Abstract
Arginine vasopressin (AVP) is a nonapeptide expressed in several brain regions. In addition to its well-characterized role in osmoregulation, AVP regulates paternal behavior, aggression, circadian rhythms, and the stress response. In the bed nucleus of the stria terminalis (BST), AVP gene expression is tightly regulated by gonadal steroid hormones. However, the degree by which AVP is regulated by gonadal steroid hormones in the suprachiasmatic nucleus (SCN) and medial amygdala (MeA) is unclear. Previous studies have shown that AVP expression in the brain of gonadectomized rats is restored with testosterone, 17β-estradiol, and 5α-dihydrotestosterone (DHT) replacement. In addition, we have demonstrated that 3β-diol, a metabolite of DHT, increased AVP promoter activity in a neuronal cell line and that the effects of 3β-diol on AVP promoter activity were mediated by estrogen receptor-β. To test whether 3β-diol has a physiological role in the regulation of central AVP expression in vivo, we gonadectomized pre- and postpubertal male rats and followed with once daily injections of estradiol benzoate (EB), DHT-propionate, 3β-diol-dipropionate, or vehicle. The SCN, BST, and MeA were analyzed for AVP mRNA expression using in situ hybridization. In the BST, intact juveniles had significantly fewer AVP-expressing cells than adults. GDX abolished all AVP mRNA expression in the BST in both age groups, whereas treatment with EB restored >80% and DHTP <10% of the AVP expression. Interestingly, 3β-diol-proprionate was more effective at inducing AVP expression in juveniles than in adults, suggesting that the regulation of AVP by 3β-diol might be age dependent.
Keywords: 5α-androstane-3β,17β-diol
arginine vasopressin (AVP) is a neurohormone comprised of nine amino acids and is expressed in several brain regions, including the bed nucleus of the stria terminalis (BST), paraventricular nucleus (PVN), supraoptic nucleus (SON), suprachiasmatic nucleus (SCN), and medial amygdala (MeA). Neurons expressing AVP in the SON and magnocellular division of the PVN are involved primarily in osmoregulation. However, in addition to its well-characterized role in osmoregulation, AVP-expressing neurons located in the BST, SCN, MeA, and the parvocellular division of the PVN extend their axons to a variety of brain regions where AVP is released intracerebrally. These latter populations of AVP-expressing neurons are of particular interest because central AVP release has been shown to regulate social behaviors, aggressive behavior, circadian rhythms, and the stress response. Importantly, dendritic release of AVP by the magnocellular neurons of the PVN in response to an acute stressor does not affect circulating levels of AVP, suggesting that cellular targets locally situated within the central AVP network could mediate the biological effects of stress and anxiety. Moreover, projections from these areas are extensive and capable of mediating a variety of behaviors. For instance, AVP-expressing neurons in the BST and MeA project to limbic regions such as the lateral septum and hippocampus, which are associated with learning, memory, and aggression. Similarly, these regions also contain AVP-expressing neurons that project to several midbrain areas, including the dorsal raphe nucleus, which contains high populations of serotonergic neurons and is important for regulating motivation and mood.
Arginine vasopressin (AVP) mRNA and protein levels in the bed nucleus of the stria terminalis (BST) and medial amygdala (MeA) are tightly regulated by gonadal steroid hormones, with castration abolishing and testosterone replacement restoring AVP expression (7–9). These data suggest that neurons in the BST and MeA contain a unique complement of regulatory components, such as steroid hormone receptors or coactivator proteins, that renders them steroid sensitive. Previous work has demonstrated that the principal regulator of AVP in these steroid-responsive regions is the testosterone metabolite 17β-estradiol (E2). Following castration, treatment with E2 restores ∼90% of the AVP expression observed in a gonadally intact animal, whereas full restoration of expression is achieved only with the concomitant treatment of E2 and 5α-dihydrotestosterone (DHT) (8). Similarly, E2 stimulated AVP promoter activity in a neuronal cell line, and this effect was mediated mainly by estrogen receptor (ER)α (25). On the basis of these data, it was postulated that both androgen and estrogen receptors mediate the effect of testosterone on AVP gene expression, since DHT exhibits ligand specificity for androgen receptors and E2 is specific for ERs.
It is well known that testosterone is converted intracellularly to E2 and DHT and that both of these metabolites are critical for maintaining a host of normal physiological functions. However, it is less well recognized that DHT is further metabolized into compounds that are functionally distinct from DHT and which are biologically significant. Of particular importance is the metabolite 5α-androstane-3β,17β-diol (3β-diol; see Fig. 1). The conversion of 3β-diol from DHT is accomplished by the steroid-metabolizing enzymes 17β-hydroxysteroid dehydrogenase and 3β-hydroxysteroid oxidoreductase (26, 28), both of which are widely expressed in many brain regions, including the hypothalamus (17). This raises the possibility that the observed actions of DHT are a result of its intracellular conversion to 3β-diol. Unlike its parent compound DHT, 3β-diol preferentially binds and activates ERβ and has a very low binding affinity for androgen receptors (33). Previously, our laboratory showed that 3β-diol stimulated AVP promoter activity in a neuronal cell line and that the effect was mediated by ERβ (19). Moreover, we showed that androgen receptors did not mediate DHT-induced stimulation of the AVP promoter (19). Taken together, these data suggest the possibility that ERs might mediate all of the observed effects of gonadal steroid hormones on AVP gene expression in the bed BST and MeA.
Fig. 1.
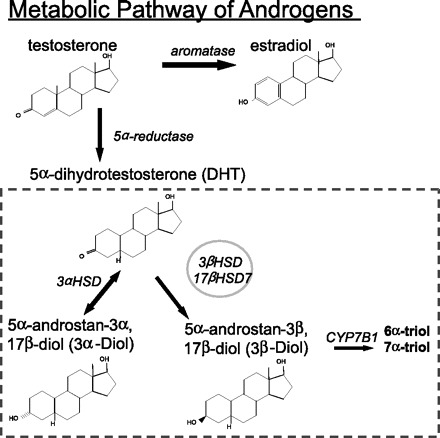
Diagram depicting the metabolic pathway of testosterone. Enzymes are shown in italics. Double-sided arrow illustrates the bidirectional catalysis of 5α-dihydrotestosterone (DHT) to 5α-androstane-3α,17β-diol (3α-diol). 3αHSD, 3α-hydroxysteroid oxidoreductase; 17βHSD7, 17β-hydroxysteroid dehydrogenase; 6α-triol, 5α-androstane-3β,6α-17β-triol; 7α-triol, 5α-androstane-3β,7α,17β-triol; CYP7B1, cytochrome P450, family 7, subfamily B, polypeptide 1.
In this study, we aimed to address whether 1) peripheral administration of 3β-diol to gonadectomized males would restore AVP expression in the steroid-responsive brain regions of the BST or MeA, 2) AVP expression was differentially affected by 3β-diol in pre- vs. postpubertal males due to their dramatically different circulating gonadal steroid hormone levels, and 3) AVP expression in the suprachiasmatic nucleus (SCN), a brain region in which the AVP response to gonadal hormones is unclear, was affected by 3β-diol treatment. Cumulatively, our data showed that AVP expression in the BST and MeA is differentially regulated by gonadal steroid hormones and 3β-diol; however, there is no effect in the SCN. Furthermore, these effects are dependent on age and specific brain region.
EXPERIMENTAL PROCEDURES
Animals.
Male Sprague-Dawley rats at 21 or 70 days of age were purchased from Harlan Laboratories. Animals were acclimated for 5 days and then orchidectomized (n = 48) or sham operated (n = 12). Hormone treatments began 1 day following orchidectomy. Animals were given food and water ad libitum. All procedures were approved by Colorado State University Animal Care and Use Committee.
Hormone treatments.
Animals were given a subcutaneous injection of vehicle (safflower oil), E2 benzoate (EB; 40 μg/kg), dihydrotestosterone proprionate (DHTP; 2.0 mg/kg), or 3β-diol proprionate (3β-diol-P; 5.0 mg/kg). These doses have been shown in previous studies to result in average circulating plasma levels of 32 pg/ml for E2 and 250 pg/ml for DHT (4). The average circulating plasma levels achieved with 3β-diol-P treatment is unknown; however, the dose provided was estimated on the basis of our previous studies in the mouse (18). Animals were injected once daily for 6 consecutive days. Animals were euthanized by rapid decapitation on day 7. Brains were removed, snap-frozen in isopentane, and stored at −80°C until they were processed by in situ hybridization.
In situ hybridization.
AVP mRNA was detected by in situ hybridization, as described previously (34). Briefly, a 33-bp oligonucleotide targeted to AVP exon 1 was end-labeled with [35S]dATP using terminal deoxynucleotidyl transferase. Brain tissue sections (16 μm) were fixed, dehydrated in graded ethanols, air-dried, hybridized (radiolabeled probe = 2 × 107 counts·min−1·ml−1), and incubated overnight at 40°C. Slides were emulsion-coated with Kodak NTB-2 and exposed for 7 days. Following exposure, slides were counterstained using cresyl violet.
Image analysis.
The total number of cells containing silver grains was counted (MeA and BST) using Image-Pro Plus software (Media Cybernetics). Cells were considered positive for AVP when the number of grains on top of the cell was three times higher than background. The entire BST and MeA were counted and sections atlas-matched (20). In the SCN, sections were atlas-matched, and density of grains covering the entire nucleus was measured and quantified relative to background density.
Statistics.
Data are presented as means ± SE. Data were analyzed by two-way ANOVA, followed by Tukey's post hoc test. Data were considered significant when P < 0.05. An asterisk represents significant differences within groups.
RESULTS
Restoration of AVP expression in the BST.
Intact juvenile rats had a significantly overall lower number of AVP-expressing cells in the BST than adult animals (Figs. 2A and 3). Consistent with results from previous studies, AVP expression in the BST was completely abolished by gonadectomy in both adult and juvenile rats (Fig. 2A). Treatment with EB restored the number of AVP-expressing cells in the BST to that of gonadally intact control animals in both age groups, validating that the provided dose was within the physiological range for both age groups (Fig. 2A). Although there was a trend for EB to restore a greater number of AVP-expressing cells in juvenile vs. adult animals (Table 1), it was not statistically significant. Interestingly, DHTP was significantly better at restoring AVP expression in the adult BST (16.7% restored) compared with juveniles (7.7% restored) (Fig. 2A and Table 1). However, this effect was opposite in animals treated with 3β-diol-P. Adult animals treated with 3β-diol-P had virtually no AVP expression in the BST (2.4% restored), whereas AVP expression in juvenile animals was restored by ∼30% (Fig. 2A and Table 1).
Fig. 2.
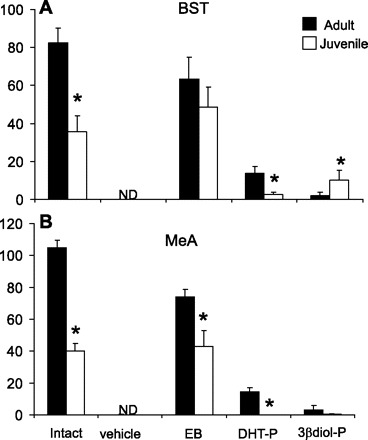
Effects of gonadal steroid hormones on total number of arginine vasopressin (AVP) mRNA-expressing cells in the bed nucleus of the stria terminalis (BST; A) and medial amygdala (MeA; B) of adult and juvenile rats. Rats were gonadally intact or gonadectomized and treated with vehicle (safflower oil), estradiol benzoate (EB), 5α-dihydrotestosterone proprionate (DHTP), or 3β-diol proprionate (3β-diol-P). ND, not detectable. *Significant difference within treatment groups (P < 0.05).
Fig. 3.
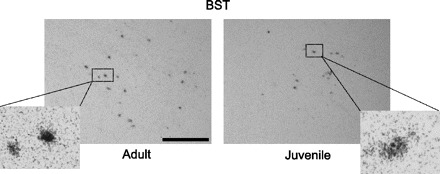
Photomicrographs showing representative regions of AVP mRNA-expressing cells in the BST in gonadally intact adult (left) and juvenile (right) rats. Cells were considered positive for AVP when the number of silver grains on top of the cells was 3 times higher than background. Insets show individual cells at higher magnification (×40). Scale bar, 10 μm.
Table 1.
%AVP expression in the BST restored by hormone treatment following orchidectomy compared with gonadally intact vehicle-treated animals (100%)
| BST | EB | DHTP | 3β-Diol-P |
|---|---|---|---|
| Adult | 77.2% | 16.7% | 2.4% |
| Juvenile | 136.5% | 7.7% | 28.7% |
AVP, arginine vasopressin; BST, bed nucleus of the stria terminalis; EB, estradiol benzoate; DHTP, dehydrotestosterone proprionate; 3β-diol-P, 3β-diol proprionate.
Restoration of AVP expression in the MeA.
Similar to the BST, gonadally intact adult rats had significantly higher numbers of AVP-expressing cells than that of juveniles, and gonadectomy abolished all expression (Figs. 2B and 4). Although EB restored the level of AVP mRNA in juvenile animals to >100% of intact controls, the total number was still significantly lower than in adult animals treated with EB (Fig. 2B and Table 2). Consistent with previous reports, EB treatment did not fully restore AVP expression in the MeA of adult animals (70.5% restored; Fig. 2B and Table 2). Unlike the results in the BST, treatment with DHTP had no effect on AVP expression in juvenile animals but did restore some expression in adults (0.0 vs. 13.8%, respectively; Fig. 2B and Table 2). Treatment with 3β-diol-P was equally efficacious at increasing AVP expression in adult MeA as it was in the BST (2.4 vs. 2.9%; Fig. 2, A and B, and Tables 1 and 2). By stark contrast to the results obtained in the BST, 3β-diol-P was virtually ineffective at restoring AVP expression in the MeA of juvenile animals (Fig. 2, A and B, and Tables 1 and 2).
Fig. 4.
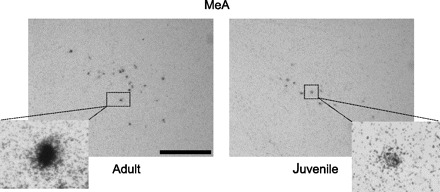
Photomicrographs showing representative regions of AVP mRNA-expressing cells in the MeA in gonadally intact adult (left) and juvenile (right) rats. Cells were considered positive for AVP when the number of silver grains on top of the cells was 3 times higher than background. Insets show individual cells at higher magnification (×40). Scale bar, 10 μm.
Table 2.
%AVP expression in the MeA restored by hormone treatment following orchidectomy compared with gonadally intact vehicle-treated animals (100%)
| MeA | EB | DHTP | 3β-Diol-P |
|---|---|---|---|
| Adult | 70.5% | 13.8% | 2.9% |
| Juvenile | 107.5% | 0.0% | 1.3% |
MeA, medial amygdala.
Effects of gonadal steroid hormones on AVP expression in the SCN.
There is very little published data describing the effects of gonadal steroid hormones on AVP expression in the SCN of adult animals and no data in prepubertal animals. Therefore, we analyzed the extent of AVP expression in the SCN by densitometry. Qualitatively, the degree of AVP expression was much greater in the SCN than in the BST or MeA, such that it was not possible to accurately count individual AVP-expressing cells. Interestingly, there were no significant differences between pre- and postpubertal gonadally intact animals (Fig. 5). Furthermore, gonadectomy did not affect the relative density, suggesting that AVP expression was unchanged (Fig. 5). Consistent with these results, treatment with EB, DHTP, and 3β-diol-P had no effect on AVP expression in either age group (Fig. 5). Overall, these data suggest that AVP expression in the SCN is not regulated by gonadal steroid hormones at any age.
Fig. 5.
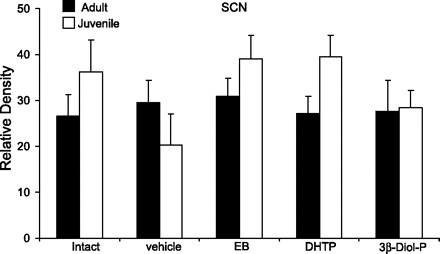
Effects of gonadal steroid hormones on the relative density of silver grains representative of AVP mRNA-expressing cells in the suprachiasmatic nucleus (SCN) of adult and juvenile rats. Rats were gonadally intact or gonadectomized and treated with vehicle (safflower oil), EB, DHTP, or 3β-diol-P.
DISCUSSION
These data have revealed important novel information about the in vivo regulation of AVP mRNA during the pubertal transition of male rats. First, we have shown that there are significantly more AVP-expressing cells in the BST and MeA of adult vs. juvenile animals, which is not evident in the SCN. These data indicate that AVP in the SCN is not regulated by gonadal steroid hormones, which raises the interesting question of what the primary regulator of AVP is in the SCN given that it is not responsive to changes in plasma osmolality (6). Furthermore, these data suggest that the observed age effect of AVP expression in the BST and MeA is likely due to the lower levels of circulating gonadal steroid hormones in prepubertal animals. Second, we have shown that the testosterone metabolite DHT was more effective at restoring AVP expression in the BST of adult vs. juvenile animals. One possibility is that juvenile animals are more sensitive than adults are to the inhibitory actions of DHT. This interpretation is in accord with the long-held hypothesis that the reproductive system of prepubertal animals is more sensitive to the negative feedback actions of testosterone, thereby preventing an inappropriate precocious maturation of adult reproductive function (23). Moreover, 3β-diol was also more effective at restoring AVP expression in the BST of juvenile animals, suggesting that the intracellular conversion of DHT to 3β-diol in this region might be higher in pre- vs. postpubertal animals. Finally, we have demonstrated that the BST and MeA are differentially regulated by gonadal steroid hormones. In the MeA, prepubertal animals were not responsive to the restorative effects of DHT or 3β-diol, unlike the adult animals.
The androgen precursor DHT is converted intracellularly by a variety of steroid-metabolizing enzymes, including 17β-hydroxysteroid dehydrogenase, 3α-hydroxysteroid oxidoreductase, and 3β-hydroxysteroid oxidoreductase (26, 28). These enzymes are ubiquitously expressed in many regions of the rat brain throughout development, including the cortex, hypothalamus, thalamus, septum, hippocampus, and cerebellum (12, 14, 21), raising the possibility that 3β-diol is an important central regulator for many systems. Interestingly, our data showed that there was a regional difference in prepubertal animals, with 3β-diol restoring nearly 30% of AVP expression in the BST, but very little in the MeA, which could be indicative of differences in enzyme activity in those regions.
Consistent with the earlier work of De Vries and colleagues (7, 8) and Wang and De Vries (32), we showed that EB restored nearly 80% of AVP expression in the BST and MeA of castrated adult animals. However, in their study DHTP failed to restore AVP expression when given alone, yet it provided an augmenting effect when combined with EB. By comparison, we have shown that DHTP treatment alone restored AVP expression in adult, but not juvenile, animals (Fig. 4 and Table 2). Overall, this suggests that the AVP gene is differentially regulated not just by gonadal steroid hormones but also by age. In agreement with this hypothesis, DHTP treatment of neonatal male rats produced a significant increase in the number of AVP-containing cells in the adult BST (13), demonstrating that circulating gonadal steroid hormones during early development play an organizational role in the adult expression patterns of AVP.
Our previous studies delineated a potential molecular mechanism for the actions of 3β-diol on the regulation of AVP. We showed that 3β-diol significantly increased AVP promoter activity and that the effect was mediated by ERβ (19). Moreover, in those studies 3β-diol was better at inducing ERβ-mediated AVP promoter activity than 17β-estradiol. On the basis of these in vitro data, we expected that 3β-diol would restore AVP expression in the BST and MeA of castrated male animals equivalently to that of its precursor hormone, DHT. However, in the MeA of adult animals, 3β-diol-P failed to restore AVP expression to the extent of DHTP, and it had no effect at all in juvenile animals. One possible explanation for these results is that our time to euthanization was too long following the last injection of 3β-diol (24 h), given that the metabolism of DHT and 3β-diol are very rapid (22). In our study, the animals were treated with a conjugated form of 3β-diol peripherally, which slows its metabolic degradation. We are confident that the dose provided (5.0 mg·kg−1·day−1) was sufficient to achieve a biological effect based on our previously reported findings (18). In that study, mice subcutaneously injected with 3β-diol (1.0 mg·kg−1·day−1) for 4 days had significantly reduced plasma corticosterone levels following a 30-min restraint stress. However, the precise circulating levels achieved in the current study and subsequent bioavailability of 3β-diol in the brain is unclear. Thus, there is a possibility that we missed the critical window of time necessary to detect peak effects of 3β-diol on AVP expression. Moreover, direct administration to specific brain regions, rather than peripheral injections, might have been more representative of the in situ actions of 3β-diol in the brain.
Another important consideration is whether the appropriate steroid hormone receptors that mediate the effects of 3β-diol are equivalently expressed in pre- and postpubertal animals. We have previously demonstrated that 3β-diol acts primarily through ERβ, and not ERα or androgen receptor, to regulate AVP promoter activity in vitro (19) and anxiety-related behaviors in vivo (17). In monkeys and rats, overall hypothalamic ERβ expression decreased in middle- and old-age animals when compared with young (2, 35). Moreover, the circadian variations in ERβ mRNA expression in the SCN are diminished in old rats (35). Thus, it is possible that ERβ mRNA or protein expression was lower in the postpubertal BST and MeA, leading to a lack of 3β-diol effect in the adult animals. However, an age-dependent variation of ERβ in the BST and MeA is contrary to our previous studies showing that 3β-diol administered directly to the paraventricular nucleus (PVN) decreased anxiety-related behaviors in adult animals.
The lack of effect of gonadal steroid hormones on AVP expression in the SCN was unexpected. Anatomically, AVP-expressing neurons in the SCN are uniquely situated to modulate a number of other steroid-responsive systems. Vasopressinergic neurons in the SCN convey circadian information to the PVN and other extrahypothalamic brain nuclei. Moreover, circadian expression of AVP in the SCN modulates the diurnal rhythm of circulating glucocorticoids, which is important for maintaining the normal physiological response to stress (15). Studies have also shown that AVP-expressing neurons form synaptic contacts with some gonadotropin-releasing hormone neurons in the preoptic area (27, 29, 30), although the biological significance remains to be determined.
Several lines of evidence have established that AVP is a critical central regulator of anxiety-related behaviors. Importantly, the pubertal transition is a time of dramatic changes in the hypothalamic-pituitary-adrenal response to stressful stimuli and the types of social behaviors exhibited. Cumulatively, our data suggest that the AVP system is differentially regulated by gonadal steroid hormones prior to pubertal onset, thereby providing some insight into the mechanisms behind pubertal alterations in hypothalamic-pituitary-adrenal function. Notably, these differences might reflect age-related changes in steroid-metabolizing enzyme activity in specific brain regions or, as in the case of the SCN, some as-yet-unidentified factor that contributes to the regulation of AVP gene expression.
GRANTS
This work was supported by US Public Health Service Grants RO1-NS-039951 (R. J. Handa) and F32-HD-046301 (T. R. Pak).
REFERENCES
- 1.Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci 17: 4895–4903, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao JZ, Ni CR, Zheng WQ. Age-related effects of estrogen on the expression of estrogen receptor alpha and beta mRNA in the ovariectomized monkey hypothalamus. Neurosci Bull 22: 97–102, 2006 [PubMed] [Google Scholar]
- 3.Blanchard RJ, Griebel G, Farrokhi C, Markham C, Yang M, Blanchard DC. AVP V1b selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacol Biochem Behav 80: 189–194, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Burgess LH, Handa RJ. Hormonal regulation of androgen receptor mRNA in the brain and anterior pituitary gland of the male rat. Brain Res Mol Brain Res 19: 31–38, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Caffe AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol 261: 237–252, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Carter DA, Murphy D. Cyclic nucleotide dynamics in the rat hypothalamus during osmotic stimulation: in vivo and in vitro studies. Brain Res 487: 350–356, 1989 [DOI] [PubMed] [Google Scholar]
- 7.de Vries GJ, Buijs RM, Sluiter AA. Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res 298: 141–145, 1984 [DOI] [PubMed] [Google Scholar]
- 8.De Vries GJ, Wang Z, Bullock NA, Numan S. Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the stria terminalis of rats. J Neurosci 14: 1789–1794, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeVries GJ, Buijs RM, Van Leeuwen FW, Caffe AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol 233: 236–254, 1985 [DOI] [PubMed] [Google Scholar]
- 10.Ebner K, Wotjak CT, Landgraf R, Engelmann M. Forced swimming triggers vasopressin release within the amygdala to modulate stress-coping strategies in rats. Eur J Neurosci 15: 384–388, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab 83: 2066–2073, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Guennoun R, Fiddes RJ, Gouézou M, Lombès M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Brain Res Mol Brain Res 30: 287–300, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Han TM, De Vries GJ. Organizational effects of testosterone, estradiol, and dihydrotestosterone on vasopressin mRNA expression in the bed nucleus of the stria terminalis. J Neurobiol 54: 502–510, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Ibanez C, Guennoun R, Liere P, Eychenne B, Pianos A, El-Etr M, Baulieu EE, Schumacher M. Developmental expression of genes involved in neurosteroidogenesis: 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase in the rat brain. Endocrinology 144: 2902–2911, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Isobe Y, Isobe M. Circadian rhythm of Arg-vasopressin contents in the suprachiasmatic nucleus in relation to corticosterone. Brain Res 800: 78–85, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Isobe Y, Nishino H. Signal transmission from the suprachiasmatic nucleus to the pineal gland via the paraventricular nucleus: analysed from arg-vasopressin peptide, rPer2 mRNA and AVP mRNA changes and pineal AA-NAT mRNA after the melatonin injection during light and dark periods. Brain Res 1013: 204–211, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci 26: 1448–1456, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett 365: 43–47, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Pak TR, Chung WC, Hinds LR, Handa RJ. Estrogen receptor-beta mediates dihydrotestosterone-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology 148: 3371–3382, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 1998
- 21.Pelletier G, Luu-The V, Labrie F. Immunocytochemical localization of type I 17 beta-hydroxysteroid dehydrogenase in the rat brain. Brain Res 704: 233–239, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Pirog EC, Collins DC. Metabolism of dihydrotestosterone in human liver: importance of 3alpha- and 3beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab 84: 3217–3221, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Reiter EO, Grumbach MM. Neuroendocrine control mechanisms and the onset of puberty. Annu Rev Physiol 44: 595–613, 1982 [DOI] [PubMed] [Google Scholar]
- 24.Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav 3: 20–26, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Shapiro RA, Xu C, Dorsa DM. Differential transcriptional regulation of rat vasopressin gene expression by estrogen receptor alpha and beta. Endocrinology 141: 4056–4064, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem 279: 10784–10795, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Thind KK, Boggan JE, Goldsmith PC. Interactions between vasopressin- and gonadotropin-releasing-hormone-containing neuroendocrine neurons in the monkey supraoptic nucleus. Neuroendocrinology 53: 287–297, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Torn S, Nokelainen P, Kurkela R, Pulkka A, Menjivar M, Ghosh S, Coca-Prados M, Peltoketo H, Isomaa V, Vihko P. Production, purification, and functional analysis of recombinant human and mouse 17beta-hydroxysteroid dehydrogenase type 7. Biochem Biophys Res Commun 305: 37–45, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol 384: 569–579, 1997 [DOI] [PubMed] [Google Scholar]
- 30.van der Beek EM, Wiegant VM, van Oudheusden HJ, van der Donk HA, van den Hurk R, Buijs RM. Synaptic contacts between gonadotropin-releasing hormone-containing fibers and neurons in the suprachiasmatic nucleus and perichiasmatic area: an anatomical substrate for feedback regulation? Brain Res 755: 101–111, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav 83: 319–328, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, De Vries GJ. Androgen and estrogen effects on vasopressin messenger RNA expression in the medial amygdaloid nucleus in male and female rats. J Neuroendocrinol 7: 827–831, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Weihua Z, Lathe R, Warner M, Gustafsson JA. An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci USA 99: 13589–13594, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson ME, Marshall MT, Bollnow MR, McGivern RF, Handa RJ. Gonadotropin-releasing hormone mRNA and gonadotropin beta-subunit mRNA expression in the adult female rat exposed to ethanol in utero. Alcohol Clin Exp Res 19: 1211–1218, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev 123: 593–601, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Wotjak CT, Naruo T, Muraoka S, Simchen R, Landgraf R, Engelmann M. Forced swimming stimulates the expression of vasopressin and oxytocin in magnocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci 13: 2273–2281, 2001 [DOI] [PubMed] [Google Scholar]


