Summary
Following tropic hormone challenge, steroidogenic tissues utilize PKA to phosphorylate unique subsets of proteins necessary to facilitate steroidogenesis. This includes the PKA-dependent expression and activation of the steroidogenic acute regulatory protein (STAR), which mediates the rate-limiting step of steroidogenesis by inducing the transfer of cholesterol from the outer to the inner mitochondrial membrane. Since both type I and type II PKA are present in steroidogenic tissues, we have utilized cAMP analog pairs that preferentially activate each PKA subtype in order to examine their impact on STAR synthesis and activity. In MA-10 mouse Leydig tumor cells Star gene expression is more dependent upon type I PKA, while the post-transcriptional regulation of STAR appears subject to type II PKA. These experiments delineate the discrete effects that type I and type II PKA exert on STAR-mediated steroidogenesis, and suggest complimentary roles for each subtype in coordinating steroidogenesis.
Keywords: steroidogenesis, STAR, cAMP-dependent protein kinase, PKA, cAMP, Leydig
Introduction
Following challenge with tropic hormone, steroidogenic tissues utilize activated cAMP-dependent protein kinase (PKA) to phosphorylate unique subsets of cellular proteins necessary to facilitate acute steroidogenesis (Pon and Orme-Johnson, 1986,Koroscil and Gallant, 1981,Neher et al., 1982). Important among these is the steroidogenic acute regulatory protein (STAR), a nuclearly-encoded, mitochondrially-targeted protein that is both expressed and activated in response to PKA (Stocco and Clark, 1996,Miller, 2007c). STAR is presently understood to play an indispensable role in acute steroidogenesis by inducing and regulating the rate-limiting step of the process, the transfer of cholesterol from the outer to the inner mitochondrial membrane, and disruption of this gene leads to the potentially lethal disease congenital lipoid adrenal hyperplasia (CLAH) (Miller, 2007b).
Steroidogenic cells govern steroid production by employing PKA signaling to regulate STAR expression and activity through multiple mechanisms. This occurs fundamentally at the level of gene expression, and Star mRNA transcription in steroidogenic tissues can increase within minutes in response to treatment with tropic hormones or cAMP analogs (Ariyoshi et al., 1998,Manna et al., 2004b). This is accomplished through a sophisticated array of transcription factors such as CRE-binding protein (CREB), c-Jun (JUN), steroidogenic factor-1 (SF-1), and GATA binding protein 4 (GATA4) that are recruited to the proximal Star promoter and confer both potent and exact cAMP-responsiveness (Manna et al., 2003,Stocco et al., 2005). Once transcribed, Star mRNA is also regulated by PKA as it is differentially polyadenylated in response to cAMP signaling. A shorter, more stable mRNA is transcribed under basal conditions, whereas cAMP stimulation induces a longer, but more ephemeral transcript (Duan and Jefcoate, 2007). PKA exerts an additional level of control over STAR once it is translated, since STAR itself is a substrate for PKA. The early studies leading to the discovery of STAR identified it as a phosphoprotein, and the sequence of STAR confirms the presence of at least two consensus PKA phosphorylation sites that are conserved among mammals (Pon et al., 1986,Arakane et al., 1997). Of these two sites, the phosphorylation of STAR within its cholesterol binding domain (serine 194 in mouse; 195 in humans) by PKA is essential in order to render the protein fully active in its capacity to support cholesterol transfer, and the mutation of serine 195 is one of many point mutations in human STAR that reportedly gives rise to congenital lipoid adrenal hyperplasia (Katsumata et al., 2000,Fleury et al., 2004).
PKA itself is a heterotetramer consisting of two regulatory subunits present as a dimer, and two catalytic subunits, each bound to one of the regulatory subunits (Taylor et al., 1990). The holoenzyme is inactive in the absence of cAMP, but the binding of cAMP to the dimer of regulatory subunits permits the release of active, monomeric catalytic subunits (Chin et al., 2002,Taylor et al., 1990). The regulatory subunits are encoded by four distinct genes that are grouped into two families, type I and type II, with each family having two different isoforms (Skalhegg and Tasken, 2000,Taylor et al., 1990). While each of the PKA regulatory subunits share the same general domain architecture, there are considerable variations among their amino acid sequences, and the individual family members show different patterns of localization within the cell as well as unique binding affinities for cAMP (Skalhegg and Tasken, 2000). Importantly, mixed family heterodimers composed of both a type I and a type II regulatory subunit do not appear to naturally occur in vivo, and thus holoenzymes are conventionally classified by the family of regulatory subunit that is present. Interestingly, both type I and type II PKA are present in the steroidogenic cells of the adrenal, ovary, and testis, as well as in steroidogenic cell lines such as MA-10 (mouse Leydig) and Y-1 (rat adrenalcortical) (Hunzicker-Dunn and Jungmann, 1978b,Gill and Garren, 1971,Lee et al., 1976,Dyson et al., 2008,Mantovani et al., 2008). The compartmentalization of these different isoforms of PKA appears to allow cAMP signaling to be focused to distinct subcellular locations; however, it is currently unknown whether steroidogenic tissues utilize distinct PKA isozymes to regulate the various aspects of STAR-mediated steroidogenesis.
One method for distinguishing the effects of type I and type II PKA has been to use cAMP analogs that preferentially activate specific subtypes of the kinase. The regulatory subunits of PKA each possess two cAMP binding sites positioned in tandem at the carboxy-terminus of the protein. While highly conserved among all the regulatory subunits, these binding sites are not equivalent in their affinities for cAMP, and are distinguished as sites A and B within each regulatory subunit. Futhermore, the relative affinities of the cAMP binding sites in regulatory subunits from type I PKA vary from those in type II. Numerous cyclic nucleotide analogs have been developed that show enhanced affinities for one or more of the cAMP-binding sites, and such analogs have been used to demonstrate a role for type I PKA in MA-10 and Y-1 cell steroidogenesis (Hipkin and Moger, 1991,Moger, 1991,Whitehouse and Abayasekara, 1994). More recently we demonstrated that type II PKA associated with the mitochondria can phosphorylate STAR, but the individual effects of type I and type II PKA were not closely examined. While earlier studies have revealed that the gross impact of type I and type II PKA on steroidogenesis varies across the steroidogenic tissues, two consistent observations have been made: first, type I PKA consistently stimulates acute steroidogenesis in all the tissues examined, and second, the compartmentalization of type I PKA within Leydig and granulosa cells appears to sensitize the response of these cells to cAMP generated after tropic hormone stimulation. We hypothesize that type I PKA signaling is closely paired to the induction and activation of STAR, and thus serves a fundamental role in regulating acute steroidogenesis. Here we have used pairs of cAMP analogs that can preferentially activate either type I or type II PKA to examine the individual roles these kinases serve in regulating the transcription, expression, and activation of STAR in MA-10 mouse Leydig tumor cells.
Materials and Methods
Chemicals and reagents
N6,2-dibutyryladenosine-3′,5′-cyclic monophosphate ((Bt)2cAMP), cAMP, phorbol 12-myristate 13-acetate (PMA), Triton X-100 protease inhibitor cocktail, and Waymouth MB/752 medium (Waymouth) were purchased from Sigma-Aldrich (St. Louis, MO). 8-piperidinoadenosine-3′,5′-cyclic monophosphate (PIP-cAMP), N6-mono-tert-butylcarbamoyl-adenosine-3′,5′-cyclic monophosphate (MBC-cAMP) and 8-(6-aminohexyl) aminoadenosine-3′,5′-cyclic monophosphate (AHA-cAMP) were purchased from BioLog (Bremen, Germany). TRIzol, PCR primers, trypsin-EDTA, tissue culture-grade antibiotics, PBS, OPTI-MEM I reduced serum medium, and horse serum were purchased from Invitrogen (Carlsbad, CA). DNase, AMV-reverse transcriptase, random hexamers, and TaqMan MasterMix were purchased from Applied Biosystems (Foster City, CA). RNasin, and other enzymes were purchased from Promega (Madison, WI).
Cell culture
Tissue culture plates and supplies were purchased from Nunc (Rochester, NY). MA-10 cells, provided by Dr. Mario Ascoli (Department of Pharmacology, University of Iowa College, Iowa City) were maintained in Waymouth medium containing 15% (v/v) heat-inactivated horse serum, and 40 μg/ml gentamicin. MA-10 cells were cultured at 37 °C in a humidified incubator with 5% CO2. In experiments with cAMP analogs or PMA stimulation, MA-10 cells were washed with PBS and replenished with serum-free Waymouth medium already containing the agonist(s).
Plasmids, transfections, and luciferase assays
The analysis of Star promoter activity was conducted in MA-10 cells cotransfected with -966 pGL3 and pRL-SV40. The promoter of the mouse Star gene has been sequenced before (Caron et al., 1997), and the 966 bp upstream of the transcriptional start site were cloned ahead of a firefly luciferase reporter in the pGL3 basic vector (Promega, WI) to generate -966 pGL3 as previously described (Wang et al., 2003). The pRL-SV40 plasmid expresses Renilla luciferase under the direction of the CMV immediate-early enhancer and promoter. MA-10 cells were transfected using Fugene HD transfection reagent (Roche Applied Science, Indianapolis, IN), and transfected cells were cultured for 36 hours prior to treatment. Stimulated cells were rinsed with PBS and cell lysates assayed for luciferase activity using a dual-luciferase reporter assay (Promega). Luminescence from each form of luciferase was measured for 10 seconds using a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). Promoter activity is reported here as normalized relative luciferase units (RLU) and represents the ratio of firefly luciferase luminescence to that of Renilla luciferase.
RNA extraction and RT-PCR
Semi-quantitative real time PCR was performed as previously described (Kowalewski et al., 2006). Briefly, total RNA was isolated from cultured cells using TRIzol reagent according to the directions supplied. Genomic DNA contamination was removed by DNase treatment. After heat inactivation of the DNase, cDNA was prepared from the total mRNA using reverse transcription reagents for TaqMan, from Applied Biosystem. The following oligos were designed as probes and primers to detect the cDNAs for Star and Gapdh:
Star (forward): 5′-CCGGGTGGATGGGTCAA-3′
Star (reverse): 5′-CACCTCTCCCTGCTGGATGTA-3′
Star (TaqMan): 5′-CGACGTCGGAGCTCTCTGCTTGG-3′
Gapdh (forward): 5′- GCAGTGGCAAAGTGGAGATTG-3′
Gapdh (reverse): 5′-GTGAGTGGAGTCATACTGGAACATG -3′
Gapdh (TaqMan): 5′-TCAACGACCCCTTCATTGACCTC -3′
TaqMan probes were synthesized with 6-carboxyflorescein as a fluorophore on their 5′ ends and 6-carboxytetramethyl-rhodamine as a quencher dye on their 3′ ends (Eurogentec North America, San Diego, CA). Duplicate reactions were performed on cDNA prepared from 50 ng of total mRNA (45 cycles; 95 C for 15 s, 60 C for 60 s) using an ABI PRISM 7000 (Applied Biosystems). The relative gene expression for Star was determined by normalizing against Gapdh expression using the comparative CT method (Livak and Schmittgen, 2001,Kowalewski et al., 2006). Real time PCR results are expressed as the fold-change in Star mRNA levels relative to untreated controls.
Western blot analysis
Whole cell lysates for Western blot analysis were prepared as previously described (Ishigaki et al., 2001). Total protein concentrations were calculated using Bio-Rad Protein microassays (Hercules, CA). SDS-PAGE was performed as previously described (Manna et al., 2002). Primary antibodies directed against JUN (sc-1694), GATA4 (sc-9053), and actin (sc-1616) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-CYP11A1 antibody was from Chemicon (Temecula, CA). Primary antibodies directed against CREB (9197), phospho-CREB (9196S), and phospho-JUN (9261S), were purchased from Cell Signaling Technology (Boston, MA). Total STAR was detected as previously described (Clark et al., 1994), while the rabbit antibody for the detection of phosphorylated STAR (phospho-STAR) (Jo et al., 2005) was a gift from Dr. Steven King (Scott Department of Urology, Baylor College of Medicine, Houston, TX). Donkey anti-rabbit IgG and Donkey anti-goat IgG conjugated to HRP, as well as SuperSignal West chemiluminescent substrates were obtained from Pierce Biotechnology (Rockford, IL). Anti-mouse IgG conjugated to HRP was purchased from Promega.
Radioimmunoassay (RIA)
The synthesis of progesterone from treated MA-10 cells was quantified by examining the recovered medium by RIA as previously described (Resko et al., 1974). Progesterone concentrations were normalized to total cellular protein and expressed as pg of progesterone per μg of protein.
PKA Activity
The levels of PKA activity in MA-10 cells were assessed using the SignaTECT PKA assay system (Promega). Following culture in 6-well dishes, MA-10 cells were washed rapidly 3 times with cold PBS and then scraped in 160 mL of extraction buffer (25 mM Tris-HCl (pH 7.4), 0.5 mM EDTA, 0.5 mM EGTA, 10 mM β-mercaptoethanol, 10 μl/ml protease inhibitor cocktail, 0.1% Triton X-100). The suspended cells were sonicated on ice as above, but for 10 s, and then centrifuged at 14,000 × g for 5 min at 4°C. From each of the resulting supernatants, 5 μl was added immediately and without dilution to 20 μl of kinase reaction mixture that contained no additional cAMP. Reactions were incubated at 30°C for 7 min and terminated as directed. Reactions were processed further in accordance with the manufacturer's instructions, and the recovered streptavidin membrane was analyzed by liquid scintillation.
Data analysis
The Western blots examining the dose response of MA-10 cells to the individual cAMP analogs were performed in duplicate. All other experiments were repeated independently a minimum of three times, as indicated in their respective figure legends. A representative Western blot for each experiment is shown, while densitometry data from these analyses were normalized against loading controls and then expressed as the mean fold change relative to the negative controls that were arbitrarily set to 1.0 (for STAR and phospho-STAR, Western data are expressed as the fold change relative to (Bt)2cAMP treated controls). RIA data are expressed as the means ± SEM. Statistical analysis of multiple groups was modeled by ANOVA and pairwise comparisons were made using Fisher's protected least significant difference test. Groups denoted with asterisks or lower case letters within the figures were determined to be statistically different from the other groups for p<0.05.
Results
The dose-dependent effects of cAMP analogs on STAR expression and steroidogenesis
Unlike many other steroidogenic tissues and cell lines (where the induction of acute steroidogenesis occurs within minutes in response to cAMP), MA-10 cells have little basal expression of STAR, and previous studies in this cell line show that cAMP analogs induce maximal STAR expression within 6 h (Stocco and Sodeman, 1991,Wang et al., 2002). Three analogs of cAMP were used in experiments to preferentially activate either type I or type II PKA. PIP-cAMP preferentially binds site A of PKAR1 and site B of PKAR2, and can complement other analogs to cooperatively activate either form of PKA (Skalhegg et al., 1992,Christensen et al., 2003). AHA-cAMP, which prefers site B of PKAR1, or MBC-cAMP, which binds to site A of PKAR2, were used in combination with PIP-cAMP to preferentially activate their respective PKA isoforms (Christensen et al., 2003,Maronde et al., 1999). All of the analogs used here are more lipophilic than cAMP and can cross the cell membrane. To establish the highest concentration at which each analog would predominantly occupy only its preferred site, the impact of the individual analogs on STAR expression and steroidogenesis was evaluated. Increasing concentrations of each analog were used to treat MA-10 cells for 6 h, after which medium and cells were collected for analysis.
Concentrations of AHA-cAMP or MBC-cAMP as high as 25 μM did not significantly increase STAR expression or steroidogenesis (Fig. 1). In contrast 50 μM of either analog increased Star mRNA by over four-fold (Fig. 1A) and produced detectable increases in STAR protein (Fig. 1B). Progesterone synthesis was also significantly increased relative to the controls when using 50 μM concentrations of either of these analogs (Fig. 1C). Notably, 50 μM AHA-cAMP treatment induced steroid than 50 μM MBC-cAMP. These increases in STAR and steroidogenesis indicate a threshold concentration between 25 and 50 μM above which both AHA-cAMP and MBC-cAMP become competent to individually activate PKA. No such threshold appeared for PIP-cAMP within the range of concentrations tested. STAR expression and steroidogenesis in MA-10 cells showed little response to PIP-cAMP at concentrations as high as 200 μM (data not shown), fitting with previous studies showing that PIP-cAMP alone does not efficiently activate PKA (Ogreid et al., 1985). Based upon these results, 100 μM PIP-cAMP was used in conjunction with AHA-cAMP or MBC-cAMP to preferentially activate type I or type II PKA.
Figure 1. The dose response relationship between AHA-cAMP, MBC-cAMP and STAR-mediated steroidogenesis in MA-10 cells.
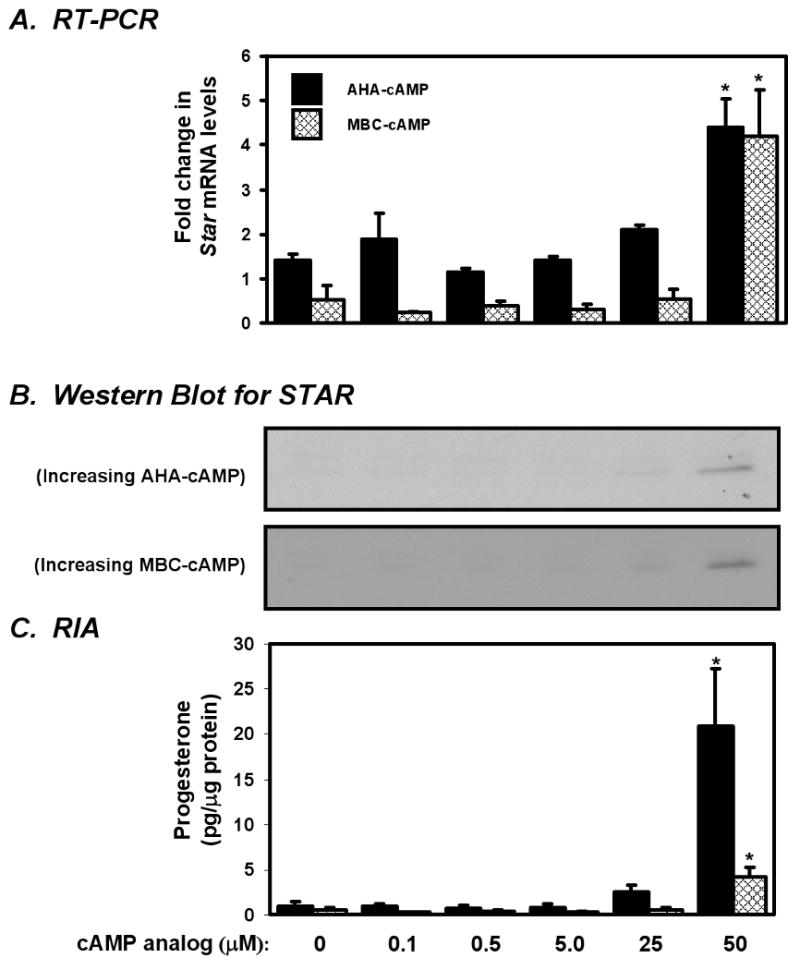
MA-10 cells were cultured for 6 h with increasing concentrations of AHA-cAMP or MBC-cAMP to determine the threshold concentration at which this analog independently activates PKA. (A) Star mRNA levels were determined by real time PCR and reported as the fold change relative to unstimulated controls. (B) STAR and CYP11A1 protein levels were examined by Western blot analysis. (C) Progesterone concentrations in recovered media were determined by RIA and reported ± SEM. Statistically significant increases in mRNA abundance and progesterone levels within dose response groups are denoted with an asterisk (*). 25 μM of either AHA-cAMP or MBC-cAMP was the highest concentration tested that did not induce STAR or steroidogenesis, and this concentration was not exceeded in subsequent experiments.
The relative effects of type I and type II PKA analog pairs on PKA activity in MA-10 cells
Using the threshold concentrations defined above, we evaluated the levels of PKA activity initiated by the analog pairs. Since the peak in PKA activity induced from the analogs would occur prior to the expression of STAR, we needed to establish the proper time frame for observing PKA activity. To do this we examined the effect of 300 μM (Bt)2cAMP on the specific kinase activity of PKA in MA-10 cells at different time points over a period of 4 h (Fig. 2A). The cAMP analog (Bt)2cAMP can activate both isoforms of PKA, and 300 μM (Bt)2cAMP can induce STAR expression and steroidogenesis in MA-10 cells to about half of their maximum potential within 4 to 6 h (Stocco and Sodeman, 1991,Makarevich et al., 2004,Wang et al., 2002). PKA activity was lowest at the initial time point and then rose steeply during the first hour, reaching a maximum by 2 h. Activity then remained steady at this level for the duration of the time course. To examine the effects of the analog pairs on PKA activity we chose to use a 2 h incubation, during which time MA-10 cells were treated with 100 μM PIP-cAMP in conjunction with either 25 μM AHA-cAMP (for type I) or 25 μM MBC-cAMP (for type II). As before 300 μM (Bt)2cAMP was included as reference (Fig. 2B). Preferential activation of type I PKA resulted in a 6.6-fold increase in PKA activity relative to the untreated controls, while the type II analog pair yielded a 4.0-fold increase. As an additional control, a group of cells was treated with PIP-cAMP, AHA-cAMP, and MBC-cAMP in order to activate both type I and II PKA simultaneously. This treatment resulted in an 11.4-fold increase in PKA activity relative to controls, levels which were effectively the same as observed with (Bt)2cAMP. Notably, the type I and type II analog pairs induced significantly less PKA activity than (Bt)2cAMP. The PKA activity induced by the type I pair was about 59% of that from (Bt)2cAMP (p<0.001). The type II analog pair induced levels substantially lower that both (Bt)2AMP (35%; p<0.0001) and the type I analog pair (60%; p<0.05). We conclude from these data that while both the type I and type II analog pairs can synergistically activate PKA, at these concentrations they are not activating equivalent levels of the kinase. Despite their lipophilicity, these analogs appear to take substantially longer to elicit their activation potential in the cell relative to what is typically observed using tropic hormone. It is presently unclear if this is due to slow penetration into the cells, or due to these analogs having reduced affinities for PKA relative to endogenous cAMP.
Figure 2. The effects of cAMP analogs on PKA activity in MA-10 cells.
(A) The effect of 300 μM (Bt)2cAMP on PKA activity on cultured MA-10 cells is shown over a period of 4 h (n=3). (B) PKA activity is shown for MA-10 cells cultured for 2 h with either the type I (100 μM PIP-cAMP, 25 μM AHA-cAMP) or type II (100 μM PIP-cAMP, 25 μM MBC-cAMP) analog pair, or a mixture of all three analogs at the same concentrations. 300 μM (Bt)2cAMP serves as a positive control. Lower case letters are used to designate groups that are significantly different from each other (a, b, c; n=4).
Activation of type I PKA using pairs of cAMP analogs induces STAR expression
To compare the relative effectiveness of the cAMP analogs pairs at inducing STAR-mediated steroidogenesis, we performed 6 h dose-response experiments using the type I analog pair (AHA-cAMP and PIP-cAMP) and the type II analog pair (MBC-cAMP and PIP-cAMP) where the concentration of PIP-cAMP was fixed at 100 μM and the complementary analog for each pair was then included at increasing concentrations up to 25 μM. As a reference, an additional group of cells was treated with 300 μM (Bt)2cAMP (Fig. 3). The type I analog pair proved effective at stimulating Star gene expression, STAR protein synthesis, and steroidogenesis in a dose-responsive manner. In comparison, the type II analog pair did not significantly increase any of these parameters. Consequently, type I PKA appeared more effective at inducing STAR-mediated steroidogenesis than type II PKA. While it is possible that the concentrations or durations of analogs used to activate type II PKA were insufficient, it should be noted that these and lower concentrations were effective at activating PKA activity as shown in figure 2.
Figure 3. The dose response relationship between pairs of cAMP analogs and STAR-mediated steroidogenesis in MA-10 cells.

MA-10 cells were cultured for 6 h in 100 μM PIP-cAMP along with increasing concentrations of either AHA-cAMP, to activate type I PKA, or MBC-cAMP, to activate type II PKA. 300 μM (Bt)2cAMP serves as a positive control. (A) Real time PCR results show Star mRNA levels relative to the unstimulated control. (B) STAR and CYP11A1 protein levels were examined by Western blot analysis. (C) Progesterone concentrations in recovered media were determined by RIA and reported ± SEM. For differences in mRNA or progesterone levels, lower case letters are used to designate groups that are significantly different from each other (a, b, c; n=5). Only the type I pair induced a steroidogenic response.
The differential effects of type I and type II PKA on STAR expression
To more specifically evaluate which elements in the synthesis and regulation of STAR were being preferentially induced by type I PKA we treated MA-10 cells for 6 h with same analog pair concentrations used in figure 2 and proceeded to examine the transcriptional activity of the Star promoter and the phosphorylation status of the STAR protein, along with the effects these compounds have on Star gene expression and steroidogenesis. The results from promoter assays following transient transfection of the -966 pGL3 construct showed that (Bt)2cAMP induced a 5.9-fold increase in the activity of the Star promoter (Fig. 4A). Treatment with the type I analog pair gave rise to a 3.9-fold increase in promoter activity while the type II pair did not significantly induce activity beyond the unstimulated controls. Stimulation with the combined mixture of type I and type II analogs elicited a 5.1-fold increase in Star promoter activity. The expression of Star mRNA closely paralleled the pattern of promoter activity generated by these treatments (Fig. 4B). Relative Star gene expression following treatment with the type I pair increased by more than 10-fold over the unstimulated controls which corresponded to about 63% of the levels seen in the (Bt)2cAMP group (p<0.003). The type II pair did not induce significant Star mRNA expression relative to unstimulated controls, and the Star mRNA levels following the use of both type I and type II analogs together was nearly identical to what was seen using the type I pair alone. The results from these promoter assays and RT-PCR studies indicate that type I PKA stimulates the transcription of the Star gene more efficiently than type II PKA.
Figure 4. The differential effects of type I and type II PKA on STAR expression and activation in MA-10 cells.
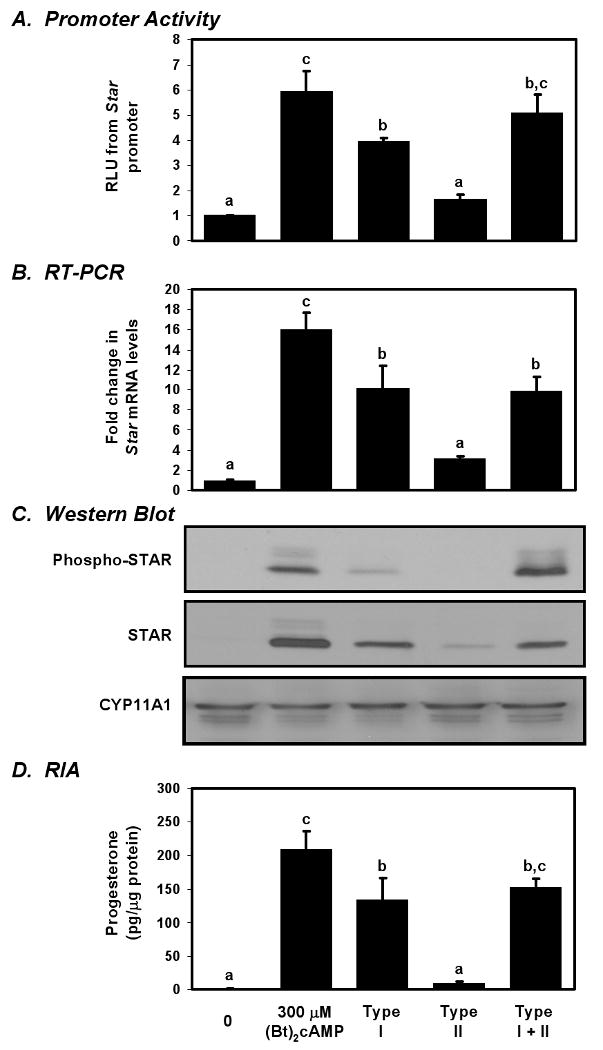
MA-10 cells were cultured for 6 h with either the type I (100 μM PIP-cAMP, 25 μM AHA-cAMP) or type II (100 μM PIP-cAMP, 25 μM MBC-cAMP) analog pair, or a mixture of all three analogs at the same concentrations. 300 μM (Bt)2cAMP serves as a positive control. (A) Star promoter activity was determined using a luciferase assay and reported as the fold change relative to unstimulated controls. (B) RT-PCR results show Star mRNA levels relative to the unstimulated control. (C) STAR, phospho-STAR, and CYP11A1 protein levels were examined by Western blot analysis. (D) Progesterone concentrations in recovered media were determined by RIA and reported ± SEM. For differences in mRNA or progesterone levels, lower case letters are used to designate groups that are significantly different from each other (a, b, c; n=4). Type I PKA better induced STAR expression while type II PKA appeared to phosphorylate STAR.
Using an antibody that discerns phosphorylated STAR, we examined the effects of the different analog pairs on STAR phosphorylation to compare to total STAR expression (Fig. 4C). The pattern of STAR expression using (Bt)2cAMP, and the individual type I and type II pairs was the similar to what we observed during the dose response experiment. Interestingly, MA-10 cells treated with a combination of both the type I and type II analog pairs expressed approximately the same amount of STAR as cells treated with only the type I pair. We were surprised, however, to see that activating type I PKA did not induce phospho-STAR levels as effectively as it could induce total STAR protein (Fig. 4C). While the type I pair consistently induced total STAR levels to over 50% of that seen in the (Bt)2cAMP positive control (p<0.0001), the levels of phospho-STAR generated in response to the type I analog pair were only 15% of the positive control (p<0.0001). More intriguing was the impact of type II PKA. Like total STAR, phospho-STAR was scarcely detectable following treatment with the type II PKA analog pair alone; however, combining the type I and type II pairs increased phospho-STAR levels above what either pair could do alone (p<0.0001), and matched the abundance seen in the (Bt)2cAMP control group. From this we surmised that while type I PKA more directly impacts STAR synthesis, type II PKA may serve a role in regulating the phosphorylation of STAR.
Steroid production in these experiments more closely paralleled the abundance of total STAR than phospho-STAR. As seen above, (Bt)2cAMP and the type I analog pair induced significant progesterone production, although the type I pair generated only 64% as much progesterone as (Bt)2cAMP (Fig. 4D; p<0.02). While activating type II PKA alone did not generate significant levels of steroidogenesis, combining the type I and type II analog pairs induced steroid production to an intermediate level between what was seen using the type I analog pair alone and the (Bt)2cAMP group. While these findings might further hint of a role for type II PKA in regulating the phosphorylation of STAR, the lack of correlation between phospho-STAR and steroidogenesis when type I PKA is activated was perplexing. Additionally, this suggests that another factor sensitive to type I PKA is capable of strongly enhancing steroidogenesis.
JUN and CREB are preferentially phosphorylated by type I PKA
Since type I PKA appeared to better induce Star gene transcription, it seemed plausible that the transcription factors governing the Star promoter could be regulated by type I PKA. As a brief test, we examined the phosphorylation status of JUN, CREB, and GATA4. While these are not the only factors regulating Star expression, these transcription factors are well studied, and known to play key roles in the Star gene transcription. MA-10 cells were stimulated with either (Bt)2cAMP or the analog pairs, and the change in phosphorylation status of each transcription factor was distinguished by Western blotting. All three transcription factors were readily observed in unstimulated MA-10 cells, and the relative expression of each transcription factor was not altered in response to any of the treatments (Fig. 5). Phospho-CREB and phospho-JUN were strongly induced following treatment with either (Bt)2cAMP or the type I analog pair. The type II analog pair induced phospho-CREB and phospho-JUN to levels higher than seen in the unstimulated controls, but was not as effective as (Bt)2cAMP or the type I pair. In contrast, all three stimulations appeared to generate equivalent amounts of phospho-GATA4. While this does not define a specific mechanism by which different subtypes of PKA coordinate transcriptional machinery at the Star promoter, these results reflect how a unique subset of transcription factors including JUN and CREB could be targeted for phosphorylation by type I PKA.
Figure 5. The effects of type I and type II PKA on transcription factors targeting the Star promoter.

MA-10 cells were cultured for 6 h with either 300 μM (Bt)2cAMP, the type I PKA agonists (100 μM PIP-cAMP, 25 μM AHA-cAMP), or the type II PKA agonists (100 μM PIP-cAMP, 25 μM MBC-cAMP). The expression of CREB, JUN, and GATA4, as well as the phosphorylation status of each transcription factor was examined by Western blot analysis. PKA activation did not alter the expression of these proteins, but type I PKA phosphorylated JUN and CREB more efficiently than type II PKA. GATA4 phosphorylation increased with both type I and type II PKA. (n=3)
STAR is preferentially phosphorylated by type II PKA
It was previously shown that activating protein kinase C (PKC) with PMA can effectively support Star transcription and translation in MA-10 cells; however, under these conditions steroid production does not occur without the additional activation of PKA due to the inability of PKC to phosphorylate and activate STAR (Jo et al., 2005). Capitalizing on this phenomenon, the same analog pairs that we have been presently using alone were used in conjunction with PMA to discriminate whether type I or type II PKA is more effective in phosphorylating STAR. A portion of our previously published data (Dyson et al., 2008) has been reproduced with permission in figure 6 in order to demonstrate the specific effects of type II PKA in activating STAR, and we have extended these findings to include new data demonstrating the impact these treatments have on Star gene expression. To minimize the impact of PKA on the Star promoter we chose to use either 0.5 μM AHA-cAMP or MBC-cAMP in conjunction with 100 μM PIP-cAMP in order to preferentially activate either type I or type II PKA, respectively. At these concentrations the type I and type II analogs pairs have little impact on Star gene expression, producing a 1.6-fold increase in Star mRNA that was not significantly different from unstimulated controls (Fig. 6A, lanes 4 and 6). Additionally, these concentrations were incapable of independently inducing STAR expression or eliciting a steroidogenic response in MA-10 cells after six hours (Fig. 6, lanes 4 and 6). In comparison, MA-10 cells treated with either 10 nM PMA or 300 μM (Bt)2cAMP produced STAR, but PMA-induced STAR was not phosphorylated, as seen in whole cell lysates (Fig. 6B). When used in combination with PMA, the type I pair did not enhance Star mRNA levels, STAR expression, phosphorylation, or activity beyond what was seen with PMA alone (Fig.6, lanes 3 and 5). The type II pair, however, resulted in the phosphorylation of PMA-induced STAR to levels comparable with those seen in the group treated with 0.3 mM (Bt)2cAMP (Fig. 6B), and stimulated about half as much progesterone (Fig. 8C). Notably, the steroidogenic response from cells treated with PMA and the type II analog pair was 6.2 times greater than when PMA was used with the type I pair (Fig. 6C, lanes 5 and 7, p<0.001). In this context, type II PKA appears more effective at phosphorylating STAR than type I PKA.
Figure 6. The induction of STAR phosphorylation in response to type I or type II PKA.

MA-10 cells were treated for six hours with pairs of cAMP analogs that preferentially activate type I or type II PKA using concentrations that alone could not induce STAR synthesis or steroidogenesis. These pairs were used either alone or in conjunction with PMA (lanes 4-7). Untreated cells (lane 1), (Bt)2cAMP stimulated cells (lane 2), and cells treated with PMA alone (lane 3) are shown as controls. (A) RT-PCR results show Star mRNA levels relative to the unstimulated control. (B) Western blots on whole cell lysates are shown for STAR, phospho-STAR, and actin. (C) The average progesterone concentrations in medium from each group after stimulation is shown ±SEM. For mRNA levels and progesterone production, lower case letters are used to designate the groups that are statistically different from each other (a, b, c; n=4; panels B and C were reproduced with permission from (Dyson et al., 2008)). PMA induces STAR but not its phosphorylation. Only type II PKA appeared to phosphorylate STAR
Discussion
Understanding the central role that cAMP-signaling plays in the adrenal and the gonads has been crucial to developing the current theories regarding how steroidogenesis is regulated. In particular, the discovery and characterization of STAR stemmed from novel breakthroughs related to PKA. This current work delineates how the two primary subtypes of PKA are involved in regulating STAR-mediated steroidogenesis in MA-10 Leydig cells. While we and others have supposed that type I PKA is primarily responsible for regulating the synthesis and activation of STAR, our current data suggest that type I and type II PKA serve distinct but complementary roles that coordinate the production of biologically active STAR in response to cAMP. Specifically, type I PKA appears to operate upstream of STAR, by activating the transcription factors necessary to induce Star transcription, while type II PKA appears to be responsible for phosphorylating and activating STAR, and this is summarized in figure 7.
Figure 7. The roles of type I and type II PKA in regulating STAR.
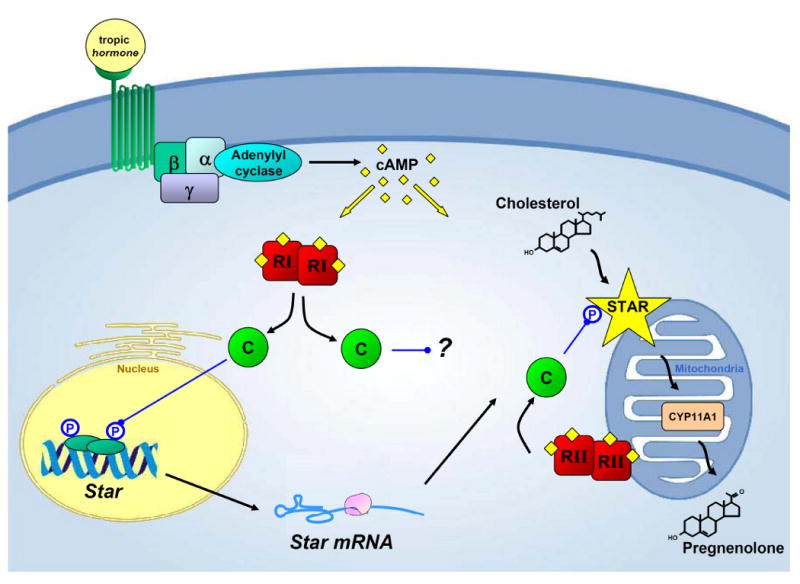
In response to tropic hormone, G-protein coupled receptors activate adenylate cyclase to generate cAMP. In turn, cAMP appears to activate both PKA subtypes, but each subtype preferentially fulfills distinct tasks necessary for steroidogenesis to proceed. Type I PKA appears more capable of inducing the Star gene, which leads to the synthesis of new STAR protein, while type II PKA serves a more prominent role in phosphorylating STAR near or at the outer mitochondrial membrane, ensuring maximal STAR activity. While the mechanism remains unclear, type I PKA also appears to serve an additional role that specifically and potently enhances steroidogenesis, possibly though modulating cholesterol trafficking in the cell. (RI/RII = type I/type II PKA regulatory subunits, C = PKA catalytic subunits, CYP11A1=p450 side chain cleavage)
The transcriptional regulation of Star largely dictates both the quantity and tissue-specific distribution pattern of STAR expression, and signaling through cAMP indisputably plays a key role in regulating this process (Clark et al., 1995,Kallen et al., 1998,Caron et al., 1997). While several cAMP-independent signaling pathways can potently enhance Star gene transcription, the recurring observation has been made that a minimal level of PKA activity is necessary to induce the Star promoter (Stocco et al., 2005). Although this tacitly suggests an obligatory role for cAMP signaling in the induction of Star transcription, it does not prove that PKA activity is a necessity. More likely, the varying degrees of cAMP-dependency demonstrate that PKA intercedes at multiple points in regulating Star promoter activity, and that some regulatory elements of the Star promoter are more sensitive to PKA than are others. One proposed explanation for such a phenomenon is that PKA can be compartmentalized within the cell prior to its activation by cAMP, substantially increasing the opportunity for the kinase to phosphorylate its nearest substrates (Stocco and Chaudhary, 1990,Hunzicker-Dunn and Jungmann, 1978a,Moger, 1991,Wong and Scott, 2004). The organization and positioning of PKA is presently known to be heavily influenced by A kinase anchoring proteins, which can target distinct forms of PKA to specific locations within the cell (Feliciello et al., 2001). Based on our findings here the transcriptional regulation of Star appears to be much more sensitive to the actions of type I PKA than type II, and this may be partly due the activation of several key transcription factors by type I PKA. Namely, CREB and JUN both appeared to be phosphorylated more effectively in response to cAMP analogs that prefer type I PKA. While we have not examined the sub-cellular organization of PKA in MA-10 cells in these studies, it has recently been shown that the compartmentalization of type I and type II PKA in neuroblastoma × glioma hybrid cells results in the differential phosphorylation of CREB and CREB-binding protein (CBP), and this mechanism is hypothesized to play a role in CRE-mediated gene expression in the nucleus accumbens (Constantinescu et al., 2002,Constantinescu et al., 2004). In the Leydig cell the interplay between in CREB, JUN and CBP are involved in conferring cAMP-responsiveness to the Star promoter, and the phosphorylation of all three of these proteins is known to accompany hormone-induced steroidogenesis (Manna and Stocco, 2007,Manna et al., 2004a,Manna et al., 2003). These results build upon earlier observations in Leydig cells where cAMP analogs preferring type I PKA synergized with LH and forskolin to activate steroidogenesis (Hipkin and Moger, 1991,Moger, 1991). Based upon these observations, it is possible that type I PKA is positioned either in or near the nucleus and that these kinases are responsible for mediating cAMP-induced signals to the nucleus.
In comparing the effects of the analog pairs, it is clear that the activation of type I PKA also results in a significant increase in STAR protein expression relative to what was seen following the activation of type II PKA. This increase, however, appears to correlate with the increased availability of Star mRNA rather than being due to a change in translational efficiency. In the work presented here we have not directly assessed the independent roles of type I and type II PKA on the translation of STAR since it is likely that endogenous levels of cAMP are sufficient to permit translation of Star mRNA. The growing number of transfection studies where Star cDNA has been successfully expressed in multiple cell types without further manipulation suggests there is no need for elevated PKA activity in order to properly translate Star mRNA (Clark et al., 1994,Bose et al., 2002,Zhao et al., 2005,King and Stocco, 1996). More compelling evidence has come from observations where MA-10 cells treated with PMA show both enhanced transcription and translation of STAR protein. PMA, which decreases cAMP levels in both adrenocortical cells and in Leydig cells (Fong and Wang, 1997,Chaudhary and Stocco, 1988), also leads to the robust activation of the PKC pathway that is now known to also drive Star gene expression (Jo et al., 2005). Thus in the experiments where PMA is used alone, STAR is effectively translated despite reduced cAMP concentration. While these observations do not eliminate the possibility that PKA is regulating STAR translation, they do suggest that STAR translation is not dependent upon further PKA activation in MA-10 cells.
An interesting result from our current work was the discovery that STAR may be predominantly phosphorylated by type II PKA rather than type I PKA. This result stands in contrast to earlier reports suggesting that type I PKA relays cAMP signaling to the steroidogenic machinery in Leydig cells (Moger, 1991). To specifically address this issue, we examined how the analog pairs affect the phosphorylation of PMA-induced STAR expression. Although both PKA subtypes are readily detected at the mitochondria of MA-10 cells (Liu et al., 2006,Dyson et al., 2008), type II PKA appears to be far more effective at phosphorylating STAR. Consequently, under conditions where STAR availability is not rate-limiting (i.e. where PMA was used to drive STAR synthesis), the activation of type II PKA permitted more steroid hormone synthesis than the stimulation of type I PKA. This observation dovetails nicely with our previous work examining the compartmentalization of PKA in Leydig cells, and supports the hypothesis that type II PKA localized to the mitochondria is important for the phosphorylation of STAR. In previous studies we and others have suggested that AKAPs may recruit PKA to the outer mitochondrial membrane to ensure the efficient phosphorylation of STAR, and we have been able to successfully demonstrate a physical association between mitochondrial AKAP121 and type II PKA regulatory subunits (Liu et al., 2003,Carlson et al., 2006,Dyson et al., 2008). Furthermore, studies by Liu et al have shown that peptides known to bind type II PKA and disrupt their ability to bind their cognate AKAPs can block hormone-induced steroidogenesis in MA-10 cells (Liu et al., 2006). Fitting with these previous observations, our present suggests that the post-translational activation of STAR is mediated by type II PKA anchored to the mitochondria.
Given the seemingly discrete effects initiated by type I and type II PKA on STAR expression and activity, it was intriguing to see that type I PKA was able to induce significant levels of steroidogenesis when STAR itself was not proportionally phosphorylated. While it is possible that the type I analog pair is non-specifically activating type II PKA, several of our observations here suggest that type I PKA is functioning in an additional capacity related to acute steroidogenesis that is yet to be defined. For example, although the type II PKA agonists more readily activated STAR and permitted greater steroidogenic output than the type I pair when used in conjunction with PMA, type II PKA did not appear to induce high levels of steroidogenesis despite having produced levels of STAR and phospho-STAR comparable to those seen following treatment with (Bt)2cAMP, suggesting that some other factor was still rate-limiting. Moreover, the combined use of the type I and type II analog pairs increased the phosphorylation of STAR, but without a corresponding increase in steroidogenesis. This latter observation is made more interesting by our PKA activity data which shows that the combined use of all the analogs increased PKA-activity above what either pair did alone. Although we activated type II PKA and allowed more STAR to be phosphorylated, the small about of phospho-STAR that was produced by the type I pair alone appeared sufficient for steroidogenesis. Consequently, the phosphorylation of STAR by type II PKA may be more of a permissive event, than a graded event. Furthermore, this leads us to question what other events type I PKA could be regulating in order to initiate steroidogenesis.
One interpretation of the data presented in figures 2 and 4 is that type I PKA regulates other events that impinge upon the ability of STAR to induce cholesterol transfer. Thus, even when STAR is phosphorylated in the group treated with all three analogs (AHA-, MBC-, and PIP-cAMP) to activate both types of PKA, and even though total PKA activity is greater, no additional type I PKA has been activated relative to the group treated with the type I analog pair alone. While we have not fully investigated this observation, preliminary studies in our lab suggest that type I PKA agonists more effectively phosphorylate hormone sensitive lipase (HSL) in MA-10 cells. In response to PKA and other signaling pathways, HSL regulates the hydrolysis of acylglycerides and cholestryl esters and serves numerous roles in regulating lipid metabolism including steroidogenesis (Belfrage et al., 1981,Stralfors and Belfrage, 1983,Yeaman, 2004). The effect HSL has on steroidogenesis has been best studied in the adrenal, where ACTH- and (Bt)2cAMP-stimulated steroidogenesis are strongly reduced in Lipe -/- mice (Kraemer et al., 2004,Kraemer et al., 2007). Additional insight into the role of HSL in steroidogenesis came following the discovery that it physically interacts with STAR in the adrenal, an interaction which increases cholesterol ester hydrolase activity of HSL (Shen et al., 2003). It is feasible that HSL is serving in a similar capacity in the Leydig cell, whereby the phosphorylation and activation of HSL by type I PKA increases the pool of free cholesterol available to STAR. Such a mechanism is made more attractive by recent studies showing that PAP7, a type I AKAP that is localized to the mitochondria, can also enhance steroidogenesis in MA-10 cells (Liu et al., 2006). PAP7 physically associates with the peripheral benzodiazepine receptor, an outer mitochondrial membrane protein that may also control the labile pools of cholesterol accessed by STAR (Miller, 2007a). From this we speculate that type I PKA may serve an important role in coordinating the cholesterol traffic to and from stores at or near the outer mitochondrial membrane by regulating a functional interaction between HSL, STAR, and PBR.
Our present evidence demonstrates that the individual actions of both type I and type II PKA in Leydig cells can influence discrete steps leading to the induction and activation of STAR. Observations such as the preferential phosphorylation of JUN and CREB, by type I PKA, and STAR, by type II PKA, suggest that these subtypes of PKA are uniquely positioned in the Leydig cell, fitting with the concept that PKA compartmentalization within the cell can affect the regulation of STAR-mediated steroidogenesis. Such compartmentalization is likely to enhance the sensitivity of the steroidogenic machinery to cAMP signaling, however, we also predict the unique organization of PKA to be a defining characteristic of steroidogenic cells. As such it will be interesting to determine if AKAPs and other scaffolding proteins that affect signal transduction networks serve in roles that are distinct or permissive for acute steroidogenesis.
Acknowledgments
We would like to acknowledge Yuping Sun for her technical assistance. This research was supported by funds from the NIH grant HD-17481 and grant # B1-0028 from the Robert A. Welch Foundation (to D.M.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss JF., 3rd Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem. 1997;272:32656–32662. doi: 10.1074/jbc.272.51.32656. [DOI] [PubMed] [Google Scholar]
- Ariyoshi N, Kim YC, Artemenko I, Bhattacharyya KK, Jefcoate CR. Characterization of the rat Star gene that encodes the predominant 3.5- kilobase pair mRNA. ACTH stimulation of adrenal steroids in vivo precedes elevation of Star mRNA and protein. J Biol Chem. 1998;273:7610–7619. doi: 10.1074/jbc.273.13.7610. [DOI] [PubMed] [Google Scholar]
- Belfrage P, Fredrikson G, Nilsson NO, Stralfors P. Regulation of adipose-tissue lipolysis by phosphorylation of hormone-sensitive lipase. Int J Obes. 1981;5:635–641. [PubMed] [Google Scholar]
- Bose H, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417:87–91. doi: 10.1038/417087a. [DOI] [PubMed] [Google Scholar]
- Carlson CR, Lygren B, Berge T, Hoshi N, Wong W, Tasken K, Scott JD. Delineation of type I protein kinase A-selective signaling events using an RI anchoring disruptor. J Biol Chem. 2006;281:21535–21545. doi: 10.1074/jbc.M603223200. [DOI] [PubMed] [Google Scholar]
- Caron KM, Ikeda Y, Soo SC, Stocco DM, Parker KL, Clark BJ. Characterization of the promoter region of the mouse gene encoding the steroidogenic acute regulatory protein. Mol Endocrinol. 1997;11:138–147. doi: 10.1210/mend.11.2.9880. [DOI] [PubMed] [Google Scholar]
- Chaudhary LR, Stocco DM. Stimulation of progesterone production by phorbol-12-myristate-13- acetate in MA-10 Leydig tumor cells. Biochimie. 1988;70:1353–1360. doi: 10.1016/0300-9084(88)90006-5. [DOI] [PubMed] [Google Scholar]
- Chin KV, Yang WL, Ravatn R, Kita T, Reitman E, Vettori D, Cvijic ME, Shin M, Iacono L. Reinventing the wheel of cyclic AMP: novel mechanisms of cAMP signaling. Ann N Y Acad Sci. 2002;968:49–64. doi: 10.1111/j.1749-6632.2002.tb04326.x. [DOI] [PubMed] [Google Scholar]
- Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Doskeland SO. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Soo SC, Caron KM, Ikeda Y, Parker KL, Stocco DM. Hormonal and developmental regulation of the steroidogenic acute regulatory protein. Mol Endocrinol. 1995;9:1346–1355. doi: 10.1210/mend.9.10.8544843. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR) J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- Constantinescu A, Gordon AS, Diamond I. cAMP-dependent protein kinase types I and II differentially regulate cAMP response element-mediated gene expression: implications for neuronal responses to ethanol. J Biol Chem. 2002;277:18810–18816. doi: 10.1074/jbc.M112107200. [DOI] [PubMed] [Google Scholar]
- Constantinescu A, Wu M, Asher O, Diamond I. cAMP-dependent protein kinase type I regulates ethanol-induced cAMP response element-mediated gene expression via activation of CREB-binding protein and inhibition of MAPK. J Biol Chem. 2004;279:43321–43329. doi: 10.1074/jbc.M406994200. [DOI] [PubMed] [Google Scholar]
- Duan H, Jefcoate CR. The predominant cAMP-stimulated 3 × 5 kb StAR mRNA contains specific sequence elements in the extended 3′UTR that confer high basal instability. J Mol Endocrinol. 2007;38:159–179. doi: 10.1677/jme.1.02153. [DOI] [PubMed] [Google Scholar]
- Dyson MT, Jones JK, Kowalewski MP, Manna PR, Alonso M, Gottesman ME, Stocco DM. Mitochondrial A-kinase anchoring protein 121 binds type II protein kinase A and enhances steroidogenic acute regulatory protein-mediated steroidogenesis in MA-10 mouse leydig tumor cells. Biol Reprod. 2008;78:267–277. doi: 10.1095/biolreprod.107.064238. [DOI] [PubMed] [Google Scholar]
- Feliciello A, Gottesman ME, Avvedimento EV. J Mol Biol. Vol. 308. 2001. The biological functions of A-kinase anchor proteins; pp. 99–114. [DOI] [PubMed] [Google Scholar]
- Fleury A, Mathieu AP, Ducharme L, Hales DB, LeHoux JG. Phosphorylation and function of the hamster adrenal steroidogenic acute regulatory protein (StAR) J Steroid Biochem Mol Biol. 2004;91:259–271. doi: 10.1016/j.jsbmb.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Fong TH, Wang SM. Dissection of the signaling mechanism for capsule detachment of lipid droplets in rat adrenocortical cells. J Cell Biochem. 1997;65:67–74. [PubMed] [Google Scholar]
- Gill GN, Garren LD. Role of the receptor in the mechanism of action of adenosine 3′:5′-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971;68:786–790. doi: 10.1073/pnas.68.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkin RW, Moger WH. Type 1 and 2 isoenzymes of cAMP-dependent protein kinase in Leydig cell steroidogenesis. Mol Cell Endocrinol. 1991;77:91–96. doi: 10.1016/0303-7207(91)90062-w. [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Jungmann RA. Rabbit ovarian protein kinases. I. Effect of an ovulatory dose of human chorionic gonadotropin or luteinizing hormone on the subcellular distribution of follicular and luteal protein kinases. Endocrinology. 1978a;103:420–430. doi: 10.1210/endo-103-2-420. [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Jungmann RA. Rabbit ovarian protein kinases. II. Effect of an ovulatory dose of human chorionic gonadotropin or luteinizing hormone on the multiplicity of follicular and luteal protein kinases. Endocrinology. 1978b;103:431–440. doi: 10.1210/endo-103-2-431. [DOI] [PubMed] [Google Scholar]
- Ishigaki Y, Li X, Serin G, Maquat LE. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell. 2001;106:607–617. doi: 10.1016/s0092-8674(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Jo Y, King SR, Khan SA, Stocco DM. Involvement of protein kinase C and cyclic adenosine 3′,5′-monophosphate-dependent kinase in steroidogenic acute regulatory protein expression and steroid biosynthesis in Leydig cells. Biol Reprod. 2005;73:244–255. doi: 10.1095/biolreprod.104.037721. [DOI] [PubMed] [Google Scholar]
- Kallen CB, Arakane F, Christenson LK, Watari H, Devoto L, Strauss JF., 3rd Unveiling the mechanism of action and regulation of the steroidogenic acute regulatory protein. Mol Cell Endocrinol. 1998;145:39–45. doi: 10.1016/s0303-7207(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Katsumata N, Tanae A, Sato N, Horikawa R, Tanaka M. Adrenal gland. Significance of new StAR gene mutation of S195A-StAR phosphorylation found in congenital adrenal lipoid hyperplasia. Clinical Endocrinology. 2000;48:141–143. [Google Scholar]
- King SR, Stocco DM. ATP and a mitochondrial electrochemical gradient are required for functional activity of the steroidogenic acute regulatory (StAR) protein in isolated mitochondria. Endocr Res. 1996;22:505–514. doi: 10.1080/07435809609043739. [DOI] [PubMed] [Google Scholar]
- Koroscil TM, Gallant S. The phosphorylation of adrenal proteins in response to adrenocorticotropic hormone. J Biol Chem. 1981;256:6700–6707. [PubMed] [Google Scholar]
- Kowalewski MP, Schuler G, Taubert A, Engel E, Hoffmann B. Expression of cyclooxygenase 1 and 2 in the canine corpus luteum during diestrus. Theriogenology. 2006;66:1423–1430. doi: 10.1016/j.theriogenology.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Kraemer FB, Shen WJ, Harada K, Patel S, Osuga J, Ishibashi S, Azhar S. Hormone-sensitive lipase is required for high-density lipoprotein cholesteryl ester-supported adrenal steroidogenesis. Mol Endocrinol. 2004;18:549–557. doi: 10.1210/me.2003-0179. [DOI] [PubMed] [Google Scholar]
- Kraemer FB, Shen WJ, Patel S, Osuga J, Ishibashi S, Azhar S. The LDL receptor is not necessary for acute adrenal steroidogenesis in mouse adrenocortical cells. Am J Physiol Endocrinol Metab. 2007;292:E408–412. doi: 10.1152/ajpendo.00428.2006. [DOI] [PubMed] [Google Scholar]
- Lee PC, Radloff D, Schweppe JS, Jungmann RA. Testicular protein kinases. Characterization of multiple forms and ontogeny. J Biol Chem. 1976;251:914–921. [PubMed] [Google Scholar]
- Liu J, Li H, Papadopoulos V. PAP7, a PBR/PKA-RIalpha-associated protein: a new element in the relay of the hormonal induction of steroidogenesis. J Steroid Biochem Mol Biol. 2003;85:275–283. doi: 10.1016/s0960-0760(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Liu J, Rone MB, Papadopoulos V. Protein-protein interactions mediate mitochondrial cholesterol transport and steroid biosynthesis. J Biol Chem. 2006;281:38879–38893. doi: 10.1074/jbc.M608820200. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Makarevich AV, Sirotkin AV, Genieser HG. Action of protein kinase A regulators on secretory activity of porcine granulosa cells in vitro. Anim Reprod Sci. 2004;81:125–136. doi: 10.1016/j.anireprosci.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol. 2002;16:184–199. doi: 10.1210/mend.16.1.0759. [DOI] [PubMed] [Google Scholar]
- Manna PR, Eubank DW, Lalli E, Sassone-Corsi P, Stocco DM. Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1. J Mol Endocrinol. 2003;30:381–397. doi: 10.1677/jme.0.0300381. [DOI] [PubMed] [Google Scholar]
- Manna PR, Eubank DW, Stocco DM. Assessment of the role of activator protein-1 on transcription of the mouse steroidogenic acute regulatory protein gene. Mol Endocrinol. 2004a;18:558–573. doi: 10.1210/me.2003-0223. [DOI] [PubMed] [Google Scholar]
- Manna PR, Huhtaniemi IT, Stocco DM. Detection of hCG Responsive Expression of the Steroidogenic Acute Regulatory Protein in Mouse Leydig Cells. Biol Proced Online. 2004b;6:83–93. doi: 10.1251/bpo76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Stocco DM. Crosstalk of CREB and Fos/Jun on a single cis-element: transcriptional repression of the steroidogenic acute regulatory protein gene. J Mol Endocrinol. 2007;39:261–277. doi: 10.1677/JME-07-0065. [DOI] [PubMed] [Google Scholar]
- Mantovani G, Lania AG, Bondioni S, Peverelli E, Pedroni C, Ferrero S, Pellegrini C, Vicentini L, Arnaldi G, Bosari S, Beck-Peccoz P, Spada A. Different expression of protein kinase A (PKA) regulatory subunits in cortisol-secreting adrenocortical tumors: relationship with cell proliferation. Exp Cell Res. 2008;314:123–130. doi: 10.1016/j.yexcr.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Maronde E, Wicht H, Tasken K, Genieser HG, Dehghani F, Olcese J, Korf HW. CREB phosphorylation and melatonin biosynthesis in the rat pineal gland: involvement of cyclic AMP dependent protein kinase type II. J Pineal Res. 1999;27:170–182. doi: 10.1111/j.1600-079x.1999.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Miller WL. Mechanism of StAR's regulation of mitochondrial cholesterol import. Mol Cell Endocrinol. 2007a;265-266:46–50. doi: 10.1016/j.mce.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Miller WL. StAR search--what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol. 2007b;21:589–601. doi: 10.1210/me.2006-0303. [DOI] [PubMed] [Google Scholar]
- Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta. 2007c;1771:663–676. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Moger WH. Evidence for compartmentalization of adenosine 3′,5′-monophosphate (cAMP)-dependent protein kinases in rat Leydig cells using site-selective cAMP analogs. Endocrinology. 1991;128:1414–1418. doi: 10.1210/endo-128-3-1414. [DOI] [PubMed] [Google Scholar]
- Neher R, Milani A, Solano AR, Podesta EJ. Compartmentalization of corticotropin-dependent steroidogenic factors in adrenal cortex: evidence for a post-translational cascade in stimulation of the cholesterol side-chain split. Proc Natl Acad Sci U S A. 1982;79:1727–1731. doi: 10.1073/pnas.79.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogreid D, Ekanger R, Suva RH, Miller JP, Sturm P, Corbin JD, Doskeland SO. Activation of protein kinase isozymes by cyclic nucleotide analogs used singly or in combination. Principles for optimizing the isozyme specificity of analog combinations. Eur J Biochem. 1985;150:219–227. doi: 10.1111/j.1432-1033.1985.tb09010.x. [DOI] [PubMed] [Google Scholar]
- Pon LA, Hartigan JA, Orme-Johnson NR. Acute ACTH regulation of adrenal corticosteroid biosynthesis. Rapid accumulation of a phosphoprotein. J Biol Chem. 1986;261:13309–13316. [PubMed] [Google Scholar]
- Pon LA, Orme-Johnson NR. Acute stimulation of steroidogenesis in corpus luteum and adrenal cortex by peptide hormones. Rapid induction of a similar protein in both tissues. J Biol Chem. 1986;261:6594–6599. [PubMed] [Google Scholar]
- Resko JA, Norman RL, Niswender GD, Spies HG. The relationship between progestins and gonadotropins during the late luteal phase of the menstrual cycle in rhesus monkeys. Endocrinology. 1974;94:128–135. doi: 10.1210/endo-94-1-128. [DOI] [PubMed] [Google Scholar]
- Shen WJ, Patel S, Natu V, Hong R, Wang J, Azhar S, Kraemer FB. Interaction of hormone-sensitive lipase with steroidogenic acute regulatory protein: facilitation of cholesterol transfer in adrenal. J Biol Chem. 2003;278:43870–43876. doi: 10.1074/jbc.M303934200. [DOI] [PubMed] [Google Scholar]
- Skalhegg BS, Landmark BF, Doskeland SO, Hansson V, Lea T, Jahnsen T. Cyclic AMP-dependent protein kinase type I mediates the inhibitory effects of 3′,5′-cyclic adenosine monophosphate on cell replication in human T lymphocytes. J Biol Chem. 1992;267:15707–15714. [PubMed] [Google Scholar]
- Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. Differential expression,regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–693. doi: 10.2741/skalhegg. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Chaudhary LR. Evidence for the functional coupling of cyclic AMP in MA-10 mouse Leydig tumour cells. Cell Signal. 1990;2:161–170. doi: 10.1016/0898-6568(90)90019-7. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Sodeman TC. The 30-kDa mitochondrial proteins induced by hormone stimulation in MA- 10 mouse Leydig tumor cells are processed from larger precursors. J Biol Chem. 1991;266:19731–19738. [PubMed] [Google Scholar]
- Stocco DM, Wang X, Jo Y, Manna PR. Multiple signaling pathways regulating steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- Stralfors P, Belfrage P. Phosphorylation of hormone-sensitive lipase by cyclic AMP-dependent protein kinase. J Biol Chem. 1983;258:15146–15152. [PubMed] [Google Scholar]
- Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Dyson MT, Jo Y, Eubank DW, Stocco DM. Involvement of 5-lipoxygenase metabolites of arachidonic acid in cyclic AMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J Steroid Biochem Mol Biol. 2003;85:159–166. doi: 10.1016/s0960-0760(03)00189-4. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Dyson MT, Mondillo C, Patrignani Z, Pignataro O, Stocco DM. Interaction between arachidonic acid and cAMP signaling pathways enhances steroidogenesis and StAR gene expression in MA-10 Leydig tumor cells. Mol Cell Endocrinol. 2002;188:55–63. doi: 10.1016/s0303-7207(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Whitehouse BJ, Abayasekara DR. Roles of type I and type II isoenzymes of cyclic AMP-dependent protein kinase in steroidogenesis in rat adrenal cells. J Mol Endocrinol. 1994;12:195–202. doi: 10.1677/jme.0.0120195. [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Yeaman SJ. Hormone-sensitive lipase--new roles for an old enzyme. Biochem J. 2004;379:11–22. doi: 10.1042/BJ20031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Duan H, Kim YC, Jefcoate CR. Rodent StAR mRNA is substantially regulated by control of mRNA stability through sites in the 3′-untranslated region and through coupling to ongoing transcription. J Steroid Biochem Mol Biol. 2005;96:155–173. doi: 10.1016/j.jsbmb.2005.02.011. [DOI] [PubMed] [Google Scholar]



