respectively. Memory cells have further been traced particularly to splenic follicles and the MZ [5, 6]. Further, these cells do not rely on the same cytokines that are required by naïve B cells, such as BLyS [19].
EXOGENOUS ACTIVATION AND REGULATORY SIGNALS MODIFY BLyS RECEPTOR SIGNATURE
Activated B cells show altered BLyS receptor expression patterns, suggesting that BLyS receptor “signatures” may specify cytokine requirements for survival, thereby delineating independent homeostatic niches. For example, BCR ligation induces an increase in BLyS binding capacity [8], and both BCR and TLR ligation have dose-dependent effects on BLyS receptor expression, in particular increasing TACI expression [131]. Further, early PC populations generated during T-dependent and TI responses express increased levels of TACI [80, 131]. The TACI knockout mouse suggests this upregulation may be important because their responses to TI-II antigens are reduced [43, 143]. Further, TACI appears key to generating isotype--switched cells secreting distal isotypes, in accord with a role in the survival or differentiation of PCs. This characteristic likely extends to humans inasmuch as TACI mutations have been reported in IgA deficiency and common variable immunodeficiency [18, 114]. Both conditions are typified by the inability to produce antibodies in response to TI antigens or infections with pneumococcal bacteria.
The other BLyS receptors, BR3 and BCMA, appear to play different roles during an immune response. Unlike early PCs, cells within the GC express elevated levels of BR3 (Crowley, unpublished) compared with the general naïve population. Increases in this receptor suggest a dependence on BLyS for initiation of GC reactions, but it has been difficult to test this directly because naïve B cells that would enter into a GC are also highly sensitive to BLyS levels. Data from the Kalled laboratory indicate that BLyS is required for GC maintenance, if not initiation [95], and the BR3 mutant A/WySnJ strain initiates but does not expand GCs (Lentz, unpublished). On the other hand, BCMA is critical in the maintenance and/or production of LLPCs [98]. This receptor binds BLyS, but has a more than100--fold greater affinity for APRIL, suggesting that APRIL may be its more relevant physiological ligand [7, 103]. Likewise, BCMA is expressed on memory B cells and may thus be important in their generation or survival (Crowley, unpublished).
MODULATION OF HOMEOSTATIC CONTROLS IN ANTIGEN-EXPERIENCED SUBSETS: POTENTIAL THERAPEUTIC APPROACHES
While a detailed understanding of the homeostatic controls operating among antigen-experienced B cell subsets has not yet been achieved, several plausible avenues for targeted strategies are already apparent. For example, subsets might be targeted based on the BLyS receptors they express. Since BCMA predominates on memory B cells and LLPCs, APRIL signaling through BCMA may be the key pairing for these pools, just as BLyS-BR3 is the major axis for naïve B cell survival. Exogenous APRIL might be administered to potentially increase the frequency of memory cells or LLPCs generated or maintained in response to immunization, thereby leading to increased antibody titers and enhanced long-lived immunity for individuals with attenuated immune responses, such as the very young and the elderly. Conversely, BCMA function might be blocked in order to reduce autoreactive specificities in the memory and LLPC pools of autoimmune individuals. Clearly, methods for targeting specific antigen-experienced subpopulations would be most useful, thereby allowing for elimination of undesirable types (e.g. autoimmune specificities) with concurrent retention of desirable types such as memory populations from prior vaccinations.
Another avenue for manipulation of antigen-experienced subsets might involve targeting of soluble or surface-bound ligands. For instance, it has recently been reported that signaling through the TACI receptor requires an oligomeric ligand [11]. APRIL contains a QKQKKQ motif required for binding to proteoglycans, and thus oligomerization [59]. If this mechanism proves to be unique to TACI, targeting these residues to reduce oligomerization of APRIL may eliminate signaling through TACI. This could allow for the regulation of TACI-expressing SLPCs while leaving BCMA-expressing LLPCs unaffected. Blocking APRIL oligomerization while leaving its receptor binding function intact could potentially be used to treat autoimmunity, as animal models have found that chromatin-reactive antibody-secreting cells express TACI exclusively [56]. Conversely, in scenarios where survival and selection of rare PC clonotypes is desirable, a TACI oligomerizing agent might be used to extend SLPC survival, perhaps leading to their acceptance into the LLPC or memory pools.
Alternative targets might involve adhesion molecules or chemokine receptors associated with the ability of pathogenic cells to home to or be retained in a specific physical niche. For instance, it has been shown that CXCR4 expression is necessary for the recruitment of newly formed PCs to the bone [47]. Blocking this receptor could thus deny newly formed PCs access to the specialized location required for LLPC differentiation [47].
CONCLUSION
The emerging understanding of mechanisms governing the homeostatic control of B cell subsets should allow refined methods for clinical manipulation of the humoral immune response. Current therapies targeting B cells are based on relatively broad strokes of ablation and reconstitution. Thus the elucidation of consequent compensatory homeostatic mechanisms should permit the fine-tuning of therapeutic outcomes. The capacity to titer cytokine levels may be vital in maintaining competitive selection, or blocking the maintenance of antigen--experienced cells, thereby preventing the survival of autoimmune cells following reconstitution. Moreover, this knowledge should afford the selection of particular previously rare or unavailable clonotypes, ultimately allowing novel vaccine strategies. Likewise, in pathological settings such as lupus, limitation of BLyS may restrict the selection of autoreactive clonotypes without adversely affecting other peripheral populations, including immunological memory.
Abbreviations
- APRIL
a proliferation-inducing ligand
- BCMA
B cell maturation antigen
- BCR
B cell receptor
- BLyS
B lymphocyte stimulator
- BM
bone marrow
- BR3
BLyS receptor 3
- FO
follicular
- GC
germinal center
- LLPC
long--lived plasma cell
- MZ
marginal zone
- PC
plasma cell
- SLPC
short-lived plasma cell
- TACI
transmembrane activator and CAML interactor
- TI
T-independent
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TR
transitional
- TRAF
TNF receptor-associated factor
References
- 1.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, Shlomchik MJ. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol. 2007;179:3351–3361. doi: 10.4049/jimmunol.179.5.3351. [DOI] [PubMed] [Google Scholar]
- 2.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 3.Allman DM, Ferguson SE, Cancro MP. Peripheral B cell maturation. I Immature peripheral B cells in adults are heat-stable antigenhi and exhibit unique signaling characteristics. J Immunol. 1992;149:2533–2540. [PubMed] [Google Scholar]
- 4.Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation. II Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow--derived B cells. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- 5.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson SM, Tomayko MM, Shlomchik MJ. Intrinsic properties of human and murine memory B cells. Immunol Rev. 2006;211:280–294. doi: 10.1111/j.0105-2896.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 7.Ansart-Pirenne H, Rouger P, Noizat-Pirenne F. [Cellular mechanisms implicated in anti-erythrocyte alloimmunization] Transfus Clin Biol. 2005;12:135–141. doi: 10.1016/j.tracli.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Baker KP, Edwards BM, Main SH, Choi GH, Wager RE, Halpern WG, Lappin PB, Riccobene T, Abramian D, Sekut L, Sturm B, Poortman C, Minter RR, Dobson CL, Williams E, Carmen S, Smith R, Rosche V, Hilbert DM, Vaughan TJ, Albert VR. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48:3253–3265. doi: 10.1002/art.11299. [DOI] [PubMed] [Google Scholar]
- 9.Becker-Merok A, Nikolaisen C, Nossent HC. B-lymphocyte activating factor in systemic lupus erythematosus and rheumatoid arthritis in relation to autoanti-body levels, disease measures and time. Lupus. 2006;15:570–576. doi: 10.1177/0961203306071871. [DOI] [PubMed] [Google Scholar]
- 10.Bosello S, Pers JO, Rochas C, Devauchelle V, De Santis M, Daridon C, Saraux A, Ferraccioli GF, Youinou P. BAFF and rheumatic autoimmune disorders: implications for disease management and therapy. Int J Immunopathol Pharmacol. 2007;20:1–8. doi: 10.1177/039463200702000101. [DOI] [PubMed] [Google Scholar]
- 11.Bossen C, Cachero TG, Tardivel A, Ingold K, Willen L, Dobles M, Scott ML, Maguelin A, Belnoue E, Siegrist CA, Chevrier S, Acha-Orbea H, Leung H, Mackay F, Tschopp J, Schneider P. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008;111:1004–1012. doi: 10.1182/blood-2007-09-110874. [DOI] [PubMed] [Google Scholar]
- 12.Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, Sharp J, Perez JL, Spencer-Green GT. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 13.Brighenti A, Andrulis M, Geissinger E, Roth S, Muller--Hermelink HK, Rudiger T. Extrafollicular proliferation of B cells in the absence of follicular hyperplasia: a distinct reaction pattern in lymph nodes correlated with primary or recall type responses. Histopathology. 2005;47:90–100. doi: 10.1111/j.1365-2559.2005.02173.x. [DOI] [PubMed] [Google Scholar]
- 14.Brink R. Regulation of B cell self-tolerance by BAFF. Semin Immunol. 2006;18:276–283. doi: 10.1016/j.smim.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55–79. doi: 10.1146/annurev.immunol.22.012703.104807. [DOI] [PubMed] [Google Scholar]
- 16.Cancro MP. The BLyS family of ligands and receptors: an archetype for niche-specific homeostatic regulation. Immunol Rev. 2004;202:237–249. doi: 10.1111/j.0105-2896.2004.00212.x. [DOI] [PubMed] [Google Scholar]
- 17.Cancro MP, Kearney JF. B cell positive selection: road map to the primary repertoire? J Immunol. 2004;173:15–19. doi: 10.4049/jimmunol.173.1.15. [DOI] [PubMed] [Google Scholar]
- 18.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, Geha RS. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 19.Crowley JE, Treml LS, Stadanlick JE, Carpenter E, Cancro MP. Homeostatic niche specification among naive and activated B cells: a growing role for the BLyS family of receptors and ligands. Semin Immunol. 2005;17:193–199. doi: 10.1016/j.smim.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 21.Davis WC, Haverson K, Saalmuller A, Yang H, Lunney JK, Hamilton MJ, Pescovitz MD. Analysis of monoclonal antibodies reacting with molecules expressed on gammadelta T-cells. Vet Immunol Immunopathol. 2001;80:53–62. doi: 10.1016/s0165-2427(01)00282-3. [DOI] [PubMed] [Google Scholar]
- 22.Denton CP, Black CM. Targeted therapy comes of age in scleroderma. Trends Immunol. 2005;26:596–602. doi: 10.1016/j.it.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Dorner T, Radbruch A. Selecting B cells and plasma cells to memory. J Exp Med. 2005;201:497–499. doi: 10.1084/jem.20050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards CJ. Immunological therapies for rheumatoid arthritis. Br Med Bull. 2005:73–74. doi: 10.1093/bmb/ldh051. 71–82. [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg R, Albert D. B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2006;2:20–27. doi: 10.1038/ncprheum0042. [DOI] [PubMed] [Google Scholar]
- 26.El Fassi D, Nielsen CH, Hasselbalch HC, Hegedus L. The rationale for B lymphocyte depletion in Graves’ disease. Monoclonal anti-CD20 antibody therapy as a novel treatment option. Eur J Endocrinol. 2006;154:623–632. doi: 10.1530/eje.1.02140. [DOI] [PubMed] [Google Scholar]
- 27.Emmerich F, Bal G, Barakat A, Milz J, Muhle C, Martinez-Gamboa L, Dorner T, Salama A. High-level serum B-cell activating factor and promoter polymorphisms in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2007;136:309–314. doi: 10.1111/j.1365-2141.2006.06431.x. [DOI] [PubMed] [Google Scholar]
- 28.Fabris M, Visentini D, De Re V, Picierno A, Maieron R, Cannizzaro R, Villalta D, Curcio F, De Vita S, Tonutti E. Elevated B cell-activating factor of the tumour necrosis factor family in coeliac disease. Scand J Gastroenterol. 2007;42:1–6. doi: 10.1080/00365520701452225. [DOI] [PubMed] [Google Scholar]
- 29.Fanale MA, Younes A. Monoclonal antibodies in the treatment of non-Hodgkin’s lymphoma. Drugs. 2007;67:333–350. doi: 10.2165/00003495-200767030-00002. [DOI] [PubMed] [Google Scholar]
- 30.Ferry H, Leung JC, Lewis G, Nijnik A, Silver K, Lambe T, Cornall RJ. B-cell tolerance. Transplantation. 2006;81:308–315. doi: 10.1097/01.tp.0000203830.79357.39. [DOI] [PubMed] [Google Scholar]
- 31.Freitas AA, Rocha BB. Lymphocyte lifespans: homeostasis, selection and competition. Immunol Today. 1993;14:25–29. doi: 10.1016/0167-5699(93)90320-K. [DOI] [PubMed] [Google Scholar]
- 32.Fulcher DA, Basten A. Reduced life span of anergic self-reactive B cells in a double-transgenic model. J Exp Med. 1994;179:125–134. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulcher DA, Lyons AB, Korn SL, Cook MC, Koleda C, Parish C, Fazekas de St Groth B, Basten A. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and availability of T cell help. J Exp Med. 1996;183:2313–2328. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galibert L, Burdin N, Barthelemy C, Meffre G, Durand I, Garcia E, Garrpme P, Rousset F, Banchereau J, Liu YJ. Negative selection of human germinal center B cells by prolonged BCR cross-linking. J Exp Med. 1996;183:2075–2085. doi: 10.1084/jem.183.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia de Vinuesa C, O’Leary P, Sze DM, Toellner KM, MacLennan IC. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur J Immunol. 1999;29:1314–1323. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Gatto D, Martin SW, Bessa J, Pellicioli E, Sudan P, Hinton HJ, Bachmann MF. Regulation of memory antibody levels: the role of persisting antigen versus plasma cell life span. J Immunol. 2007;178:67–76. doi: 10.4049/jimmunol.178.1.67. [DOI] [PubMed] [Google Scholar]
- 37.Gaudin E, Rosado M, Agenes F, McLean A, Freitas AA. B-cell homeostasis, competition, resources, and positive selection by self-antigens. Immunol Rev. 2004;197:102–115. doi: 10.1111/j.0105-2896.2004.0095.x. [DOI] [PubMed] [Google Scholar]
- 38.Gavin AL, Ait-Azzouzene D, Ware CF, Nemazee D. DeltaBAFF, an alternate splice isoform that regulates receptor binding and biopresentation of the B cell survival cytokine, BAFF. J Biol Chem. 2003;278:38220–38228. doi: 10.1074/jbc.M306852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gavin AL, Duong B, Skog P, Ait-Azzouzena D, Greaves DR, Scott ML, Nemazzee D. deltaBAFF, a splice isoform of BAFF, opposes full-length BAFF activity in vivo in transgenic mouse models. J Immunol. 2005;175:319–328. doi: 10.4049/jimmunol.175.1.319. [DOI] [PubMed] [Google Scholar]
- 40.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldenberg DM. Epratuzumab in the therapy of oncological and immunological diseases. Expert Rev Anticancer Ther. 2006;6:1341–1353. doi: 10.1586/14737140.6.10.1341. [DOI] [PubMed] [Google Scholar]
- 42.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 43.Gordon NC, Pan B, Hymowitz SG, Yin J, Kelley RF, Cochran AG, Yan M, Dixit VM, Fairbrother WJ, Starovasnik MA. BAFF/BLyS receptor 3 comprises a minimal TNF receptor-like module that encodes a highly focused ligand-binding site. Biochemistry. 2003;42:5977–5983. doi: 10.1021/bi034017g. [DOI] [PubMed] [Google Scholar]
- 44.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D, Lofton-Day C, Moore M, Littau A, Grossman A, Haugen H, Foley K, Blumberg H, Harrison K, Kindsvogel W, Clegg CH. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 45.Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, Sordat B, Rimoldi D, Tschopp J. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998;188:1185–1190. doi: 10.1084/jem.188.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardy RR, Shinton SA. Characterization of B lymphopoiesis in mouse bone marrow and spleen. Methods Mol Biol. 2004;271:1–24. doi: 10.1385/1-59259-796-3:001. [DOI] [PubMed] [Google Scholar]
- 47.Hargreaves DC, Hyman PL, Lu TT, Ngo VN, Bidgol A, Suzuki G, Zou YR, Littman DR, Cyster JG. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harless SM, Lentz VM, Sah AP, Hsu BL, Clise-Dwyer K, Hilbert DM, Hayes CE, Cancro MP. Competition for BLyS-mediated signaling through Bcmd/BR3 regulates peripheral B lymphocyte numbers. Curr Biol. 2001;11:1986–1989. doi: 10.1016/s0960-9822(01)00598-x. [DOI] [PubMed] [Google Scholar]
- 49.Harless Smith S, Cancro MP. BLyS: the pivotal determinant of peripheral B cell selection and lifespan. Curr Pharm Des. 2003;9:1833–1847. doi: 10.2174/1381612033454405. [DOI] [PubMed] [Google Scholar]
- 50.Harless Smith S, Cancro MP. Integrating B cell homeostasis and selection with BLyS. Arch Immunol Ther Exp. 2003;51:209–218. [PubMed] [Google Scholar]
- 51.Hauer J, Puschner S, Ramakrishnan P, Simon U, Bongers M, Federle C, Engelmann H. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the non-canonical NF-kappaB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci USA. 2005;102:2874–2879. doi: 10.1073/pnas.0500187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hauser AE, Shlomchik MJ, Haberman AM. In vivo imaging studies shed light on germinal-centre development. Nat Rev Immunol. 2007;7:499–504. doi: 10.1038/nri2120. [DOI] [PubMed] [Google Scholar]
- 53.Hendriks J, Planelles L, de Jong-Odding J, Hardenberg G, Pals ST, Hahne M, Spaargaren M, Medema JP. Heparan sulfate proteoglycan binding promotes APRIL-induced tumor cell proliferation. Cell Death Differ. 2005;12:637–648. doi: 10.1038/sj.cdd.4401647. [DOI] [PubMed] [Google Scholar]
- 54.Ho F, Lortan JE, MacLennan IC, Khan M. Distinct short-lived and long-lived antibody-producing cell populations. Eur J Immunol. 1986;16:1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- 55.Hofer T, Muehlinghaus G, Moser K, Yoshida T, Mei EH, Hebel K, Hauser A, Hoyer B, Luger OE, Dorner T, Manz RA, Hiepe F, Radbruch A. Adaptation of humoral memory. Immunol Rev. 2006;211:295–302. doi: 10.1111/j.0105-2896.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- 56.Hondowicz BD, Alexander St, Quinn WJ, 3rd, Pagan AJ, Metzgar MH, Cancro MP, Erikson J. The role of BLyS/BLyS receptors in anti-chromatin B cell regulation. Int Immunol. 2007;19:465–475. doi: 10.1093/intimm/dxm011. [DOI] [PubMed] [Google Scholar]
- 57.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002;168:5993–5996. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- 59.Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero Tg, Qiang F, Gorelik L, Kalled SL, Acha--Orbea H, Rennert PD, Tschopp J, Schneider P. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201:1375–1383. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaczmarek I, Deutsch MA, Sadoni S, Brenner P, Schmauss D, Daebritz SH, Weiss M, Meiser BM, Reichart B. Successful management of antibody--mediated cardiac allograft rejection with combined immunoadsorption and anti-CD20 monoclonal antibody treatment: case report and literature review. J Heart Lung Transplant. 2007;26:511–515. doi: 10.1016/j.healun.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 61.Kalled SL. The role of BAFF in immune function and implications for autoimmunity. Immunol Rev. 2005;204:43–54. doi: 10.1111/j.0105-2896.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 62.Kato A, Truong-Tran AQ, Scott AL, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta--dependent mechanism. J Immunol. 2006;177:7164–7172. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DM, Grewal IS, Cochran AG, Gordon NC, Yin J, Starovasnik MA, Dixit VM. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002;17:515–524. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 64.Keystone EC. B cells in rheumatoid arthritis: from hypothesis to the clinic. Rheumatology. 2005;44(suppl 2):ii8–ii12. doi: 10.1093/rheumatology/keh617. [DOI] [PubMed] [Google Scholar]
- 65.Kincade PW, He Q, Ishihara K, Miyake K, Lesley J, Hyman R. CD44 and other cell interaction molecules contributing to B lymphopoiesis. Curr Top Microbiol Immunol. 1993;184:215–222. doi: 10.1007/978-3-642-78253-4_17. [DOI] [PubMed] [Google Scholar]
- 66.Kincade PW, Lee G, Pietrangeli CE, Hayashi S, Gimble JM. Cells and molecules that regulate B lymphopoiesis in bone marrow. Annu Rev Immunol. 1989;7:111–143. doi: 10.1146/annurev.iy.07.040189.000551. [DOI] [PubMed] [Google Scholar]
- 67.Koller MD. Targeted therapy in rheumatoid arthritis. Wien Med Wochenschr. 2006;156:53–60. doi: 10.1007/s10354-005-0245-6. [DOI] [PubMed] [Google Scholar]
- 68.Lentz VM, Cancro MP, Nashold FE, Hayes CE. Bcmd governs recruitment of new B cells into the stable peripheral B cell pool in the A/WySnJ mouse. J Immunol. 1996;157:598–606. [PubMed] [Google Scholar]
- 69.Lentz VM, Hayes CE, Cancro MP. Bcmd decreases the life span of B-2 but not B-1 cells in A/WySnJ mice. J Immunol. 1998;160:3743–3747. [PubMed] [Google Scholar]
- 70.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 71.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40--independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu C, Noorchashm H, Sutter JA, Naji M, Prak EL, Boyer J, Green T, Rickels MR, Tomaszewski JE, Koeberlein B, Wang Z, Paessler ME, Velidedeoglu E, Rostami SY, Yu M, Baker CF, Naji A. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med. 2007;13:1295–1298. doi: 10.1038/nm1673. [DOI] [PubMed] [Google Scholar]
- 73.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Looney RJ, Anolik J, Sanz I. B cells as therapeutic targets for rheumatic diseases. Curr Opin Rheumatol. 2004;16:180–185. doi: 10.1097/00002281-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 75.Lopez-Fraga M, Fernandez R, Albar JP, Hahne M. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2001;2:945–951. doi: 10.1093/embo-reports/kve198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mackay F, Ambrose C. The TNF family members BAFF and APRIL: the growing complexity. Cytokine Growth Factor Rev. 2003;14:311–324. doi: 10.1016/s1359-6101(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 77.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 78.Mackay F, Sierro F, Grey ST, Gordon TP. The BAFF/APRIL system: an important player in systemic rheumatic diseases. Curr Dir Autoimmun. 2005;8:243–265. doi: 10.1159/000082106. [DOI] [PubMed] [Google Scholar]
- 79.MacLennan IC, Gray D. Antigen-driven selection of virgin and memory B cells. Immunol Rev. 1986;91:61–85. doi: 10.1111/j.1600-065x.1986.tb01484.x. [DOI] [PubMed] [Google Scholar]
- 80.Mantchev GT, Cortesao CS, Rebrovich M, Cascalho M, Bram RJ. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J Immunol. 2007;179:2282–2288. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- 81.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 82.Mariette X, Roux S, Zhang J, Bengoufa D, Lavie F, Zhou T, Kimberly R. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren’s syndrome. Ann Rheum Dis. 2003;62:168–171. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McHeyzer-Williams MG, Ahmed R. B cell memory and the long-lived plasma cell. Curr Opin Immunol. 1999;11:172–179. doi: 10.1016/s0952-7915(99)80029-6. [DOI] [PubMed] [Google Scholar]
- 84.McLean AR, Rosado MM, Agenes F, Vasconcellos R, Freitas AA. Resource competition as a mechanism for B cell homeostasis. Proc Natl Acad Sci USA. 1997;94:5792–5797. doi: 10.1073/pnas.94.11.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Medina KL, Singh H. Gene regulatory networks orchestrating B cell fate specification, commitment, and differentiation. Curr Top Microbiol Immunol. 2005;290:1–14. doi: 10.1007/3-540-26363-2_1. [DOI] [PubMed] [Google Scholar]
- 86.Miller JP, Stadanlick JE, Cancro MP. Space, selection, and surveillance: setting boundaries with BLyS. J Immunol. 2006;176:6405–6410. doi: 10.4049/jimmunol.176.11.6405. [DOI] [PubMed] [Google Scholar]
- 87.Minges Wols HA, Ippolito JA, Yu Z, Palmer JL, White FA, Le PT, Witte PL. The effects of microenvironment and internal programming on plasma cell survival. Int Immunol. 2007;19:837–846. doi: 10.1093/intimm/dxm051. [DOI] [PubMed] [Google Scholar]
- 88.Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169:4213–4221. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
- 89.Moore PA, Belvedere O, Orr A, Pieri K, La Fleur DW, Feng S, Sppet D, Chartes M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 90.Moreaux J, Cremer FW, Reme T, Raab M, Mahtouk K, Kaukel P, Pantesco V, De Vos J, Jourdan E, Jauch A, Legouffe E, Moos M, Fiol G, Goldschmidt H, Rossi JF, Hose D, Klein B. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood. 2005;106:1021–1030. doi: 10.1182/blood-2004-11-4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakamura A, Akiyama K, Takai T. Fc receptor targeting in the treatment of allergy, autoimmune diseases and cancer. Expert Opin Ther Targets. 2005;9:169–190. doi: 10.1517/14728222.9.1.169. [DOI] [PubMed] [Google Scholar]
- 92.Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, Sosnovtseva S, Carrell JA, Feng P, Giri JG, Hilbert DM. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001;97:198–204. doi: 10.1182/blood.v97.1.198. [DOI] [PubMed] [Google Scholar]
- 93.Nemazee D, Buerki K. Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras. Proc Natl Acad Sci USA. 1989;86:8039–8043. doi: 10.1073/pnas.86.20.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nemazee D, Russell D, Arnold B, Haemmerling G, Allison J, Miller JF, Morahan G, Buerki K. Clonal deletion of autospecific B lymphocytes. Immunol Rev. 1991;122:117–132. doi: 10.1111/j.1600-065x.1991.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 95.Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML, Thompson JS, Whenway J, Chtanova T, Groom J, Sutton IJ, Xin C, Tangye SG, Kalled SL, Mackay F, Mackay CR. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. 2004;173:807–817. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- 96.Ni CZ, Oganesyan G, Welsh K, Zhu X, Reed Jc, Satterthwait AC, Cheng G, Ely KR. Key molecular contacts promote recognition of the BAFF receptor by TNF receptor-associated factor 3: implications for intracellular signaling regulation. J Immunol. 2004;173:7394–3400. doi: 10.4049/jimmunol.173.12.7394. [DOI] [PubMed] [Google Scholar]
- 97.Nikbin B, Bonab MM, Khosravi F, Talebian F. Role of B cells in pathogenesis of multiple sclerosis. Int Rev Neurobiol. 2007;79:13–42. doi: 10.1016/S0074-7742(07)79002-5. [DOI] [PubMed] [Google Scholar]
- 98.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oritani K, Kincade PW. Lymphopoiesis and matrix glycoprotein SC1/ECM2. Leuk Lymphoma. 1998;32:1–7. doi: 10.3109/10428199809059241. [DOI] [PubMed] [Google Scholar]
- 100.Osmond DG. B cell development in the bone marrow. Semin Immunol. 1990;2:173–180. [PubMed] [Google Scholar]
- 101.Osmond DG, Gordon J. Characterization of small lymphocytes in the bone marrow of mice after prolonged treatment with anti-IgM antibodies. Cell Immunol. 1979;42:188–193. doi: 10.1016/0008-8749(79)90233-8. [DOI] [PubMed] [Google Scholar]
- 102.Ouarzane M, Zouali M. Novel opportunities for therapeutic targeting in systemic autoimmune diseases. Methods Mol Biol. 2007;361:285–297. doi: 10.1385/1-59745-208-4:285. [DOI] [PubMed] [Google Scholar]
- 103.Patel DR, Wallweber HJ, Yin J, Shriver SK, Marsters SA, Gordon NC, Starovasnik MA, Kelley RF. Engineering an APRIL-specific B cell maturation antigen. J Biol Chem. 2004;279:16727–16735. doi: 10.1074/jbc.M312316200. [DOI] [PubMed] [Google Scholar]
- 104.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pelayo R, Welner RS, Nagai Y, Kincade PW. Life before the pre-B cell receptor checkpoint: specification and commitment of primitive lymphoid progenitors in adult bone marrow. Semin Immunol. 2006;18:2–11. doi: 10.1016/j.smim.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 106.Pescovitz MD. B cells: a rational target in alloantibody-mediated solid organ transplantation rejection. Clin Transplant. 2006;20:48–54. doi: 10.1111/j.1399-0012.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 107.Pescovitz MD. Rituximab, an anti-CD20 monoclonal antibody: history and mechanism of action. Am J Transplant. 2006;6:859–866. doi: 10.1111/j.1600-6143.2006.01288.x. [DOI] [PubMed] [Google Scholar]
- 108.Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and the canonical NF-kappaB activation pathways by NF--kappaB-inducing kinase. Immunity. 2004;21:477–489. doi: 10.1016/j.immuni.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 109.Ramanujam M, Wang X, Huang W, Liu Z, Schiffer L, Tao H, Frank D, Rice J, Diamond B, Yu KO, Porcelli S, Davidson A. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;116:724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, Diamond B, Madaio MP, Davidson A. Mechanism of action of transmembrane activator and calcium modulator ligand inter-actor-Ig in murine systemic lupus erythematosus. J Immunol. 2004;173:3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 111.Rolink A, Melchers F. B-cell development in the mouse. Immunol Lett. 1996;54:157–161. doi: 10.1016/s0165-2478(96)02666-1. [DOI] [PubMed] [Google Scholar]
- 112.Rolink AG, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur J Immunol. 1998;28:3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 113.Sabahi R, Anolik JH. B-cell-targeted therapy for systemic lupus erythematosus. Drugs. 2006;66:1933–1948. doi: 10.2165/00003495-200666150-00004. [DOI] [PubMed] [Google Scholar]
- 114.Salzer U, Chapel HM, Webster AD, Pan--Hammarstrom Q, Schmitt-Graeff A, Schlesier M, Peter HH, Rockstroh JK, Schneider P, Schaffer AA, Hammarstrom L, Grimbacher B. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 115.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 116.Schittek B, Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990;346:749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 117.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–289. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 118.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shivakumar L, Ansell S. Targeting B-lymphocyte stimulator/B-cell activating factor and a proliferation--inducing ligand in hematologic malignancies. Clin Lymphoma Myeloma. 2006;7:106–108. doi: 10.3816/CLM.2006.n.046. [DOI] [PubMed] [Google Scholar]
- 120.Sprent J, Basten A. Circulating T and B lymphocytes of the mouse. II Lifespan. Cell Immunol. 1973;7:40–59. doi: 10.1016/0008-8749(73)90181-0. [DOI] [PubMed] [Google Scholar]
- 121.Sprent J, Bruce J. Physiology of B cells in mice with X-linked immunodeficiency (xid). III Disappearance of xid B cells in double bone marrow chimeras. J Exp Med. 1984;160:711–723. doi: 10.1084/jem.160.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stoddart A, Fleming HE, Paige CJ. The role of the preBCR, the interleukin-7 receptor, and homotypic interactions during B-cell development. Immunol Rev. 2000;175:47–58. [PubMed] [Google Scholar]
- 123.Stohl W. BlySfulness does not equal blissfulness in systemic lupus erythematosus: a therapeutic role for BLyS antagonists. Curr Dir Autoimmun. 2005;8:289–304. doi: 10.1159/000082108. [DOI] [PubMed] [Google Scholar]
- 124.Stohl W. A therapeutic role for BLyS antagonists. Lupus. 2004;13:317–322. doi: 10.1191/0961203304lu1019oa. [DOI] [PubMed] [Google Scholar]
- 125.Stohl W. Therapeutic targeting of B lymphocyte stimulator (BLyS) in the rheumatic diseases. Endocr Metab Immune Disord Drug Targets. 2006;6:351–358. doi: 10.2174/187153006779025801. [DOI] [PubMed] [Google Scholar]
- 126.Stohl W, Xu D, Kim KS, Koss MN, Jorgensen TN, Deocharan B, Metzger TE, Bixler SA, Hong YS, Ambrose CM, Mackay F, Morel L, Putterman C, Kotzin BL, Kalled SL. BAFF overexpression and accelerated glomerular disease in mice with an incomplete genetic predisposition to systemic lupus erythematosus. Arthritis Rheum. 2005;52:2080–2091. doi: 10.1002/art.21138. [DOI] [PubMed] [Google Scholar]
- 127.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 128.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 129.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Toubi E, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. The reduction of serum B-lymphocyte activating factor levels following quinacrine add-on therapy in systemic lupus erythematosus. Scand J Immunol. 2006;63:299–303. doi: 10.1111/j.1365-3083.2006.01737.x. [DOI] [PubMed] [Google Scholar]
- 131.Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, Cancro MP, Khan WN. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 132.Treml LS, Crowley JE, Cancro MP. BLyS receptor signatures resolve homeostatically independent compartments among naive and antigen-experienced B cells. Semin Immunol. 2006;18:297–304. doi: 10.1016/j.smim.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 133.Tremoulet AH, Albani S. Novel therapies for rheumatoid arthritis. Expert Opin Investig Drugs. 2006;15:1427–1441. doi: 10.1517/13543784.15.11.1427. [DOI] [PubMed] [Google Scholar]
- 134.Vallerskog T, Heimburger M, Gunnarsson I, Zhou W, Wahren-Herlenius M, Trollmo C, Malmstrom V. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2006;8:R167. doi: 10.1186/ar2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.von Bulow GU, Bram RJ. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science. 1997;278:138–141. doi: 10.1126/science.278.5335.138. [DOI] [PubMed] [Google Scholar]
- 136.von Bulow GU, Russell H, Copeland NG, Gilbert DJ, Jenkins NA, Bram RJ. Molecular cloning and functional characterization of murine transmembrane activator and CAML interactor (TACI) with chromosomal localization in human and mouse. Mamm Genome. 2000;11:628–632. doi: 10.1007/s003350010125. [DOI] [PubMed] [Google Scholar]
- 137.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–582. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 138.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 139.Xia XZ, Treanor J, Senaldi G, Khare SD, Boone T, Kelley M, Theill LE, Colombero A, Solovyev I, Lee F, McCabe S, Elliott R, Miner K, Hawkins N, Guo J, Stolina M, Yu G, Wang J, Delaney J, Meng SY, Boyle WJ, Hsu H. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192:137–143. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu LG, Shu HB. TNFR-associated factor-3 is associated with BAFF-R and negatively regulates BAFF-R-mediated NF-kappa B activation and IL-10 production. J Immunol. 2002;169:6883–6889. doi: 10.4049/jimmunol.169.12.6883. [DOI] [PubMed] [Google Scholar]
- 141.Xu S, Lam KP. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol Cell Biol. 2001;21:4067–4074. doi: 10.1128/MCB.21.12.4067-4074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S, Cancro M, Grewal IS, Dixit VM. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 143.Yan M, Wang H, Chan B, Roose-Girma M, Erickson S, Baker T, Tumas D, Grewal IS, Dixit VM. Activation and accumulation of B cells in TACI--deficient mice. Nat Immunol. 2001;2:638–643. doi: 10.1038/89790. [DOI] [PubMed] [Google Scholar]
Added in proof
- 144.Alinari L, Lapalombella R, Andritsos L, Baiocchi RA, Lin TS, Byrd JC. Alemtuzumab (Campath--1H) in the treatment of chronic lymphocytic leukemia. Oncogene. 2007;26:3644–3653. doi: 10.1038/sj.onc.1210380. [DOI] [PubMed] [Google Scholar]
- 145.Ansell SM, Horwitz SM, Engert A, Khan KD, Lin T, Strair R, Keler T, Graziano R, Blanset D, Yellin M, Fischkoff S, Assad A, Borchmann P. Phase I/II study of an anti-CD30 monoclonal antibody (MDX--060) in Hodgkin’s lymphoma and anaplastic large-cell lymphoma. J Clin Oncol. 2007;25:2764–2769. doi: 10.1200/JCO.2006.07.8972. [DOI] [PubMed] [Google Scholar]
- 146.Ding C, Jones G. Belimumab Human Genome Sciences/Cambridge Antibody Technology//GlaxoSmithKline. Curr Opin Invest Drugs. 2006;7:464–472. [PubMed] [Google Scholar]
- 147.El-Habbash MM, Alwindi AM. Progress in immunotherapy rituximab. Saudi Med J. 2007;28:1635–1644. [PubMed] [Google Scholar]
- 148.Fietz T, Thiel E. Antibody therapy in non--Hodgkin’s lymphoma: the role of rituximab, 90Y-ibritu-momab tiuxetan, and alemtuzumab. Recent Results Cancer Res. 2007;176:153–163. doi: 10.1007/978-3-540-46091-6_13. [DOI] [PubMed] [Google Scholar]
- 149.Gottlieb AB, Kang S, Linden KG, Lebwohl M, Menter A, Abdulghani AA, Goldfarb M, Chieffo N, Totoritis MC. Evaluation of safety and clinical activity of multiple doses of the anti-CD80 monoclonal antibody, galiximab, in patients with moderate to severe plaque psoriasis. Clin Immunol. 2004;111:28–37. doi: 10.1016/j.clim.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 150.Law CL, Gordon KA, Collier J, Klussman K, McEarchern JA, Cerveny CG, Mixan BJ, Lee WP, Lin Z, Valdez P, Wahl AF, Grewal IS. Preclinical antilymphoma activity of a humanized anti--CD40 monoclonal antibody, SGN-40. Cancer Res. 2005;65:8331–8338. doi: 10.1158/0008-5472.CAN-05-0095. [DOI] [PubMed] [Google Scholar]
- 151.Munafo A, Priestley A, Nestorov I, Visich J, Rogge M. Safety, pharmacokinetics and pharmacodynamics of atacicept in healthy volunteers. Eur J Clin Pharmacol. 2007;63:647–656. doi: 10.1007/s00228-007-0311-7. [DOI] [PubMed] [Google Scholar]
- 152.Piccaluga PP, Rondoni M, Paolini S, Rosti G, Martinelli G, Baccarani M. Imatinib mesylate in the treatment of hematologic malignancies. Expert Opin Biol Ther. 2007;7:1597–1611. doi: 10.1517/14712598.7.10.1597. [DOI] [PubMed] [Google Scholar]
- 153.Reichert JM. Technology evaluation: lumiliximab, Biogen Idec. Curr Opin Mol Ther. 2004;6:675–683. [PubMed] [Google Scholar]
- 154.Schnell R, Borchmann P. SGN-30 (Seattle genetics) Curr Opin Mol Ther. 2006;8:164–172. [PubMed] [Google Scholar]


