Abstract
Ground squirrels in hibernation torpor have been shown to have striking increases in global SUMOylation on tissue immunoblots. Here, we find evidence that global SUMOylation is also involved in ischemic tolerance in primary cortical neuronal cultures (from rats and mice) and SHSY5Y human neuroblastoma cells. Cultured cortical neurons preconditioned by sublethal oxygen/glucose deprivation (OGD) were less vulnerable to severe OGD than non-preconditioned neurons. Preconditioned neurons maintained elevated SUMO-1 conjugation levels (and, to a lesser extent those of SUMO-2/3) on western blots in contrast to non-preconditioned cells. Further, cortical neurons and SHSY5Y cells in which transfected SUMO-1 or SUMO-2 were over-expressed showed increased survival after severe OGD. In contrast, cell cultures subjected to depletion of endogenous SUMO-1 protein by RNAi had reduced survival after exposure to this form of in vitro ischemia and an attenuated protective response to preconditioning. These findings suggest that maintenance of a globally elevated SUMO-1 (and maybe SUMO-2/3) conjugation level as revealed by immunoblot assays is a component of ischemic tolerance.
Keywords: cortical neurons, ischemic preconditioning, neuroprotection, post-translational modification, SHSY5Y, SUMO
Stroke is a major cause of death and severe, long-term disability. Although there have been recent advances, further development of cytoprotective treatments for acute ischemic stroke remains an unmet need (Ginsberg 2008). The factors responsible for cerebral ischemic tolerance are not well understood, although previous studies have identified a wide variety of putative mediators (Dirnagl et al. 2003; Meller et al. 2008).
Mammalian hibernators during torpor undergo a remarkable phenotypic switch that induces a state of natural tolerance to profound brain hypoperfusion. Entrance into torpor involves changes in physiology, morphology, and behavior in response to periods of unfavorable environmental conditions (Carey et al. 2003). During torpor, hibernating animals lower their energy consumption, blood flow and body temperature to otherwise lethal levels, but because of special adaptive changes, suffer no CNS damage or cellular loss (Hochachka 1986; Frerichs and Hallenbeck 1998; Kondo et al. 2006). Since protein synthesis is almost completely suppressed during torpor, we speculated that post-translational protein modification might be involved in this adaptive process. Indeed, we found that massive post-translational protein modification by both SUMO-1 and SUMO-2/3 (appearing as unusually strong high molecular weight immunopositive smears on western blots) occurs during hibernation in the brains of 13-lined ground squirrels (Spermophilus tridecemilineatus) (Lee et al. 2007).
Three enzymes are involved in the conjugation of SUMO-1, SUMO-2, or SUMO-3 to proteins. The first step is catalyzed by an E1 activase heterodimer, Aos1/Uba2. The next step in the enzymatic cascade requires the single E2 conjugase, Ubc9, which can identify a teterapeptide motif in proteins (ψ, K, X, E/D) and is capable of conjugating SUMO to the target lysine epsilon amino group of some proteins without E3 ligases. There are also approximately 10 E3 ligases (identified, so far) that contribute with Ubc9 to substrate specificity (Johnson 2004). Covalent attachment of the small ubiquitin-related modifier (SUMO) is involved in many cellular processes including gene expression, remodeling of chromatin structure, signal transduction, and maintenance of the genome (Hay 2005; Geiss-Friedlander and Melchior 2007). Transcription factors are the major targets of SUMO conjugation producing mostly negative effects on gene expression (Chinnadurai 2007). The increase of SUMO conjugation during hibernation torpor was suggested to attenuate the activity of a wide range of transcription factors as part of the shift to a hypo-metabolic state during torpor, and to protect nuclear proteins from degradation especially at the time of arousal (Lee et al. 2007). Recently, two groups reported that a dramatic or a modest increase of SUMO conjugation levels, especially SUMO-2, occurred in the brain in a transient global ischemia model (Yang et al. 2008a) and a focal cerebral ischemia model (Cimarosti et al. 2008; Yang et al. 2008b). However, it is still far from clear whether the increase in the SUMOylation levels is merely a stress response or whether it serves as a protective mechanism.
Oxygen-glucose deprivation (OGD) provides an in vitro model of ischemia (Goldberg and Choi 1993; Hillion et al. 2005). We have been using this model to clarify and advance the findings in our animal models. We reported that over-expressing the SUMO conjugating enzyme, Ubc9, (which increased SUMO-1 conjugation levels) increased the resistance to OGD in a human neuroblastoma cell line, SHSY5Y cells; blocking SUMO-1 conjugation by over-expressing a dominant negative mutant of Ubc9 sensitized the cells to OGD (Lee et al. 2007). In addition, OGD preconditioned SHSY5Y cells increased SUMO-1 conjugation levels and increased resistance to otherwise cell-damaging severe OGD exposures (Lee et al. 2007). These experiments showed that SUMO-1 conjugation levels correlated well with the resistance to OGD.
Is SUMOylation involved in ischemic tolerance? In the current study, we examined in primary cultures of cortical neurons (from rats and mice) whether either SUMO-1 or SUMO-2/3 conjugation levels have any relationship to ischemic tolerance. Cortical neurons, which had been preconditioned and had acquired tolerance to OGD, showed significantly higher SUMOylation levels during OGD or following restoration of oxygen/glucose (ROG) compared with the cells that had not been preconditioned. We also examined the direct effects of SUMO-1, SUMO-2, or SUMO-3 conjugation on cellular resistance to ischemia using SHSY5Y cells and cortical neurons with genetically engineered SUMOylation levels. We found in both SHSY5Y cells and cortical neurons that SUMO-1 conjugation levels and to a lesser extent SUMO-2/3 conjugation levels contribute importantly to ischemic tolerance.
Materials and methods
Cell Culture, isolation of rat or mouse cortical neurons and cortical neuronal culture
The human neuroblastoma cell line SHSY5Y (American Type Culture Collection, Manassas, VA, USA) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin (DMEM complete) in 5% CO2 at 37°C. For stable transfectants, tetracycline (1 μg/mL) was added to the culture 24 h before subjecting the cells to OGD. Embryos of Sprague-Dawley rats or B6 mice (C57BL/6) were used to prepare cortical neuronal cultures. Cortices were dissected from embryos (E18) and dissociated with papain (Worthington Biochemicals, Lakewood, NJ, USA), and either plated out at 1 000 000 cells per well on poly-L-lysine-coated six-well plates in Neurobasal-A/B27 media or directly used for transfection with the Amaxa system. Cells were used after 5–7 days in culture.
Transfection of SHSY5Y cells, generation of tet-inducible stable transfectants, and depletion of endogenous proteins by shRNA
Transfections of SHSY5Y cells were performed by electroporation with nucleofector (cell line nucleofector V) (Amaxa, Inc.), according to the manufacturer’s instructions. A tetracycline (tet)-regulated expression system (T-Rex system; Invitrogen, Carlsbad, CA, USA) was used for generating tet-inducible stable transfectants as described previously (Lee et al. 2007). Briefly, we first generated an SHSY5Y cell line stably expressing the tet repressor from pcDNA6/TR (T-Rex-SHSY5Y) (selected by blasticidine 5–50 μg/mL) (parent cells). Genes of interest (SUMO-1, SUMO-2 and SUMO-3 in this study) were subcloned into pcDNA4/TO, introduced into the T-Rex-SHSY5Y cell line, and established as stable transfectants by dual selection using zeocin (100 μg/mL) and blasticidin (5 μg/mL). The original constructs of SUMO-1, SUMO-2 and SUMO-3 were kind gifts from Mary Dasso (NICHD/NIH).
The plasmids expressing shRNAs were constructed by synthesizing two cDNA oligonucleotides bearing the target sequence, HindIII linker, and U6 terminator, annealing them and ligating them into pSHAG-1 (Cold Spring Harbor Laboratory, Cold Spring, NY, USA) cut with BseRI and BamHI. For long-term (stable) suppression of gene expression by shRNA, the region encoding the U6 promoter and shRNA in pSHAG-1 was subcloned into the NotI and BamHI sites of pREP4 (Invitrogen) which also carries a hygromycin resistance gene. The target sequences for the human SUMO-1, SUMO-2 and SUMO-3 were as follows: 5′AGTCATTGGACAGGATAGCAGTGAGATTCAC-3′ (#1) and 5′-GGTCAGAGAATTGCTGATAATCATACTCC-3′ (#2) for human SUMO-1; 5′-AGCCCAAGGAAGGAGTCAAGACTGAGAACAA-3′ (#1), 5′-ATTGTGAACGACAGGGATTGTCAATGAGGCAG-3′ (#2) and 5′-AGATACAATTGATGTGTTCCAACAGCAGACGG-3′ (#3) for human SUMO-2; 5′-CCAAGGAGGGTGTGAAGACAGAGAATGACCA-3′ (#1), 5′-CAAGCTGATGAAGGCCTACTGCGAGAGGCAG-3′ (#2) and 5′-CGAGGACACCATCGACGTGTTCCAGCAGCAGA-3′ (#3) for human SUMO-3. SHSY5Y cells were transfected with these constructs (shRNA/pREP4) and were grown in DMEM containing 200 μg/mL hygromycin B for 2 days followed by 3–4 days in DMEM containing 100 μg/mL hygromycin B for the selection of transfectants.
Transfection of cortical neurons for over-expression of SUMO-1, SUMO-2 and SUMO-3 and for depletion of endogenous SUMO-1 and SUMO-2 by siRNA
Cortical neurons isolated from rat embryos (E18) were transfected directly (13 × 106 cells per transfection) with 10 μg of SUMO-1, SUMO-2 or SUMO-3 construct along with 1 μg of green fluorescent protein (GFP) vector (for the analysis of transfection efficiency) by the Amaxa system (rat neuron nucleofector kit, program G-013), and plated in two wells of poly-L-lysine/laminine-double coated six-well plates in DMEM complete medium. Four hours after transfections, the media were replaced with fresh DMEM complete. Twenty four hours later media were replaced with fresh Neurobasal-A supplemented with B27, 5% fetal calf serum and 5 μM cytosine arabinoside. Transfection efficiency and protein expression levels were usually checked at 3–5 days after the transfection. For siRNA transfection, cortical neurons isolated from mouse E18 embryos were plated out first at 1000 000 cells per well of poly-L-lysine-coated six-well plates in Neurobasal-A/B27 media. At day 4 of the culture, cortical neurons were transfected with siRNA using lipofectamine RNAi Max (Invitrogen) according to the manufacturer’s instructions. The target sequences for mouse SUMO-1 and SUMO-2 are as follows: CAGCAGTTATTAATAAAGTTA (Qiagen, Valencia, CA, USA; cat# S101 437863) for SUMO-1 and CTCGCCATGGCCGACGACAAA (Qiagen cat# S101 437905) for SUMO-2. For controls, we used AllStars negative control siRNA (Qiagen cat# 1027281). Typically, 40 nM of siRNA was used for each transfection. Protein expression levels were checked 3 days after transfections.
Oxygen and glucose deprivation (OGD) and the assessment of cell death
OGD for SHSY5Y cells was performed as described previously (Lee et al. 2007). Briefly, cells which were plated at 500 000 cells per well of collagen-coated six-well plates (BD Biosciences, Bedford, MA, USA) and grown for 24 h, were washed twice in DMEM medium without glucose (Invitrogen) supplemented with 2% fetal bovine serum (OGD medium) and placed in modular incubator chambers (Billups-Rothenberg, Del Mar, CA, USA). The chambers were flushed with a gas mixture of 95% N2/5% CO2 for 30 min at room temperature at 4 L/min. After flushing, the chambers were sealed and maintained at 37°C. A harmful OGD was performed for 12 h in SHSY5Y cells unless mentioned otherwise, and cell deaths were assessed right after the OGD (without ROG). In case of shRNA transfected SHSY5Y cells (which had been selected by hygromycin B), shorter (4 h for preconditioning and 8 h for harmful) OGD was performed and cell deaths were measured after ROG 12 h. OGD for cortical neuronal culture was performed similarly except phosphate-buffered saline (supplemented with 1 mM CaCl2 and 2 mM MgCl2) was used for washing and during OGD, and shorter OGD times were used (30 or 60 min for preconditioning and 4 h for harmful OGD); OGD was always followed by ROG for 16 h before cell death assay. Cortical neurons were subjected to harmful OGD under all conditions at 5–7 days-in-culture (non-transfected cells and cells transfected by Amaxa or lipfectamine systems).
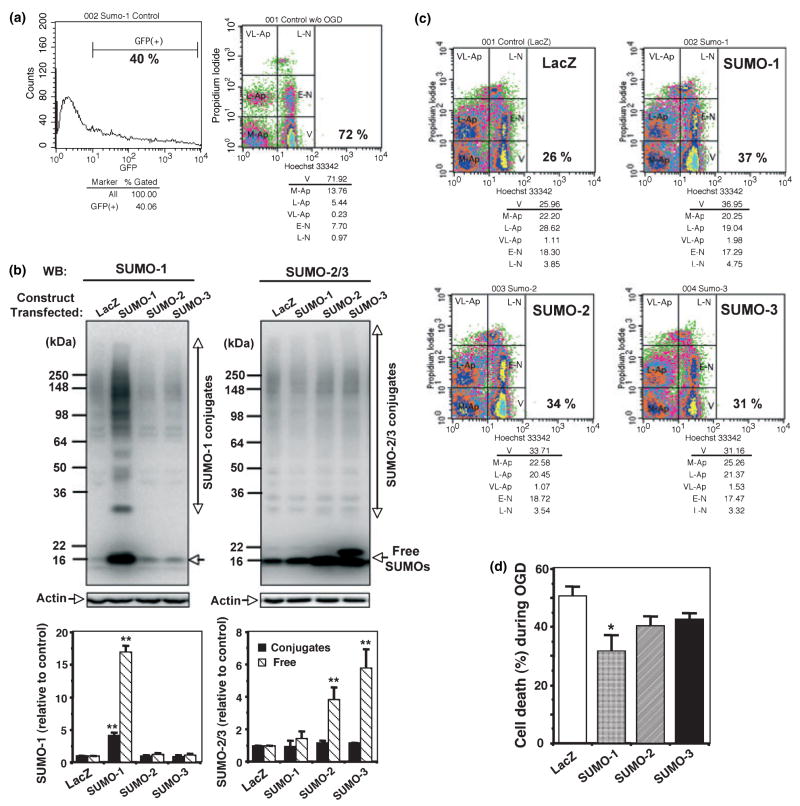
Cell death was assessed by nuclear staining with Hoechst 33342 and propidium iodide followed by counting directly under the fluorescent microscope (Fig. 1a): live cells and dead or dying cells were scored by the criteria shown in the inset in the center: live cells (smooth blue nuclei) shown by arrowheads and dead (mostly apoptotic) cells (early apoptosis: condensed or fragmented blue nuclei, late apoptosis: condensed or fragmented red nuclei) shown by thin arrows. At least 1000 cells total in several different fields were counted in each condition, or followed by fluorescence-activated cell sorting analysis as described previously (Lee et al. 2007): typically, 100 000 cells were analyzed. Hoechst 33342 stains all nuclei and propidium iodide (PI) stains nuclei of cells with disrupted plasma membranes. Depending on fluorescence intensities for Hoechst 33342 and PI, cell status was differentiated into six categories as follows: V, viable cells; M-Ap, mid-apoptotic cells; L-Ap, late apoptotic cells; VL-Ap, very late apoptotic cells, E-N, early necrotic cells; L–N, late necrotic cells. A lactate dehydrogenase (LDH) release assay (in vitro toxicology assay kit, Sigma Chemicals) was also used for a cell death assessment of cortical neuronal cultures.
Fig. 1.
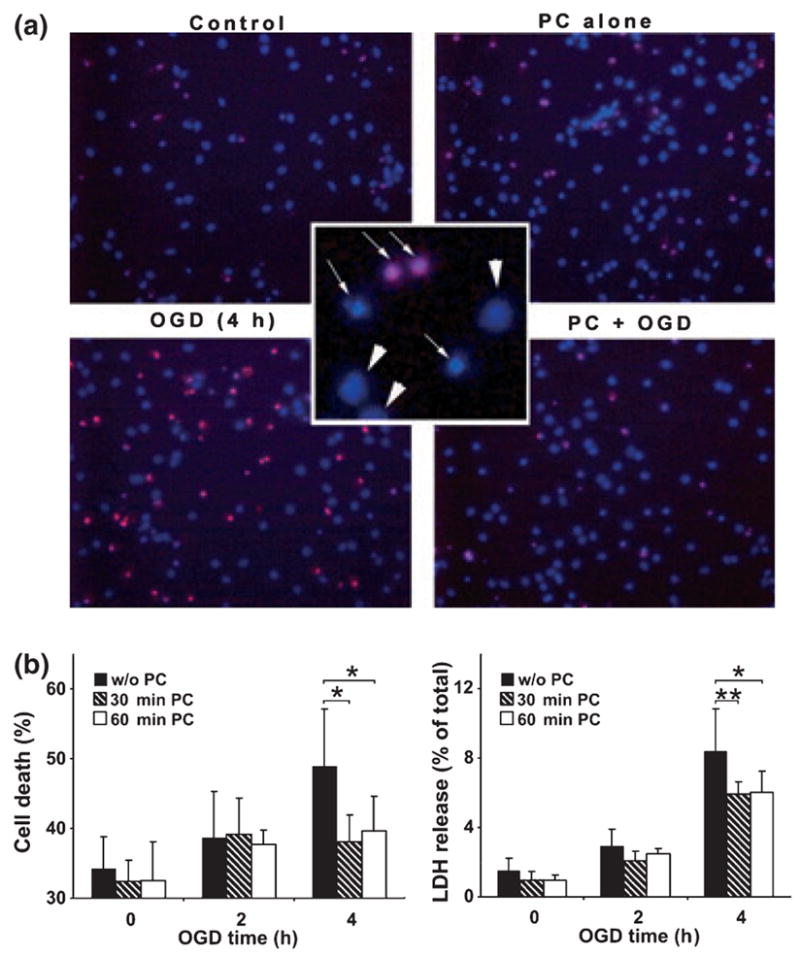
Preconditioning (PC) of cultured rat cortical neurons made these cells more resistant to a harmful OGD. Cortical neurons were subjected to a brief OGD (30 min or 60 min) as preconditioning and 24 h later to a harmful OGD (4 h). After 16 h ROG, cell death was assessed. (a) Representative fields of cortical neuronal culture subjected to the various conditions indicated and stained with Hoechst 33342 and PI. In the center inset, nuclear stains show examples of live cells designated by arrowheads and dead cells shown by thin arrows. (b) Quantitative data of cell death (total apoptotic and necrotic) shown as percentage of total cell population in different experimental conditions (left panel) and by LDH release as percentage of total LDH (right panel). Data are shown as means ± SD (n = 4). **p < 0.01, *p < 0.05 compared with cells subjected to a harmful OGD without PC.
Western Blot Analysis
Cortical neurons or SHSY5Y cells were washed once with phosphate-buffered saline and lysates were made directly in the culture plates on ice by adding 50–100 μL of 2x SDS lysis buffer (100 mM Tris–HCl, pH 6.8, 2% SDS, 50 mM EDTA) and scraping. Cell lysates were heated immediately at 99°C for 10 min. Samples can be stored in this state at −80°C without any decrease in SUMO conjugation levels. Lysates which were still viscous were sonicated for 5–10 s at 50% power. Protein concentrations were determined by BCA assay. After the addition of β-mercaptoethanol and dye (BPB:bromophenyl blue), 10–20 μg of protein was used for each SDS-PAGE/WB analysis. We used SUMO antibodies (both −1, and −2/3) at 1 : 2000 dilution in 5% milk/PBST, either 2 h at room temperature or overnight at 4°C. For the chemiluminescence detection, we used “Immobilon Western” (Millipore) followed by digital imaging using Fluor Chem (Alpha Innotech, San Leandro, CA, USA). Anti-SUMO-1 and anti-SUMO-2/3 antibodies were generated in rabbits using purified recombinant SUMO-1 or SUMO-2 protein as an antigen. Affinity purified sera from these animals were used for this study. They are very specific to each SUMO protein (both free and conjugated), with very little, if any, cross reactivity among them. The full protein SUMO-1 amino acid sequence is identical for human, rat, mouse, and ground squirrel (we sequenced SUMO-1 in the ground squirrel) as is the full protein SUMO-2 amino acid sequence. Anti-β-actin antibody was from Sigma Chemical. The intensities of bands were analyzed by Image-J (NIH). In order to measure SUMO-conjugation levels, the region corresponding to molecular weights 30 to 250 kDa in each lane was cropped and the total intensity was analyzed (Lee et al. 2007). The densities were normalized with corresponding actin levels and expressed as the ratio to control (untreated cells).
Statistical analysis
All values were expressed as mean ± SD (standard deviation). Statistic analyses between different groups were done by one way analysis of variance (ANOVA). Trend analyses were implemented via Jonckheere-Terpstra test (JT test) in order to test decreasing order in mean change in SUMOylation among preordered OGD time, for with PC or without PC respectively (Figs 2 and 3). Bonferroni correction was applied for multiple comparisons as necessary. All reported p-values are two-sided and Bonferroni-corrected, and they are considered as significant if the p-values are less than 0.05.
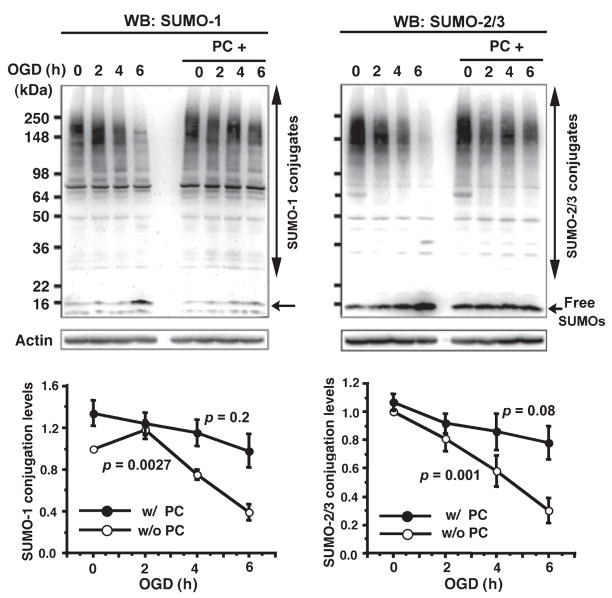
Fig. 2.
SUMO-1 and SUMO-2/3 conjugation levels in rat cortical neurons during OGD. SUMO-1 (left panel) and SUMO-2/3 (right panel) protein levels (conjugates indicated by a long vertical double ended arrow and the free form indicated by a short horizontal arrow around 16 kDa) were analyzed in the total cell extracts from rat cortical neurons right after OGD treatment for 0, 2, 4 and 6 h with (filled circle) or without (open circle) preconditioning (PC) (60 min OGD). Upper panels are representative PAGE/WB pictures and lower panels are quantitative analyses of densities of conjugates and free forms. Data are shown as means ± SD (n = 3). Jonckheere-Terpstra tests were applied in order to analyze trends in SUMOylation levels during OGD. p values are indicated on the figure.
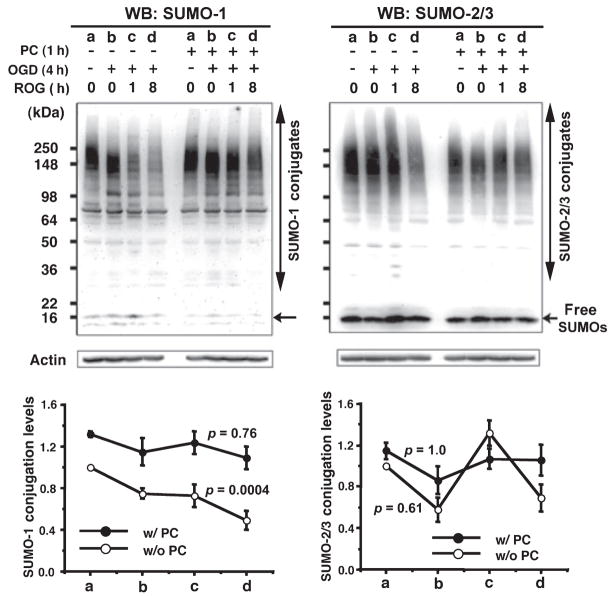
Fig. 3.
SUMO-1 and SUMO-2/3 conjugation levels in rat cortical neurons during ROG after a harmful OGD. SUMO-1 (left panel) and SUMO-2/3 (right panel) protein levels were analyzed in the total cell extracts from rat cortical neurons treated as follows with or without PC: a, control; b, OGD 4 h; c, OGD 4 h followed by 1 h ROG; d, OGD 4 h followed by 8 h ROG. Upper panels are representative PAGE/WB pictures and lower panels are quantitative analyses of the densities of conjugates and free forms. Open circles designate without PC and filled circles designate with PC. Data are shown as means ± SD (n = 3). Jonckheere-Terpstra tests were applied in order to analyze trends in SUMOylation levels during OGD. p values are indicated on the figure.
Results
OGD preconditioning modulates SUMO-1 and SUMO-2/3 conjugation levels in rat cortical neuronal cultures
Is protein SUMOylation a part of the mechanism involved in the tolerance to ischemia acquired by ischemic preconditioning? In order to answer this question we used an in vitro system (primary cortical neurons subjected to OGD with and without preconditioning). Since embryonic cortical neuronal cultures contain very few if any astrocytes which support neuronal survival, cultured cortical neurons undergo progressive apoptotic cell death such that around 32–34% of the cells have become apoptotic by 7 days in culture. Cell deaths were measured by Hoechst 33342/PI staining followed by counting directly under the fluorescent microscope. Fig. 1a shows representative fields of cortical neuronal culture subjected to the various conditions indicated (control, PC alone, OGD 4 h, and PC plus OGD 4 h) followed by ROG for 16 h, and nuclear staining with Hoechst 33342/PI. Live cells and dead or dying cells were scored by the criteria shown in the inset in the center: live cells (smooth blue nuclei) shown by arrowheads and dead (mostly apoptotic) cells (early apoptosis: condensed or fragmented blue nuclei, late apoptosis: condensed or fragmented red nuclei) shown by thin arrows. At least 1000 cells total in several different fields were counted in each condition, and quantitative data on cell death are shown as a percentage of the total cell population counted in the different experimental conditions (Fig. 1b, left panel). The cortical neurons that were subjected to 4 h OGD (harmful OGD) followed by 16 h ROG underwent further cell death (ranging up to 60%), but preconditioning (PC) by brief 30 or 60 min OGD 24 h before the harmful OGD induced tolerance with 50% suppression of neuronal cell death (p < 0.05). Late apoptotic or necrotic cells release LDH, which has been used as a biomarker of cell death. We measured the LDH release during ROG since we replaced the OGD media with fresh culture media after OGD had been completed (Fig. 1b, right panel). LDH release during ROG after 4 h OGD was very modest, but again preconditioning (either 30 min or 60 min) protected significantly (27% suppressions of cell death; p < 0.01 for PC 30 min and p < 0.05 for PC 60 min, respectively) from neuronal cell death as gauged by this biomarker. Next we examined the cellular SUMOylation levels in these experimental conditions. Both SUMO-1 and SUMO-2/3 conjugation levels in cortical neurons decreased markedly during OGD (without ROG) (Fig. 2). The decrease, however, was largely prevented by preconditioning (PC) (1 h OGD 24 h before subjecting to OGD indicated on the figure). Jonckheere-Terpstra tests applied to without preconditioning (p = 0.0027 for SUMO-1, p = 0.001 for SUMO-2/3) and with preconditioning (p = 0.2 for SUMO-1, p = 0.08 for SUMO-2/3), indicated that there were significant decreasing patterns on SUMO conjugation levels in order of OGD time (0, 2, 4 and 6 h) without preconditioning but not with preconditioning. Note that while conjugation levels declined during OGD without PC, the amount of free SUMO-1 or SUMO-2/3 increased, suggesting that little or no protein degradation was involved. After a harmful OGD, SUMO-1 conjugation levels progressively decrease during a subsequent ROG period and this decrease was also prevented by PC (Fig. 3, left panel). There was a significant decreasing pattern on SUMO-1 conjugation level during OGD/ROG without preconditioning (p = 0.0004) but not with preconditioning (p = 0.76). On the other hand, SUMO-2/3 conjugation increased during the first hour of ROG and then decreased in non-preconditioned cells (Fig. 3, right panel). Interestingly, PC prevented the initial increase and kept the conjugation level almost unchanged for up to 8 h of ROG (Fig. 3, right panel).
Over-expression of SUMO-1 made SHSY5Y cells more resistant to OGD
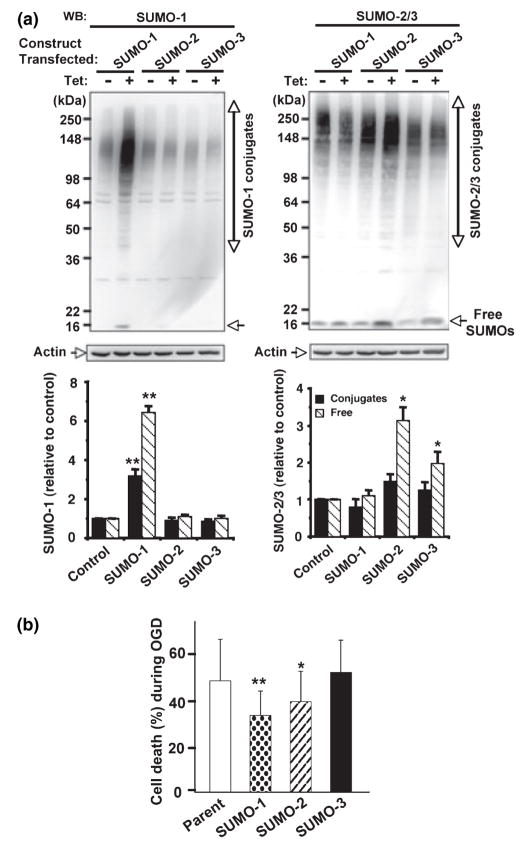
Is the SUMOylation level critical for cells to be resistant to ischemia? We established three SHSY5Y cell lines which stably express (but only when tetracycline was added) exogenous SUMO-1, SUMO-2 or SUMO-3 protein, respectively. As shown in Fig. 4a, SUMO-1 was highly expressed in the presence of tetracycline (tet), and the expression levels of SUMO-2 and SUMO-3 were also increased, though to a lesser extent than SUMO-1. Since endogenous SUMO-2/3 is abundant in SHSY5Y cells, the difference in protein levels between endogenous and over-expressed proteins may not be apparent especially in conjugated form. When these stable transfectants along with parent cells were subjected to a harmful OGD (12 h, with no subsequent ROG) in the presence of tetracycline, SUMO-1 over-expressing cells were significantly more resistant to OGD compared with parent cells (Fig. 4b). SUMO-2 over-expressing cells also gained some tolerance to OGD though less than SUMO-1 over-expressing cells (Fig. 4b). The differences in sensitivity to OGD between parent cells and SUMO-1 transfectants, or parent cells and SUMO-2 transfectants were statistically significant (36% and 24% suppressions of cell death; p < 0.01 and 0.05, respectively).
Fig. 4.
Over-expression of SUMO-1 and SUMO-2 made SHSY5Y cells more resistant to OGD. (a) SUMO-1 (left panel) and SUMO-2/3 (right panel) protein levels were analyzed in total cell extracts from stable SUMO-1, -2 or -3 transfectants grown in the presence (+) or the absence (−) of tetracycline (1 μM). Upper panels are representative PAGE/WB pictures and lower panels are quantitative analyses of densities of conjugates (solid bars) and free forms (striped bars). Data are shown as means ± SD (n = 4). **p < 0.01, *p < 0.05 compared with endogenous levels. (b) The three transfectants along with their parent cells were subjected to OGD (12 h) in the presence of tetracycline, and cell deaths during OGD (without ROG) were measured by nuclear staining with Hoechst 33342 and PI followed by FACS analysis. Data are shown as means ± SD (n = 6). **p < 0.01, *p < 0.05 compared with parent cells.
Depletion of endogenous SUMO-1 protein made SHSY5Y cells more sensitive to OGD and these cells could not to be preconditioned
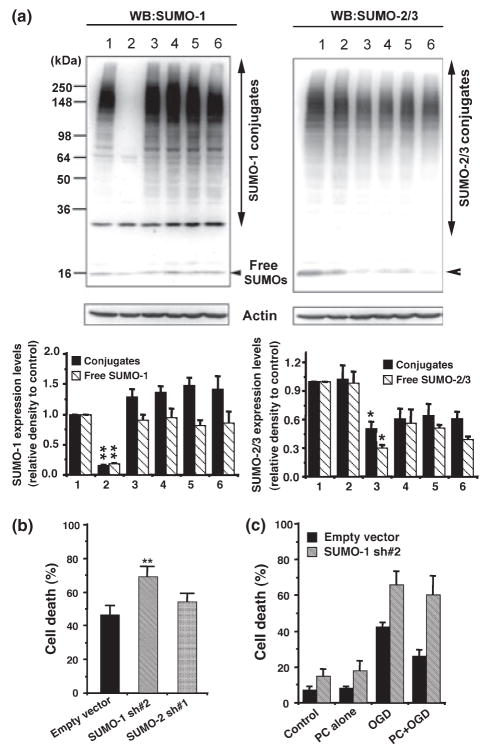
Next we constructed several plasmids each expressing shRNAs which target human SUMO-1, SUMO-2 or SUMO-3 to deplete (or to reduce) the endogenous proteins. As shown in Fig. 5a, endogenous SUMO-1 proteins (both free and conjugated) were almost completely depleted by SUMO-1 sh#2, and the endogenous SUMO-2/3 levels were decreased by 50% by SUMO-2 sh#1. We are not sure whether the remaining anti-SUMO-2/3 reactive proteins are leftover SUMO-2 (incomplete depletion of endogenous SUMO-2) or all endogenous SUMO-3. None of the shRNA constructs targeting SUMO-3 reduced the SUMO-2/3 protein level more than did SUMO-2 sh#1 alone. The SUMO-1-depleted cells showed a significant increase in sensitivity to OGD (p < 0.01), but cells with depressed SUMO-2/3 levels did not show a corresponding clear increase in sensitivity to OGD (Fig. 5b). Collectively, these results show that SUMO-1 conjugation levels are critical for cells to be resistant to OGD/ischemia. In addition, the SUMO-1-depleted cells could not be preconditioned (Fig. 5c), suggesting that SUMO-1 conjugation is needed for the induction of tolerance to OGD. As shown in Fig. 5c, the control cells that had been transfected with empty vector gained resistance to OGD by preconditioning (a sublethal 4 h OGD), but SUMO-1-depleted cells gained little or no resistance to OGD by the same pre-treatment. The importance of SUMO-2 and SUMO-3 in the induction of tolerance (resistance) to OGD are, however, still unclear since we could not effectively deplete these proteins.
Fig. 5.
The depletion of SUMO-1 made SHSY5Y cells more sensitive to OGD/ROG and the cells that were depleted of SUMO-1 were not protected by preconditioning. (a) SUMO-1 (left panel) and SUMO-2/3 (right panel) protein levels in total cell lysates from SHSY5Y cells transfected with various shRNA constructs as follows: 1, empty vector; 2, SUMO-1 sh#2; 3, SUMO-2 sh#1; 4, SUMO-2 sh#1 plus SUMO-3 sh#1; 5, SUMO-2 sh#1 plus SUMO-3 sh#2; 6, SUMO-2 sh#1 plus SUMO-3 sh#3. Upper panel: a representative PAGE/WB picture, lower panels: quantitative analyses; solid bars:conjugates; striped bar: free forms. Data are shown as means ± SD (n = 6). **p < 0.01, *p < 0.05 compared with endogenous levels. (b) Sensitivities to OGD/ROG among shRNA transfected SHSY5Y cells. Data here are shown as means ± SD (n = 6). **p < 0.01 compared with the cells transfected with empty vector. (c) SHSY5Y cells transfected with empty vector or SUMO-1 sh#2 were subjected to a harmful OGD/ROG with or without preconditioning (4 h OGD), and assayed for cell death. Cell death in control and PC alone are also included. Data are shown as means ± SD (n = 3).
Over-expression of SUMO-1 made rat cortical neurons more resistant to OGD
Cortical neurons are known to be very difficult to transfect. After extensive efforts we could achieve up to 40% transfection efficiencies with ~70% viability for cortical neurons by means of the Amaxa system (Fig. 6a). We always co-transfected a gene of interest with GFP, so that transfection efficiency could be measured in each experiment. The transfection efficiencies were in the range of 28~40% and the average is about 32%. Our initial plan for transfecting GFP along with a gene of interest was to gate out transfectants (GFP-positive cells) from the whole population and analyze them for cell death and viability. However, we (also (Schmid and Sakamoto 2001)) found that GFP, which is expressed in the cytoplasm, was leaking out of cells that had compromised plasma membranes (e.g. dying cells), so GFP-positive cells after cytotoxic treatment (OGD in our case) are mostly viable cells. Another option is to use GFP-SUMO fusion constructs, but it may not be wise for physiological studies since the GFP molecule (~30 kDa) is much bigger than free SUMOs (~16 kDa). So we analyzed cell deaths after OGD/ROG in whole populations. In spite of the ~30% transfection efficiency, over-expression of both the conjugated and the free form of SUMO-1 were clearly detected by WB in total cell lysates (Fig. 6b, left panel). In the case of SUMO-2 and SUMO-3, over-expression of the free form, but not conjugates, was observed (Fig. 6b, right panel). Can we detect any difference in sensitivities to OGD among these transfectants in the presence of ~30% transfection efficiency? Fig. 6c shows a set of representative fluorescence-activated cell sorting pseudo-colored dot-density plots for various transfectants that had been subjected to a 4 h OGD followed by ROG for 16 h. Viabilities of all transfectants were around 60–70% without OGD (control live), and after the OGD followed by ROG the viabilities varied among transfectants. We carried out four independent experiments and calculated the cell death (%) in each transfectant as follows: Cell death (%) by OGD/ROG = [Control live (%)-OGD/ROG live (%)]/control live (%)× 100, and plotted them as shown in Fig. 6d. The differences among transfectants are modest, but it is clear that SUMO-1 transfectants are the most resistant to OGD (Fig. 6d). SUMO-1 transfectants are significantly more resistant (p < 0.05) to OGD compared with control (LacZ) transfectants and the difference would likely have been more profound if it had been analyzed only in the transfected cell subset.
Fig. 6.
Over-expression of SUMO-1 made rat cortical neurons more resistant to OGD. (a) Transfection efficiency (GFP-positive cells over total cell population) (left panel), and viability (right panel).V: viable cells. (b) SUMO-1 (left panel) and SUMO-2/3 (right panel) protein levels in total cell extracts from various transfectants. Upper panels: PAGE/WB, lower panels: quantitative analyses. Data are shown as means ± SD (n = 4). **p < 0.01 compared with endogenous levels. (c) Representative pseudocolored dot-density plots of FACS analysis for various transfectants that had been subjected to OGD/ROG. Identity of transfectant and the percentage of viable cells are written in each dot gram. (d) Sensitivity to OGD/ROG among transfectants. Cell death (%) during OGD was calculated as follows: [Control live (%)−OGD live (%)]/control live (%) ×100. Data are shown as means ± SD (n = 4). *p < 0.05 compared with control (Lac Z-transfected) cells.
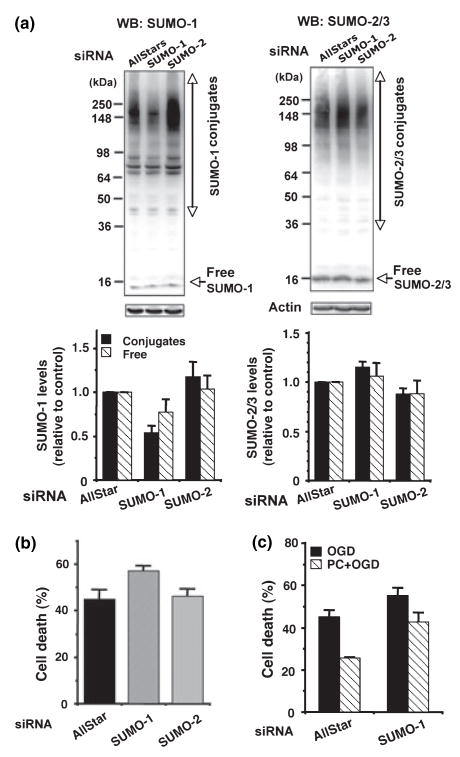
Knock-down of endogenous SUMO-1 protein made mouse cortical neurons more sensitive to OGD and less able to be preconditioned
Since more siRNAs are commercially available for mouse genes, we used mouse cortical neurons for RNAi study. We have tested four different target sequences for SUMO-1 and SUMO-2 each, and picked those that worked best. Three siRNAs out of four reduced endogenous SUMO-1 levels (50% reduction for conjugates, 25% reduction for free form), but none of the SUMO-2 siRNAs worked very well (~15% reduction at most for conjugates) (Fig. 7a). As shown in Fig. 7a and b 50% reduction of endogenous SUMO-1 made mouse cortical neurons more sensitive to OGD/ROG. Next we examined if the cortical neurons whose SUMO-1levels had been reduced by 50% can be preconditioned. As shown in Fig. 7c, the cells which had been transfected with control siRNA gained resistance to OGD/ROG by preconditioning with a sublethal 30 min OGD as with rat cortical neurons (Fig. 1b). Cells in which SUMO-1 was partially depleted by siRNA also gained some resistance to OGD/ROG by the same pre-treatment that did not reach the level achieved by cells transfected with the control siRNA. Indeed the degree of tolerance attenuation and the degree of SUMO-1 depletion were quite comparable in these cells.
Fig. 7.
The down-regulation of SUMO-1 made mouse cortical neurons more sensitive to OGD/ROG and the SUMO-1-depleted neurons show reduced protection by preconditioning. (a) SUMO-1 (left panel) and SUMO-2/3 (right panel) protein levels in total cell lysates from mouse cortical neurons treated with various siRNAs. Upper panels: representative PAGE/WB pictures. Lower panels: quantitative analyses of densities. Data are shown as means ± SD (n = 3). (b) Sensitivities to OGD/ROG among siRNA-treated cells. Cell death was measured with Hoechst 33342 and PI followed by FACS analysis. Data here are shown as means ± SD (n = 3). (c) Mouse cortical neurons that had been transfected with AllStar siRNA or SUMO-1 siRNA were subjected to a harmful OGD/ROG with or without preconditioning (30 min OGD). Cell deaths were measured as mentioned above. Data here are shown as means ± SD (n = 3).
Discussion
The question we addressed in this study was whether or not SUMOylation (by SUMO-1 and/or SUMO-2/3) is involved in the acquisition of tolerance to ischemia. We have extended our earlier studies (Lee et al. 2007) by more directly addressing whether increased SUMOylation is critical for induction of cellular tolerance to in vitro ischemia in both human neuroblastoma cell lines and primary cultures of rat and mouse cortical neurons.
In cortical neuronal cultures, SUMO-1 conjugation levels decreased during OGD (almost no conjugates remained by 6 h OGD) (Fig. 2, left panel) and also decreased during ROG (Fig. 3, left panel). Preconditioning of the neuronal cultures prevented decreases in SUMO-1 conjugation both during and after OGD (Figs 2 and 3, left panels). In this in vitro system, the SUMO-1 conjugation levels seemed to be well correlated with the resistance to ischemia (OGD/ROG) (Fig. 1). The findings that over-expression of exogenous SUMO-1 in SHSY5Y (Fig. 4) cells and cortical neurons (Fig. 6) made the cells significantly more resistant to OGD or OGD/ROG and that the depletion of endogenous SUMO-1 sensitized the cells to OGD/ROG (Figs 5 and 7) provide further support for SUMO-1 participation in cytoprotection from ischemia. Our finding that SUMO-1-depleted cells were not protected by preconditioning (Fig. 5c) makes the case stronger. On the other hand, the role of SUMO-2/3 conjugations in ischemic tolerance is still not clear. In our in vitro experiments in primary cultures of rat cortical neurons, we observed a decrease of SUMO-2/3 conjugation during a harmful OGD that resembled the fall in SUMO-1 conjugation (Fig. 2), but a transient increase of SUMO-2/3 conjugation was seen at an early ROG time point (1 h) after OGD (Fig. 3, right panel). The decrease of SUMO-2/3 conjugation during harmful OGD and the transient increase of SUMO2/3 conjugation in early ROG were both inhibited in the cultured cortical neurons that had been preconditioned (Figs 2 and 3, right panel). In addition, the over-expression of SUMO-2 or SUMO-3 in cortical neurons or SHSY5Y cells showed very modest or no effect on OGD- or OGD/ROG-induced cell death (Figs. 4 and 6), and knock-down of SUMO-2 had very little effect (Figs 5 and 7). These findings may relate to technical limitations in the over-expression and knock down of SUMO-2 and SUMO-3, however.
In recent publications, Yang et al. showed that an increase in protein SUMOylation by SUMO-2/3 was maximally induced in both the hippocampus and cerebral cortex of mice three hours after a 10 min global ischemia exposure (Yang et al. 2008a). Yang et al. also reported that SUMO-2/3 protein conjugation was increased during ROG from 30 min to 6 h after transient focal cerebral ischemia in the ipsilateral parietal cortex of rats (Yang et al. 2008b). Cimarosti et al. reported an increase in SUMOylation by SUMO-2/3 in the ipsilateral infarcted striatum of rats during reperfusion 6 h after a transient middle cerebral artery occlusion (MCAO), but no significant increase of SUMO-2/3 conjugation was found in any of the brain regions of mice that had been subjected to MCAO without reperfusion (Cimarosti et al. 2008). SUMO-1 conjugation levels in different animal models of ischemia have been variously reported, either as unchanged in a mouse global ischemia model (Yang et al. 2008a) and a rat transient focal ischemia model (Yang et al. 2008b) or as modestly increased in rat transient MCAO (ipsilateral striatum at 6–24 h after reperfusion) and in mouse permanent MCAO (ipsilateral cortex and hippocampus without apparent ischemic damage at 24 h after occlusion) (Cimarosti et al. 2008). The variability of SUMO-1 conjugation levels reported in these ischemia/reperfusion models might be partly due to the sensitivity of detection, since levels of SUMO-1 protein (especially the free form) are much lower than levels of SUMO-2/3 in brain tissue as well as in cultured cortical neurons and SHSY5Y cells (endogenous levels in Figs 4 and 6, also data not shown). However, none of these animal studies were designed to examine the involvement of SUMO conjugations in ischemic tolerance. They only reported the SUMO conjugation levels and their distributions in the brains of animals exposed to ischemic surgery, which might have reflected only stress responses.
We initially found that massive conjugation of both SUMO-1 and SUMO-2/3 occurred in the torpor phase of hibernating ground squirrels (Lee et al. 2007), which are naturally tolerant to oligemic conditions. During hibernation torpor, ground squirrels can tolerate brain blood flows that average 10% of baseline, flow levels characteristic of a stroke’s ischemic core (reviewed in (Ginsberg 2003), and a severely reduced capacity to deliver oxygen for many weeks and recover without any evidence of cellular damage in the brain (Frerichs et al. 1994). Hibernation torpor has also been shown to provide neuroprotection against a superimposed lethal ischemic insult. Hippocampal slices from hibernating ground squirrels have increased resistance to CA1 pyramidal cell death from OGD at 36°C, 20°C and 7°C compared with slices from non-hibernating ground squirrels and rats (Frerichs and Hallenbeck 1998). Hibernation/torpor therefore provides an excellent model for natural tolerance to ischemia.
It remains possible that, like the intricately controlled phosphorylation or ubiquitin conjugation systems, it is modification of one or a few specific SUMOylation substrates that is critical for protection. However, SUMOylation has broad, potentially protective effects that include DNA repair, genetic and epigenetic transcriptional regulation, regulation of protein subcellular location, maintenance of both genome integrity and subnuclear architecture, and stabilization of hypoxia-inducible factor 1α during hypoxia (Johnson 2004; Gill 2005; Cheng et al. 2007; Heun 2007). These latter considerations, our earlier work in ground squirrel torpor, and this study collectively support the hypothesis that increased SUMOylation is critical for induction of cellular tolerance to ischemia.
Does SUMOylation by SUMO-1 or by SUMO-2/3 lead to different consequences in ischemia? Most proteins that are known to be SUMOylated are SUMOylated by both SUMO-1 and SUMO-2/3, but some proteins are SUMOylated by only one of the SUMO isoforms (Saitoh and Hinchey 2000; Vertegaal et al. 2006). We observed in our in vitro system that SUMOylation patterns by SUMO-1 and SUMO-2 are not exactly the same, especially during the ROG period (Figure 3). Animal studies of Yang et al. (Yang et al. 2008a,b) and Cimarosti et al. (2008) showed clear differences in SUMOylation levels by SUMO-1 and SUMO-2/3 during ischemia/reperfusion. We suspect that SUMO-1 conjugation is more likely responsible for ischemic tolerance, but we still believe that many overlapping or compensating functions among SUMO paralogs exist in developing ischemic tolerance.
Many proteins have been reported as SUMOylation substrates, and many of them are likely to be involved in the pathobiology of brain ischemia (Rajan et al. 2005; Scheschonka et al. 2007). It would be of great interest and importance to identify those SUMO substrates. Protein SUMOylation is clearly involved in the cellular responses to stroke, but in the absence of small molecules that specifically activate or inhibit SUMO conjugation, resolution of questions concerning the cytoprotective or stress signaling properties of this form of post-translational modification will require further studies in engineered cells and transgenic animals.
Acknowledgments
This research was supported by the Intramural Research Program of the NINDS/NIH.
The authors thank Dace Klimanis for technical assistance and Maria Spatz for valuable suggestions.
Abbreviations
- GFP
green fluorescent protein
- LDH
lactate dehydrogenase
- OGD
oxygen-glucose deprivation
- PC
preconditioning
- PI
propidium iodide
- ROG
restoration of oxygen/glucose
- SUMO
small ubiquitin-related modifier
- tet
tetracycline
References
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Cheng J, Kang X, Zhang S, Yeh ET. SUMO-specific protease 1 is essential for stabilization of HIF1alpha during hypoxia. Cell. 2007;131:584–595. doi: 10.1016/j.cell.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai G. Transcriptional regulation by C-terminal binding proteins. Int J Biochem Cell Biol. 2007;39:1593–1607. doi: 10.1016/j.biocel.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, Lindberg C, Bomholt SF, Ronn LC, Henley JM. Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology. 2008;54:280–289. doi: 10.1016/j.neuropharm.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab. 1998;18:168–175. doi: 10.1097/00004647-199802000-00007. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab. 1994;14:193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Gill G. Something about SUMO inhibits transcription. Curr Opin Genet Dev. 2005;15:536–541. doi: 10.1016/j.gde.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. Adventures in the pathophysiology of brain ischemia: penumbra, gene expression, neuroprotection: the 2002 Thomas Willis Lecture. Stroke. 2003;34:214–223. doi: 10.1161/01.str.0000048846.09677.62. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Heun P. SUMOrganization of the nucleus. Curr Opin Cell Biol. 2007;19:350–355. doi: 10.1016/j.ceb.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Hillion JA, Takahashi K, Maric D, Ruetzler C, Barker JL, Hallenbeck JM. Development of an ischemic tolerance model in a PC12 cell line. J Cereb Blood Flow Metab. 2005;25:154–162. doi: 10.1038/sj.jcbfm.9600003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW. Defense strategies against hypoxia and hypothermia. Science. 1986;231:234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- Kondo N, Sekijima T, Kondo J, Takamatsu N, Tohya K, Ohtsu T. Circannual control of hibernation by HP complex in the brain. Cell. 2006;125:161–172. doi: 10.1016/j.cell.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Miyake S, Wakita H, McMullen DC, Azuma Y, Auh S, Hallenbeck JM. Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J Cereb Blood Flow Metab. 2007;27:950–962. doi: 10.1038/sj.jcbfm.9600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller R, Thompson SJ, Lusardi TA, et al. Ubiquitin proteasome-mediated synaptic reorganization: a novel mechanism underlying rapid ischemic tolerance. J Neurosci. 2008;28:50–59. doi: 10.1523/JNEUROSCI.3474-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell. 2005;121:37–47. doi: 10.1016/j.cell.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Saitoh H, Hinchey J. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J Biol Chem. 2000;275:6252–6258. doi: 10.1074/jbc.275.9.6252. [DOI] [PubMed] [Google Scholar]
- Scheschonka A, Tang Z, Betz H. Sumoylation in neurons: nuclear and synaptic roles? Trends Neurosci. 2007;30:85–91. doi: 10.1016/j.tins.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Schmid I, Sakamoto KM. Analysis of DNA content and green fluorescent protein expression. Curr Protoc Cytom. 2001;16:16. doi: 10.1002/0471142956.cy0716s16. (Chapter 7, Unit 7) [DOI] [PubMed] [Google Scholar]
- Vertegaal AC, Andersen JS, Ogg SC, Hay RT, Mann M, Lamond AI. Distinct and overlapping sets of SUMO-1 and SUMO-2 target proteins revealed by quantitative proteomics. Mol Cell Proteomics. 2006;5:2298–2310. doi: 10.1074/mcp.M600212-MCP200. [DOI] [PubMed] [Google Scholar]
- Yang W, Sheng H, Warner DS, Paschen W. Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metab. 2008a;28:269–279. doi: 10.1038/sj.jcbfm.9600523. [DOI] [PubMed] [Google Scholar]
- Yang W, Sheng H, Warner DS, Paschen W. Transient focal cerebral ischemia induces a dramatic activation of small ubiquitin-like modifier conjugation. J Cereb Blood Flow Metab. 2008b;28:892–896. doi: 10.1038/sj.jcbfm.9600601. [DOI] [PubMed] [Google Scholar]