Abstract
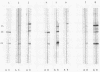
The human immunodeficiency virus (HIV) was isolated from the blood of 192 of 410 seropositive individuals. Original isolations were made in peripheral blood mononuclear cell (PBMC) cultures, and only one-fifth of the HIV isolates could be adapted to replicate in continuous T-cell lines. Of the 192 HIV isolates, 42 had the characteristic p24 antigen marker of the acquired immunodeficiency syndrome-associated retrovirus type 2 strain of HIV (HIV ARV-2) and 150 resembled the human T-cell lymphotropic virus type III strain of HIV (HIV HTLV-III). Significantly, primary PBMC cultures from two patients yielded multiple variants. When these variants were exposed to continuous T-cell lines, only one of them continued to replicate. The remaining variants were lost and could not be reisolated following passage back into PBMC cultures. We conclude the following from these studies: PBMC cultures are more efficient at isolating HIV than continuous T-cell lines are; some patients harbor more than one genetic variant of HIV in the blood at the same time; and continuous T-cell lines are likely to yield only a portion of the HIV variants originally present in the blood.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Genetic variation in AIDS viruses. Cell. 1986 Jul 4;46(1):1–4. doi: 10.1016/0092-8674(86)90851-2. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Gonda M. A., Shaw G. M., Popovic M., Hoxie J. A., Gallo R. C., Wong-Staal F. Genomic diversity of the acquired immune deficiency syndrome virus HTLV-III: different viruses exhibit greatest divergence in their envelope genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4813–4817. doi: 10.1073/pnas.82.14.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B. H., Shaw G. M., Taylor M. E., Redfield R. R., Markham P. D., Salahuddin S. Z., Wong-Staal F., Gallo R. C., Parks E. S., Parks W. P. Genetic variation in HTLV-III/LAV over time in patients with AIDS or at risk for AIDS. Science. 1986 Jun 20;232(4757):1548–1553. doi: 10.1126/science.3012778. [DOI] [PubMed] [Google Scholar]
- Higgins J. R., Pedersen N. C., Carlson J. R. Detection and differentiation by sandwich enzyme-linked immunosorbent assay of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus- and acquired immunodeficiency syndrome-associated retroviruslike clinical isolates. J Clin Microbiol. 1986 Sep;24(3):424–430. doi: 10.1128/jcm.24.3.424-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J. Recovery of AIDS-associated retroviruses from patients with AIDS or AIDS-related conditions and from clinically healthy individuals. J Infect Dis. 1985 Oct;152(4):734–738. doi: 10.1093/infdis/152.4.734. [DOI] [PubMed] [Google Scholar]
- Montelaro R. C., Parekh B., Orrego A., Issel C. J. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J Biol Chem. 1984 Aug 25;259(16):10539–10544. [PubMed] [Google Scholar]
- Nathanson N., Georgsson G., Pálsson P. A., Najjar J. A., Lutley R., Pétursson G. Experimental visna in Icelandic sheep: the prototype lentiviral infection. Rev Infect Dis. 1985 Jan-Feb;7(1):75–82. doi: 10.1093/clinids/7.1.75. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Salinovich O., Payne S. L., Montelaro R. C., Hussain K. A., Issel C. J., Schnorr K. L. Rapid emergence of novel antigenic and genetic variants of equine infectious anemia virus during persistent infection. J Virol. 1986 Jan;57(1):71–80. doi: 10.1128/jvi.57.1.71-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Shaw G. M., Hahn B. H., Salahuddin S. Z., Popovic M., Markham P., Redfield R., Gallo R. C. Genomic diversity of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Aug 23;229(4715):759–762. doi: 10.1126/science.2992084. [DOI] [PubMed] [Google Scholar]