Abstract
In middle-aged women, follicular depletion is a critical factor mediating the menopausal transition; however, all levels of the hypothalamic-pituitary-gonadal (HPG) axis contribute to the age-related decline in reproductive function. To help elucidate the complex interactions between the ovary and brain during middle-age that lead to the onset of the menopause, we utilize animal models which share striking similarities in reproductive physiology. Our results show that during middle-age, prior to any overt irregularities in estrous cyclicity, the ability of 17β-estradiol (E2) to modulate the cascade of neurochemical events required for preovulatory gonadotropin-releasing hormone (GnRH) release and a luteinizing hormone (LH) surge is diminished. Middle-aged female rats experience a delay in and an attenuation of LH release in response to E2. Additionally, although we do not observe a decrease in GnRH neuron number until a very advanced age, E2-mediated GnRH neuronal activation declines during the earliest stages of age-related reproductive decline. Numerous hypothalamic neuropeptides and neurochemical stimulatory inputs (i.e., glutamate, norepinephrine (NE), and vasoactive intestinal peptide (VIP) that drive the E2-mediated GnRH/LH surge appear to dampen with age or lack the precise temporal coordination required for a specific pattern of GnRH secretion, while inhibitory signals such as gamma aminobutyric acid (GABA) and opioid peptides remain unchanged or elevated during the afternoon of proestrus. These changes, occurring at the level of the hypothalamus, lead to irregular estrous cycles and, ultimately, the cessation of reproductive function. Taken together, our studies indicate that the hypothalamus is an important contributor to age-related female reproductive decline.
Keywords: aging, brain, estradiol, female, neuroendocrine, reproduction
1. Introduction
Although the mean life span of humans continues to increase, the mean age at which women begin the perimenopausal transition has remained constant at 45.5-47.5 years, a process that lasts about 4 years (Burger et al., 2002; Hidayet et al., 1999; McKinlay et al., 1992; Treloar, 1981). Therefore, most women can expect to spend over one third of their lives in the postmenopausal state where the associated chronic decrease in E2 will have far-reaching health implications on bone (Ebeling et al., 1996; Seifert-Klauss et al., 2002), neurodegeneration and stroke (Suzuki et al., 2006; Suzuki et al., 2007; Wise et al., 2005), cognition (Halbreich et al., 1995; Lacreuse, 2006; Paganini-Hill and Henderson, 1994; Rapp et al., 2003; Roberts et al., 1997; Shaywitz et al., 1999; Voytko and Tinkler, 2004), cardiovascular disease (Do et al., 2000; Hall et al., 2002; Luoto et al., 2000; Matthews et al., 2001), and immune function (Keller et al., 2001; Pfeilschifter et al., 2002; Porter et al., 2001). For these reasons, factors underlying the timing of the onset of the menopause and the repercussions of attenuated circulating E2 on women’s health are of considerable interest. We utilize animal models to gain insight about the complex interactions between the ovary and the brain leading up to the onset of the menopause and the hypoestrogenic state. During the past five years, we have come to appreciate that during middle-age, the changes that occur in reproductive cyclicity and hormone patterns among women, nonhuman primates, and rodents are strikingly similar (Bellino and Wise, 2003; Downs and Urbanski, 2006; Wise et al., 1999; Wise et al., 2002; Wu et al., 2005). In this review we include a discussion of the differences and similarities of rodent and nonhuman primate model systems for human menopause onset. This review focuses primarily on our work; however, we cite numerous studies from our colleagues that have greatly benefited our understanding of neuroendocrine factors contributing to female reproductive senescence.
2. Aging of the female hypothalamic-pituitary-ovarian axis
Age-related female reproductive decline involves deficits at all levels of the HPG axis (Peng and Huang, 1972; Rubin, 2000; Wise et al., 1999; Wise et al., 2002). In women, depletion of the postmitotic pool of ovarian follicles and the associated decline in circulating E2 concentrations are traditionally recognized as being the ultimate markers of menopause (vom Saal, 1994). In addition to the ovary, the pituitary is likely to contribute to reproductive decline since the LH response to a pharmacological doses of GnRH administered in the early follicular phase decreases in older compared with younger cycling women (Fujimoto et al., 1993; Weiss et al., 2004). However, accumulating evidence indicates that during the earliest stages of reproductive decline, prior to any changes in ovarian cyclicity and circulating E2, changes at the level of the brain play an important role in the initiation of reproductive senescence (Gore et al., 2004; Lapolt and Lu, 2001; Lu et al., 1994; Rubin, 2000; Wise, 1993; Wise et al., 1997; Wise et al., 2002; Yin and Gore, 2006).
One of the earliest markers of reproductive decline is a dampened and delayed GnRH-driven proestrous LH surge (Lu, 1983; Nass et al., 1984; van der Schoot, 1976; Wise, 1982). Prior to any observable changes in estrous cycle length or regularity, we observed a consistent delay in the onset of the LH surge and an attenuation in peak LH concentrations (Fig. 1). These age-related changes in LH release likely reflect alterations at the level of the hypothalamus rather than the pituitary because they occur at an age prior to any change in pituitary responsiveness to GnRH. However, since we have never observed a change in GnRH neuron number during middle-age (Krajnak et al., 2001; Lloyd et al., 1994), an age-related change in GnRH neuronal activity is more likely to contribute to the alterations in LH release that we observe. To determine if a change in GnRH neuronal function during middle-age occurs, and if such a change could result in a dampening and delay of the LH surge, we quantified the expression of the immediate early gene, cFos, as an indicator of GnRH neuronal activity. We used this immunocytochemical technique, which has been used to identify increased neuronal activity in individual neurons (Hoffman et al., 1993) because GnRH is not detectable in peripheral blood, GnRH neurons are few in number, and their populations are diffuse. In Fig. 2, we show that GnRH neuronal activation in middle-aged, regularly cycling female rats was significantly lower compared to their young counterparts during the time of the proestrous LH surge (Lloyd et al., 1994). Others have confirmed and extended our results by using three-dimensional reconstructions of GnRH populations in young and middle-aged rats showing similar age-related declines in GnRH neuronal activation either during the proestrous or E2-induced LH surge (Rubin et al., 1994; Rubin et al., 1995). These findings strongly indicate that afferent inputs to GnRH neurons change during the earliest stages of reproductive aging.
FIG. 1.
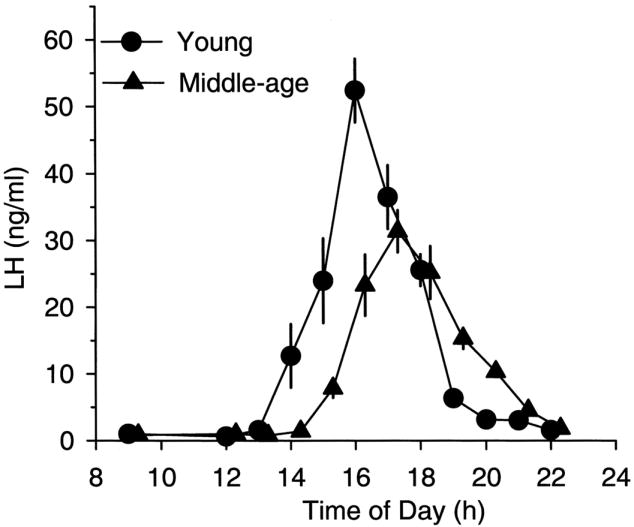
The luteinizing hormone (LH) surge is blunted and delayed in middle-aged, compared to young, rats. Young and middle-aged regularly cycling rats were sequentially bled from right atrial cannulae during the day of proestrus. Plasma was radioimmunoassayed for LH. The first significant increase in LH was delayed by 1 hour and attenuated significantly in middle-aged rats. [Reprinted with permission from Wise, P. M., 1982. Alterations in proestrous LH, FSH, and prolactin surges in middle-aged rats. Proc Soc Exp Biol Med 169(3), 348-354.]
FIG. 2.
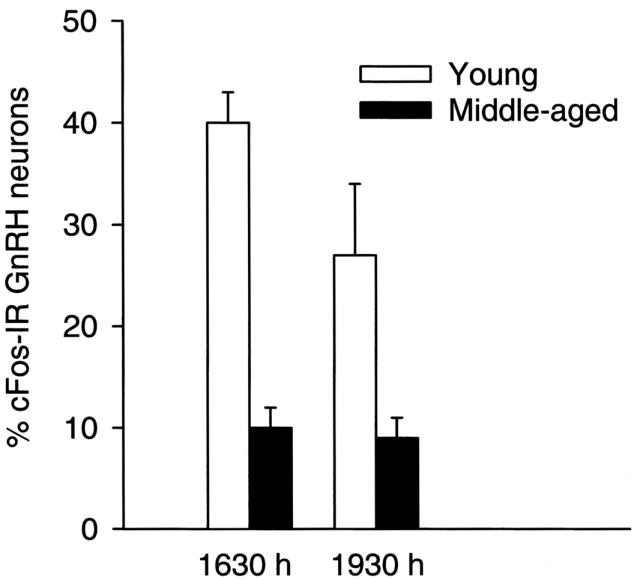
Percentage of gonadotropin-releasing hormone (GnRH) neurons that express Fos during the proestrous LH surge decreases with age. Young and middle-aged regularly cycling rats were perfused with paraformaldehyde and their brains sectioned for dual immunocytochemical localization of GnRH and Fos. Age significantly decreased the level of activation of GnRH neurons. [Reprinted with permission from Lloyd JM, Hoffman GE, Wise PM 1994 Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology 134:1800–1805. Copyright The Endocrine Society.]
3. Neuronal factors involved in the onset of reproductive senescence
The driving force behind GnRH/LH output relies upon the complex balance between, and timing of, stimulatory and inhibitory inputs to GnRH neurons. By middle-age the orchestration of this balance has deteriorated, which is manifested in reduced GnRH release, leading to a reduction in magnitude and delay in the LH surge (Wise, 1982; Wise et al., 2002). Much attention has been paid to the role of declining stimulating factors that may contribute to the observed age-effects on the GnRH/LH surge. For example, excitatory input from the amino acid, glutamate, is a critical stimulatory factor that directly influences GnRH neuronal activity through the N-methyl-d-aspartate (NMDA) and non-NMDA receptors (Gore, 2004). However, during aging glutamatergic input to brain regions containing GnRH neurons appear to decrease (Brann et al., 2005; Neal-Perry et al., 2005). Additionally, glutamatergic mediated GnRH release (Zuo et al., 1996) and glutamate receptor subunits, expressed on GnRH neurons, appear to decline with age (Gore et al., 2000; Smith and Jennes, 2001), possibly contributing to the delay and decline in the GnRH/LH surge.
Another key stimulator of GnRH release is norepinepherine (NE), which acts through the α1-adrenergic receptor and is important in the induction of the GnRH/LH surge (Barraclough and Wise, 1982; Barraclough et al., 1984). We observed that NE exhibits a diurnal rhythm in which the activity turnover rate is increased just prior to and during the LH surge in young but not middle-aged rats (Rance et al., 1981; Wise, 1982; Wise, 1984). Moreover, the regions where we observed an age-related dampening of the diurnal variation in NE turnover occurred in key anterior hypothalamic nuclei that regulate the timing of the LH surge (Wise, 1984). Additionally, with age, the density of the α1-adrenergic receptors lacks the diurnal variation observed in several hypothalamic nuclei of young rats (Weiland et al., 1989; Weiland and Wise, 1990). The loss of rhythmic monoamine turnover (Wise, 1984) and adrenergic receptor density (Weiland et al., 1989; Weiland and Wise, 1990), in conjunction with a reduced sensitivity of NE neurons to E2 (which may contribute to lower catecholamine release during the LH surge in middle-aged rats) (Temel et al., 2002), strongly suggest that the subtle control of NE is compromised during the earliest stages of reproductive decline.
However, in order for stimulating factors such as NE to have their full effect on GnRH neurons to initiate the GnRH/LH surge, a withdrawal of inhibitory factors on GnRH release appears to be necessary (Kalra and Kalra, 1984). Additionally, pharmacological blockade of inhibitory tone on GnRH during early proestrus results in an advance in the LH surge. Therefore, investigating age-effects on inhibitory factors contributing to restraining GnRH release seem equally important as age-effects on GnRH stimulatory factors.
Gamma-aminobutyric acid (GABA) and opioid peptides act as inhibitory neurotransmitters and as important modulators of the timing and amplitude of the GnRH/LH surge on proestrus (Cashion et al., 2004; Kalra and Kalra, 1984). During the estrous cycle, GABA and opioid peptides act to restrain GnRH secretion, but as proestrus nears, their activity lessens, allowing for stimulating neuromodulators to maximally influence GnRH neurons. We (Cashion et al., 2004), and others (Grove-Strawser et al., 2007) observe a loss of rhythmic GAD67 gene expression, the rate-limiting enzyme for GABA synthesis, with age. Studies suggest that multiple neurotransmitters that modulate GnRH synthesis and secretion may account for changes in GnRH dynamics. The long list of neurotransmitters and neuropeptides that alter the release of GnRH continues to grow while the hierarchy of neuromodulators remains unclear.
The sensitivity to E2 positive feedback declines with age in the female rat, resulting in an attenuated LH surge that is delayed (Wise, 1984; Wise et al., 1996). Similar findings in perimenopausal women indicate that the ability of exogenous E2 to induce a surge is diminished (van Look et al., 1977). It is likely that the important hypothalamic nodal point for the proper convergence of circadian signals from the SCN and E2 stimulation is the anteroventral periventricular (AVPV) nucleus and that a decline in the responsiveness of GnRH/LH to E2 with age may be at least partially attributable to age-related changes within the AVPV. We know that the AVPV expresses estrogen receptors (Petersen et al., 2003) and if lesioned, GnRH/LH surges are abolished (Wiegand and Terasawa, 1982). Moreover, we have shown that E2-induced activation of the medial AVPV and GnRH neurons decline in parallel in middle-aged rats (Le et al., 2001). This suggests that an age-related deficit in E2 responsiveness at the level of the medial AVPV may facilitate, in part, the age-related delay in and attenuation of the E2-induced GnRH/LH surge.
4. Aging of circadian signals contribute to reproductive decline
Timing is a critical factor in initiating the preovulatory GnRH/LH surge (Chappell, 2005; de la Iglesia and Schwartz, 2006). A series of seminal studies by John Everett and Charles Sawyer first established that two requirements were necessary to induce a preovulatory LH surge in the female rat: 1) a neurogenic signal during a “critical period”; and 2) a rise in circulating E2 (Everett et al., 1949; Everett and Sawyer, 1950; Everett and Sawyer, 1953; Sawyer et al., 1949). These studies indicated that the neuronal signal(s) leading to the LH surge needed to occur within a precisely timed window, and if they did not, the surge was delayed 24 hours, implicating a circadian neuronal signal as a prerequisite. An extension of the 4-day estrous cycle of the rat is intrinsically unstable due to uncoupling of the temporally coordinated rise in E2, the light/dark cycle, circadian signal and the neurogenic critical period (Schwartz, 1969). The mechanisms underlying how these two necessities integrate within the hypothalamus to elicit an appropriately timed GnRH/LH surge and how this mechanism changes with age remains an area of active research. Subsequent evidence indicated that the circadian pacemaker, located in the suprachiasmatic nuclei (SCN), regulates the timing of the preovulatory and steroid-induced LH surge. Researchers found:
direct neuronal pathways from the SCN to GnRH neuronal populations exist (Krajnak et al., 2001; van der Beek et al., 1997), and E2-receptive neurons in the AVPV are synaptic targets of the SCN (Watson et al., 1995).
rodents that are ovariectomized and treated with E2 have daily afternoon surges of LH that occur at a specific time relative to the light/dark cycle, indicating that the circadian signal is produced daily, but requires high E2 levels to be expressed (Legan and Karsch, 1975; Legan et al., 1975).
lesions of the SCN abolish estrous cycles in gonadally intact females (Gray et al., 1978; Stetson and Watson-Whitmyre, 1976; Terasawa et al., 1980) and eliminate daily afternoon surges of LH in E2-treated ovariectomized females (Kawakami et al., 1980). Thus, we can conclude that the rhythm that regulates the LH surge and estrous cycle is generated endogenously by a circadian pacemaker located in the SCN and that the AVPV is a likely intermediary.
Coupling of a circadian neuronal signal(s) from the mammalian biological clock, or SCN, with GnRH neuromodulators induces the E2-driven GnRH/LH surge (Chappell, 2005). Several lines of evidence indicate that the rhythmic expression of the neuropeptide, vasoactive intestinal polypeptide (VIP), primarily from the ventrolateral SCN, acts to directly convey time-of-day information to GnRH neurons in the female rat (van der Beek, 1996). Data show that: 1) GnRH neurons receive direct innervations from VIP afferents (van der Beek et al., 1993), 2) approximately 40% of GnRH neurons express the VIP2 receptor (Smith et al., 2000), and 3) GnRH neurons that are innervated by VIP fibers are preferentially activated during an E2-induced GnRH/LH surge (van der Beek et al., 1994). Furthermore, an uncoupling of the rhythmic VIP signal to GnRH neurons occurs during middle-age, which appears to partially account for the delay and attenuation of the LH surge in E2-treated female rats. This line of reasoning is supported by our observations that a dampening of the VIP rhythm occurs with age (Fig. 3); conversely, the arginine vasopressin (AVP) rhythm is preserved during aging (Krajnak et al., 1998). These differential age-dependent effects suggest that the integrity of the SCN does not deteriorate in a uniform manner. We observed a clear attenuation of the VIP mRNA rhythm, while the number of AVP cells, predominantly located in the dorsomedial aspect of the SCN, and the amount of AVP mRNA per cell were unaffected with age. In view of the fact that the rhythm of VIP expression is lost with advancing age, we sought to determine if a decrease in VIP and suppression of its rhythm would lead to a decrease/delay in the E2-mediated LH surge. We found that the effects of age on the LH surge could be mimicked in young, ovariectomized, E2-treated rats when the VIP signal was suppressed by direct infusion of VIP antisense oligonucleotides into the SCN (Fig. 4). Rats treated with antisense oligonucleotides to VIP displayed a decrease and delay in their E2-mediated LH surge (Harney et al., 1996). Our observations were later supported when the immunoneutralization of VIP by the central administration of antiserum to VIP reduced and delayed the E2-induced LH surge in rats (van der Beek et al., 1999).
FIG. 3.
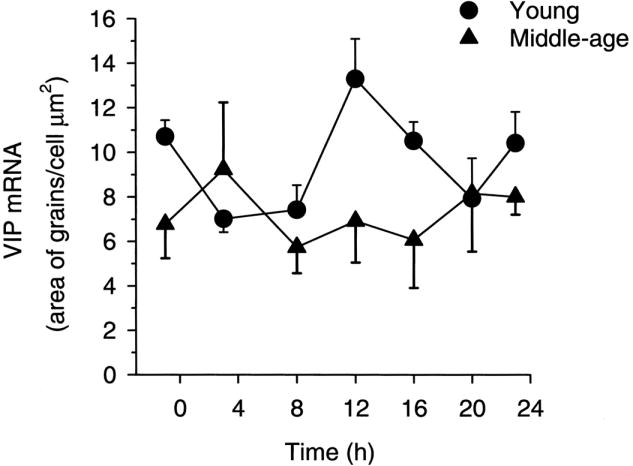
VIP mRNA levels/cell in young and middle-aged ovariectomized, estradiol-treated rats as measured by in situ hybridization exhibits age-related changes in rhythmicity. Young, middle-aged, and old rats were killed at 7 times of day over a 24-hour period. Young rats exhibited a diurnal rhythm in gene expression. The rhythm was no longer detectable in middle-aged or old rats. [Reprinted from Krajnak K, Kashon ML, Rosewell KL, Wise PM 1998 Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA, but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci 18:4767–4774.]
FIG. 4.
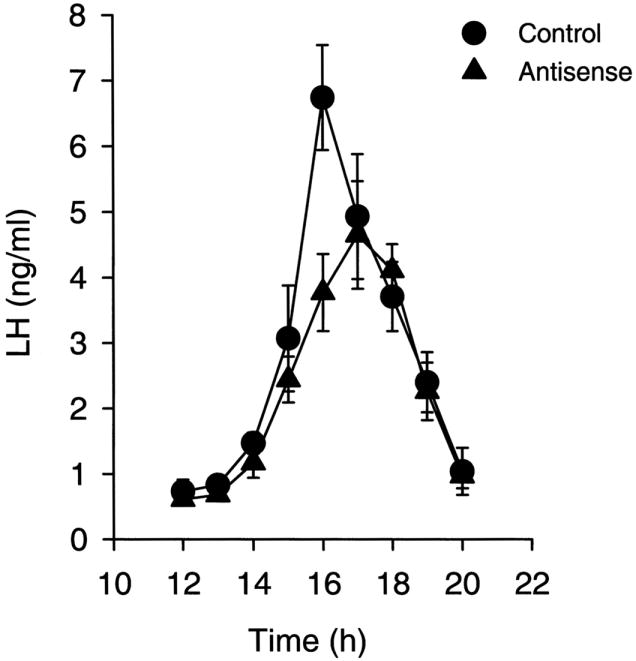
The steroid-induced LH is blunted and delayed in rats that were treated with antisense oligos to VIP or control scrambled oligos directly at the suprachiasmatic nucleus (SCN). Ovariectomized, estradiol-treated young rats were administered antisense or scrambled oligos and sequentially bled. The steroid-induced surge of LH exhibited changes that are remarkably like those observed during aging. [Reprinted with permission from Harney JP, Scarbrough K, Rosewell KL, Wise PM 1996 In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nucleus causes aging-like changes in the estradiol-induced LH and prolactin surge. Endocrinology 137:3696–3701. Copyright The Endocrine Society.]
We next sought to determine if advancing age could alter the innervation of VIP fibers onto GnRH neurons and the sensitivity of GnRH neurons to VIP (Fig. 5). Using immunohistochemical techniques, we found that the number of GnRH neurons that are likely to receive direct VIP input due to their close apposition does not decrease with age. However, the number of activated GnRH neurons determined by Fos expression closely apposed by VIP fibers was significantly decreased during the peak of an E2-induced LH surge (Krajnak et al., 2001). Taken together, these results provide further evidence for direct VIP innervation from the SCN to GnRH neurons and that the age-related attenuation and delay in the LH surge is not due to a decline in VIP input to GnRH, but may rather be due to a decreased sensitivity of GnRH neurons to VIP.
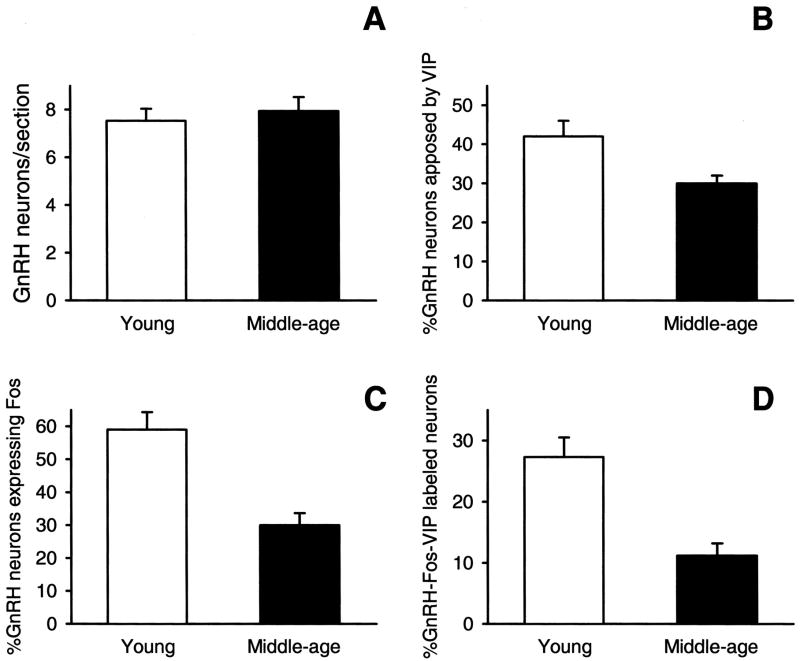
FIG. 5.
(A) Number of GnRH-immunopositive neurons per section; (B) percent of GnRH and VIP-immunopositive neurons; (C) percent of GnRH and Fos-immunopositive neurons; and (D) percent of GnRH, Fos, and VIP immunoreactive neurons in the preoptic area of young and middle-aged females during the peak of a steroid-induced LH surge exhibit age-related changes. Aging is associated with no change in the number of GnRH immunopositive neurons or the percent of GnRH and VIP immunopositive neurons. However, percent of activated GnRH neurons and the percent of activated GnRH that were closely apposed to VIP neurons decreased with age. [Reprinted from Krajnak K, Rosewell KL, Wise PM 2001 Fos-induction in gonadotropin-releasing hormone neurons receiving vasoactive intestinal polypeptide innervation is reduced in middle-aged female rats. Biol Reprod 64:1160–1164.]
5. Experimental models to study the role of the brain in female reproductive aging
Rodents
The majority of information on which we base our conclusions regarding the brain’s role in the onset of reproductive senescence originates from studies performed in rodents. Some controversy exists as to whether the rodent provides a suitable model for studying reproductive aging in women. Questions surrounding the appropriateness of the rodent model to provide insights into the onset of menopause in women stem from two primary observations. First, the negative feedback effects of E2 on gonadotropins in the aging rat are different than those observed in women. As the levels of ovarian-derived E2 drop precipitously in the postmenopausal woman, circulating LH and follicle-stimulating hormone (FSH) become unrestrained and are both markedly elevated (Yen, 1999). Conversely, circulating LH concentrations remain relatively normal in aged, acyclic, repeatedly pseudopregnant rats (Lu, 1983) despite an age-related decline in E2. These data suggest that a decline or disconnect in hypothalamic drive to GnRH neurons during reproductive aging may be fundamentally critical in rats but less so in women. Second, the age-related dynamics of the depletion of the ovarian follicular reserve may be different between rodents and women. As women approach middle-age, the depletion of the follicular pool appears to accelerate, so by the end of a woman’s reproductive life she is left with virtually no ovarian follicles (Richardson et al., 1987). In contrast, although an equivalent study in rats has not been performed, some follicles remain in old acyclic rats (Lu et al., 1979).
Despite key differences in the roles of the brain, pituitary and ovary in the postreproductive states of acyclic female rats and postmenopausal women, there are remarkable parallels between middle-aged female rats and middle-aged pre- and peri-menopausal women (Rubin, 2000; Wise et al., 2002). First, one of the earliest hallmarks of impending reproductive decline in both women and female rats is a rise in FSH, especially near the time of ovulation (Reyes et al., 1977; DePaolo and Chappel, 1986; DePaolo, 1987; Klein et al., 1996). Second, the interpulse interval and pulse duration of LH release increases similarly in older regularly cycling women (Matt et al., 1998) and middle-aged female rats (Scarbrough and Wise, 1990). Third, reproductive cycle length becomes highly variable in women and middle-aged female rats as they progress towards reproductive decline (Fitzgerald et al., 1994; Sherman et al., 1976). Fourth, pre- and peri-menopausal women have normal, or even elevated, circulating E2 concentrations (Klein et al., 1996; Santoro et al., 1996) as do middle-aged rats as they begin the transition to irregular estrous cyclicity (Butcher and Page, 1981; Lu, 1983). Additionally, the capacity of E2 to induce GnRH/LH surges is diminished in middle-aged rats and perimenopausal women (van Look et al., 1977; Weiss et al., 2004; Wise, 1984). And finally, aging alters the timing and the amplitude of the LH surge such that the onset and peak are delayed and the amplitude is diminished (Wise, 1982; Wise, 1984). These key pieces of evidence emphasize that changes at the level of the brain may drive the earliest stages of the transition to reproductive decline in both women and female rats, including the accelerated follicular atresia that occurs in middle-aged women (Faddy et al., 1983; Richardson et al., 1987).
Nonhuman Primates
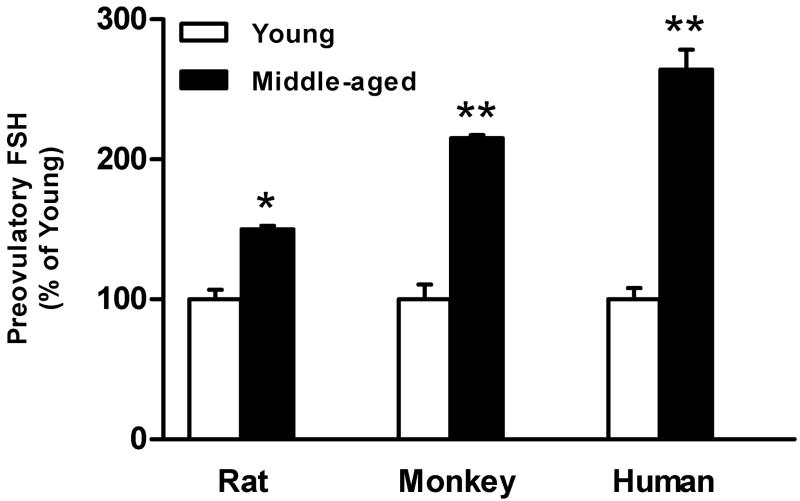
Like women, female rhesus monkeys experience regular 28-day menstrual cycles and sloughing of the endometrial lining. With advanced age, female rhesus monkeys undergo the transitions through menopause, culminating in the loss of menstrual cycles and the associated decline in E2 (Downs and Urbanski, 2006). Moreover, aged female rhesus monkeys experience a marked elevation in circulating FSH concentrations prior to any overt changes in menstrual cyclicity or circulating E2 concentrations (Downs and Urbanski, 2006), which acts as a hallmark of impending reproductive decline in women (Reyes et al., 1977). Despite these similarities that make the rhesus monkey a valuable model of menopause in women, nonhuman primate research can be prohibitive because of cost and the time required to investigate aging systems in such a long-lived species. Although these caveats have limited the extent of nonhuman primate studies regarding the role of the brain in the onset of reproductive aging, some important contributions have been made utilizing the monkey as a model system. Pulsatile GnRH release increases during the menopausal transition in the nonhuman primate (Gore et al., 2004), and parallel changes in pulsatile LH release have been detected (Woller et al., 2002). Whether these age-related changes precede age-related ovarian hormonal changes is yet to be determined. Taken together, some, but not all, parameters are shared between rats, nonhuman primates and human females during the earliest stages of reproductive decline prior to the onset of any detectable changes in reproductive cyclicity or decline in E2, of which the robust monotropic rise in FSH (Fig. 6) is one of the earliest detectable.
FIG. 6.
The preovulatory rise in circulating FSH is significantly elevated across species during middle-age prior to the onset of irregular reproductive cycles. The marked increase in plasma FSH during middle-age, which is a commonality shared between rats, monkeys and human females, is one of the earliest observable neuroendocrine markers indicative of impending reproductive decline. Mean group FSH values are expressed as percent of young ± SEM. *p<0.05, **p<0.01. [Adapted from: DePaolo, L. V. and Chappel S. C., 1986.Alterations in the secretion and production of follicle-stimulating hormone precede age-related lengthening of estrous cycles in rats. Endocrinology 118(3), 1127-1133, Downs, J. L. and Urbanski, H. F., 2006. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta). Biol Reprod 75(4), 539-546, and Reyes, F. I., Winter, J. S. and Faiman, C., 1977. Pituitary-ovarian relationships preceding the menopause. I. A cross-sectional study of serum follice-stimulating hormone, luteinizing hormone, prolactin, estradiol, and progesterone levels. Am J Obstet Gynecol 129(5), 557-564.]
5. Summary
The complex and redundant mechanisms governing female reproduction emphasize that no single piece of the HPG axis can completely explain the changes that occur to mediate age-related reproductive decline. Evidence indicate female reproductive aging involves all levels of the HPG axis from studies in several species, including rats, monkeys, and women. The balance of stimulatory and inhibitory GnRH neuromodulators becomes temporally disorganized and deteriorates with advancing age. We propose that this imbalance leads to an insensitivity to E2 and an uncoupling from critical circadian signals and is therefore what causes the attenuation and delay of the E2-induced GnRH/LH surge leading to reproductive senescence. Future studies that exploit the advantages of different animal models in cooperation with clinical investigations will undoubtedly shed further light on the key factors that orchestrate the onset of menopause.
Acknowledgments
This work was supported by National Institutes of Health grants AG-02224 and AG-17164 to P.M. Wise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barraclough CA, Wise PM. The role of catecholamines in the regulation of pituitary luteinizing hormone and follicle-stimulating hormone secretion. Endocr Rev. 1982;3(1):91–119. doi: 10.1210/edrv-3-1-91. [DOI] [PubMed] [Google Scholar]
- Barraclough CA, Wise PM, Selmanoff MK. A role for hypothalamic catecholamines in the regulation of gonadotropin secretion. Recent Prog Horm Res. 1984;40:487–529. doi: 10.1016/b978-0-12-571140-1.50016-5. [DOI] [PubMed] [Google Scholar]
- Bellino FL, Wise PM. Nonhuman primate models of menopause workshop. Biol Reprod. 2003;68(1):10–18. doi: 10.1095/biolreprod.102.005215. [DOI] [PubMed] [Google Scholar]
- Brann DW, Zamorano PL, De Sevilla L, Mahesh VB. Expression of glutamate receptor subunits in the hypothalamus of the female rat during the afternoon of the proestrous luteinizing hormone surge and effects of antiprogesin treatment and aging. Neuroendocrinology. 2005;81:120–128. doi: 10.1159/000086405. [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–275. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- Butcher R, Page R. Role of the aging ovary in cessation of reproduction. In: Schwartz N, Hunzicker-Dunn M, editors. Dynamics of ovarian function. Raven Press; New York: 1981. pp. 253–271. [Google Scholar]
- Cashion AB, Smith MJ, Wise PM. Glutamic acid decarboxylase 67 (GAD67) gene expression in discrete regions of the rostral preoptic area change during the oestrous cycle and with age. J Neuroendocrinol. 2004;16(8):711–716. doi: 10.1111/j.1365-2826.2004.01225.x. [DOI] [PubMed] [Google Scholar]
- Chappell PE. Clocks and the black box: Circadian influences on gonadotropin-releasing hormone secretion. J Neuroendocrinol. 2005;17(2):119–130. doi: 10.1111/j.1365-2826.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Schwartz WJ. Minireview: Timely ovulation: Circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147(3):1148–1153. doi: 10.1210/en.2005-1311. [DOI] [PubMed] [Google Scholar]
- DePaolo LV. Age-associated increases in serum follicle-stimulating hormone levels on estrus are accompanied by a reduction in the ovarian secretion of inhibin. Exp Aging Res. 1987;13(12):3–7. doi: 10.1080/03610738708259293. [DOI] [PubMed] [Google Scholar]
- Do KA, Green A, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Longitudinal study of risk factors for coronary heart disease across the menopausal transition. Am J Epidemiol. 2000;151(6):584–593. doi: 10.1093/oxfordjournals.aje.a010246. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75(4):539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Ebeling PR, Atley LM, Guthrie JR, Burger HG, Dennerstein L, Hopper JL, Wark JD. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996;81(9):3366–3371. doi: 10.1210/jcem.81.9.8784098. [DOI] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH, Markee JE. A neurogenic timing factor in control of the ovulatory discharge of luteinizing hormone in the cyclic rat. Endocrinology. 1949;44(3):234–250. doi: 10.1210/endo-44-3-234. [DOI] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” Of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47(3):198–218. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- Everett JW, Sawyer CH. Estimated duration of the spontaneous activation which causes release of ovulating hormone from the rat hypophysis. Endocrinology. 1953;52(1):83–92. doi: 10.1210/endo-52-1-83. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Edwards RG. Ovarian follicle dynamics in mice: A comparative study of three inbred strains and an F1 hybrid. J Endocrinol. 1983;96(1):23–33. doi: 10.1677/joe.0.0960023. [DOI] [PubMed] [Google Scholar]
- Fitzgerald CT, Seif MW, Killick SR, Elstein M. Age related changes in the female reproductive cycle. Br J Obstet Gynaecol. 1994;101(3):229–233. doi: 10.1111/j.1471-0528.1994.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Fujimoto VY, Spencer SJ, Rabinovici J, Plosker S, Jaffe RB. Endogenous catecholamines augment the inhibitory effect of opioids on luteinizing hormone secretion during the midluteal phase. Am J Obstet Gynecol. 1993;169(6):1524–1530. doi: 10.1016/0002-9378(93)90429-m. [DOI] [PubMed] [Google Scholar]
- Gore AC, Yeung G, Morrison JH, Oung T. Neuroendocrine aging in the female rat: The changing relationship of hypothalamic gonadotropin-releasing hormone neurons and N-methyl-d-aspartate receptors. Endocrinology. 2000;141(12):4757–4767. doi: 10.1210/endo.141.12.7841. [DOI] [PubMed] [Google Scholar]
- Gore AC. Gonadotropin-releasing hormone neurons: multiple inputs, multiple outputs. Endocrinology. 2004;145(9):4016–4017. doi: 10.1210/en.2004-0855. [DOI] [PubMed] [Google Scholar]
- Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145(10):4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- Gray GD, Soderstein P, Tallentire D, Davidson JM. Effects of lesions in various structures of the suprachiasmatic-preoptic region on LH regulation and sexual behavior in female rats. Neuroendocrinology. 1978;25(3):174–191. doi: 10.1159/000122739. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Jimenez-Linan M, Rubin BS. Middle-aged female rats lack the dynamic changes in GAD(67) mrna levels observed in young females on the day of a luteinizing hormone surge. J Neuroendocrinol. 2007;19(9):708–716. doi: 10.1111/j.1365-2826.2007.01579.x. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Lumley LA, Palter S, Manning C, Gengo F, Joe SH. Possible acceleration of age effects on cognition following menopause. J Psychiatr Res. 1995;29(3):153–163. doi: 10.1016/0022-3956(95)00005-p. [DOI] [PubMed] [Google Scholar]
- Hall G, Collins A, Csemiczky G, Landgren BM. Lipoproteins and BMI: A comparison between women during transition to menopause and regularly menstruating healthy women. Maturitas. 2002;41(3):177–185. doi: 10.1016/s0378-5122(01)00258-4. [DOI] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137(9):3696–3701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- Hidayet NM, Sharaf SA, Aref SR, Tawfik TA, Moubarak II. Correlates of age at natural menopause: A community-based study in alexandria. East Mediterr Health J. 1999;5(2):307–319. [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG. C-fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14(3):173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. Opioid-adrenergic-steroid connection in regulation of luteinizing hormone secretion in the rat. Neuroendocrinology. 1984;38(5):418–426. doi: 10.1159/000123928. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Arita J, Yoshioka E. Loss of estrogen-induced daily surges of prolactin and gonadotropins by suprachiasmatic nucleus lesions in ovariectomized rats. Endocrinology. 1980;106(4):1087–1092. doi: 10.1210/endo-106-4-1087. [DOI] [PubMed] [Google Scholar]
- Keller ET, Zhang J, Yao Z, Qi Y. The impact of chronic estrogen deprivation on immunologic parameters in the ovariectomized rhesus monkey (Macaca mulatta) model of menopause. J Reprod Immunol. 2001;50(1):41–55. doi: 10.1016/s0165-0378(00)00087-5. [DOI] [PubMed] [Google Scholar]
- Klein NA, Illingworth PJ, Groome NP, McNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: A study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81(7):2742–2745. doi: 10.1210/jcem.81.7.8675606. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998;18(12):4767–4774. doi: 10.1523/JNEUROSCI.18-12-04767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Rosewell KL, Wise PM. Fos-induction in gonadotropin-releasing hormone neurons receiving vasoactive intestinal polypeptide innervation is reduced in middle-aged female rats. Biol Reprod. 2001;64(4):1160–1164. doi: 10.1095/biolreprod64.4.1160. [DOI] [PubMed] [Google Scholar]
- Lacreuse A. Effects of ovarian hormones on cognitive function in nonhuman primates. Neuroscience. 2006;138(3):859–867. doi: 10.1016/j.neuroscience.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Lapolt PS, Lu JKH. Factors influencing the onset of female reproductive senescence. In: Hof PR, Mobbs CV, editors. Functional neurobiology of aging. Academic Press; San Diego: 2001. pp. 761–768. [Google Scholar]
- Le WW, Wise PM, Murphy AZ, Coolen LM, Hoffman GE. Parallel declines in fos activation of the medial anteroventral periventricular nucleus and LHRH neurons in middle-aged rats. Endocrinology. 2001;142(11):4976–4982. doi: 10.1210/endo.142.11.8470. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96(1):57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96(1):50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- Lloyd JM, Hoffman GE, Wise PM. Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling, middle-aged rats. Endocrinology. 1994;134(4):1800–1805. doi: 10.1210/endo.134.4.8137745. [DOI] [PubMed] [Google Scholar]
- Lu JK, Anzalone CR, LaPolt PS. Relation of neuroendocrine function to reproductive decline during aging in the female rat. Neurobiol Aging. 1994;15(4):541–544. doi: 10.1016/0197-4580(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Lu JKH. Changes in ovarian function and gonadotropin and prolactin secretion in aging female rats. In: Meites J, editor. Neuroendocrinology of aging. Plenum Press; New York: 1983. pp. 103–122. [Google Scholar]
- Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21(1):193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- Luoto R, Sharrett AR, Schreiner P, Sorlie PD, Arnett D, Ephross S. Blood pressure and menopausal transition: The atherosclerosis risk in communities study (1987-95) J Hypertens. 2000;18(1):27–33. doi: 10.1097/00004872-200018010-00005. [DOI] [PubMed] [Google Scholar]
- Matt DW, Kauma SW, Pincus SM, Veldhuis JD, Evans WS. Characteristics of luteinizing hormone secretion in younger versus older premenopausal women. Am J Obstet Gynecol. 1998;178(3):504–510. doi: 10.1016/s0002-9378(98)70429-6. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Kuller LH, Sutton-Tyrrell K, Chang YF. Changes in cardiovascular risk factors during the perimenopause and postmenopause and carotid artery atherosclerosis in healthy women. Stroke. 2001;32(5):1104–1111. doi: 10.1161/01.str.32.5.1104. [DOI] [PubMed] [Google Scholar]
- McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14(2):103–115. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- Nass TE, LaPolt PS, Judd HL, Lu JK. Alterations in ovarian steroid and gonadotrophin secretion preceding the cessation of regular oestrous cycles in ageing female rats. J Endocrinol. 1984;100(1):43–50. doi: 10.1677/joe.0.1000043. [DOI] [PubMed] [Google Scholar]
- Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. Attenuation of preoptic area glutamate release correlates with reduced luteinizing hormone secretion in middle-aged female rats. Endocrinology. 2005;146:4331–4339. doi: 10.1210/en.2005-0575. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol. 1994;140(3):256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- Peng MT, Huang HH. Aging of hypothalamic-pituitary-ovarian function in the rat. Fertil Steril. 1972;23(8):535–542. doi: 10.1016/s0015-0282(16)39131-2. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69(6):1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23(1):90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- Porter VR, Greendale GA, Schocken M, Zhu X, Effros RB. Immune effects of hormone replacement therapy in post-menopausal women. Exp Gerontol. 2001;36(2):311–326. doi: 10.1016/s0531-5565(00)00195-9. [DOI] [PubMed] [Google Scholar]
- Rance N, Wise PM, Selmanoff MK, Barraclough CA. Catecholamine turnover rates in discrete hypothalamic areas and associated changes in median eminence luteinizing hormone-releasing hormone and serum gonadotropins on proestrus and diestrous day 1. Endocrinology. 1981;108(5):1795–1802. doi: 10.1210/endo-108-5-1795. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23(13):5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes FI, Winter JS, Faiman C. Pituitary-ovarian relationships preceding the menopause. I. A cross-sectional study of serum follicle-stimulating hormone, luteinizing hormone, prolactin, estradiol, and progesterone levels. Am J Obstet Gynecol. 1977;129(5):557–564. [PubMed] [Google Scholar]
- Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: Evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65(6):1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Gilardi KV, Lasley B, Rapp PR. Reproductive senescence predicts cognitive decline in aged female monkeys. Neuroreport. 1997;8(8):2047–2051. doi: 10.1097/00001756-199705260-00048. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lee CE, King JC. A reduced proportion of luteinizing hormone (LH)-releasing hormone neurons express fos protein during the preovulatory or steroid-induced LH surge in middle-aged rats. Biol Reprod. 1994;51(6):1264–1272. doi: 10.1095/biolreprod51.6.1264. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Mitchell S, Lee CE, King JC. Reconstructions of populations of luteinizing hormone releasing hormone neurons in young and middle-aged rats reveal progressive increases in subgroups expressing fos protein on proestrus and age-related deficits. Endocrinology. 1995;136(9):3823–3830. doi: 10.1210/endo.136.9.7649089. [DOI] [PubMed] [Google Scholar]
- Rubin BS. Hypothalamic alterations and reproductive aging in female rats: Evidence of altered luteinizing hormone-releasing hormone neuronal function. Biol Reprod. 2000;63(4):968–976. doi: 10.1095/biolreprod63.4.968. [DOI] [PubMed] [Google Scholar]
- Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81(4):1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- Sawyer CH, Everett JW, Markee JE. A neural factor in the mechanism by which estrogen induces the release of luteinizing hormone in the rat. Endocrinology. 1949;44(3):218–233. doi: 10.1210/endo-44-3-218. [DOI] [PubMed] [Google Scholar]
- Scarbrough K, Wise PM. Age-related changes in pulsatile luteinizing hormone release precede the transition to estrous acyclicity and depend upon estrous cycle history. Endocrinology. 1990;126(2):884–890. doi: 10.1210/endo-126-2-884. [DOI] [PubMed] [Google Scholar]
- Schwartz NB. A model for the regulation of ovulation in the rat. Recent Prog Horm Res. 1969;25:1–55. doi: 10.1016/b978-0-12-571125-8.50004-1. [DOI] [PubMed] [Google Scholar]
- Seifert-Klauss V, Mueller JE, Luppa P, Probst R, Wilker J, Hoss C, Treumann T, Kastner C, Ulm K. Bone metabolism during the perimenopausal transition: A prospective study. Maturitas. 2002;41(1):23–33. doi: 10.1016/s0378-5122(01)00248-1. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281(13):1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- Sherman BM, West JH, Korenman SG. The menopausal transition: Analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab. 1976;42(4):629–636. doi: 10.1210/jcem-42-4-629. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Jennes L, Wise PM. Localization of the VIP2 receptor protein on GnRH neurons in the female rat. Endocrinology. 2000;141(11):4317–4320. doi: 10.1210/endo.141.11.7876. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Jennes L. Neural signals that regulate GnRH neurones directly during the oestrous cycle. Reproduction. 2001;122:1–10. doi: 10.1530/rep.0.1220001. [DOI] [PubMed] [Google Scholar]
- Stetson MH, Watson-Whitmyre M. Nucleus suprachiasmaticus: The biological clock in the hamster? Science. 1976;191(4223):197–199. doi: 10.1126/science.942799. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29(2):209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104(14):6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel S, Lin W, Lakhlani S, Jennes L. Expression of estrogen receptor-alpha and cfos in norepinephrine and epinephrine neurons of young and middle-aged rats during the steroid-induced luteinizing hormone surge. Endocrinology. 2002;143(10):3974–3983. doi: 10.1210/en.2002-220430. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Wiegand SJ, Bridson WE. A role for medial preoptic nucleus on afternoon of proestrus in female rats. Am J Physiol. 1980;238(6):E533–539. doi: 10.1152/ajpendo.1980.238.6.E533. [DOI] [PubMed] [Google Scholar]
- Treloar AE. Menstrual cyclicity and the pre-menopause. Maturitas. 1981;3(34):249–264. doi: 10.1016/0378-5122(81)90032-3. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R, Buijs RM. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J Neuroendocrinol. 1993;5(2):137–144. doi: 10.1111/j.1365-2826.1993.tb00373.x. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, van Oudheusden HJ, Buijs RM, van der Donk HA, van den Hurk R, Wiegant VM. Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-releasing hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology. 1994;134(6):2636–2644. doi: 10.1210/endo.134.6.8194489. [DOI] [PubMed] [Google Scholar]
- van der Beek EM. Circadian control of reproduction in the female rat. Prog Brain Res. 1996;111:295–320. doi: 10.1016/s0079-6123(08)60415-x. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: Combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. 1997;384(4):569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Swarts HJ, Wiegant VM. Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology. 1999;69(4):227–237. doi: 10.1159/000054423. [DOI] [PubMed] [Google Scholar]
- van der Schoot P. Changing pro-oestrous surges of luteinizing hormone in ageing 5-day cyclic rats. J Endocrinol. 1976;69(2):287–288. doi: 10.1677/joe.0.0690287. [DOI] [PubMed] [Google Scholar]
- van Look PF, Lothian H, Hunter WM, Michie EA, Baird DT. Hypothalamic-pituitary-ovarian function in perimenopausal women. Clin Endocrinol (Oxf) 1977;7(1):13–31. doi: 10.1111/j.1365-2265.1977.tb02936.x. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Finch CE, Nelson JF. Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates. In: Knobil E, Neill J, editors. The physiology of reproduction. Raven Press; New York: 1994. pp. 861–1010. [Google Scholar]
- Voytko ML, Tinkler GP. Cognitive function and its neural mechanisms in nonhuman primate models of aging, alzheimer disease, and menopause. Front Biosci. 2004;9:1899–1914. doi: 10.2741/1370. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Langub MC, Jr, Engle MG, Maley BE. Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Res. 1995;689(2):254–264. doi: 10.1016/0006-8993(95)00548-5. [DOI] [PubMed] [Google Scholar]
- Weiland NG, Cohen IR, Wise PM. Age-associated alterations in catecholaminergic concentrations, neuronal activity, and alpha 1 receptor densities in female rats. Neurobiol Aging. 1989;10(4):323–329. doi: 10.1016/0197-4580(89)90043-2. [DOI] [PubMed] [Google Scholar]
- Weiland NG, Wise PM. Aging progressively decreases the densities and alters the diurnal rhythms of alpha 1-adrenergic receptors in selected hypothalamic regions. Endocrinology. 1990;126(5):2392–2397. doi: 10.1210/endo-126-5-2392. [DOI] [PubMed] [Google Scholar]
- Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292(24):2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- WHO. Research on the menopause in the 1990s. Report of a WHO scientific group. World Health Organ Tech Rep Ser 866. 1996:1–107. [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34(6):395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wise PM. Norepinephrine and dopamine activity in microdissected brain areas of the middle-aged and young rat on proestrus. Biol Reprod. 1982;27(3):562–574. doi: 10.1095/biolreprod27.3.562. [DOI] [PubMed] [Google Scholar]
- Wise PM. Alterations in proestrous LH, FSH, and prolactin surges in middle-aged rats. Proc Soc Exp Biol Med. 1982;169(3):348–354. doi: 10.3181/00379727-169-41356. [DOI] [PubMed] [Google Scholar]
- Wise PM. Estradiol-induced daily luteinizing hormone and prolactin surges in young and middle-aged rats: Correlations with age-related changes in pituitary responsiveness and catecholamine turnover rates in microdissected brain areas. Endocrinology. 1984;115(2):801–809. doi: 10.1210/endo-115-2-801. [DOI] [PubMed] [Google Scholar]
- Wise PM. Neuroendocrine ageing: Its impact on the reproductive system of the female rat. J Reprod Fertil Suppl. 1993;46:35–46. [PubMed] [Google Scholar]
- Wise PM, Krajnak KM, Kashon ML. Menopause: The aging of multiple pacemakers. Science. 1996;273(5271):67–70. doi: 10.1126/science.273.5271.67. [DOI] [PubMed] [Google Scholar]
- Wise PM, Kashon ML, Krajnak KM, Rosewell KL, Cai A, Scarbrough K, Harney JP, McShane T, Lloyd JM, Weiland NG. Aging of the female reproductive system: A window into brain aging. Recent Prog Horm Res. 1997;52:279–303. discussion 303-275. [PubMed] [Google Scholar]
- Wise PM, Smith MJ, Dubal DB, Wilson ME, Krajnak KM, Rosewell KL. Neuroendocrine influences and repercussions of the menopause. Endocr Rev. 1999;20(3):243–248. doi: 10.1210/edrv.20.3.0364. [DOI] [PubMed] [Google Scholar]
- Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, Böttner M, Rosewell KL. Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the women’s health initiative. Endocr Rev. 2005;26(3):308–312. doi: 10.1210/er.2004-0014. [DOI] [PubMed] [Google Scholar]
- Woller MJ, Everson-Binotto G, Nichols E, Acheson A, Keen KL, Bowers CY, Terasawa E. Aging-related changes in release of growth hormone and luteinizing hormone in female rhesus monkeys. J Clin Endocrinol Metab. 2002;87(11):5160–5167. doi: 10.1210/jc.2002-020659. [DOI] [PubMed] [Google Scholar]
- Wu JM, Zelinski MB, Ingram DK, Ottinger MA. Ovarian aging and menopause: Current theories, hypotheses, and research models. Exp Biol Med (Maywood) 2005;230(11):818–828. doi: 10.1177/153537020523001106. [DOI] [PubMed] [Google Scholar]
- Yen SSC. The human menstrual cycle: Neuroendocrine regulation. In: Yen SSC, Jaffe RB, Barbieri RL, editors. Reproductive endocrinology: Physiology, pathophysiology, and clinical management. W. B. Saunders; Philadelphia: 1999. pp. 191–217. [Google Scholar]
- Yin W, Gore AC. Neuroendocrine control of reproductive aging: Roles of GnRH neurons. Reproduction. 2006;131(3):403–414. doi: 10.1530/rep.1.00617. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Mahesh VB, Zamorano PL, Brann DW. Decreased gonadotropin-releasing hormone neurosecretory response to glutamate agonists in middle-aged female rats on proestrus afternoon: A possible role in reproductive aging? 1996;137(6):2334–2338. doi: 10.1210/endo.137.6.8641183. [DOI] [PubMed] [Google Scholar]