Summary
The organisation of neuronal processes into series of layers is a hallmark of many brain regions. Homophilic cell adhesion molecules of the cadherin family have been implicated in layer choice. How they contribute to the targeting of neurons to distinct layers remains unclear. Here we systematically explore the role of a classical cadherin, Drosophila N-cadherin (CadN), in the targeting of five classes of related neurons to a series of consecutive layers in the fly visual system. We show that CadN is required in lamina neurons at discrete developmental steps but not used in a layer-specific fashion. Local CadN expression patterns correlate with specific growth cone movements and CadN expression on one growth cone in a specific layer is essential for targeting of processes of another neuron to this layer. We propose that dynamic regulation of CadN enables this widely expressed protein to mediate specific local interactions during neural circuit assembly.
Introduction
During development, neurons elaborate precise patterns of synaptic connections. These are often arranged in layers or strata with different neurons forming connections in different layers. Classical cadherins have been shown to promote layer-specific targeting of axons in both vertebrates (Inoue and Sanes, 1997) (Poskanzer et al., 2003) and invertebrates (Lee et al., 2001) and, thus, may play an evolutionarily conserved role in this process. Our previous studies have focused on understanding how Drosophila N-cadherin (CadN) regulates the layer-specific targeting of growth cones of R7 neurons in a multilayered structure in the optic lobe called the medulla. CadN is required for R7 targeting to a specific medulla layer, designated M6 (Lee et al., 2001). As CadN is a homophilic cell adhesion protein (Iwai et al., 1997) a simple model for R7 layer choice would be one in which CadN is selectively expressed on R7 growth cones and recognizes neuronal processes in the M6 layer that also selectively express CadN. Indeed, specific immunoglobulin superfamily proteins may act as such laminar cues in the inner plexiform layer of the vertebrate retina (Yamagata and Sanes, 2008). However, CadN is broadly expressed in the developing medulla neuropil, indicating that it functions in a different way. To further define the role of cadherins in layer choice, we sought to assess CadN requirements in multiple neurons that target to different layers in the medulla neuropil.
The medulla neuropil comprises ten layers (M1–M10), each divided into ~750 orthogonally arranged columns (Fischbach and Dittrich, 1989) (Meinertzhagen and Hanson, 1993). Similar to, for example, the layers and sublayers of the inner plexiform layer of the mammalian retina (Masland, 2001), medulla layers are closely spaced and contain processes, but not cell bodies, of many types of neurons. Developmental studies allude to a stereotyped and dynamic interplay between different neurites during medulla development (Bazigou et al., 2007) (Ting et al., 2005). Each medulla column contains processes of at least 50–60 different neurons, including projections of R7, another photoreceptor, R8, and five lamina neurons, L1–5 (Fischbach and Dittrich, 1989). L1–5, R7 and R8, which terminate and arborize in specific subsets of the six layers of the outer medulla, provide an example of precise cell-type specific layer choice by a group of neurons within a small shared target region (see Figure 1A).
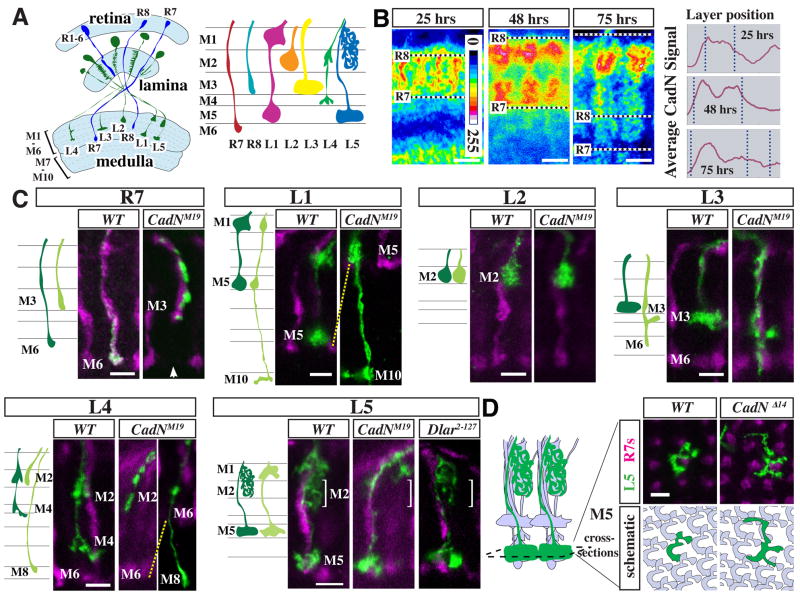
Figure 1. CadN requirements for lamina neuron targeting.
(A) Schematic of R cell (blue) and lamina neuron (green) projections in the adult visual system. Left panel, light blue areas mark the retina and the neuropils of lamina and medulla. Right panel, R cell and lamina neuron terminals in the outer six layers of the medulla. (B) CadN distribution in the developing medulla. Single confocal sections of CadN protein staining (mAb DN-Ex#8) at indicated times after puparium formation (APF) are shown in pseudocolor (see included scale for values). Graphs show layer distribution of the anti-CadN staining intensity (y-scale shown is 0–200) averaged over five adjacent columns. Dotted lines mark corresponding layer positions in confocal images and graphs. Positions of R8 and R7 growth cones are indicated. Scale bars, 5 μm. (C) Single cell CadN mutant phenotypes of L1–L5 and, for comparison, R7. Schematics and confocal images show medulla terminals of wild-type (dark green in cartoon) and CadNM19 mutant (light green) neurons of the indicated cell types. L1–L5 and R7 MARCM clones (anti-GFP staining, green) were generated with dacFLP and GMR-FLP, respectively. R7 and R8 terminals (labeled with mAb 24B10) are shown in purple. CadNM19 and CadNΔ14 (not shown) phenotypes are indistinguishable from CadNM19 on the level of individual cells. Layer choice of lamina neurons mutant for Dlar was wild-type (L5 is shown as an example). Arrow in R7 panel points to the gap in the R7 layer of the column with the mutant R7. L1 and L2 images are of late pupal stages (> 80 hrs APF). All others show adult cells. For quantification of phenotypes, see Table 1. (D) CadN is required for tiling of L5 terminals in M5. Confocal images (top) show L5 MARCM clones (green) and R7 axons (purple) in cross-sections of the M5 layer at 90 hrs APF. The cartoon on the right illustrates the grouping of L1–L5, R7 and R8 terminals into columns. In the schematic (bottom) wild-type L5s are grey and one wild type (left) or mutant cell (right) is green. Wild-type L5 terminals tile the M5 layer with little overlap of L5 processes from adjacent columns whereas CadN mutant L5s extend past column boundaries. Scale bars, 5 μm.
In this study, we have used single cell genetic analyses to examine CadN functions in the layer-specific targeting of all five lamina neurons. We show that some, but not all, lamina neurons require CadN for layer-specific termination of axons. CadN requirements differ between cell types, but are not layer specific. Developmental analyses show that CadN acts at specific targeting steps and, together with protein expression studies, further suggest that growth cones respond to local differences in CadN distribution. In a specific case, we show that layer-specific targeting requires CadN mediated interactions between the interstitial branches of one lamina neuron and the growth cone of another. In contrast to CadN, the receptor tyrosine phosphatase Dlar, which is required for R7 layer choice (Clandinin et al., 2001; Maurel-Zaffran et al., 2001) and has been shown to modulate the activity of classical cadherins in vertebrate cell culture systems (Lilien and Balsamo, 2005), is dispensable for L1–5 targeting. Together these data indicate that cell-type specific CadN-mediated interactions contribute to layer-specific targeting in the medulla.
Results
N-cadherin is strongly expressed in lamina neurons during layer development
CadN protein is dynamically expressed in a distinct stage-specific fashion in the developing medulla neuropil (Figure 1B). For example, at 25 hrs after pupal formation (APF), CadN is strongly expressed between the R7 and R8 growth cones that mark the boundaries of the outer medulla at this stage. This CadN-rich region later divides into two layers separated by a zone of weaker CadN staining (Figure 1B, 48 hrs APF) (Lee et al., 2001). At even later stages, CadN labeling was most prominent in a single layer in the distal part of the outer medulla (Figure 1B, 75 hrs APF). These patterns of CadN protein were suggestive of differential CadN expression in processes of different cell-types, in specific subcellular domains or at specific cell-contacts.
We next sought to determine the cellular origin of these patterns. As CadN is largely localized to processes, we assessed CadN expression in early to mid-stage pupal optic lobes in flies heterozygous for a mutant form of CadN, encoded by CadNM12, which accumulates in cell bodies (Iwai et al., 1997) (Figure S1A). This showed strong labeling of lamina neuron cell bodies compared to most cell bodies in the medulla cortex. To directly test whether CadN was present on the growth cones of lamina neurons in the medulla neuropil, we examined CadN protein patterns in genetically mosaic animals (for details see below) in which CadN was specifically removed from individual lamina neurons (Figure S1B,C). Wild-type lamina neuron growth cones overlapped with specific CadN-rich structures, whereas the corresponding CadN immunoreactivity in columns with mutant L1, L2, L3 or L5 growth cones was reduced. For L4, staining results were less clear. But as L4 requires CadN for targeting in a cell autonomous fashion (see below), it must also express CadN. These results showed that CadN is expressed on the growth cones of developing lamina neurons and that lamina neurons are a major source of the distinct CadN patterns in the medulla neuropil. This raised the possibility that CadN is required for lamina neuron targeting and that CadN may mediate interactions between lamina neuron growth cones within developing columns.
N-cadherin requirements for lamina neuron targeting are cell-type not layer specific
To assess phenotypes of mutant L1–L5 neurons, we used mosaic analysis with a repressible cell marker (MARCM) (Lee and Luo, 1999) with dacFLP (Millard et al., 2007). This approach permits the generation and visualization of individual homozygous mutant lamina neurons surrounded by heterozygous tissue (Figures 1C; S2A,C,D). The terminals of wild-type L1–L5 neurons in the medulla each have a distinct morphology (Figure 1A,C) (Fischbach and Dittrich, 1989). CadN mutant lamina neurons were identified as L1–L5 by marker expression and, in some cases, morphological criteria (see Supplementary Experimental Procedures and Figure S2D). We used two different CadN alleles: CadNM19, a truncation mutant that is generally considered a null mutation based on genetic criteria (Iwai et al., 1997) and CadNΔ14, a deletion that disrupts both CadN and the adjacent highly related N-cadherin2 (CadN2) gene (Prakash et al., 2005). In addition to CadN, we also examined lamina neuron phenotypes of null mutants of the DLar receptor tyrosine phosphatase, which has an R7 phenotype similar to CadN (Clandinin et al., 2001; Maurel-Zaffran et al., 2001).
Removal of CadN from individual lamina neurons resulted in distinct cell-type specific targeting phenotypes (Figure 1C, Table 1). Mutant L1, L3 and L4 axons projected to inappropriate layers: mistargeted L1s typically terminated in M10 rather than M5, L3s in M5 and M6 rather than M3, and L4s in either M2 or, less frequently, M8 instead of M4. Mutant L2 and L5 axons stopped in the correct layers M2 and M5. However, without CadN, the L5 interstitial branches invariably failed to extend from M1 into M2. The penetrance of these phenotypes ranged from 22% to 100% (Table 1). All phenotypes were observed with both CadN alleles, although the L3 layer choice defect was stronger in CadNΔ14. This difference may reflect a specific requirement for CadN2 in L3 but could also be due to residual CadN activity in CadNM19 or differences in genetic background. In contrast to CadN, single cell Dlar mutants did not affect layer choice of L1–L5 (Table 1, Figure 1C and data not shown).
Table 1. Quantification of lamina neuron targeting phenotypes.
Percentage of mistargeted axons and branches and the total number of cells and animals examined are listed for each genotype and layer choice phenotype. For comparison, R7 and R8 are also included. Phenotypes are illustrated in Figure 2. DLar alleles used were Dlar2–127 and DlarC12. R8 targeting was scored in ey3.5FLP clones with Rh5-lacZ as a marker. A small percentage of both mutant and control R8s (numbers in parentheses) appeared to extend significantly deeper into the medulla (~M5) than the remaining R8s.
| Cell | Allele | Mistargeting [%] | Cells | Animals |
|---|---|---|---|---|
| L1 | CadN | 0 | 142 | 14 |
| CadNΔ14 | 29 | 332 | 16 | |
| CadNM19 | 22 | 189 | 10 | |
| Dlar | 0 | 155 | 9 | |
| L2 | CadN | 0 | 125 | 14 |
| CadNΔ14 | 0 | 121 | 16 | |
| CadNM19 | 0 | 62 | 10 | |
| Dlar | 0 | 90 | 9 | |
| L3 | CadN | 1 | 252 | 21 |
| CadNΔ14 | 81 | 375 | 31 | |
| CadNM19 | 25 | 338 | 26 | |
| Dlar | 0 | 76 | 16 | |
| L4 | CadN | 0 | 70 | 21 |
| CadNΔ14 | 81 | 129 | 15 | |
| CadNM19 | 65 | 268 | 17 | |
| Dlar | 0 | 105 | 14 | |
| L5 terminal | CadN | 0 | 25 | 4 |
| CadNΔ14 | 0 | 138 | 6 | |
| CadNM19 | 0 | 88 | 5 | |
| Dlar | 0 | 99 | 5 | |
| L5 branch | CadN | 0 | 25 | 4 |
| CadNΔ14 | 100 | 138 | 6 | |
| CadNM19 | 100 | 88 | 5 | |
| Dlar | 0 | 99 | 5 | |
| R7 | CadN | 0 | 87 | 4 |
| CadNΔ14 | 100 | 128 | 4 | |
| CadNM19 | 100 | 59 | 2 | |
| Dlar | 95 | 321 | 10 | |
| R8 | CadN | (3) | 187 | 2 |
| CadNΔ14 | (4) | 501 | 6 | |
| CadNM19 | (3) | 111 | 3 |
Interestingly, while targeting of the L5 branches to M2 requires CadN, the targeting of the L5 growth cone to M5 does not (Figure 1C). CadN is, however, necessary for tiling of L5 processes, that is restricting L5 terminal processes to a single column within the M5 layer (Figure 1D). Thus, CadN can act differently in axon terminals and interstitial branches of one cell within the same target region.
In summary, all lamina neurons, with the exception of L2, require CadN cell-autonomously for layer-specific targeting. There is no simple correlation between the layer specificity of a given neuron and its CadN phenotype. For example, CadN is necessary for extension of L5 interstitial branches but not L2 terminals to M2, for termination of L3 but not R8 (Choe et al., 2006)(Table 1) axons in M3, and for termination of L1 but not L5 axons in M5. Thus, CadN requirements for layer choice differ between cell-types but are not layer-specific.
N-cadherin is required at specific steps of lamina neuron targeting
To determine the developmental origin of these complex yet specific CadN requirements, we compared growth cone shape and position of wild-type and CadNΔ14 lamina neurons in an otherwise wild-type background at different pupal stages. We focused on L3 and L5, which can be identified with cell-type specific markers and have distinct CadNΔ14 phenotypes.
Layer specificity in the medulla emerges through a highly choreographed sequence of growth cone movements and interstitial branching (Figures 2, S3A). Figure 3A summarizes the development of wild-type projections of L3 and L5 and, for comparison, R7 and R8 photoreceptor neurons. As previously shown, R7 and R8 growth cones initially target to temporary layers in early pupal development followed by later progression to their final layers (Ting et al., 2005) (Figure 2A). Similarly, L1–L5 growth cones already occupy distinct positions at early stages (e.g. 25 hrs APF), but undergo further shape changes and short-range movements before reaching their adult form (Figures 2A–C; S3A; 1C). At 25 hrs APF, wild-type L3 growth cones are long and thin, and extend along one side of a developing medulla column with their centers approximately halfway between the R7 and R8 temporary layers (Figure 2A,B). Between 25 hrs APF and 48 hrs APF, L3 processes asymmetrically spread into the column to acquire a more bulbous morphology and a precise laminar position (Figure 2B). By contrast, L5 growth cones are located just distal to and overlapping with the R7 terminals from 25 hrs APF to adult (Figure 2C, Figure 1C). The targeting of the interstitial L5 branch follows a very different time course. L5 branches appear in M1 at ~ 48 hrs APF (Figure 2C). They become more complex within M1 over the ensuing 24 hrs before these processes extend into M2 between 75 hrs APF and 90 hrs APF (Figure 2C). L1, L2 and L4 projections also develop in a distinct cell type specific sequence (Figure S3A). Thus, lamina neuron targeting occurs in discrete steps at distinct stages of pupal development.
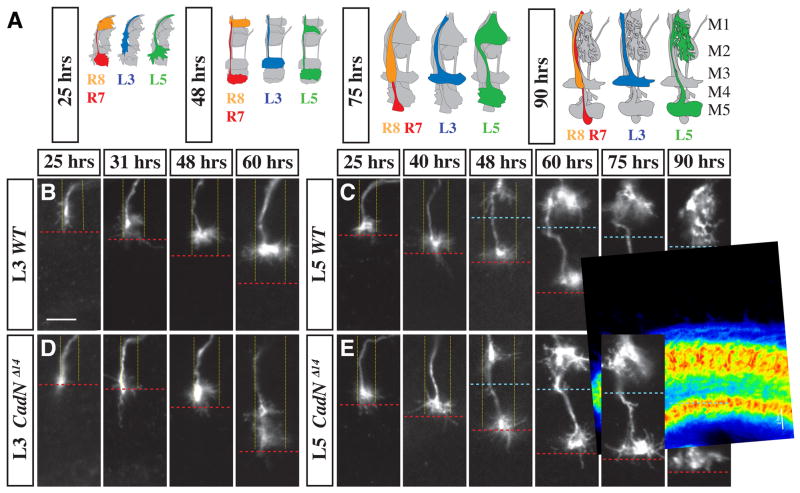
Figure 2. CadN is required for specific steps of L3 and L5 targeting.
(A) Schematic of wild-type L3, L5, R7 and R8 growth cones at different developmental stages (indicated as hrs APF). In each cartoon, all R cell and lamina neuron processes in a medulla column are shown in grey with either L3 (blue), L5 (green) or both R7 (red) and an R8 (orange) highlighted. (B–E) Medulla terminals of single wild-type (B,C) and CadNΔ14 mutant (D,E) (L3 [B,D] and L5 [C, E]) neurons at indicated times APF. Note the CadN-dependent lateral spread of L3 growth cones within their layer at early- to midpupal stages and the late pupal extension of L5 processes into M2. Column boundaries (fine yellow dotted lines) and M2/M3 layer boundary (blue dashed line) are based on anti-CadN staining and the R7 layer position (red dashed lines) on mAb24B10 staining (not shown). L3 and L5 clones were visualized by MARCM with dacFLP and the 9–9 GAL4 marker plus two copies of UAS mCD8GFP (L3) or the 6–60 GAL4 marker with UAS mCD8GFP and UAS N-sybGFP (L5). Scale bar, 5 μm.
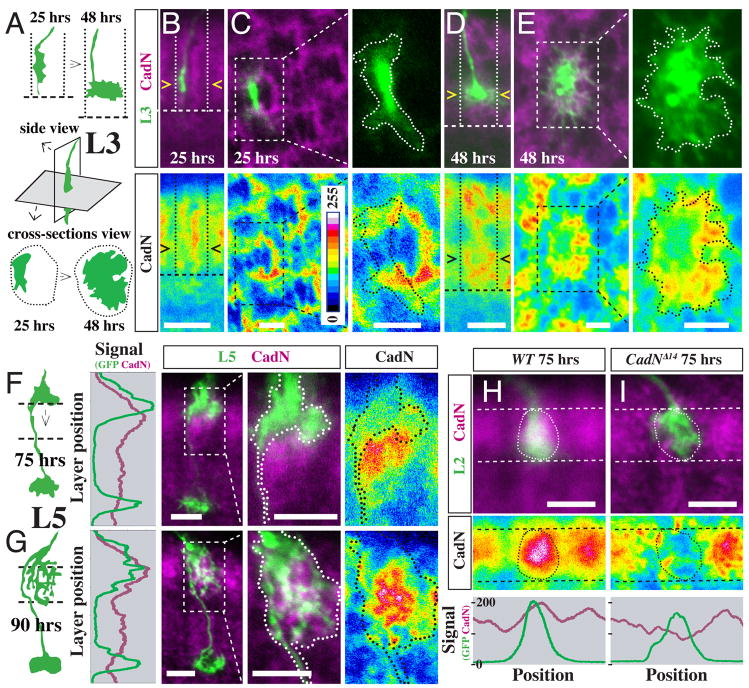
Figure 3. Local patterns of CadN protein expression correlate with CadN-dependent steps in L3 and L5 targeting.
(A–E) Asymmetric CadN expression precedes lateral L3 growth cone expansion in the developing M3 layer. (A) Schematic of wild-type L3 growth cone position and shape at 25 and 48 hrs APF in lateral and cross section views. Dotted lines, column boundaries; dashed lines, the R7 layer. (B–E) CadN protein (purple [upper panels] or pseudocolor [lower panels]) and position of L3 growth cones (green [upper panels]) at 25 hrs APF (B,C) and 48 hrs APF (D,E) APF. Confocal images show side views (B,D) or column cross sections (C,E). L3 growth cones are outlined in higher magnification views in (C,E). L3s and L5s (see below) were labeled by MARCM as in Figure 2. (F,G) Strong CadN protein expression in M2 precedes directed extension of L5 interstitial branches into this layer. Schematics, graphs and confocal images of L5s and CadN patterns before (F, 75 hrs APF) and after (G, 90 hrs APF) branch extension into M2. For the graph, CadN (purple) and GFP (green) channel signals were averaged across the column. (H,I) CadN expression on L2 growth cones at 75 hrs APF. MARCM clones of wild-type (H) and CadNΔ14 mutant (I) L2 terminals (green, circled) and CadN protein in the M2 layer (dashed lines) are shown. Graphs show the average CadN (purple) and GFP (green) signals between the dashed lines. Scale bars, 5 μm (B,D,F,G,H,I) or 2.5 μm (C,E). Intensity scale in (B) applies to all pseudocolor images.
We next examined when CadN was required during L3 and L5 targeting. At 25 hrs APF, the centers of CadNΔ14 L3 growth cones were located in the R7 temporary layer about 2 μm more proximal than wild-type L3s (Figure 2D). While both wild-type and mutant L3 terminals have a similar elongated morphology at this stage, the subsequent lateral extension and layer refinement of wild-type growth cones does not take place in the mutant (Figure 2B,D). Instead, the mutant L3 terminals continue to exhibit incorrect layer choice, and remain elongated along one side of the column with only some diffuse lateral processes (Figure 2D; see also Figure 1C). The CadN mutant defects in L5 occur at a later stage than in L3. Layer choice of mutant L5 terminals was indistinguishable from wild type throughout development (Figure 2C,E). However, while wild type interstitial branches extended into M2 between 75 hrs APF and 90 hrs APF (Figure 2C), those of mutant L5 neurons did not (Figure 2E, Figure 1C). These results indicate that CadN is required to execute specific developmental steps in different lamina neurons.
Local N-cadherin expression patterns correlate with movements of L3 and L5 processes at specific developmental steps
How does CadN function at these targeting steps? As a homophilic cell adhesion molecule (Iwai et al., 1997), CadN on a growth cone presumably binds to CadN molecules on the processes of other cells. We therefore compared CadN protein expression patterns in the vicinity of L3 and L5 growth cones before and after CadN-dependent changes. For L3, we focused on the asymmetric lateral expansion of its terminal between 25 hrs APF and 48 hrs APF (Figure 3A). At 25 hrs APF, L3 growth cones are restricted to one side of a CadN-rich column (Figure 3B,C). Column cross-sections at this stage show L3 growth cones next to a semicircle of strong CadN staining with little overlap between these two structures (Figure 3C). By 48 hrs APF, L3 growth cones have expanded and now cover the entire CadN-rich area in their layer (Figure 3D,E). Similarly, during late L5 development (Figure 3F,G), strong CadN expression in the M2 layer was observed shortly before (75 hrs APF, Figure 3F) and after (90 hrs APF, Figure 3G) extension of L5 processes into this layer. While L5 interstitial branches barely overlap with the strongest CadN staining at 75 hrs APF, they largely fill the CadN-rich area at 90 hrs APF. Specific CadN expression patterns may also play a role during L4 targeting (Figure S3B). Thus, during discrete developmental periods, L3 and L5 processes expand in the direction of specific CadN-rich structures. Together with the CadN mutant phenotypes, these results suggested that CadN-mediated local interactions between processes of specific lamina neurons and neurites of CadN expressing cells forming these CadN-rich domains play a crucial role in directing layer-specific targeting.
N-cadherin in L2 is required for layer choice of L5 interstitial branches
We sought to identify cell(s) involved in the specific CadN-dependent interactions of lamina neuron growth cones. As CadN is strongly expressed in lamina neurons (see Figure S1), we speculated that CadN-mediated interactions between lamina neuron axons could play a role in target layer specificity. While the cell(s) forming the CadN-rich semicircle in the incipient M3 layer (Figure 3C) remains to be identified, the strong CadN staining within the M2 layer (Figure 3H,I) requires CadN expression on L2 growth cones; CadN staining in M2 at 75 hrs APF largely overlapped with wild-type L2 growth cones and was strongly reduced in columns containing a mutant L2 (Figure 3H,I). This result raised the possibility that CadN expressed on the surface of L2 was required for L5 branches to extend into M2. Since CadN mutant L2 growth cones still project to the M2 layer, it was possible to directly test this hypothesis.
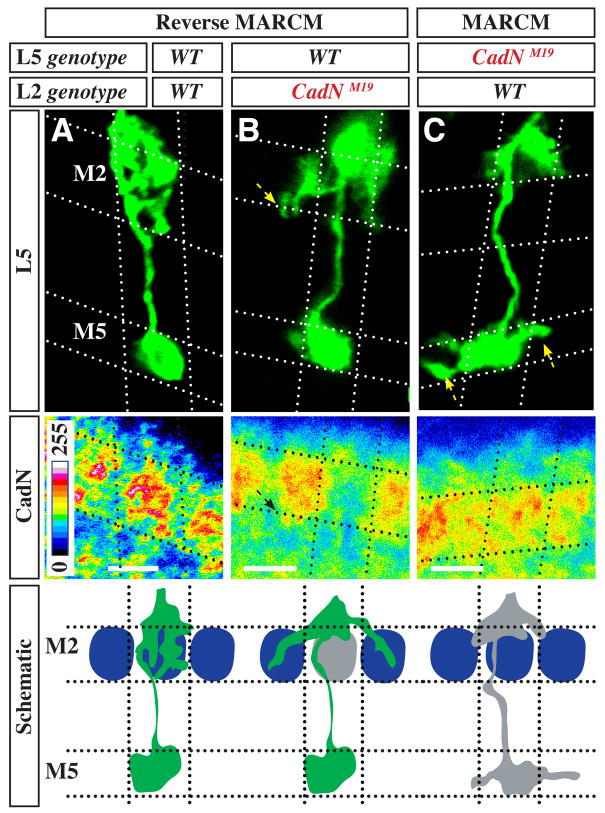
To assess the targeting of wild-type L5 interstitial branches in the presence of CadN mutant L2 cells, we used reverse MARCM (Lee et al., 2000) (see Figure S2B) with dacFLP and an L5-specific marker (Figure 4A,B). As in the MARCM experiments described above, this approach generates mosaic optic lobes in which CadN is specifically removed from individual lamina neurons. However, now only genotypically wild-type, rather than mutant, L5 cells are labeled. Mutant L5 phenotypes therefore indicate a non-autonomous CadN requirement in another lamina neuron. Out of 825 wild type L5 neurons in these experiments, 44 were observed in which almost no interstitial branches extended into M2 within the same column. Instead, these neurons sent branches laterally into adjacent columns (Figure 4B). By contrast, none of 220 control L5s in an entirely wild-type medulla (not shown) showed targeting defects. As L2 is the only lamina neuron that terminates in the M2 layer, these results strongly indicated that CadN on L2 growth cones is necessary for layer choice of L5 interstitial branches. This conclusion was further supported by the observation that in all cases of columns with a phenotypically mutant L5 neuron, CadN protein staining in the M2 layer was strongly reduced (Figure 4B). Such an M2 specific reduction of CadN levels is only seen when L2, but not L5 (Figure 4C) or another lamina neuron (data not shown), lacks CadN. No reduction in CadN expression within M2 was observed for 179 randomly selected columns with phenotypically wild-type L5s (Figure 4A). The simplest interpretation of these results is that CadN on L5 branches recognizes CadN on the surface of L2 growth cones and utilizes this homophilic binding to promote a specific targeting step. In this model, CadN interactions between L5 and L2 growth cones sharing the same column both prevents L5 branches from entering adjacent columns and promotes extension of these branches within the column from layer M1 into M2. In summary, these results show that the projection of L2 growth cones to a specific layer provides a critical spatial cue for the subsequent targeting of processes of another afferent, L5, to this layer. CadN-mediated cell recognition may be sufficient to mediate this targeting step or, alternatively, CadN may function to enable L5 processes to respond to an as yet unidentified M2-specific targeting cue.
Figure 4. Targeting of L5 branches to M2 requires CadN in L2.
(A–C) Extension of L5 processes into M2 requires CadN in both L2 and L5. L5s of indicated genotypes (green, upper panels) were visualized by reverse MARCM (A,B) or MARCM (C) with dacFLP. Reverse MARCM was used to label genotypically wild-type L5s in the presence of CadN mutant L2s, while MARCM was used to label genotypically mutant L5s in the presence of wild type L2s. Genotypes of L2s in columns with a labeled mutant L5 were inferred from CadN staining in M2 (pseudocolor, lower panels) and the specificity of dacFLP expression to lamina precursor cells. Dotted lines mark approximate column and layer boundaries. Results are summarized in cartoon form in bottom panel. When both L5 interstitial branches (green) and L2 growth cones (blue oval) express CadN, L5 processes extend into the M2 layer of the same column. If L2 lacks CadN (grey oval), the vast majority of wild-type L5 processes do not extend into the M2 layer within the same column. Instead, some of these processes extended into adjacent columns (B, arrows), which rarely, if ever, occurs in the wild-type. Processes of CadN mutant L5s (grey) sometimes enter adjacent columns in M1 but do not project into M2. Tiling of L5 terminals in the M5 layer requires CadN in L5 (C, arrows) but not in L2 (compare L5 terminal in B). Scale bars, 5 μm.
Discussion
The formation of the distinct layer-specific projections of lamina neurons and R cells in the medulla is a striking example of precise neuronal targeting. In this study we have shown that a classical cadherin, CadN, is dynamically regulated in the developing medulla and required for targeting of multiple lamina neurons to specific layers. Our results support a model in which cell-type specific modulation of CadN’s homophilic binding activity plays a key role in regulating discrete targeting steps.
Our developmental analyses provide insights into the cellular strategies of layer formation in the medulla. Lamina neurons, as well as R7 and R8 (Ting et al., 2005), acquire their final layer position and shape in a stepwise fashion. After projection of R8, R7 and L1–5 to their initial temporary layers, the medulla not only continues to expand but further changes in the relative positions of afferent growth cones to one another take place. Thus, the precise packing of the terminals of L1–L5, R7 and R8 in the adult medulla is neither a consequence of targeting to pre-existing layers nor of the exact sequence of afferent innervation. Instead, our results suggest that the layers of the adult medulla emerge through the co-ordinated execution of multiple neuron-specific targeting programs.
We present evidence that interactions between lamina neurons (i.e., L2 and L5) are essential for layer-specific targeting. Similarly, previous genetic experiments indicated that specific interactions between R1-R6 growth cones are required for their targeting in the lamina (Clandinin and Zipursky, 2000). These afferent interactions in the lamina and medulla involve neurons that are not synaptic partners (Meinertzhagen and Hanson, 1993) (S. Takemura and I. A. Meinertzhagen, personal communication). These findings argue that, in addition to afferents recognizing their targets, interactions between different classes of afferents play a key role in local patterning of terminals within the target region.
CadN is required in each lamina neuron for specific aspects of targeting, acting automously in L1 and L3-L5, and non-autonomously in L2. Previous work has identified CadN requirements in several classes of neurons in different regions of the developing nervous system (Hummel and Zipursky, 2004; Iwai et al., 1997; Lee et al., 2001; Prakash et al., 2005; Zhu and Luo, 2004). These include roles for CadN in extension of axons to targets (Prakash et al., 2005), aggregation of neuronal processes of the same class (Hummel and Zipursky, 2004), and maintenance of axonal contacts (Zhu and Luo, 2004). What makes the CadN phenotypes in lamina neurons and R cells in the medulla so striking is that they occur within the context of a small shared volume of neuropil, where several afferent growth cones are in direct contact or filopodial reach of each other. The discrete CadN phenotypes in these neurons therefore strongly suggest that CadN expression or function is modulated in a cell-type specific fashion such that growth cones in close proximity use CadN in different ways.
How can CadN regulate discrete targeting steps in different neurons within a common neuropil? Clues come from both cell culture and in vivo studies. For instance, it has long been recognized that differences in the level of expression of the same cadherin in different populations of cells can mediate their separation in vitro (Steinberg and Takeichi, 1994). In vivo, differential adhesion is, for example, important in the developing Drosophila ovary, where local differences in E-cadherin levels determine the position of the oocyte (Godt and Tepass, 1998). Similarly, local differences in CadN expression in the medulla could direct L5 interstitial branches, and perhaps other lamina neuron processes, at specific targeting steps. When L2 lacks CadN and CadN expression in the M2 layer of a column is thus reduced, almost all wild-type L5 branches from this column fail to target to the M2 layer within the column and some branches instead extend into this layer in adjacent columns, where CadN levels remain high. While spatial signals in addition to CadN may obvioulsy be involved, the simplest interpretation of these results is that L5 branches target to the region with the highest CadN levels in their vicinity. In this scenario, additional extrinsic or intrinsic factors would determine the timing of L5 branch extension.
Distinct behavior of different CadN-expressing growth cones could reflect specific regulation of CadN activity. For example, multiple cell surface proteins can modulate cadherin function. These include receptor tyrosine phosphatases such as Lar (Lilien and Balsamo, 2005), protocadherins such Arcadlin (Yasuda et al., 2007), Nectins (Togashi et al., 2006), and axon guidance receptors of the Robo family (Rhee et al., 2007). Indeed, DLar, the fly ortholog of Lar, is required for R7 targeting (Clandinin et al., 2001; Maurel-Zaffran et al., 2001), but, as we report here, it is not necessary for the targeting of lamina neurons.
In addition to its roles in layer specifcity, CadN is required for restricting L5 terminal processes to a single column. While it is possible that CadN promotes homotypic repulsive interactions between L5 growth cones within the M5 layer, as recently proposed for Dscam2 in the tiling of L1 terminals (Millard et al., 2007), we think it is more likely that this L5 tiling phenotype results from a loss of adhesion between L5 terminals and other processes within the M5 layer. Interestingly, L1 and L5 terminals form synapses with each other and share extensive regions of membrane apposition (S. Takemura and I. A. Meinertzhagen, personal communication). Thus, L1 may contribute to the tiling of L5 terminals, just as L2 is important for columnar restriction of L5 interstitial branches
The repeated use of a broadly expressed cell surface molecule at distinct developmental steps within a tissue is not unique to the role CadN in the medulla. For instance, the epidermal growth factor and Notch signaling pathways contribute to cell fate decisions in the Drosophila retina in multiple ways (Doroquez and Rebay, 2006). Indeed, it has been argued that the vast majority of intercellular patterning in tissue development relies on dynamic regulation of a small core set of signaling systems acting alone or in combination with one another. We propose that CadN has a similar widespread role in patterning of axons and dendrites in the developing nervous system. Presumably, CadN mediates specific growth cone behaviors through its combination with other signals, which are determined by cell type, growth cone position and developmental stage. Given the widespread expression of classical cadherins in the developing brain (Takeichi, 2007), we speculate that during evolution the genetic programs regulating brain wiring have repeatedly recruited this ancient module to promote selective interactions between neuronal processes in many different contexts.
Experimental Procedures
Lamina neuron clones were visualized using MARCM (Lee and Luo, 1999) or reverse MARCM (Lee et al., 2000) with dacFLP (Millard et al., 2007) and a variety of GAL4 markers and GFP reporters. Single cell R7 phenotypes were analyzed as described (Nern et al., 2005). R8 targeting was scored in ey3.5FLP (Bazigou et al., 2007) mosaics. Unless indicated otherwise, phenotypes were scored at the adult stage. For developmental analyses, white prepupae were collected and incubated for the indicated number of hours at 25 °C. Histology of fly brains was essentially as described (Lee et al., 2001). Samples were imaged on a Zeiss LSM 510 Meta confocal microscope with 40x/1.3 NA or 63x/1.4 NA oil objectives. Unless otherwise indicated, images show single confocal sections. Different channels were scanned sequentially. For presentation purposes, most images shown are cropped and linear adjustments to brightness and contrast were made in some cases (Figures 1C [some panels, one or both channels], 3D,E [both channels], 4A,B,C and S3B [GFP only]). For detailed Experimental Procedures see Supplemental Materials.
Supplementary Material
Acknowledgments
We thank members of the Zipursky lab for comments on the manuscript and Tom Clandinin, Ulrike Heberlein, Liquin Luo, Iris Salecker, Tadashi Uemura, the Bloomington Stock center and the Developmental Studies Hybridoma Bank for reagents. A.N. was supported by postdoctoral fellowships from the European Molecular Biology Organization and the Human Frontiers Science Program. S.L.Z. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bazigou E, Apitz H, Johansson J, Loren CE, Hirst EM, Chen PL, Palmer RH, Salecker I. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. 2007;128:961–975. doi: 10.1016/j.cell.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Choe KM, Prakash S, Bright A, Clandinin TR. Liprin-alpha is required for photoreceptor target selection in Drosophila. Proc Natl Acad Sci U S A. 2006;103:11601–11606. doi: 10.1073/pnas.0601185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, Lee CH, Herman T, Lee RC, Yang AY, Ovasapyan S, Zipursky SL. Drosophila LAR regulates R1-R6 and R7 target specificity in the visual system. Neuron. 2001;32:237–248. doi: 10.1016/s0896-6273(01)00474-3. [DOI] [PubMed] [Google Scholar]
- Clandinin TR, Zipursky SL. Afferent growth cone interactions control synaptic specificity in the Drosophila visual system. Neuron. 2000;28:427–436. doi: 10.1016/s0896-6273(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Doroquez DB, Rebay I. Signal integration during development: mechanisms of EGFR and Notch pathway function and cross-talk. Crit Rev Biochem Mol Biol. 2006;41:339–385. doi: 10.1080/10409230600914344. [DOI] [PubMed] [Google Scholar]
- Fischbach KF, Dittrich APM. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell and Tissue Research. 1989;258:441. [Google Scholar]
- Godt D, Tepass U. Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature. 1998;395:387–391. doi: 10.1038/26493. [DOI] [PubMed] [Google Scholar]
- Hummel T, Zipursky SL. Afferent induction of olfactory glomeruli requires N-cadherin. Neuron. 2004;42:77–88. doi: 10.1016/s0896-6273(04)00158-8. [DOI] [PubMed] [Google Scholar]
- Inoue A, Sanes JR. Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron. 1997;19:77–89. doi: 10.1016/s0896-6273(00)80349-9. [DOI] [PubMed] [Google Scholar]
- Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Lee T, Winter C, Marticke SS, Lee A, Luo L. Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron. 2000;25:307–316. doi: 10.1016/s0896-6273(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Maurel-Zaffran C, Suzuki T, Gahmon G, Treisman JE, Dickson BJ. Cell-autonomous and -nonautonomous functions of LAR in R7 photoreceptor axon targeting. Neuron. 2001;32:225–235. doi: 10.1016/s0896-6273(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Hanson TE. The Development of Drosophila melanogaster. (CSH Laboratory Press); 1993. The Development of the Optic Lobe. [Google Scholar]
- Millard SS, Flanagan JJ, Pappu KS, Wu W, Zipursky SL. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature. 2007;447:720–724. doi: 10.1038/nature05855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nern A, Nguyen LV, Herman T, Prakash S, Clandinin TR, Zipursky SL. An isoform-specific allele of Drosophila N-cadherin disrupts a late step of R7 targeting. Proc Natl Acad Sci U S A. 2005;102:12944–12949. doi: 10.1073/pnas.0502888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer K, Needleman LA, Bozdagi O, Huntley GW. N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. J Neurosci. 2003;23:2294–2305. doi: 10.1523/JNEUROSCI.23-06-02294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S, Caldwell JC, Eberl DF, Clandinin TR. Drosophila N-cadherin mediates an attractive interaction between photoreceptor axons and their targets. Nat Neurosci. 2005;8:443–450. doi: 10.1038/nn1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee J, Buchan T, Zukerberg L, Lilien J, Balsamo J. Cables links Robo-bound Abl kinase to N-cadherin-bound beta-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat Cell Biol. 2007;9:883–892. doi: 10.1038/ncb1614. [DOI] [PubMed] [Google Scholar]
- Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci U S A. 1994;91:206–209. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- Ting CY, Yonekura S, Chung P, Hsu SN, Robertson HM, Chiba A, Lee CH. Drosophila N-cadherin functions in the first stage of the two-stage layer-selection process of R7 photoreceptor afferents. Development. 2005;132:953–963. doi: 10.1242/dev.01661. [DOI] [PubMed] [Google Scholar]
- Togashi H, Miyoshi J, Honda T, Sakisaka T, Takai Y, Takeichi M. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J Cell Biol. 2006;174:141–151. doi: 10.1083/jcb.200601089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Tanaka H, Sugiura H, Okamura K, Sakaguchi T, Tran U, Takemiya T, Mizoguchi A, Yagita Y, Sakurai T, et al. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2beta and p38 MAP kinases. Neuron. 2007;56:456–471. doi: 10.1016/j.neuron.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Luo L. Diverse functions of N-cadherin in dendritic and axonal terminal arborization of olfactory projection neurons. Neuron. 2004;42:63–75. doi: 10.1016/s0896-6273(04)00142-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.