Abstract
Neuroblasts in the sub ventricular zone (SVZ) proliferate markedly after brain ischemia, and migrate to the site of injury along with blood vessels. However, a large fraction of stroke-generated neuroblasts die shortly after being born, in part, because of local inflammation. In spontaneously hypertensive rats (Sirs) subjected to permanent middle cerebral artery occlusion, we primed E-selectin-specific regulatory T cells (Trigs) by repetitive intranasal administration of recombinant E-selectin to target local secretion of immunomodulating, antiinflammatory cytokines to activating blood vessel segments. E-selectin-tolerized SHRs had decreased infarction volumes, and increased numbers of Tregs in the cervical lymph nodes and ischemic brain. The brain Tregs were distributed primarily in periinfarct regions. E-selectin tolerization did not alter cellular proliferation in the ipsilateral SVZ after stroke, but the expression of tumor necrosis factor on vascular niche blood vessels was suppressed and both doublecortin protein levels and the number of newly generated neuroblasts or neurons were increased in the brain. This enhanced survival of neural progenitor cells and neurons was paralleled by improved functional performance. These studies suggest that E-selectin-specific Tregs can modulate the efficacy of neurogenesis after ischemia and promote repair after brain injury.
Keywords: brain ischemia, Foxp3, functional recovery, neurognesis, Treg, vascular niche
Introduction
Stroke is the leading cause of long-term disability, in part, because of the inability of the injured central nervous system to effectively regenerate itself. Endogenous neural stem/progenitor cells (NSPCs) in the subventricular zone (SVZ) proliferate markedly after stroke in the adult mammalian brain (Arvidsson et al, 2002). Newly generated NSPCs in the SVZ migrate as neuroblasts to the site of injury along with blood vessels, and some differentiate into mature neurons (Arvidsson et al, 2002; Ohab et al, 2006; Yamashita et al, 2006). However, approximately 80% of the stroke-generated neuroblasts and neurons in the ischemic region die during the first 2 weeks after their formation (Arvidsson et al, 2002). Promoting the survival of new progeny, therefore, is an important priority for enhancing neurogenesis after stroke.
A role for neuroinflammation in regulating neurogenesis is emerging. After stroke, various inflammatory cytokines secreted from immune cells or endothelial cells are upregulated in injured brain regions where they contribute to the death of neurons and neural progenitor cells (Hallenbeck, 2002; Iosif et al, 2008; Iosif et al, 2006). Antiinflammatory agents such as nonsteroidal antiinflammatory drugs or minocycline can restore neurogenesis in focal ischemic rat models by reducing infiltration of immune cells and promoting survival of newly generated neurons in the ipsilateral SVZ and dentate gyrus (Hoehn et al, 2005; Liu et al, 2007). Thus, modulating immune conditions in the brain may help to promote newly generated neuronal cell survival.
There are also data showing that induction of a regulatory immune response to brain antigens by mucosal administration of antigen before stroke can improve outcome (Frenkel et al, 2005; Gee et al, 2008). Mucosal tolerance to E-selectin provides cytoprotection against ischemic or hemorrhagic brain injury through the generation of regulatory T cells (Tregs) targeted to activated blood vessels in the ischemic brain (Chen et al, 2003; Nakayama et al, 2007; Takeda et al, 2004; Wakita et al, 2008). E-selectin is a glycoprotein adhesion molecule that is specifically expressed on endothelial cells (Bevilacqua et al, 1989), but only when the endothelium activates (Bevilacqua, 1993). Endothelial activation has a role in the initiation of stroke (Hallenbeck et al, 1988; Libby et al, 1995) and occurs after brain injury from a stroke (Fassbender et al, 1999). E-selectin can, therefore, serve as an immunologic tolerization antigen that can focus immunomodulation to regions of the vascular tree in which thrombosis or hemorrhage are threatened. Intranasal instillation of recombinant E-selectin will induce mucosal tolerance to that antigen with the generation of E-selectin-specific Tregs (Weiner, 2001). E-selectin- specific Tregs may protect neurons or neuronal progenitor cells from damage through ‘bystander suppression’ in which immunomodulatory cytokines such as transforming growth factor-β and interleukin- 10 are released locally (Miller et al, 1991).
In this study we examined whether E-selectin tolerization induces Tregs in lymphoid organs and brains in a rat focal ischemia model. We used a forkhead/winged helix transcription factor, Foxp3, antibody to identify Tregs by flow cytometry and immunohistochemistry (Hori et al, 2003). Next, we examined whether E-selectin tolerization alters adult neurogenesis in the SVZ, neurologic functions after permanent middle cerebral artery occlusion (pMCAO), or the properties of the neurovascular niche for newly generated neuroblasts or neurons in brain ischemia.
Materials and methods
Animals and Tolerization Schedule
This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the NIH and was approved by the NINDS Care and Use Committee. We used 8-week-old spontaneously hypertensive rats (SHRs; Charles River Laboratory, Wilmington, MA, USA) weighing 180 to 220 g (n = 110). SHRs were selected to better model a common comorbidity in clinical stroke, hypertension, as recommended by modified STAIR criteria (Ford, 2008). The active treatment group received recombinant human E-selectin (Novavax, Rockville, MD, USA), whereas the control group received phosphate-buffered saline (PBS). Phosphate-buffered saline (25 μl) or E-selectin (2.5 μg/25 μl) instilled into each nostril under brief anesthesia every other day for 10 days, with this tolerization schedule repeated once after 11 days (Supplementary Figure 1).
Permanent Middle Cerebral Artery Occlusion Surgery
Spontaneously hypertensive rats were anesthetized with 1.5% to 2.0% isoflurane by facemask. Rectal temperature was maintained at 36.5±0.5°C using a thermal blanket during surgery, and for 4 h thereafter. The rats were placed in the lateral position, and a vertical skin incision was made at the midpoint between the left orbit and the external auditory canal. A small burr hole was made with a microdrill through the outer surface of the skull at the junction between the medial wall and the roof of the inferotemporal fossa. The dura was opened to expose the MCAand the MCA was occluded between the inferior cerebral vein and the lateral olfactory tract by bipolar electrocoagulator (ICC 200; ERBE USA Inc., Atlanta, GA, USA). The sham-operated animals underwent the same procedure, but with no MCAO.
BrdU Labeling
To label mitotic cells, 5-bromo-2′-deoxyuridine-5′-monophosphate (BrdU), the thymidine analog incorporated into the DNA of dividing (S-phase) cells, was administered intraperitoneally (50 mg/kg body weight; Sigma-Aldrich, St Louis, MO, USA). We gave injections of BrdU twice daily for 5 consecutive days from 7 to 11 days after surgery. Thereafter, cellular proliferation and neurogenesis were quantified on day 14 or 28.
Cell Preparation and Fluorescence Flow Cytometry Analysis of CD4 + Foxp3 + cells
Fluorescence flow cytometry analyses were performed to determine percentage of Foxp3 + cells in CD4 + cells. Four days after sham-operation, or 2, 4, and 7 days after pMCAO surgery, spleen, superficial or deep cervical lymph nodes (SCLNs or DCLNs), ipsilateral forebrains, and peripheral blood (PB) were isolated, and passed through a 40-μm nylon cell strainer. The red blood cells in PB and spleen were lysed using ACK lysing buffer. Mononuclear cells were isolated by a Percoll gradient (70%/20% or 70%/37%), and collected from the interface.
After Percoll separation, 10,000 cells per tube were incubated with 1 μg mouse IgG2a anti-rat PE-conjugated CD4 (BD Biosciences, San Jose, CA, USA). Subsequently, the cells were fixed with 4% paraformaldehyde, then washed in permeabilization buffer (eBioscience, San Diego, CA, USA), and incubated with 1 μg rat IgG2a anti-mouse/rat AlexaFluor 488 conjugated Foxp3 (eBioscience). The CD4 + cells or Foxp3 + cells were discriminated from cell debris using DAPI staining. The cells were analyzed using the FACSVantage (BD Biosciences). The percentage of Foxp3 + cells in CD4 + cells was calculated using CellQuest Acquisition and Analysis Software (BD Biosciences). More detailed methods are described in Supplementary method section.
Western Blot Analysis
Western blot analyses were performed using the following antibodies: rabbit polyclonal anti-doublecortin (Dcx) antibody (1:1000; Cell Signaling, Danvers, MA, USA), and mouse monoclonal anti-β-actin antibody (1:10,000; Sigma-Aldrich) as a loading control.
Frozen brains (ipsilateral forebrains excluded olfactory bulb and cerebellum) 2 or 4 weeks after surgery were crushed on dry ice, added to 2% SDS, 100 mmol/L Tris HCl, 50 mmol/L EDTA, and 1 mmol/L PMSF, and homogenized and sonicated on ice, and centrifuged at 10,000 g for 10 mins at 4°C. The supernatant was boiled with 5% β-mercaptoethanol and 2% glycerol. Equal amounts of protein (10 μg) were separated on 10% SDS-PAGE and blotted onto PVDF membranes (NEN Life Science Products Inc., Boston, MA, USA). The membranes were then blocked with 5% nonfat dry milk in PBS and incubated with primary antibodies, and followed by incubation with appropriate horseradish peroxidase-conjugated secondary antibodies. For the chemiluminescence detection, we used Immobilon Western (Millipore, Billerica, MA, USA) followed by digital imaging using Fluor Chem (Alpha Innotech, San Leandro, CA, USA). Intensities of bands were analyzed by the AlphaEaseFC software.
Tissue Preparation and Measurement of Total Infarction Volume
Two or four weeks after MCAO, animals were fixed by transcardiac perfusion with 4% paraformaldehyde under deep anesthesia. The brains of animals were removed, incubated in 20% sucrose, and then frozen rapidly on dry ice. The brains were then cut into 30-μm serial coronal sections from the level of the anterior pole of the caudate nucleus through the cerebral hemisphere, mounted on the slides, and processed for staining.
To evaluate the ischemic lesions, 30-μm coronal sections at bregma levels + 2.20, + 1.00, −0.20, −1.20, −2.30, −3.60, −4.80, and −6.00mm were stained with Hematoxylin and Eosin. The areas of the infarction, the ipsilateral hemisphere, and the contralateral hemisphere in the eight coronal sections were measured on an image of each section by using the NIH Image analysis software. The total infarction volume of the ipsilateral hemisphere (% infarction volume) was calculated as a percentage of the volume of the contralateral hemisphere.
Immunohistochemistry
The primary antibodies used for the immunohistochemical staining were: mouse anti-BrdU (1:500; Millipore), mouse anti-rat proliferating cell nuclear antigen (PCNA, 1:10; DAKO, Carpinteria, CA, USA) for detection of mitotic cells, rabbit anti-Dcx (1:500; Cell Signaling) and guinea pig anti- Dcx (1:5000; Millipore) for detection of neuroblasts, mouse anti-neuron-specific nuclear protein (NeuN, 1:500; Millipore) for detection of mature neurons, mouse anti-rat endothelial cell antigen 1 (RECA-I, 1:500; Wako, Osaka, Japan) and rabbit anti-von Willebrand factor (vWF, 1:200; DAKO) for detection of blood vessels, and mouse anti-TNF (1:100; Alpco Diagnostics, Salem, NH, USA) for detection of tumor necrosis factor-α (TNF), and rat anti-Foxp3 (1:500; eBioscience) for detection of Tregs.
To quantify cellular proliferation in the SVZ or the Foxp3 + cells invasion into the ischemic brain, single immunohistochemical staining for BrdU, PCNA, and Foxp3 was conducted. Immunostaining of BrdU required DNA denaturing. The 30-μm coronal sections were rinsed in PBS containing 0.3% tritonX for 30 mins at room temperature for permeabilization, and treated for 30 min in 2N HCl at 37°C, and the reaction was neutralized in 0.1 mol/L boric acid for 10 mins. Thereafter, the sections were washed in PBS, treated with 0.3% hydrogen peroxidase for 30 mins, preincubated in 10% normal donkey serum (NDS) for 60 mins at room temperature, and incubated with primary antibody diluted in 10% normal donkey serum for 3 days or longer at 4°C to obtain a homogeneous staining throughout the entire thickness of the sections. The sections were then incubated for 2 h with the biotinylated secondary antibody, followed by avidin– biotin–peroxidase complex. The peroxidase labeling was visualized with diaminobenzidine.
Double or triple immunostaining was performed by immunofluorescence. After the required DNA denaturing for BrdU staining described above, the sections were rinsed in PBS containing 0.3% tritonX for 30 mins, preincubated with 10% normal goat serum, and then incubated with two or three primary antibodies diluted with 10% normal goat serum for 3 days or longer at 4°C. Subsequently, sections were incubated in fluorescent-labeled secondary antibodies (Alexa 488/546/633; Molecular Probes, Eugene, OR, USA) for 2 h. After washing, they were mounted with VECTASHIELD, and observed under a confocal laser scanning microscope (Zeiss, LSM510).
Cell Quantification
To estimate the total number of BrdU + or PCNA+ cells in the ipsilateral SVZ, 10 serial sections, spaced 300 μm apart, through the anterior to posterior SVZ (bregma level 1.70 to −1.30mm) were quantified with the unbiased optical fractionator approach (StereoInvestigator; MicroBright-Field, Colchester, VT, USA). More detailed methods are described in Supplementary method section.
Foxp3 +, vWF+/TNF +, BrdU +/Dcx +, and BrdU +/NeuN + cells in the periinfarct region were counted manually in 5 serial 30-μm-thick sections, spaced 300 μm apart from bregma level 1.20 to −0.30mm. Owing to their low numbers and densities, counts of cells in ipsilateral periinfarct region are given as cells per sections. Periinfarct region including cerebral cortex, white matter, and striatum, was defined by a 400 μm boundary extending from the edge of the infarction core, medial and lateral to the infarct. The number of Dcx + cells attached to vWF+ or TNF+ blood vessels was counted manually in 3 serial 30-μm-thick sections, spaced 300 μm apart from bregma level 1.20 to 0.30mm. Twenty vWF+ or TNF+ blood vessels in each animal were randomly sampled in the periinfarct region.
Colocalization of BrdU +/Dcx +, BrdU +/NeuN +, or vWF+/TNF + cells and colabeling of Dcx/vWF or Dcx/TNF was validated in a confocal laser scanning microscope using ×40 objective, and was verified by scanning the cell in its entirety within the section by focusing through the z axis.
Evaluation of Neurologic Functions
To assess the effect of E-selectin tolerization on neurologic functions after stroke, pMCAO animals with or without E-selectin tolerization were subjected to a series of behavioral tests. The elevated body swing test (EBST) and tactile adhesive removal test (TAR) were conducted on 2 days before surgery and on days 4, 7, 14, 21, and 28 after surgery. The researcher conducting the behavioral testing and scoring was completely masked to the experimental conditions.
The EBST was used to evaluate asymmetric motor behavior in rats (Borlongan and Sanberg, 1995). Animals were held by the base of the tail and elevated approximately 10cm from the tabletop. The direction of body swing, defined as an upper body turn of > 10° to either side, was recorded for 1 min during each of three trials per day. The numbers of left and right turns were counted, and the percentage of turns made to the side contralateral to the lesioned hemisphere (% right-biased body swing) was determined.
The TAR is a test of unilateral somatosensory dysfunction (Schallert et al, 1982). Two small pieces of adhesive-backed paper circular dots (surface area, 113.1mm2) were used as bilateral tactile stimuli occupying the distal-radial region on the wrist of each forelimb. The time, to a maximum 3 min, that it took for each animal to remove each stimulus from the forelimb (removal time) was recorded in three trials per day.
Statistics
Unpaired Student’s t-test, two-way factorial ANOVA, or one factor ANOVAwith post hoc Bonferroni/Dunn test was used to analyze most data as indicated in the figure legends. Repeated-measures of ANOVA with post hoc Bonferroni/Dunn test was used to analyze the data of behavioral tests. All values are presented as mean±s.d. P < 0.05 was considered to be statistically significant. More detailed methods are described in Supplementary method section.
Results
Animals
One PBS-treated pMCAO animal died of unknown causes 2 days after surgery. Hemorrhage from MCA during vessel occlusion occurred in two PBS-treated and one E-selectin-tolerized pMCAO animals. These 4 animals were excluded, and all other animals (n = 106) were eligible for the analyses.
Infarction Volume
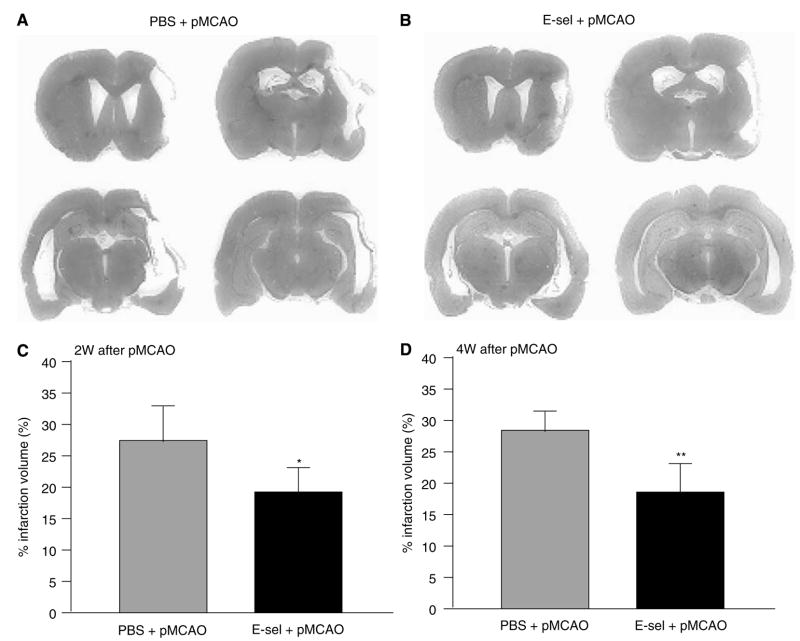
Sham-operated controls either with or without E-selectin tolerization did not show any detectable pathologic changes in brains with Hematoxylin and Eosin staining. In all pMCAO animals, infarction, which showed cavity formation both at 2 and 4 weeks after pMCAO, primarily involved the ipsilateral cerebral cortex (Figures 1A and 1B), and appeared to spare the hippocampus, striatum, and SVZ. Total infarction volume after pMCAO was decreased significantly at 2 weeks (27.4±6.1% of contralateral hemisphere volume in PBS +pMCAO animals and 18.6±5.0% in the E-selectin-tolerized pMCAO group, P < 0.05) and 4 weeks (28.1±7.9% in the PBS-treated pMCAO group and 17.8±5.4% in E-sel +pMCAO animals, P < 0.01; Figures 1C and 1D).
Figure 1.
Total infarction volume was decreased in E-selectin-tolerized animals exposed to pMCAO. Brain slices of a PBS-treated (A) and an E-selectin-tolerized (B) animal at 2 weeks after pMCAO were stained with Hematoxylin and Eosin. The total infarction volume measured in 8 coronal sections in PBS-treated (gray bar) and E-selectin-tolerized (black bar) animals at 2 weeks (C) and 4 weeks (D) after pMCAO. (n=8 and 7 for PBS+pMCAO and E-sel+pMCAO groups, respectively, at 2 weeks after pMCAO; n=7 and 6 for PBS+pMCAO and E-sel+pMCAO groups, respectively, at 4 weeks after pMCAO. *P<0.05, **P<0.01 compared with the PBS-treated pMCAO group by unpaired Student’s t-test.)
Flow Cytometry Analysis for CD4 + Foxp3 + cells
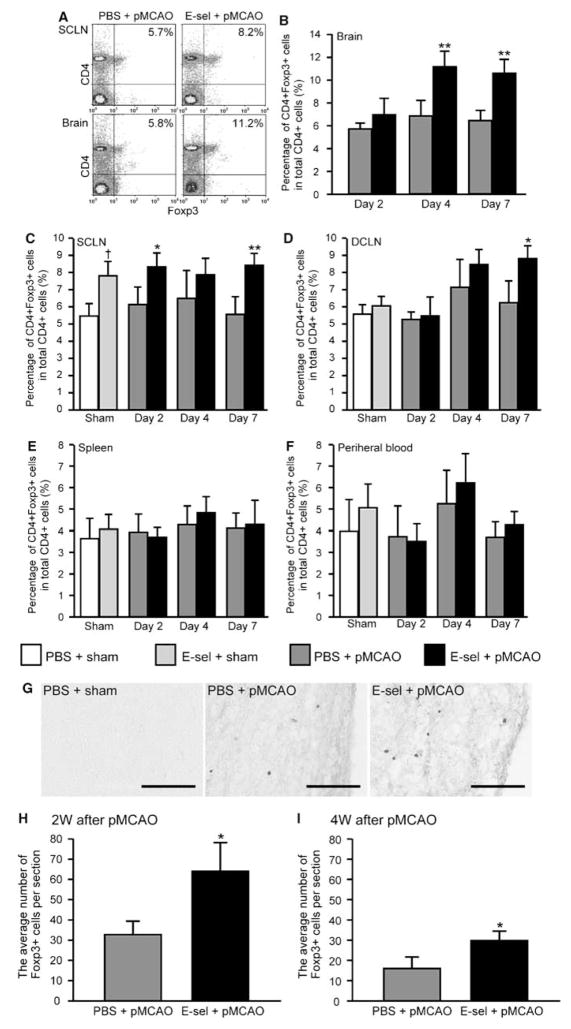
In nonischemic animals, E-selectin tolerization increased the percentage of the CD4 + Foxp3 + T-cell subset in total CD4 + cells compared with PBS-treated sham-operated controls in SCLN (7.8±1.0% versus 6.0±0.6%; Figure 2C). However, the number of mononuclear cells obtained from nonischemic brain was below a threshold that would permit analysis by FACS. In DCLN, spleen, and PB, there were no significant differences in the percentage of the CD4 + Foxp3 + T-cell subset between sham-operated animals with and without E-selectin tolerization (Figures 2D–F).
Figure 2.
Effects of E-selectin tolerization on infiltration of CD4+Foxp3+ Tregs in the ischemic brain and CLN. Cell sorting analysis for each treatment group with anti-CD4 and anti-FoxP3 antibodies. (A) Cell sorting in superficial cervical lymph nodes (SCLNs) and brains from representative PBS-treated or E-selectin-tolerized pMCAO animals. Right upper quadrants indicate the percentage of CD4+FoxP3+ cells in total CD4+ cells. The time profile of the percentage of CD4+FoxP3+ cells in total CD4+ cells is depicted in brains (B), SCLNs (C), DCLNs (D), spleens (E), and peripheral blood (F). (n=4 to 5; †P<0.05, compared with PBS + sham values;*P<0.05, **P<0.01 compared with PBS + pMCAO group by two-way factorial ANOVA followed by Bonferroni/Dunn post hoc test.) (G) Photomicrographs of the immunohistochemical staining for Foxp3 antibody in the periinfarct region 2 weeks after surgery. The cavity as shown in the right side of photomicrographs in PBS-treated pMCAO and E-selectin-tolerized pMCAO animals is infarction (H, I). Average number of Foxp3+ cells per section in the periinfarct region with or without E-selectin tolerization at 2 or 4 weeks after pMCAO (n=6 and 5 for PBS+pMCAO and E-sel+pMCAO animals, respectively; *P<0.01; compared with corresponding PBS+pMCAO group by unpaired Student’s t-test). Scale bar=100 μm.
In pMCAO animals, the percentage of the CD4 + Foxp3 + T-cell subset in the E-selectin-tolerized pMCAO group increased significantly in the SCLN, DCLN, and brain compared with the PBS-treated pMCAO group (Figures 2A–D). As early as 2 days after pMCAO, the percentage of CD4 + Foxp3 + T cells increased significantly in SCLNs in E-selectin-tolerized pMCAO animals (Figure 2C; P < 0.05). From 4 and 7 days after pMCAO, but not 2 days after pMCAO, the percentage of the CD4 + Foxp3 + T-cell subset increased significantly in brains of pMCAO animals that had undergone E-selection tolerization compared with PBS-tolerized animals (Figure 2B; P < 0.01, P < 0.05). In DCLNs, the difference was significant by 7 days after pMCAO (Figure 2D; P < 0.05). In spleen and PB, E-selectin tolerization did not increase the percentage of CD4 + Foxp3 + cells at any time point from 2 to 7 days after surgery (Figures 2E and 2F).
To investigate the distribution of Foxp3 + cells in the brain, immunostaining for Foxp3 antibody was conducted. There were no Foxp3 + cells in the brains of nonischemic animals (Figure 2G). In pMCAO animals, Foxp3 + cells were consistently observed to be distributed primarily in the periinfarct region (Figure 2G), but not in the ischemic core. The number of Foxp3 + cells was significantly higher in the E-selectin-tolerized pMCAO group compared with the PBS-treated pMCAO group both 2 and 4 weeks (32.7±7.2 and 16.1±4.1 cells in PBS-treated pMCAO group and 64.2±14.0 and 29.1±4.8 cells in E-selectin-tolerized pMCAO group, P < 0.01) after pMCAO (Figures 2H and 2I).
Cell Proliferation Assay in the Ipsilateral Subventricular Zone
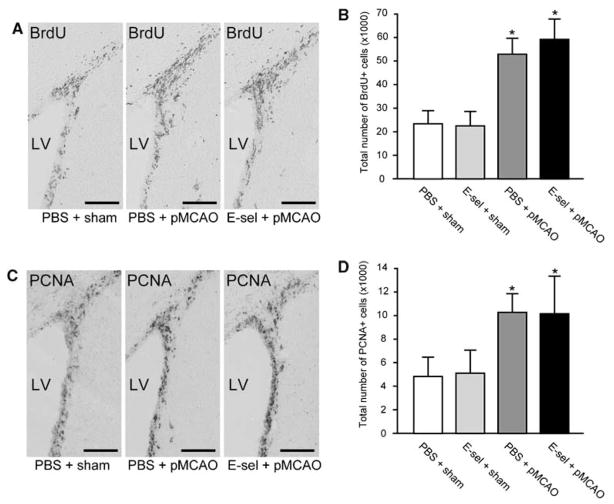
To evaluate celluar proliferation in the SVZ, we quantified the total number of BrdU+ or PCNA+ cells in the ipsilateral SVZ at 2 weeks after surgery with the unbiased optical fractionator approach. Immunostaining revealed BrdU + or PCNA+ cells in the SVZ (Figures 3A and 3C). In nonischemic animals, the total number of BrdU + and PCNA+ cells in the ipsilateral SVZ did not differ significantly between PBS-treated (23938±6016 and 5078±1459) and E-selectin-tolerized (23076±6566 and 5396±1833) group 2 weeks after sham surgery (Figures 3B and 3D). The total number of BrdU + and PCNA+ cells in PBS-treated pMCAO group (52830±5486 and 10501±1494) and E-selectin-tolerized pMCAO group (57405±9183 and 10197±3202) in the ipsilateral SVZ significantly increased 2- to 2.5-fold compared with PBS-treated sham-operated controls (P < 0.01) as shown in Figures 3B and 3D. But differences in the number of BrdU+ or PCNA+ cells between PBS-treated and E-selectin-tolerized pMCAO groups were not significant, suggesting that E-selectin tolerization did not primarily alter the postischemic cellular proliferation in the ipsilateral SVZ after pMCAO.
Figure 3.
Infarction increased cellular proliferation in the ipsilateral SVZ. Photomicrographs of the immunohistochemical staining with BrdU (A) and PCNA (C) antibodies in the ipsilateral SVZ 2 weeks after surgery. Stereologic quantification of BrdU+ (B) and PCNA+ (D) cells in the ipsilateral SVZ at 2 weeks after surgery (n=5 for each group; *P<0.01; compared with PBS+sham group by one-way ANOVA followed by Bonferroni/Dunn post hoc test). Scale bar=100 μm. LV=lateral ventricle.
Doublecortin Protein Level in Brains
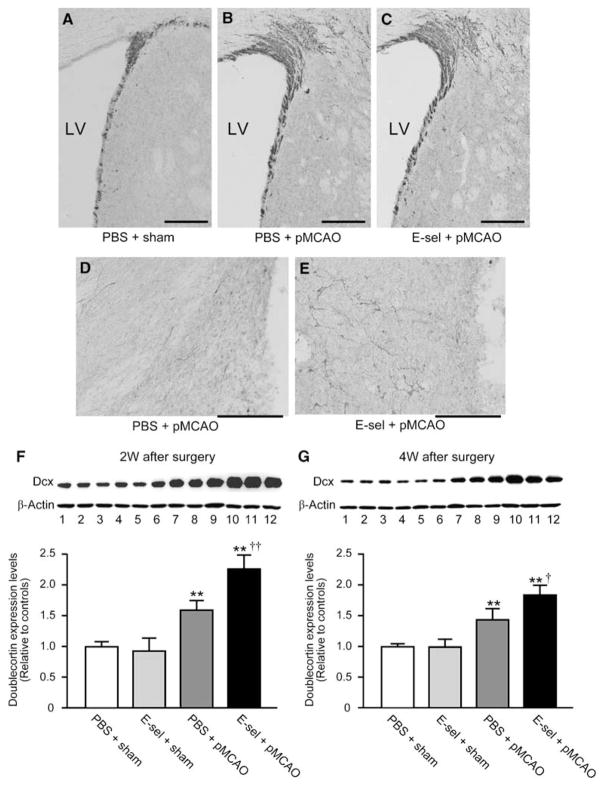
Neuroblasts were identified by the microtubule-associated protein Dcx, a specific marker for migratory neuroblasts in the adult brain. Immunostaining with Dcx antibody detected Dcx+ cells in the SVZ of all animals (Figures 4A–C). Dcx+ cells were identified also in the periinfarct region of pMCAO animals (Figures 4D and 4E), but not in corresponding regions of the brain in sham-operated controls. In the ipsilateral SVZ, more Dcx+ cells were detected in pMCAO animals (Figures 4B and 4C) than in nonischemic animals (Figure 4A); the area of the SVZ also appeared expanded in pMCAO animals compared with nonischemic animals. However, there was no difference in the number of Dcx+ cells in the ipsilateral SVZ between E-selectin-tolerized and PBS-treated pMCAO animals. In the periinfarct region, the E-selectin-tolerized pMCAO group (Figure 4E) had a higher number of Dcx + cells than the PBS-treated pMCAO group (Figure 4D). In the infarct core, there were no Dcx + cells in pMCAO animals.
Figure 4.
Dcx protein levels were increased in the ischemic hemisphere of E-selectin-tolerized animals. Photomicrographs of the immunohistochemical staining for Dcx antibody in the ipsilateral SVZ (A–C) and periinfarct region (D, E) at 2 weeks after surgery. The cavity as shown on the right side of photomicrographs (D, E) is infarction. Scale bar=100 μm in A–E. LV=lateral ventricle. Representative western blot of doublecortin levels in brains exposed to PBS/sham, E-selectin/sham, PBS/pMCAO, or E-selectin/pMCAO (lanes 1 to 3, 4 to 6, 7 to 9, and 10 to 12, respectively) at 2 weeks (F) and 4 weeks (G) after surgery. Reblotting with anti-β-actin was performed as a loading control. Comparison of quantitative densitometric analyses of doublecortin protein levels in the brains. (n=4 for each group; *P<0.05, **P<0.01, compared with PBS+sham values, and †P<0.05, ††P<0.01, compared between PBS+pMCAO versus E-sel+pMCAO values; One way ANOVA followed by Bonferroni/Dunn post hoc test).
The Dcx protein level in the brain was compared by western blotting with rabbit polyclonal Dcx antibody. Consistent with the Dcx protein molecular weight, a 45 kDa band was detected in all animals (Figures 4F and 4G). The ratio of the Dcx protein level relative to PBS-treated sham-operated controls significantly increased in PBS-tolerized pMCAO (1.6±0.1 and 1.4±0.2) and E-selection-tolerized pMCAO (2.3±0.3 and 1.7±0.2) groups at both 14- and 28-day time points after surgery. Statistical comparison of PBS-treated pMCAO and E-selectin-tolerized pMCAO animals showed that the E-selectin-tolerized group had significantly increased Dcx protein levels in the ischemic hemisphere (P < 0.01 at 2 weeks and P < 0.05 at 4 weeks after pMCAO).
Postmitotic Neuroblasts and Neurons in Periinfarct Region
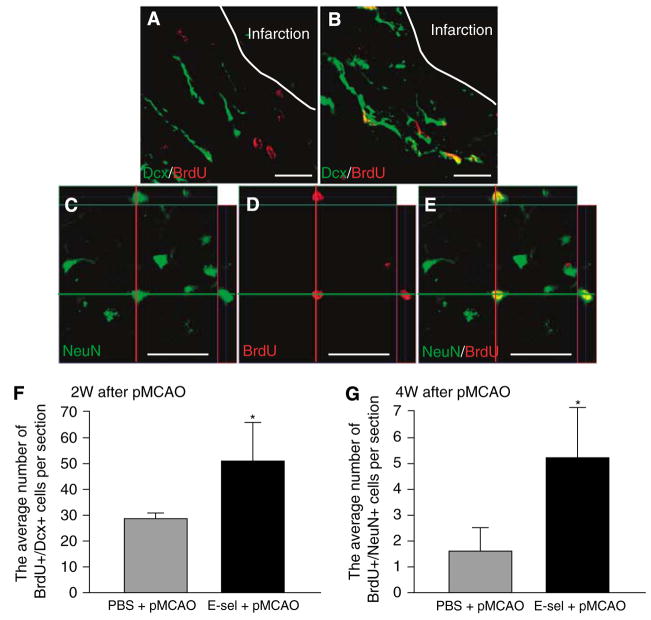
The results described above shows that E-selectin tolerization is associated with an increase in the number of Dcx+ neuroblasts that primarily occurs in the periinfarct region rather than in the ipsilateral SVZ. Next, we investigated the fate of BrdU+ postmitotic cells in ischemic brain. In the brain of sham-operated animals either with or without E-selectin tolerization, BrdU+/Dcx+ cells, indicating postmitotic neuroblasts, were distributed only in the SVZ. In pMCAO animals, BrdU+/Dcx+ cells were identified both in the SVZ and periinfarct region (Figures 5A and 5B). The number of BrdU+/Dcx+ cells in periinfarct region at 2 weeks after pMCAO was significantly higher in the E-selectin-tolerized pMCAO group (51.5±15.2 cells) than in PBS-treated pMCAO animals (29.3±2.0 cells, P < 0.01; Figure 5F).
Figure 5.
The formation of neuroblasts and neurons was enhanced in the periinfarct regions of E-selectin-tolerized animals. Confocal immunofluorescence double-labeling images with anti-Dcx (green) and anti-BrdU (red) antibodies in the periinfarct region of a PBS-treated pMCAO animal (A) and an E-selectin-tolerized pMCAO animal (B) 2 weeks after surgery. Confocal immunofluorescence double-labeling images with anti-NeuN (green) and anti-BrdU (red) antibodies in the periinfarct region of an E-selectin-tolerized pMCAO animal (C–E) 4 weeks after surgery. Orthogonal reconstruction from confocal Z-series represented as viewed in x–z (top) and y–z (right) clearly showed the colocalization of these two signals in the same cell (C–E). Confocal images of a BrdU+/NeuN+ cell showing NeuN (C) and BrdU (D) separately and as a merged image (E). Scale bar in A and B=20 μm, in C–E=50μm. Average number of BrdU+/Dcx+ cells (F) and BrdU+/NeuN+ (G) cells per section in the periinfarct region at 2 and 4 weeks after surgery, respectively (n=6 for each group; *P<0.01; compared with PBS+pMCAO group by unpaired Student’s t-test).
In pMCAO animals, a few BrdU +/NeuN + cells, indicating postmitotic neurons, could be detected in the periinfarct region 4 weeks after pMCAO (Figures 5C–E). Quantitative analysis revealed an increase of almost threefold in the number of BrdU+/NeuN+ cells in the periinfarct region of E-selectin-tolerized pMCAO animals (5.3±1.9 cells) relative to PBS-treated pMCAO controls (1.7±1.0 cells, P< 0.01; Figure 5G).
Neurovascular Niche for Neuroblasts
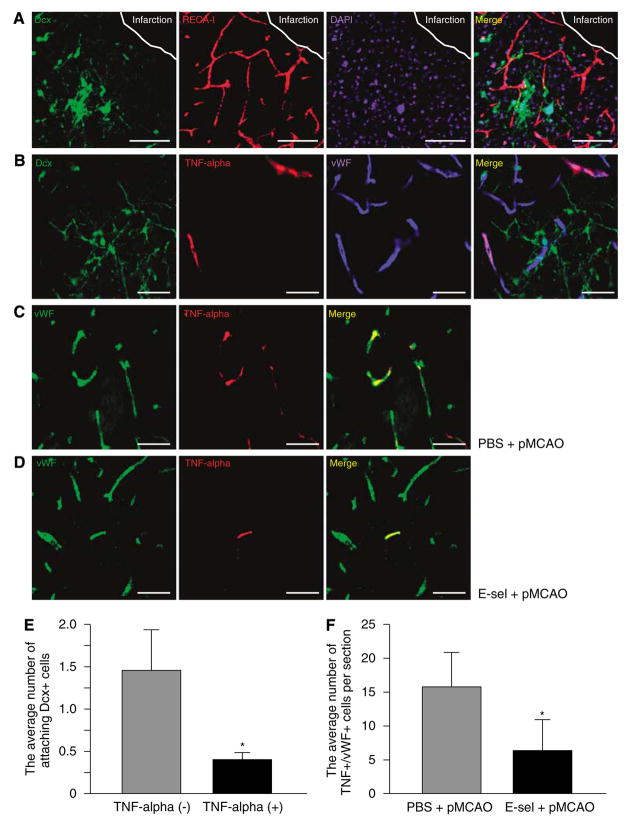
Previous studies have shown that after strokes, migrating neuroblasts can be visualized adjacent to blood vessels in the ischemic brain (Ohab et al, 2006; Yamashita et al, 2006). Consistent with these studies, a large portion of Dcx + cells were noted to be closely associated with RECA-I + blood vessels (Figure 6A) in the periinfarct region of pMCAO animals. As E-selectin tolerization decreased TNF+ blood vessels in extensive cerebral hypoperfused rats in our previous report (Wakita et al, 2008), we conducted immunostaining with anti-TNF and anti-vWF antibodies to assess the relationship between TNF expression by blood vessels and the localization of neuroblasts. Seven days after pMCAO, TNF was expressed in neurons, astrocytes, immune cells, and endothelial cells that surrounded the infarct core (data not shown), but a large proportion of the TNF + cells at 14 and 28 days after pMCAO were vWF+ angiogenic blood vessels. TNF +/vWF + cells were primarily distributed in the periinfarct region of pMCAO animals (Figures 6B–D), but were not in the ipsilateral SVZ or in the sham-operated brain.
Figure 6.
Immunomodulation of the vascular niche in the periinfarct region occurred in E-selectin-tolerized animals. (A) Confocal images showing Dcx (green), RECA-I (red), and DAPI (blue) separately and as a merged image in the E-selectin-tolerized pMCAO animal 2 weeks after surgery. (B) Confocal images showing Dcx (green), TNF (red), vWF (blue) separately and as a merged image in the E-selectin-tolerized pMCAO animal 2 weeks after surgery. Confocal images showing vWF (green) and TNF (red) antibodies and as a merged image in the PBS-treated pMCAO (C) and E-selectin-tolerized pMCAO (D) animals 2 weeks after surgery. Scale bar in A=100 μm, in B–D=50μm. Average number of attaching Dcx+ cells per blood vessel with or without expression of TNF (E) and TNF+/vWF+ blood vessels per section (F) in the periinfarct region (n=6 for each group and n=8 for each group, respectively; *P<0.01; unpaired Student’s t-test).
Within vascular niches for Dcx + cells in periinfarct regions, it was clear that more Dcx + cells existed around TNF– blood vessels than around TNF-expressing blood vessels (Figure 6B). Quantitative analysis revealed that the number of Dcx + cells attached to blood vessels is significantly higher in TNF– blood vessels (1.5±0.4 cells per vessel) than in TNF-expressing blood vessels (0.4±0.1 cells per vessel; Figure 6E; P < 0.01), suggesting that TNF expression by blood vessels could have a detrimental effect on neuroblast survival. Among pMCAO animals, E-selectin tolerization significantly decreased the number of TNF +/vWF + blood vessels (16.1±5.3 vessels per section in PBS-treated pMCAO group and 6.6±3.5 in E-selectin-tolerized pMCAO group,) in periinfarct regions (Figures 6C, 6D, and 6F; P < 0.01).
Neurologic Outcome
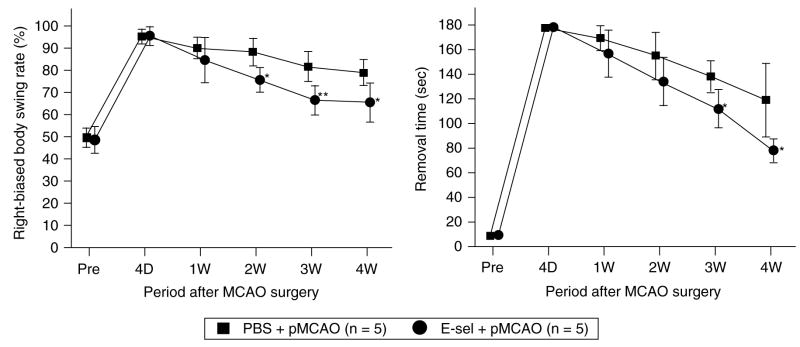
We investigated whether E-selectin tolerization would lead to improvement of neurologic functions after pMCAO. In the EBST, the presurgical baseline level of right-biased body swing rate was 49.6±4.3% in PBS-treated group and 49.4±5.6% in E-selectin-tolerized group. Four days after pMCAO, the frequency of body swings to the right increased to 95.1±2.8% in the PBS-treated pMCAO group and 96.2±3.5% in the E-selectin-tolerized pMCAO group. During the 4-week observation period, E-selectin-tolerized pMCAO animals showed significant recovery of the right-biased body swing rate compared with PBS-treated pMCAO animals (P < 0.01). Post hoc tests showed significant differences in right-biased body swing rates at 2, 3, and 4 weeks after pMCAO (Figure 7A).
Figure 7.
Sensorimotor functions after pMCAO were improved in E-selectin-tolerized animals. Time courses of results of behavioral tests in PBS-treated pMCAO and E-selectin-tolerized pMCAO groups before surgery and during a 28-day period after surgery. (A) Percentage right-biased swing (elevated body swing test). Assessed by the EBST, animals displayed more frequent turns toward contralateral side (right) after pMCAO. E-selectin-tolerized pMCAO animals showed significant recovery of the right-biased body swing rate compared with PBS-treated pMCAO animals (group effect, F1,8=10.5, P=0.0118; group × trial interaction, F5, 40=3.9, P=0.0059). (B) Removal time (adhesive removal test). Assessed by the TAR, pMCAO caused animals to use much longer time to remove the adhesive tape occupying the wrist of the contralateral forelimb. E-selectin-tolerized pMCAO animals showed significant recovery of removal time compared with PBS-treated pMCAO animals (group effect, F1,8=18.0, P=0.0028; group×trial interaction, F5,40=3.0, P=0.021). n=5 for each group; *P<0.05, **P<0.01, compared with PBS+pMCAO values; repeated measures of ANOVA followed by Bonferroni/Dunn post hoc test.
In the TAR, the presurgical baseline time for removal was 7.9±3.1 secs in the PBS-treated group and 10.2±3.3 secs in the E-selectin-tolerized group. Four days after surgery, the removal time increased to 178.1±1.9 secs in the PBS-treated pMCAO group and 179.0±1.5 secs in the E-selectin-tolerized pMCAO group. During the 4-week observation period, E-selectin-tolerized pMCAO animals showed significant recovery of removal time compared with PBS-treated pMCAO animals (P < 0.01). Post hoc tests showed significant differences in the removal times at both 3 and 4 weeks after pMCAO (Figure 7B).
Discussion
In this series of experiments, we have shown that after mucosal tolerization to E-selectin in SHRs exposed to pMCAO CD4 + Foxp3 + Tregs transmigrate primarily to the periinfarct region of ischemic brain, infarction volume is decreased, TNF expression in the local neurovascular niche is reduced, and the survival of newly generated neuroblasts or neurons in the periinfarct region is increased. Under these conditions, an improvement in sensorimotor function after pMCAO also occurs.
Increased Transmigration of Tregs Into the Ischemic Brain in E-Selectin-Tolerized Animals
In our study, the percentage of CD4 + Foxp3 + cells in the total CD4 + cell population (Treg subset) was increased in SCLNs of E-selectin-tolerized sham-operated animals, and in brains, SCLNs, and DCLNs of E-selectin-tolerized pMCAO animals. Furthermore, the Foxp3 + cells were distributed primarily in the periinfarct region; Tregs were not increased in the nonischemic regions. Initial descriptions indicated that Tregs expressed very high levels of the interleukin-2Rα chain (CD25) and consequently these cells are often referred to as CD4 + CD25 +. Treg cells limit inflammation and inhibit autoimmune diseases. The forkhead/winged helix transcription factor gene, Foxp3, is strongly linked to the regulatory function of CD4 + CD25 + cells, and has become a useful intracellular marker for their identification (Hori et al, 2003). Tregs comprise approximately 5% to 10% of CD4 + T cells (Ng et al, 2001) and the results of our flow cytometry analyses are in accord with this.
The observed time profiles of the percentage of CD4 + Foxp3 + T cells among brains, SCLNs, DCLNs conform to the immunologic model of mucosal tolerance. Mucosal tolerance can be achieved through induction of Tregs by repetitive administration of low-dose antigen (Chen et al, 1994). Lymphocytes that are tolerized to an antigen and have become antigen-specific Tregs tend to migrate to the locale of the protein molecule to which they have been primed. With introduction of antigen via the nasal mucosa route, it has been shown that the SCLNs and DCLNs are crucial for tolerance induction (Wolvers et al, 1999). In these studies, intrana-sally instilled fluorescence-labeled antigen was detected primarily in CLNs and to a lesser extent in other lymph nodes. Removal of CLNs after intranasal tolerization prevented the induction of immune tolerance (Wolvers et al, 1999). Our data, which show increased CD4 + Foxp3 + Tregs in SCLNs of E-selectin-tolerized animals both before ischemia and as early as 2 days after pMCAO, are in line with these reports showing an intrinsic capacity of CLNs to support immunologic tolerance.
During endothelial activation after ischemia, E-selectin-specific Tregs can be restimulated to produce immunosuppressive cytokines by presentation of E-selectin by local antigen-presenting cells (Balabanov et al, 1999) and can move to the site of inflammation and suppress the effector functions of proinflammatory cells within the affected tissues (Green et al, 2003). In our study, CD4 + Foxp3 + Tregs were more abundant in the ischemic brains of the E-selectin-tolerized group, suggesting that E-selectinspecific Tregs may move to the site of expressed E-selectin, undergo restimulation, and transmigrate into the brain. Lymphocyte restimulation can also occur in CLNs; dendritic cells that have processed E-selectin from ischemic brain accumulate in CLNs through an anatomic communication that drains antigens from the brain to the CLN via an olfactory route (Walter et al, 2006). Based on our data that the percentage of CD4 + Foxp3 + Tregs in SCLNs and DCLNs increased during a 7-day period after pMCAO, it is likely that restimulation of E-selectinspecific Tregs did occur in CLNs.
The Survival of Periinfarct Region Neuroblasts was Increased in E-Selectin-Tolerized Animals
Neurogenesis persists in the adult mammalian brain and can be enhanced by ischemic injury (Arvidsson et al, 2002; Hoehn et al, 2005; Jin et al, 2001; Nakatomi et al, 2002; Ohab et al, 2006). We detected that both BrdU+ and PCNA+ cells became markedly increased in the ipsilateral SVZ 2 weeks after pMCAO compared with corresponding cell counts in the SVZ of nonischemic animals indicating that stroke can induce SVZ cell proliferation. After BrdU administration from 7 to 11 days after surgery, we quantified the number of BrdU + or PCNA+ cells in the SVZ 2 weeks after surgery. Therefore, BrdU+ cells reflect the number of cells that underwent mitosis during a 7 to 11 day period after surgery, and cells positive for PCNA, which is a protein involved in DNA replication, represent the number of mitotic cells at 2 weeks after surgery in our study. Our findings are consistent with previous reports, in which SVZ cell proliferation stimulated by MCAO increased around 4 days to 2 weeks in the ipsilateral SVZ (Arvidsson et al, 2002; Jin et al, 2001). However, there were no differences in the number of BrdU+ or PCNA+ cells in the SVZ between pMCAO animals with and without E-selectin tolerization. Thus, E-selectin tolerization appears not to alter the rates of postischemic cellular proliferation in the SVZ.
In phase with SVZ cellular proliferation after stroke, Dcx + cells increased in the ipsilateral SVZ in our pMCAO animals compared with nonischemic animals. Proliferating NSPCs differentiate into Dcx + neuroblasts in the SVZ, and then migrate to the injured site (Arvidsson et al, 2002; Jin et al, 2001; Yamashita et al, 2006). Neuroblast migration mainly occurs over the first 2 weeks, but can be sustained for several months after MCAO (Thored et al, 2007). In our study, immunoblotting showed a significant increase in the Dcx protein level in the ischemic forebrain compared with the nonischemic brain signifying generation of neuroblasts in response to stroke. A further increase in the Dcx protein level occurred in E-selectin-tolerized animals compared with PBS-treated pMCAO controls. This increase in Dcx protein persisted for at least 4 weeks after pMCAO. There was also a significant increase in the number of BrdU+/Dcx + cells, newly generated neuroblasts, in the periinfarct region of E-selectin-tolerized animals. As the number of mitotic cells and Dcx + neuroblasts in the ipsilateral SVZ did not differ between animals with and without E-selectin tolerization, E-selectin tolerization could be inferred to promote the survival of neuroblasts in periinfarct regions rather than to augment cellular proliferation in the SVZ. Previous papers reporting that new neurons formed in the ischemic brain during the prolonged phase of stroke (Arvidsson et al, 2002; Lois and Alvarez-Buylla, 1993; Ohab et al, 2006; Yamashita et al, 2006) are in agreement with our results, in which BrdU +/NeuN+ cells appeared at 4 weeks after pMCAO. The threefold increase in these newly generated neurons in E-selectin-tolerized animals exposed to pMCAO may be because of enhanced survival of newly generated neuroblasts in the periinfarct region.
E-Selectin Tolerization Appears to Immunomodulate the Vascular Niche for Neuroblasts
In our previous study (Wakita et al, 2008), E-selectin tolerization induced mucosal tolerance and decreased the number of activated TNF immunoreactive blood vessels in ischemic white matter. Also in our present results, attenuation of TNF expression on blood vessels and an increase of Tregs in the periinfarct region after pMCAO were observed in E-selectin-tolerized animals. These results suggest that E-selectin tolerization can modify the local activation of endothelial cells induced by ischemia through the immunomodulating capacity of Tregs.
Within the normal adult SVZ and dentate gyrus, neurogenesis occurs in close association with endothelial cells in an environment termed the neurovascular niche (Alvarez-Buylla and Lim, 2004). Recently, it has been reported that the majority of neuroblasts from the SVZ migrate along with blood vessels, especially newly born endothelial cells, to an ischemic brain region (Ohab et al, 2006; Thored et al, 2007; Yamashita et al, 2006) suggesting that endothelial cell trophic factors could be important for the survival, migration, and differentiation of contiguous neuroblasts during neurogenesis (Ohab et al, 2006). Consistent with these studies, we found that fewer Dcx + neuroblasts associated with TNF-expressing blood vessels than with vessels devoid of TNF in periinfarct regions. We infer from these observations that TNF expressed on activated endothelial cells may influence the migration and/or survival of neuroblasts in the ischemic brain.
TNF, which is upregulated in response to brain injury (Hallenbeck, 2002), has been reported to play a differential role in modulating adult neurogenesis after stroke by acting through TNF-R1 or TNF-R2 of NSPCs (Iosif et al, 2006). In terms of proliferation of NSPC after stroke, TNF and TNF-R1 signals attenuate the cellular proliferation in two neurogenic regions (Iosif et al, 2008; Iosif et al, 2006). It is reported that TNF-R1 knockout mice showed enhanced stroke-activated NSPC proliferation and neuroblast formation in the SVZ, and that TNF treatment reduced the size and numbers of neurospheres, which are largely composed of NSPCs, through a TNF-R1-dependent mechanism (Iosif et al, 2008). In contrast, TNF-R2 knockout mice showed slightly reduced hippocampal cellular proliferation in status epilepticus (Iosif et al, 2006). These results show that TNF-R1 can act as a negative regulator of NSPC proliferation in SVZ, whereas TNF-R2 can improve NSPC proliferation.
Besides regulating proliferation of NSPCs, TNF also exerts detrimental effects on their differentiation into neuronal lineage and on neuronal survival. It was observed that, when TNF production by lipopo-lysaccharide- activated microglia was blocked by pentoxifyline, the reduction in the number of differentiated neurons in the NSPC culture was not observed (Liu et al, 2005). In contrast with these studies, intraventricular administration of a blocking antibody to TNF reduced hippocampal neuroblasts generated after MCAO. This may indicate a possible TNF-mediated neuroprotective action via TNF-R2, which is blocked by the neutralizing antibody. In our study, E-selectin tolerization did not alter cellular proliferation in the SVZ, but the number of newly generated neuroblasts adjacent to TNF– blood vessels in periinfarct regions was increased in E-selectin-tolerized animals. Collectively, these studies and our findings suggest that TNF expressed on angiogenic blood vessels after pMCAO is likely to have a detrimental effect on the survival of neuroblasts in periinfarct region, possibly through activation of TNF-R1 signaling (Iosif et al, 2008).
Neurologic Functions were Improved After Permanent Middle Cerebral Artery Occlusion in E-selectin-Tolerized Animals
We previously reported that E-selectin tolerization had protected against memory dysfunction in a extensive cerebral hypoperfusion rat model. In this study, we evaluated sensorimotor function after stroke with EBST and TAR, and both tests detected sensorimotor deficits over a 4-week period in SHRs subjected to pMCAO. A significant beneficial effect on neurologic function especially from 2 to 4 weeks after pMCAO was noted in E-selectin-tolerized animals. The question as to whether neurogenesis improves functional recovery after stroke is still uncertain. However, several papers support the hypothesis that increased neurogenesis is related to functional neurologic improvement after stroke, and treatment of stroke with growth factors or drugs promotes functional recovery concomitant with increased adult neurogenesis (Nakatomi et al, 2002; Ohab et al, 2006). Proliferating NSPCs or their progeny may exert salutary effects that do not depend on their acquisition of neuronal properties, such as by secreting trophic factors that provide neuroprotection for ischemic brain (Maysami et al, 2008; Rafuse et al, 2005). Furthermore, newly generated neurons that have migrated from the ipsilateral SVZ have been noted to form synapses and show an electrical potential consistent with functional integration into the local neuronal network in periinfarct regions of focally ischemic rodent brain (Lai et al, 2008; Yamashita et al, 2006). In our study, the neurologic improvement with E-selectin tolerization occurred from 2 to 4 weeks after pMCAO, during the period in which more newly generated neuroblasts and neurons were recruited into periinfarct region. Enhanced neurogenesis, therefore, may well contribute to functional recovery after stroke.
We have instilled E-selectin intranasally to prime E-selectin-specific Tregs before we induced brain ischemia in this study. This experimental paradigm cannot, therefore, directly address the potential clinical usefulness of this form of immunomodulation as a stroke treatment and/or repair innovation. However, E-selectin tolerization is currently being developed as a novel approach for prevention of secondary stroke. This work is relevant to patients being treated extensively with regularly scheduled E-selectin tolerization in whom a stroke supervenes. It is encouraging that E-selectin-tolerized animals show attenuation of ischemic brain damage and augmentation both of brain remodeling and of functional recovery in preclinical stroke models. For patients on an E-selectin mucosal tolerization regimen to have in place mechanisms that can lead to protection and repair from the moment of the stroke ictus on into the recovery period would seem to be a distinct advantage. Finally, the enhanced remodeling and repair observed in E-selectin-tolerized animals in this study, supports further examination of measures that target immunomodulation to the neurovascular niche in brain ischemia.
Supplementary Material
Acknowledgments
The authors thank Joliet Bembry and Dace Klimanis for technical assistance, Maria Spatz, Yang-ja Lee-Wickner, and Jacqueline Shukaliak for valuable suggestions, and NINDS animal facility staffs for skillful postoperative care of our rats every day. They also thank Sandra Taubenkibel for excellent secretarial assistance.
This research was supported by the Intramural Research Program of the NINDS/NIH.
References
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–6. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Beaumont T, Dore-Duffy P. Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. J Neurosci Res. 1999;55:578–87. doi: 10.1002/(SICI)1097-4547(19990301)55:5<578::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, Stengelin S, Gimbrone MA, Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989;243:1160–5. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Sanberg PR. Elevated body swing test: a new behavioral parameter for rats with 6-hydroxydo-pamine- induced hemiparkinsonism. J Neurosci. 1995;15:5372–8. doi: 10.1523/JNEUROSCI.15-07-05372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ruetzler C, Pandipati S, Spatz M, McCarron RM, Becker K, Hallenbeck JM. Mucosal tolerance to Eselectin provides cell-mediated protection against ischemic brain injury. Proc Natl Acad Sci USA. 2003;100:15107–12. doi: 10.1073/pnas.2436538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Bertsch T, Mielke O, Muhlhauser F, Hennerici M. Adhesion molecules in cerebrovascular diseases. Evidence for an inflammatory endothelial activation in cerebral large- and small-vessel disease. Stroke. 1999;30:1647–50. doi: 10.1161/01.str.30.8.1647. [DOI] [PubMed] [Google Scholar]
- Ford GA. Clinical pharmacological issues in the development of acute stroke therapies. Br J Pharmacol. 2008;2008:S112–9. doi: 10.1038/sj.bjp.0707654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. J Neurol Sci. 2005;233:125–32. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–82. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-beta-TGF-beta receptor interactions in type 1 diabetes. Proc Natl Acad Sci USA. 2003;100:10878–83. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck JM. The many faces of tumor necrosis factor in stroke. Nat Med. 2002;8:1363–8. doi: 10.1038/nm1202-1363. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM, Dutka AJ, Kochanek PM, Siren A, Pezeshkpour GH, Feuerstein G. Stroke risk factors prepare rat brainstem tissues for modified local Shwartzman reaction. Stroke. 1988;19:863–9. doi: 10.1161/01.str.19.7.863. [DOI] [PubMed] [Google Scholar]
- Hoehn BD, Palmer TD, Steinberg GK. Neurogenesis in rats after focal cerebral ischemia is enhanced by indomethacin. Stroke. 2005;36:2718–24. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ahlenius H, Ekdahl CT, Darsalia V, Thored P, Jovinge S, Kokaia Z, Lindvall O. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab. 2008;28:1574–87. doi: 10.1038/jcbfm.2008.47. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–12. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–5. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai B, Mao XO, Xie L, Jin K, Greenberg DA. Electrophysiological neurodifferentiation of subventricular zone-derived precursor cells following stroke. Neurosci Lett. 2008;442:305–8. doi: 10.1016/j.neulet.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Sukhova G, Lee RT, Galis ZS. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S9–12. doi: 10.1097/00005344-199500252-00003. [DOI] [PubMed] [Google Scholar]
- Liu YP, Lin HI, Tzeng SF. Tumor necrosis factor-alpha and interleukin-18 modulate neuronal cell fate in embryonic neural progenitor culture. Brain Res. 2005;1054:152–8. doi: 10.1016/j.brainres.2005.06.085. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–52. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci USA. 1993;90:2074–7. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maysami S, Lan JQ, Minami M, Simon RP. Proliferating progenitor cells: a required cellular element for induction of ischemic tolerance in the brain. J Cereb Blood Flow Metab. 2008;28:1104–13. doi: 10.1038/jcbfm.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, Lider O, Weiner HL. Antigen-driven bystander suppression after oral administration of antigens. J Exp Med. 1991;174:791–8. doi: 10.1084/jem.174.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–41. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Illoh K, Ruetzler C, Auh S, Sokoloff L, Hallenbeck J. Intranasal administration of E-selectin to induce immunological tolerization can suppress subarachnoid hemorrhage-induced vasospasm implicating immune and inflammatory mechanisms in its genesis. Brain Res. 2007;1132:177–84. doi: 10.1016/j.brainres.2006.09.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–44. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafuse VF, Soundararajan P, Leopold C, Robertson HA. Neuroprotective properties of cultured neural progenitor cells are associated with the production of sonic hedgehog. Neuroscience. 2005;131:899–916. doi: 10.1016/j.neuroscience.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Schallert T, Upchurch M, Lobaugh N, Farrar SB, Spirduso WW, Gilliam P, Vaughn D, Wilcox RE. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol Biochem Behav. 1982;16:455–62. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- Takeda H, Spatz M, Ruetzler C, McCarron R, Becker K, Hallenbeck J. Induction of mucosal tolerance to E-selectin targets immunomodulation to activating vessel segments and prevents ischemic and hemorrhagic stroke. Ernst Schering Res Found Workshop. 2004;47:117–32. doi: 10.1007/978-3-662-05426-0_7. [DOI] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–9. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Wakita H, Ruetzler C, Illoh KO, Chen Y, Takanohashi A, Spatz M, Hallenbeck JM. Mucosal tolerization to E-selectin protects against memory dysfunction and white matter damage in a vascular cognitive impairment model. J Cereb Blood Flow Metab. 2008;28:341–53. doi: 10.1038/sj.jcbfm.9600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter BA, Valera VA, Takahashi S, Ushiki T. The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol Appl Neurobiol. 2006;32:388–96. doi: 10.1111/j.1365-2990.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- Weiner HL. The mucosal milieu creates tolerogenic dendritic cells and T(R)1 and T(H)3 regulatory cells. Nat Immunol. 2001;2:671–2. doi: 10.1038/90604. [DOI] [PubMed] [Google Scholar]
- Wolvers DA, Coenen-de Roo CJ, Mebius RE, van der Cammen MJ, Tirion F, Miltenburg AM, Kraal G. Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp-39. J Immunol. 1999;162:1994–8. [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–36. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.