Abstract
The FMR1 gene is involved in three different syndromes, the Fragile X syndrome (FXS), premature ovarian insufficiency (POI) and the Fragile X-associated tremor/ataxia syndrome (FXTAS) at older age. Fragile X syndrome is caused by an expansion of a CGG repeat above 200 units in the FMR1 gene resulting in the absence of the FMR1 mRNA and protein. The FMR1 protein is proposed to act as a regulator of mRNA transport and of translation of target mRNAs at the synapse. FXS is seen as a loss of function disorder. POI and FXTAS are found in individuals with an expanded repeat between 50–200 CGGs and are associated with increased FMR1 mRNA levels. The presence of elevated FMR1 mRNA in FXTAS suggests that FXTAS may represent a toxic RNA gain-of-function effect. The molecular basis of POI is yet unknown. The role of the FMR1 gene in these disorders is discussed.
Introduction
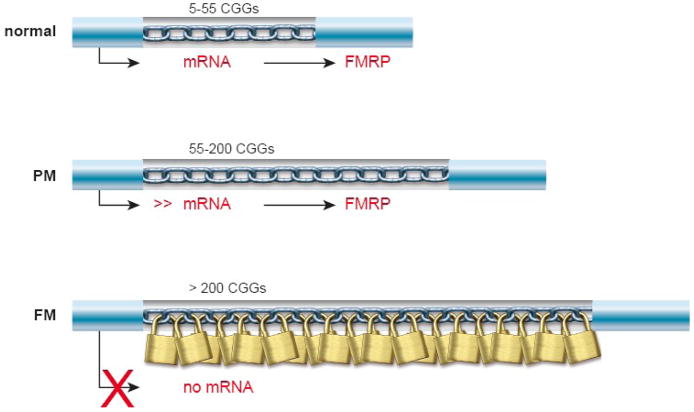
Fragile X syndrome (FXS) is the most prevalent cause of inheritable mental retardation often presenting as an autism spectrum disorder with a frequency of 1:4000 males and 1:6000 females (for review [1]). FXS is an X-linked disorder and is notable for its unusual inheritance pattern, showing increased penetrance as the mutant gene passes to subsequent generations (the Sherman paradox) [2]. In 1991 the responsible gene was identified by positional cloning and named the fragile X mental retardation-1 gene (FMR1) [3] FXS is almost exclusively caused by an expansion of a CGG repeat in the 5′ untranslated region of the FMR1 gene and was the first example of a trinucleotide repeat expansion mutation (Figure 1). In the normal population, the CGG repeat is polymorphic and ranges from 5–55 CGGs with an average length of 30 CGG units [4]. In Fragile X patients, however, the CGG repeat is found to be expanded beyond 200 repeats known as the full mutation (FM), that is usually hypermethylated and the methylation extends to the adjacent promoter region of the FMR1 gene [3, 5, 6]. As a consequence the gene is transcriptionally silenced and the gene product, the fragile X mental retardation protein (FMRP), is absent.
Figure 1. The CGG repeat in the FMR1 gene.
Schematic representation of normal, PM (premutation) and FM (full mutation) alleles of the FMR1 gene and the effect of the expansion on transcription and translation. Methylation due to extensive elongation of the CGG repeat in the 5′-ÚTR of the FMR1 gene is depicted as a lock.
Unmethylated expansions of 55–200 CGG units, called premutations (PM), are unstable in meiosis and are found in both males and females and may expand to a full mutation only upon maternal transmission to the next generation. The risk of transition is dependent on the size of the premutation, which accounts for the Sherman paradox [4]. The smallest CGG repeat number known to expand to a full mutation is 59 repeats to date [7].
FMR1 protein
The cognitive impairment in FXS is caused by the absence of the fragile X mental retardation protein (FMRP) in neurons. FMRP expression is widespread with abundant expression in neurons and with testicular expression in spermatogonia [8–13]. The subcellular distribution of FMRP is largely cytoplasmic, with high concentrations of FMRP found associated with (poly)ribosomes attached to the endoplasmic reticulum and with free ribosomes in the cytoplasm, at the bases of dendrites and within dendritic spines [10, 12, 14, 15]. Interestingly, both in vitro and in vivo studies illustrated the presence of FMRP in the nucleus [12, 14, 16–19]. The association of FMRP with ribosomes is mRNA dependent via large ribonucleoprotein (RNP) particles, which contain several other proteins including FXR1P and FXR2P, nucleolin, YB-1, NUFIP1, CYFIP1 and CYFIP2 [20–23].
Expansion of the fragile X mutation
Many models have been proposed to explain the expansion of trinucleotide repeats. One of the first proposed mechanisms involved in repeat instability at the molecular level was slippage of the replication fork during replication. Unpaired bases form loops, which result in expansions or contractions in a next round of replication, depending on whether the looped repeats are located in the newly synthesized or template strand [24]. However, slippage alone cannot explain all aspects of repeat expansions, especially large expansions and contractions. Strong experimental support came from studies in a yeast model deficient in RAD27. RAD27, like its mammalian homologue FEN1, is involved in removing DNA loops, such as those arising during displacement synthesis of the Okazaki fragments. As predicted by the above model, propagation of a CGG repeat in the RAD27 null background results in a highly significant increase of repeat expansions [25].
Recognition of the unusual structural properties of trinucleotide repeats yielded new insights. Disease-causing repeats are almost exclusively formed by (CNG)n –triplets. Single-stranded (CNG)n can form hairpin-like structures that can include both Watson-Crick and mismatched base pairs. Due to their different sequences, the leading and lagging strand have different tendencies to form hairpins. The secondary structures are likely to affect recognition and subsequent repair or recombination of this structure [26, 27]. Unusual DNA structures may stall DNA polymerases. Studies in yeast replication mutants showed a marked increase in frequency of repeat instability. A complex model based on replication fork stalling and restarting is described in detail by Mirkin [28].
Unlike other trinucleotide repeat disorders such as DM1 there is absence of repeat instability in somatic cells. For full mutations it has been proposed that methylation stabilizes the CGG repeat and prevents expansion or contraction. However, males with an unmethylated full mutation do not show somatic instability of the repeat [29]. Recently human embryonic stem cells containing a full mutation have been described [30]. The original embryonic cells show clear instability of the repeat, but after subcloning the unmethylated repeat becomes stable while the cells are still displaying all the characteristics typical of embryonic stem cells. Premutation alleles (that are not methylated) are also stable. What is causing this different behavior of the repeat in different repeat disorders is still unknown.
As observed for other dynamic mutations, the degree of expansion in humans depends on the gender of the transmitting parent. Repeat expansion from the premutation to the full mutation only occurs during maternal transmission. The maternal transmission bias could result from sex-specific differences in gametogenesis leading to the production of full expansion oocytes in females and premutation size repeats in sperm in carrier males. This is compatible with the observation that in sperm from full-mutation male patients only a premutation is found [31]. If the zygote starts development with a fully expanded FMR1 repeat, then somatic mosaicism must result form reductional repeat instability limited to a window in early development. Such reductional instability may be even more pronounced in the fetal testes. This contraction hypothesis is supported by evidence that in a 17-week old male fetus carrying a FM, germ cells harbor a premutation, while in the other cells a full mutation is present [32]. However, the timing of repeat expansion during development remains uncertain. A prezygotic model predicts that an expansion of PM to FM could occur during maternal meiosis, with the FM contracting to a PM in gametes of male offspring. PM gametes might have some selection advantage either due to the presence of FMRP, or against the presence of an expanded CGG-repeat [32]. Furthermore, FMs are responsible for a delay in replication of the FMR1 gene during the cell cycle [33]. Thus, primordial germ cells with a PM might have a proliferative advantage, thereby overgrowing FM cells. A second model considers a postzygotic expansion, direct after separation of the germ line. It assumes that the FM allele has never been present in male or female gametes [34]. In contrast to this hypothesis, oocytes of a foetal female FM carrier show only FM alleles [32]. A final conclusion can only be drawn after analysis of oocytes from a PM carrier. This material is not available for obvious reasons. Expanded (CGG)n knock-in mouse models have been developed [35, 36], that show intergenerational repeat instability [36, 37]. When the CGG repeat in the mouse expands into a full mutation they might be used to study the timing of repeat instability.
Epigenetic changes in the FMR1 gene
Methylation of the expanded FMR1 CGG repeat occurs early in embryonic development and is a dynamic process. In early germ cells from female FM fetuses, the FMR1 repeat is fully expanded and unmethylated [32], whereas in chorionic villus samples from FM fetuses, the expanded repeat in this extraembryonal tissue is methylated to an increasing degree as development progresses [38]. The difficulty in studying this biological phenomenon is the absence of suitable material for study. Transgenic mouse models bearing more than 200 CGG repeats (human origin) within the murine Fmr1 gene have been generated but they do not show epigenetic changes up till now [36, 37]. Recently, Eiges et al. developed an embryonic stem cell line that was shown to contain the FM of FMR1 with repeats up to 1000 triplets [30]. In this pluripotent undifferentiated stem line, the FMR1 gene is unmethylated and transcriptionally active. When differentiated as embryoid bodies, transcription of FMR1 is nearly eliminated. The embryoid bodies show a substantial increase in FMR1 DNA methylation, similar to cells derived from fragile X patients. Chromatin immunoprecipitation studies show, the epigenetic switch of histone H3 deacetylation and histone H3K9 methylation which is also seen in fragile X patients [39, 40]. The molecular mechanism behind these changes is not clear and the question remains how the repeat length itself is responsible for DNA methylation and histone modifications in the FMR1 gene. These embryonic stem cells might help to unravel the mechanism and timing of gene silencing and to identify the proteins involved.
Almost all fragile X patients carry an expanded FM and show methylation of the promoter region of the FMR1 gene and the coding region is intact. Rare individuals with expanded, but unmethylated repeats have been described. Such individuals are not showing the full spectrum of the phenotype of fragile X syndrome, demonstrating that methylation and not repeat elongation per se causes the typical features of FXS [29, 41]. This observation prompted to investigate the possibility of epigenetic reactivation of the FMR1 gene. It was shown that treatment of fragile X cell lines with 5-azadeoxycytidine (5-azadC) leads to reactivation of the FMR1 gene (figure 2) [42] and DNA demethylation [43]. As normal methylation in the cell is followed by deacetylation it was hypothesized that a combination of demethylating and acetylating drugs might enhance demethylation of the CGG repeat. Indeed, a synergistic effect of the two types of drugs was demonstrated to enhance the reactivation of the FMRP production approximately five-fold. Histone acetylating drugs, such as butyrate, can potentiate the reactivation induced by 5-azadC [39, 40]. Unfortunately, 5-azadC cannot be administered to fragile X patients because of its toxicity and histone acetylating drugs alone do not reactivate the FMR1 gene, as DNA methylation appears to be the dominant epigenetic modification responsible for gene silencing.
Figure 2. Schematic representation of the chromatin structure of the FMR1 gene.
In the normal situation the active gene has an open chromatin structure. When the CGG repeat (red line) is expanded, deacetylation and methylation of the promoter and CGG region takes place leading to a packaged and less accessible chromatin structure causing inactivation of the FMR1 gene. Treatment with 5-azadC results in demethylation and acetylation leading to an open chromatin structure and transcription will be (partly) restored.
Structural domains of FMRP
Two types of RNA binding domains have been identified in FMRP, including two KH domains and an RGG box containing a conserved Arg-Gly-Gly triplet [44, 45]. The biological significance of the RNA-binding capacities and (poly)ribosomal association of FMRP-associated RNP particles is demonstrated in cells from a severely affected fragile X patient, who has a missense mutation (I304N) in the second KH domain within the FMR1 gene [46]. The mutation disrupts the normal folding of the KH domain and the mutant I304N protein no longer associates with active polyribosomes [47–49].
In addition, a nuclear localization signal (NLS), a nuclear export signal (NES), two coiled coils and a G-quartet binding structure have been identified. The presence of both a NES and NLS suggests that FMRP may shuttle into and out of the nucleus. The nuclear export mediated by the NES of FMRP is exportin1-dependent [50]. In accordance with the shuttling hypothesis, the protein has been observed in the nuclear pore during transfer between the nucleus and cytoplasm [10]. Nuclear FMRP has been shown to exit the nucleus through its bound mRNA [19].
In human cell lines, FMRP co-localizes primarily with polyribosomes and ribosomes at/in the endoplasmic reticulum membrane. There is strong evidence to support an important role for FMRP in regulation of translation of specific target mRNAs. Evidence was presented that FMRP in vitro may function as a repressor of translation of its own mRNA [51]. It is interesting to note that the Ile304Asn mutant FMRP still is able to interact with polyA-mRNA but loses its function in vitro as a translational repressor due to a loss of homo-oligomerization. FMRP-associated target mRNAs contain a sequence that can form an intramolecular G quartet structure. FMRP binds also to its own mRNA via a G quartet that is found at the C terminal end of the part coding for the open reading frame [52, 53]. A subset of mRNAs containing a G-quartet has been identified that are potential targets for FMRP, including important neuronal proteins like microtubule associated protein 1B (MAP1B) and semaphorin [53–55]. A validated list of targeted FMRP mRNAs can be found in Bassell and Warren [56]. But FMRP also can bind to mRNAs that do not contain a G quartet. It is still not fully clear what the role of the different binding regions is and whether they all are important for FMRP functioning.
FMRP and mRNP transport
In 1987 Steward demonstrated that mRNAs were transported into dendrites of cultured hippocampal neurons [57]. Since then a large number of dendritic localized mRNAs have been identified and it is suggested that the translation of those mRNAs can be regulated in a spatially restricted manner in response to stimulation (for review [58]).
The dynamics of the transport of mRNP particles in neurons has been studied by different experimental approaches and a supramolecular complex was identified containing mRNAs, translational factors and ribosomal subunits [59–61]. FMRP containing RNP particles have been observed as RNA granules traveling in the dendritic branching [61, 62] (figure 3). The migration of mRNP particles over long distances within processes towards the growth cone is established by movement along microtubules [59, 63, 64]. A similar model has been proposed in which FMRP binds specific mRNAs and mediates the targeting/transport of these transcripts into the dendrite using intact microtubules. During transport they remain translationally inactive until appropriate synaptic input allows translation. Recently, Dictenburg et al have shown that FMRP is involved in a stimulus-induced dendritic mRNA transport [65]. FMRP-positive mRNP granules were associated with KIF5, which is a neurospecific motor protein. In the absence of FMRP, a number of mRNAs are diminished in their association with KIF5 supporting the role of FMRP as a molecular adaptor to bind mRNA targets during kinesin-mediated transport along microtubules to synaptic sites.
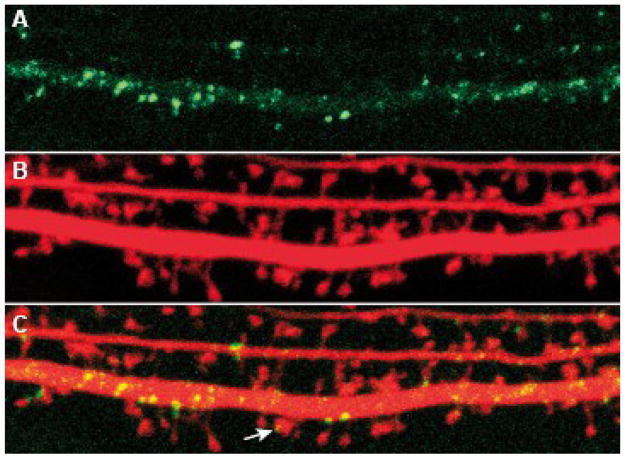
Fig. 3. Localization of EGFP-FMRP in dendritic spines.
Primary hippocampal mouse neurons of E18 Fmr1 KO mice were co-transfected with β-actin-EGFP-Fmr1 (A) and β-actin-mCherry (B). The dendrite, including many spines, of one neuron is depicted. Note the presence of EGFP-FMRP in a spinehead in the overlay (arrow in C). Images were acquired using a Zeiss LSM510 confocal microscope (scalebar= 5 μm). Courtesy by Femke de Vrij.
It is not known whether certain mRNAs can not be transported into the dendrite in the absence of FMRP. However, it is evident that FMRP plays a role at the synapse in controlling the translation of certain mRNAs. A role of FMRP in translationally silencing during transport into the dendrites have been proposed [66, 67].
FMRP and spines
Transport and regulated translation of mRNAs in dendrites is important for neuronal function, including modulation of synaptic plasticity. This is essential in memory consolidation and learning [68, 69]. Altered spine morphology (long and thin dendritic spines) has been observed in post-mortem brains of fragile X patients [70–72] and in Fmr1 KO mice [73–75]. The presence of the protein machinery near synaptic connections allows neurons to rapidly respond to signals at particular synapses through local translation of (specific) mRNAs. Weiler et al showed that FMRP is synthesized in response to mGluR activation using glutamate [15]. Controlled efficient translation of mRNAs in the vicinity of the synapse is important and herein FMRP plays a crucial role. It was proposed that FMRP located at the synapse is repressing translation of mRNAs encoding proteins that regulate endocytic events of AMPA. Upon synaptic stimulation FMRP may dissociate from these mRNA targets to allow translation and facilitation of AMPA receptor internalization. The model predicts that in the absence of FMRP the upregulated translation of a subset of mRNAs would result in the perturbation of AMPA receptor internalization dynamics.
A model was proposed in which FMRP is regulating mRNA translation upon mGluR stimulation [76] (figure 4). This mGluR theory states that AMPA receptor internalization triggered by mGluR5 stimulation [77], is exaggerated in Fmr1 KO mice [78]. Recently it was shown that FMRP deficient dendrites indeed show aberrant AMPA receptor internalization resulting in a significantly reduced number of AMPA receptors at the postsynaptic membrane [79]. Moreover, Fmr1 KO mice that are crossbred with mice that have a 50% reduction in mGluR5 expression were shown to be rescued in several phenotypic aspects [80], This illustrates that reducing the mGluR5 signaling pathway potential can rescue some phenotypic features such as Inhibitory Avoidance Extinction test and the occurrence of audiogenic seizures while the macroorchidism was not rescued [80].. Since the amount of AMPA receptors at the postsynaptic membrane is correlated with protrusion shape, this might also explain the immature protrusion morphology that has been found in different brain areas of both fragile X patients and Fmr1 KO mice [70, 73–75, 81–83]. An antagonist of mGluR5 receptors might reduce the mGluR5 signaling pathway potential and would theoretically counteract the increased internalization of AMPA receptor internalization in Fmr1 KO neurons. Behavioral studies have shown that Fmr1 KO mice treated with the mGluR5 antagonist MPEP (2-methyl-6-(phenylethynyl)-pyridine hydrochloride) clearly display less sensitivity to audiogenic seizures and show more wild type-like behavior in an open field test compared with untreated mice [84]. A similar rescue of the phenotype of the Prepulse Inhibition (PPI) paradigm in Fmr1 KO mice was seen upon treatment with different mGluR antagonists including MPEP and fenobam [75] (figure 4). Treatment with these two mGluR antagonists also rescued the immature spine morphology in cultured hippocampal neurons [75]. Since spine shape is correlated with the number of AMPA receptors at the postsynaptic membrane [85], these data are in line with the rescue effect of MPEP on AMPA receptor trafficking as shown by Nakamoto et al [79]. Recently, Phase I clinical studies in fragile X patients have been started with different mGluR antagonists, including fenobam and AFQ056. [86]. Thus far, no site-effects have been described both in mice and men.
Figure 4. The mGluR theory.
Hypothetical model for the action of FMRP at the synapse, adapted from reference [76]. Treatment with MPEP, an mGluR5 antagonist, results in the rescue of some phenotypic features because mGluR5 stimulation is reduced and subsequently local translation at the synapse is no longer exaggerated. Ultimately, the number of internalized AMPA receptors is reduced and restored to normal levels. A. Stimulation of mGluR5, a metabotropic glutamate receptor, induces local mRNA translation. This results in novel protein synthesis that on its turn stimulates the internalization of the ionotropic AMPA receptor, essential for in long-term plasticity. FMRP acts as a negative regulator of transcription [51, 76], reducing the internalization of the ionotropic glutamate receptor. B. In neurons from fragile X patients the absence of FMRP leads to an increase internalization of the ionotropic glutamate receptors which results in enhanced LTD. C. Rescue of normal translation due to the mGluR antagonist MPEP, slowing down the internalization of the ionotropic glutamate receptors.
Translation of FMRP target mRNAs at the synapse
Protein kinases are crucial for the regulation of neuronal development and synaptic transmission upon response to extracellular or intracellular signals. The mGluR theory is in line with in the translation control pathways within the dendritic spines: a simplified version is depicted in figure 5. Compelling evidence supports the postsynaptic FMRP signaling model. Ceman et al [19] showed that unphosphorylated FMRP associated with actively translating polyribosomes while a fraction of phosphorylated FMRP is associated with apparently stalled polyribosomes. The data suggest that dephosphorylation of FMRP may regulate FMRP and that the release of FMRP-induced translational suppression may involve a dephosphorylation signal. Rapid dephosphorylation of FMRP allows target mRNAs to be translated, whereas rephosphorylation represses translation. Subsequent experiments identified protein phosphatase 2A (PP2A) as an FMRP phosphatise. Rapid FMRP dephosphorylation has been reported after mGluR stimulation (<1 min) in neurons caused by enhanced PP2A enzymatic activity. On the other hand, extended mGluR activation (1–5 min) resulted in mammalian target of rapamycin (mTOR)-mediated PP2A suppression and FMRP rephosphorylation [66, 67]. Ribosomal protein S6 kinase (S6K1) was identified as an important kinase involved in FMRP phosphorylation in the mouse hippocampus. It was shown that activity-dependent phosphorylation of FMRP by S6K1 requires signaling inputs from mammalian target of rapamycin (mTOR), ERK1/2, and PP2A. FMRP rephosphorylation is known to partly involve mTOR-dependent PP2A suppression (and thus suppression of dephosphorylation) and phosphorylation by S6K1. TSC1 and TSC2 proteins have been shown to negatively regulate cell growth through inhibition of the mTOR pathway.
Figure 5. Simplified model of the translation control pathways at the dendritic spines.
Stimulation of mGluR leads to active PP2A (<1min) which dephosphorylates FMRP, and this results in rapid translation of FMRP-associated mRNAs. Within 5 min, mTOR is activated, inhibiting PP2A and activating S6K1, leading to FMRP phosphorylation and translational inhibition of FMRP target messages. Based on [66, 67].
Deficiency of one of these proteins results in Tuberous Sclerosis, a syndrome which may include intellectual disabilities. In cells lacking either TSC1 or TSC2 protein, the downstream targets of mTOR, including p70 S6 kinase (S6K) and ribosomal protein S6, are constitutively phosphorylated [87] resulting in activation of protein synthesis. Hu et al [88] showed that MEK and ERK signaling appears normal, and phosphoinositide 3-kinase (PI3K)-protein kinase B (PKB; or Akt) signaling is compromised in Fmr1 knock-out mice. Enhancing Ras-PI3K-PKB signaling restores synaptic delivery of GluR1-containing AMPA-receptors and normal LTP in Fmr1 knock-out mice
Further research is needed to characterize the cascade of signaling upon mGluR activation and the mechanism whereby FMRP phosphorylation regulates translation of target mRNAs.
Primary ovarian insufficiency
Furthermore, it became apparent that 20% of female PM carriers manifest premature ovarian failure (POF: cessation of menstruation at or before 40 years of age)[89]. It has been proposed that primary ovarian insufficiency (FXPOI) is a more accurate term for the disorder, to describe the broad range of clinical manifestations associated with what used to be classified as POF [90]. Hundscheid et al. [91] reported evidence for a paternal-parent-of-origin effect on FXPOI (fragile X associated primary ovarian insufficiency) in female PM carriers; however, subsequent studies by others did not support this observation [92, 93]. Differences between the different data sets may be related to the observed discrepancy. Women with the PM, even if they are still cycling, have higher levels of follicle stimulation hormone (FSH) than do healthy women [94]. It has recently been suggested that Anti-Müllerian Hormone (AMH) may be a better marker of ovarian decline. AMH is expressed in granulose cells of growing follicles, thus serves as a marker for the size of the primordial follicle pool in women. Indeed, female PM carriers, with (CGG) repeat lengths beyond 70, had lower AMH levels than did female PM carriers with (CGG) repeat shorter than 70 repeats. Thus, lower AMH levels are suggestive of early ovarian decline in women with (CGG)>70 [95]. Penetrance and age of onset of FXPOI, as well as the increase of FSH levels, correlate with (CGG) repeat length [96]. However, a non-linear relationship has been described for age at menopause and premutation size, in which premutations in the mid-size range are at greatest risk for POF, while larger repeat tracts are associated with a lower risk [97].
Fragile X-associated tremor/ataxia syndrome
Over the past few years, it has become apparent that PM carriers are also at risk of developing a progressive neurodegenerative disorder, which is clinically and neuropathologically entirely distinct from FXS [98–100]. This syndrome is called Fragile X-associated tremor/ataxia syndrome (FXTAS). Although both disorders involve repeat expansions in the FMR1 gene, the clinical presentation and molecular mechanisms underlying each disease are completely distinct. The most common clinical features of FXTAS are a progressive action tremor and ataxia. More advanced or severe cases may show a progressive cognitive decline that ranges from executive and memory deficits to dementia (for review of clinical symptoms see [101]). The major neuropathological hallmark and postmortem criterion for definitive FXTAS is eosinophilic, ubiquitin-positive intranuclear inclusions located in broad distribution throughout the brain in neurons and astrocytes [102, 103] A study of the penetrance of the tremor and ataxia among PM carriers, ascertained through families with known probands with FXS, revealed more than one third of carriers, aged 50 years and older, show symptoms of FXTAS. The penetrance of this disorder exceeds 50% for men over 70 years of age [104]. Since the prevalence of the PM alleles in the general population is approximately 1/800 for males and 1/250 for females. It has been estimated that 1 in 3000 men older than 50 years in the general population will develop symptoms of FXTAS [105, 106]. It has been suggested that penetrance increases with CGG repeat length.
An RNA gain of function mechanism has been suggested for FXTAS [98, 99, 107] based on the observation of elevated levels of CGG containing FMR1 mRNA [108], along with either no detectable change in FMRP or slightly reduced FMRP levels, observed in peripheral blood leukocytes [108, 109] and brain regions [110, 111]} of PM carriers. The mechanism underlying the elevated FMR1 mRNA levels is unknown. The observation that increased levels of transcripts were also measured when constructs bearing the FMR1 5′ UTR with PM-sized (CGG)n, fused to a luciferase reporter were transfected into two different cell lines [112], suggests that the expanded (CGG)n itself, rather than the reduced FMRP levels, is responsible for increased transcription [109, 113, 114] (figure 6).
Figure 6. A schematic representation of the RNA gain of function mechanism proposed for the pathogenesis of FXTAS.
The FMR1 gene is transcribed in the nucleus and transported to the ribosomes. The expanded CGG repeat present in the 5′ UTR of the FMR1 mRNA hampers translation, leading to lower levels of FMRP. The presence of the expanded CGG repeat results in enhanced transcription via a thusfar unknown mechanism and leads to elevated FMR1 mRNA levels. In an attempt to get rid of the excess of FMR1 mRNAs, the cell might attract chaperones or elements of the ubiquitin/proteasome system. Also CGG-binding proteins might be recruited. These processes could lead to the formation of intranuclear inclusions. Sequestration of proteins into the inclusion might prevent them from exerting their function, thereby disturbing normal cellular function, which in the end might cause neurodegeneration. However, it cannot be excluded that the formation of inclusions has a neuroprotective effect, such that neurons that are capable of capturing the toxic transcripts in the inclusions are the cells that survive.
Mouse models for FXTAS
Knock-in mouse models have been generated in which the murine (CGG)8 repeat within the endogenous Fmr1 gene was replaced by a human (CGG)98 or (CGG)118 repeat using a homologous recombination technique in embryonic stem (ES) cells [35, 36]. An expansion bias was observed, with the largest expansion being 43 CGGs [37]. Although it was expected that these longer CGG repeat expansions would eventually lead to methylation of the Fmr1 gene, to date, and despite CGG repeat tracts well over 200 CGGs long, no methylation of the promoter region has been detected. Biochemical analysis of the brains of these mice revealed elevated Fmr1 mRNA levels and reduced Fmrp expression. Importantly, elevated Fmr1 mRNA levels were detected throughout development from 1–72 weeks of age [115]. Since Fmr1 mRNA levels were already elevated by 1 week of age, the potential exists for as yet undocumented developmental consequences of excess transcript production in human FXTAS. On average, a two-fold increase of Fmr1 mRNA levels in brain tissue was reported.
Both CGG repeat mouse strains showed an inverse correlation between CGG repeat length and Fmrp expression in brain using quantitative Western blot analysis [36, 116]. The degree of change in Fmrp expression in the brain appears to be brain region-specific with significant reduced expression throughout the brain and relatively high expression in the hippocampus [36, 37]. Thus, in spite of the elevated levels of Fmr1 transcripts reduced Fmrp expression was found. This apparent paradox was explained by a hypothetical model in which the CGG expansion in the 5′ UTR of the transcript hampers the initiation of translation (figure 6).
The knock-in model shows ubiquitin positive intranuclear inclusion formation in several neurons [115]. A comprehensive study for the presence of these intranuclear neuronal inclusions in different brain regions at different ages demonstrated the occurrence of intranuclear neuronal inclusions throughout the brain with high percentages of inclusions in specific brain structures, including olfactory nucleus, parafascicular thalamic nucleus, medial mammillary nucleus, colliculus inferior, cerebellum, amygdala and pontine nucleus at 72 weeks of age [115, 116]. Furthermore, the average size of the inclusions within one specific brain area correlated significantly with the age of CGG repeat mice. In younger mice, smaller sized inclusions were found compared to older mice. Interestingly, the gradual increase in the size of the inclusions and the percent of ubiquitin-positive neurons over lifetime probably parallels the progressive development of the neurological phenotype of FXTAS in humans [100]. In an attempt to further characterize the constituents of the inclusions, a systematic analysis was performed to localize a panel of protein candidates (related to FMRP or other disorders with inclusions) using double-labeling immunohistochemistry. Next to ubiquitin, molecular chaperone Hsp40, 20S proteasome complex and DNA repair-ubiquitin-associated HR23B accumulated in the inclusions [115, 117]. Importantly, Fmrp could not be detected in the inclusions, although FMR1 mRNA is detectable.
Further evidence of the role of rCGG came from fly models and from transgenic mice [107, 118]. An rCGG transgenic fly study in which a DNA fragment containing 90 CGG repeats with a downstream EGFP coding sequence used as a reporter system, reported the first evidence that rCGG outside of the context of FMR1 could induce neurodegeneration and inclusion formation [107]. Given that the fragile X PM rCGG repeat itself is sufficient to cause neurodegeneration in Drosophila it has been hypothesized that specific rCGG binding may sequester rCGG-repeat-binding proteins from their normal function [119]. Using biochemical and genetic approaches, three proteins, Pur α, hnRNP A2/B1 (two protein isoforms from one gene), and CUGBP1 were found to bind rCGG repeats either directly (Pur α and hnRNP A2/B1) or indirectly (CUGBP1, through the interaction with hnRNP A2/B1) [120, 121]. These proteins are RNA-binding proteins, and have been shown to play a role in transcription, mRNA trafficking, splicing and translation. Furthermore, in vivo Purα was found to be present in the inclusions of both human and fly tissues, suggesting that expression of the fragile X PM rCGG repeats can sequester the rCGG-binding proteins from their normal cellular function(s) and cause neurodegeneration [120] (figure 6). This idea is further supported by the fact that over-expression of either Pur α or hnRNP A2/B1 ameliorated neurodegeneration in the fly model of FXTAS. For Myotonic Dystrophy (DM1) it is proposed that CUG-binding proteins (CUG-BP) are sequestered by expanded DMPK-(CUG)n mRNAs and this is causing aberrant expression of other transcripts that are normally regulated by these proteins [122]. MBNL1 is a specific CUG-BP, homologous to the Drosophila muscleblind protein, which is essential in terminal differentiation of muscle and photoreceptor cells. It accumulates in the nuclear foci in DM1 cells, such that MBNL1 cannot exert its normal function during a critical period of cell differentiation and mRNA splicing [123]. In a similar way proteins can be sequestered by the expanded CGG repeat in FMR1 mRNA and the lack of these proteins can contribute to the FXTAS phenotype.
PM carriers (with or without FXTAS) may develop a variety of neuropsychological symptoms, including mood and anxiety disorders [100, 124–127]. Elevated stress hormone levels have been suggested as a possible explanation. Additional evidence for an elevated stress response and thus aberrant hypothalamo-pituitary-adrenal (HPA) axis function in PM carriers comes from reports demonstrating ubiquitin-positive intranuclear inclusions in the pituitary gland of autopsy brain material from patients with FXTAS [128, 129]. Similarly, studies in expanded CGG mice at 72 weeks of age point to an increased anxiety phenotype in the open field behavior test [130]. Further characterization of the HPA axis physiology in CGG repeat mice revealed dramatically elevated corticosterone levels in serum in response to a mild stressor, as well as intranuclear ubiquitin-positive inclusions in both the pituitary and adrenal gland of 100-week-old mice [116].
Future directions
For Fragile X syndrome research will focus on the cellular function of FMRP, especially in spines of neurons. Based on the current knowledge the first attempts have been started to treat Fragile X patients with drugs that are counteracting the lack of FMRP and the control of protein synthesis at the synapse. The first signs in animal studies are very promising. A next question is the repeat instability leading to FM. The availability of human embryonic stem cells opens the door for studies about the timing and the mechanism underlying repeat instability. This knowledge can form the basis for intervention in repeat instability from generation to generation.
For FXTAS the main question remains however; namely how the (CGG)n RNA leads to formation of inclusions and how this relates to cellular dysfunction originates, ultimately leading to neurodegeneration and clinical problems. Future studies should focus on the identification and role of (CGG)n-BP, and the cellular consequences of their depletion. If these proteins are indeed sequestered away from their normal cellular function, as the toxic RNA gain-of-function model predicts, their function might provide important links between the mutant RNA and clinical symptoms.
The mouse model will remain valuable since the onset as well as the course of disease can be studied. Furthermore, the (CGG)n mice will furthermore be valuable in attempts at reversing the neurodegenerative phenotype. Therapeutic strategies will likely focus on diminishing (CGG)n mRNA levels. The initial studies conducted in Drosophila are promising in this light, as reversing the negative effect of the expanded (CGG)n RNA by co-expressing an anti-sense repeat or by overexpressing the proteins sequestered by the repeat RNA, rescued the neurodegenerative phenotype. Hence, strategies involving the siRNA pathway might prove beneficial in the future. In addition, when more (CGG)n-BPs will be identified, this might provide new therapeutic targets.
Conclusion
FMR1 gene expression is involved in three important disorders with distinct entities. The fragile X syndrome, a neurodevelopmental disorder and the most prevalent cause of heritable mental retardation is caused by the total lack of the FMR1 gene product, FMRP. Thus, in FM carriers the disease is caused by a loss-of-function mechanism. In contrast, PM carriers show increased transcription of the FMR1 gene that results in elevated levels of FMR1 mRNAs and causes a new progressive neurological syndrome, called FXTAS. Although most data is reported about male FM and PM carriers, but female FM and PM carriers can be affected as well. The molecular basis of FXTAS is unknown; however, a toxic RNA gain-of-function mechanism has been proposed and is currently under study. The third disorder is Premature Ovarian Insufficiency which is seen in female PM carrier. The molecular basis of POI is yet unknown.
Acknowledgments
This work was supported in part by grant HD38038 from the US National Institute of Child Health and Human Development to B.A.O., and by the National Institutes of Health (UL1 RR024922; RL1 NS062411) and ZonMw (912-07-022) to R.W. The authors wish to thank Tom de Vries Lentsch for graphical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hagerman RJ. The physical and behavioural phenotype. In: Hagerman RJ, Hagerman P, editors. Fragile-X syndrome: diagnosis, treatment and research. The Johns Hopkins University Press; Baltimore: 2002. pp. 3–109. [Google Scholar]
- 2.Sherman SL, Jacobs PA, Morton NE, Froster-Iskenius U, Howard-Peebles PN, Nielsen KB, Partington MW, Sutherland GR, Turner G, Watson M. Further segregation analysis of the fragile X syndrome with special reference to transmitting males. Hum Genet. 1985;69:289–299. doi: 10.1007/BF00291644. [DOI] [PubMed] [Google Scholar]
- 3.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, Eussen BE, Van Ommen GJB, Blonden LAJ, Riggins GJ, Chastain JL, Kunst CB, Galjaard H, Caskey CT, Nelson DL, Oostra BA, Warren ST. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 4.Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick R, Jr, Warren ST, Oostra BA, Nelson DL, Caskey CT. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 5.Oberlé I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas MF, Mandel JL. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- 6.Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, Warren ST. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1:397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- 7.Nolin SL, Brown WT, Glicksman A, Houck GE, Jr, Gargano AD, Sullivan A, Biancalana V, Brondum-Nielsen K, Hjalgrim H, Holinski-Feder E, Kooy F, Longshore J, Macpherson J, Mandel JL, Matthijs G, Rousseau F, Steinbach P, Vaisanen ML, Von Koskull H, Sherman SL. Expansion of the Fragile X CGG Repeat in Females with Premutation or Intermediate Alleles. Am J Hum Genet. 2003;72:454–464. doi: 10.1086/367713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verheij C, Bakker CE, de Graaff E, Keulemans J, Willemsen R, Verkerk AJ, Galjaard H, Reuser AJ, Hoogeveen AT, Oostra BA. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature. 1993;363:722–724. doi: 10.1038/363722a0. [DOI] [PubMed] [Google Scholar]
- 9.Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 1993;4:335–340. doi: 10.1038/ng0893-335. [DOI] [PubMed] [Google Scholar]
- 10.Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: Nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamanini F, Willemsen R, van Unen L, Bontekoe C, Galjaard H, Oostra BA, Hoogeveen AT. Differential expression of FMR1, FXR1 and FXR2 proteins in human brain and testis. Hum Mol Genet. 1997;6:1315–1322. doi: 10.1093/hmg/6.8.1315. [DOI] [PubMed] [Google Scholar]
- 12.Bakker CE, de Diego Otero Y, Bontekoe C, Raghoe P, Luteijn T, Hoogeveen AT, Oostra BA, Willemsen R. Immunocytochemical and biochemical characterization of FMRP, FXR1P, and FXR2P in the mouse. Exp Cell Res. 2000;258:162–170. doi: 10.1006/excr.2000.4932. [DOI] [PubMed] [Google Scholar]
- 13.De Diego Otero Y, Bakker CE, Raghoe P, Severijnen LWFM, Hoogeveen A, Oostra BA, Willemsen R. Immunocytochemical characterization of FMRP, FXR1P and FXR2P during embryonic development in the mouse. Gene Funct Dis. 2000;1:28–37. doi: 10.1006/excr.2000.4932. [DOI] [PubMed] [Google Scholar]
- 14.Willemsen R, Bontekoe C, Tamanini F, Galjaard H, Hoogeveen AT, Oostra BA. Association of FMRP with ribosomal precursor particles in the nucleolus. Biochem Biophys Res Comm. 1996;225:27–33. doi: 10.1006/bbrc.1996.1126. [DOI] [PubMed] [Google Scholar]
- 15.Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA. 1997;94:5395–5400. doi: 10.1073/pnas.94.10.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberhart DE, Malter HE, Feng Y, Warren ST. The fragile X mental retardation protein is a ribosonucleoprotein containing both nuclear localization and nuclear export signals. Hum Mol Genet. 1996;5:1083–1091. doi: 10.1093/hmg/5.8.1083. [DOI] [PubMed] [Google Scholar]
- 17.Fridell RA, Benson RE, Hua J, Bogerd HP, Cullen BR. A nuclear role for the fragile X mental retardation protein. EMBO J. 1996;15:5408–5414. [PMC free article] [PubMed] [Google Scholar]
- 18.Sittler A, Devys D, Weber C, Mandel JL. Alternative splicing of exon 14 determines nuclear or cytoplasmic localisation of FMR1 protein isoforms. Hum Mol Genet. 1996;5:95–102. doi: 10.1093/hmg/5.1.95. [DOI] [PubMed] [Google Scholar]
- 19.Kim M, Bellini M, Ceman S. Fragile X mental retardation protein FMRP binds mRNAs in the nucleus. Mol Cell Biol. 2008;29:214–228. doi: 10.1128/MCB.01377-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceman S, Brown V, Warren ST. Isolation of an FMRP-Associated Messenger Ribonucleoprotein Particle and Identification of Nucleolin and the Fragile X-Related Proteins as Components of the Complex. Mol Cell Biol. 1999;19:7925–7932. doi: 10.1128/mcb.19.12.7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceman S, Nelson R, Warren ST. Identification of Mouse YB1/p50 as a Component of the FMRP-Associated mRNP Particle. Biochem Biophys Res Commun. 2000;279:904–908. doi: 10.1006/bbrc.2000.4035. [DOI] [PubMed] [Google Scholar]
- 22.Bardoni B, Schenck A, Mandel JL. A novel RNA-binding nuclear protein that interacts with the fragile X mental retardation (FMR1) protein. Hum Mol Genet. 1999;8:2557–2566. doi: 10.1093/hmg/8.13.2557. [DOI] [PubMed] [Google Scholar]
- 23.Schenck A, Bardoni B, Moro A, Bagni C, Mandel JL. A highly conserved protein family interacting with the fragile X mental retardation protein (FMRP) and displaying selective interactions with FMRP-related proteins FXR1P and FXR2P. Proc Natl Acad Sci U S A. 2001;98:8844–8849. doi: 10.1073/pnas.151231598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells RD. Molecular basis of genetic instability of triplet repeats. J Biol Chem. 1996;271:2875–2878. doi: 10.1074/jbc.271.6.2875. [DOI] [PubMed] [Google Scholar]
- 25.White PJ, Borts RH, Hirst MC. Stability of the human fragile X (CGG) (n) triplet repeat array in saccharomyces cerevisiae deficient in aspects of DNA metabolism. Mol Cell Biol. 1999;19:5675–5684. doi: 10.1128/mcb.19.8.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson CE, Tam M, Wang YH, Montgomery SE, Dar AC, Cleary JD, Nichol K. Slipped-strand DNAs formed by long (CAG)*(CTG) repeats: slipped-out repeats and slip-out junctions. Nucleic Acids Res. 2002;30:4534–4547. doi: 10.1093/nar/gkf572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirkin SM. Expandable DNA repeats and human disease. Nature. 2007;447:932–940. doi: 10.1038/nature05977. [DOI] [PubMed] [Google Scholar]
- 28.Mirkin SM. DNA structures, repeat expansions and human hereditary disorders. Curr Opin Struct Biol. 2006;16:351–358. doi: 10.1016/j.sbi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Smeets H, Smits A, Verheij CE, Theelen J, Willemsen R, Losekoot M, Van de Burgt I, Hoogeveen AT, Oosterwijk J, Oostra BA. Normal phenotype in two brothers with a full FMR1 mutation. Hum Mol Genet. 1995;4:2103–2108. doi: 10.1093/hmg/4.11.2103. [DOI] [PubMed] [Google Scholar]
- 30.Eiges R, Urbach A, Malcov M, Frumkin T, Schwartz T, Amit A, Yaron Y, Eden A, Yanuka O, Benvenisty N, Ben-Yosef D. Developmental Study of Fragile X Syndrome Using Human Embryonic Stem Cells Derived from Preimplantation Genetically Diagnosed Embryos. Cell Stem Cell. 2007;1:568–577. doi: 10.1016/j.stem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Reyniers E, Vits L, De Boulle K, Van Roy B, Van Velzen D, de Graaff E, Verkerk AJMH, Jorens HZ, Darby JK, Oostra BA, Willems PJ. The full mutation in the FMR-1 gene of male fragile X patients is absent in their sperm. Nat Genet. 1993;4:143–146. doi: 10.1038/ng0693-143. [DOI] [PubMed] [Google Scholar]
- 32.Malter HE, Iber JC, Willemsen R, De Graaff E, Tarleton JC, Leisti J, Warren ST, Oostra BA. Characterization of the full fragile X syndrome mutation in fetal gametes. Nat Genet. 1997;15:165–169. doi: 10.1038/ng0297-165. [DOI] [PubMed] [Google Scholar]
- 33.Hansen RS, Canfield TK, Lamb MM, Gartler SM, Laird CD. Association of fragile X syndrome with delayed replication of the FMR1 gene. Cell. 1993;73:1403–1409. doi: 10.1016/0092-8674(93)90365-w. [DOI] [PubMed] [Google Scholar]
- 34.Wöhrle D, Hennig I, Vogel W, Steinbach P. Mitotic stability of fragile X mutations in differentiated cells indicates early post-conceptional trinucleotide repeat expansion. Nat Genet. 1993;4:140–142. doi: 10.1038/ng0693-140. [DOI] [PubMed] [Google Scholar]
- 35.Bontekoe CJ, Bakker CE, Nieuwenhuizen IM, van Der Linde H, Lans H, de Lange D, Hirst MC, Oostra BA. Instability of a (CGG)(98) repeat in the Fmr1 promoter. Hum Mol Genet. 2001;10:1693–1699. doi: 10.1093/hmg/10.16.1693. [DOI] [PubMed] [Google Scholar]
- 36.Entezam A, Biacsi R, Orrison B, Saha T, Hoffman GE, Grabczyk E, Nussbaum RL, Usdin K. Regional FMRP deficits and large repeat expansions into the full mutation range in a new Fragile X premutation mouse model. Gene. 2007;395:125–134. doi: 10.1016/j.gene.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brouwer JR, Mientjes EJ, Bakker CE, Nieuwenhuizen IM, Severijnen LA, Van der Linde HC, Nelson DL, Oostra BA, Willemsen R. Elevated Fmr1 mRNA levels and reduced protein expression in a mouse model with an unmethylated Fragile X full mutation. Exp Cell Res. 2007;313:244–253. doi: 10.1016/j.yexcr.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willemsen R, Bontekoe CJ, Severijnen LA, Oostra BA. Timing of the absence of FMR1 expression in full mutation chorionic villi. Hum Genet. 2002;110:601–605. doi: 10.1007/s00439-002-0723-5. [DOI] [PubMed] [Google Scholar]
- 39.Chiurazzi P, Pomponi MG, Pietrobono R, Bakker CE, Neri G, Oostra BA. Synergistic effect of histone hyperacetylation and DNA demethylation in the reactivation of the FMR1 gene. Hum Mol Genet. 1999;8:2317–2323. doi: 10.1093/hmg/8.12.2317. [DOI] [PubMed] [Google Scholar]
- 40.Coffee B, Zhang F, Warren ST, Reines D. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat Genet. 1999;22:98–101. doi: 10.1038/8807. [DOI] [PubMed] [Google Scholar]
- 41.Tabolacci E, Moscato U, Zalfa F, Bagni C, Chiurazzi P, Neri G. Epigenetic analysis reveals a euchromatic configuration in the FMR1 unmethylated full mutations. Eur J Hum Genet. 2008;16:1487–1498. doi: 10.1038/ejhg.2008.130. [DOI] [PubMed] [Google Scholar]
- 42.Chiurazzi P, Pomponi MG, Willemsen R, Oostra BA, Neri G. In vitro reactivation of the FMR1 gene involved in fragile X syndrome. Hum Mol Genet. 1998;7:109–113. doi: 10.1093/hmg/7.1.109. [DOI] [PubMed] [Google Scholar]
- 43.Pietrobono R, Pomponi MG, Tabolacci E, Oostra B, Chiurazzi P, Neri G. Quantitative analysis of DNA demethylation and transcriptional reactivation of the FMR1 gene in fragile X cells treated with 5-azadeoxycytidine. Nucleic Acids Res. 2002;30:3278–3285. doi: 10.1093/nar/gkf434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashley C, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–568. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- 45.Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993;74:291–298. doi: 10.1016/0092-8674(93)90420-u. [DOI] [PubMed] [Google Scholar]
- 46.De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, Van den Bos F, de Graaff E, Oostra BA, Willems PJ. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993;3:31–35. doi: 10.1038/ng0193-31. [DOI] [PubMed] [Google Scholar]
- 47.Siomi H, Choi M, Siomi MC, Nussbaum RL, Dreyfuss G. Essential role for KH domains in RNA binding: impaired RNA binding by a mutation in the KH domain of FMR1 that causes fragile X syndrome. Cell. 1994;77:33–39. doi: 10.1016/0092-8674(94)90232-1. [DOI] [PubMed] [Google Scholar]
- 48.Verheij C, De Graaff E, Bakker CE, Willemsen R, Willems PJ, Meijer N, Galjaard H, Reuser AJJ, Oostra BA, Hoogeveen AT. Characterization of FMR1 proteins isolated from different tissues. Hum Mol Genet. 1995;4:895–901. doi: 10.1093/hmg/4.5.895. [DOI] [PubMed] [Google Scholar]
- 49.Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol Cell. 1997;1:109–118. doi: 10.1016/s1097-2765(00)80012-x. [DOI] [PubMed] [Google Scholar]
- 50.Tamanini F, Bontekoe C, Bakker CE, van Unen L, Anar B, Willemsen R, Yoshida M, Galjaard H, Oostra BA, Hoogeveen AT. Different targets for the fragile X-related proteins revealed by their distinct nuclear localizations. Hum Mol Genet. 1999;8:863–869. doi: 10.1093/hmg/8.5.863. [DOI] [PubMed] [Google Scholar]
- 51.Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- 52.Schaeffer C, Bardoni B, Mandel JL, Ehresmann B, Ehresmann C, Moine H. The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. Embo J. 2001;20:4803–4813. doi: 10.1093/emboj/20.17.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X Mental Retardation Protein Targets G Quartet mRNAs Important for Neuronal Function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- 54.Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray Identification of FMRP-Associated Brain mRNAs and Altered mRNA Translational Profiles in Fragile X Syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 55.Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA Cargoes Associating with FMRP Reveal Deficits in Cellular Functioning in Fmr1 Null Mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 56.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis L, Banker GA, Steward O. Selective dendritic transport of RNA in hippocampal neurons in culture. Nature. 1987;330:477–479. doi: 10.1038/330477a0. [DOI] [PubMed] [Google Scholar]
- 58.Martin KC, Zukin RS. RNA trafficking and local protein synthesis in dendrites: an overview. J Neurosci. 2006;26:7131–7134. doi: 10.1523/JNEUROSCI.1801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knowles RB, Sabry JH, Martone ME, Deerinck TJ, Ellisman MH, Bassell GJ, Kosik KS. Translocation of RNA granules in living neurons. J Neurosci. 1996;16:7812–7820. doi: 10.1523/JNEUROSCI.16-24-07812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bassell GJ, Oleynikov Y, Singer RH. The travels of mRNAs through all cells large and small. Faseb J. 1999;13:447–454. doi: 10.1096/fasebj.13.3.447. [DOI] [PubMed] [Google Scholar]
- 61.De Diego Otero Y, Severijnen LA, Van Cappellen G, Schrier M, Oostra B, Willemsen R. Transport of Fragile X Mental Retardation Protein via Granules in Neurites of PC12 Cells. Mol Cell Biol. 2002;22:8332–8341. doi: 10.1128/MCB.22.23.8332-8341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antar LN, Dictenberg JB, Plociniak M, Afroz R, Bassell GJ. Localization of FMRP-associated mRNA granules and requirement of microtubules for activity-dependent trafficking in hippocampal neurons. Genes Brain Behav. 2005;4:350–359. doi: 10.1111/j.1601-183X.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 63.Kohrmann M, Luo M, Kaether C, DesGroseillers L, Dotti CG, Kiebler MA. Microtubule-dependent recruitment of Staufen-green fluorescent protein into large RNA-containing granules and subsequent dendritic transport in living hippocampal neurons. Mol Biol Cell. 1999;10:2945–2953. doi: 10.1091/mbc.10.9.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang HL, Singer RH, Bassell GJ. Neurotrophin regulation of beta-actin mRNA and protein localization within growth cones. J Cell Biol. 1999;147:59–70. doi: 10.1083/jcb.147.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dictenberg JB, Swanger SA, Antar LN, Singer RH, Bassell GJ. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile X syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, Warren ST. S6K1 phosphorylates and regulates FMRP with the neuronal protein synthesis-dependent mTOR signaling cascade. J Biol Chem. 2008;283:18478–18482. doi: 10.1074/jbc.C800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kiebler MA, DesGroseillers L. Molecular insights into mRNA transport and local translation in the mammalian nervous system. Neuron. 2000;25:19–28. doi: 10.1016/s0896-6273(00)80868-5. [DOI] [PubMed] [Google Scholar]
- 69.Steward O. mRNA at synapses, synaptic plasticity, and memory consolidation. Neuron. 2002;36:338–340. doi: 10.1016/s0896-6273(02)01006-1. [DOI] [PubMed] [Google Scholar]
- 70.Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile X syndrome. Am J Med Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- 71.Rudelli RD, Brown WT, Wisniewski K, Jenkins EC, Laure-Kamionowska M, Connell F, Wisniewski HM. Adult fragile X syndrome. Clinico-neuropathologic findings. Acta Neuropathol. 1985;67:289–295. doi: 10.1007/BF00687814. [DOI] [PubMed] [Google Scholar]
- 72.Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: A quantitative examination. Am J Med Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 73.Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: Maturation and pruning deficits. Proc Natl Acad Sci USA. 1997;94:5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in fmr1 knock-out mice. J Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Vrij FMS, Levenga J, Van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in FMR1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 78.Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 79.Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST. Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci U S A. 2007;104:15537–15542. doi: 10.1073/pnas.0707484104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of Fragile X Syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galvez R, Greenough WT. Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am J Med Genet A. 2005;135:155–160. doi: 10.1002/ajmg.a.30709. [DOI] [PubMed] [Google Scholar]
- 82.Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006;26:7151–7155. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, Vanderwerf F, Bakker CE, Willemsen R, Ikeda T, Kakizawa S, Onodera K, Nelson DL, Mientjes E, Joosten M, De Schutter E, Oostra BA, Ito M, De Zeeuw CI. Deletion of FMR1 in Purkinje Cells Enhances Parallel Fiber LTD, Enlarges Spines, and Attenuates Cerebellar Eyelid Conditioning in Fragile X Syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 85.Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berry-Kravis EM, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open-label single-dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 2009 doi: 10.1136/jmg.2008.063701. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 88.Hu H, Qin Y, Bochorishvili G, Zhu Y, van Aelst L, Zhu JJ. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J Neurosci. 2008;28:7847–7862. doi: 10.1523/JNEUROSCI.1496-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sherman SL. Premature Ovarian Failure among Fragile X Premutation Carriers: Parent-of-Origin Effect? Am J Hum Genet. 2000;67:11–13. doi: 10.1086/302985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, Corrigan EC, Simpson JL, Nelson LM. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 91.Hundscheid RDL, Sistermans EA, Thomas CMG, Braat DDM, Straatman H, Kiemeney LALM, Oostra BA, Smits APT. Imprinting effect in premature ovarian failure confined to paternally inherited fragile X premutations. Am J Hum Genet. 2000;66:413–418. doi: 10.1086/302774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Murray A, Ennis S, Morton N. No Evidence for Parent of Origin Influencing Premature Ovarian Failure in Fragile X Premutation Carriers. Am J Hum Genet. 2000;67:253–254. doi: 10.1086/302963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vianna-Morgante AM, Costa SS. Premature Ovarian Failure Is Associated with Maternally and Paternally Inherited Premutation in Brazilian Families with Fragile X. Am J Hum Genet. 2000;67:254–255. doi: 10.1086/302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile x premutation carriers. J Clin Endocrinol Metab. 2004;89:4569–4574. doi: 10.1210/jc.2004-0347. [DOI] [PubMed] [Google Scholar]
- 95.Rohr J, Allen EG, Charen K, Giles J, He W, Dominguez C, Sherman SL. Anti-Mullerian hormone indicates early ovarian decline in fragile X mental retardation (FMR1) premutation carriers: a preliminary study. Hum Reprod. 2008;23:1220–1225. doi: 10.1093/humrep/den050. [DOI] [PubMed] [Google Scholar]
- 96.Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2004;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 97.Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet. 2006;14:253–255. doi: 10.1038/sj.ejhg.5201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 99.Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Brunberg JA, Greco C, Des Portes V, Jardini T, Levine R, Berry-Kravis E, Brown WT, Schaeffer S, Kissel J, Tassone F, Hagerman PJ. Fragile X Premutation Tremor/Ataxia Syndrome: Molecular, Clinical, and Neuroimaging Correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jacquemont S, Farzin F, Hall D, Leehey M, Tassone F, Gane L, Zhang L, Grigsby J, Jardini T, Lewin F, Berry-Kravis E, Hagerman PJ, Hagerman RJ. Aging in individuals with the FMR1 mutation. Am J Ment Retard. 2004;109:154–164. doi: 10.1352/0895-8017(2004)109<154:AIIWTF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hagerman PJ, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome-an older face of the fragile X gene. Nat Clin Pract Neurol. 2007;3:107–112. doi: 10.1038/ncpneuro0373. [DOI] [PubMed] [Google Scholar]
- 102.Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, Leehey MA, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 103.Iwahashi CK, Yasui DH, An HJ, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB, Hagerman RJ, Hagerman PJ. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129:256–271. doi: 10.1093/brain/awh650. [DOI] [PubMed] [Google Scholar]
- 104.Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, Zhang L, Jardini T, Gane LW, Harris SW, Herman K, Grigsby J, Greco CM, Berry-Kravis E, Tassone F, Hagerman PJ. Penetrance of the fragile x-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 105.Tassone F, Adams J, Berry-Kravis EM, Cohen SS, Brusco A, Leehey MA, Li L, Hagerman RJ, Hagerman PJ. CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS) Am J Med Genet. 2007;144B:566–569. doi: 10.1002/ajmg.b.30482. [DOI] [PubMed] [Google Scholar]
- 106.Leehey MA, Berry-Kravis E, Goetz CG, Zhang L, Hall DA, Li L, Rice CD, Lara R, Cogswell J, Reynolds A, Gane L, Jacquemont S, Tassone F, Grigsby J, Hagerman RJ, Hagerman PJ. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology. 2008;70:1397–1402. doi: 10.1212/01.wnl.0000281692.98200.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin P, Zarnescu DC, Zhang F, Pearson CE, Lucchesi JC, Moses K, Warren ST. RNA-Mediated Neurodegeneration Caused by the Fragile X Premutation rCGG Repeats in Drosophila. Neuron. 2003;39:739–747. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- 108.Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: A new mechanism of involvement in the Fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10:1449–1454. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- 110.Tassone F, Hagerman RJ, Garcia-Arocena D, Khandjian EW, Greco CM, Hagerman PJ. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J Med Genet. 2004;41:E43. doi: 10.1136/jmg.2003.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA withinthe intranuclear inclusions of fragile X-associated Tremor/Ataxia syndrome (FXTAS) RNA biology. 2004;1:103–105. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- 112.Chen LS, Tassone F, Sahota P, Hagerman PJ. The (CGG)n repeat element within the 5′untranslated region of the FMR1 message provides both positive and negative cis effects on in vivo translation of a downstream reporter. Hum Mol Genet. 2003;12:3067–3074. doi: 10.1093/hmg/ddg331. [DOI] [PubMed] [Google Scholar]
- 113.Tassone F, Hagerman RJ, Chamberlain WD, Hagerman PJ. Transcription of the FMR1 gene in individuals with fragile X syndrome. Am J Med Genet. 2000;97:195–203. doi: 10.1002/1096-8628(200023)97:3<195::AID-AJMG1037>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 114.Willemsen R, Oostra BA, Bassell GJ, Dictenberg J. The fragile X syndrome: From molecular genetics to neurobiology. Ment Retard Dev Disabil Res Rev. 2004;10:60–67. doi: 10.1002/mrdd.20010. [DOI] [PubMed] [Google Scholar]
- 115.Willemsen R, Hoogeveen-Westerveld M, Reis S, Holstege J, Severijnen L, Nieuwenhuizen I, Schrier M, VanUnen L, Tassone F, Hoogeveen A, Hagerman P, Mientjes E, Oostra BA. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12:949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- 116.Brouwer JR, Severijnen E, de Jong FH, Hessl D, Hagerman RJ, Oostra BA, Willemsen R. Altered hypothalamus-pituitary-adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology. 2008;33:863–873. doi: 10.1016/j.psyneuen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bergink S, Severijnen LA, Wijgers N, Sugasawa K, Yousaf H, Kros JM, van Swieten J, Oostra BA, Hoeijmakers JH, Vermeulen W, Willemsen R. The DNA repair-ubiquitin-associated HR23 proteins are constituents of neuronal inclusions in specific neurodegenerative disorders without hampering DNA repair. Neurobiol Dis. 2006;23:708–716. doi: 10.1016/j.nbd.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 118.Hashem V, Galloway JN, Mori M, Willemsen R, Oostra BA, Paylor R, Nelson DL. Ectopic expression of CGG containing mRNA is neurotoxic in mammals. 2008 doi: 10.1093/hmg/ddp182. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jin P, Warren ST. New insights into fragile X syndrome: from molecules to neurobehaviors. Trends Biochem Sci. 2003;28:152–158. doi: 10.1016/S0968-0004(03)00033-1. [DOI] [PubMed] [Google Scholar]
- 120.Jin P, Duan R, Qurashi A, Qin Y, Tian D, Rosser TC, Liu H, Feng Y, Warren ST. Pur alpha Binds to rCGG Repeats and Modulates Repeat-Mediated Neurodegeneration in a Drosophila Model of Fragile X Tremor/Ataxia Syndrome. Neuron. 2007;55:556–564. doi: 10.1016/j.neuron.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sofola OA, Jin P, Qin Y, Duan R, Liu H, de Haro M, Nelson DL, Botas J. RNA-Binding Proteins hnRNP A2/B1 and CUGBP1 Suppress Fragile X CGG Premutation Repeat-Induced Neurodegeneration in a Drosophila Model of FXTAS. Neuron. 2007;55:565–571. doi: 10.1016/j.neuron.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Timchenko LT, Miller JW, Timchenko NA, DeVore DR, Datar KV, Lin L, Roberts R, Caskey CT, Swanson MS. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 1996;24:4407–4414. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miller JW, Urbinati CR, Teng-Umnuay P, Stenberg MG, Byrne BJ, Thornton CA, Swanson MS. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. Embo J. 2000;19:4439–4448. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, Barbato I, Rice C, Gould E, Hall DA, Grigsby J, Wegelin JA, Harris S, Lewin F, Weinberg D, Hagerman PJ, Hagerman RJ. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139B:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- 125.Bacalman S, Farzin F, Bourgeois JA, Cogswell J, Goodlin-Jones BL, Gane LW, Grigsby J, Leehey MA, Tassone F, Hagerman RJ. Psychiatric Phenotype of the Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS) in Males: Newly Described Fronto-Subcortical Dementia. J Clin Psychiatry. 2006;67:87–94. doi: 10.4088/jcp.v67n0112. [DOI] [PubMed] [Google Scholar]
- 126.Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, Hessl D, Hagerman PJ, Cogswell JB, Bennett RE, Cook K, Hall DA, Bounds LS, Paulich MJ, Reynolds A. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- 127.Hunter JE, Allen EG, Abramowitz A, Rusin M, Leslie M, Novak G, Hamilton D, Shubeck L, Charen K, Sherman SL. Investigation of Phenotypes Associated with Mood and Anxiety Among Male and Female Fragile X Premutation Carriers. Behav Genet. 2008;38:493–502. doi: 10.1007/s10519-008-9214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Louis E, Moskowitz C, Friez M, Amaya M, Vonsattel JP. Parkinsonism, dysautonomia, and intranuclear inclusions in a fragile X carrier: A clinical-pathological study. Mov Disord. 2006;27:193–201. doi: 10.1002/mds.20753. [DOI] [PubMed] [Google Scholar]
- 129.Greco CM, Soontrapornchai K, Wirojanan J, Gould JE, Hagerman PJ, Hagerman RJ. Testicular and pituitary inclusion formation in fragile X associated tremor/ataxia syndrome. J Urol. 2007;177:1434–1437. doi: 10.1016/j.juro.2006.11.097. [DOI] [PubMed] [Google Scholar]
- 130.Van Dam D, Errijgers V, Kooy RF, Willemsen R, Mientjes E, Oostra BA, De Deyn PP. Cognitive decline, neuromotor and behavioural disturbances in a mouse model for Fragile-X-associated tremor/ataxia syndrome (FXTAS) Behavioural Brain Research. 2005;162:233–239. doi: 10.1016/j.bbr.2005.03.007. [DOI] [PubMed] [Google Scholar]