Abstract
Whereas adipose tissue possesses a local renin-angiotensin system, the synthesis and regulated release of renin has not been addressed. To that end, we utilized differentiating 3T3-L1 cells and analyzed renin expression and secretion. Renin mRNA expression and protein enzymatic activity were not detectable in preadipocytes. However, upon differentiation, renin mRNA and both intracellular and extracellular renin activity were upregulated. In differentiated adipocytes, forskolin treatment resulted in a 28-fold increase in renin mRNA, whereas TNFα treatment decreased renin mRNA fourfold. IL-6, insulin, and angiotensin (Ang) II were without effect. In contrast, forskolin and TNFα each increased renin protein secretion 12- and sevenfold, respectively. Although both forskolin and TNFα induce lipolysis in adipocytes, fatty acids, prostaglandin E2, and lipopolysaccharide had no effect on renin mRNA or secretion. To evaluate the mechanism(s) by which forskolin and/or TNFα are able to regulate renin secretion, a general lipase inhibitor (E600) and PKA inhibitor (H89) were used. Both inhibitors attenuated forskolin-induced renin release, whereas they had no effect on TNFα-regulated secretion. In contrast, E600 potentiated forskolin-stimulated renin mRNA levels, whereas H89 had no effect. Neither inhibitor had any influence on TNFα regulation of renin mRNA. Relative to lean controls, renin expression was reduced 78% in the epididymal adipose tissue of obese male C57Bl/6J mice, consistent with TNFα-mediated downregulation of renin mRNA in the culture system. In conclusion, the expression and secretion of renin are regulated under a complex series of hormonal and metabolic determinants in mature 3T3-L1 adipocytes.
Keywords: secretion, tumor necrosis factor-α, forskolin, renin-angiotensin system, angiotensin II
a local renin-angiotensin system (RAS) has recently been described in adipose tissue (1, 18, 20, 25, 29, 32, 35, 37, 44), where its functional significance has been linked to a number of processes, including metabolism, differentiation, oxidative stress, and inflammation (1, 11, 19, 25, 37). All the necessary components of the RAS, including renin, angiotensin-converting enzyme, and angiotensinogen (AGT) (20, 35), are expressed in adipose tissue, although the specific cell type(s) responsible has not been completely defined. Although the role of the local adipose RAS is complex, it is clear that angiotensin (Ang) II, the terminal product of the catalytic cascade system, has many effects within adipose tissue. Indeed, antagonism of the RAS using pharmacological or molecular means improves insulin sensitivity (15) and decreases inflammation. Moreover, Ang II increases lipogenesis in cultured human and murine cells (19), and AGT-knockout mice display decreased weight gain and decreased adipocyte cell size (30).
AGT mRNA expression, protein synthesis, and regulation within adipose tissue has been investigated extensively, particularly with regard to the issue of fat-derived AGT contribution to circulating levels (29). Renin enzymatic levels, however, have not been consistently detected in adipose tissue, and although renin expression within adipose tissue has been demonstrated (20, 35), it has not been a consistent observation (9). Nevertheless, renin appears to be an integral part in some parts of adipose biology. Mice lacking renin are resistant to diet-induced obesity (44). In addition, when inserted into mice, the human renin gene showed tissue-specific regulation, including adipose tissue expression (40). Since renin action on AGT is the rate-limiting step in the production of Ang II, the ultimate RAS effector, local expression and regulated release from adipose tissue may be an important regulatory mechanism for production of local Ang II.
Given the wide disparity of conclusions concerning adipose renin biology, we utilized the 3T3-L1 system to systematically investigate renin expression and enzymatic activity. During the course of this study, we made the novel observation that forskolin and TNFα function as major regulators of renin synthesis and secretion and report herein findings that characterize a linkage between proinflammatory cytokines and the local RAS system.
MATERIALS AND METHODS
Cell culture.
3T3-L1 preadipocytes, maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum, were differentiated to adipocytes, as described previously (41). Briefly, cells were treated with 174 μM insulin, 0.5 mM dexamethasone, and 0.25 μM methylisobutylxanthine in DMEM supplemented with 10% fetal bovine serum at 2 days postconfluence (designated “day 0”) and again with insulin on day 2. Insulin was withdrawn on day 4, and cells were maintained in DMEM-10% FBS until mature (days 6–8). Unless otherwise indicated, experiments were conducted with day 8 adipocytes.
Animals.
C57BL/6J male mice were maintained on a high-fat (HF) or normal chow (LF) diet (F3282; BioServ), ∼35 and 3.5% fat by weight, respectively, at weaning, and maintained for an additional 7–10 wk. HF feeding of C57Bl/6J mice results in diet-induced obesity and insulin resistance (42). Animal protocols were approved by The University of Minnesota Institutional Animal Care and Use Committee.
Protein isolation.
To isolate intracellular protein for renin activity measurement, cells in culture were rinsed with 1× PBS to remove residual media. Ice-cold protease inhibitor buffer (PIB) consisting of 0.15 M sodium phosphate buffer (pH 7.45), 1% BSA, 0.05% NaN3, and serine, metallo-, and thiol protease inhibitors [15 mM EDTA, 2.3 mM captopril, 2 mM 8-hydroxyquinoline, 10 mM sodium tetrathionate, 20 mM benzamidine, 10 mM N-ethylmaleimide, 3 mM PEFABLOC (Sigma), 20 μM leupeptin, and 450 μM aprotinin] was added directly to culture plate, and cells were scraped off the plate. Cell suspension was homogenized with a 1-ml dounce glass homogenizer and centrifuged at 100,000 g to remove cellular debris. Supernatant was collected and frozen at −70°C for renin activity assay.
Renin concentration measurement.
Fifty microliters of sample (either soluble adipose protein or culture medium), 25 μl of renin-free rat AGT [obtained from 48-h bilaterally nephrectomized rats, as described previously (23)], and 25 μl of sodium phosphate buffer (0.15 M, pH 7.45, 2.5% BSA) were incubated at 37°C for 0, 0.5, and 1 h in duplicate to generate Ang I. As described previously, AGT levels in the assay are at near-saturating conditions such that Ang I generation over time is directly proportional to renin concentration (21–23). Ang I was subsequently quantified by radioimmunoassay, as described previously (21). Briefly, I125-labeled Ang I was mixed with a polyclonal primary antibody directed toward Ang I (27) and incubated at 4°C overnight. Secondary antibody (anti-rabbit IgG; Sigma-Aldrich) in a mixture of 4% polyethylene glycol (molecular weight = 8,000) with 0.5% rice starch was added for 10 min at 4°C to precipitate primary antibody-Ang I complex. Samples were centrifuged for 20 min at 2,000 g, and the supernatant was discarded. Radioactivity in the pellets was measured and compared with known Ang I standards to calculate the initial renin concentration.
Shallow gradient isoelectric focusing of renin and cathepsin D.
Shallow gradient isoelectric focusing was performed as described previously (21). Gels were frozen, cut into 65 slices, and incubated in PIB overnight at 4°C to elute the separated proteins. Fifty microliters of the extracted protein from each gel slice was assayed for renin activity at pH 7.45 as described. Cathepsin D activity was measured from 10 ul of the extracted protein at pH 4.1 using porcine tetradecapeptide as a substrate, as described previously (21).
RNA isolation and quantitative real-time reverse transcriptase polymerase chain reaction.
Cultured adipocytes and adipose tissue were harvested in Trizol (Invitrogen) for total RNA isolation according to the manufacturer's protocol and DNase treated (Ambion) prior to cDNA synthesis. cDNA synthesis was performed using iScript cDNA synthesis kit (Bio-Rad). Five nanograms of cDNA in duplicate was diluted to 20 μl of total volume in iQ SYBR Green Supermix (Bio-Rad) for quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) analysis of renin and transcription initiation factor IIE (TFIIE) message levels. qRT-PCR was performed using a Bio-Rad My iQ iCycler with subsequent data acquisition and analysis with Bio-Rad iQ5 software. Real-time RT-PCR primers to renin (forward TGAAGAAGGCTGTGCGGTAGT, reverse TCCCAGGTCAAAGGAAATGTC) and TFIIE (forward CCAGGCTTTAGGGGACCAGATAC, reverse CATCCATTGACTCCACAGTGACAC) were designed using MacVector Software. All qRT-PCR data were normalized to TFIIE.
Statistical analysis.
Renin enzymatic activity during 3T3-L1 adipose conversion was analyzed by Kruskal-Wallis one-way analysis of variance on ranks. Differences between groups were further analyzed via the Student-Newman-Keuls method for all pairwise multiple comparisons; n is between 3 and 6 for each sample. Inducible renin release from cultured preadipocytes and adipocytes was analyzed by Kruskal-Wallis one-way analysis of variance on ranks followed by Dunn's method for multiple comparisons vs. control group. Data are representative of multiple experiments; n = 3 for each treatment. Renin release dose-response curves were analyzed via sigmoidal dose-response curve calculation of simple ligand binding for calculation of K0.5; n = 6 for each data point. Statstical analysis of TNFα and forskolin treatment of day 8 3T3-L1 adipocytes on renin release, intracellular renin levels, and renin mRNA expression were analyzed by two-way ANOVA followed by the Holm-Sidak all-pairwise multiple comparison procedure. Culture media renin activity data for both TNFα and forskolin treatment were square root transformed to pass the normality test. Statistical analysis was performed on the ΔCT values for renin message data; n = 6 per data point. Attenuation of regulated renin release enzyme activity measurements was analyzed by Kruskal-Wallis one-way analysis of variance on ranks followed by the Student-Newman-Keuls method for all-pairwise multiple comparisons. Relative renin expression data were analyzed by one-way ANOVA followed by Holm-Sidak all-pairwise multiple comparison procedure to analyze differences between groups; n = 4/treatment, and P < 0.05 is significant. The expression of renin in epididymal adipose tissue was analyzed by Student's t-test.
RESULTS
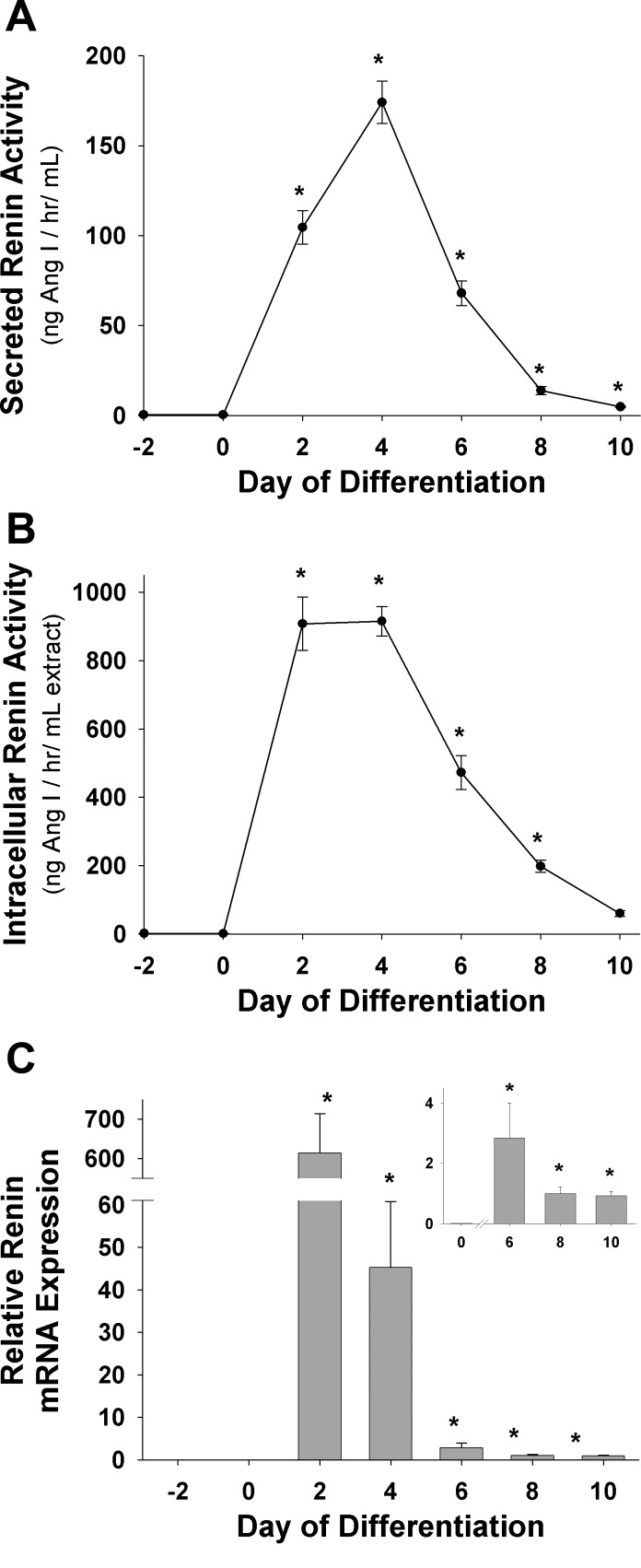
The RAS has been implicated in a variety of adipose tissue functions, and the significance of adipose-derived renin to either a local or systemic RAS is controversial. Some reports have detected renin protein enzymatic activity from adipose tissue in addition to renin mRNA (35), whereas others have failed to detect renin mRNA expression (9). To address the role of renin enzyme production and release from adipocytes, the 3T3-L1 culture system was investigated for secreted (medium) and intracellular renin protein enzymatic activity and message (Fig. 1). Renin message and protein activity were undetectable in preadipocytes (day −2 and day 0; Fig. 1); however, during adipose conversion, renin mRNA levels increased dramatically (days 2–4), with significant increases in intracellular renin (>1,000-fold, P < 0.05) and media renin (>500-fold, P < 0.05) (Fig. 1). Renin mRNA, intracellular renin, and secreted renin all declined in abundance over the next 3–5 days to new steady-state levels markedly above those in preadipocytes. Intracellular renin activity remained significantly elevated above preadipocyte levels (∼300-fold) in day 8 fully mature adipocytes (Fig. 1B). Treatment of preadipocytes with insulin, dexamethasone, or methylisobutylxanthine (MIX) individually displayed no significant increases in renin message level or protein activity above untreated preadipocytes (data not shown).
Fig. 1.
Renin enzymatic activity during 3T3-L1 adipose conversion. 3T3-L1 preadipocytes were maintained and differentiated as described in materials and methods. Media, cells, and total RNA were collected every 48 h and assayed for secreted or intracellular renin activity via enzymatic radioimmunoassay and renin message via quantitative real-time RT-PCR (qRT-PCR). A: secreted (medium) renin enzymatic activity. B: intracellular renin enzyme activity from cultured adipocyte cell extracts. C: relative renin mRNA levels during adipose conversion normalized to transcription initiation factor IIE (TFIIE). Data are representative of 3 separate experiments for each plot; n = 3–6 for each sample. Data were analyzed by Kruskal-Wallis 1-way analysis of variance on ranks followed by Student-Newman-Keuls method for all pairwise multiple comparison procedures. *P < 0.05 vs. day 0 (preadipocytes). Ang I, angiotensin I.
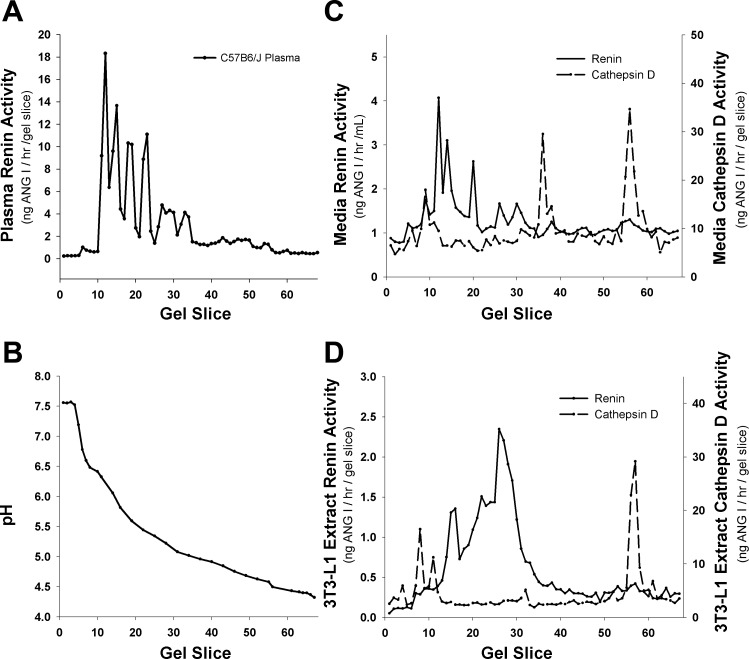
Although AGT is the only known substrate for renin, other enzymes are able to cleave AGT to Ang I and thus potentially obscure measurement of renin activity in the 3T3-L1 cells and media. Although serine, metallo-, and thiol proteases are inhibited in the renin assay, aspartyl proteases are not inhibited. Since cathepsin D is an aspartyl protease with ∼40% sequence similarity to renin and is capable of cleaving AGT to Ang I, we demonstrated that the renin activity measured in 3T3-L1 cells is derived from renin and not cathepsin D. We utilized shallow gradient isoelectric focusing to separate the two enzymes followed by enzyme assay. In this assay, the activity profile as a function of gel slice (pH) represents a diagnostic fingerprint for distinguishing various enzyme activities. Since C57BL/6J mice only have the single ren1 gene, a C57BL/6J mouse plasma focusing profile is shown as a positive control for murine ren1 (Fig. 2A) and demonstrates that renin glycoforms (22) focus as a cluster of activities between pH 5.0 and 6.5 (Fig. 2B). Renin activity in the medium from 3T3-L1 adipocytes (Fig. 2C) focused with an isoelectric point activity profile similar to that of mouse plasma renin. In contrast, the cathepsin D activity profile was distinct from that shown by renin and focused with a distinctive pattern of activities at pH 6.5, pH 5.0, and pH 4.5. Since the renin activity profile does not overlap with that of cathepsin D (Fig. 2C), the 3T3-L1 renin concentration measurements are independent of cathepsin D activity, as has been reported previously in rat and human (21). Similarly, cell extract renin activity profile (Fig. 2D) focused with the same pH range as C57BL/6J plasma renin, whereas the cathepsin D activity profile focused outside the pH range of C57BL/6J plasma renin, media renin, and cell extract renin.
Fig. 2.
Shallow gradient isoelectric focusing profiles of mouse and 3T3-L1 renin and 3T3-L1 cathepsin D. A: focusing profile of C57BL/6J mouse plasma renin activity. B: representative pH gradient from individual gel slices. C: renin and cathepsin D focusing profiles in 3T3-L1 day 8 culture medium. D: renin and cathepsin D focusing profiles in 3T3-L1 day 8 cellular extracts. Data are representative of at least 3 experiments.
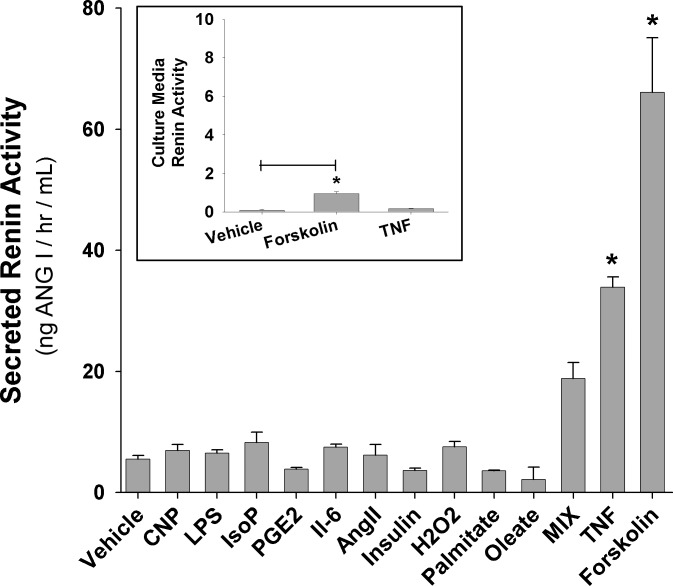
The wide disparity of reports on the mRNA or secreted levels of renin suggested that the control of renin production and release may be a regulated process. To test this hypothesis, day 8 adipocytes were treated for 24 h with a variety of potential regulatory molecules followed by media analysis of renin enzymatic activity. Treatment of adipocytes with 1 μM C-type natriuretic peptide (CNP), 1 μg/ml lipopolysaccharide, 10 μM isoproternol, 100 ng/ml IL-6, 100 nM prostaglandin E2, 1 × 10−5 M Ang II, 100 μM H2O2, 500 μM oleate, 500 μM palmitate (each fatty acid delivered on 100 μM bovine serum albumin), or 100 nM insulin each was ineffective in altering media renin levels above vehicle controls (Fig. 3). Although not statistically significant, MIX did slightly increase media renin levels, suggesting that some component of the cAMP and/or lipolysis system may be regulatory. Pursuing this concept further, 100 μM forskolin and 1 nM TNFα, two agents known to increase fat cell triglyceride hydrolysis, dramatically increased renin enzymatic activity in the culture media of day 8 adipocytes 12.6- and 7.5-fold, respectively (P < 0.05; Fig. 3).
Fig. 3.
Inducible renin release from cultured adipocytes. Day 8 cultured 3T3-L1 adipocytes were treated for 24 h with various compounds to stimulate renin release. Vehicle bar is representative for each individual treatment since vehicle treatment was not statistically different from no treatment for any compound, 1 μM C-type natriuretic peptide (CNP), 1 μg/ml lipopolysaccharide (LPS), 10 μM isoproterenol (IsoP), 100 nM prostaglandin E2 (PGE2), 100 ng/ml IL-6, 1 × 10−5 M angiotensin II (Ang II), 100 nM insulin, 100 μM hydrogen peroxide (H2O2), 100 μM palmitate, 100 μM oleate (fatty acids delivered on 100 μM bovine serum albumin), 0.115 mg/ml methylisobutylxanthine (MIX), 100 μM forskolin, and 1 nM TNFα. *P < 0.05 vs. vehicle control; n = 4–6 for each sample. Insert: effect of 100 μM forskolin and 1 nM TNFα on culture medium renin activity of cultured preadipocytes. *P < 0.05 vs. vehicle control; n = 6.
Although adipose conversion in culture is routinely >85% (measured by macroscopic lipid accumulation), it can vary experimentally, leading to differing levels of residual preadipocytes. Although preadipocytes did not show detectable renin activity or message (day 0; Fig. 1), it may be possible for preadipocytes to synthesize or secrete renin upon 24-h TNFα or forskolin stimulation. Therefore, we treated preadipocytes with TNFα and forskolin to measure their effects on culture media renin activity. TNFα was unable to increase culture media renin enzymatic activity above that of untreated preadipocytes (both were nearly undetectable; Fig. 3, insert). Forskolin treatment did show a significant increase in culture media enzymatic activity vs. untreated preadipocytes; however, absolute renin activity was negligible compared with forskolin-treated mature adipocytes (∼100-fold less; Fig. 3, insert). Therefore, residual preadipocytes in the day 8 adipocyte population do not significantly contribute to renin activity.
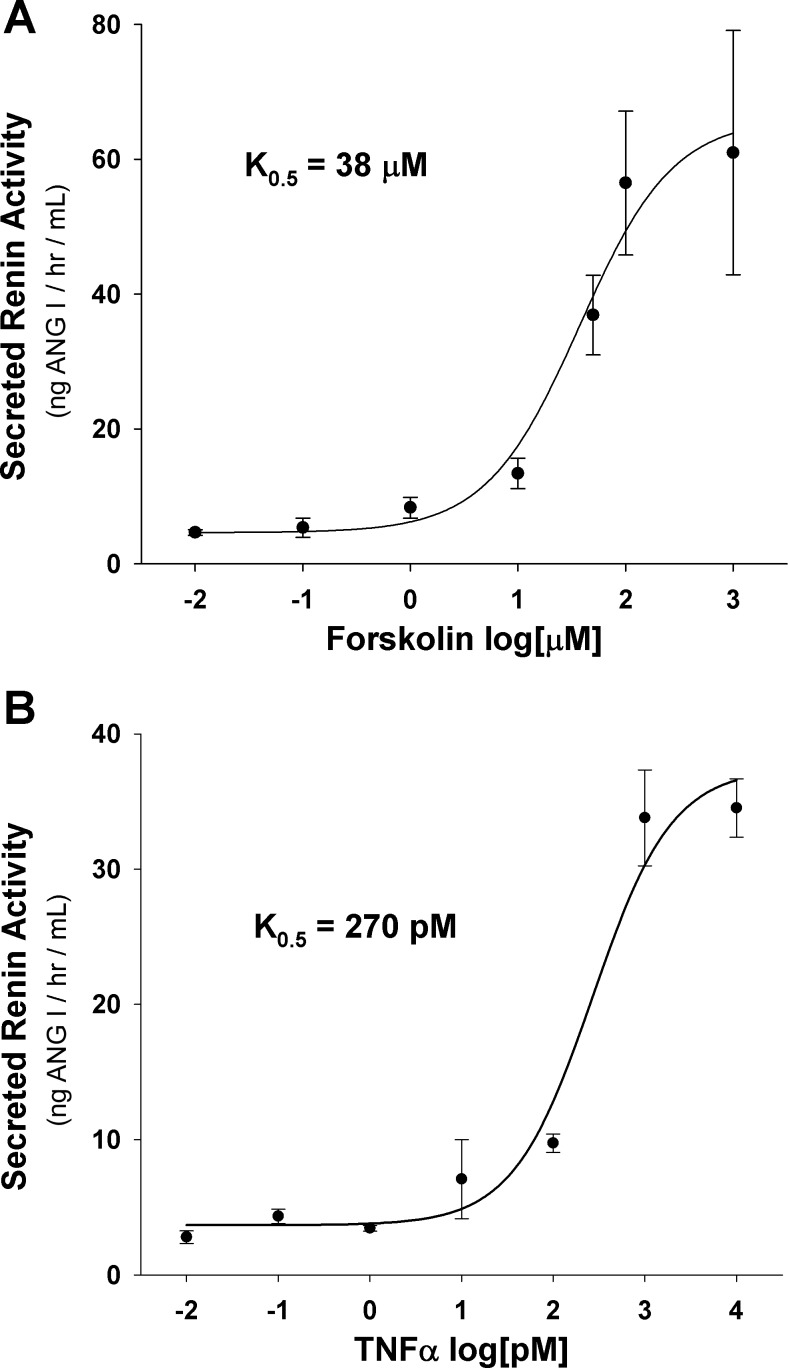
To further characterize the effects of forskolin and TNFα on renin release from cultured adipocytes, we evaluated the concentration dependence for each compound (Fig. 4). Day 8 adipocytes were treated with varying concentrations of either forskolin or TNFα for 24 h, and culture media renin activity was assayed. TNFα displayed a maximal culture media renin activity of ∼35 ng Ang I·h−1·ml−1 at 1 nM, with a K0.5 of 270 pM (Fig. 4B). Forskolin treatment displayed a more robust increase in media renin activity than TNFα of 66 ng Ang I·h−1·ml−1, at a dose of 100 μM, with a K0.5 of 38 μM (Fig. 4A).
Fig. 4.
Renin release dose-response curves. Day 8 3T3-L1 adipocytes were treated for 24 h with forskolin or TNFα at increasing concentrations to induce renin release into culture media. A: forskolin dose-response curve. B: TNFα dose-response curve; n = 6/data point.
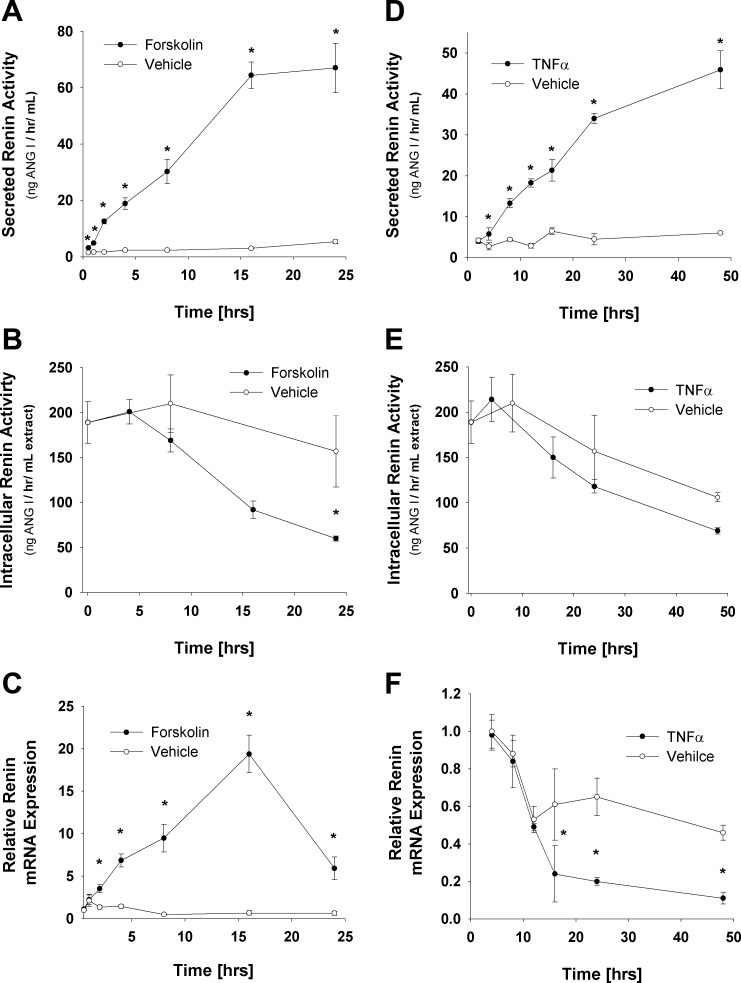
Forskolin treatment displayed a time-dependent increase in media renin activity, with a maximum induction of ∼13-fold at 24 h (Fig. 5A) as well as a decrease in intracellular renin protein levels of 42%, P < 0.05, at 24 h relative to vehicle (Fig. 5B). qRT-PCR analysis of renin message during forskolin stimulation revealed a significant increase in renin message of 28-fold (P < 0.05) after 16 h of treatment and then declined to a 9.5-fold increase after 24 h (Fig. 5C). In parallel studies, TNFα treatment increased media levels of renin 7.4-fold (P < 0.05) at 48 h (Fig. 5D). Whereas forskolin displayed significant increases (2.1-fold, P < 0.05) in media renin activity at 30 min, TNFα induction of renin media activity was significantly delayed and did not increase above vehicle control until 4 h of treatment (Fig. 5D). In addition, although intracellular levels did trend toward a decrease in the TNFα-treated samples (Fig. 5E), no statistical difference was observed, perhaps due to the decline in intracellular renin levels between day 8 and day 10 adipocytes (Fig. 1B). In contrast to forskolin, TNFα treatment resulted in a 4.2-fold decrease (P < 0.05) in renin message by 16 h, which continued through 48 h, the furthest time point evaluated (Fig. 5C).
Fig. 5.
Effect of forskolin and TNFα treatment on renin enzymatic activity and message levels in 3T3-L1-cultured adipocytes. Day 8 3T3-L1 adipocytes were treated with either 100 μM forskolin or 1 nM TNFα for indicated periods of time, and medium renin enzymatic activity, intracellular renin enzymatic activity, and renin mRNA levels were analyzed. A: forskolin-regulated secreted renin enzymatic activity. B: forskolin-regulated intracellular renin enzymatic activity. C: qRT-PCR analysis of renin message levels normalized to TFIIE following forskolin treatment. D: TNFα-regulated secreted renin enzymatic activity. E: TNFα-regulated intracellular renin enzymatic activity. F: qRT-PCR analysis of renin message levels normalized to TFIIE following TNFα treatment. Statistical analysis via 2-way ANOVA followed by Holm-Sidak all-pairwise multiple comparison procedure to analyze differences between groups. *P < 0.05 vs. same time point vehicle control. Data are representative of multiple experiments; n = 6/data point.
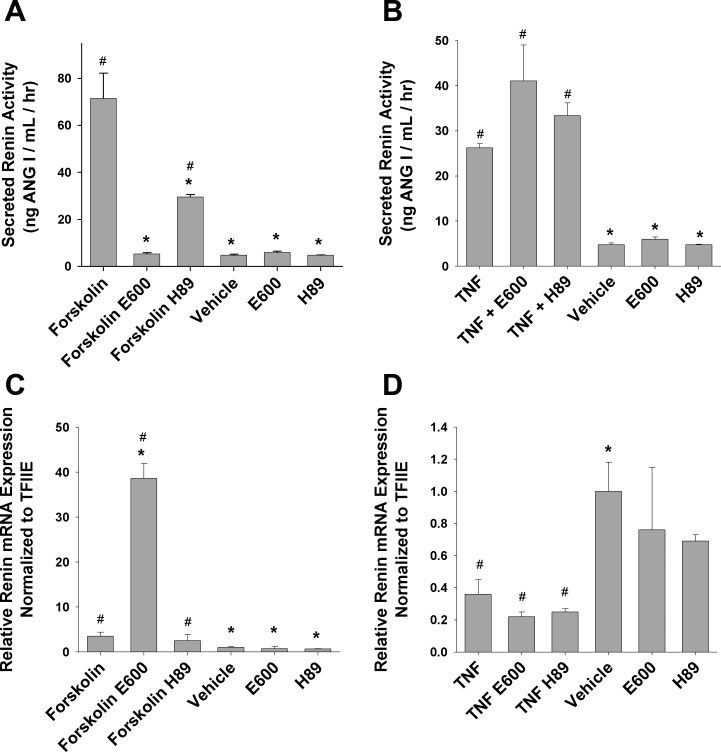
Given the ability of forskolin and TNFα to regulate adipocyte lipolysis, albeit by different mechanisms, we utilized pharmacological inhibition as an experimental tool to probe the mechanism of regulated renin release. First, since forskolin activates adenylyl cyclase, H89, a protein kinase A inhibitor, was used to disrupt cAMP activation and subsequent intracellular signaling. Moreover, some reports have suggested a role of cAMP-mediated intracellular signaling with TNFα stimulation (26, 36, 48). Second, E600, a general lipase inhibitor, was used to block the production of fatty acids from triacylglycerol hydrolysis. H89 decreased forskolin-induced renin release by 59% (P < 0.05) at 24 h of treatment, whereas E600 completely attenuated rennin secretion (Fig. 6A). Neither inhibitor had any effect on TNFα-induced renin release (Fig. 6B).
Fig. 6.
Attenuation of regulated renin release. Day 8 3T3-L1 adipocytes were treated for 1 h with either 10 μM H89 (PKA inhibitor) or 100 μM E600 (general lipase inhibitor) followed by 20-h incubation with 100 μM forskolin or 1 nM TNFα in the presence of inhibitor. H89 and E600 alone were preincubated for 1 h and changed to H89 and E600 for an additional 24 h. A: secreted renin enzymatic activity during treatment. B: secreted renin enzymatic activity during the indicated treatment. C: relative renin mRNA expression normalized to TFIIE during the indicated treatment. D: relative renin mRNA expression normalized to TFIIE during the indicated treatment. *P < 0.05 vs. TNF or forskolin treatment; #P < 0.05 vs. vehicle.
Renin message levels were also analyzed after 24-h treatment with or without E600 and H89. Twenty-four-hour forskolin treatment increased renin expression above vehicle control 3.5-fold (Fig. 6C). Whereas H89 had no effect on forskolin-induced induction of renin message (Fig. 6C), E600 in contrast increased renin expression ∼40-fold above vehicle control (Fig. 6C). Twenty-four-hour TNFα treatment decreased renin expression by >60% (Fig. 6D), whereas TNFα plus H89 or E600 treatment did not significantly affect the reduction in renin message (Fig. 6D). E600 and H89 alone had no effect on renin expression with 24-h treatment (Fig. 6, C and D).
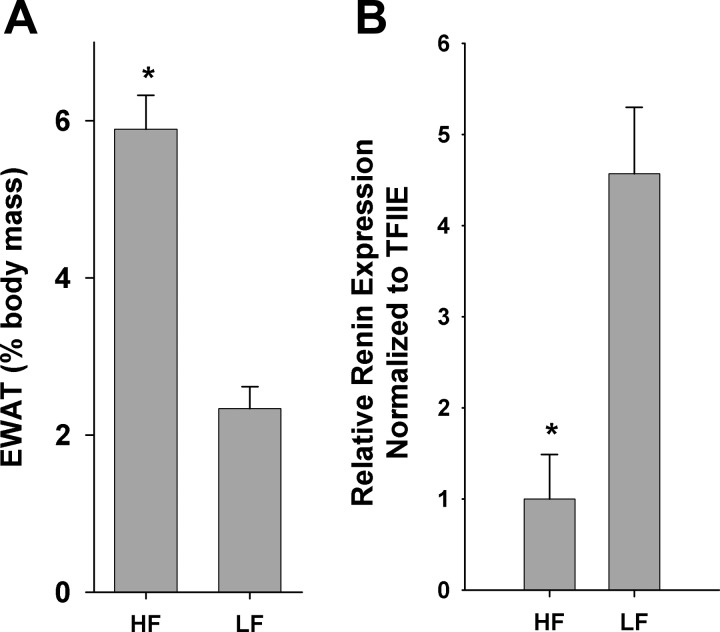
Obesity and insulin resistance are associated with increased basal inflammation as well as sympathetic drive (14). Therefore, it is likely that obesity may modulate renin message levels in the pathologically obese/insulin-resistant state. To address the potential interaction of obesity and insulin resistance on renin message levels, obese insulin-resistant C57BL/6J mice maintained on a HF diet (42) were compared with LF-fed mice. Twelve-week-old male mice on a HF diet (n = 9) displayed a 2.5-fold increase in epididymal white adipose tissue as a percentage of total body mass over normal-chow counterparts (n = 9) (Fig. 7A). Total body weight was also significantly increased 1.43-fold in the HF mice, P < 0.001 (data not shown). qRT-PCR analysis revealed a 78.1% decrease (P < 0.01) in the epididymal white adipose tissue renin expression of obese male mice compared with normal-chow controls (Fig. 7B).
Fig. 7.
Obesity/insulin resistance effects on epididymal adipose tissue renin expression. Epididymal adipose tissue depots were harvested from 12-wk-old male C57BL/6J mice maintained on either a high-fat (HF) or normal chow (LF) diet. A: epididymal white adipose tissue (EWAT) mass expressed as %total body weight. B: qRT-PCR analysis of relative renin expression normalized to TFIIE. *P < 0.001.
DISCUSSION
A variety of studies support an interaction between a local adipose RAS and obesity, oxidative stress, insulin resistance, and inflammation (13, 19, 25, 32, 37, 39, 44). Considerable effort has been directed toward adipose tissue-derived AGT effects within adipose tissue as well as its contribution to the systemic RAS (1, 29, 30). Previous reports have shown that AGT-null mice have hypotrophic adipose tissue (30), whereas overexpression of AGT is able to attenuate adipose tissue dysfunction as well as partially reconstitute plasma AGT levels (29, 30). Moreover, adipose tissue expresses Ang II receptors, implying a local signaling function of AGT (5) through Ang II production. Despite the widespread interest in adipose tissue RAS function, little data exist with regard to the role of renin (9, 10, 20, 35, 38). Herein, we report that renin is synthesized and secreted by cultured 3T3-L1 cells in a regulated manner.
To assess renin expression, we utilized the differentiating 3T3-L1 cell culture system. Although 3T3-L1 adipocytes do not faithfully mirror all the functions of adipocytes in vivo, they do represent a convenient, reproducible, cell culture-based system for analysis of adipose genes and protein (3). Using the 3T3-L1 system, we found that renin message and enzymatic expression are greatly increased during adipocyte differentiation (Fig. 1). Renin expression was maximal on about day 4 and declined by days 8–10 to a new steady-state level. The biphasic nature of adipose renin expression during differentiation is shared with many other adipocyte proteins, such as the adipocyte fatty acid-binding protein (aP2), pyruvate carboxylase, and fatty acid synthase (3, 6), commonly referred to as “late” adipose markers. Despite the decline in renin expression late in the differentiation program, renin expression and activity in adipocytes is much greater than in undifferentiated preadipocytes (Fig. 1).
Macrophage infiltration and subsequent TNFα production play an important role in the pathogenesis of obesity-induced inflammation (2, 14, 31, 33, 43). Adipose tissue in both diet-induced and molecular genetic models of obesity has significant increases in macrophages, and adipose tissue TNFα production is due primarily to macrophage synthesis (47). In addition, renin expression has been demonstrated in rat peritoneal macrophage cells (17), suggesting that adipose tissue renin may be derived from macrophages. However, we were unable to measure renin mRNA expression in RAW 264.7 macrophage cells by qRT-PCR, and renin enzymatic activity was undetectable in cell extracts or culture media (data not shown). These results suggest that, in adipose tissue, the adipocyte is a primary locus for renin expression.
To ensure that the renin activity measured in the 3T3-L1 system was due to renin protein and not to a similar enzyme, we evaluated the potential contamination of cathepsin D. Early literature measuring tissue renin enzymatic activity was often performed at an acidic pH, allowing for the lysosomal enzyme acitivity of cathepsin D to contribute to perceived renin activity (21). Previously published reports in the rat and human have demonstrated that a neutral pH assay buffer is able to effectively distinguish between renin and cathepsin D activity. Here we demonstrate that the assay for murine renin is independent of contaminating cathepsin D activity. This was shown by the diagnostic nonoverlapping isoelectric focusing activity profiles for murine renin and cathepsin D. In addition, since the renin detected in media and cellular extracts from 3T3-L1 cells has the same focusing profile as C57Bl/6J mouse plasma renin, and it is distinct from that exhibited by cathepsin D, it is highly unlikely that other contaminating proteases are interfering with the renin measurement. Consistent with this, plasma from a C57Bl/6J mouse that had undergone a bilateral nephrectomy exhibited a 96% reduction in renin activity (data not shown). In addition, unlike other species, some mice, including those from which the 3T3-L1 cell system was developed, possess two renin genes, ren1 and ren2. Ren2 has previously been shown to be nonglycosylated and will display a single peak of activity in an isoelectric focusing gel, whereas the differentially glycosylated ren1 will display multiple peaks (22). C57Bl/6J mice possess only a single renin gene, ren1, which was measured as a positive control for ren1 profiling (Fig. 2A). Here we show that ren1 activity is expressed in our 3T3-L1 system, as evidenced by multiple renin activity peaks (Fig. 2), but is clearly distinct from the cathepsin D profile. It is also possible that ren2 is synthesized as a relatively minor component.
The RAS has been implicated in a variety of adipose tissue functions (25, 29, 30, 37, 39). Therefore, we evaluated a variety of regulatory factors thought to be involved in obesity-associated pathologies or previously linked to kidney regulation of renin synthesis/secretion to assess which, if any, may regulate renin synthesis and/or secretion from differentiated adipocytes (Fig. 3). Since cAMP and cGMP are known to regulate kidney renin, we treated cells with CNP to induce cGMP synthesis as well as forskolin and MIX to increase cAMP production. In addition, LPS and TNFα are important regulators of inflammation associated with pathological obesity, whereas isoproterenol treatment stimulates β-adrenergic receptors activated in adipose tissue. Furthermore, we treated cells with hydrogen peroxide to increase oxidative stress as well as prostaglandins and Ang II, known regulators of kidney renin levels (34). Of these treatment groups, forskolin and TNFα were identified as potent agonists of renin release from mature adipocytes (Fig. 3). Both compounds displayed sigmoid activity plots, suggesting a single binding event. Forskolin displayed a K0.5 of 38 μM and TNFα a K0.5 of 270 pM, both of which are consistent with the induction of other regulated processes in cultured adipocytes (Fig. 4) (7, 24, 28).
The kinetics of renin secretion in the culture medium from intracellular stores and concomitant changes in renin mRNA expression in response to either forskolin or TNFα followed distinctly different progressions. Forskolin induced a rapid release of renin into the culture medium and was accompanied by a decrease in intracellular renin and increase in renin mRNA expression (Fig. 5). In contrast, TNFα treatment resulted in a relatively slow appearance of renin activity in the medium and was accompanied by a decrease in renin mRNA expression. As such, although both forskolin and TNFα lead to increased renin secretion, their differing kinetics and opposite effects on renin mRNA expression imply distinctly different regulatory processes.
Although little is known about the regulation of renin release from adipocytes, it is interesting that increased cAMP strongly stimulates adipocyte renin release analogous to kidney juxtaglomerular cells (34). TNFα has also been shown to decrease renin expression in juxtaglomerular cells primarily by transcriptional inhibition, and TNFα−/− mice have threefold higher basal renin mRNA expression (45). Furthermore, modulation of cAMP activity via PKA inhibition (H89) supports cAMP-mediated renin release in forskolin-treated adipocytes, whereas in contrast the lack of H89 effect in TNFα-treated adipocytes (Fig. 6) suggests that renin release may be cAMP independent. Since both forskolin and TNFα induce lipolysis in adipocytes, albeit by different mechanisms, a potential linkage to renin release may be related to production of free fatty acids. However, fatty acids themselves (oleate or palmitate) were ineffective in regulating renin release (Fig. 3). Interestingly, E600 (a pan-specific triglyceride hydrolase inhibitor) completely attenuated forskolin-induced renin release, whereas it had no effect on TNFα-mediated renin release (Fig. 6). As such, it is unclear at this time what mechanism(s) is involved in the E600 inhibition of renin release. Further experiments may elucidate the signaling mechanisms by which cAMP and TNFα induce renin release from cultured adipocytes.
In pathological obesity, adipose tissue is associated with increased inflammatory cytokine burden (2, 14, 31, 33, 43). TNFα has previously been shown to increase AGT and Ang II in cultured adipocytes (12), and in this report we extend the effects of TNFα to increased secretion of renin. Concomitant with increased renin secretion was a 4.2-fold (P < 0.05) decrease in renin message in TNFα-treated cultured adipocytes. A decrease in renin message was also observed in high-fat-fed obese, insulin-resistant mice compared with normal chow-fed control animals. Moreover, suppression of renin expression by chronic TNFα exposure may decrease the effectiveness of other renin-stimulating processes. Although the mechanistic basis of TNFα-mediated renin secretion and suppression of message remains unclear, one explanation is that, in an obese insulin-resistant state, chronically elevated TNFα levels alter the normal role of a local adipose RAS system though decreased renin expression and in part contribute to obesity-associated pathology.
One intriguing hypothesis regarding the role of a local RAS system in adipose tissue is participation in adipose tissue expansion and differentiation. Adipose tissue is highly vascularized and is dependent on neovascularization (possibly by vascular endothelial growth factor and/or Ang II) for expansion (4, 8, 46). Consistent with this, Ang II receptor blockers decrease the angiogenic effects of Ang II (16). Therefore, TNFα- or cAMP-mediated induction of renin release may be involved in the production of Ang II and induction of adipose angiogenesis during normal adipose tissue expansion. If the RAS is involved in normal adipose tissue expansion, as evidenced by decreased adipose tissue mass in AGT−/−, ACE−/−, and renin−/− mice (18, 30, 44), then pathological decreases in renin expression and subsequent decreased production of Ang II may be in part responsible for failure of normal adipose expansion in the obese state. Modulation of the RAS, specifically at the rate-limiting step (renin action on AGT), may be a potential therapeutic option to limit further adipose tissue expansion. Future studies will focus on the mechanism of renin secretion and role(s) for the local RAS system in adipose biology.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-053189 (to D. A. Bernlohr), the Minnesota Obesity Center (National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-050456), and a University of Minnesota Academic Health Center Seed Grant (to S. A. Katz). J. D. Fowler is a trainee of the Medical Scientist Training Program (National Institute of General Medical Sciences Grant T32-GM-008244) at the University of Minnesota.
Acknowledgments
We thank members of the Katz and Bernlohr laboratories for helpful discussions during the preparation of this manuscript.
Present address of J. E. Herrera: R & D Systems, 614 McKinley Pl. NE, Minneapolis, MN 55413.
REFERENCES
- 1.Ailhaud G, Fukamizu A, Massiera F, Negrel R, Saint-Marc P, Teboul M. Angiotensinogen, angiotensin II and adipose tissue development. Int J Obes Relat Metab Disord 24, Suppl 4: S33–S35, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol 28: 1654–1659, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernlohr DA, Bolanowski MA, Kelly TJ Jr, Lane MD. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem 260: 5563–5567, 1985. [PubMed] [Google Scholar]
- 4.Cao Y Angiogenesis modulates adipogenesis and obesity. J Clin Invest 117: 2362–2368, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassis LA, Police SB, Yiannikouris F, Thatcher SE. Local adipose tissue renin-angiotensin system. Curr Hypertens Rep 10: 93–98, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christy RJ, Yang VW, Ntambi JM, Geiman DE, Landschulz WH, Friedman AD, Nakabeppu Y, Kelly TJ, Lane MD. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev 3: 1323–1335, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Dobashi K, Asayama K, Shirahata A. Differential effects of cyclic AMP on induction of nitric oxide synthase in 3T3-L1 cells and brown adipocytes. Free Radic Biol Med 35: 94–101, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res 93: e88–e97, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gálvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, Ruiz-Gayo M, Huber M, Wehland M, Kreutz R, Fernandez-Alfonso MS. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J Endocrinol 197: 55–64, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Goossens GH, Jocken JW, Blaak EE, Schiffers PM, Saris WH, van Baak MA. Endocrine role of the renin-angiotensin system in human adipose tissue and muscle: effect of beta-adrenergic stimulation. Hypertension 49: 542–547, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Guo C, Yuan L, Liu X, Du A, Huang Y, Zhang L. Effect of ARB on expression of CD68 and MCP-1 in adipose tissue of rats on long-term high fat diet. J Huazhong Univ Sci Technolog Med Sci 28: 257–260, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Harte A, McTernan P, Chetty R, Coppack S, Katz J, Smith S, Kumar S. Insulin-mediated upregulation of the renin angiotensin system in human subcutaneous adipocytes is reduced by rosiglitazone. Circulation 111: 1954–1961, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hattori Y, Akimoto K, Gross SS, Hattori S, Kasai K. Angiotensin-II-induced oxidative stress elicits hypoadiponectinaemia in rats. Diabetologia 48: 1066–1074, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des 14: 1225–1230, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Henriksen EJ Improvement of insulin sensitivity by antagonism of the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 293: R974–R980, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Huang W, Wu YL, Zhong J, Jiang FX, Tian XL, Yu LF. Angiotensin II type 1 receptor antagonist suppress angiogenesis and growth of gastric cancer xenografts. Dig Dis Sci 53: 1206–1210, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Iwai N, Inagami T, Ohmichi N, Kinoshita M. Renin is expressed in rat macrophage/monocyte cells. Hypertension 27: 399–403, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Jayasooriya AP, Mathai ML, Walker LL, Begg DP, Denton DA, Cameron-Smith D, Egan GF, McKinley MJ, Rodger PD, Sinclair AJ, Wark JD, Weisinger HS, Jois M, Weisinger RS. Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc Natl Acad Sci USA 105: 6531–6536, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones BH, Standridge MK, Moustaid N. Angiotensin II increases lipogenesis in 3T3-L1 and human adipose cells. Endocrinology 138: 1512–1519, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson C, Lindell K, Ottosson M, Sjostrom L, Carlsson B, Carlsson LM. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab 83: 3925–3929, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Katz SA, Opsahl JA, Forbis LM. Myocardial enzymatic activity of renin and cathepsin D before and after bilateral nephrectomy. Basic Res Cardiol 96: 659–668, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Katz SA, Opsahl JA, Lunzer MM, Forbis LM, Hirsch AT. Effect of bilateral nephrectomy on active renin, angiotensinogen, and renin glycoforms in plasma and myocardium. Hypertension 30: 259–266, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Katz SA, Opsahl JA, Wernsing SE, Forbis LM, Smith J, Heller LJ. Myocardial renin is neither necessary nor sufficient to initiate or maintain ventricular hypertrophy. Am J Physiol Regul Integr Comp Physiol 278: R578–R586, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, Fasshauer M. Isoproterenol, TNFalpha, and insulin downregulate adipose triglyceride lipase in 3T3-L1 adipocytes. Mol Cell Endocrinol 240: 43–49, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Kurata A, Nishizawa H, Kihara S, Maeda N, Sonoda M, Okada T, Ohashi K, Hibuse T, Fujita K, Yasui A, Hiuge A, Kumada M, Kuriyama H, Shimomura I, Funahashi T. Blockade of angiotensin II type-1 receptor reduces oxidative stress in adipose tissue and ameliorates adipocytokine dysregulation. Kidney Int 70: 1717–1724, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Langin D, Arner P. Importance of TNFalpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metab 17: 314–320, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Li T, Zimmerman BG. Relation between inhibition of renal angiotensin II production and hemodynamic effect of captopril in anesthetized rabbit. J Hypertens 10: 795–805, 1992. [PubMed] [Google Scholar]
- 28.Lim JY, Kim WH, Park SI. GO6976 prevents TNF-alpha-induced suppression of adiponectin expression in 3T3-L1 adipocytes: putative involvement of protein kinase C. FEBS Lett 582: 3473–3478, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Massiera F, Bloch-Faure M, Ceiler D, Murakami K, Fukamizu A, Gasc JM, Quignard-Boulange A, Negrel R, Ailhaud G, Seydoux J, Meneton P, Teboul M. Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J 15: 2727–2729, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Massiera F, Seydoux J, Geloen A, Quignard-Boulange A, Turban S, Saint-Marc P, Fukamizu A, Negrel R, Ailhaud G, Teboul M. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology 142: 5220–5225, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo Y, Hashizume T, Shioji S, Akasaka T. Metabolic syndrome is strongly associated with chronic subclinical inflammation in patients achieving optimal low-density lipoprotein-cholesterol levels in secondary prevention of cardiovascular disease. Circ J 72: 2046–2050, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Mogi M, Iwai M, Horiuchi M. Emerging concept of adipogenesis regulation by the renin-angiotensin system. Hypertension 48: 1020–1022, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Nieto-Vazquez I, Fernández-Veledo S, Krämer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem 114: 183–194, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Persson PB Renin: origin, secretion and synthesis. J Physiol 552: 667–671, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinterova L, Krizanova O, Zorad S. Rat epididymal fat tissue express all components of the renin-angiotensin system. Gen Physiol Biophys 19: 329–334, 2000. [PubMed] [Google Scholar]
- 36.Rahn LT, Mei J, Karlsson M, Manganiello V, Degerman E. Down-regulation of cyclic-nucleotide phosphodiesterase 3B in 3T3-L1 adipocytes induced by tumour necrosis factor alpha and cAMP. Biochem J 346: 337–343, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saint-Marc P, Kozak LP, Ailhaud G, Darimont C, Negrel R. Angiotensin II as a trophic factor of white adipose tissue: stimulation of adipose cell formation. Endocrinology 142: 487–492, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Schling P, Mallow H, Trindl A, Loffler G. Evidence for a local renin angiotensin system in primary cultured human preadipocytes. Int J Obes Relat Metab Disord 23: 336–341, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Sharma AM, Janke J, Gorzelniak K, Engeli S, Luft FC. Angiotensin blockade prevents type 2 diabetes by formation of fat cells. Hypertension 40: 609–611, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Sinn PL, Zhang X, Sigmund CD. JG cell expression and partial regulation of a human renin genomic transgene driven by a minimal renin promoter. Am J Physiol Renal Physiol 277: F634–F642, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem 255: 4745–4750, 1980. [PubMed] [Google Scholar]
- 42.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37: 1163–1167, 1988. [DOI] [PubMed] [Google Scholar]
- 43.Sutherland JP, McKinley B, Eckel RH. The metabolic syndrome and inflammation. Metab Syndr Relat Disord 2: 82–104, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi N, Li F, Hua K, Deng J, Wang CH, Bowers RR, Bartness TJ, Kim HS, Harp JB. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab 6: 506–512, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Todorov V, Muller M, Schweda F, Kurtz A. Tumor necrosis factor-α inhibits renin gene expression. Am J Physiol Regul Integr Comp Physiol 283: R1046–R1051, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr 100: 227–235, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes 51: 2929–2935, 2002. [DOI] [PubMed] [Google Scholar]