Abstract
Previous physiological studies suggest that avian pulmonary capillaries behave like almost rigid tubes. We made morphometric measurements to determine the diameter of the capillaries in chicken lungs when the transmural pressure was altered over a wide range. The diameter of avian pulmonary capillaries increased by only 13% when the pressure inside them was raised from 0–25 cm H2O. In contrast, other studies have shown that the mean width of the pulmonary capillaries in dogs increased by about 125% and in cats by 128% for the same pressure change. Furthermore, raising the pressure 35 cmH2O outside the capillaries compared to the pressure inside the capillaries in chicken lungs caused little change in diameter whereas under the same conditions in mammal lungs the capillaries are completely collapsed. We conclude that the epithelial bridges between the blood capillaries in the bird lung provide strong support to the capillaries both in expansion and compression.
Keywords: Avian respiratory physiology, bird lung, lung zones, morphometry, pulmonary vascular resistance, pulmonary hypertension
1. Introduction
There are only two classes of vertebrates, Aves and Mammalia, that are both endothermic homeotherms and capable of very high sustained levels of aerobic exercise. Consequently, these animals require an efficient respiratory system to extract oxygen from the environment to maintain aerobic metabolism. Although functionally similar, the mechanics and structure of the respiratory systems in birds and mammals are remarkably different. The gross anatomical details have been described elsewhere (King, 1966; Duncker, 1972, 1974). In brief, the avian respiratory system has separate structures for ventilation and gas exchange; the former is comprised of air sacs that act as bellows and the latter is a rigid lung attached to the ventral aspect of the vertebral column. Air flow through the lung is unidirectional in the bird, whereas there is reciprocating ventilation in the mammalian lung. Finally, in the bird, gas exchange at the capillary level is cross-current, unlike the mammalian lung where gas exchange has a uniform pool schema (Piiper and Scheid, 1972).
The microstructure of the respiratory anatomy in the lung parenchyma is likewise organized differently in the bird when compared to that of the mammal. The functional unit of the avian lung is the parabronchus, in which blind-ended terminal air spaces called air capillaries are heavily intertwined with blood capillaries. These air capillaries are much smaller in diameter than the alveoli of mammals (Duncker, 1972, 1974). The increase in the surface area over which gas exchange occurs aids to increase the diffusing capacity of the lung, and ventilation-perfusion inequality is reported to be small in exercising birds (Powell and Wagner, 1982; Hempleman and Powell, 1986; Schmitt et al., 2002). Recent studies have shown that the blood gas barrier of the avian lung is generally thinner and more uniform than that of mammals, which also contributes to its gas-exchanging efficiency (Watson et al., 2007). Based upon our previous research on the microanatomy of the avian lung, we proposed that the support of the blood capillaries by the surrounding air capillaries contributes to the strength of the capillary wall and thus allows a very thin blood gas barrier (West et al., 2006, Watson et al., 2007).
These anatomical differences between the avian and mammalian lung also translate to physiological differences. Temporary unilateral occlusion of the pulmonary artery in ducks results in an approximate doubling of blood flow and pulmonary vascular resistance (PVR) through the non-occluded lung due to an apparent lack of recruitment and distension in the pulmonary capillaries (Powell et al., 1985). Another recent study that used an in situ preparation to examine pressure-flow relationships in the bird lung found that when pulmonary venous pressure was held constant, pulmonary artery pressure initially rose linearly with flow rate and then, at higher flow rates, it rose even faster (West et al., 2007). This near-linear increase in pressure with flow rate, coupled with electron micrographs showing clearly patent blood capillaries even when the air capillary pressure greatly exceeded blood capillary pressure suggested that the pulmonary capillaries in the avian lung behave like near-rigid tubes, unlike the compliant capillaries in the mammalian lung. This concept was initially introduced some 30 years ago (Duncker, 1972).
In the present study we determined the distensibility of avian pulmonary capillaries with respect to those in mammalian lungs. Morphometric techniques were used to quantify the diameter of the capillaries under varying conditions of compression and expansion. Morphometric studies have not previously been carried out over such a large, well-characterized pressure range. We hypothesized that the blood capillaries in the avian lung would be less distensible than those in mammalian lungs and, over a range of transcapillary pressures, they would behave as near-rigid tubes.
2. Materials and Methods
2.1 Experimental Preparation and Tissue Sampling
The animal protocol for this experiment was approved by the Animal Care Committees of the University of California, San Diego. Chicken lungs were fixed using tracheal instillation. Animal surgery, perfusion, dissection and sample preparation were similar to procedures previously performed in our laboratory (Tsukimoto et al., 1991; West et al., 2007). For each of the four pressure groups (Table 1), three female white leghorn chickens (Gallus gallus domesticus; mean weight ± SEM = 1.62 ± 0.04 kg) were anesthetized with IV sodium pentobarbital (40 mg/kg). The animal was placed in the supine position, and the chest was opened in the midline to expose the heart for insertion of catheters into the main pulmonary artery via the right ventricle and the left atrium via the left ventricle (Fig. 1). Mean pulmonary arterial pressure and left atrial pressure were adjusted using reservoirs and measured with saline manometers. Perfusion fluid was a mixture of blood with heparinized saline/dextran solution (11.1 g NaCl/L, 350 mOsm; 3% T-70 dextran and 10 IU/ml heparin) as needed. Within a few seconds of stabilization of the vascular pressures, 1 L of fixative (3% glutaraldehyde and 3% T-70 dextran in 0.1 M phosphate buffer; total osmolarity 500 mOsm; pH 7.4), was poured into the trachea at a pressure of 15 or 20 cm H2O (Table 1), during which time the pulmonary artery and left atrial pressures were maintained constant. After fixation, the thorax with the lungs attached were dissected free and stored in 3% gluteraldehyde at 4°C for 7 days. This procedure was performed for four vascular pressure groups as shown in Table 1.
Table 1.
Mean diameters ± standard error and parameters for each pressure group. Pressures are in cm H2O. Pa, pulmonary artery pressure; Pv, pulmonary venous pressure; PA, tracheal (airway) pressure; Ptm, capillary transmural pressure. A, B, C, and D refer to the data in Figs. 2, 3 and 4.
| Pa | Pv | PA | Ptm | Diameter (μm) | |
|---|---|---|---|---|---|
| A | −15 | −15 | 20 | −35 | 3.9 ± 0.14 |
| B | 5 | 5 | 20 | −15 | 4.4 ± 0.13 |
| C | 20 | 20 | 15 | 5 | 4.7 ± 0.13 |
| D | 45 | 45 | 20 | 25 | 5.4 ± 0.14 |
Figure 1.
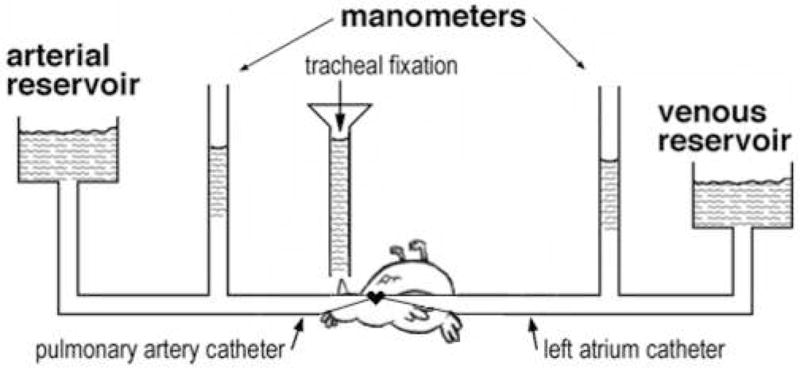
Diagram of the experimental preparation.
Lung samples were taken from the left lung from each chicken at about 1/3 the distance from the most caudal aspect of the lung. A section of tissue 0.5 cm thick was excised from the entire width of each lung transverse to the cranial-caudal axis. This section was then systematically cut into ca. 10 vertical slices and trimmed into blocks following a lung vertical section sampling procedure for morphometric analysis (Michel and Cruz-Orive, 1988). Only samples from the dorsal region of the lung were used in this study to ensure that they came from the paleopulmo.
2.2 Electron Microscopy
Blocks were rinsed overnight in 0.1 M phosphate buffer (350 mOsm, pH 7.4) and post-fixed for 2 hours in osmium tetroxide (1% osmium tetroxide in 0.125 sodium cacodylate buffer; 400 mOsm, pH 7.4). The samples were then passed through stepwise dehydration in increasing concentrations of ethanol (50–100%), rinsed with 100% propylene oxide, and embedded in Araldite.
Three blocks from each animal were randomly selected for analysis. One micron thick sections were cut from each block with an LKB Ultratome III, stained with 0.1% toluidine blue aqueous solution and examined by light microscopy to verify fixation quality. If fixation was inadequate in a selected block, it was excluded from analysis and another block from the same individual was randomly selected. The blocks were then cut into ultrathin sections (50–70 nm) and contrast stained with saturated uranyl acetate and bismuth subnitrate. Sections were examined at an accelerating voltage of 60 kV using a Zeiss EM10C transmission electron microscope. For each chicken, a total of 30 micrographs were taken on film plates at a magnification of 1,600× (10 micrographs taken by systematic random sampling from a single ultrathin section from each of 3 blocks). Micrographs of a carbon grating replica were taken with each film for calibration and to confirm that the measured magnification was within 5% of nominal magnification. Negatives were converted to high resolution digital images with a Microtek Scanmaker i900 flatbed scanner (Microtek USA, Carson, CA) and saved on compact discs.
2.3 Analysis
Digital images were optimized for on-screen viewing by adjusting the contrast and brightness of the image using Adobe Photoshop CS2 or ACDSee v5.0.1 loaded onto a PC. During all digital manipulations, care was taken to maintain the aspect ratio of the image to eliminate distortion.
All measurements were made using ImageJ v1.37 software (U.S. National Institutes of Health, Bethesda, Maryland, USA) with the “grid” and “cell count” plugins (http://rsb.info.nih.gov/ij/plugins/index.html). The capillary surface area to volume ratio (v/s) gives an inverse estimate of capillary lumen diameter. V/s of the blood capillaries was estimated using a multipurpose test grid composed of 30 test points and 13 test lines similar to figure 1E in Weibel et al. (2007). The spacing of the test lines (d) for the grid was calibrated with a carbon grating replica and the grid was overlaid on each micrograph. Test points that fell on capillary lumen were quantified to yield an estimate of capillary volume density (Pc). Surface area was estimated by counting the number of times the test lines intersected the capillary surface trace (Ic). With this count data, we calculated v/s and estimated the average diameter (d) of the capillaries at each pressure (Weibel et al., 2007).
Diameter data were not normally distributed, and within pressure group differences were not significant (n = 3 chickens for each group; Kruskal-Wallis Oneway Analysis of Variance on Ranks; at least p > 0.15 for each group). Thus we used a Kruskal-Wallis Oneway Analysis of Variance on Ranks Test and Dunn’s Method (post-hoc) to determine significant differences between pressure groups. In all cases, P<0.05 was required for statistical significance.
3. Results
3.1 Distensibility
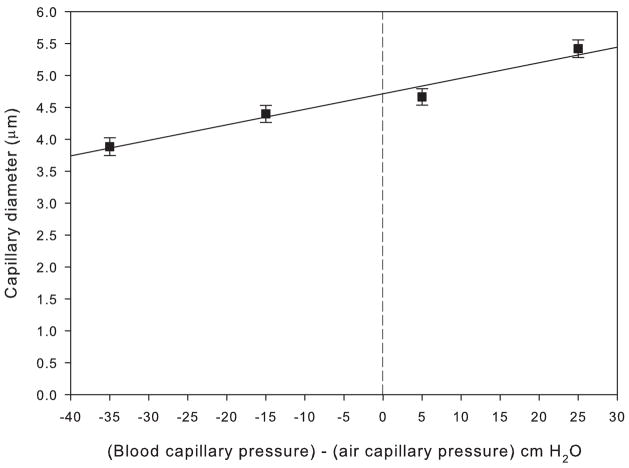
The results of the morphometric analysis of chicken lungs fixed at different vascular pressures are shown in Table 1 and Fig. 2. The transmural pressure of the capillaries has been calculated as capillary hydrostatic pressure minus air capillary pressure, the latter being the height of the tracheal reservoir (Fig. 1). Over the range of transmural pressures from −35 cm H2O (resulting in capillary compression) to +25 cm H2O (expansion), capillary diameter increased by approximately 37% in a linear fashion described by a regression line y = mx + b where m = 0.024, b = 4.7 and y = diameter, m = the slope of the line and b = the y intercept at x = 0. The slope of the regression line was significantly different from zero (t = 7.5, df = 3, p < 0.05). Mean diameters of the capillaries fixed at −35 cm H2O, −15 cm H2O, +5 cm H2O, and +25 cm H2O were 3.9 μm, 4.4 μm, 4.7 μm, and 5.4 μm, respectively (Table 1). The mean change in diameter from −35 to +25 was significantly different (Q = 7.6, p < 0.05), but the change in diameter between the intermediate pressures was not (Q = 1.5, p > 0.05). Thus there was a significant change in capillary diameter under extreme conditions of vascular compression and expansion.
Figure 2.
Plot of pulmonary capillary diameter (μm) vs. (blood capillary pressure) – (air capillary pressure) (cm H2O) with a fitted least-squares regression line for the chicken pulmonary capillaries. The four points correspond to the four rows in Table 1.
It may be argued that a 37% increase in diameter is inconsistent with the reference to “minimal” distensibility in the title of the paper. However, it should be noted that the pressure range that we studied is very large. For example, in the human lung the capillary pressure half way up the lung has been calculated to rise from about 9 cm H2O to 41 cm H2O as a result of maximal exercise (Maina and West, 2005). Using the regression line in Fig. 2, this corresponds to an increase in diameter of the capillaries in the chicken lung by only 16%. For a pressure increase from 0 to 25 cmH2O, the increase in diameter was 13%.
3.2 Comparison with mammals
Our data were obtained using electron microscopic and morphometric techniques that have not previously been used to measure distensibility of the pulmonary capillaries in the lungs of birds or mammals. However, there are two published studies that measure the “mean width” of the mammalian pulmonary capillaries orthogonal to the septum axis in rapidly frozen dog lungs at different vascular and alveolar pressures (Glazier et al., 1969; Mazzone, 1980). In these studies, the measurement of width was made at equal intervals along the septum so that some measurements were located on the capillaries whereas others were on the tissue between the capillaries. Thus these studies give a measurement of distensibility, but underestimate capillary diameter (Mazzone, 1980). There is also a single study that used light micrographs to measure the thickness of the capillary bed “sheet” of cat lungs perfused with silicone-elastomer. These data were collected by measuring the distance between opposing basement membranes along the “cut edge” of the interalveolar wall (Sobin et al., 1972).
The data from the present study on birds and the data of similar measurements taken from the mammalian studies mentioned above are combined in Fig. 3. Only one of the mammalian studies measured distensibility when the alveolar pressure exceeded capillary pressure (Mazzone 1980), but the steepness of the least-squares regression lines show that the distensibility of mammalian pulmonary capillaries is much larger than that of the bird (Fig. 3). Statistical analysis confirms that the slopes of the avian and mammalian lines are not parallel and that the slope of the avian regression line is significantly different from that of the mammals (t = 10.7, df = 203, p<0.05). For example, the change in mean width in the Mazzone (1980) study in dogs over a transcapillary pressure range from −10 to +25 cm H2O was about a 5 fold increase (410%) whereas for the same pressure difference in the chicken the difference was about 19%. In the Mazzone (1980) study, a pressure increase from 0 to 25 cmH2O resulted in an increase in mean width of 125%, and in the Sobin et al. (1972) study the increase was 128%.
Figure 3.
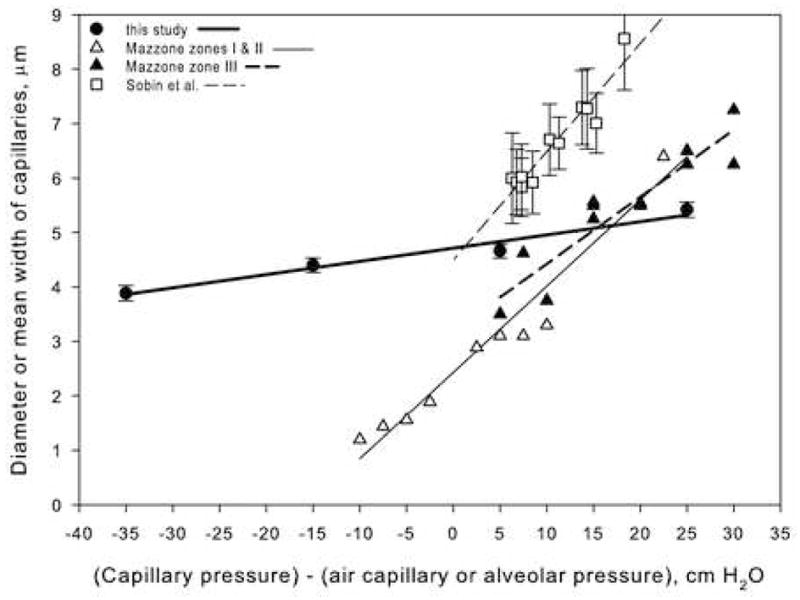
Comparison of bird and mammalian (dog and cat) pulmonary capillary diameter changes under different vascular and airway pressures. Dog data from Mazzone (1980); cat data from Sobin et al. (1972).
Taken together, these morphometric results show that over a large range of pressures, the avian capillaries are profoundly less distensible than those of mammals, and indeed behave as near-rigid tubes. These data are in agreement with our previous data of pressure-flow relationships, where we found that pulmonary vascular resistance (PVR) did not change in a significant or predictable fashion under conditions of large pulmonary artery and venous pressure changes (West et al. 2006). This behavior is unlike the PVR of the pulmonary vasculature in mammalian lungs, which decreases substantially under high pulmonary vascular pressures due to distension and recruitment of the pulmonary capillaries (Borst et al., 1956; Glazier et al., 1969).
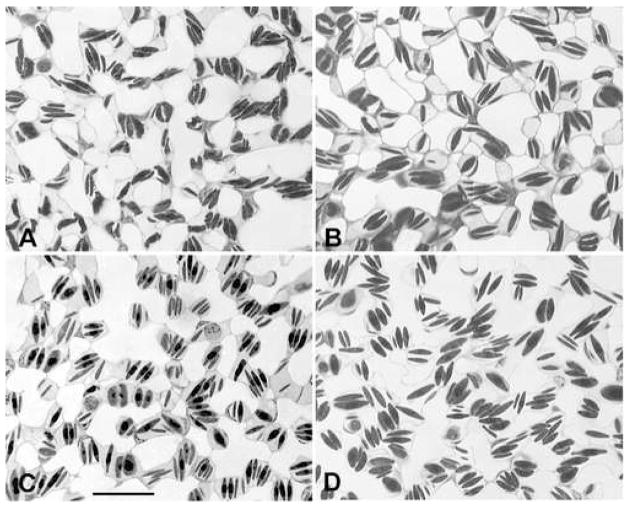
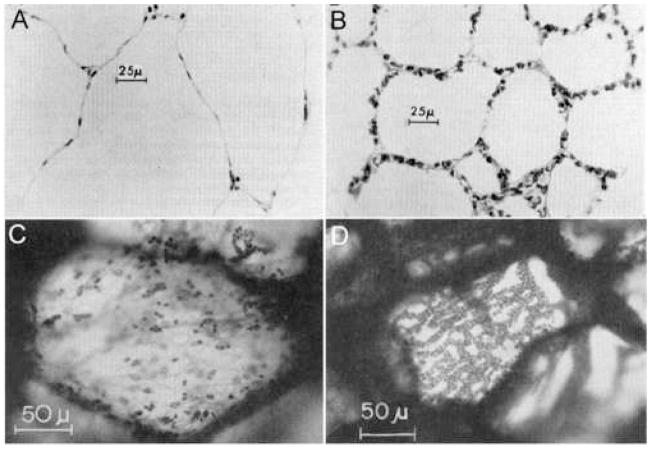
Morphological evidence supports the quantitative evidence that the bird pulmonary capillaries behave as near-rigid tubes. Light micrographs of the chicken pulmonary capillaries were taken for each of the four vascular pressure groups, and there appears to be little change in pulmonary capillary caliber over the wide range of pressures (Fig. 4). In contrast, mammalian (dog) pulmonary capillary images taken when the alveolar pressure exceeded the capillary pressure by 24 cm H2O (Fig 5A) and when capillary pressure exceeded alveolar pressure by 32 cm H2O (Fig 5B) showed a marked change in capillary caliber. In Fig 5A, the pulmonary capillaries are completely flattened with a few erythrocytes trapped in the capillaries whereas in Fig 5B, the capillaries are clearly patent (Glazier et al., 1969). Similarly, when the pulmonary capillary bed of the cat lung is viewed orthogonal to the bed at 7 cm above the zone I–II junction, the capillaries are compressed (Fig. 5C) and when viewed 17 cm below the zone III junction, the capillary bed is distended and multiple erythrocytes are seen within each capillary (Fig. 5D, Vreim et al., 1974).
Figure 4.
Light micrographs of the chicken lung fixed intratracheally at the different pressures shown in Table 1. All four panels are taken at the same magnification. Bar, 20 μm.
Figure 5.
Histological images of mammalian pulmonary capillaries shown at (A) 24 cm above zone I–II junction in the dog lung, (B) 32 cm below zone II–III junction in the dog lung, (C) 7 cm above zone I–II junction in the cat lung, and (D) 17 cm below zone II–III junction in the cat lung. In A and C the capillaries are collapsed, but in B and D they are clearly patent. Dog images from Glazier et al. (1969); cat images from Vreim et al. (1974).
4. Discussion
4.1 Near-rigid pulmonary capillaries
The morphological and morphometric evidence presented here show that the avian pulmonary capillaries exhibit little deformation when subject to extreme changes in transmural pressure when compared to mammals. Our results are consistent with studies performed by Powell et al. (1985) where total unilateral occlusion of the pulmonary artery in ducks resulted in an approximate doubling of pulmonary vascular resistance (PVR) in the non-occluded lung. Wideman (2001) found similar results.
We believe that the epithelial bridges (Fig. 6A) that separate the air capillaries from one another are responsible for the strength and rigidity of the blood capillaries (Klika et al., 1997; Scheuermann et al., 1997; Watson et al., 2007). In some instances blood capillaries adjoin other capillaries and, infrequently, blood capillaries are seen on opposite sides of the cytoplasm of epithelial cells. The epithelial bridges in the bird lung are structures made of squamous cells that are not involved with gas exchange but form part of the walls of the air capillaries. They are contiguous with the epithelium layer of the blood capillaries and form thin sheet-like barriers (Figs. 6 and 7). The epithelial bridges are extremely thin (Fig. 6) and it is difficult to believe that they could withstand large mechanical stresses. Presumably the explanation of how they can be responsible for the rigidity of the pulmonary capillaries is that the mechanical loads are distributed in such a way that these structures are not overstressed.
Figure 6.
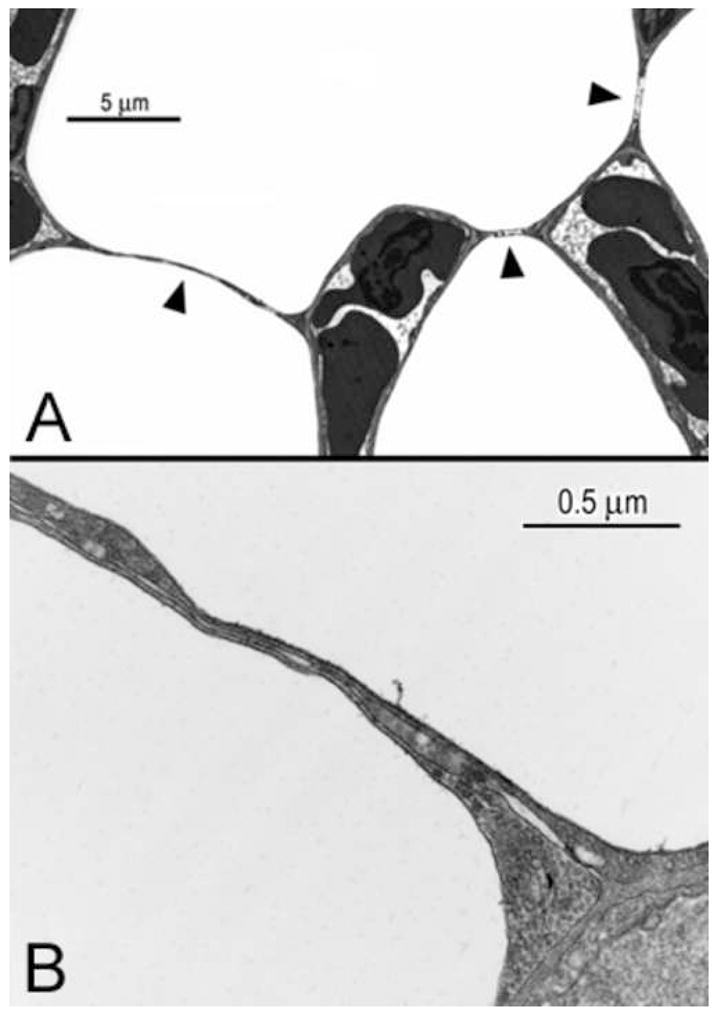
High magnification electron micrographs of the epithelial bridges that connect the blood capillaries with each other and are not involved with gas exchange. In (A) the bridges are marked by arrowheads; (B), the extracellular matrix (ECM) is clearly visible in the bridge and appears to be contiguous with the ECM of the blood capillary.
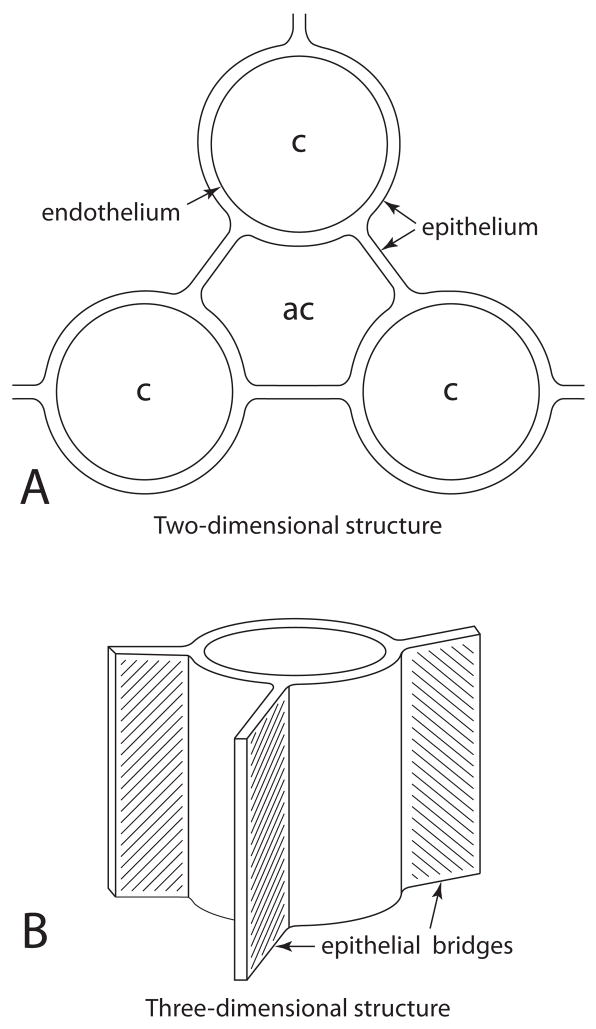
Figure 7.
Schematic of epithelial bridges shown in three dimensions. (A) A plane cut through the blood capillary perpendicular to its long axis shows the epithelial bridges as thin cables, but (B) a view of the bridges parallel to the long axis of the capillary shows the contiguous epithelium as a sheet.
In addition, the air capillaries have a very small radius of curvature and therefore surface tension forces are potentially very large. However the bird lung contains surfactant, which reduces surface tension, and also a trilaminar lipoprotein material, the function of which is unclear (Pattle, 1978; Klika et al., 1997). It is possible that surface tension forces contribute to the mechanical support of the blood capillaries but the micromechanics in such small structures are very complex.
High magnification electron micrographs clearly show an extra-cellular matrix layer within at least part of the sheets, which may confer strength to the epithelial bridges (Fig. 6C). Although Powell and Mazzone (1985) found folds and pleats in both the blood and air capillaries in rapidly-frozen goose lungs and hypothesized that the blood capillaries may therefore be compliant, our data show very limited compliance over a wide range of transmural pressures. Macklem et al. (1979) found that the paleopulmo parabronchi of ducks were slightly compliant over a limited range, but also observed that compression of the lung did not cause collapse of the air capillaries.
4.2 Experimental limitations
Our experimental protocol was straightforward and tracheal instillation of fixative has previously been used by numerous other investigators. An assumption is that the tissue is fixed at the pressure given by the reservoir shown in Fig. 1. In order to ensure this the fixative was poured in repidly. The small variation in diameter at any given pressure (Fig. 2) is evidence that this procedure was successful. The large differences between the pressure groups were great enough to ensure no overlap in data. Since the slope of the regression line of our data explained 97% of the variation, there appears to be little variability for estimating capillary diameter.
A limitation in this study is the lack of similar published data. We are not aware of any previous studies that have measured distensibility in the pulmonary blood capillaries of birds by morphometric techniques. Of the handful of studies that have reported distensibility data in mammals, none have used the morphometric technique that we have employed in this study. Because the mammalian studies of pulmonary capillary distensibility may underestimate the width of the capillaries (see Results section), the comparison of the absolute data is not completely accurate. However we were able to compare relative data (e.g., the slopes of the lines; percent increases in diameter). It would be of interest to examine the distensibility of blood capillaries in other bird species that have blood-gas barriers that are thinner than that of the chicken.
A brief comment on the plotting of the dog and cat data in Fig 3 is warranted. The zone III data can be accurately related to capillary pressure because the arterial-venous pressure difference was small and therefore the capillary pressure is given by the distance down the lung. For the zone II data, the situation is not so clear and we have used the distance below the zone I–II junction as the capillary pressure. This assumption is supported by the fact that the zone I–II and zone III points are nearly contiguous and also that the slopes of the regression lines are similar. This assumption is consistent with the notion that the collapse point responsible for the zone II behavior is at the downstream end of the capillary, or even in the small veins. There is evidence to support the latter in the chicken lung (West et al., 2007) and no evidence against it in mammals.
4.3 Pulmonary Hypertension Syndrome
Pulmonary Hypertension Syndrome (PHS) is a disease that occurs in some strains of fast-growing chickens and turkeys which eventually leads to pulmonary hypertension, right heart failure, ascites and death. The etiology of the illness has been studied extensively (Currie, 1999; Wideman et al., 2007) because worldwide it is estimated to cost the broiler industry over a billion dollars each year.
During the last 40 years, genetic selection for rapid growth combined with changes in feed have resulted in a growth rate that has more than doubled (Julian, 1998). Presumably the increased cardiac output associated with the fast growth and therefore the increased pulmonary blood flow results in a large increase of pulmonary artery pressure due in part to a lack of pulmonary compensatory responses such as capillary recruitment and distension. Our data have shown that the distensibility of the pulmonary capillaries is minimal in chickens, and we suggest that this may be an important contributing factor to Pulmonary Hypertension Syndrome.
Acknowledgments
This study was supported by NIH grants 5 RO1 HL60968-06 and 5 T32 HL07212-29.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Borst HG, McGregor M, Whittenberger JL, Berglund E. Influence of pulmonary arterial and left atrial pressures on pulmonary vascular resistance. Circ Res. 1956;4:393–399. doi: 10.1161/01.res.4.4.393. [DOI] [PubMed] [Google Scholar]
- Currie RJW. Acites in poultry: recent investigations. Avian Path. 1999;28:313–326. doi: 10.1080/03079459994560. [DOI] [PubMed] [Google Scholar]
- Duncker HR. Structure of avian lungs. Respir Physiol. 1972;14:44–63. doi: 10.1016/0034-5687(72)90016-3. [DOI] [PubMed] [Google Scholar]
- Duncker HR. Structure of the avian respiratory tract. Respir Physiol. 1974;22:1–19. doi: 10.1016/0034-5687(74)90044-9. [DOI] [PubMed] [Google Scholar]
- Glazier JB, Hughes JMB, Maloney JE, West JB. Measurements of capillary dimensions and blood volume in rapidly frozen lungs. J Appl Physiol. 1969;26:65–76. doi: 10.1152/jappl.1969.26.1.65. [DOI] [PubMed] [Google Scholar]
- Hempleman SC, Powell FL. Influence of pulmonary blood flow and O2 flux on DO2 in avian lungs. Respir Physiol. 1986;63:285–292. doi: 10.1016/0034-5687(86)90096-4. [DOI] [PubMed] [Google Scholar]
- Julian RJ. Rapid growth problems: ascites and skeletal deformities in broilers. Poult Sci. 1998;77:1773–1780. doi: 10.1093/ps/77.12.1773. [DOI] [PubMed] [Google Scholar]
- King AS. Structural and functional aspects of the avian lungs and air sacs. In: Felts WJL, Harrison RJ, editors. International Review of General and Experimental Zoology. Academic Press; New York: 1966. pp. 171–267. [Google Scholar]
- Klika E, Scheuermann DW, De Groodt-Lasseel MH, Bazantova I, Switka A. Anchoring and support system of pulmonary gas-exchange tissue in four bird species. Acta Anat. 1997;159:30–41. doi: 10.1159/000147962. [DOI] [PubMed] [Google Scholar]
- Macklem PT, Bouverot P, Scheid P. Measurement of the distensibility of the parabronchi in duck lungs. Respir Physiol. 1979;38:23–35. doi: 10.1016/0034-5687(79)90004-5. [DOI] [PubMed] [Google Scholar]
- Maina JN, West JB. Thin and strong! The bioengineering dilemma in the structural and functional design of the blood-gas barrier. Physiol Rev. 2005;85:811–844. doi: 10.1152/physrev.00022.2004. [DOI] [PubMed] [Google Scholar]
- Mazzone RW. Influence of vascular and transpulmonary pressures on the functional morphology of the pulmonary microcirculation. Microvasc Res. 1980;20:295–306. doi: 10.1016/0026-2862(80)90030-8. [DOI] [PubMed] [Google Scholar]
- Michel RP, Cruz-Orive LM. Application of the Cavalieri principle and vertical sections method to lung: estimation of volume and pleural surface area. J Microsc. 1988;150:117–136. doi: 10.1111/j.1365-2818.1988.tb04603.x. [DOI] [PubMed] [Google Scholar]
- Pattle RE. Lung surfactant and lung lining in birds. In: Piiper J, editor. Proceedings in life sciences: Respiratory function in birds, adult and embryonic. Satellite symposium of the 27th International Congress of Physiological Sciences; Paris, Springer-Verlag, Berlin. 1978. pp. 23–32. [Google Scholar]
- Piiper J, Scheid P. Maximum gas transfer efficacy of models for fish gills, avian lungs and mammalian lungs. Respir Physiol. 1972;14:115–124. doi: 10.1016/0034-5687(72)90022-9. [DOI] [PubMed] [Google Scholar]
- Powell FL, Hastings RH, Mazzone RW. Pulmonary vascular resistance during unilateral pulmonary artery occlusion in ducks. Am J Physiol. 1985;249:R34–R43. doi: 10.1152/ajpregu.1985.249.1.R39. [DOI] [PubMed] [Google Scholar]
- Powell FL, Mazzone RW. Morphometrics of rapidly frozen goose lung. Resp Physiol. 1985;51:319–332. doi: 10.1016/0034-5687(83)90026-9. [DOI] [PubMed] [Google Scholar]
- Powell FL, Wagner PD. Ventilation-perfusion inequality in avian lungs. Resp Physiol. 1982;48:233–241. doi: 10.1016/0034-5687(82)90083-4. [DOI] [PubMed] [Google Scholar]
- Schmitt PM, Powell FL, Hopkins SR. Ventilation-perfusion inequality during normoxic and hypoxic exercise in the emu. J Appl Physiol. 2002;93:1980–1986. doi: 10.1152/japplphysiol.01108.2001. [DOI] [PubMed] [Google Scholar]
- Scheuermann DW, Klika E, De Groodt-Lasseel MH, Bazantova I, Switka A. An electron microscopic study of the parabronchial epithelium in the mature lung of four bird species. Anat Rec. 1997;249:213–225. doi: 10.1002/(SICI)1097-0185(199710)249:2<213::AID-AR8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Sobin SS, Fung YC, Tremer HM, Rosenquist TH. Elasticity of the pulmonary alveolar microvascular sheet in the cat. Circ Res. 1972;30:440–450. doi: 10.1161/01.res.30.4.440. [DOI] [PubMed] [Google Scholar]
- Tsukimoto K, Mathieu-Costello O, Prediletto R, Elliott AR, West JB. Ultrastructural appearances of pulmonary capillaries at high transmural pressures. J Appl Physiol. 1991;71:573–582. doi: 10.1152/jappl.1991.71.2.573. [DOI] [PubMed] [Google Scholar]
- Vreim CE, Staub NC. Pulmonary vascular pressures and capillary blood volumes in anesthetized cats. J Appl Physiol. 1974;36:275–279. doi: 10.1152/jappl.1974.36.3.275. [DOI] [PubMed] [Google Scholar]
- Watson RR, Fu Z, West JB. Morphometry of the extremely thin pulmonary blood-gas barrier in the chicken lung. Am J Physiol Lung Cell Mol Physiol. 2007;292:L769–L777. doi: 10.1152/ajplung.00355.2006. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Hsia CC, Ochs M. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol. 2007;102:459–467. doi: 10.1152/japplphysiol.00808.2006. [DOI] [PubMed] [Google Scholar]
- West JB, Watson RR, Fu Z. The honeycomb-like structure of the bird lung allows a uniquely thin blood-gas barrier. Respir Physiol Neurobiol. 2006;152:115–118. doi: 10.1016/j.resp.2005.12.009. [DOI] [PubMed] [Google Scholar]
- West JB, Watson RR, Fu Z. Major differences in the pulmonary circulation between birds and mammals. Respir Physiol Neurobiol. 2007;157:382–90. doi: 10.1016/j.resp.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman RF., Jr Pathophysiology of heart/lung disorders: pulmonary hypertension syndrome in broiler chickens. World’s Poult Sci J. 2001;57:289–307. [Google Scholar]
- Wideman RF, Jr, Chapman ME, Hamal KR, Bowen OT, Lorenzoni AG, Erf GF, Anthony NB. An inadequate pulmonary vascular capacity and susceptibility to pulmonary arterial hypertension in broilers. Poult Sci. 2007;86:984–998. doi: 10.1093/ps/86.5.984. [DOI] [PubMed] [Google Scholar]